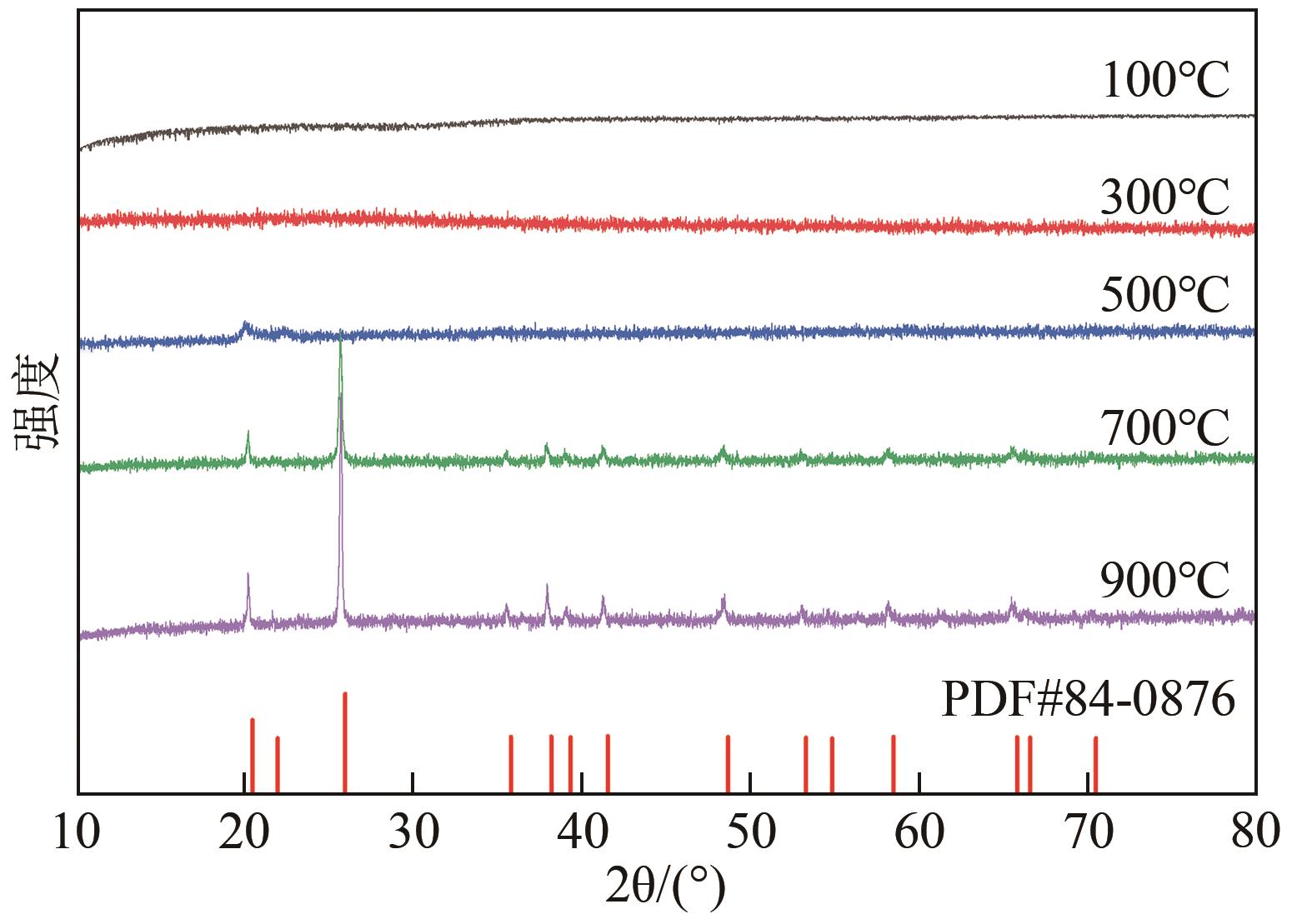
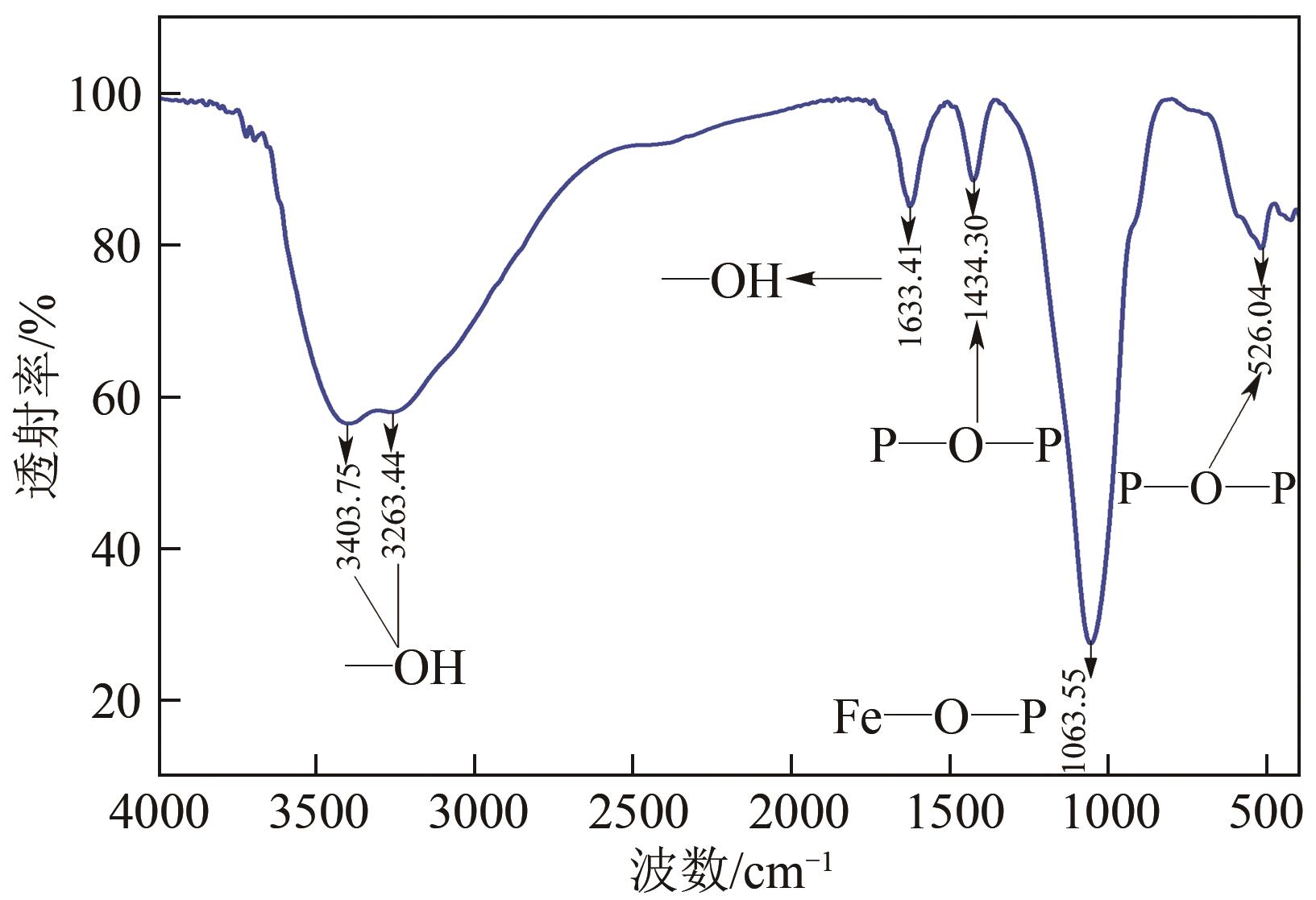
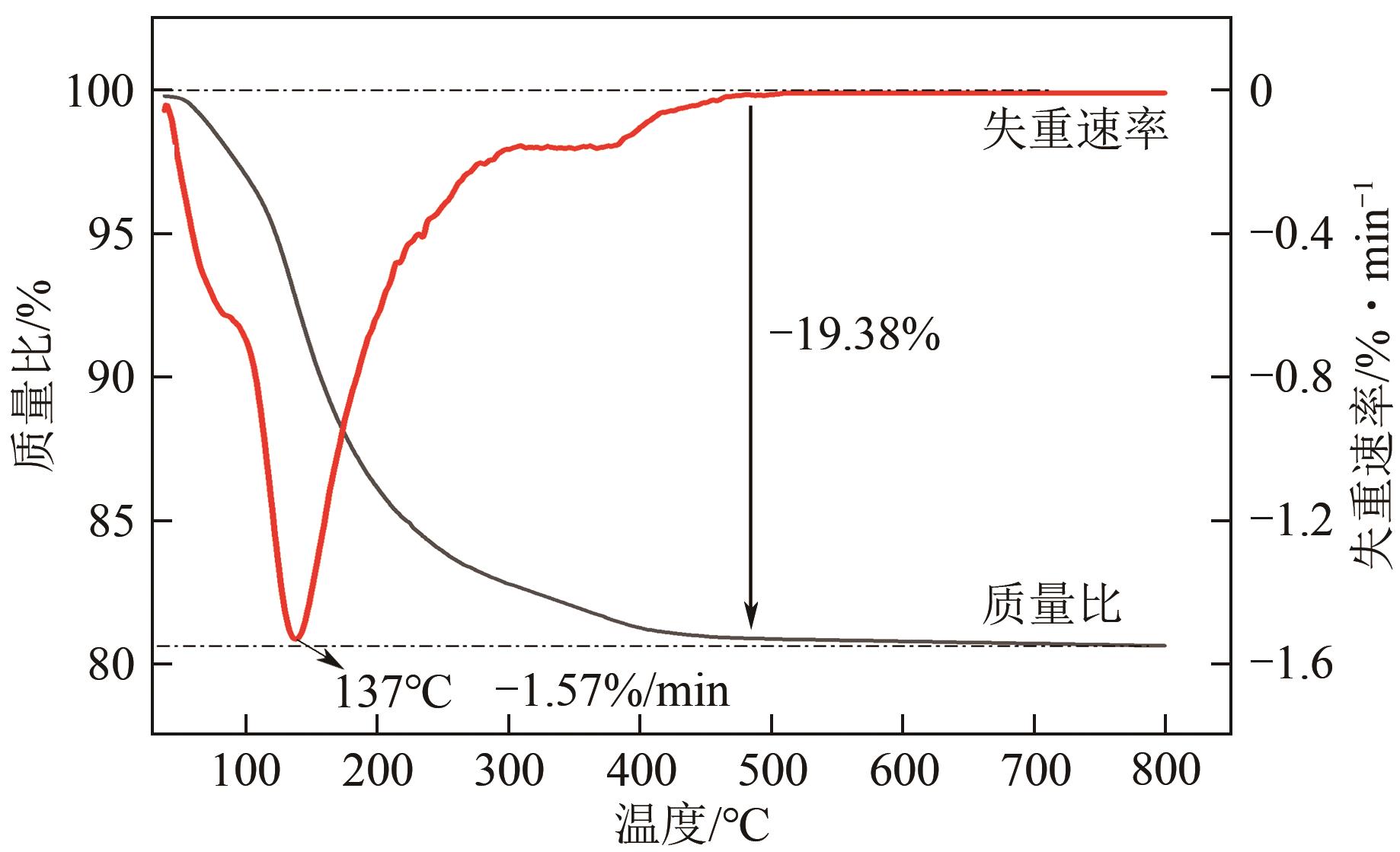
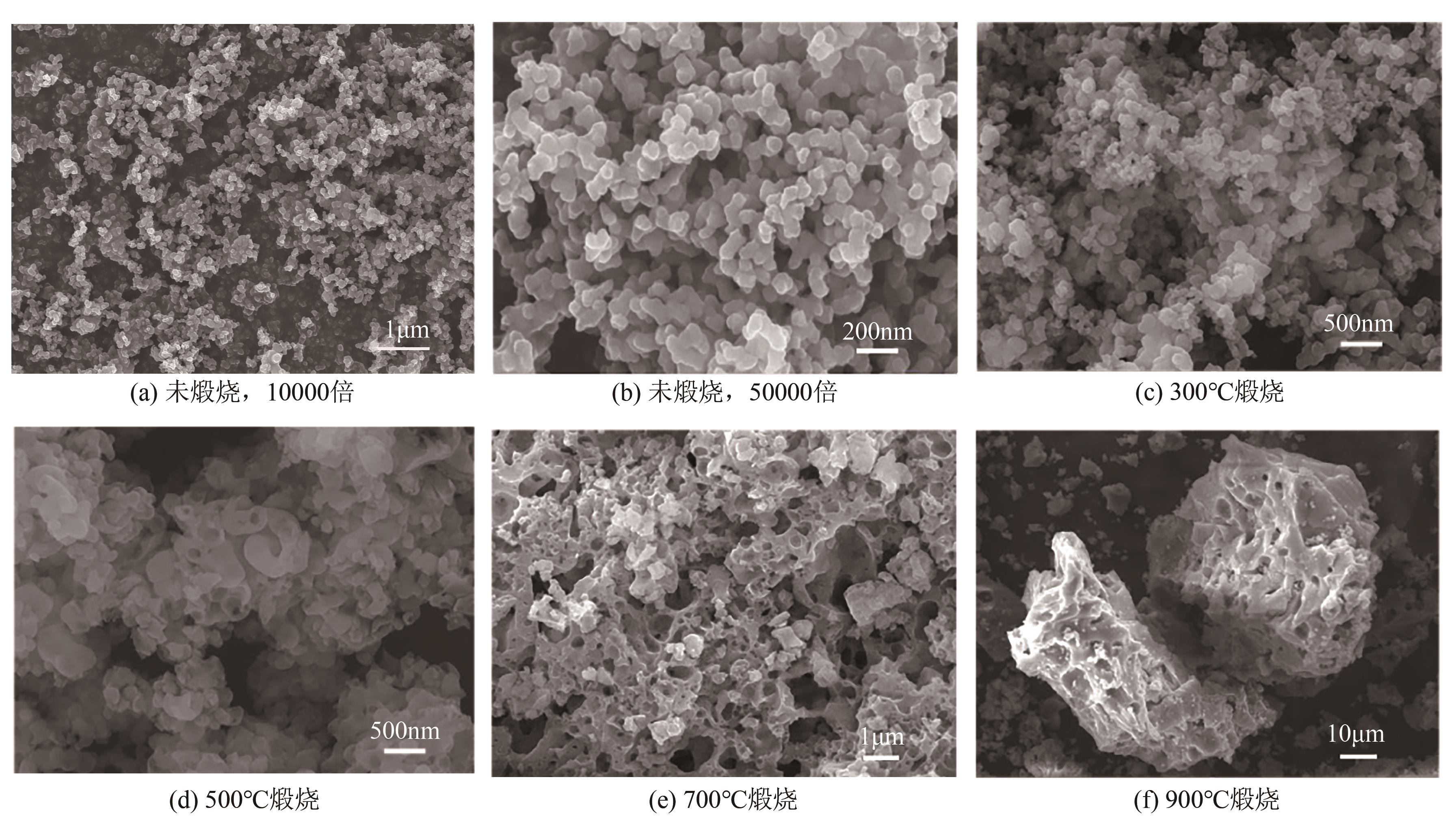
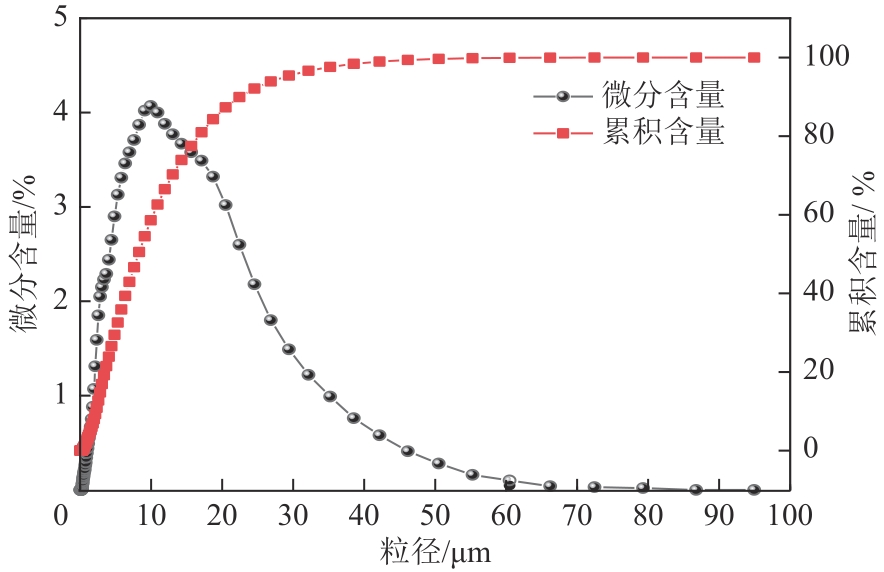
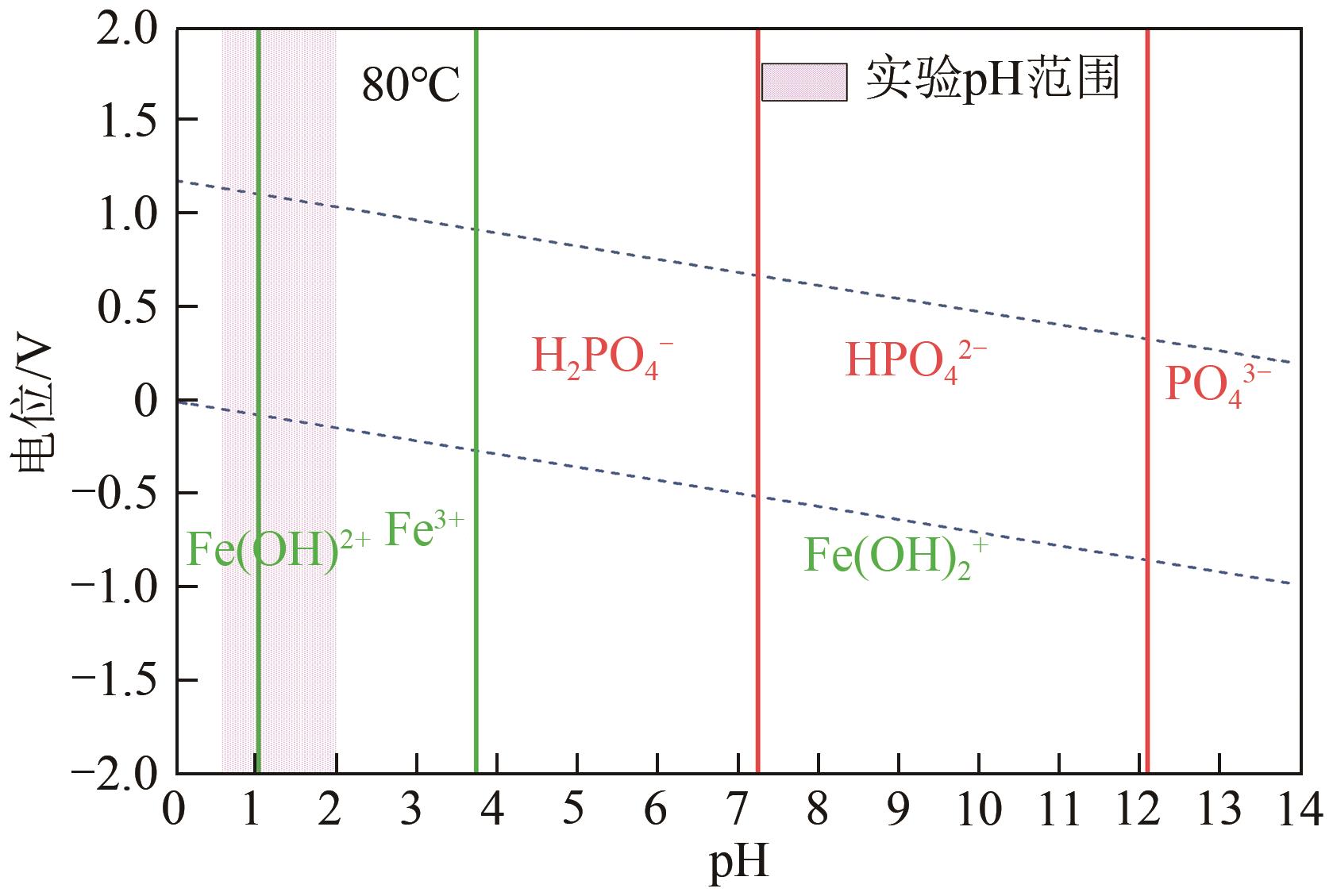
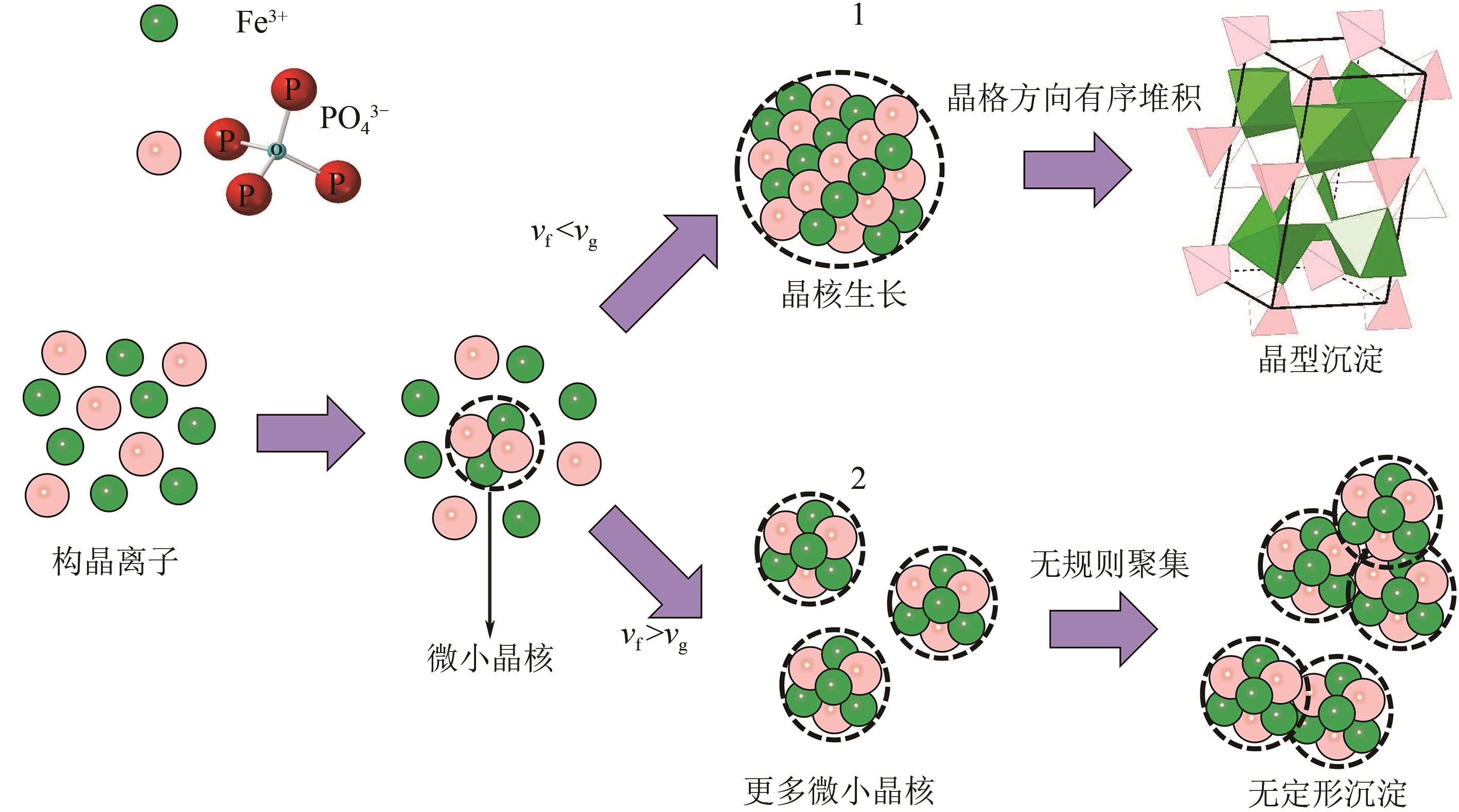
Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (8): 4523-4533.DOI: 10.16085/j.issn.1000-6613.2024-0152
• Materials science and technology • Previous Articles
Preparation of battery-grade iron phosphate using the by-product ferrous sulfate of titanium dioxide
LI Binde( ), WANG Bixia(
), WANG Bixia( ), YUAN Wenlong, DANG Xiao’e, MA Hongzhou
), YUAN Wenlong, DANG Xiao’e, MA Hongzhou
- School of Metallurgical Engineering, Xi’an University of Architecture and Technology, Xi’an 710055, Shaanxi, China
-
Received:2024-01-19Revised:2024-05-09Online:2024-09-02Published:2024-08-15 -
Contact:WANG Bixia
钛白副产硫酸亚铁制备电池级磷酸铁
- 西安建筑科技大学冶金工程学院,陕西 西安 710055
-
通讯作者:王碧侠 -
作者简介:李斌德(2000—),男,硕士研究生,研究方向为储能材料制备。E-mail:3010747603@qq.com。 -
基金资助:国家自然科学基金(51974221);陕西省自然科学基础研究项目(2021JM-374)
CLC Number:
Cite this article
LI Binde, WANG Bixia, YUAN Wenlong, DANG Xiao’e, MA Hongzhou. Preparation of battery-grade iron phosphate using the by-product ferrous sulfate of titanium dioxide[J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4523-4533.
李斌德, 王碧侠, 袁文龙, 党晓娥, 马红周. 钛白副产硫酸亚铁制备电池级磷酸铁[J]. 化工进展, 2024, 43(8): 4523-4533.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-0152
| 组成 | 质量分数/% |
|---|---|
| FeSO4·7H2O | ≥90 |
| Fe | 17.52 |
| Fe(Ⅲ) | 1.37 |
| Ti | 0.39 |
| Mg | 0.48 |
| Mn | 0.12 |
| Al | 0.0095 |
| 组成 | 质量分数/% |
|---|---|
| FeSO4·7H2O | ≥90 |
| Fe | 17.52 |
| Fe(Ⅲ) | 1.37 |
| Ti | 0.39 |
| Mg | 0.48 |
| Mn | 0.12 |
| Al | 0.0095 |
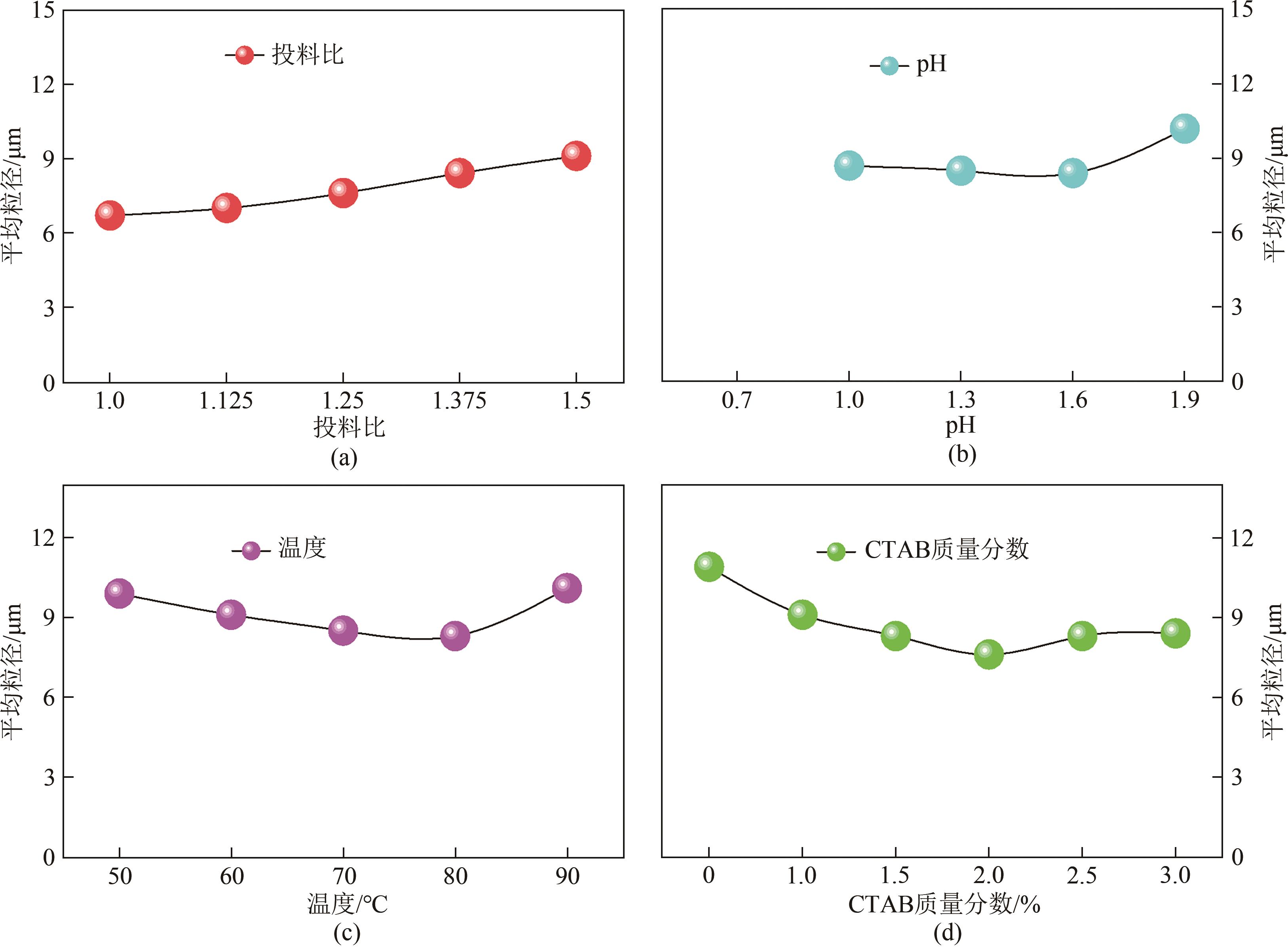
| 因素 | 代码 | 水平 | ||
|---|---|---|---|---|
| -1 | 0 | 1 | ||
| 铁磷投料比 | A | 1.28 | 1.33 | 1.38 |
| 温度/℃ | B | 75 | 80 | 85 |
| pH | C | 1.5 | 1.6 | 1.7 |
| 因素 | 代码 | 水平 | ||
|---|---|---|---|---|
| -1 | 0 | 1 | ||
| 铁磷投料比 | A | 1.28 | 1.33 | 1.38 |
| 温度/℃ | B | 75 | 80 | 85 |
| pH | C | 1.5 | 1.6 | 1.7 |
| 序号 | 铁磷投料比 | pH | 温度/℃ | Fe/% | P/% | Fe/P | 产率/% |
|---|---|---|---|---|---|---|---|
| 1 | 1.28 | 1.5 | 80 | 28.62 | 16.78 | 0.9457 | 86.34 |
| 2 | 1.38 | 1.6 | 75 | 29.73 | 16.06 | 1.0261 | 91.15 |
| 3 | 1.33 | 1.7 | 85 | 30.60 | 15.80 | 1.0738 | 93.65 |
| 4 | 1.33 | 1.6 | 80 | 29.79 | 16.59 | 0.9956 | 90.29 |
| 5 | 1.33 | 1.7 | 75 | 29.91 | 16.03 | 1.0348 | 91.86 |
| 6 | 1.33 | 1.5 | 85 | 30.14 | 16.24 | 1.0290 | 87.79 |
| 7 | 1.28 | 1.6 | 75 | 28.74 | 16.90 | 0.9432 | 87.30 |
| 8 | 1.33 | 1.6 | 80 | 29.68 | 16.41 | 1.0032 | 91.22 |
| 9 | 1.33 | 1.6 | 80 | 29.66 | 16.52 | 0.9955 | 91.43 |
| 10 | 1.33 | 1.5 | 75 | 29.55 | 16.47 | 0.9952 | 86.58 |
| 11 | 1.38 | 1.5 | 80 | 29.67 | 16.18 | 1.0169 | 90.93 |
| 12 | 1.28 | 1.7 | 80 | 29.90 | 16.56 | 1.0016 | 92.45 |
| 13 | 1.38 | 1.6 | 85 | 30.48 | 15.99 | 1.0574 | 92.04 |
| 14 | 1.28 | 1.6 | 85 | 29.69 | 16.69 | 0.9862 | 89.09 |
| 15 | 1.38 | 1.7 | 80 | 30.83 | 15.79 | 1.0825 | 93.68 |
| 序号 | 铁磷投料比 | pH | 温度/℃ | Fe/% | P/% | Fe/P | 产率/% |
|---|---|---|---|---|---|---|---|
| 1 | 1.28 | 1.5 | 80 | 28.62 | 16.78 | 0.9457 | 86.34 |
| 2 | 1.38 | 1.6 | 75 | 29.73 | 16.06 | 1.0261 | 91.15 |
| 3 | 1.33 | 1.7 | 85 | 30.60 | 15.80 | 1.0738 | 93.65 |
| 4 | 1.33 | 1.6 | 80 | 29.79 | 16.59 | 0.9956 | 90.29 |
| 5 | 1.33 | 1.7 | 75 | 29.91 | 16.03 | 1.0348 | 91.86 |
| 6 | 1.33 | 1.5 | 85 | 30.14 | 16.24 | 1.0290 | 87.79 |
| 7 | 1.28 | 1.6 | 75 | 28.74 | 16.90 | 0.9432 | 87.30 |
| 8 | 1.33 | 1.6 | 80 | 29.68 | 16.41 | 1.0032 | 91.22 |
| 9 | 1.33 | 1.6 | 80 | 29.66 | 16.52 | 0.9955 | 91.43 |
| 10 | 1.33 | 1.5 | 75 | 29.55 | 16.47 | 0.9952 | 86.58 |
| 11 | 1.38 | 1.5 | 80 | 29.67 | 16.18 | 1.0169 | 90.93 |
| 12 | 1.28 | 1.7 | 80 | 29.90 | 16.56 | 1.0016 | 92.45 |
| 13 | 1.38 | 1.6 | 85 | 30.48 | 15.99 | 1.0574 | 92.04 |
| 14 | 1.28 | 1.6 | 85 | 29.69 | 16.69 | 0.9862 | 89.09 |
| 15 | 1.38 | 1.7 | 80 | 30.83 | 15.79 | 1.0825 | 93.68 |
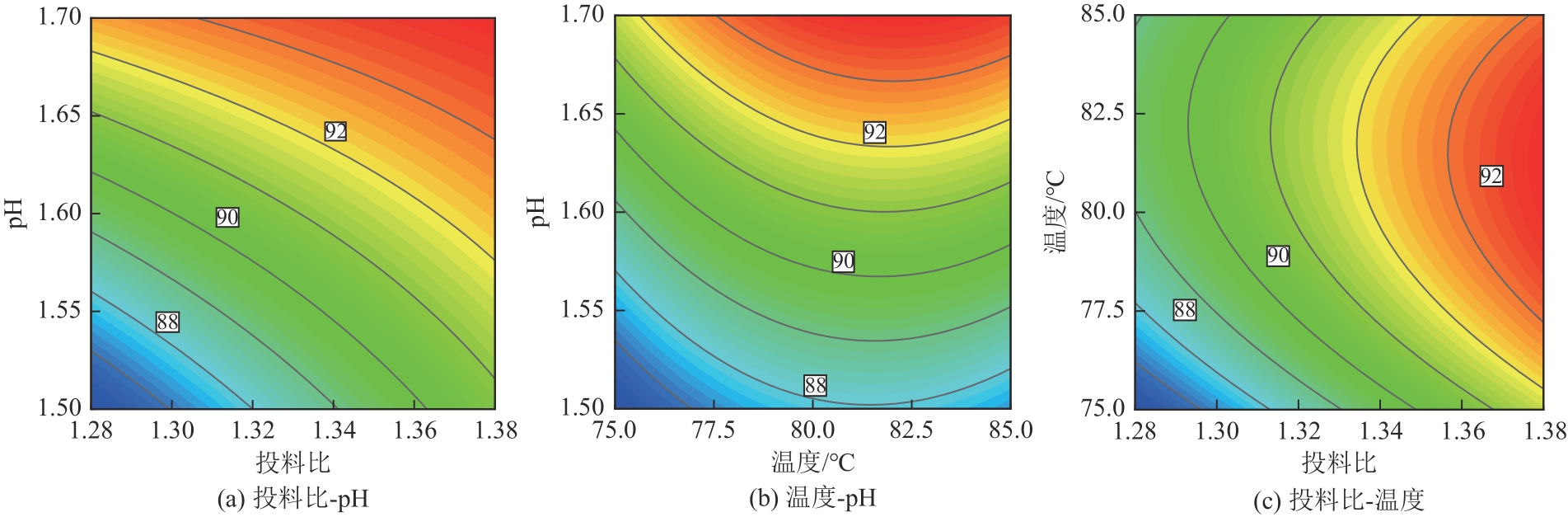
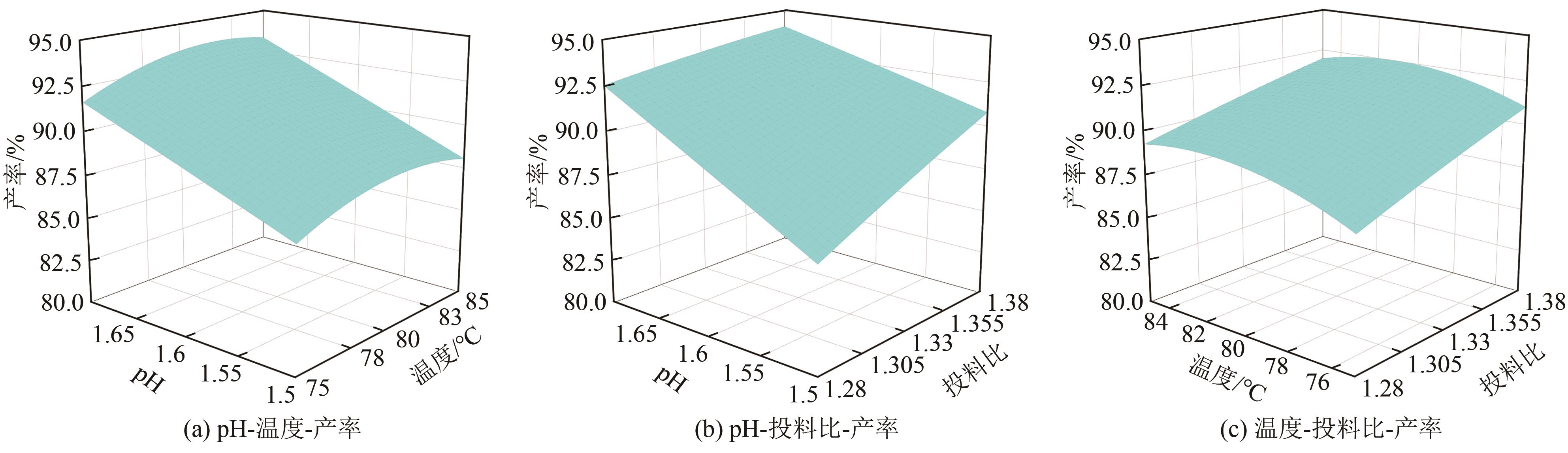
| 方差来源 | 平方和 | 自由度 | 均方和 | F | P | 显著性 |
|---|---|---|---|---|---|---|
| 模型 | 80.63 | 9 | 8.96 | 29.49 | 0.0008 | ★★ |
| A | 19.91 | 1 | 19.91 | 65.54 | 0.0005 | ★★ |
| B | 4.03 | 1 | 4.03 | 13.28 | 0.0148 | ★ |
| C | 50.00 | 1 | 50.00 | 164.60 | <0.0001 | ★★ |
| AB | 0.20 | 1 | 0.20 | 0.67 | 0.4514 | |
| AC | 2.82 | 1 | 2.82 | 9.29 | 0.0285 | ★ |
| BC | 0.084 | 1 | 0.084 | 0.28 | 0.6213 | |
| A2 | 0.039 | 1 | 0.039 | 0.13 | 0.7354 | |
| B2 | 3.56 | 1 | 3.56 | 11.73 | 0.0187 | ★ |
| C2 | 2.792×10-3 | 1 | 2.792×10-3 | 9.192×10-3 | 0.9273 | |
| 残差 | 1.52 | 5 | 0.30 | |||
| 失拟差 | 0.78 | 3 | 0.26 | 0.71 | 0.6301 | |
| 净误差 | 0.74 | 2 | 0.37 | |||
| 总误差 | 82.14 | 14 |
| 方差来源 | 平方和 | 自由度 | 均方和 | F | P | 显著性 |
|---|---|---|---|---|---|---|
| 模型 | 80.63 | 9 | 8.96 | 29.49 | 0.0008 | ★★ |
| A | 19.91 | 1 | 19.91 | 65.54 | 0.0005 | ★★ |
| B | 4.03 | 1 | 4.03 | 13.28 | 0.0148 | ★ |
| C | 50.00 | 1 | 50.00 | 164.60 | <0.0001 | ★★ |
| AB | 0.20 | 1 | 0.20 | 0.67 | 0.4514 | |
| AC | 2.82 | 1 | 2.82 | 9.29 | 0.0285 | ★ |
| BC | 0.084 | 1 | 0.084 | 0.28 | 0.6213 | |
| A2 | 0.039 | 1 | 0.039 | 0.13 | 0.7354 | |
| B2 | 3.56 | 1 | 3.56 | 11.73 | 0.0187 | ★ |
| C2 | 2.792×10-3 | 1 | 2.792×10-3 | 9.192×10-3 | 0.9273 | |
| 残差 | 1.52 | 5 | 0.30 | |||
| 失拟差 | 0.78 | 3 | 0.26 | 0.71 | 0.6301 | |
| 净误差 | 0.74 | 2 | 0.37 | |||
| 总误差 | 82.14 | 14 |
| 序号 | 铁磷投料比 | pH | 温度/℃ | 预测值/% | 实验值/% | 偏差/% |
|---|---|---|---|---|---|---|
| 1 | 1.33 | 1.6 | 80 | 90.98 | 91.22 | 0.24 |
| 2 | 1.33 | 1.6 | 80 | 90.98 | 90.29 | 0.69 |
| 3 | 1.33 | 1.6 | 80 | 90.98 | 91.43 | 0.45 |
| 序号 | 铁磷投料比 | pH | 温度/℃ | 预测值/% | 实验值/% | 偏差/% |
|---|---|---|---|---|---|---|
| 1 | 1.33 | 1.6 | 80 | 90.98 | 91.22 | 0.24 |
| 2 | 1.33 | 1.6 | 80 | 90.98 | 90.29 | 0.69 |
| 3 | 1.33 | 1.6 | 80 | 90.98 | 91.43 | 0.45 |
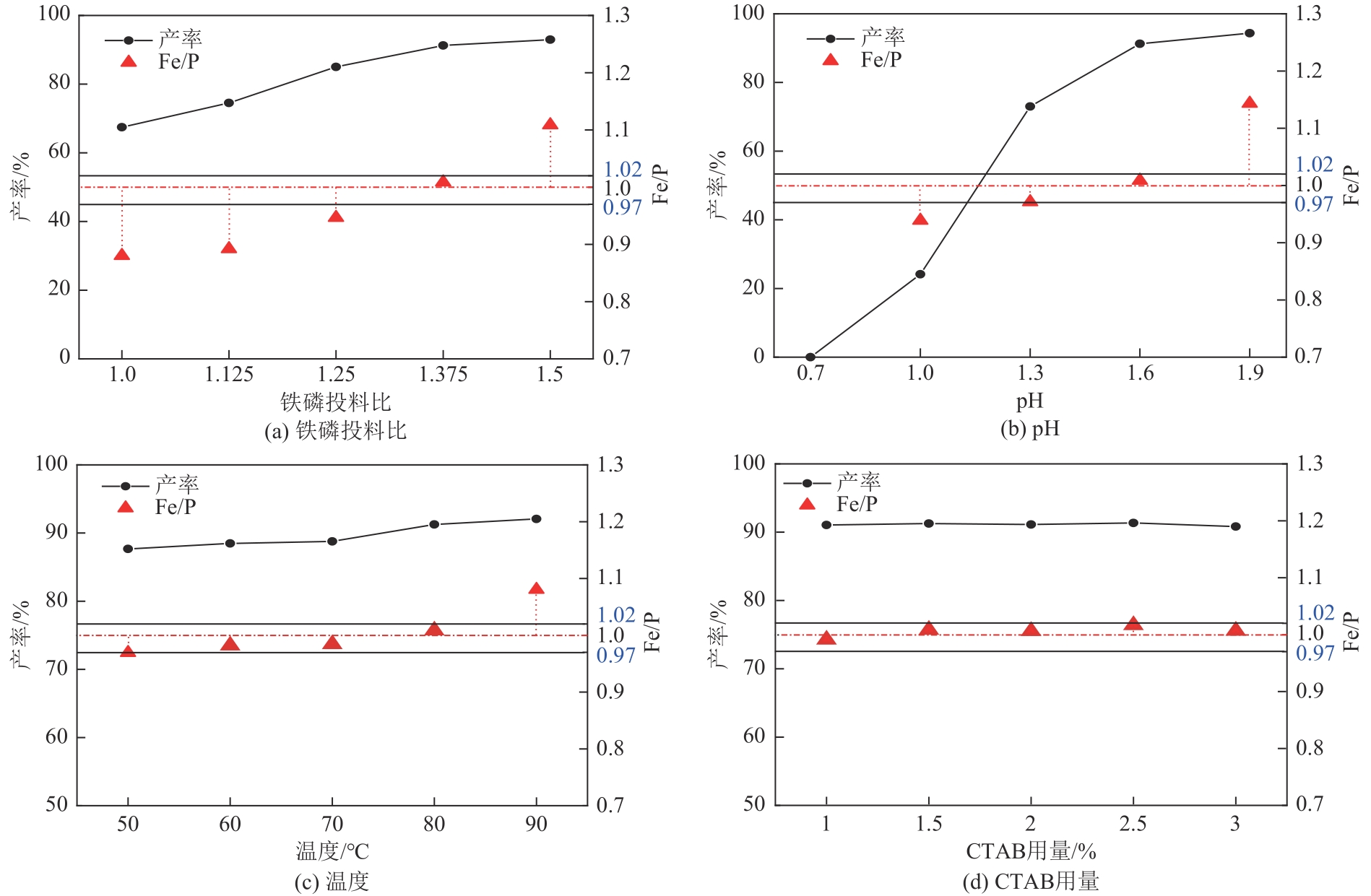
| 检验项目 | 电池级指标要求 | 产品检验结果 |
|---|---|---|
| Fe | 29.0~30.0 | 29.66 |
| P | 16.2~17.2 | 16.52 |
| Fe/P | 0.97~1.02 | 0.9955 |
| Ca | ≤0.005 | 0.0048 |
| Mg | ≤0.005 | 0.0039 |
| K | ≤0.01 | 0.0031 |
| Na | ≤0.01 | 0.001 |
| Cu | ≤0.005 | 0.00093 |
| Zn | ≤0.005 | 0.0038 |
| Ni | ≤0.005 | 0.00036 |
| Mn | ≤0.01 | 0.00033 |
| Ti | ≤0.005 | 0.00053 |
| Al | ≤0.005 | 0.0047 |
| SO | ≤0.01 | 0.0077 |
| Cl- | ≤0.01 | 未检出 |
| F- | ≤0.01 | 0.0055 |
| 检验项目 | 电池级指标要求 | 产品检验结果 |
|---|---|---|
| Fe | 29.0~30.0 | 29.66 |
| P | 16.2~17.2 | 16.52 |
| Fe/P | 0.97~1.02 | 0.9955 |
| Ca | ≤0.005 | 0.0048 |
| Mg | ≤0.005 | 0.0039 |
| K | ≤0.01 | 0.0031 |
| Na | ≤0.01 | 0.001 |
| Cu | ≤0.005 | 0.00093 |
| Zn | ≤0.005 | 0.0038 |
| Ni | ≤0.005 | 0.00036 |
| Mn | ≤0.01 | 0.00033 |
| Ti | ≤0.005 | 0.00053 |
| Al | ≤0.005 | 0.0047 |
| SO | ≤0.01 | 0.0077 |
| Cl- | ≤0.01 | 未检出 |
| F- | ≤0.01 | 0.0055 |
| 1 | ZUBI Ghassan, Rodolfo DUFO-LÓPEZ, CARVALHO Monica, et al. The lithium-ion battery: State of the art and future perspectives[J]. Renewable and Sustainable Energy Reviews, 2018, 89: 292-308. |
| 2 | ZHU Yongming, RUAN Zewen, TANG Shenzhi, et al. Research status in preparation of FePO4: A review[J]. Ionics, 2014, 20(11): 1501-1510. |
| 3 | LU Liming, JIANG Guoqiang, GU Chunyan, et al. Revisiting polyanionic LiFePO4 battery material for electric vehicles[J]. Functional Materials Letters, 2021, 14(4): 2130006. |
| 4 | GUO Ju, LIANG Chengbo, CAO Jianxin, et al. Synthesis and electrochemical performance of lithium iron phosphate/carbon composites based on controlling the secondary morphology of precursors[J]. International Journal of Hydrogen Energy, 2020, 45(58): 33016-33027. |
| 5 | LIU Yuanyuan, LIU Hao, ZHAO Xinxin, et al. Effect of spherical particle size on the electrochemical properties of lithium iron phosphate[J]. Journal of Wuhan University of Technology-Mater. Sci. Ed., 2019, 34(3): 549-557. |
| 6 | LETHOLE N L, CHAUKE H R, NGOEPE P E. Thermodynamic stability and pressure dependence of FePO4 polymorphs[J]. Computational and Theoretical Chemistry, 2019, 1155: 67-74. |
| 7 | ZHAO Xinyue, LUO Mingwu, PENG Kunyao, et al. Low-temperature synthesis of amorphous FePO4@rGO composites for cost-effective sodium-ion batteries[J]. ACS Applied Materials & Interfaces, 2021, 13(48): 57442-57450. |
| 8 | ALSAMET Mohammed A M M, BURGAZ Engin. Synthesis and characterization of nano-sized LiFePO4 by using consecutive combination of sol-gel and hydrothermal methods[J]. Electrochimica Acta, 2021, 367: 137530. |
| 9 | LU Yangcheng, ZHANG Tongbao, LIU Yang, et al. Preparation of FePO4 nano-particles by coupling fast precipitation in membrane dispersion microcontactor and hydrothermal treatment[J]. Chemical Engineering Journal, 2012, 210: 18-25. |
| 10 | SONG Haojie, SUN Yali, JIA Xiaohua. Hydrothermal synthesis of iron phosphate microspheres constructed by mesoporous polyhedral nanocrystals[J]. Materials Characterization, 2015, 107: 182-188. |
| 11 | ZHOU Wenzheng, LIU Chunying, WEN Zhangfan, et al. Effects of defect chemistry and kinetic behavior on electrochemical properties for hydrothermal synthesis of LiFePO4/C cathode materials[J]. Materials Chemistry and Physics, 2019, 227: 56-63. |
| 12 | YANG Shiliu, HU Mingjun, XI Liujiang, et al. Solvothermal synthesis of monodisperse LiFePO4 micro hollow spheres as high performance cathode material for lithium ion batteries[J]. ACS Applied Materials & Interfaces, 2013, 5(18): 8961-8967. |
| 13 | SMIRNOV K S, YASHTULOV N A, KUZ’MICHEVA G M, et al. Synthesis and electrochemical properties of lithium iron phosphate[J]. Russian Journal of Applied Chemistry, 2011, 84(10): 1744-1747. |
| 14 | ZHANG Xuekai, ZHOU Kanggen, ZENG Dewen, et al. Preparation of battery-grade FePO4·2H2O using the stripping solution generated from resource recycling of bauxite residue[J]. Bulletin of Environmental Contamination and Toxicology, 2022, 109(1): 86-94. |
| 15 | ZHANG Weiguang, ZHANG Tingan, CAI Liuliu, et al. Preparation of doped iron phosphate by selective precipitation of iron from titanium dioxide waste acid[J]. Metals, 2020, 10(6): 789. |
| 16 | WANG Xuan, WANG Xianyou, ZHANG Rui, et al. Hydrothermal preparation and performance of LiFePO4 by using Li3PO4 recovered from spent cathode scraps as Li source[J]. Waste Management, 2018, 78: 208-216. |
| 17 | LI Jianlong, WU Jinhua, LI Yi, et al. Facile strategies to utilize FeSO4·7H2O waste slag for LiFePO4/C cathode with high performances[J]. Journal of the Taiwan Institute of Chemical Engineers, 2019, 99: 74-81. |
| 18 | GUO Ju, FENG Yulong, MO Xinliang, et al. Preparation of LiFePO4 using iron(Ⅱ) sulfate as product from titanium dioxide slag purification process and its electrochemical properties[J]. International Journal of Electrochemical Science, 2021, 16(11): 211141. |
| 19 | GUO Ju, YU Mei, WU Fuyong. Preparation of high purity iron phosphate based on the advanced liquid-phase precipitation method and its enhanced properties[J]. Journal of Solid State Chemistry, 2020, 287: 121346. |
| 20 | WANG Zhongyu, LU Yangcheng. Facile construction of high-performance amorphous FePO4/carbon nanomaterials as cathodes of lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2019, 11(14): 13225-13233. |
| 21 | ZHANG Yi, YI Zhifeng, WANG Jingshi, et al. Sub-50nm amorphous iron phosphate dihydrate nanoplates fabricated via liquid exfoliation from recycled steelmaking phosphate slag[J]. Materials Letters, 2018, 233: 290-293. |
| 22 | LOU Wenbo, ZHANG Yang, ZHANG Ying, et al. A facile way to regenerate FePO4·2H2O precursor from spent lithium iron phosphate cathode powder: Spontaneous precipitation and phase transformation in an acidic medium[J]. Journal of Alloys and Compounds, 2021, 856: 158148. |
| 23 | 袁文龙. 钛白副产硫酸亚铁净化除杂制备磷酸铁的工艺研究[D]. 西安:西安建筑科技大学,2023. |
| YUAN Wenlong. Study on the process of preparing iron phosphate by purifying by-product ferrous sulfate of titanium dioxide[D]. Xi’an: Xi’an University of Architecture and Technology, 2023. | |
| 24 | LUNDAGER MADSEN H E. Redox process catalysed by growing crystal-strengite, FePO4·2H2O, crystallizing from solution with iron(Ⅱ) and hydroxylamine[J]. Journal of Crystal Growth, 2014, 401: 275-278. |
| 25 | 费文玲, 秦兰, 陈庆庆, 等. 响应曲面法优化千金藤素环糊精微球制备工艺研究[J]. 当代化工, 2022, 51(8): 1845-1849. |
| FEI Wenling, QIN Lan, CHEN Qingqing, et al. Optimization of preparation technology of cepharanthine cyclodextrin microspheres by response surface methodology[J]. Contemporary Chemical Industry, 2022, 51(8): 1845-1849. | |
| 26 | 苏勇杰, 张勇, 陈喆, 等. 圆片状超细二水磷酸铁的制备与表征[J]. 武汉工程大学学报, 2018, 40(1): 66-70. |
| SU Yongjie, ZHANG Yong, CHEN Zhe, et al. Preparation and characterization of ultrafine disc shaped ferric phosphate dihydrate[J]. Journal of Wuhan Institute of Technology, 2018, 40(1): 66-70. | |
| 27 | LI Yongqiang, ZHOU Yue, MA Wenlong, et al. Facile fabrication of the hybrid of amorphous FePO4·2H2O and GO toward high performance sodium-ion batteries[J]. Journal of Physics and Chemistry of Solids, 2023, 176: 111243. |
| 28 | 马晓玲, 吴田, 王梦. 纳米级FePO4·2H2O的制备与表征[J]. 广州化工, 2012,40(5):60-62. |
| MA Xiaoling, WU Tian, WANG Meng. Preparation and characterization of nano-sized FePO4·2H2O[J]. Guangzhou Chemical Industry, 2012,40(5):60-62. | |
| 29 | KU Jun H, Ji Heon RYU, KIM Sun Ha, et al. Reversible lithium storage with high mobility at structural defects in amorphous molybdenum dioxide electrode[J]. Advanced Functional Materials, 2012, 22(17): 3658-3664. |
| 30 | ZHAO Peizheng, LIU Hongbo, ZHENG Honghe, et al. Facile synthesis of FePO4·2H2O submicrometer-discs[J]. Materials Letters, 2014, 123: 128-130. |
| 31 | NIELSEN A E. Kinetics of precipitation[M]. UK: Oxford Pergamon Press, 1964: 350. |
| 32 | DIRKSEN J A, RING T A. Fundamentals of crystallization: Kinetic effects on particle size distributions and morphology[J]. Chemical Engineering Science, 1991, 46(10): 2389-2427. |
| 33 | 李文升, 樊勇利, 童书辉, 等. 高振密球形FePO4·xH2O的合成研究[J]. 电源技术, 2013, 37(6): 950-952. |
| LI Wensheng, FAN Yongli, TONG Shuhui, et al. Study on the synthesis of spherical FePO4·xH2O with high vibrational density[J]. Chinese Journal of Power Sources, 2013, 37(6): 950-952. |
| [1] | HU Feiyan, PENG Jiahuan, LI Heng, XU Zhaohua, SUN Ning. Preparation and sodium storage application of one-dimensional porous TiO2@carbon nanofibers composite [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1934-1943. |
| [2] | LIU Yang, WANG Yungang, XIU Haoran, ZOU Li, BAI Yanyuan. Optimal carbonization process of walnut shell based on dynamic analysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 94-103. |
| [3] | LI Huaquan, WANG Minghua, QIU Guibao. Behavior of sulfuric acid acidolysis of perovskite concentrates [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 536-541. |
| [4] | ZHAO Yao, ZHOU Zhihui, WU Hongdan, HU Chuanzhi, ZHANG Guochun, WU Ruipeng. Response surface analysis and optimization of membrane permeation vaporization by Silicalite-1 molecular sieve [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2586-2594. |
| [5] | HE Shanming, PAN Jiechang, XU Guozuan, LI Wenjun, LIANG Yong. Thermodynamic analysis and experimental verification of chromium and vanadium removal by ferrous salt precipitation from crude sodium tungstate solution [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2171-2179. |
| [6] | HUANG Zhiguo, SUN Zhigao. Preparation and properties of nano phase change microcapsules for heat storage [J]. Chemical Industry and Engineering Progress, 2023, 42(11): 5842-5851. |
| [7] | KONG Qian, SUN Jinchao, GE Jiaqi, ZHANG Peng, MA Yanlong, LIU Baijun. Effect of precipitant on the hydrocracking performance of NiW/TiO2-ASA catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 265-271. |
| [8] | QIU Qili, JIANG Xuguang. Application of municipal solid waste incineration fly ash in the field of pollutant control [J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3855-3864. |
| [9] | DUAN Boyan, NI Hui, LI Zhipeng, JIANG Zedong, ZHU Yanbing, LI Qingbiao. Process optimization and design of eliminating domestic agar phosphate precipitation [J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3824-3831. |
| [10] | CUI Weiyi, DING Guomin, TAN Naidi. Research progress on titanium dioxide based catalysts for catalytic oxidation of formaldehyde [J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6310-6318. |
| [11] | LI Shuai, LIU Mingyan, MA Yongli. Prediction of scaling location of fluid in geothermal well based on BP artificial neural network [J]. Chemical Industry and Engineering Progress, 2022, 41(11): 5761-5770. |
| [12] | WEN Qianmin, QIN Yongli, ZHENG Junjian, WEI Qiaoyan, ZHANG Yuanyuan, JIANG Yongrong. Research advances in the fixation of heavy metals in acid mine wastewater by sulfate reducing bacteria [J]. Chemical Industry and Engineering Progress, 2022, 41(10): 5578-5587. |
| [13] | SUN Tong, XU Dongdong, SONG Minhang, JIN Xing, HUANG Yun. Research progress of the burners in synthesis of TiO2 by combustion method [J]. Chemical Industry and Engineering Progress, 2022, 41(1): 17-29. |
| [14] | HU Nan, CHEN Lin, LI Huizhen, ZHANG Siyao, ZHANG Zhijun. Enrichment and recovery of engineered nanoparticle using flotation with intensified foam drainage [J]. Chemical Industry and Engineering Progress, 2022, 41(1): 485-492. |
| [15] | ZHANG Xuan, ZHENG Lijun. Process of single phase photocatalysts for hydrogen production [J]. Chemical Industry and Engineering Progress, 2021, 40(S1): 215-222. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||