Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (S1): 94-103.DOI: 10.16085/j.issn.1000-6613.2023-0809
• Chemical processes and equipment • Previous Articles Next Articles
Optimal carbonization process of walnut shell based on dynamic analysis
LIU Yang( ), WANG Yungang(
), WANG Yungang( ), XIU Haoran, ZOU Li, BAI Yanyuan
), XIU Haoran, ZOU Li, BAI Yanyuan
- Key Laboratory of Thermo-Fluid Science and Engineering (Ministry of Education), Xi'an Jiaotong University, Xi'an 710049, Shaanxi, China
-
Received:2023-05-15Revised:2023-06-21Online:2023-11-30Published:2023-10-25 -
Contact:WANG Yungang
基于动力学分析的核桃壳最佳炭化工艺
- 西安交通大学热流科学与工程教育部重点实验室,陕西 西安 710049
-
通讯作者:王云刚 -
作者简介:刘阳(1995—),男,博士研究生,研究方向为生物质高效利用等。E-mail:707187439@qq.com。 -
基金资助:国家重点研发计划(2021YFC3001803);陕西省重点研发计划(2018ZDXM-SF-033);王宽诚基金会项目
CLC Number:
Cite this article
LIU Yang, WANG Yungang, XIU Haoran, ZOU Li, BAI Yanyuan. Optimal carbonization process of walnut shell based on dynamic analysis[J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 94-103.
刘阳, 王云刚, 修浩然, 邹立, 白彦渊. 基于动力学分析的核桃壳最佳炭化工艺[J]. 化工进展, 2023, 42(S1): 94-103.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-0809
| 原料 | 工业分析(质量分数)/% | 元素分析(质量分数)/% | Qnet·ar/MJ·kg -1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aad | Vad | Mad | FCad | C | H | O | N | S | ||
| 核桃壳 | 0.28 | 70.73 | 7.77 | 21.22 | 48.4 | 6.18 | 44.29 | 0.33 | 0.01 | 17.44 |
| 原料 | 工业分析(质量分数)/% | 元素分析(质量分数)/% | Qnet·ar/MJ·kg -1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aad | Vad | Mad | FCad | C | H | O | N | S | ||
| 核桃壳 | 0.28 | 70.73 | 7.77 | 21.22 | 48.4 | 6.18 | 44.29 | 0.33 | 0.01 | 17.44 |
| 参数 | 数值 | |||
|---|---|---|---|---|
| 5℃·min -1 | 10℃·min -1 | 20℃·min -1 | 30℃·min -1 | |
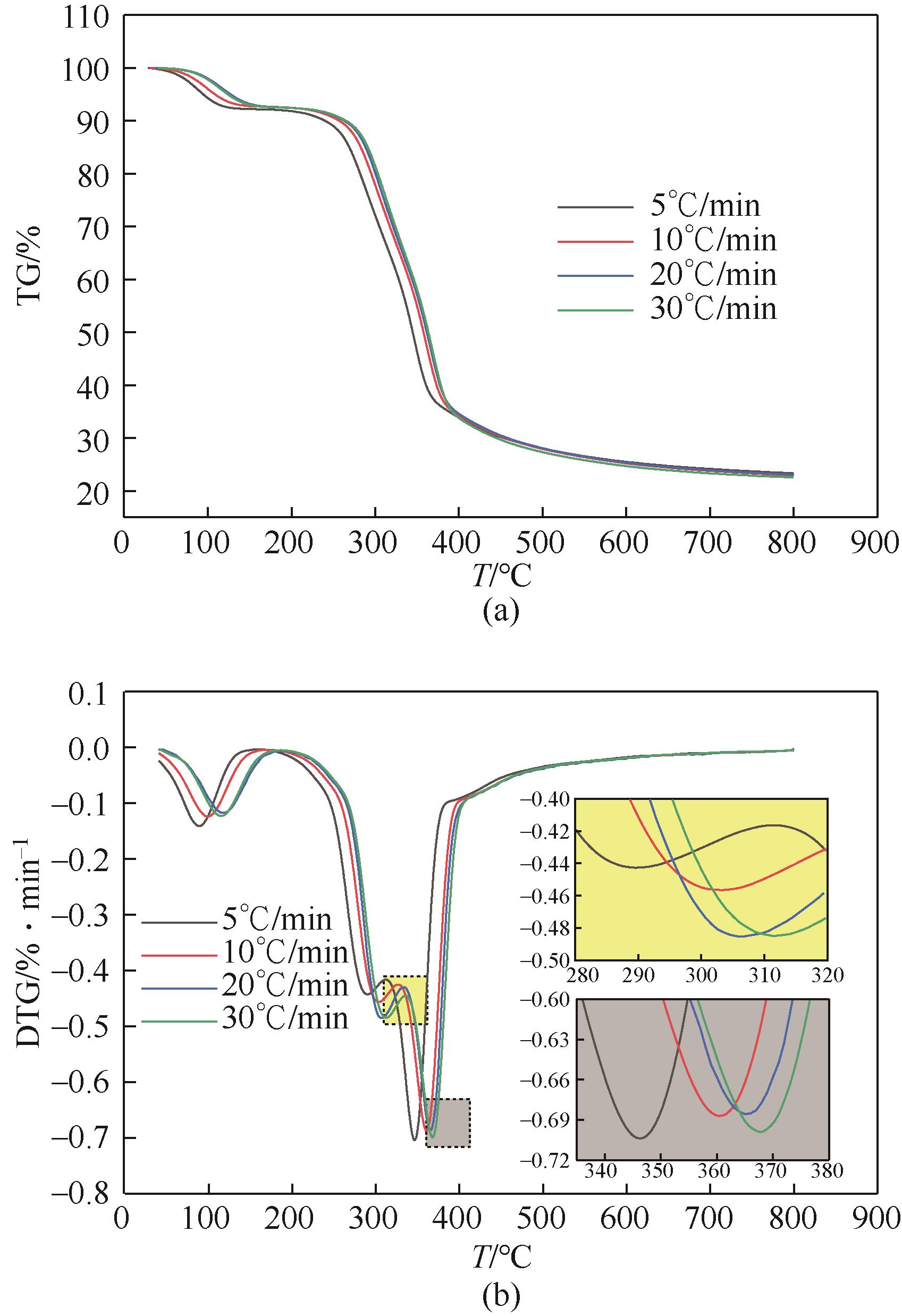
| Td/℃ | 228.5 | 247.8 | 259.2 | 267.3 |
| Te/℃ | 389.9 | 395.1 | 405.3 | 416.6 |
| TP/℃ | 346.3 | 361.7 | 365.2 | 367.7 |
| ΔWmax/% | 51.1 | 52.7 | 52.3 | 52.9 |
| η∞/% | 76.6 | 77.0 | 76.9 | 77.4 |
| (dW/dt)max/%·min -1 | 0.71 | 0.69 | 0.69 | 0.70 |
| (dW/dt)mean/%·min -1 | 0.1 | 0.1 | 0.1 | 0.1 |
| P/10 -5%3·min -2·℃ -1 | 9.8 | 10.1 | 9.5 | 9.3 |
| 参数 | 数值 | |||
|---|---|---|---|---|
| 5℃·min -1 | 10℃·min -1 | 20℃·min -1 | 30℃·min -1 | |
| Td/℃ | 228.5 | 247.8 | 259.2 | 267.3 |
| Te/℃ | 389.9 | 395.1 | 405.3 | 416.6 |
| TP/℃ | 346.3 | 361.7 | 365.2 | 367.7 |
| ΔWmax/% | 51.1 | 52.7 | 52.3 | 52.9 |
| η∞/% | 76.6 | 77.0 | 76.9 | 77.4 |
| (dW/dt)max/%·min -1 | 0.71 | 0.69 | 0.69 | 0.70 |
| (dW/dt)mean/%·min -1 | 0.1 | 0.1 | 0.1 | 0.1 |
| P/10 -5%3·min -2·℃ -1 | 9.8 | 10.1 | 9.5 | 9.3 |
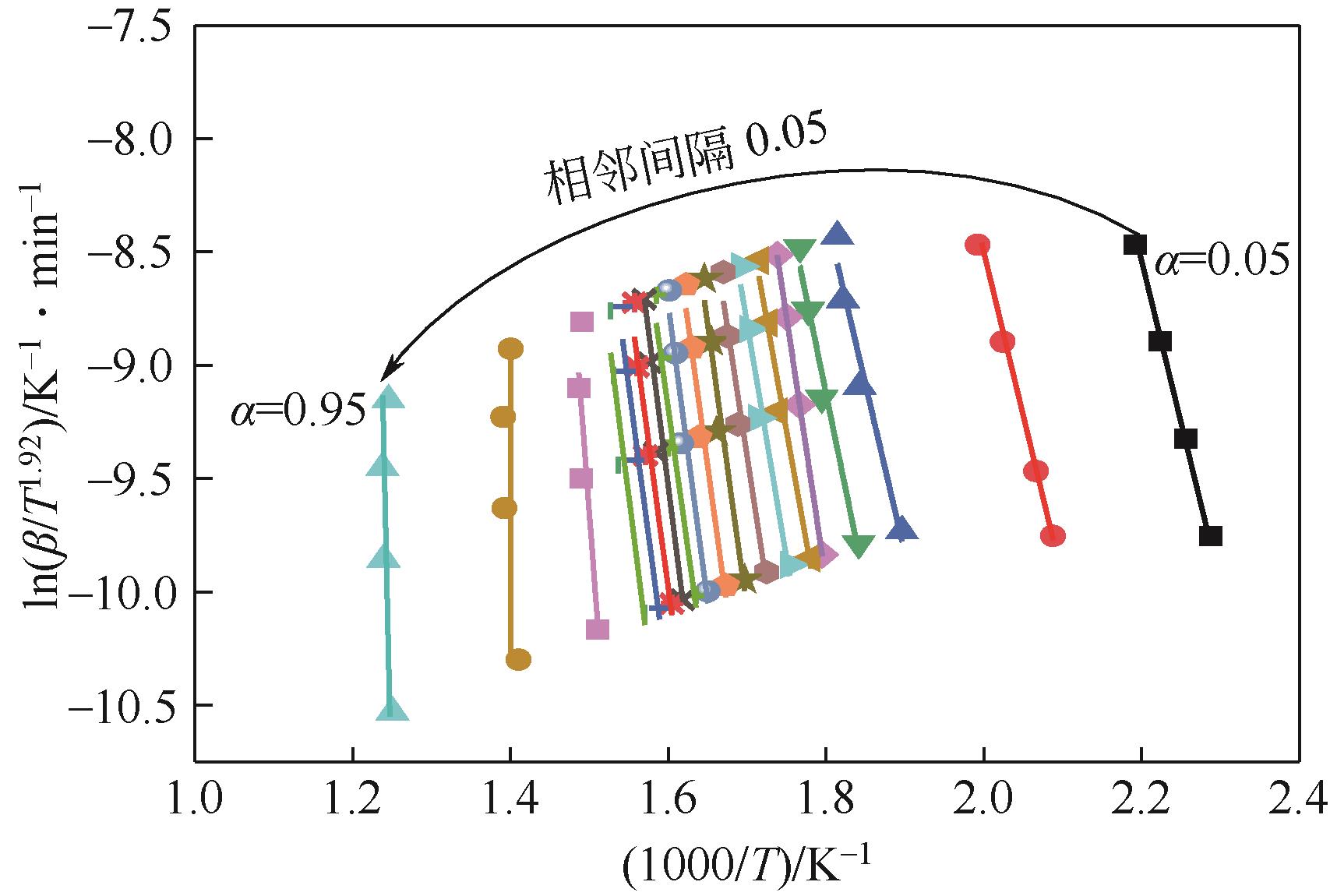
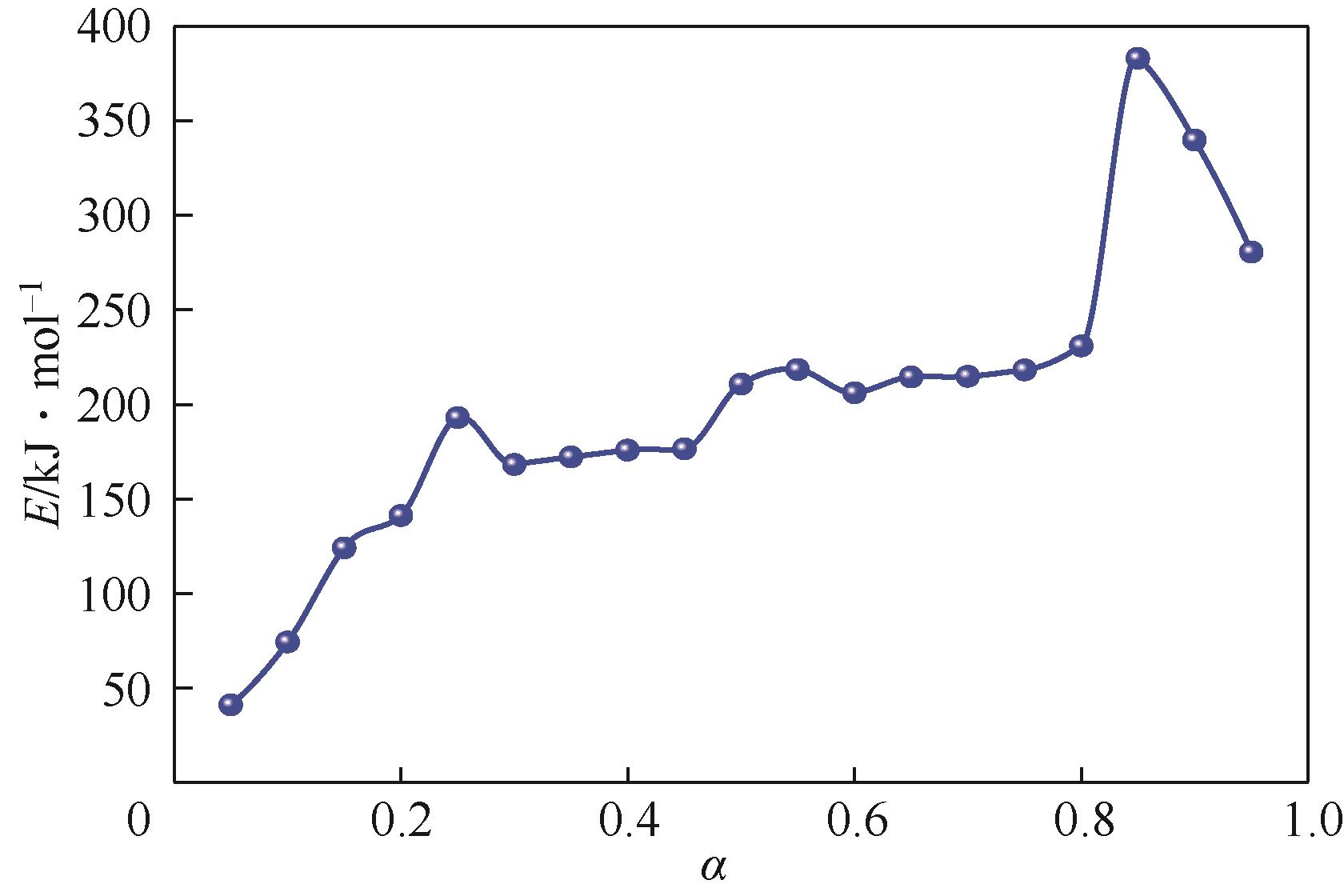
| 转化率 | 拟合曲线 | 活化能E/kJ·mol -1 | 线性相关系数R2 |
|---|---|---|---|
| 0.05 | y=10.47x+21.07 | 40.92942 | 0.96724 |
| 0.1 | y=11.32x+18.37 | 74.23701 | 0.96137 |
| 0.15 | y=14.93x+18.52 | 123.99989 | 0.96492 |
| 0.2 | y=16.99x+21.47 | 141.1927 | 0.97774 |
| 0.25 | y=23.23x+31.85 | 192.93921 | 0.99983 |
| 0.3 | y=19.52x+24.87 | 168.19976 | 0.97851 |
| 0.35 | y=20.73x+26.42 | 172.21079 | 0.96724 |
| 0.4 | y=22.85x+29.43 | 175.81415 | 0.96137 |
| 0.45 | y=25.21x+32.78 | 176.44426 | 0.9587 |
| 0.5 | y=25.37x+32.41 | 210.77128 | 0.95368 |
| 0.55 | y=26.29x+33.33 | 218.43556 | 0.95423 |
| 0.6 | y=24.82x+30.51 | 206.15721 | 0.95912 |
| 0.65 | y=25.81x+30.69 | 214.45144 | 0.96901 |
| 0.7 | y=25.86x+31.39 | 214.83034 | 0.9575 |
| 0.75 | y=26.27x+31.62 | 218.23444 | 0.95142 |
| 0.8 | y=10.47x+21.07 | 230.85888 | 0.91083 |
| 0.85 | y=27.79x+33.49 | 383.15627 | 0.94912 |
| 0.9 | y=40.92x+47.70 | 339.92115 | 0.95142 |
| 0.95 | y=53.78x+57.11 | 280.62832 | 0.99983 |
| 转化率 | 拟合曲线 | 活化能E/kJ·mol -1 | 线性相关系数R2 |
|---|---|---|---|
| 0.05 | y=10.47x+21.07 | 40.92942 | 0.96724 |
| 0.1 | y=11.32x+18.37 | 74.23701 | 0.96137 |
| 0.15 | y=14.93x+18.52 | 123.99989 | 0.96492 |
| 0.2 | y=16.99x+21.47 | 141.1927 | 0.97774 |
| 0.25 | y=23.23x+31.85 | 192.93921 | 0.99983 |
| 0.3 | y=19.52x+24.87 | 168.19976 | 0.97851 |
| 0.35 | y=20.73x+26.42 | 172.21079 | 0.96724 |
| 0.4 | y=22.85x+29.43 | 175.81415 | 0.96137 |
| 0.45 | y=25.21x+32.78 | 176.44426 | 0.9587 |
| 0.5 | y=25.37x+32.41 | 210.77128 | 0.95368 |
| 0.55 | y=26.29x+33.33 | 218.43556 | 0.95423 |
| 0.6 | y=24.82x+30.51 | 206.15721 | 0.95912 |
| 0.65 | y=25.81x+30.69 | 214.45144 | 0.96901 |
| 0.7 | y=25.86x+31.39 | 214.83034 | 0.9575 |
| 0.75 | y=26.27x+31.62 | 218.23444 | 0.95142 |
| 0.8 | y=10.47x+21.07 | 230.85888 | 0.91083 |
| 0.85 | y=27.79x+33.49 | 383.15627 | 0.94912 |
| 0.9 | y=40.92x+47.70 | 339.92115 | 0.95142 |
| 0.95 | y=53.78x+57.11 | 280.62832 | 0.99983 |
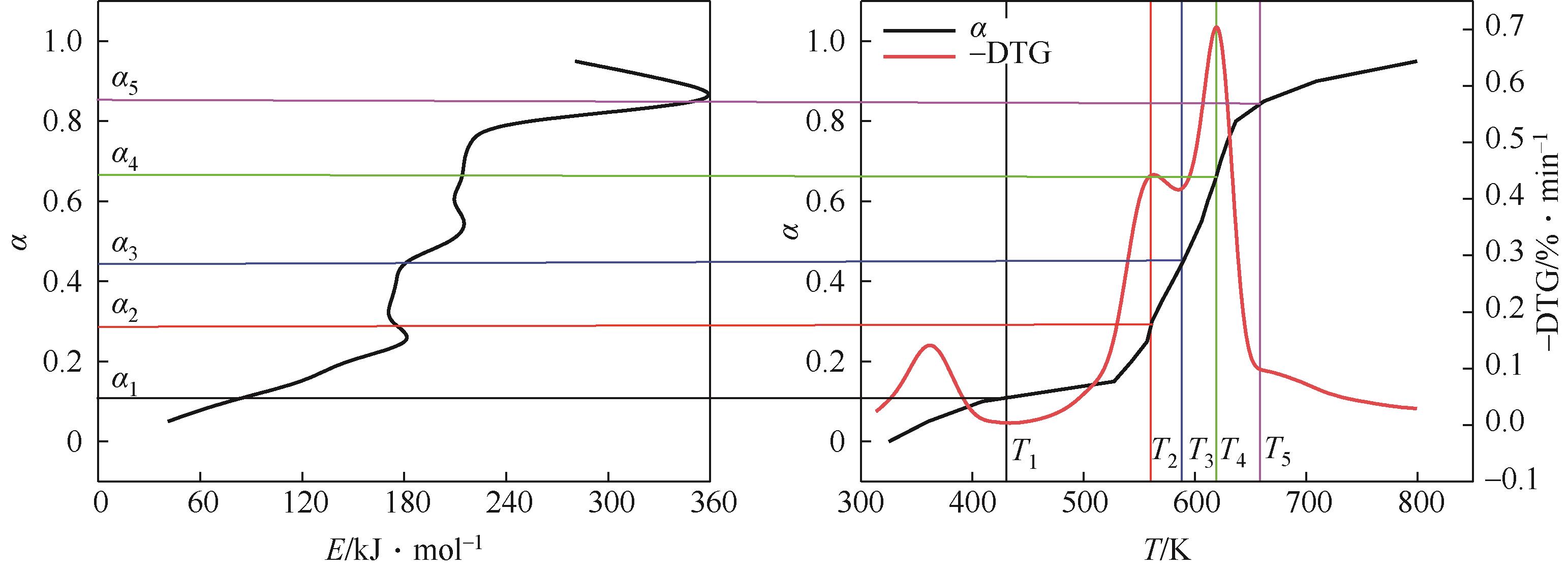
| 转化率范围 | 温度范围 | 反应 | 有效活化能Ea |
|---|---|---|---|
| α<0.11 | T0~T1 | 水分蒸发,低温易分解组分进行分解 | 从40.9kJ/mol上升至 86.2kJ/mol |
| 0.11≤α<0.44 | T1~T3 | 半纤维素分解出挥发分 | 从86.2kJ/mol上升至181.7kJ/mol再下降至173.6kJ/mol后续基本没有变化 |
| 0.44≤α<0.85 | T3~T5 | 纤维素分解出挥发分、木质素分解成炭 | 从173.6kJ/mol迅速上升至352.2kJ/mol |
| α≥0.85 | T5~Te | 木质素分解成炭 | 从352.2kJ/mol下降至280.4kJ/mol |
| 转化率范围 | 温度范围 | 反应 | 有效活化能Ea |
|---|---|---|---|
| α<0.11 | T0~T1 | 水分蒸发,低温易分解组分进行分解 | 从40.9kJ/mol上升至 86.2kJ/mol |
| 0.11≤α<0.44 | T1~T3 | 半纤维素分解出挥发分 | 从86.2kJ/mol上升至181.7kJ/mol再下降至173.6kJ/mol后续基本没有变化 |
| 0.44≤α<0.85 | T3~T5 | 纤维素分解出挥发分、木质素分解成炭 | 从173.6kJ/mol迅速上升至352.2kJ/mol |
| α≥0.85 | T5~Te | 木质素分解成炭 | 从352.2kJ/mol下降至280.4kJ/mol |
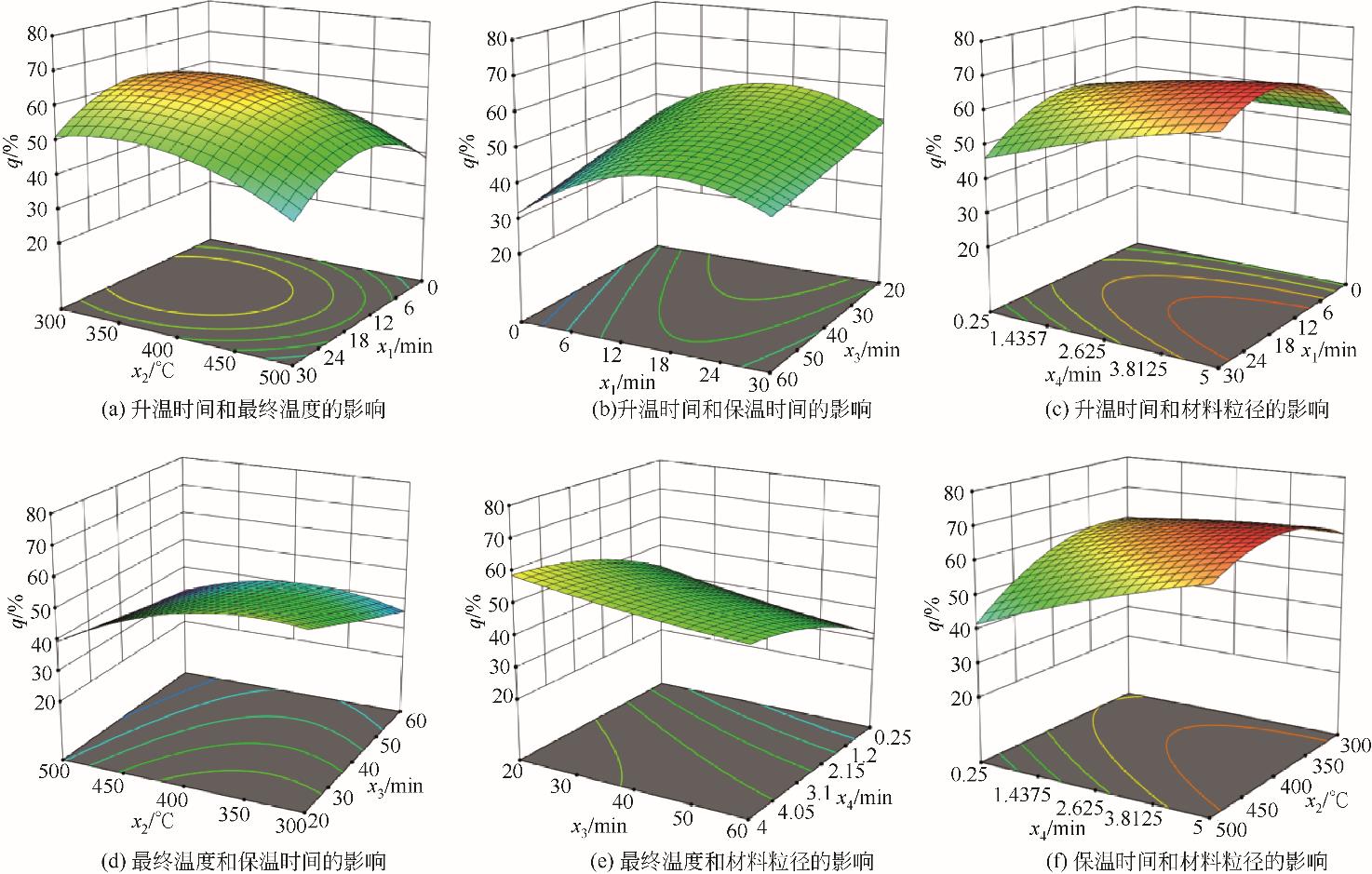
| 因素 | 水平 | ||
|---|---|---|---|
| 低水平 (-1) | 中间水平 (0) | 高水平 (+1) | |
| X1 | 0 | 15 | 30 |
| X2 | 300 | 400 | 500 |
| X3 | 20 | 40 | 60 |
| X4 | 0.25 | 2.625 | 5 |
| 因素 | 水平 | ||
|---|---|---|---|
| 低水平 (-1) | 中间水平 (0) | 高水平 (+1) | |
| X1 | 0 | 15 | 30 |
| X2 | 300 | 400 | 500 |
| X3 | 20 | 40 | 60 |
| X4 | 0.25 | 2.625 | 5 |
| 方差来源 | 平方和 | 自由度 | 均方差 | F值 | P值 | 显著性 |
|---|---|---|---|---|---|---|
| R2=0.9913, Adj R2=0.9792, 信噪比=27.2898 | ||||||
| 模型 | 5387.31 | 14 | 384.81 | 81.80 | < 0.0001 | 显著 |
| X1 | 39.79 | 1 | 39.79 | 8.46 | 0.0156 | |
| X2 | 4338.08 | 1 | 4338.08 | 922.14 | < 0.0001 | |
| X3 | 119.20 | 1 | 119.20 | 25.34 | 0.0005 | |
| X4 | 110.84 | 1 | 110.84 | 23.56 | 0.0007 | |
| X1X2 | 4.62 | 1 | 4.62 | 0.9826 | 0.3449 | |
| X1X3 | 0.54 | 1 | 0.54 | 0.1148 | 0.7417 | |
| X1X4 | 2.04 | 1 | 2.04 | 0.4347 | 0.5246 | |
| X2X3 | 0.73 | 1 | 0.73 | 0.1554 | 0.7017 | |
| X2X4 | 23.38 | 1 | 23.38 | 4.97 | 0.0499 | |
| X3X4 | 0.02 | 1 | 0.02 | 0.0042 | 0.9498 | |
| X12 | 6.09 | 1 | 6.09 | 1.29 | 0.2818 | |
| X22 | 406.73 | 1 | 406.73 | 86.46 | < 0.0001 | |
| X32 | 5.57 | 1 | 5.57 | 1.18 | 0.3021 | |
| X42 | 3.09 | 1 | 3.09 | 0.6565 | 0.4367 | |
| 误差 | 47.04 | 10 | 4.70 | — | — | |
| 失拟项 | 28.93 | 3 | 6.58 | 7.43 | 0.0771 | 不显著 |
| 纯误差 | 1.32 | 4 | 0.97 | — | — | |
| 校正总和 | 5434.35 | 24 | — | — | — | |
| 方差来源 | 平方和 | 自由度 | 均方差 | F值 | P值 | 显著性 |
|---|---|---|---|---|---|---|
| R2=0.9913, Adj R2=0.9792, 信噪比=27.2898 | ||||||
| 模型 | 5387.31 | 14 | 384.81 | 81.80 | < 0.0001 | 显著 |
| X1 | 39.79 | 1 | 39.79 | 8.46 | 0.0156 | |
| X2 | 4338.08 | 1 | 4338.08 | 922.14 | < 0.0001 | |
| X3 | 119.20 | 1 | 119.20 | 25.34 | 0.0005 | |
| X4 | 110.84 | 1 | 110.84 | 23.56 | 0.0007 | |
| X1X2 | 4.62 | 1 | 4.62 | 0.9826 | 0.3449 | |
| X1X3 | 0.54 | 1 | 0.54 | 0.1148 | 0.7417 | |
| X1X4 | 2.04 | 1 | 2.04 | 0.4347 | 0.5246 | |
| X2X3 | 0.73 | 1 | 0.73 | 0.1554 | 0.7017 | |
| X2X4 | 23.38 | 1 | 23.38 | 4.97 | 0.0499 | |
| X3X4 | 0.02 | 1 | 0.02 | 0.0042 | 0.9498 | |
| X12 | 6.09 | 1 | 6.09 | 1.29 | 0.2818 | |
| X22 | 406.73 | 1 | 406.73 | 86.46 | < 0.0001 | |
| X32 | 5.57 | 1 | 5.57 | 1.18 | 0.3021 | |
| X42 | 3.09 | 1 | 3.09 | 0.6565 | 0.4367 | |
| 误差 | 47.04 | 10 | 4.70 | — | — | |
| 失拟项 | 28.93 | 3 | 6.58 | 7.43 | 0.0771 | 不显著 |
| 纯误差 | 1.32 | 4 | 0.97 | — | — | |
| 校正总和 | 5434.35 | 24 | — | — | — | |
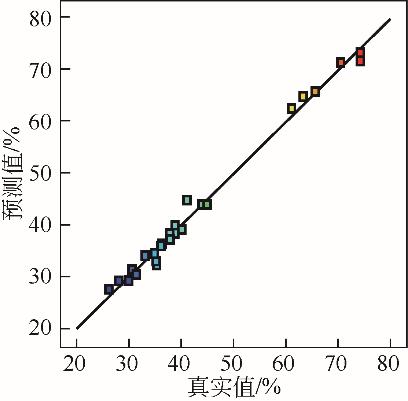
| X1 | X2 | X3 | X4 | q | |
|---|---|---|---|---|---|
| 实验值 | 预测值 | ||||
| 14.8min | 324.7℃ | 60min | 5mm | 68.8% | 72.3% |
| 14.8min | 324.7℃ | 60min | 5mm | 70.9% | 72.3% |
| 14.8min | 324.7℃ | 60min | 5mm | 68.6% | 72.3% |
| X1 | X2 | X3 | X4 | q | |
|---|---|---|---|---|---|
| 实验值 | 预测值 | ||||
| 14.8min | 324.7℃ | 60min | 5mm | 68.8% | 72.3% |
| 14.8min | 324.7℃ | 60min | 5mm | 70.9% | 72.3% |
| 14.8min | 324.7℃ | 60min | 5mm | 68.6% | 72.3% |
| 1 | Umi Fazara MD ALI, AZMI Nur Hidayah, Khairuddin MD ISA, et al. Optimization study on preparation of amine functionalized sea mango (cerbera odollam) activated carbon for carbon dioxide (CO2) adsorption[J]. Combustion Science and Technology, 2018, 190: 1259-1282. |
| 2 | 王申宛, 郑晓燕, 校导, 等. 生物炭的制备、改性及其在环境修复中应用的研究进展[J]. 化工进展, 2020, 39(S2): 352-361. |
| WANG Shenwan, ZHENG Xiaoyan, XIAO Dao, et al. Research progress of production, modification and application in environment remediation of biochar[J]. Chemical Industry and Engineering Progress, 2020, 39(S2): 352-361. | |
| 3 | Endah AGUSTINA S, FIRMANSYAH. Design and performance test of drum kiln for durian peel carbonization[J]. IOP Conference Series: Earth and Environmental Science, 2020, 542(1): 012040. |
| 4 | 常秋连, 李文博, 赵鹏. 煤焦油渣炭化过程中孔结构及表面分形特征[J]. 化工进展, 2020, 39(10): 4305-4313. |
| CHANG Qiulian, LI Wenbo, ZHAO Peng. Structure and surface fractal characteristics of coal tar residue during carbonization[J]. Chemical Industry and Engineering Progress, 2020, 39(10): 4305-4313. | |
| 5 | 朱金陵, 何晓峰, 王志伟, 等. 玉米秸秆颗粒热解制炭的试验研究[J]. 太阳能学报, 2010, 31(7): 789-793. |
| ZHU Jinling, HE Xiaofeng, WANG Zhiwei, et al. Experimental study on pyrolysising and producting charcoal with corn straw pellet[J]. Acta Energiae Solaris Sinica, 2010, 31(7): 789-793. | |
| 6 | 朱赫男, 王志朴, 邢文龙, 等. 污泥与生物质共热解制备生物质炭工艺优化及吸附性能[J]. 化工进展, 2018, 37(S1): 199-204. |
| ZHU Henan, WANG Zhipu, XING Wenlong, et al. Process optimization and adsorption performance of biochars prepared by co-pyrolysis of sludge and biomasses[J]. Chemical Industry and Engineering Progress, 2018, 37(S1): 199-204. | |
| 7 | 胡福昌, 陈顺伟, 康志雄, 等. 竹材列管移动床连续干馏炭化的工业试验[J]. 林产化学与工业, 2005, 25(2): 47-51. |
| HU Fuchang, CHEN Shunwei, KANG Zhixiong, et al. An industrial test on continuous carbonization of bamboo in multitubular moving bed[J]. Chemistry & Industry of Forest Products, 2005, 25(2): 47-51. | |
| 8 | 杨莉, 付婧, 文子伟, 等. 6种低温生物质炭的制备及结构表征[J]. 吉林农业大学学报, 2021, 43(5): 565-573. |
| YANG Li, FU Jing, WEN Ziwei, et al. Preparation and structure characterization of six kinds of low temperature biochar[J]. Journal of Jilin Agricultural University, 2021, 43(5): 565-573. | |
| 9 | 徐大勇, 张苗, 杨伟伟, 等. 氧化铝改性污泥生物炭粒制备及其对Pb(Ⅱ)的吸附特性[J]. 化工进展, 2020, 39(3): 1153-1166. |
| XU Dayong, ZHANG Miao, YANG Weiwei, et al. Preparation of alumina modified sludge biocharcoal particles and their adsorption characteristics for Pb(Ⅱ)[J]. Chemical Industry and Engineering Progress, 2020, 39(3): 1153-1166. | |
| 10 | GHYSELS Stef, RONSSE Frederik, DICKINSON Dane, et al. Production and characterization of slow pyrolysis biochar from lignin-rich digested stillage from lignocellulosic ethanol production[J]. Biomass and Bioenergy, 2019, 122: 349-360. |
| 11 | 罗煜, 赵立欣, 孟海波, 等. 不同温度下热裂解芒草生物质炭的理化特征分析[J]. 农业工程学报, 2013, 29(13): 208-217. |
| LUO Yu, ZHAO Lixin, MENG Haibo, et al. Physio-chemical characterization of biochars pyrolyzed from miscanthus under two different temperatures[J]. Transactions of the Chinese Society of Agricultural Engineering, 2013, 29(13): 208-217. | |
| 12 | 高美. 生物质炭化成型燃料的制备及其燃烧性能的研究[D]. 哈尔滨: 黑龙江科技学院, 2010. |
| GAO Mei. The research of the fabrication and combustion characteristic of the biomass carbonized forming fuel [D].: Harbin: Heilongjiang University of Science and Technology, 2010. | |
| 13 | MEDIC D, DARR M, SHAH A, et al. Effects of torrefaction process parameters on biomass feedstock upgrading[J]. Fuel, 2012, 91(1): 147-154. |
| 14 | PIMCHUAI Anuphon, DUTTA Animesh, BASU Prabir. Torrefaction of agriculture residue to enhance combustible properties[J]. Energy & Fuels, 2010, 24(9): 4638-4645. |
| 15 | Po-Chih KUO, WU Wei, CHEN Wei-Hsin. Gasification performances of raw and torrefied biomass in a downdraft fixed bed gasifier using thermodynamic analysis[J]. Fuel, 2014, 117: 1231-1241. |
| 16 | 姚红宇, 唐光木, 葛春辉, 等. 炭化温度和时间与棉杆炭特性及元素组成的相关关系[J]. 农业工程学报, 2013, 29(7): 199-206. |
| YAO Hongyu, TANG Guangmu, GE Chunhui, et al. Characteristics and elementary composition of cotton stalk-char in different carbonization temperature and time[J]. Transactions of the Chinese Society of Agricultural Engineering, 2013, 29(7): 199-206. | |
| 17 | MA Yuhui, WANG Jing, ZHANG Yushan. TG-FTIR study on pyrolysis of Enteromorpha prolifera[J]. Biomass Conversion and Biorefinery, 2018, 8(1): 151-157. |
| 18 | FAN Fangyu, ZHENG Yunwu, HUANG Yuanbo, et al. Combustion kinetics of biochar prepared by pyrolysis of macadamia shells[J]. BioResources, 2017, 12(2): 68-71. |
| 19 | 梁嘉晋. 纤维素和半纤维素热解机理及其产物调控途径的研究[D]. 广州: 华南理工大学, 2016. |
| LIANG Jiajin. Mechanism researches of cellulose and hemicellulose pyrolysis and their products regulation[D]. Guangzhou: South China University of Technology, 2016. | |
| 20 | 钱卫. 低阶烟煤中低温热解及热解产物研究[D]. 北京: 中国矿业大学(北京), 2012. |
| QIAN Wei. Experimental study on medium-low temperature pyrolysis of low rank bituminous coal and characterization of pyrolysis-derived products[D]. Beijing: China University of Mining & Technology, Beijing, 2012. | |
| 21 | VYAZOVKIN Sergey, BURNHAM Alan K, CRIADO José M, et al. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data[J]. Thermochimica Acta, 2011, 520(1/2): 1-19. |
| 22 | 吴正锐. 黄松甸木耳菌糠的热解特性分析及动力学研究[D]. 吉林: 东北电力大学, 2020. |
| WU Zhengrui. Study on pyrolysis charcteristic and pyrolysis kinetics analysis of spent jew’s-ear substrate in huangsongdian[D]. Jilin: Northeast Dianli University, 2020. | |
| 23 | 任宁, 王昉, 张建军, 等. 热分析动力学研究方法的新进展[J]. 物理化学学报, 2020, 36(6): 12-18. |
| REN Ning, WANG Fang, ZHANG Jianjun, et al. Progress in thermal analysis kinetics[J]. Acta Physico-Chimica Sinica, 2020, 36(6): 12-18. | |
| 24 | TSAMBA Alberto J, YANG Weihong, BLASIAK Wlodzimierz. Pyrolysis characteristics and global kinetics of coconut and cashew nut shells[J]. Fuel Processing Technology, 2006, 87(6): 523-530. |
| 25 | YANG Haiping, YAN Rong, CHEN Hanping, et al. Characteristics of hemicellulose, cellulose and lignin pyrolysis[J]. Fuel, 2007, 86(12/13): 1781-1788. |
| [1] | ZHAO Yao, ZHOU Zhihui, WU Hongdan, HU Chuanzhi, ZHANG Guochun, WU Ruipeng. Response surface analysis and optimization of membrane permeation vaporization by Silicalite-1 molecular sieve [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2586-2594. |
| [2] | WANG Xue, XU Qiyong, ZHANG Chao. Hydrothermal carbonization of the lignocellulosic biomass and application of the hydro-char [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2536-2545. |
| [3] | HE Shanming, PAN Jiechang, XU Guozuan, LI Wenjun, LIANG Yong. Thermodynamic analysis and experimental verification of chromium and vanadium removal by ferrous salt precipitation from crude sodium tungstate solution [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2171-2179. |
| [4] | LI Yeqing, YANG Xingru, LIANG Zhuo, JIANG Hao, XU Quan, ZHOU Hongjun, FENG Lu. Impact of exogenous additives on hydrothermal dechlorination performance of polyvinyl chloride [J]. Chemical Industry and Engineering Progress, 2022, 41(5): 2706-2712. |
| [5] | WANG Yujuan, TANG Jianfeng, HUA Yihuai, CHEN Jing, SANG Wei, LIU Yunfei. Influence of different start-up conditions on response characteristics of natural gas decarbonization device [J]. Chemical Industry and Engineering Progress, 2022, 41(4): 1770-1780. |
| [6] | XU Jie, HUANG Qunxing, MENG Xiangdong, GAO Huaping. Effect of calcium-based additive on phosphorus form and bioavailability during hydrothermal carbonization of sewage sludge [J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3507-3514. |
| [7] | Jianfeng TANG, Yujuan WANG, Yue WANG, Yihuai HUA, Jie CHU, Wei SANG, Jing CHEN. Applicability of Aspen HYSYS for simulation of amine decarbonization regeneration process [J]. Chemical Industry and Engineering Progress, 2021, 40(2): 747-754. |
| [8] | Xiaoyuan ZHENG, Zhengwei JIANG, Wei CHEN, Yutong YE, Zhi YING, Shasha JI, Bo WANG. Migration and transformation of phosphorus in sewage sludge during hydrothermal carbonization process [J]. Chemical Industry and Engineering Progress, 2020, 39(5): 2017-2025. |
| [9] | Zheng TANG,Song ZHAO,Yajie QIAN,Gang XUE,Hanzhong JIA,Pin GAO. Formation mechanisms and environmental applications of persistent free radicals in biochar: a review [J]. Chemical Industry and Engineering Progress, 2020, 39(4): 1521-1527. |
| [10] | Entian LI,Yang XU,Pei YAO,Yuanyuan ZHU,Yihan ZHANG,Xiashi ZHU. Remove naphthalene from solvent oil by vinyl imidazole ionic liquid and β-cyclodextrin [J]. Chemical Industry and Engineering Progress, 2020, 39(4): 1321-1328. |
| [11] | Liubin SHI, Mingde TANG, Yong TANG, Lulu HE, Zhangfa TONG, Lishuo LI. Preparation and characterization of micro-nano hierarchical hollow rod-like calcium carbonate by high pressure carbonization [J]. Chemical Industry and Engineering Progress, 2020, 39(11): 4742-4748. |
| [12] | Qiulian CHANG, Wenbo LI, Peng ZHAO. Structure and surface fractal characteristics of coal tar residue during carbonization [J]. Chemical Industry and Engineering Progress, 2020, 39(10): 4305-4313. |
| [13] | YUAN Yanwen, ZHAO Lixin, MENG Haibo, CONG Hongbin, HUO Lili, TANG Sen. Research on the preparation of Fischer-Tropsch synthesis gas by biomass carbonization pyrolysis gas catalytic reforming [J]. Chemical Industry and Engineering Progress, 2019, 38(s1): 152-158. |
| [14] | Liangcai WANG,Huanhuan MA,Jianbin ZHOU. Effect of carbonization process on physiochemical properties of digestate [J]. Chemical Industry and Engineering Progress, 2019, 38(03): 1545-1551. |
| [15] | Jing FANG, Mengyu DIAO, Chunli LI, Bihan XUAN. Study on thermodynamic analysis and energy saving of heat integrated distillation column [J]. Chemical Industry and Engineering Progress, 2019, 38(02): 834-841. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||