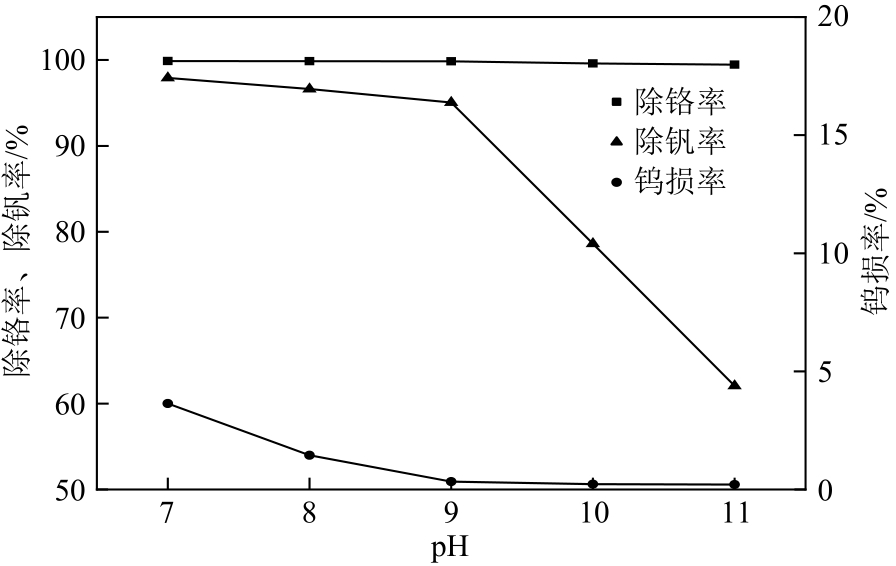
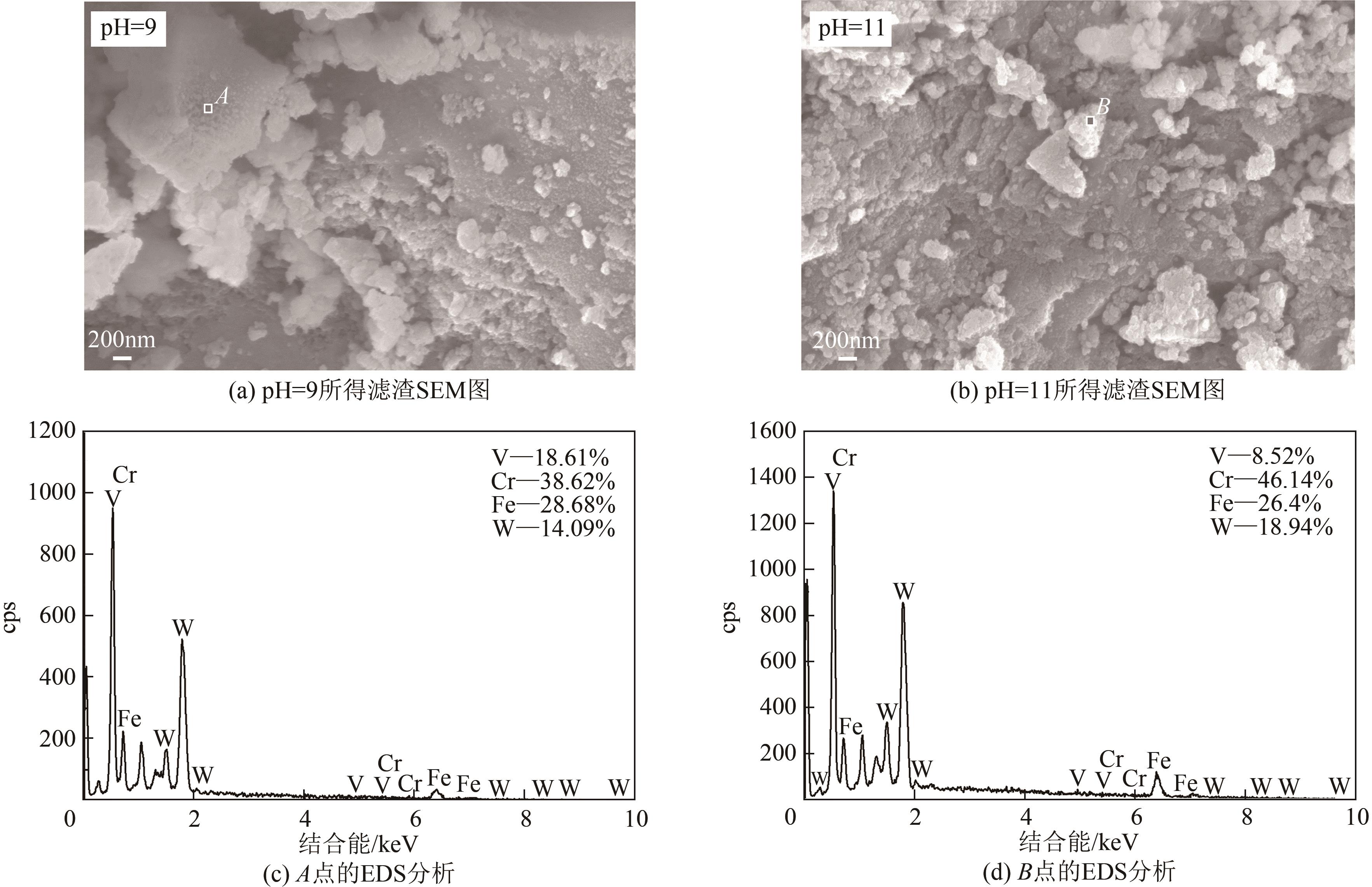
Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (4): 2171-2179.DOI: 10.16085/j.issn.1000-6613.2022-1170
• Resources and environmental engineering • Previous Articles Next Articles
Thermodynamic analysis and experimental verification of chromium and vanadium removal by ferrous salt precipitation from crude sodium tungstate solution
HE Shanming1,2( ), PAN Jiechang1, XU Guozuan2, LI Wenjun1, LIANG Yong1(
), PAN Jiechang1, XU Guozuan2, LI Wenjun1, LIANG Yong1( )
)
- 1.Department of Materials Metallurgy Chemistry, Jiangxi University of Science and Technology, Ganzhou 341000, Jiangxi, China
2.Chongyi Zhangyuan Tungsten Industry Co. , Ltd. , Ganzhou 341300, Jiangxi, China
-
Received:2022-06-22Revised:2022-08-09Online:2023-05-08Published:2023-04-25 -
Contact:LIANG Yong
粗钨酸钠溶液亚铁盐沉淀法除铬、钒的热力学分析及实验验证
贺山明1,2( ), 潘界昌1, 徐国钻2, 李文君1, 梁勇1(
), 潘界昌1, 徐国钻2, 李文君1, 梁勇1( )
)
- 1.江西理工大学材料冶金化学学部,江西 赣州 341000
2.崇义章源钨业股份有限公司,江西 赣州 341300
-
通讯作者:梁勇 -
作者简介:贺山明(1984—),男,博士,副教授,研究方向为湿法冶金及二次金属资源综合利用。E-mail:heshanming200809@163.com。 -
基金资助:国家自然科学基金(52064023);国家级大学生创新创业训练计划(202110407008)
CLC Number:
Cite this article
HE Shanming, PAN Jiechang, XU Guozuan, LI Wenjun, LIANG Yong. Thermodynamic analysis and experimental verification of chromium and vanadium removal by ferrous salt precipitation from crude sodium tungstate solution[J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2171-2179.
贺山明, 潘界昌, 徐国钻, 李文君, 梁勇. 粗钨酸钠溶液亚铁盐沉淀法除铬、钒的热力学分析及实验验证[J]. 化工进展, 2023, 42(4): 2171-2179.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-1170
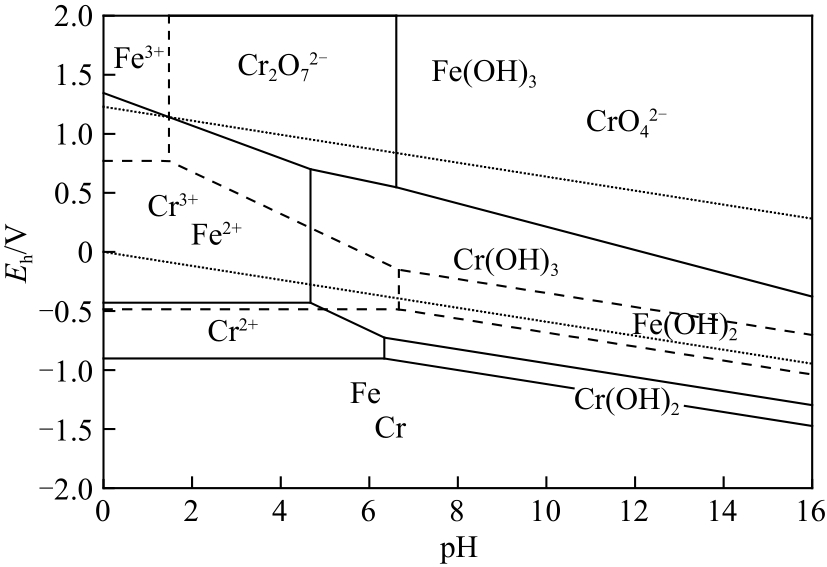
| 序号 | 化学反应 | lgK | 平衡方程 |
|---|---|---|---|
| (1) | Cr3++OH- | 9.969 | [CrOH2+][Cr3+]-1[OH-]-1=109.969 |
| (2) | Cr3++2OH- | 15.384 | [Cr(OH)2+][Cr3+]-1[OH-]-2=1015.384 |
| (3) | Cr3++4OH- | 38.054 | [Cr(OH)4-][Cr3+]-1[OH-]-4=1038.054 |
| (4) | HCrO4- | -6.49 | [H+][CrO42-][HCrO4-]-1=10-6.49 |
| (5) | 2HCrO4- | 1.52 | [Cr2O72-][HCrO4-]-2=101.52 |
| (6) | Fe2++OH- | 6.892 | [FeOH+][Fe2+]-1[OH-]-1=106.892 |
| (7) | Fe2++2OH- | 9.77 | [Fe(OH)2(aq)][Fe2+]-1[OH-]-2=109.77 |
| (8) | Fe2++3OH- | 12.143 | [Fe(OH)3-][Fe2+]-1[OH-]-3=1012.143 |
| (9) | Fe3++OH- | 11.825 | [FeOH2+][Fe3+]-1[OH-]-1=1011.825 |
| (10) | Fe3++2OH- | 21.103 | [Fe(OH)2+][Fe3+]-1[OH-]-2=1021.103 |
| (11) | Fe3++3OH- | 29.67 | [Fe(OH)3(aq)][Fe3+]-1[OH-]-3=1029.67 |
| (12) | Fe3++4OH- | 35.561 | [Fe(OH)4-][Fe3+]-1[OH]-4=1035.561 |
| (13) | 2Fe3++2OH- | 20.779 | [Fe2(OH)24+][Fe+3]-2[OH-]-2=1020.779 |
| (14) | VO2++2H2O | -29.38 | [VO43-][H+]4[VO2+]-1=10-29.38 |
| (15) | VO43-+H+ | 13.36 | [HVO42-][VO43-]-1[H+]-1=1013.36 |
| (16) | VO43-+2H+ | 21.31 | [H2VO4-][VO43-]-1[H+]-2=1021.31 |
| (17) | 2VO43-+2H+ | 27.38 | [V2O74-][H+]-2[VO43-]-2=1027.38 |
| (18) | 2VO43-+4H+ | 45.4 | [H2V2O72-][VO43-]-2[H+]-4=1045.4 |
| (19) | 3HVO42-+2H+ | 20.96 | [HV3O104-][HVO42-]-3[H+]-2=1020.96 |
| (20) | 4VO43-+8H+ | 95.11 | [V4O124-][VO43-]-4[H+]-8=1095.11 |
| (21) | 4HVO42-+2H+ | 20.55 | [V4O136-][HVO42-]-4[H+]-2=1020.55 |
| (22) | 4HVO42-+3H+ | 31.33 | [HV4O135-][HVO42-]-4[H+]-3=1031.33 |
| (23) | 5VO43-+10H+ | 118.69 | [V5O155-][VO43-]-5[H+]-10=10118.69 |
| (24) | 2VO43-+3H+ | 37.17 | [HV2O73-][VO43-]-2[H+]-3=1037.17 |
| (25) | 6HVO42-+6H+ | 60.79 | [V6O186-][HVO42-]-6[H+]-6=1060.79 |
| (26) | 10VO43-+24H+ | 264.82 | [V10O286-][VO43-]-10[H+]-24=10264.82 |
| (27) | 10VO43-+25H+ | 270.89 | [HV10O285-][VO43-]-10[H+]-25=10270.89 |
| (28) | 10VO43-+26H+ | 274.49 | [H2V10O284-][VO43-]-10[H+]-26=10274.49 |
| (29) | 10HVO42-+17H+ | 150.38 | [H3V10O283-][HVO42-]-10[H+]-17=10150.38 |
| (30) | Cr(OH)3(s) | -29.554 | [Cr3+][OH-]3=10-29.554 |
| (31) | Fe(OH)2(s) | -15.042 | [Fe2+][OH-]2=10-15.042 |
| (32) | Fe(OH)3(s) | -37.94 | [Fe3+][OH-]3=10-37.94 |
| (33) | Fe(VO3)2(s)+2H2O | -15.524 | [Fe2+][H2VO4-]2=10-15.524 |
| 序号 | 化学反应 | lgK | 平衡方程 |
|---|---|---|---|
| (1) | Cr3++OH- | 9.969 | [CrOH2+][Cr3+]-1[OH-]-1=109.969 |
| (2) | Cr3++2OH- | 15.384 | [Cr(OH)2+][Cr3+]-1[OH-]-2=1015.384 |
| (3) | Cr3++4OH- | 38.054 | [Cr(OH)4-][Cr3+]-1[OH-]-4=1038.054 |
| (4) | HCrO4- | -6.49 | [H+][CrO42-][HCrO4-]-1=10-6.49 |
| (5) | 2HCrO4- | 1.52 | [Cr2O72-][HCrO4-]-2=101.52 |
| (6) | Fe2++OH- | 6.892 | [FeOH+][Fe2+]-1[OH-]-1=106.892 |
| (7) | Fe2++2OH- | 9.77 | [Fe(OH)2(aq)][Fe2+]-1[OH-]-2=109.77 |
| (8) | Fe2++3OH- | 12.143 | [Fe(OH)3-][Fe2+]-1[OH-]-3=1012.143 |
| (9) | Fe3++OH- | 11.825 | [FeOH2+][Fe3+]-1[OH-]-1=1011.825 |
| (10) | Fe3++2OH- | 21.103 | [Fe(OH)2+][Fe3+]-1[OH-]-2=1021.103 |
| (11) | Fe3++3OH- | 29.67 | [Fe(OH)3(aq)][Fe3+]-1[OH-]-3=1029.67 |
| (12) | Fe3++4OH- | 35.561 | [Fe(OH)4-][Fe3+]-1[OH]-4=1035.561 |
| (13) | 2Fe3++2OH- | 20.779 | [Fe2(OH)24+][Fe+3]-2[OH-]-2=1020.779 |
| (14) | VO2++2H2O | -29.38 | [VO43-][H+]4[VO2+]-1=10-29.38 |
| (15) | VO43-+H+ | 13.36 | [HVO42-][VO43-]-1[H+]-1=1013.36 |
| (16) | VO43-+2H+ | 21.31 | [H2VO4-][VO43-]-1[H+]-2=1021.31 |
| (17) | 2VO43-+2H+ | 27.38 | [V2O74-][H+]-2[VO43-]-2=1027.38 |
| (18) | 2VO43-+4H+ | 45.4 | [H2V2O72-][VO43-]-2[H+]-4=1045.4 |
| (19) | 3HVO42-+2H+ | 20.96 | [HV3O104-][HVO42-]-3[H+]-2=1020.96 |
| (20) | 4VO43-+8H+ | 95.11 | [V4O124-][VO43-]-4[H+]-8=1095.11 |
| (21) | 4HVO42-+2H+ | 20.55 | [V4O136-][HVO42-]-4[H+]-2=1020.55 |
| (22) | 4HVO42-+3H+ | 31.33 | [HV4O135-][HVO42-]-4[H+]-3=1031.33 |
| (23) | 5VO43-+10H+ | 118.69 | [V5O155-][VO43-]-5[H+]-10=10118.69 |
| (24) | 2VO43-+3H+ | 37.17 | [HV2O73-][VO43-]-2[H+]-3=1037.17 |
| (25) | 6HVO42-+6H+ | 60.79 | [V6O186-][HVO42-]-6[H+]-6=1060.79 |
| (26) | 10VO43-+24H+ | 264.82 | [V10O286-][VO43-]-10[H+]-24=10264.82 |
| (27) | 10VO43-+25H+ | 270.89 | [HV10O285-][VO43-]-10[H+]-25=10270.89 |
| (28) | 10VO43-+26H+ | 274.49 | [H2V10O284-][VO43-]-10[H+]-26=10274.49 |
| (29) | 10HVO42-+17H+ | 150.38 | [H3V10O283-][HVO42-]-10[H+]-17=10150.38 |
| (30) | Cr(OH)3(s) | -29.554 | [Cr3+][OH-]3=10-29.554 |
| (31) | Fe(OH)2(s) | -15.042 | [Fe2+][OH-]2=10-15.042 |
| (32) | Fe(OH)3(s) | -37.94 | [Fe3+][OH-]3=10-37.94 |
| (33) | Fe(VO3)2(s)+2H2O | -15.524 | [Fe2+][H2VO4-]2=10-15.524 |
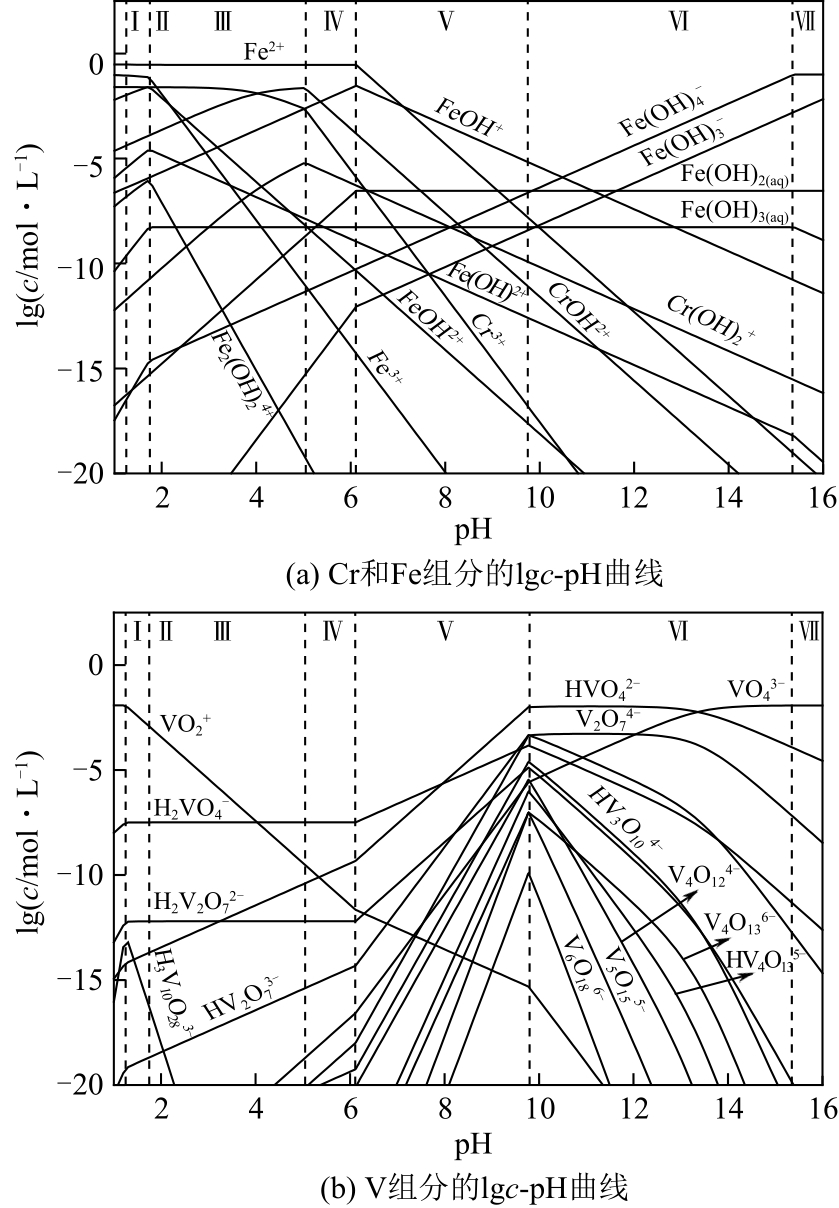
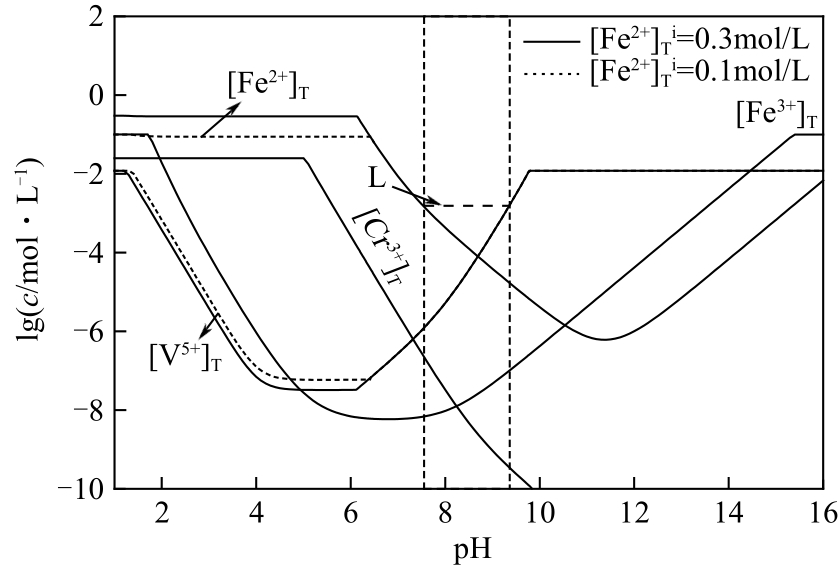
| 序号 | 反应方程式 |
|---|---|
| (34) | [Cr6+]T |
| (35) | [Cr3+]T |
| (36) | [Fe2+]T |
| (37) | [Fe3+]T |
| (38) | [V5+]T |
| (39) | [Cr6+]T |
| (40) | [Cr3+]T |
| (41) | [Fe2+]T |
| (42) | [Fe3+]T |
| (43) | [V5+]T |
| (44) | [Fe2+]Ti-[Fe2+]T |
| 序号 | 反应方程式 |
|---|---|
| (34) | [Cr6+]T |
| (35) | [Cr3+]T |
| (36) | [Fe2+]T |
| (37) | [Fe3+]T |
| (38) | [V5+]T |
| (39) | [Cr6+]T |
| (40) | [Cr3+]T |
| (41) | [Fe2+]T |
| (42) | [Fe3+]T |
| (43) | [V5+]T |
| (44) | [Fe2+]Ti-[Fe2+]T |
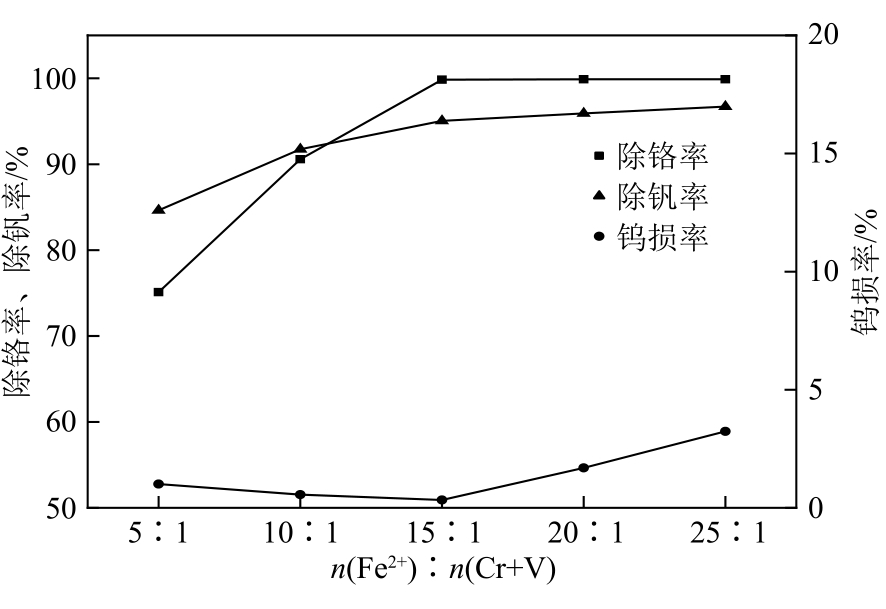
| 21 | LI Xin, LI Ming, LIANG Bin. Removal of vanadium from acidic chromium solution by ferric sulfate[J]. Iron Steel Vanadium Titanium, 2016, 37(4): 20-24. |
| 22 | 杨得军, 王少娜, 陈晓芳, 等. 铬盐无钙焙烧工艺铬酸钠中性液铁盐除钒[J]. 中国有色金属学报, 2014, 24(1): 279-285. |
| YANG Dejun, WANG Shaona, CHEN Xiaofang, et al. Removing vanadium from sodium chromate neutral liquid by non-calcium roasting technology with chromium salt[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(1): 279-285. | |
| 23 | 刘芳. 还原沉淀法对含铬重金属废水的处理研究[J]. 环境污染与防治, 2014, 36(4): 54-59. |
| LIU Fang. Treatment of chromium containing heavy metal wastewater by reduction and sedimentation process[J]. Environmental Pollution & Control, 2014, 36(4): 54-59. | |
| 1 | MISHRA D, SINHA S, SAHU K K, et al. Recycling of secondary tungsten resources[J]. Transactions of the Indian Institute of Metals, 2017, 70(2): 479-485. |
| 2 | 周新华, 王力民, 彭英健. 我国硬质合金再生产业现状与发展[J]. 硬质合金, 2016, 33(5): 356-364. |
| ZHOU Xinhua, WANG Limin, PENG Yingjian. Status and development of cemented carbide recycling industry in China[J]. Cemented Carbide, 2016, 33(5): 356-364. | |
| 3 | 李洪桂. 稀有金属冶金学[M]. 北京: 冶金工业出版社, 1990: 22. |
| LI Honggui. Rare metals metallurgy[M]. Beijing: Metallurgical Industry Press, 1990: 22. | |
| 4 | 谢盛德, 李春海, 雷吟春. 废钨回收中钨酸钠结晶料的除铬处理研究与实践[J]. 稀有金属与硬质合金, 2012, 40(3): 8-12. |
| XIE Shengde, LI Chunhai, LEI Yinchun. Research and practice of chromium removal from sodium tungstate crystal material during waste tungsten recovery[J]. Rare Metals and Cemented Carbides, 2012, 40(3): 8-12. | |
| 5 | 雷吟春, 欧阳海波, 曹育龙, 等. 镁盐共沉淀法深度除铬及WO3的回收研究[J]. 稀有金属与硬质合金, 2019, 47(6): 1-4. |
| LEI Yinchun, OUYANG Haibo, CAO Yulong, et al. Study on deep chromium removal by magnesium salt coprecipitation and recovery of WO3 [J]. Rare Metals and Cemented Carbides, 2019, 47(6): 1-4. | |
| 6 | 张乐怡, 吴红梅, 姜晓庆, 等. 活性氧化铝的改性及除铬(Ⅵ)性能研究[J]. 天津化工, 2020, 34(1): 11-14. |
| ZHANG Leyi, WU Hongmei, JIANG Xiaoqing, et al. Modification of active alumina with sodium hydroxide for adsorption of chromium (Ⅵ) ions[J]. Tianjin Chemical Industry, 2020, 34(1): 11-14. | |
| 7 | 肖超, 曾理, 李义兵, 等. 磷酸盐沉淀法除铬热力学研究[J]. 有色金属科学与工程, 2017, 8(5): 103-108. |
| XIAO Chao, ZENG Li, LI Yibing, et al. Thermodynamic study on chromium removal by phosphate precipitation[J]. Nonferrous Metal Science and Engineering, 2017, 8(5): 103-108. | |
| 8 | 宋阜, 朱宾权. 离子交换法分离富集钨酸钠溶液中的钒[J]. 稀有金属与硬质合金, 2006, 34(3): 5-7, 11. |
| SONG Fu, ZU Binquan. Extraction of vanadium from sodium tungstate by ion exchange[J]. Rare Metals and Cemented Carbides, 2006, 34(3): 5-7, 11. | |
| 9 | LUO Lin, KEJUN Liu, SHIBAYAMA Atsushi, et al. Recovery of tungsten and vanadium from tungsten alloy scrap[J]. Hydrometallurgy, 2004, 72(1): 1-8. |
| 10 | 陈星宇, 肖露萍, 赵中伟. 钨钼冶炼过程中除钒[J]. 中国有色金属学报, 2014, 24(7): 1883-1887. |
| CHEN Xingyu, XIAO Luping, ZHAO Zhongwei. Vanadium removal in tungsten or molybdenum smelting process[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(7): 1883-1887. | |
| 11 | 张家靓, 赵中伟. W(Ⅵ)-V(Ⅴ)-H2O体系钨钒分离的热力学分析[J]. 中国有色金属学报, 2014, 24(6): 1656-1662. |
| ZHANG Jialiang, ZHAO Zhongwei. Thermodynamic analysis for separation of tungsten and vanadium in W(Ⅵ)-V(Ⅴ)-H2O system[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(6): 1656-1662. | |
| 12 | SADIQ Muhammad. Thermodynamic solubility relationships of inorganic vanadium in the marine environment[J]. Marine Chemistry, 1988, 23(12): 87-96. |
| 13 | 张文娟, 马保中, 王成彦. 基于磷酸盐沉淀分离电镀污泥中铬铁的热力学研究[J]. 稀有金属, 2018, 42(10): 1084-1092. |
| ZHANG Wenjuan, MA Baozhong, WANG Chengyan. Thermodynamic study on selective separation of chromium and iron from electroplating sludge based on phosphate precipitation[J]. Chinese Journal of Rare Metals, 2018, 42(10): 1084-1092. | |
| 14 | VAGHETTI J C P, LIMA E C, ROYER B, et al. Application of Brazilian-pine fruit coat as a biosorbent to removal of Cr(Ⅵ) from aqueous solution—Kinetics and equilibrium study[J]. Biochemical Engineering Journal, 2008, 42(1): 67-76. |
| 15 | 赵中伟. 钨冶炼的理论与应用[M]. 北京: 清华大学出版社, 2013: 75. |
| ZHAO Zhongwei. Tungsten metallurgy: Fundamentals and applications[M]. Beijing: Tsinghua University Press, 2013: 75. | |
| 16 | CHANG Liyang. Alternative chromium reduction and heavy metal precipitation methods for industrial wastewater[J]. Environmental Progress, 2003, 22(3):174-182. |
| 17 | SPEIGHT J G. Lange's handbook of chemistry[M]. 16th ed. New York: McGraw-Hill Professional, 2005: 1237. |
| 18 | 杨显万. 高温水溶液热力学数据计算手册[M]. 北京: 冶金工业出版社, 1983: 645. |
| YANG Xianwan. Handbook of thermodynamic data of high temperature aqueous solution[M]. Beijing: Metallurgical Industry Press, 1983: 645. | |
| 19 | PEACOCK C L, SHERMAN D M. Vanadium(Ⅴ) adsorption onto goethite (α-FeOOH) at pH 1.5 to 12: A surface complexation model based on ab initio molecular geometries and EXAFS spectroscopy[J]. Geochimica et Cosmochimica Acta, 2004, 68(8): 1723-1733. |
| 20 | COLTON J V, MICHAEL P S, MOJTABA A, et al. Adsorption of (poly)vanadate onto ferrihydrite and hematite: An in situ ATR-FTIR study[J]. ACS Earth and Space Chemistry, 2020, 4(4): 641-649. |
| 21 | 李信, 李明, 梁斌. 利用硫酸铁除去沉钒后酸性铬溶液中钒的试验研究[J]. 钢铁钒钛, 2016, 37(4): 20-24. |
| [1] | Jing FANG, Mengyu DIAO, Chunli LI, Bihan XUAN. Study on thermodynamic analysis and energy saving of heat integrated distillation column [J]. Chemical Industry and Engineering Progress, 2019, 38(02): 834-841. |
| [2] | ZHAO Ming, CHEN Xing, LIANG Junyu, ZHANG Xiaolei, ZHANG Huiyan, XIAO Rui. Thermodynamic analysis of a novel IGCC system based on LAES technology [J]. Chemical Industry and Engineering Progree, 2015, 34(s1): 75-79. |
| [3] | TIAN Yuanming, LI Jinhuan, FAN Yuanjie, ZHANG Yuxin, SHEN Yang, LI Qunsheng. Solubility measurement and correlation for 4-acetamidobenzoic acid in methanol+ethanol mixtures [J]. Chemical Industry and Engineering Progree, 2015, 34(3): 647-651. |
| [4] | QI Yu, SUN Wei. Simulation and calculation of biomass yield based on the theory of Gibbs dissipation [J]. Chemical Industry and Engineering Progree, 2015, 34(04): 1068-1073. |
| [5] | ZHU Lin,ZHANG Zheng,FAN Junming. Processing parameter analysis on integrated coal gasification system with chemical looping air separation [J]. Chemical Industry and Engineering Progree, 2014, 33(08): 1997-2003. |
| [6] | XUE Xiaojun,JIA Guangxin,HE Junhui,LI Ting. Thermodynamics of ethanol synthesis from dimethyl ether and syngas [J]. Chemical Industry and Engineering Progree, 2014, 33(05): 1160-1163. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||