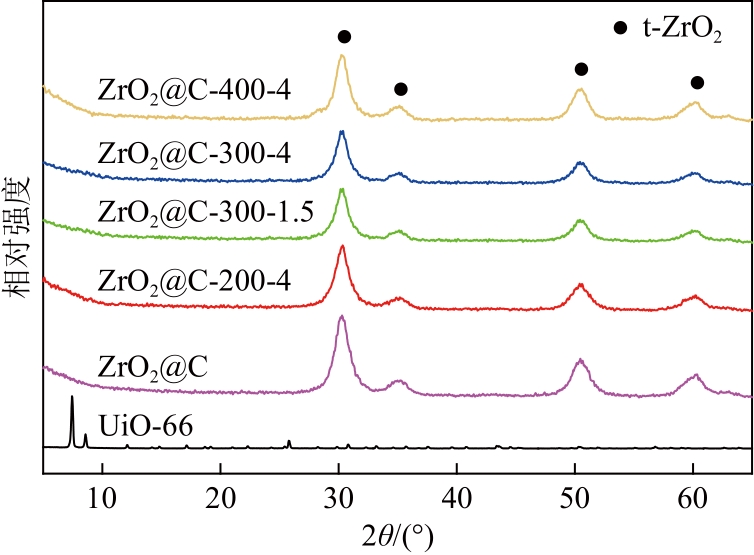
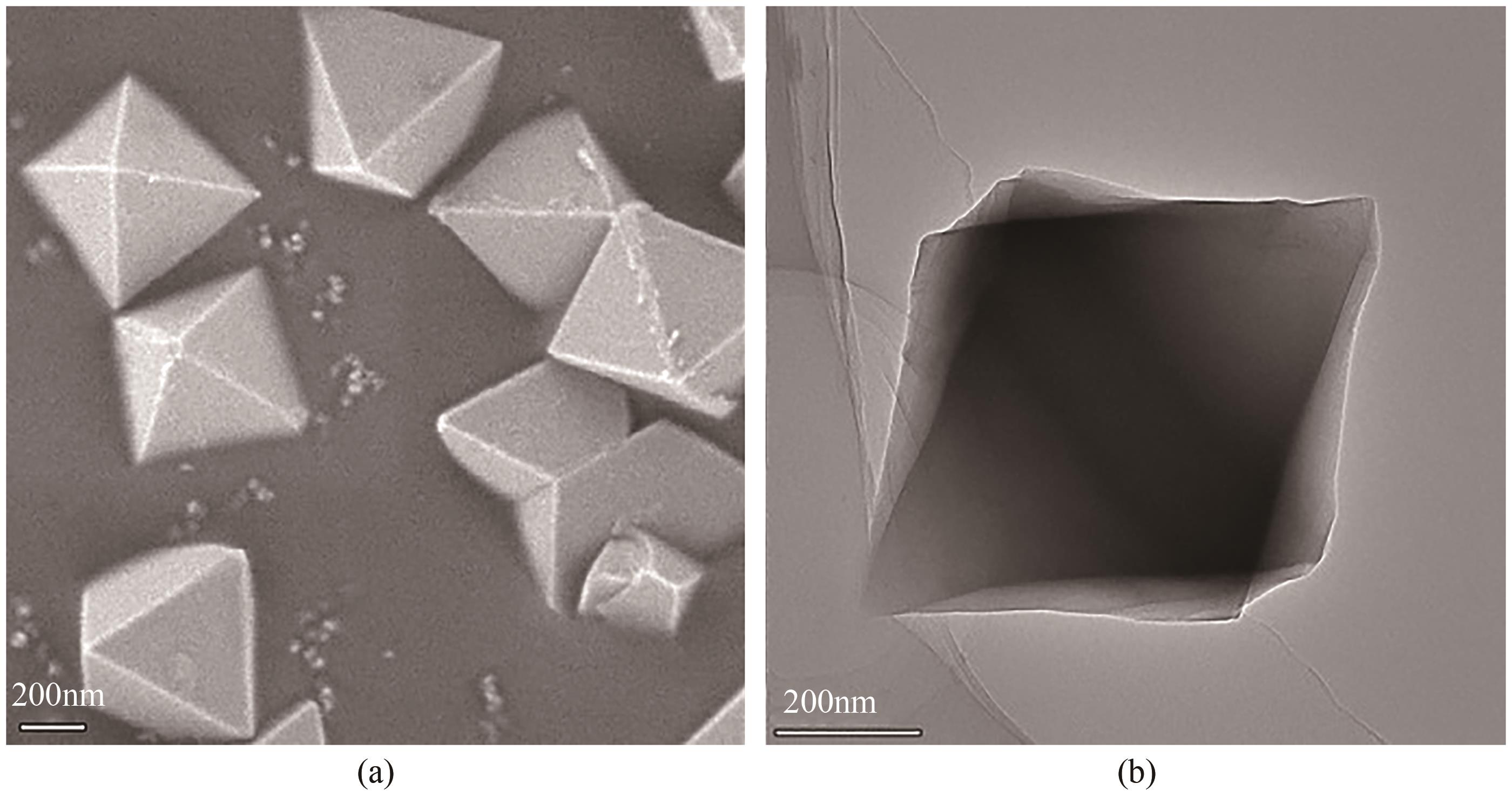
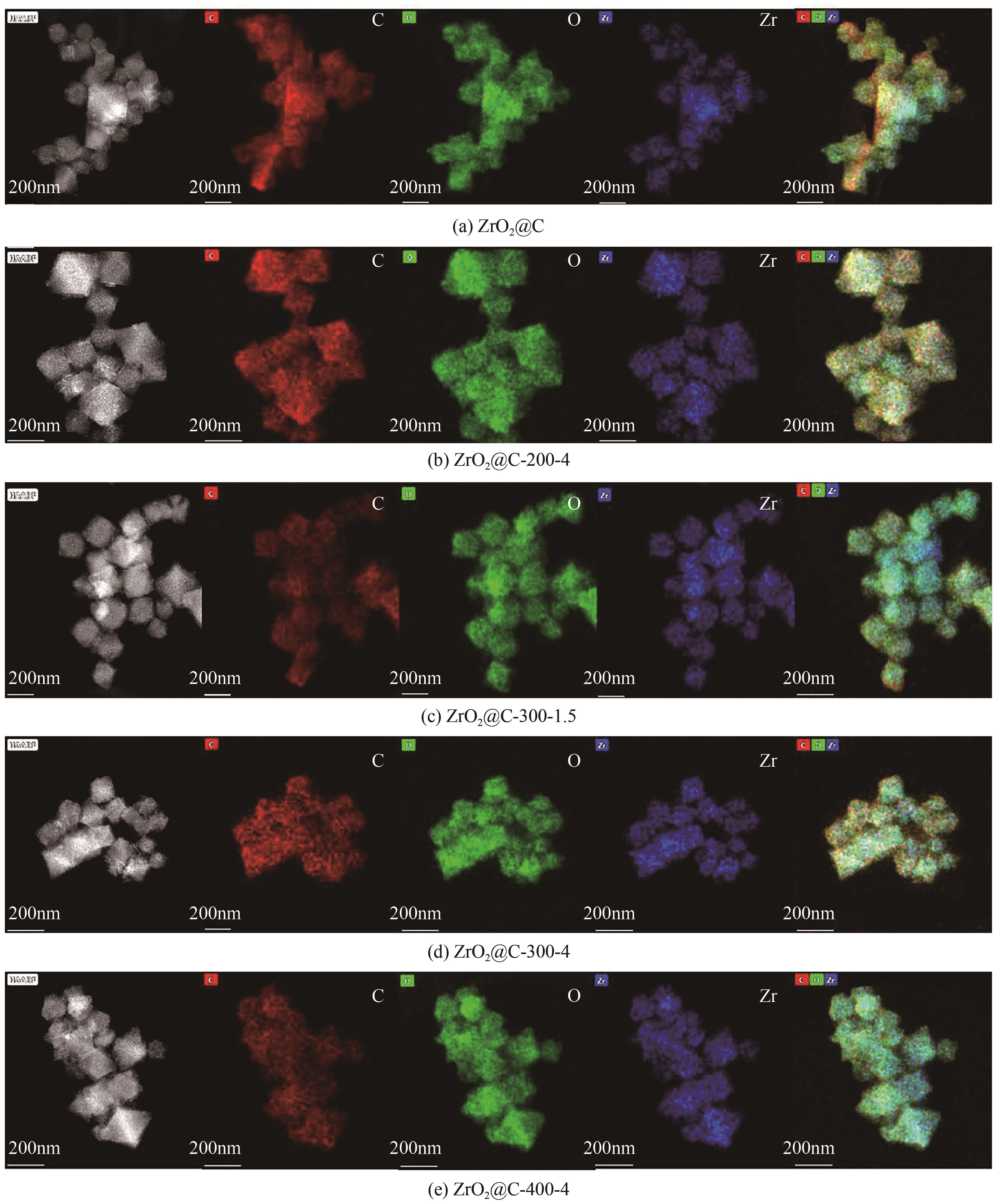
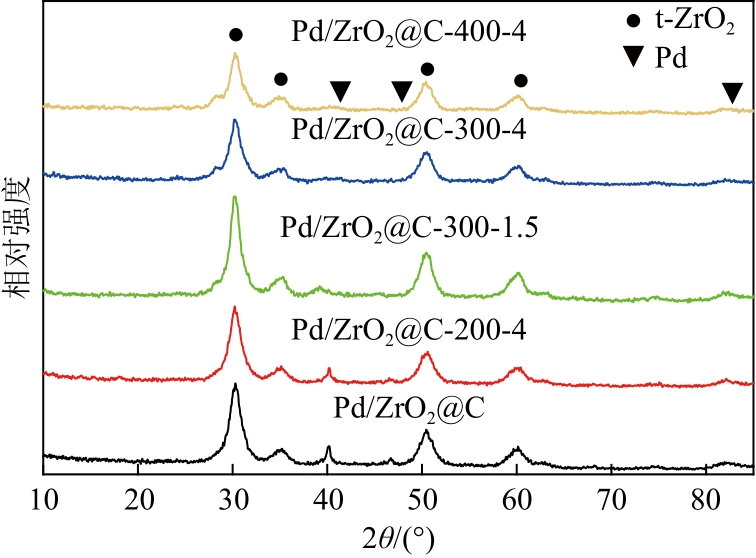
Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (8): 4421-4431.DOI: 10.16085/j.issn.1000-6613.2023-1200
• Industrial catalysis • Previous Articles
Synthesis of p-aminophenol by transfer hydrogenation of nitrobenzene using formic acid as hydrogen source
WANG Yufei1( ), JIA Yu1, ZHANG Yisheng1, XUE Wei1,2, LI Fang1,2(
), JIA Yu1, ZHANG Yisheng1, XUE Wei1,2, LI Fang1,2( ), WANG Yanji1,2,3
), WANG Yanji1,2,3
- 1.Hebei Provincial Key Laboratory of Green Chemical Technology and High Efficient Energy Saving, School of Chemical Engineering and Technology, Hebei University of Technology, Tianjin 300400, China
2.Tianjin Key Laboratory of Chemical Process Safety, Tianjin 300400, China
3.Hebei Industrial Technology Research Institute of Green Chemical Industry, Cangzhou 061000, Hebei, China
-
Received:2023-07-14Revised:2023-12-10Online:2024-09-02Published:2024-08-15 -
Contact:LI Fang
甲酸为氢源硝基苯转移加氢合成对氨基苯酚
王雨菲1( ), 贾宇1, 张议升1, 薛伟1,2, 李芳1,2(
), 贾宇1, 张议升1, 薛伟1,2, 李芳1,2( ), 王延吉1,2,3
), 王延吉1,2,3
- 1.河北工业大学化工学院河北省绿色化工与高效节能重点实验室,天津 300400
2.天津市本质安全化工技术重点实验室,天津 300400
3.河北省绿色化学工业产业研究院,河北 沧州 061000
-
通讯作者:李芳 -
作者简介:王雨菲(1999—),女,硕士研究生,研究方向为绿色化工。E-mail:961283910@qq.com。 -
基金资助:国家自然科学基金(U21A20306);河北省自然科学基金(B2022202077)
CLC Number:
Cite this article
WANG Yufei, JIA Yu, ZHANG Yisheng, XUE Wei, LI Fang, WANG Yanji. Synthesis of p-aminophenol by transfer hydrogenation of nitrobenzene using formic acid as hydrogen source[J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4421-4431.
王雨菲, 贾宇, 张议升, 薛伟, 李芳, 王延吉. 甲酸为氢源硝基苯转移加氢合成对氨基苯酚[J]. 化工进展, 2024, 43(8): 4421-4431.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-1200
| 样品 | C质量 分数/% | 比表面积 /m2·g-1 | 孔容积 /cm3·g-1 | 孔径/nm |
|---|---|---|---|---|
| ZrO2@C | 24.38 | 240.95 | 0.23 | 3.82 |
| ZrO2@C-200-4 | 22.74 | 246.94 | 0.25 | 4.01 |
| ZrO2@C-300-1.5 | 7.56 | 206.56 | 0.20 | 3.92 |
| ZrO2@C-300-4 | 2.77 | 173.74 | 0.26 | 6.02 |
| ZrO2@C-400-4 | 0.50 | 118.44 | 0.26 | 8.84 |
| 样品 | C质量 分数/% | 比表面积 /m2·g-1 | 孔容积 /cm3·g-1 | 孔径/nm |
|---|---|---|---|---|
| ZrO2@C | 24.38 | 240.95 | 0.23 | 3.82 |
| ZrO2@C-200-4 | 22.74 | 246.94 | 0.25 | 4.01 |
| ZrO2@C-300-1.5 | 7.56 | 206.56 | 0.20 | 3.92 |
| ZrO2@C-300-4 | 2.77 | 173.74 | 0.26 | 6.02 |
| ZrO2@C-400-4 | 0.50 | 118.44 | 0.26 | 8.84 |
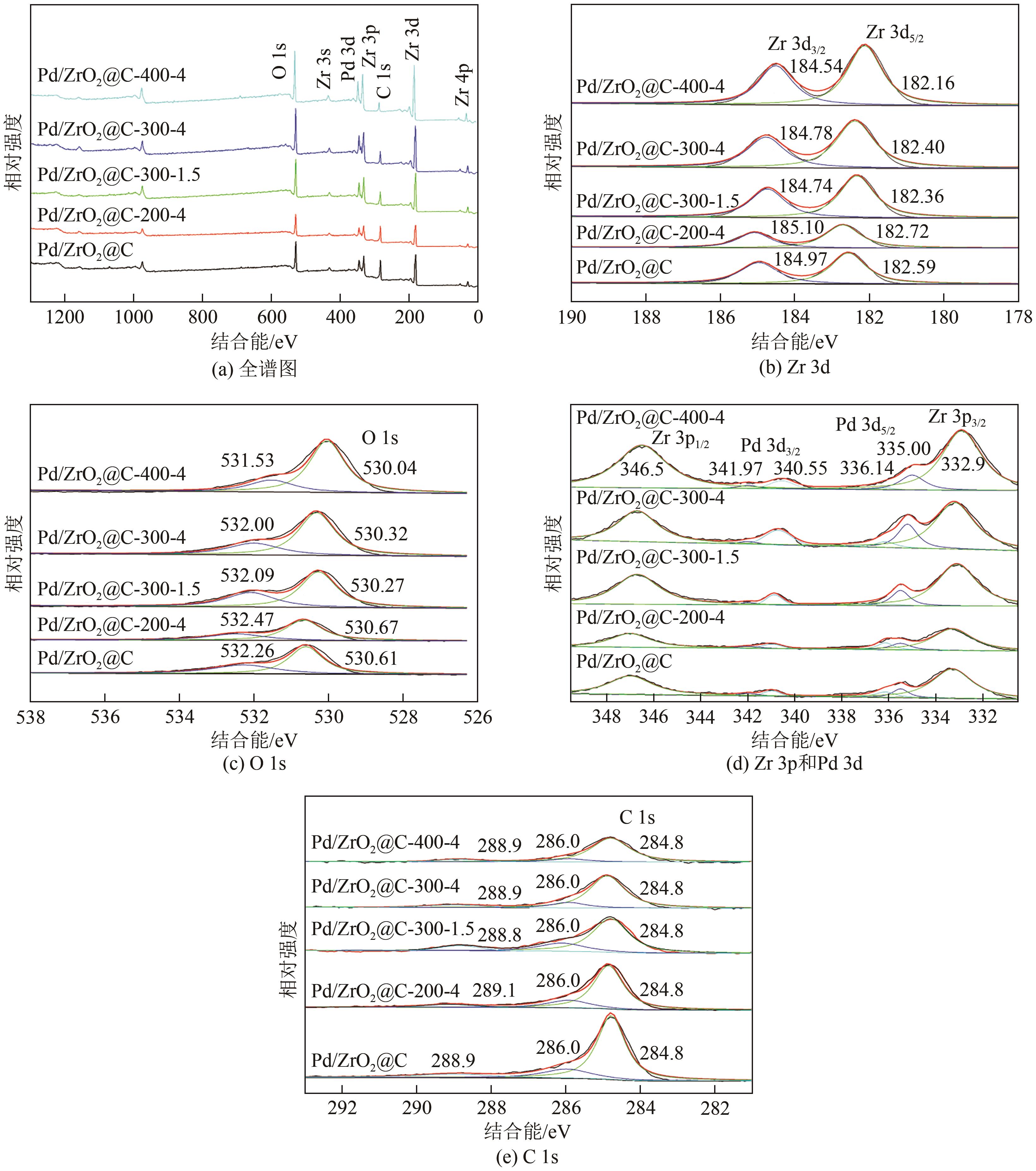
| 样品 | O 1s/% | Zr 3d/% | O/Zr |
|---|---|---|---|
| ZrO2@C | 30.65 | 10.88 | 2.82 |
| ZrO2@C-200-4 | 33.67 | 11.64 | 2.89 |
| ZrO2@C-300-1.5 | 43.74 | 15.34 | 2.85 |
| ZrO2@C-300-4 | 51.55 | 18.34 | 2.81 |
| ZrO2@C-400-4 | 59.73 | 21.97 | 2.72 |
| 样品 | O 1s/% | Zr 3d/% | O/Zr |
|---|---|---|---|
| ZrO2@C | 30.65 | 10.88 | 2.82 |
| ZrO2@C-200-4 | 33.67 | 11.64 | 2.89 |
| ZrO2@C-300-1.5 | 43.74 | 15.34 | 2.85 |
| ZrO2@C-300-4 | 51.55 | 18.34 | 2.81 |
| ZrO2@C-400-4 | 59.73 | 21.97 | 2.72 |
| 样品 | 比表面积/m2·g-1 | 孔容积/cm3·g-1 | 孔径/nm |
|---|---|---|---|
| Pd/ZrO2@C | 234.37 | 0.26 | 4.51 |
| Pd/ZrO2@C-200-4 | 223.02 | 0.29 | 5.15 |
| Pd/ZrO2@C-300-1.5 | 168.93 | 0.24 | 5.62 |
| Pd/ZrO2@C-300-4 | 160.99 | 0.21 | 5.10 |
| Pd/ZrO2@C-400-4 | 115.89 | 0.25 | 8.69 |
| 样品 | 比表面积/m2·g-1 | 孔容积/cm3·g-1 | 孔径/nm |
|---|---|---|---|
| Pd/ZrO2@C | 234.37 | 0.26 | 4.51 |
| Pd/ZrO2@C-200-4 | 223.02 | 0.29 | 5.15 |
| Pd/ZrO2@C-300-1.5 | 168.93 | 0.24 | 5.62 |
| Pd/ZrO2@C-300-4 | 160.99 | 0.21 | 5.10 |
| Pd/ZrO2@C-400-4 | 115.89 | 0.25 | 8.69 |
| 样品 | Pd物种分布/% | Pd2+/Pd0 | |
|---|---|---|---|
| Pd0 | Pd2+ | ||
| Pd/ZrO2@C | 57.0 | 43.0 | 0.75 |
| Pd/ZrO2@C-200-4 | 55.7 | 44.3 | 0.80 |
| Pd/ZrO2@C-300-1.5 | 80.2 | 19.8 | 0.25 |
| Pd/ZrO2@C-300-4 | 81.7 | 18.3 | 0.22 |
| Pd/ZrO2@C-400-4 | 83.3 | 16.7 | 0.20 |
| 样品 | Pd物种分布/% | Pd2+/Pd0 | |
|---|---|---|---|
| Pd0 | Pd2+ | ||
| Pd/ZrO2@C | 57.0 | 43.0 | 0.75 |
| Pd/ZrO2@C-200-4 | 55.7 | 44.3 | 0.80 |
| Pd/ZrO2@C-300-1.5 | 80.2 | 19.8 | 0.25 |
| Pd/ZrO2@C-300-4 | 81.7 | 18.3 | 0.22 |
| Pd/ZrO2@C-400-4 | 83.3 | 16.7 | 0.20 |
| 催化剂 | TOF/h-1 |
|---|---|
| Pd/ZrO2@C | 306.4 |
| Pd/ZrO2@C-200-4 | 331.1 |
| Pd/ZrO2@C-300-1.5 | 252.7 |
| Pd/ZrO2@C-300-4 | 182.9 |
| Pd/ZrO2@C-400-4 | 160.0 |
| 催化剂 | TOF/h-1 |
|---|---|
| Pd/ZrO2@C | 306.4 |
| Pd/ZrO2@C-200-4 | 331.1 |
| Pd/ZrO2@C-300-1.5 | 252.7 |
| Pd/ZrO2@C-300-4 | 182.9 |
| Pd/ZrO2@C-400-4 | 160.0 |
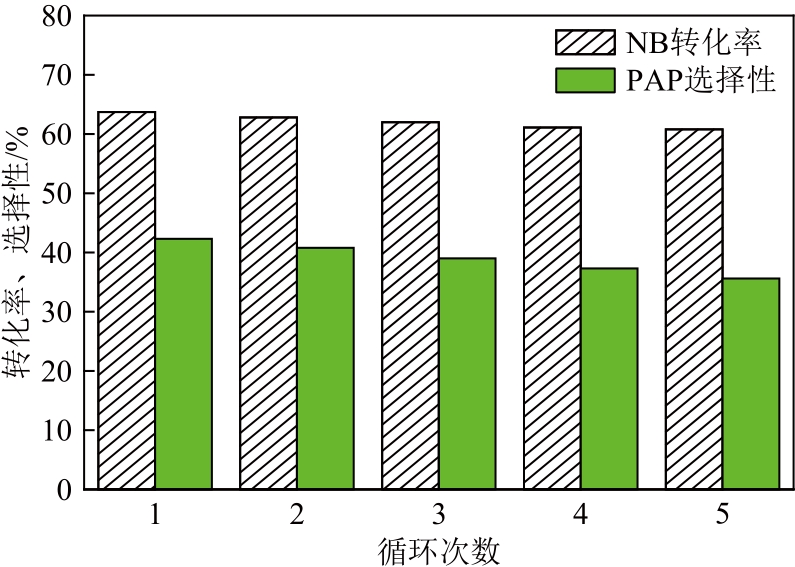
| 催化剂 | S/% | Pd/% | 比表面积 /m2·g-1 | 孔容 /cm3·g-1 | 孔径 /nm |
|---|---|---|---|---|---|
| 新鲜催化剂 | 5.90 | 0.33 | 81.24 | 0.31 | 11.72 |
| 循环5次后 的催化剂 | 4.03 | 0.30 | 80.15 | 0.23 | 8.05 |
| 催化剂 | S/% | Pd/% | 比表面积 /m2·g-1 | 孔容 /cm3·g-1 | 孔径 /nm |
|---|---|---|---|---|---|
| 新鲜催化剂 | 5.90 | 0.33 | 81.24 | 0.31 | 11.72 |
| 循环5次后 的催化剂 | 4.03 | 0.30 | 80.15 | 0.23 | 8.05 |
| 1 | WU Shutao, HUANG Xun, ZHANG Hongliang, et al. Efficient electrochemical hydrogenation of nitroaromatics into arylamines on a CuCo2O4 spinel cathode in an alkaline electrolyte[J]. ACS Catalysis, 2022, 12(1): 58-65. |
| 2 | ABDELHAMID Hani Nasser. High performance and ultrafast reduction of 4-nitrophenol using metal-organic frameworks[J]. Journal of Environmental Chemical Engineering, 2021, 9(1): 104404. |
| 3 | ATTIA Yasser A, MOHAMED Yasser M A. Silicon-grafted Ag/AgX/rGO nanomaterials (X = Cl or Br) as dip-photocatalysts for highly efficient p-nitrophenol reduction and paracetamol production[J]. Applied Organometallic Chemistry, 2019, 33(3): 1-11. |
| 4 | YU Yeonhwa, JUNG Euiyoung, KIM Hyun Jin, et al. Protein particles decorated with Pd nanoparticles for the catalytic reduction of p-nitrophenol to p-aminophenol[J]. ACS Applied Nano Materials, 2020, 3(10): 10487-10496. |
| 5 | ZHANG Tingting, JIANG Jingyang, WANG Yanhua. Green route for the preparation of p-aminophenol from nitrobenzene by catalytic hydrogenation in pressurized CO2/H2O system[J]. Organic Process Research & Development, 2015, 19(12): 2050-2054. |
| 6 | ZOU Luyao, CUI Yuanyuan, DAI Weilin. Highly efficient Au/TiO2 catalyst for one-pot conversion of nitrobenzene to p-aminophenol in water media[J]. Chinese Journal of Chemistry, 2014, 32(3): 257-262. |
| 7 | DONG Zhen, WANG Tao, ZHAO Jie, et al. Ni-Silicides nanoparticles as substitute for noble metals for hydrogenation of nitrobenzene to p-aminophenol in sulfuric acid[J]. Applied Catalysis A: General, 2016, 520: 151-156. |
| 8 | RODE Chandrashekhar V, VAIDYA Manisha J, CHAUDHARI Raghunath V. Synthesis of p-aminophenol by catalytic hydrogenation of nitrobenzene[J]. Organic Process Research & Development, 1999, 3(6): 465-470. |
| 9 | 王淑芳, 高杨, 王延吉, 等. 负载型纳米Pt催化剂的制备及其催化合成对氨基苯酚[J]. 石油化工, 2009, 38(4): 361-366. |
| WANG Shufang, GAO Yang, WANG Yanji, et al. Preparation of supported nano-Pt catalyst for synthesis of p-aminophenol[J]. Petrochemical Technology, 2009, 38(4): 361-366. | |
| 10 | 王淑芳, 王延吉, 高杨, 等. SAPO-5分子筛的制备及其催化合成对氨基苯酚[J]. 催化学报, 2010, 31(6): 637-644. |
| WANG Shufang, WANG Yanji, GAO Yang, et al. Preparation of SAPO-5 and its catalytic synthesis of p-aminophenol[J]. Chinese Journal of Catalysis, 2010, 31(6): 637-644. | |
| 11 | WANG Shufang, JIN Yadan, HE Beibei, et al. Synthesis of bifunctional Pt/MgAPO-5 catalysts and their catalytic performance in the hydrogenation of nitrobenzene to p-aminophenol[J]. Science China Chemistry, 2010, 53(7): 1514-1519. |
| 12 | 宋丽娟, 王利, 张晓彤, 等. 一种非酸介质中硝基苯选择加氢制对氨基苯酚的催化剂:CN102600891A[P]. 2012-07-25. |
| SONG Lijuan, WANG Li, ZHANG Xiaotong, et al. A catalyst for selective hydrogenation of nitrobenzene to p-aminophenol in a non-acid medium:CN102600891A[P]. 2012-07-25. | |
| 13 | Jeeva RATNAM K, Sudarshan REDDY R, SEKHAR N S, et al. Bamberger rearrangement on solid acids[J]. Applied Catalysis A: General, 2008, 348(1): 26-29. |
| 14 | DESHPANDE Abhay, FIGUERAS F, LAKSHMI KANTAM M, et al. Environmentally friendly hydrogenation of nitrobenzene to p-aminophenol using heterogeneous catalysts[J]. Journal of Catalysis, 2010, 275(2): 250-256. |
| 15 | 黄伟, 储政, 任磊, 等. 碳基固体酸在硝基苯加氢制备对氨基苯酚中的应用[J]. 化工进展, 2023, 42(1): 272-281. |
| HUANG Wei, CHU Zheng, REN Lei, et al. Application of carbon-based solid acid in hydrogenation of nitrobenzene to p-aminophenol[J]. Chemical Industry and Engineering Progress, 2023, 42(1): 272-281. | |
| 16 | 刘迎新, 刘晓爽, 曾茂, 等. 硝基苯催化加氢合成对氨基苯酚的研究进展[J]. 石油化工, 2018, 47(1): 79-85. |
| LIU Yingxin, LIU Xiaoshuang, ZENG Mao, et al. Progress in synthesis of p-aminophenol by catalytic hydrogenation of nitrobenzene[J]. Petrochemical Technology, 2018, 47(1): 79-85. | |
| 17 | GU Jing, ZHANG Zhiyang, DING Liping, et al. Platinum nanoparticles encapsulated in HZSM-5 crystals as an efficient catalyst for green production of p-aminophenol[J]. Catalysis Communications, 2017, 97: 98-101. |
| 18 | 蒋文艳, 魏光涛, 刘子涵, 等. 催化转移加氢及其强化研究进展[J]. 现代化工, 2019, 39(3): 26-30. |
| JIANG Wenyan, WEI Guangtao, LIU Zihan, et al. Research progress in catalytic transfer hydrogenation and its intensification[J]. Modern Chemical Industry, 2019, 39(3): 26-30. | |
| 19 | 郑纯智, 张继炎, 王日杰. 催化转移加氢及其在有机合成中的应用[J]. 工业催化, 2004, 12(3): 29-35. |
| ZHENG Chunzhi, ZHANG Jiyan, WANG Rijie. Catalytic transfer hydrogenation and its application in organic synthesis[J]. Industrial Catalysis, 2004, 12(3): 29-35. | |
| 20 | MENG Qinglei, YANG Xiaolong, WANG Xian, et al. Preparation strategy using pre-nucleation coupled with in situ reduction for a high-performance catalyst towards selective hydrogen production from formic acid[J]. Catalysts, 2022, 12(3): 325. |
| 21 | 王显. 甲酸氢能转换过程中抗中毒催化剂的理性设计与精准制备[D]. 合肥: 中国科学技术大学, 2021. |
| WANG Xian. Rational design and precise preparation of anti-poisoning catalyst in the conversion of formic acid to hydrogen energy[D]. Hefei: University of Science and Technology of China, 2021. | |
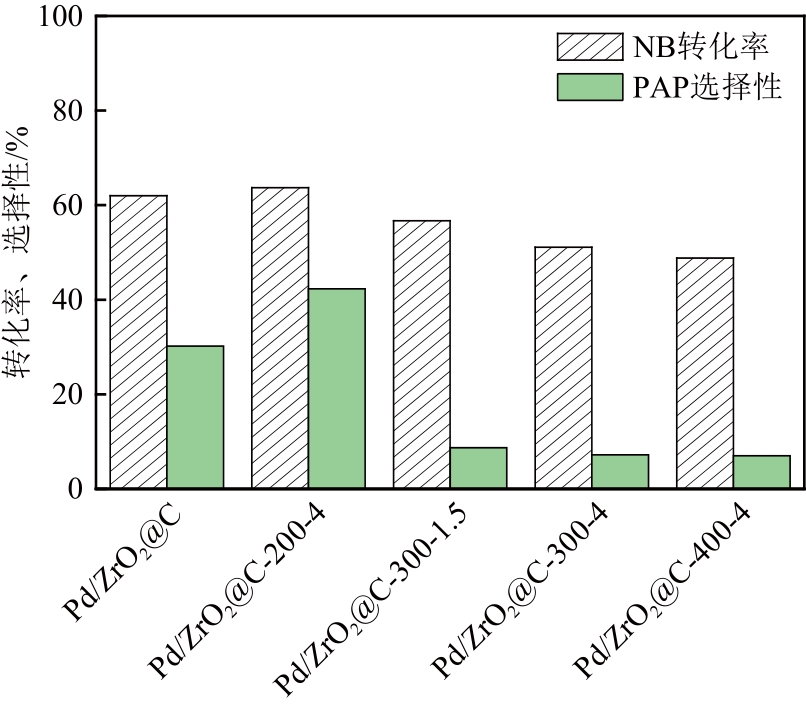
| 22 | JIA Yu, LI Fang, ZHANG Yisheng, et al. A novel process for the synthesis of p-aminophenol by transfer hydrogenation of nitrobenzene using formic acid as hydrogen source[J]. Asia-Pacific Journal of Chemical Engineering, 2022, 17(5): 1-10. |
| 23 | DHAKSHINAMOORTHY Amarajothi, GARCIA Hermenegildo. Metal-organic frameworks as solid catalysts for the synthesis of nitrogen-containing heterocycles[J]. Chemical Society Reviews, 2014, 43(16): 5750-5765. |
| 24 | Raja DAS, PACHFULE Pradip, BANERJEE Rahul, et al. Metal and metal oxide nanoparticle synthesis from metal organic frameworks (MOFs): Finding the border of metal and metal oxides[J]. Nanoscale, 2012, 4(2): 591-599. |
| 25 | SHEN Kui, CHEN Xiaodong, CHEN Junying, et al. Development of MOF-derived carbon-based nanomaterials for efficient catalysis[J]. ACS Catalysis, 2016, 6(9): 5887-5903. |
| 26 | CAO Wenxiu, LUO Wenhao, GE Hongguang, et al. UiO-66 derived Ru/ZrO2@C as a highly stable catalyst for hydrogenation of levulinic acid to γ-valerolactone[J]. Green Chemistry, 2017, 19(9): 2201-2211. |
| 27 | ZHANG Weina, LU Guang, CUI Chenlong, et al. A family of metal-organic frameworks exhibiting size-selective catalysis with encapsulated noble-metal nanoparticles[J]. Advanced Materials, 2014, 26(24): 4056-4060. |
| 28 | ZHANG Xue, QIAO Jing, LIU Chang, et al. A MOF-derived ZrO2/C nanocomposite for efficient electromagnetic wave absorption[J]. Inorganic Chemistry Frontiers, 2020, 7(2): 385-393. |
| 29 | RAHMAN Md Anisur, ROUT S, THOMAS Joseph P, et al. Defect-rich dopant-free ZrO2 nanostructures with superior dilute ferromagnetic semiconductor properties[J]. Journal of the American Chemical Society, 2016, 138(36): 11896-11906. |
| 30 | QIAO Jing, ZHANG Xue, XU Dongmei, et al. Design and synthesis of TiO2/Co/carbon nanofibers with tunable and efficient electromagnetic absorption[J]. Chemical Engineering Journal, 2020, 380: 122591. |
| 31 | FENG Wei, WANG Yaming, CHEN Junchen, et al. Reduced graphene oxide decorated with in-situ growing ZnO nanocrystals: Facile synthesis and enhanced microwave absorption properties[J]. Carbon, 2016, 108: 52-60. |
| 32 | Qing LYU, MENG Qinglei, LIU Weiwei, et al. Pd-PdO interface as active site for HCOOH selective dehydrogenation at ambient condition[J]. The Journal of Physical Chemistry C, 2018, 122(4): 2081-2088. |
| 33 | WEI Qinhong, MA Qingxiang, ZUO Pingping, et al. Hollow structure and electron promotion effect of mesoporous Pd/CeO2 catalyst for enhanced catalytic hydrogenation[J]. ChemCatChem, 2018, 10(5): 1019-1026. |
| [1] | WU Zeliang, GUAN Qihui, CHEN Shixia, WANG Jun. Advances in selective hydrogenation of alkynes to alkenes [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4366-4381. |
| [2] | ZHANG Yesu, QUAN Yanhong, DING Xinxin, REN Jun. Synthesis and application of chainlike MFI type zeolites [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4382-4392. |
| [3] | FU Tao, LI Li, GAO Lining, ZHU Fuwei, CAO Weiye, CHEN Huaxin. Cement-based boron-doped graphite phase carbon nitride material degrades NO [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4403-4410. |
| [4] | GUO Peng, LI Hongwei, LI Guixian, JI Dong, WANG Dongliang, ZHAO Xinhong. Mechanisms and coping strategies on deactivation of anode catalysts for direct methanol fuel cells [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3812-3823. |
| [5] | WANG Yingjie, ZHU Xinli. Highly dispersed Ni-Cu/SiO2 synthesized by sol-gel method for prompting direct deoxygenation of m-cresol to toluene [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3824-3833. |
| [6] | ZHANG Shirui, FAN Zhenlian, SONG Huiping, ZHANG Lina, GAO Hongyu, CHENG Shuyan, CHENG Fangqin. Research progress of fly ash supported photocatalytic materials [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 4043-4058. |
| [7] | YANG Xin, ZHONG Chengwei, YANG Zhishan, ZHU Weiwei, WANG Wenhao, YU Jiang. Catalytic remediation of polycyclic aromatic hydrocarbons contaminated soil by synthetic siderite and its derivatives [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 4118-4127. |
| [8] | ZHOU Aiguo, ZHENG Jiale, YANG Chuanruo, YANG Xiaoyi, ZHAO Junde, LI Xingchun. Industrial progress in direct air CO2 capture technology [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 2928-2939. |
| [9] | CHEN Fuqiang, ZHONG Zhaoping, QI Renzhi. Research progress on copper-based catalysts for electrochemical reduction of carbon dioxide to formic acid [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3051-3060. |
| [10] | ZENG Zhuang, LI Kezhi, YUAN Zhiwei, DU Jintao, LI Zhuoshi, WANG Yue. Advances in modified Fischer-Tropsch synthesis catalysts for CO/CO2 hydrogenation to higher alcohols [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3061-3079. |
| [11] | WAN Chengfeng, LI Zhida, ZHANG Chunyue, LU Lu. Highly efficient electrocatalytic water splitting by MXene supported CoP nanorods [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3232-3239. |
| [12] | YANG Lei, QIU Guangwei, LI Siyan, GE Hongcheng, SUN Yuanyuan, WANG Fei, FAN Xiaoguang. Insulin controlled release carriers based on temperature and glucose dual-response copolymer microcapsules [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3277-3284. |
| [13] | LI Siwen, LEI Min, LIU Yushuang, DONG Zhaoqi, XUE Lili, ZHAO Jianshe. Research progress of ionic liquid-based heteropolyacids in fuel oxidation desulfurization [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3322-3335. |
| [14] | XIE Zhongkai, SHI Weidong. Research progress of charge polarized photocatalysts in photoconversion carbon dioxide into multi-carbon chemicals [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2714-2722. |
| [15] | ZHOU Yuntao, WANG Hongxing, LI Xingang, CUI Lifeng. Application and research progress of CeO2 support in CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2723-2738. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||