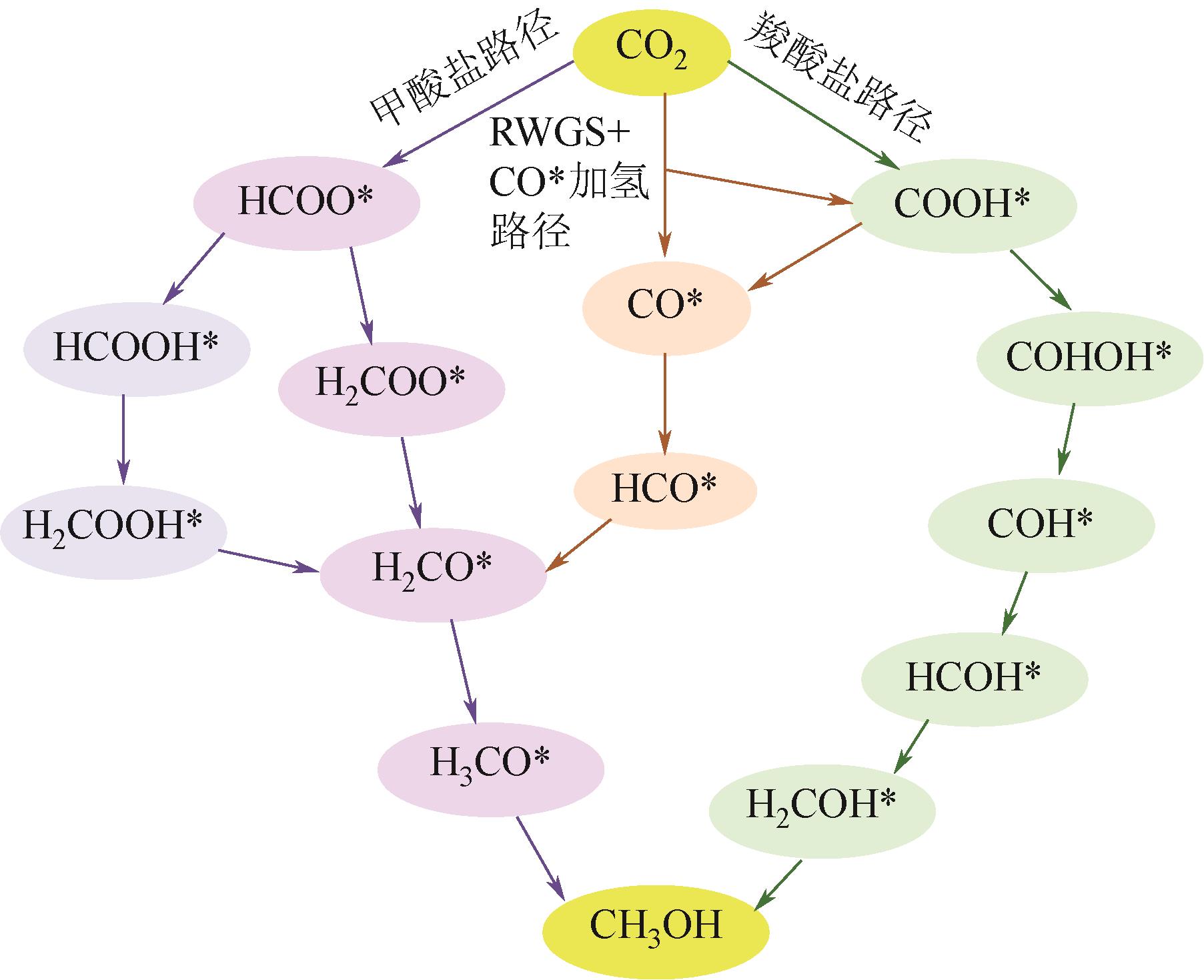
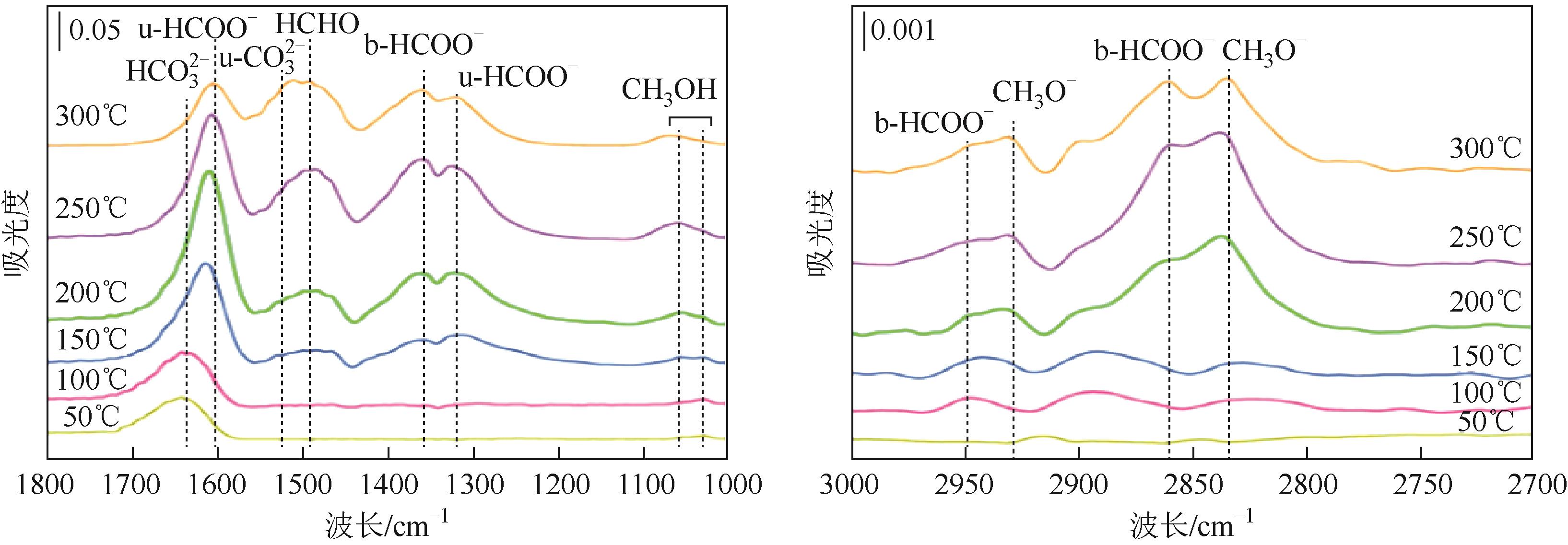
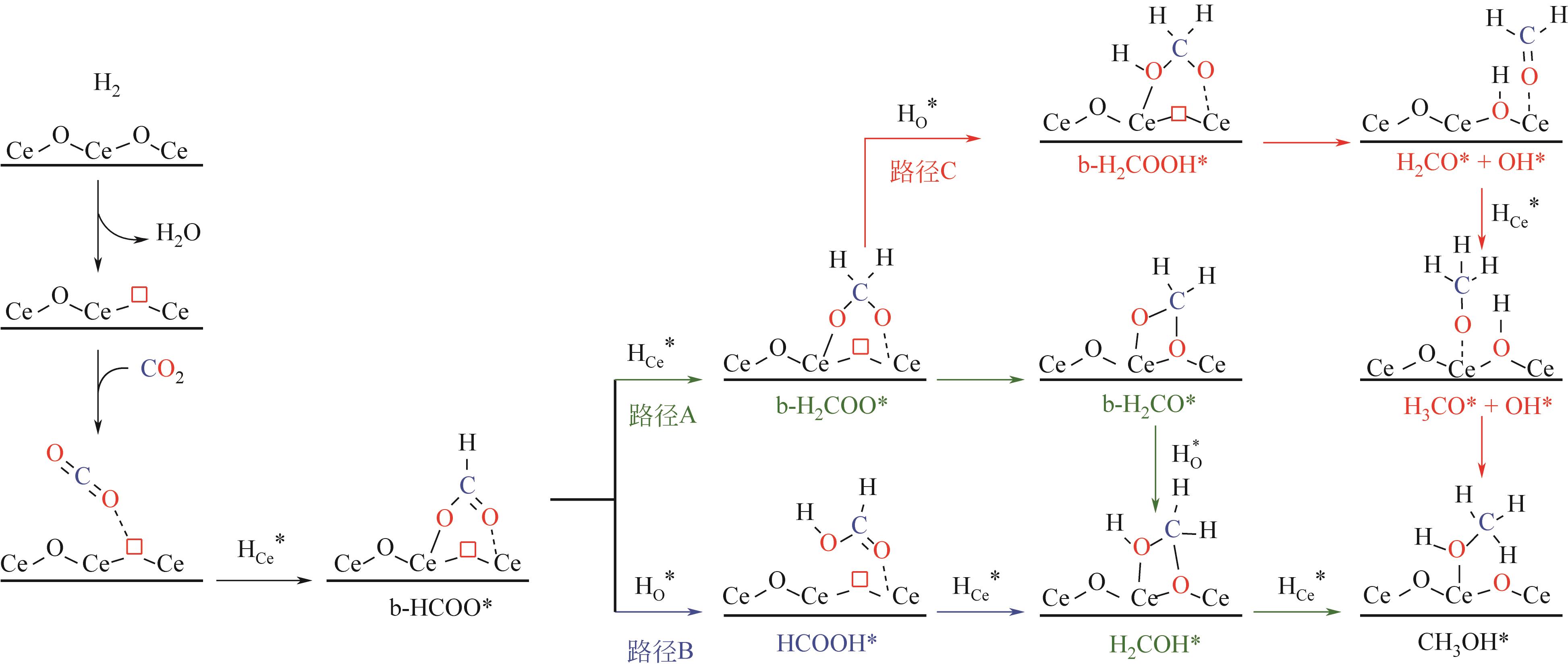
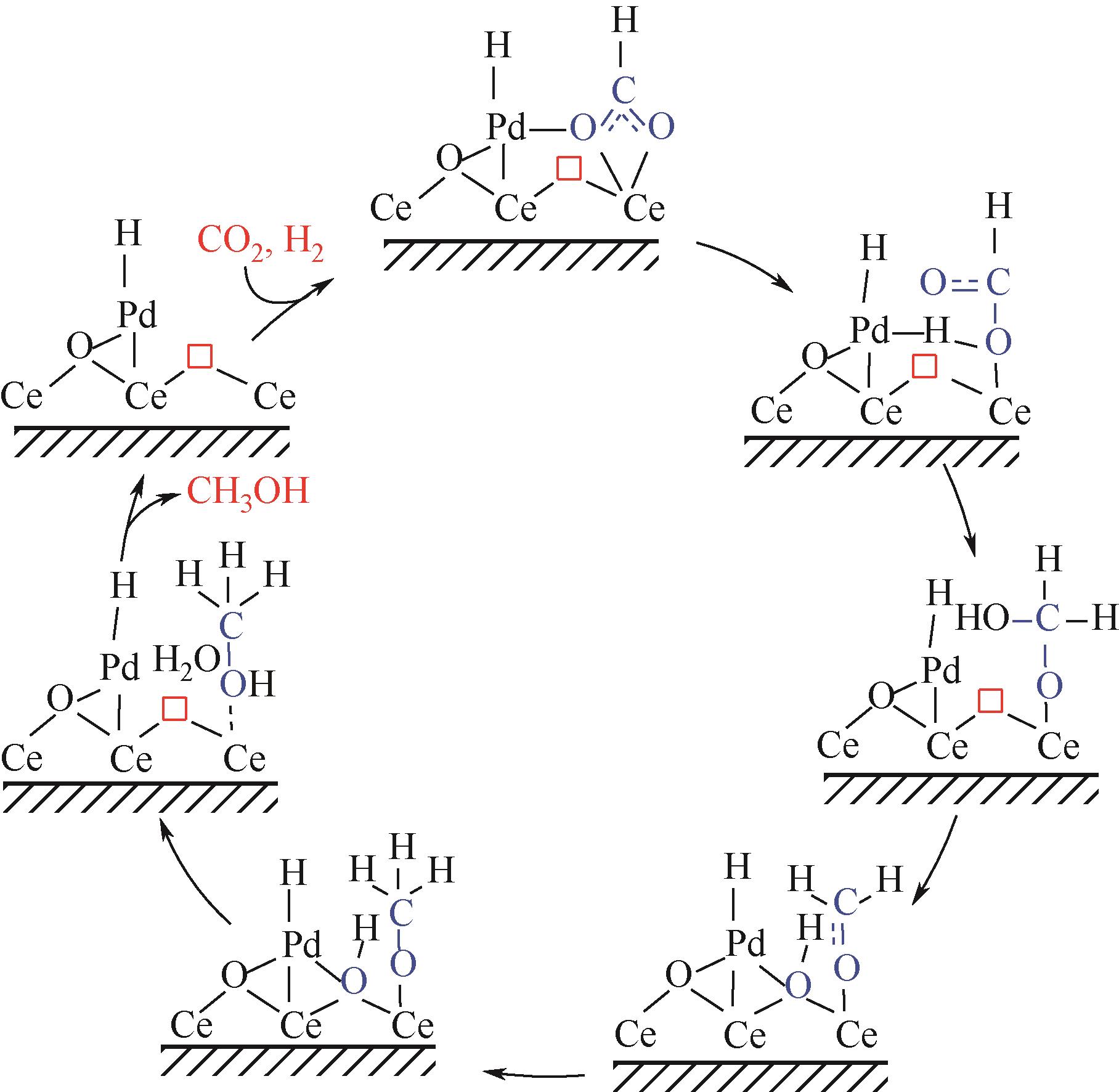
Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (5): 2723-2738.DOI: 10.16085/j.issn.1000-6613.2023-2207
• Carbon dioxide capture and utilization • Previous Articles
Application and research progress of CeO2 support in CO2 hydrogenation to methanol
ZHOU Yuntao1,2( ), WANG Hongxing1,2, LI Xingang1(
), WANG Hongxing1,2, LI Xingang1( ), CUI Lifeng2(
), CUI Lifeng2( )
)
- 1.State Key Laboratory of Chemical Engineering, Collaborative Innovation Center of Chemical Science and Engineering, Tianjin Key Laboratory of Applied Catalysis Science and Engineering, School of Chemical Engineering & Technology, Tianjin University, Tianjin 300354, China
2.Shandong Hualu Hengsheng Chemical Co. , Ltd. , Dezhou 253024, Shandong, China
-
Received:2023-12-15Revised:2024-03-01Online:2024-06-15Published:2024-05-15 -
Contact:LI Xingang, CUI Lifeng
CeO2载体在CO2加氢制甲醇中的应用和研究进展
周运桃1,2( ), 王洪星1,2, 李新刚1(
), 王洪星1,2, 李新刚1( ), 崔丽凤2(
), 崔丽凤2( )
)
- 1.化学工程联合国家重点实验室,天津化学化工协同创新中心,天津市应用催化科学与工程重点实验室,天津大学化工学院,天津 300354
2.山东华鲁恒升化工股份有限公司,山东 德州 253024
-
通讯作者:李新刚,崔丽凤 -
作者简介:周运桃(1988—),男,博士,研究方向为催化反应。E-mail:zhouyuntao1005@163.com。 -
基金资助:国家重点研发计划(2022YFB4101800)
CLC Number:
Cite this article
ZHOU Yuntao, WANG Hongxing, LI Xingang, CUI Lifeng. Application and research progress of CeO2 support in CO2 hydrogenation to methanol[J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2723-2738.
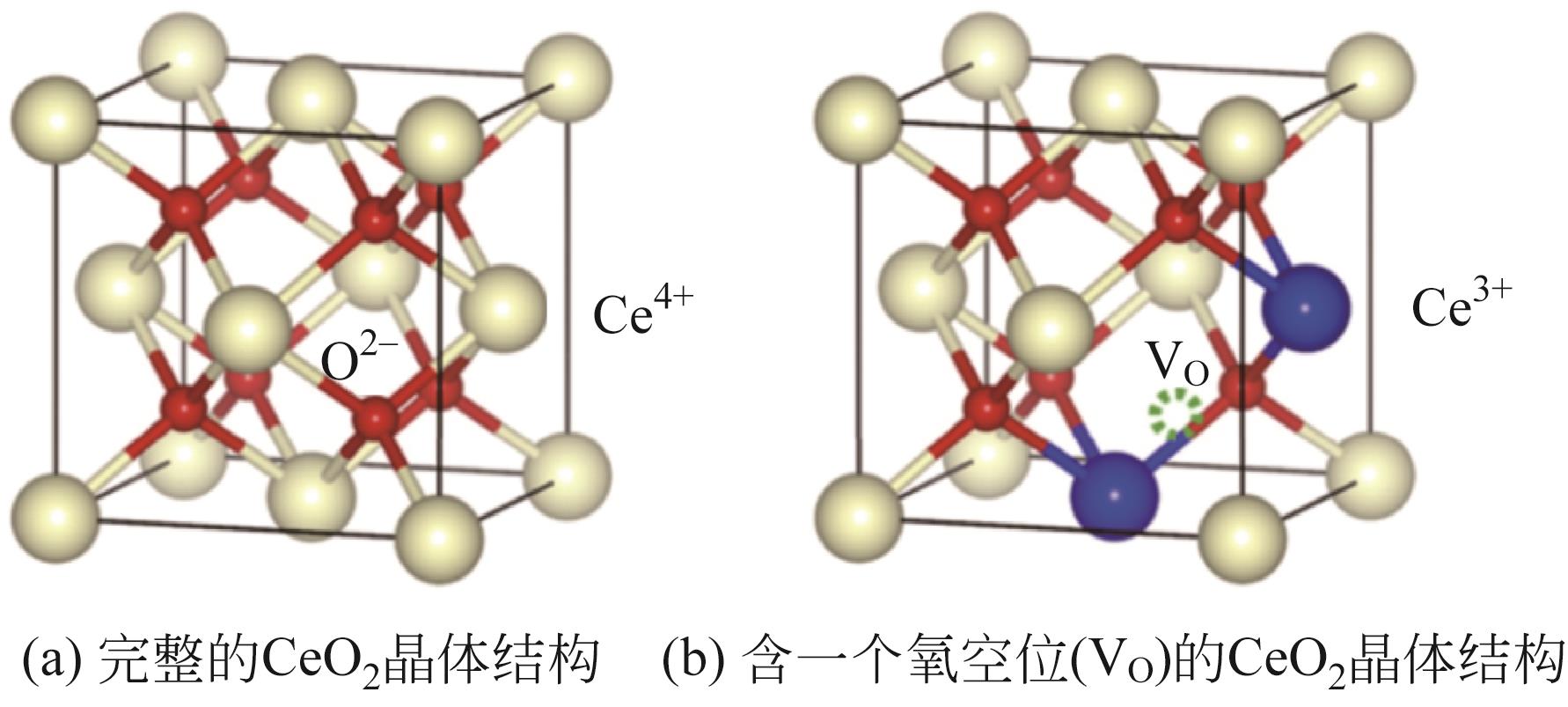
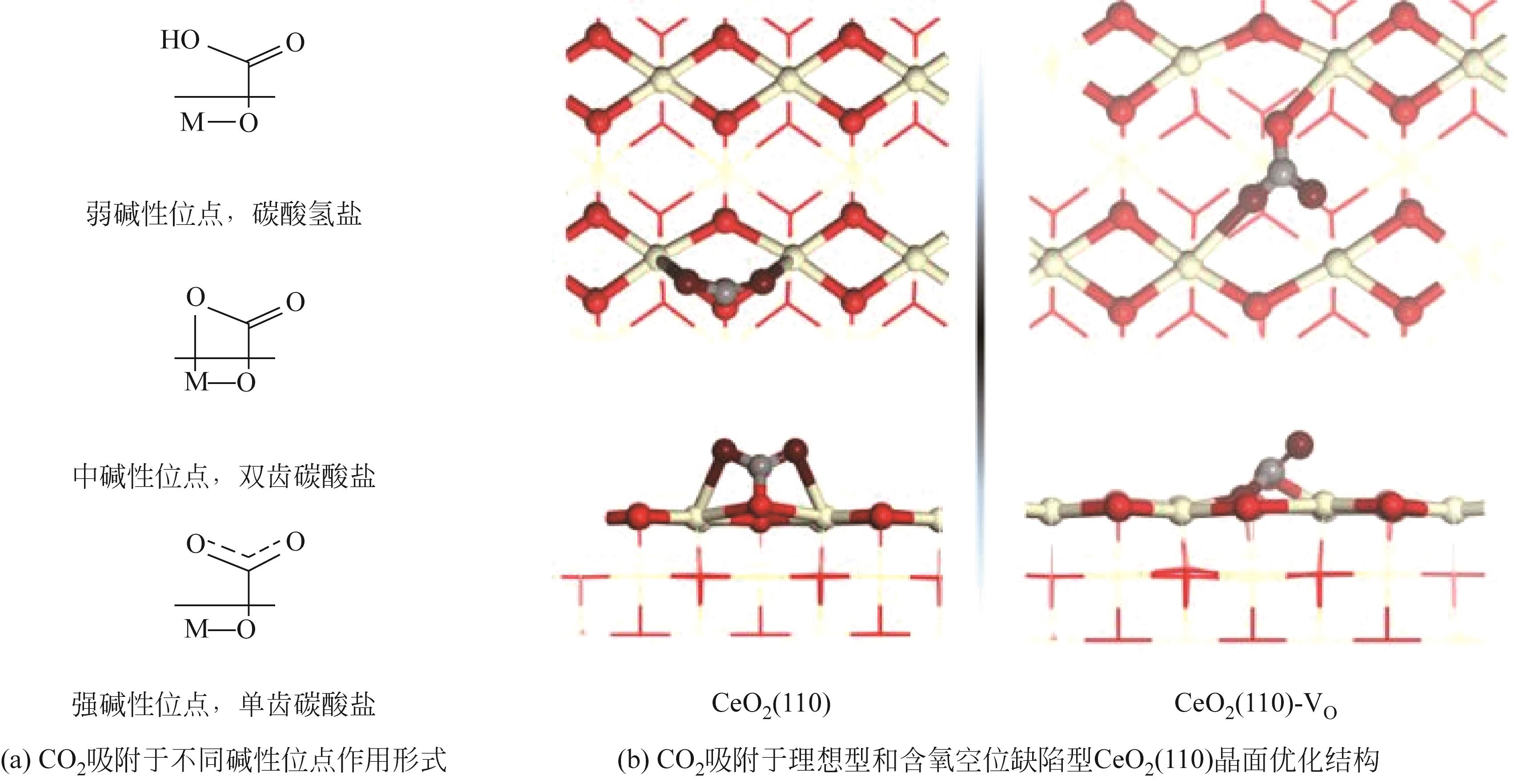
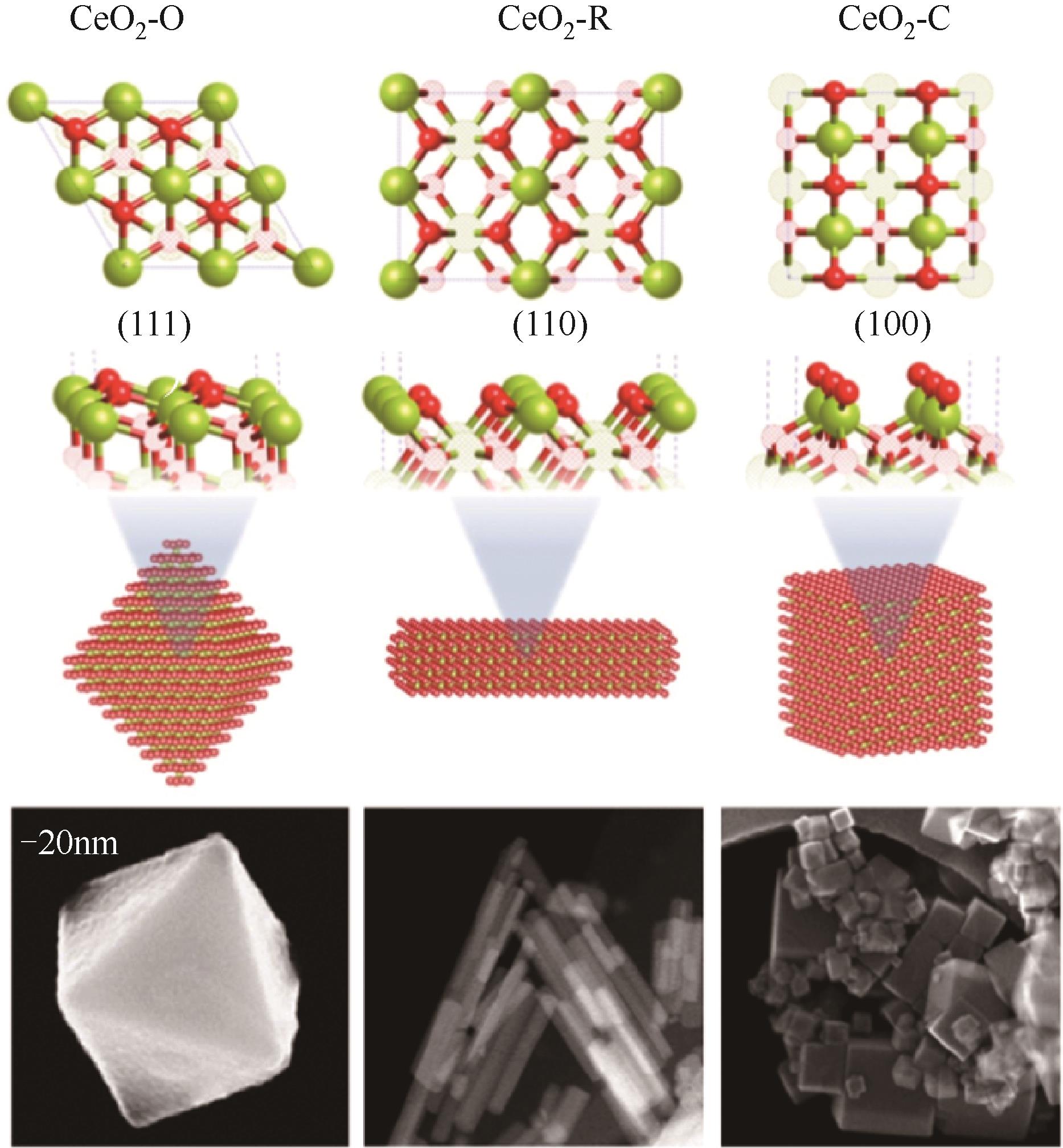
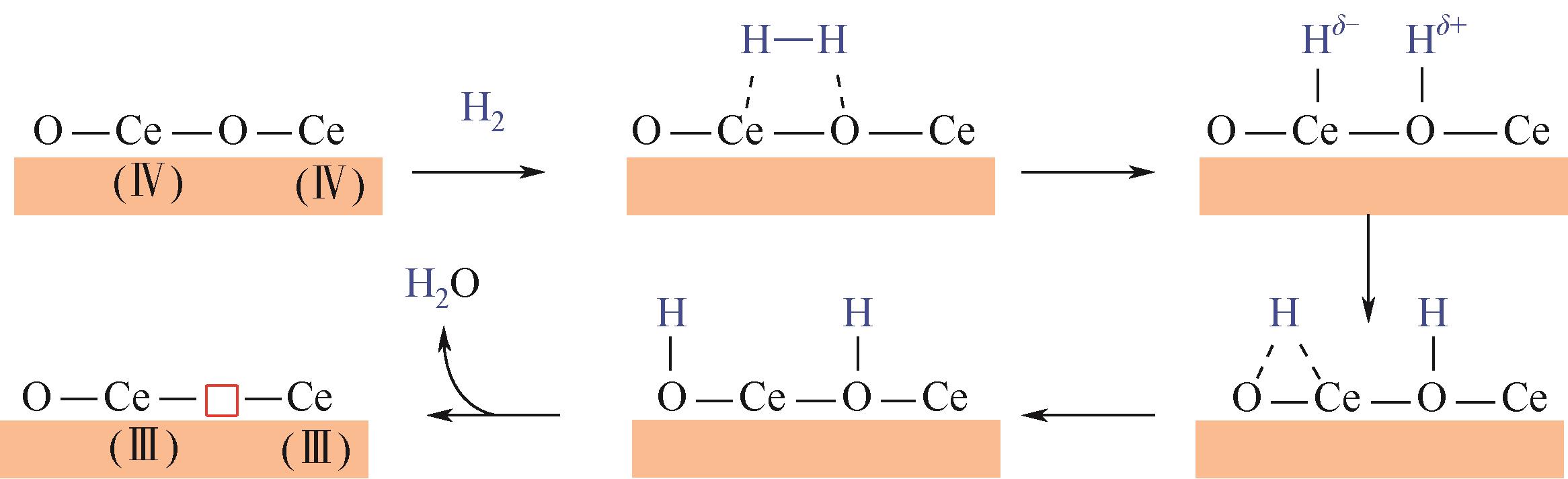
周运桃, 王洪星, 李新刚, 崔丽凤. CeO2载体在CO2加氢制甲醇中的应用和研究进展[J]. 化工进展, 2024, 43(5): 2723-2738.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-2207
| 催化剂 | H2/CO2 | 温度/℃,压力/MPa | GHSV/WHSV | CO2转化率/% | 甲醇选择性/% | STY/g·kgcat-1·h-1 |
|---|---|---|---|---|---|---|
| 2Pd/CeO2-R[ | 3 | 240,3.0 | 2.0L/(gcat·h) | 约6.2① | 约30① | 12.0 |
| 2Pd/CeO2-P[ | 3 | 240,3.0 | 2.0L/(gcat·h) | — | 约29① | 9.5 |
| 2Pd/CeO2-C[ | 3 | 240,3.0 | 2.0L/(gcat·h) | — | 约25① | 5.0 |
| 2Pd/CeO2-O[ | 3 | 240,3.0 | 2.0L/(gcat·h) | 约6.3① | 约26① | 2.5 |
| Cu/CeO2-R[ | 3 | 240,2.0 | 3.0L/(gcat·h) | 约2.2① | 89.5 | 21.1 |
| Cu/CeO2-C[ | 3 | 240,2.0 | 3.0L/(gcat·h) | 约1.5① | 84 | 13.5 |
| Cu/CeO2-S[ | 3 | 240,2.0 | 3.0L/(gcat·h) | 约0.6① | 89 | 5.7 |
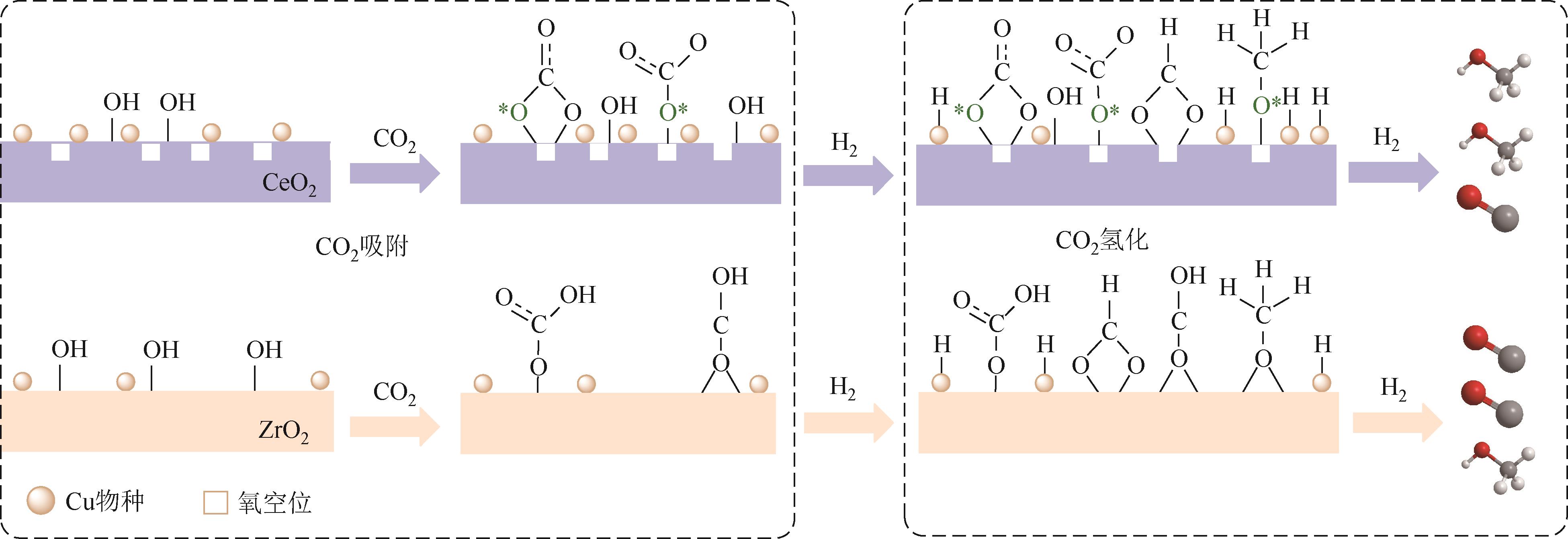
| 1Cu2Ni/CeO2-R[ | 3 | 260,3.0 | 6.0L/h | 18.3 | 73.3 | 230.2 |
| 1Cu2Ni/CeO2-S[ | 3 | 260,3.0 | 6.0L/h | 约17.5① | 约69.5① | 208.8 |
| 1Cu2Ni/CeO2-P[ | 3 | 260,3.0 | 6.0L/h | 约14.0① | 约68.5① | 164.6 |
| Cu/CeO2-SG[ | 3 | 240,3.0 | 2.4L/(gcat·h) | 6.4 | 89.1 | 48.9 |
| Cu/CeO2-SCP[ | 3 | 240,3.0 | 2.4L/(gcat·h) | 5.9 | 84.6 | 42.8 |
| Cu/CeO2-CP[ | 3 | 240,3.0 | 2.4L/(gcat·h) | 3.8 | 80.5 | 26.2 |
| Cu-CF[ | 3 | 260,3.0 | 18.0L/(gcat·h) | 约9.8① | 约41.0① | 249.0 |
| Cu-CP[ | 3 | 260,3.0 | 18.0L·/(gcat·h) | 约9.0① | 约35.0① | 197.0 |
| Cu-IM[ | 3 | 260,3.0 | 18.0L/(gcat·h) | 约5.1① | 约42.5① | 143.0 |
| 5Ag/ZrCeO x-IM[ | 3 | 250,2.0 | 1.8L/h | 7 | 70 | 31.9 |
| 5Ag/ZrCeO x-CH[ | 3 | 250,2.0 | 1.8L/h | 8 | 60 | 30.9 |
| 催化剂 | H2/CO2 | 温度/℃,压力/MPa | GHSV/WHSV | CO2转化率/% | 甲醇选择性/% | STY/g·kgcat-1·h-1 |
|---|---|---|---|---|---|---|
| 2Pd/CeO2-R[ | 3 | 240,3.0 | 2.0L/(gcat·h) | 约6.2① | 约30① | 12.0 |
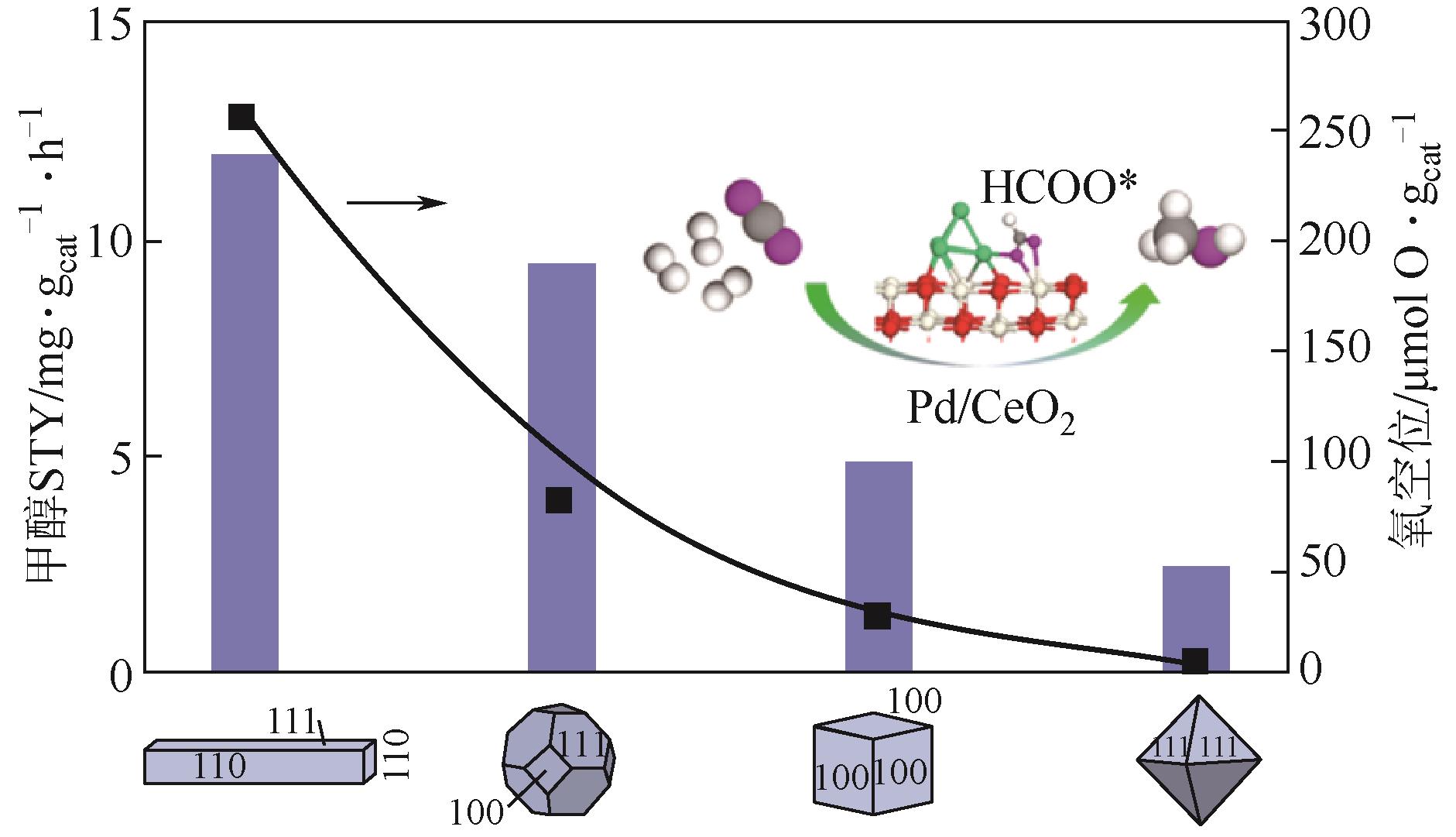
| 2Pd/CeO2-P[ | 3 | 240,3.0 | 2.0L/(gcat·h) | — | 约29① | 9.5 |
| 2Pd/CeO2-C[ | 3 | 240,3.0 | 2.0L/(gcat·h) | — | 约25① | 5.0 |
| 2Pd/CeO2-O[ | 3 | 240,3.0 | 2.0L/(gcat·h) | 约6.3① | 约26① | 2.5 |
| Cu/CeO2-R[ | 3 | 240,2.0 | 3.0L/(gcat·h) | 约2.2① | 89.5 | 21.1 |
| Cu/CeO2-C[ | 3 | 240,2.0 | 3.0L/(gcat·h) | 约1.5① | 84 | 13.5 |
| Cu/CeO2-S[ | 3 | 240,2.0 | 3.0L/(gcat·h) | 约0.6① | 89 | 5.7 |
| 1Cu2Ni/CeO2-R[ | 3 | 260,3.0 | 6.0L/h | 18.3 | 73.3 | 230.2 |
| 1Cu2Ni/CeO2-S[ | 3 | 260,3.0 | 6.0L/h | 约17.5① | 约69.5① | 208.8 |
| 1Cu2Ni/CeO2-P[ | 3 | 260,3.0 | 6.0L/h | 约14.0① | 约68.5① | 164.6 |
| Cu/CeO2-SG[ | 3 | 240,3.0 | 2.4L/(gcat·h) | 6.4 | 89.1 | 48.9 |
| Cu/CeO2-SCP[ | 3 | 240,3.0 | 2.4L/(gcat·h) | 5.9 | 84.6 | 42.8 |
| Cu/CeO2-CP[ | 3 | 240,3.0 | 2.4L/(gcat·h) | 3.8 | 80.5 | 26.2 |
| Cu-CF[ | 3 | 260,3.0 | 18.0L/(gcat·h) | 约9.8① | 约41.0① | 249.0 |
| Cu-CP[ | 3 | 260,3.0 | 18.0L·/(gcat·h) | 约9.0① | 约35.0① | 197.0 |
| Cu-IM[ | 3 | 260,3.0 | 18.0L/(gcat·h) | 约5.1① | 约42.5① | 143.0 |
| 5Ag/ZrCeO x-IM[ | 3 | 250,2.0 | 1.8L/h | 7 | 70 | 31.9 |
| 5Ag/ZrCeO x-CH[ | 3 | 250,2.0 | 1.8L/h | 8 | 60 | 30.9 |
| 催化剂 | H2/CO2 | 温度/℃,压力/MPa | GHSV/WHSV | CO2转化率/% | 甲醇选择性/% | STY/g·kgcat-1·h-1 |
|---|---|---|---|---|---|---|
| 1Pd-10Cu/CeO2[ | 3 | 250,3.0 | 3.0L/(gcat·h) | 16.1 | 26.7 | 28.3 |
| 5Pd5Zn/CeO2[ | 3 | 220,3.0 | 2.4L/(gcat·h) | 6.3 | 100 | 54.1 |
| 0.5Ca5Pd5Zn/CeO2[ | 3 | 220,3.0 | 2.4L/(gcat·h) | 7.7 | 100 | 66.1 |
| PdZn/CeO2[ | 3 | 220,2.0 | 2.4L/(gcat·h) | 14.1 | 94.5 | 114.3 |
| CuNi2/CeO2-NT[ | 3 | 260,3.0 | 6.0L/h | 17.8 | 78.8 | 579.9 |
| 催化剂 | H2/CO2 | 温度/℃,压力/MPa | GHSV/WHSV | CO2转化率/% | 甲醇选择性/% | STY/g·kgcat-1·h-1 |
|---|---|---|---|---|---|---|
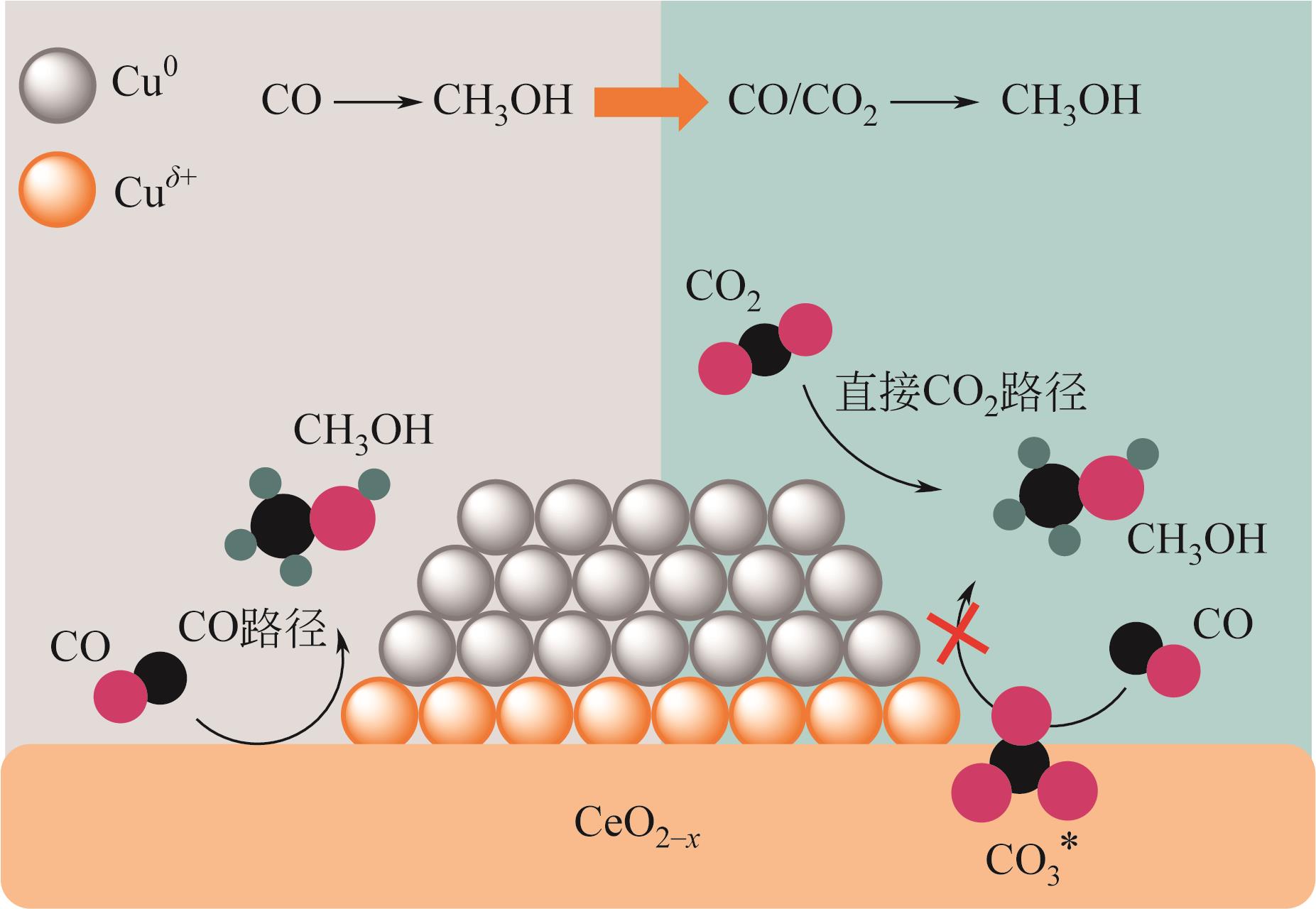
| 1Pd-10Cu/CeO2[ | 3 | 250,3.0 | 3.0L/(gcat·h) | 16.1 | 26.7 | 28.3 |
| 5Pd5Zn/CeO2[ | 3 | 220,3.0 | 2.4L/(gcat·h) | 6.3 | 100 | 54.1 |
| 0.5Ca5Pd5Zn/CeO2[ | 3 | 220,3.0 | 2.4L/(gcat·h) | 7.7 | 100 | 66.1 |
| PdZn/CeO2[ | 3 | 220,2.0 | 2.4L/(gcat·h) | 14.1 | 94.5 | 114.3 |
| CuNi2/CeO2-NT[ | 3 | 260,3.0 | 6.0L/h | 17.8 | 78.8 | 579.9 |
| 催化剂 | H2/CO2 | 温度/℃,压力/MPa | GHSV/WHSV | CO2转化率/% | 甲醇选择性/% | STY/g·kgcat-1·h-1 |
|---|---|---|---|---|---|---|
| Cu/CeO2[ | 3 | 220,3.0 | 2.4L/(gcat·h) | 约2.5① | 91 | 19.5 |
| Cu/ZrO2[ | 3 | 220,3.0 | 2.4L/(gcat·h) | 约3.0① | 80 | 20.6 |
| Cu/ZnO[ | 3 | 220,3.0 | 2.4L/(gcat·h) | 约3.5① | 74 | 22.2 |
| Cu/CeO2[ | 3 | 220,3.0 | 10L/h | 4.5 | 约85① | 136.8 |
| Cu/ZrO2[ | 3 | 220,3.0 | 10L/h | 6.5 | 约77① | 179.0 |
| Cu/CeO2[ | 3 | 250,3.0 | 30L/(gcat·h) | 1.0 | 53 | 45.5 |
| Cu/SiO2[ | 3 | 250,3.0 | 60L/(gcat·h) | 1.1 | 16 | 30.2 |
| Au/CeO2[ | 9 | 225,0.1 | 20L/h | — | 62.2 | — |
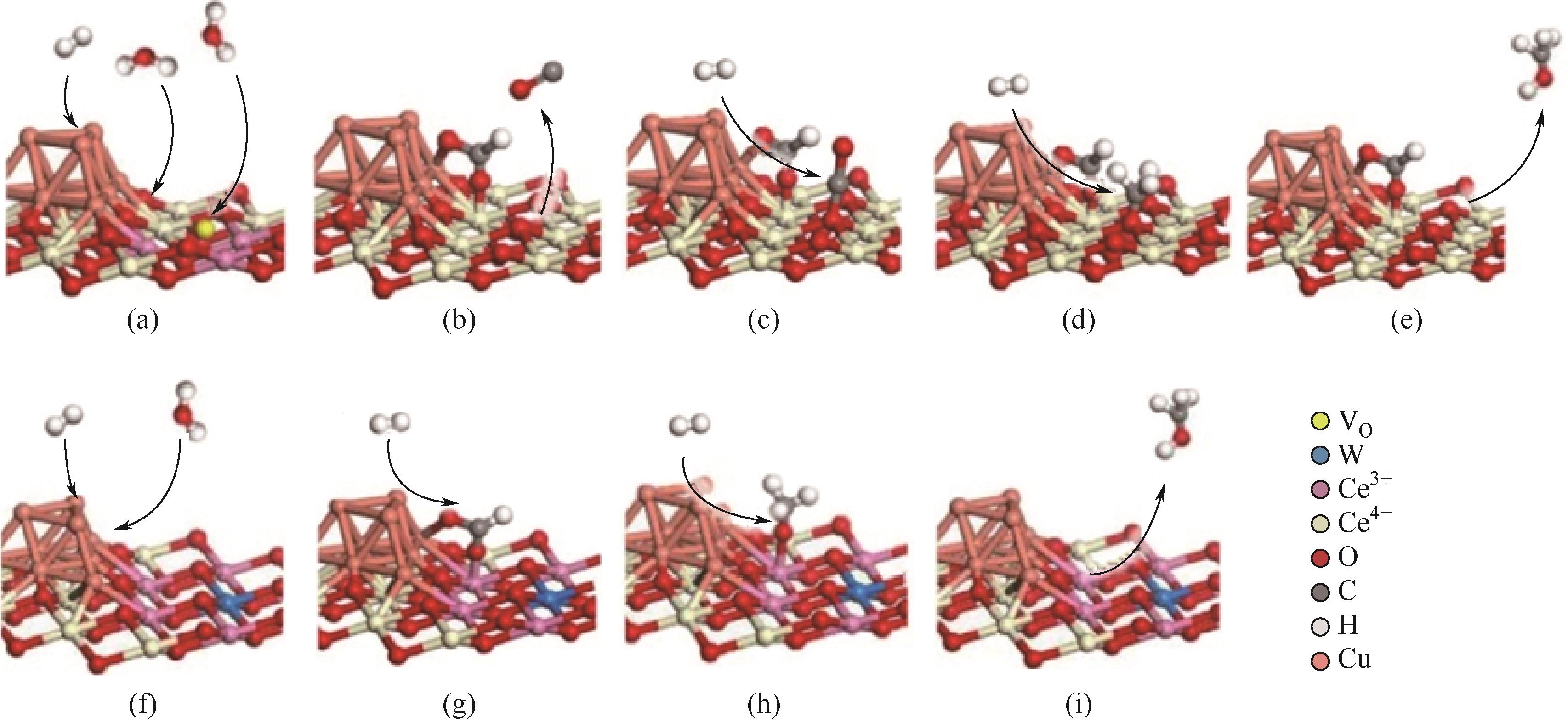
| Au/ZnO[ | 9 | 225,0.1 | 20L/h | — | 88.9 | — |
| Ni5Ga3/SiO2[ | 3 | 270,1.0 | 8.0L/(gcat·h) | 1.5 | 12.5 | 50.0 |
| Ni5Ga3/ZrO2[ | 3 | 270,1.0 | 8.0L/(gcat·h) | 3.5 | 18.0 | 160.0 |
| Ni5Ga3/CeO2[ | 3 | 270,1.0 | 8.0L/(gcat·h) | 6.1 | 3.4 | 54.0 |
| 催化剂 | H2/CO2 | 温度/℃,压力/MPa | GHSV/WHSV | CO2转化率/% | 甲醇选择性/% | STY/g·kgcat-1·h-1 |
|---|---|---|---|---|---|---|
| Cu/CeO2[ | 3 | 220,3.0 | 2.4L/(gcat·h) | 约2.5① | 91 | 19.5 |
| Cu/ZrO2[ | 3 | 220,3.0 | 2.4L/(gcat·h) | 约3.0① | 80 | 20.6 |
| Cu/ZnO[ | 3 | 220,3.0 | 2.4L/(gcat·h) | 约3.5① | 74 | 22.2 |
| Cu/CeO2[ | 3 | 220,3.0 | 10L/h | 4.5 | 约85① | 136.8 |
| Cu/ZrO2[ | 3 | 220,3.0 | 10L/h | 6.5 | 约77① | 179.0 |
| Cu/CeO2[ | 3 | 250,3.0 | 30L/(gcat·h) | 1.0 | 53 | 45.5 |
| Cu/SiO2[ | 3 | 250,3.0 | 60L/(gcat·h) | 1.1 | 16 | 30.2 |
| Au/CeO2[ | 9 | 225,0.1 | 20L/h | — | 62.2 | — |
| Au/ZnO[ | 9 | 225,0.1 | 20L/h | — | 88.9 | — |
| Ni5Ga3/SiO2[ | 3 | 270,1.0 | 8.0L/(gcat·h) | 1.5 | 12.5 | 50.0 |
| Ni5Ga3/ZrO2[ | 3 | 270,1.0 | 8.0L/(gcat·h) | 3.5 | 18.0 | 160.0 |
| Ni5Ga3/CeO2[ | 3 | 270,1.0 | 8.0L/(gcat·h) | 6.1 | 3.4 | 54.0 |
| 催化剂 | H2/CO2 | 温度/℃,压力/MPa | GHSV/WHSV | CO2转化率/% | 甲醇选择性/% | STY/g·kgcat-1·h-1 |
|---|---|---|---|---|---|---|
| Cu/ZnO-CeO2[ | 3 | 280,3.0 | 12.0L/h | 15.6 | 64.5 | 431.7 |
| Cu/AlCeO-7[ | 3 | 260,3.0 | 14.4L/(gcat·h) | 约17① | 约45① | 381.3 |
| Cu0.3Zr0.3Ce0.7[ | 3 | 240,3.0 | 30.0L/(gcat·h) | 4.1 | 55.3 | 192.0 |
| Cu30Ce35Zr35O[ | 3 | 250,3.0 | 7.5L/(gcat·h) | 14.3 | 53.8 | 165.0 |
| 5-Cu-CeO2/ZrO2[ | 3 | 260,5.0 | 60.0L/(gcat·h) | 1.7 | 42 | 147.1 |
| CuO/Ce0.4Zr0.6O2[ | 3 | 280,3.0 | 10.0L/h | 13.2 | 71.8 | 338.9 |
| CuO/Mn0.2CeO x[ | 3 | 260,1.5 | 6.0L/(gcat·h) | 14.2 | 82.3 | 250.0 |
| CuO/La0.25CeO x[ | 3 | 260,1.5 | 6.0L/(gcat·h) | 15.7 | 83.3 | 281.0 |
| Cu/CeO2[ | 3 | 250,3.5 | 15.0L/(gcat·h) | 1.3 | 51 | 40.4 |
| Cu/CeW0.25O x[ | 3 | 250,3.5 | 15.0L/(gcat·h) | 13 | 87 | 394.7 |
| 催化剂 | H2/CO2 | 温度/℃,压力/MPa | GHSV/WHSV | CO2转化率/% | 甲醇选择性/% | STY/g·kgcat-1·h-1 |
|---|---|---|---|---|---|---|
| Cu/ZnO-CeO2[ | 3 | 280,3.0 | 12.0L/h | 15.6 | 64.5 | 431.7 |
| Cu/AlCeO-7[ | 3 | 260,3.0 | 14.4L/(gcat·h) | 约17① | 约45① | 381.3 |
| Cu0.3Zr0.3Ce0.7[ | 3 | 240,3.0 | 30.0L/(gcat·h) | 4.1 | 55.3 | 192.0 |
| Cu30Ce35Zr35O[ | 3 | 250,3.0 | 7.5L/(gcat·h) | 14.3 | 53.8 | 165.0 |
| 5-Cu-CeO2/ZrO2[ | 3 | 260,5.0 | 60.0L/(gcat·h) | 1.7 | 42 | 147.1 |
| CuO/Ce0.4Zr0.6O2[ | 3 | 280,3.0 | 10.0L/h | 13.2 | 71.8 | 338.9 |
| CuO/Mn0.2CeO x[ | 3 | 260,1.5 | 6.0L/(gcat·h) | 14.2 | 82.3 | 250.0 |
| CuO/La0.25CeO x[ | 3 | 260,1.5 | 6.0L/(gcat·h) | 15.7 | 83.3 | 281.0 |
| Cu/CeO2[ | 3 | 250,3.5 | 15.0L/(gcat·h) | 1.3 | 51 | 40.4 |
| Cu/CeW0.25O x[ | 3 | 250,3.5 | 15.0L/(gcat·h) | 13 | 87 | 394.7 |
| 1 | FORSTER Piers M, SMITH Christopher J, WALSH Tristram, et al. Indicators of global climate change 2022: Annual update of large-scale indicators of the state of the climate system and human influence[J]. Earth System Science Data, 2023, 15(6): 2295-2327. |
| 2 | CHENG Lijing, ABRAHAM John, TRENBERTH Kevin E, et al. Another year of record heat for the oceans[J]. Advances in Atmospheric Sciences, 2023, 40(6): 963-974. |
| 3 | LAN Xin, TANS Pieter, KIRK Thoning. Trends in globally-averaged CO2 determined from NOAA Global Monitoring Laboratory measurements[EB/OL]. (2023-11-30) [2023-12-10]. . |
| 4 | LIN Qingyang, ZHANG Xiao, WANG Tao, et al. Technical perspective of carbon capture, utilization, and storage[J]. Engineering, 2022, 14: 27-32. |
| 5 | DE Sudipta, DOKANIA Abhay, RAMIREZ Adrian, et al. Advances in the design of heterogeneous catalysts and thermocatalytic processes for CO2 utilization[J]. ACS Catalysis, 2020, 10(23): 14147-14185. |
| 6 | KUMARAVEL Vignesh, BARTLETT John, PILLAI Suresh C. Photoelectrochemical conversion of carbon dioxide (CO2) into fuels and value-added products[J]. ACS Energy Letters, 2020, 5(2): 486-519. |
| 7 | GAO Peng, ZHANG Lina, LI Shenggang, et al. Novel heterogeneous catalysts for CO2 hydrogenation to liquid fuels[J]. ACS Central Science, 2020, 6(10): 1657-1670. |
| 8 | KALIYAPERUMAL Alamelu, GUPTA Pooja, PRASAD Yadavalli Satya Sivaram, et al. Recent progress and perspective of the electrochemical conversion of carbon dioxide to alcohols[J]. ACS Engineering Au, 2023, 3(6): 403-425. |
| 9 | GANJI Parameswaram, CHOWDARI Ramesh Kumar, LIKOZAR Blaž. Photocatalytic reduction of carbon dioxide to methanol: Carbonaceous materials, kinetics, industrial feasibility, and future directions[J]. Energy & Fuels, 2023, 37(11): 7577-7602. |
| 10 | MINYUKOVA Tatyana P, DOKUCHITS Eugene V. Hydrogen for CO2 processing in heterogeneous catalytic reactions[J]. International Journal of Hydrogen Energy, 2023, 48(59): 22462-22483. |
| 11 | SHIH Choon Fong, ZHANG Tao, LI Jinghai, et al. Powering the future with liquid sunshine[J]. Joule, 2018, 2(10): 1925-1949. |
| 12 | DANG Shanshan, YANG Haiyan, GAO Peng, et al. A review of research progress on heterogeneous catalysts for methanol synthesis from carbon dioxide hydrogenation[J]. Catalysis Today, 2019, 330: 61-75. |
| 13 | ZHONG Jiawei, YANG Xiaofeng, WU Zhilian, et al. State of the art and perspectives in heterogeneous catalysis of CO2 hydrogenation to methanol[J]. Chemical Society Reviews, 2020, 49(5): 1385-1413. |
| 14 | ROY Soumyabrata, CHEREVOTAN Arjun, PETER Sebastian C. Thermochemical CO2 hydrogenation to single carbon products: Scientific and technological challenges[J]. ACS Energy Letters, 2018, 3(8): 1938-1966. |
| 15 | BAI Shaotao, DE SMET Gilles, LIAO Yuhe, et al. Homogeneous and heterogeneous catalysts for hydrogenation of CO2 to methanol under mild conditions[J]. Chemical Society Reviews, 2021, 50(7): 4259-4298. |
| 16 | MURTHY Pradeep S, LIANG Weibin, JIANG Yijiao, et al. Cu-based nanocatalysts for CO2 hydrogenation to methanol[J]. Energy & Fuels, 2021, 35(10): 8558-8584. |
| 17 | VIEIRA Luiz H, RASTEIRO Letícia F, SANTANA Cássia S, et al. Noble metals in recent developments of heterogeneous catalysts for CO2 conversion processes[J]. ChemCatChem, 2023, 15(14): e202300493. |
| 18 | LIANG Binglian, MA Junguo, SU Xiong, et al. Investigation on deactivation of Cu/ZnO/Al2O3 catalyst for CO2 hydrogenation to methanol[J]. Industrial & Engineering Chemistry Research, 2019, 58(21): 9030-9037. |
| 19 | WANG Jijie, LI Guanna, LI Zelong, et al. A highly selective and stable ZnO-ZrO2 solid solution catalyst for CO2 hydrogenation to methanol[J]. Science Advances, 2017, 3(10): e1701290. |
| 20 | YANG Chengsheng, PEI Chunlei, LUO Ran, et al. Strong electronic oxide-support interaction over In2O3/ZrO2 for highly selective CO2 hydrogenation to methanol[J]. Journal of the American Chemical Society, 2020, 142(46): 19523-19531. |
| 21 | MARTIN Oliver, MARTÍN Antonio J, MONDELLI Cecilia, et al. Indium oxide as a superior catalyst for methanol synthesis by CO2 hydrogenation[J]. Angewandte Chemie International Edition, 2016, 55(21): 6261-6265. |
| 22 | LI Huazheng, QIU Chenglong, REN Shoujie, et al. Na+-gated water-conducting nanochannels for boosting CO2 conversion to liquid fuels[J]. Science, 2020, 367(6478): 667-671. |
| 23 | SONOWAL Karanika, NANDAL Neha, BASYACH Purashri, et al. Photocatalytic reduction of CO2 to methanol using Zr(Ⅳ)-based MOF composite with g-C3N4 quantum dots under visible light irradiation[J]. Journal of CO2 Utilization, 2022, 57: 101905. |
| 24 | WANG Fei, WEI Min, EVANS David G, et al. CeO2-based heterogeneous catalysts toward catalytic conversion of CO2 [J]. Journal of Materials Chemistry A, 2016, 4(16): 5773-5783. |
| 25 | CHANG Kuan, ZHANG Haochen, CHENG Mu-jeng, et al. Application of ceria in CO2 conversion catalysis[J]. ACS Catalysis, 2020, 10(1): 613-631. |
| 26 | ZHANG Sai, TIAN Zhimin, MA Yuanyuan, et al. Adsorption of molecules on defective CeO2 for advanced catalysis[J]. ACS Catalysis, 2023, 13(7): 4629-4645. |
| 27 | GRACIANI Jesús, MUDIYANSELAGE Kumudu, XU Fang, et al. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from CO2 [J]. Science, 2014, 345(6196): 546-550. |
| 28 | HUANG Weixin, GAO Yuxian. Morphology-dependent surface chemistry and catalysis of CeO2 nanocrystals[J]. Catalysis Science & Technology, 2014, 4(11): 3772-3784. |
| 29 | TROVARELLI Alessandro, LLORCA Jordi. Ceria catalysts at nanoscale: How do crystal shapes shape catalysis?[J]. ACS Catalysis, 2017, 7(7): 4716-4735. |
| 30 | MA Yuanyuan, GAO Wei, ZHANG Zhiyun, et al. Regulating the surface of nanoceria and its applications in heterogeneous catalysis[J]. Surface Science Reports, 2018, 73(1): 1-36. |
| 31 | LI Ping, CHEN Xiaoyin, LI Yongdan, et al. A review on oxygen storage capacity of CeO2-based materials: Influence factors, measurement techniques, and applications in reactions related to catalytic automotive emissions control[J]. Catalysis Today, 2019, 327: 90-115. |
| 32 | HUANG Xiubing, ZHANG Kaiyue, PENG Baoxiang, et al. Ceria-based materials for thermocatalytic and photocatalytic organic synthesis[J]. ACS Catalysis, 2021, 11(15): 9618-9678. |
| 33 | VIVIER Laurence, DUPREZ Daniel. Ceria-based solid catalysts for organic chemistry[J]. ChemSusChem, 2010, 3(6): 654-678. |
| 34 | LI Ge, WANG Ping, HE Miao, et al. Cerium-based nanomaterials for photo/electrocatalysis[J]. Science China Chemistry, 2023, 66(8): 2204-2220. |
| 35 | CAI Jun, LI Danyang, JIANG Lei, et al. Review on CeO2-based photocatalysts for photocatalytic reduction of CO2: Progresses and perspectives[J]. Energy & Fuels, 2023, 37(7): 4878-4897. |
| 36 | WANG Jianda, XIAO Xiao, LIU Yong, et al. The application of CeO2-based materials in electrocatalysis[J]. Journal of Materials Chemistry A, 2019, 7(30): 17675-17702. |
| 37 | YU Jun, DU Xinjuan, LIU Hongzhi, et al. Mini review on active sites in Ce-based electrocatalysts for alkaline water splitting[J]. Energy & Fuels, 2021, 35(23): 19000-19011. |
| 38 | MONTINI Tiziano, MELCHIONNA Michele, MONAI Matteo, et al. Fundamentals and catalytic applications of CeO2-based materials[J]. Chemical Reviews, 2016, 116(10): 5987-6041. |
| 39 | SINGH Rajan, PANDEY Vaibhav, PANT Kamal Kishore. Promotional role of oxygen vacancy defects and Cu-Ce interfacial sites on the activity of Cu/CeO2 catalyst for CO2 hydrogenation to methanol[J]. ChemCatChem, 2022, 14(24): e202201053. |
| 40 | GAO Peng, LI Feng, ZHAO Ning, et al. Influence of modifier (Mn, La, Ce, Zr and Y) on the performance of Cu/Zn/Al catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol[J]. Applied Catalysis A: General, 2013, 468: 442-452. |
| 41 | SINGH Rajan, TRIPATHI Komal, PANT Kamal Kishore. Investigating the role of oxygen vacancies and basic site density in tuning methanol selectivity over Cu/CeO2 catalyst during CO2 hydrogenation[J]. Fuel, 2021, 303: 121289. |
| 42 | WANG Weiwei, QU Zhenping, SONG Lixin, et al. CO2 hydrogenation to methanol over Cu/CeO2 and Cu/ZrO2 catalysts: Tuning methanol selectivity via metal-support interaction[J]. Journal of Energy Chemistry, 2020, 40: 22-30. |
| 43 | FAN Liping, ZHANG Jing, MA Kexin, et al. Ceria morphology-dependent Pd-CeO2 interaction and catalysis in CO2 hydrogenation into formate[J]. Journal of Catalysis, 2021, 397: 116-127. |
| 44 | XIE Yu, CHEN Jianjun, WU Xi, et al. Frustrated Lewis pairs boosting low-temperature CO2 methanation performance over Ni/CeO2 nanocatalysts[J]. ACS Catalysis, 2022, 12(17): 10587-10602. |
| 45 | ZHANG Sai, XIA Zhaoming, ZOU Yong, et al. Interfacial frustrated Lewis pairs of CeO2 activate CO2 for selective tandem transformation of olefins and CO2 into cyclic carbonates[J]. Journal of the American Chemical Society, 2019, 141(29): 11353-11357. |
| 46 | LIU Bin, LI Congming, ZHANG Guoqiang, et al. Oxygen vacancy promoting dimethyl carbonate synthesis from CO2 and methanol over Zr-doped CeO2 nanorods[J]. ACS Catalysis, 2018, 8(11): 10446-10456. |
| 47 | ZHANG Wei, MA Xuelu, XIAO Hai, et al. Mechanistic investigations on thermal hydrogenation of CO2 to methanol by nanostructured CeO2(100): The crystal-plane effect on catalytic reactivity[J]. The Journal of Physical Chemistry C, 2019, 123(18): 11763-11771. |
| 48 | KUMARI Neetu, HAIDER M ALI, AGARWAL Manish, et al. Role of reduced CeO2(110) surface for CO2 reduction to CO and methanol[J]. The Journal of Physical Chemistry C, 2016, 120(30): 16626-16635. |
| 49 | SCHMITT Rafael, NENNING Andreas, KRAYNIS Olga, et al. A review of defect structure and chemistry in ceria and its solid solutions[J]. Chemical Society Reviews, 2020, 49(2): 554-592. |
| 50 | GUO Chen, WEI Shuxian, ZHOU Sainan, et al. Initial reduction of CO2 on Pd-, Ru-, and Cu-doped CeO2(111) surfaces: Effects of surface modification on catalytic activity and selectivity[J]. ACS Applied Materials & Interfaces, 2017, 9(31): 26107-26117. |
| 51 | Marçal CAPDEVILA-CORTADA, Gianvito VILÉ, TESCHNER Detre, et al. Reactivity descriptors for ceria in catalysis[J]. Applied Catalysis B: Environmental, 2016, 197: 299-312. |
| 52 | ZHANG Yang, ZHAO Shuna, FENG Jing, et al. Unraveling the physical chemistry and materials science of CeO2-based nanostructures[J]. Chem., 2021, 7(8): 2022-2059. |
| 53 | JIANG Feng, WANG Shanshan, LIU Bing, et al. Insights into the influence of CeO2 crystal facet on CO2 hydrogenation to methanol over Pd/CeO2 catalysts[J]. ACS Catalysis, 2020, 10(19): 11493-11509. |
| 54 | OUYANG Bi, TAN Weiling, LIU Bing. Morphology effect of nanostructure ceria on the Cu/CeO2 catalysts for synthesis of methanol from CO2 hydrogenation[J]. Catalysis Communications, 2017, 95: 36-39. |
| 55 | WEI Yuechang, ZHANG Yilin, ZHANG Peng, et al. Boosting the removal of diesel soot particles by the optimal exposed crystal facet of CeO2 in Au/CeO2 catalysts[J]. Environmental Science & Technology, 2020, 54(3): 2002-2011. |
| 56 | WANG Zheng, HUANG Zhenpeng, BROSNAHAN John T, et al. Ru/CeO2 catalyst with optimized CeO2 support morphology and surface facets for propane combustion[J]. Environmental Science & Technology, 2019, 53(9): 5349-5358. |
| 57 | WERNER Kristin, WENG Xuefei, CALAZA Florencia, et al. Toward an understanding of selective alkyne hydrogenation on ceria: On the impact of O vacancies on H2 interaction with CeO2(111)[J]. Journal of the American Chemical Society, 2017, 139(48): 17608-17616. |
| 58 | WANG Zhiqiang, CHU Deren, ZHOU Hui, et al. Role of low-coordinated Ce in hydride formation and selective hydrogenation reactions on CeO2 surfaces[J]. ACS Catalysis, 2022, 12(1): 624-632. |
| 59 | WANG Zhiqiang, LIU Huihui, WU Xinping, et al. Hydride generation on the Cu-doped CeO2(111) surface and its role in CO2 hydrogenation reactions[J]. Catalysts, 2022, 12(9): 963. |
| 60 | LEE Jaeha, TIEU Peter, FINZEL Jordan, et al. How Pt influences H2 reactions on high surface-area Pt/CeO2 powder catalyst surfaces[J]. JACS Au, 2023, 3(8): 2299-2313. |
| 61 | AZHARI Noerma J, ERIKA Denanti, MARDIANA St, et al. Methanol synthesis from CO2: A mechanistic overview[J]. Results in Engineering, 2022, 16: 100711. |
| 62 | ZHAO Yafan, YANG Yong, MIMS Charles, et al. Insight into methanol synthesis from CO2 hydrogenation on Cu(111): Complex reaction network and the effects of H2O[J]. Journal of Catalysis, 2011, 281(2): 199-211. |
| 63 | LIU Lingna, YAO Hedan, JIANG Zhao, et al. Theoretical study of methanol synthesis from CO2 hydrogenation on PdCu3(111) surface[J]. Applied Surface Science, 2018, 451: 333-345. |
| 64 | NIE Xiaowa, JIANG Xiao, WANG Haozhi, et al. Mechanistic understanding of alloy effect and water promotion for Pd-Cu bimetallic catalysts in CO2 hydrogenation to methanol[J]. ACS Catalysis, 2018, 8(6): 4873-4892. |
| 65 | WU Wenlong, WANG Yanan, LUO Lei, et al. CO2 hydrogenation over copper/ZnO single-atom catalysts: Water-promoted transient synthesis of methanol[J]. Angewandte Chemie, 2022, 134(48): e202213024. |
| 66 | JIANG Lei, LI Kongzhai, PORTER William N, et al. Role of H2O in catalytic conversion of C1 molecules[J]. Journal of the American Chemical Society, 2024, 146(5): 2857-2875. |
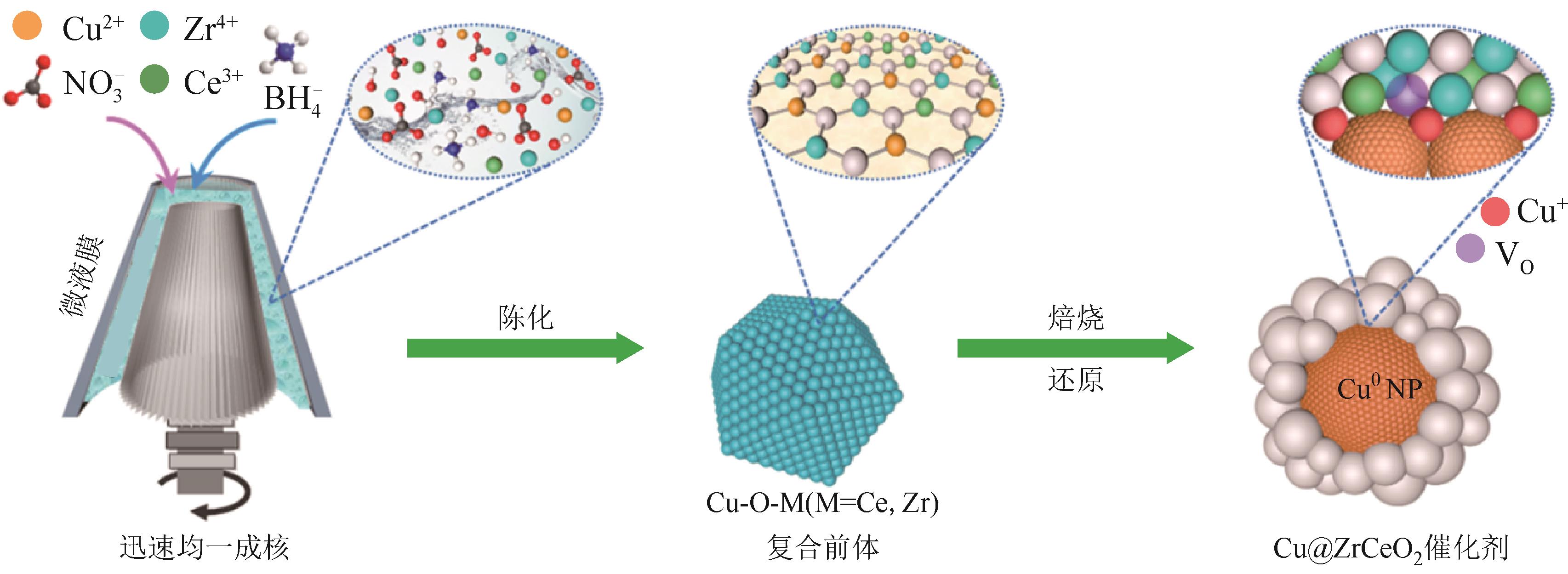
| 67 | WANG Hao, ZHANG Guangcheng, FAN Guoli, et al. Fabrication of Zr-Ce oxide solid solution surrounded Cu-based catalyst assisted by a microliquid film reactor for efficient CO2 hydrogenation to produce methanol[J]. Industrial & Engineering Chemistry Research, 2021, 60(45): 16188-16200. |
| 68 | NIE Mengdong, CUI Aixin, WU Man, et al. CuO/LaCeO x catalysts with enhanced metal-support interactions for CO2 methanolization[J]. Journal of CO2 Utilization, 2023, 75: 102579. |
| 69 | MALIK Ali Shan, ZAMAN Sharif F, AL-ZAHRANI Abdulrahim A, et al. Development of highly selective PdZn/CeO2 and Ca-doped PdZn/CeO2 catalysts for methanol synthesis from CO2 hydrogenation[J]. Applied Catalysis A: General, 2018, 560: 42-53. |
| 70 | REZVANI Azita, ABDEL-MAGEED Ali M, ISHIDA Tamao, et al. CO2 reduction to methanol on Au/CeO2 catalysts: Mechanistic insights from activation/deactivation and SSITKA measurements[J]. ACS Catalysis, 2020, 10(6): 3580-3594. |
| 71 | TAN Qingqing, SHI Zhisheng, WU Dongfang. CO2 hydrogenation over differently morphological CeO2-supported Cu-Ni catalysts[J]. International Journal of Energy Research, 2019, 43(10): 5392-5404. |
| 72 | PASUPULETY Nagaraju, DRISS Hafedh, RAFIQUI Mohammed Raoof A, et al. Methanol synthesis using CO2 and H2 on nano silver-ceria zirconia catalysts: Influence of preparation method[J]. Journal of Nanoscience and Nanotechnology, 2019, 19(6): 3197-3204. |
| 73 | CHENG Zhuo, SHERMAN Brent J, Cynthia S LO. Carbon dioxide activation and dissociation on ceria(110): A density functional theory study[J]. The Journal of Chemical Physics, 2013, 138(1): 014702. |
| 74 | MURAVEV Valery, PARASTAEV Alexander, VAN DEN BOSCH Yannis, et al. Size of cerium dioxide support nanocrystals dictates reactivity of highly dispersed palladium catalysts[J]. Science, 2023, 380(6650): 1174-1179. |
| 75 | CHOI Eun Jeong, LEE Yong Hee, LEE Dae-Won, et al. Hydrogenation of CO2 to methanol over Pd-Cu/CeO2 catalysts[J]. Molecular Catalysis, 2017, 434: 146-153. |
| 76 | OJELADE Opeyemi A, ZAMAN Sharif F, DAOUS Muhammad A, et al. Optimizing Pd: Zn molar ratio in PdZn/CeO2 for CO2 hydrogenation to methanol[J]. Applied Catalysis A: General, 2019, 584: 117185. |
| 77 | TAN Qingqing, SHI Zhisheng, WU Dongfang. CO2 hydrogenation to methanol over a highly active Cu-Ni/CeO2-nanotube catalyst[J]. Industrial & Engineering Chemistry Research, 2018, 57(31): 10148-10158. |
| 78 | WU Congyi, CHENG Danyang, WANG Meng, et al. Understanding and application of strong metal-support interactions in conversion of CO2 to methanol: A review[J]. Energy & Fuels, 2021, 35(23): 19012-19023. |
| 79 | ZHU Jiadong, SU Yaqiong, CHAI Jiachun, et al. Mechanism and nature of active sites for methanol synthesis from CO/CO2 on Cu/CeO2 [J]. ACS Catalysis, 2020, 10(19): 11532-11544. |
| 80 | VOURROS A, GARAGOUNIS I, KYRIAKOU V, et al. Carbon dioxide hydrogenation over supported Au nanoparticles: Effect of the support[J]. Journal of CO2 Utilization, 2017, 19: 247-256. |
| 81 | RASTEIRO Letícia F, DE SOUSA Rafael A, VIEIRA Luiz H, et al. Insights into the alloy-support synergistic effects for the CO2 hydrogenation towards methanol on oxide-supported Ni5Ga3 catalysts: An experimental and DFT study[J]. Applied Catalysis B: Environmental, 2022, 302: 120842. |
| 82 | YANG Xiaofang, KATTEL Shyam, SENANAYAKE Sanjaya D, et al. Low pressure CO2 hydrogenation to methanol over gold nanoparticles activated on a CeO x /TiO2 interface[J]. Journal of the American Chemical Society, 2015, 137(32): 10104-10107. |
| 83 | ABDEL-MAGEED Ali M, KLYUSHIN Alexander, REZVANI Azita, et al. Negative charging of Au nanoparticles during methanol synthesis from CO2/H2 on a Au/ZnO catalyst: Insights from operando IR and near-ambient-pressure XPS and XAS measurements[J]. Angewandte Chemie International Edition, 2019, 58(30): 10325-10329. |
| 84 | CHANG Shuai, NA Wei, ZHANG Jiaqi, et al. Effect of the Zn/Ce ratio in Cu/ZnO-CeO2 catalysts on CO2 hydrogenation for methanol synthesis[J]. New Journal of Chemistry, 2021, 45(48): 22814-22823. |
| 85 | LI Shaozhong, GUO Limin, ISHIHARA Tatsumi. Hydrogenation of CO2 to methanol over Cu/AlCeO catalyst[J]. Catalysis Today, 2020, 339: 352-361. |
| 86 | ZHANG Jingpeng, SUN Xiaohang, WU Congyi, et al. Engineering Cu+/CeZrO x interfaces to promote CO2 hydrogenation to methanol[J]. Journal of Energy Chemistry, 2023, 77: 45-53. |
| 87 | SHI Zhisheng, TAN Qingqing, WU Dongfang. Ternary copper-cerium-zirconium mixed metal oxide catalyst for direct CO2 hydrogenation to methanol[J]. Materials Chemistry and Physics, 2018, 219: 263-272. |
| 88 | ZABILSKIY Maxim, MA Kaibo, BECK Arik, et al. Methanol synthesis over Cu/CeO2-ZrO2 catalysts: The key role of multiple active components[J]. Catalysis Science & Technology, 2021, 11(1): 349-358. |
| 89 | WANG Weiwei, QU Zhenping, SONG Lixin, et al. An investigation of Zr/Ce ratio influencing the catalytic performance of CuO/Ce1- x Zr x O2 catalyst for CO2 hydrogenation to CH3OH[J]. Journal of Energy Chemistry, 2020, 47: 18-28. |
| 90 | NIE Mengdong, GUO Tuo, QIANG Fangyuan, et al. Effect of Mn content in CuO/MnCeO x catalysts on CO2 hydrogenation for methanol synthesis[J]. Reaction Chemistry & Engineering, 2023, 8(6): 1383-1394. |
| 91 | YAN Yong, WONG Roong Jien, MA Zhirui, et al. CO2 hydrogenation to methanol on tungsten-doped Cu/CeO2 catalysts[J]. Applied Catalysis B: Environmental, 2022, 306: 121098. |
| 92 | LI Shaozhong, WANG Yu, YANG Bin, et al. A highly active and selective mesostructured Cu/AlCeO catalyst for CO2 hydrogenation to methanol[J]. Applied Catalysis A: General, 2019, 571: 51-60. |
| 93 | ATTADA Yerrayya, VELISOJU Vijay Kumar, MOHAMED Hend Omar, et al. Dual experimental and computational approach to elucidate the effect of Ga on Cu/CeO2-ZrO2 catalyst for CO2 hydrogenation[J]. Journal of CO2 Utilization, 2022, 65: 102251. |
| 94 | MA Nana, CHENG Weiyi, WEI Changgeng, et al. Mechanism of methanol synthesis from CO2 on Cu/CeO2 and Cu/W-CeO2: A DFT investigation into the nature of W-doping[J]. Journal of Materials Chemistry A, 2024, 12(4): 2323-2334. |
| 95 | RODRIGUEZ José A, LIU Ping, GRACIANI Jesús, et al. Inverse oxide/metal catalysts in fundamental studies and practical applications: A perspective of recent developments[J]. The Journal of Physical Chemistry Letters, 2016, 7(13): 2627-2639. |
| 96 | SENANAYAKE Sanjaya D, STACCHIOLA Dario, RODRIGUEZ Jose A. Unique properties of ceria nanoparticles supported on metals: Novel inverse ceria/copper catalysts for CO oxidation and the water-gas shift reaction[J]. Accounts of Chemical Research, 2013, 46(8): 1702-1711. |
| 97 | KAPIAMBA Kashala Fabrice, OTOR Hope O, VIAMAJALA Sridhar, et al. Inverse oxide/metal catalysts for CO2 hydrogenation to methanol[J]. Energy & Fuels, 2022, 36(19): 11691-11711. |
| 98 | SENANAYAKE Sanjaya D, RAMÍREZ Pedro J, WALUYO Iradwikanari, et al. Hydrogenation of CO2 to methanol on CeO x /Cu(111) and ZnO/Cu(111) catalysts: Role of the metal-oxide interface and importance of Ce3+ sites[J]. The Journal of Physical Chemistry C, 2016, 120(3): 1778-1784. |
| 99 | MONCADA Jorge, CHEN Xiaobo, DENG Kaixi, et al. Structural and chemical evolution of an inverse CeO x /Cu catalyst under CO2 hydrogenation: Tunning oxide morphology to improve activity and selectivity[J]. ACS Catalysis, 2023, 13(23): 15248-15258. |
| 100 | LOU Yang, JIANG Feng, ZHU Wen, et al. CeO2 supported Pd dimers boosting CO2 hydrogenation to ethanol[J]. Applied Catalysis B: Environmental, 2021, 291: 120122. |
| 101 | ZHENG Ke, LI Yufeng, LIU Bing, et al. Ti-doped CeO2 stabilized single-atom rhodium catalyst for selective and stable CO2 hydrogenation to ethanol[J]. Angewandte Chemie International Edition, 2022, 61(44): e202210991. |
| 102 | CHEN Jie, ZHA Yajun, LIU Bing, et al. Rationally designed water enriched nano reactor for stable CO2 hydrogenation with near 100% ethanol selectivity over diatomic palladium active sites[J]. ACS Catalysis, 2023, 13(10): 7110-7121. |
| [1] | WU Da, JIANG Shujiao, WEI Qiang, YUAN Shenghua, YANG Gang, ZHANG Cheng. Research progress on efficient utilization technology of residue in energy transition [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2343-2353. |
| [2] | JIANG Andi, DING Xuexing, WANG Shipeng, DING Junhua, LI Ning. Research progress on thermodynamic performance of supercritical CO2 dry gas seal [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2354-2369. |
| [3] | GUI Xin, CHEN Huiyong, BAI Boyang, JIA Yongliang, MA Xiaoxun. Catalytic hydrogenation of pyrene over Mo-doped NiC/Al-MCM-41 [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2386-2395. |
| [4] | DING Sijia, JIANG Shujiao, YANG Zhanlin, PENG Shaozhong, JIANG Qianmin. Design of heavy oil hydrodenitrogenation catalysts based on hydrogenation performance determined by structure of nitrogen compounds [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2436-2448. |
| [5] | DUAN Xiang, TIAN Ye, DONG Wenwei, SONG Song, LI Xingang. Research progress on reaction networks and catalytic reaction mechanisms of phthalic anhydride synthesis [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2587-2599. |
| [6] | FANG Yao, LIU Lei, GAO Zhihua, HUANG Wei, ZUO Zhijun. Advances in anode catalysts for photo-assisted direct methanol fuel cells [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2611-2628. |
| [7] | ZHANG Jinpeng, QU Ting, JING Jieying, LI Wenying. Composite catalyst of sorption enhanced water gas shift for hydrogen production: A review [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2629-2644. |
| [8] | LI Na, ZHAO Wantong, LING Lixia, WANG Baojun, ZHANG Riguang. Confined environment of RhCu catalyst to regulate the reaction performance for synthesis gas conversion to CH x [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2684-2695. |
| [9] | FENG Yongqiang, WANG Jieru, WANG Chaoxian, LI Fang, SU Wanting, SUN Yu, ZHAO Binran. Influence of Ni, Fe, and Cu loaded on γ-Al2O3 in CO2/CH4 conversion via dielectric barrier discharge plasma [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2705-2713. |
| [10] | XIE Zhongkai, SHI Weidong. Research progress of charge polarized photocatalysts in photoconversion carbon dioxide into multi-carbon chemicals [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2714-2722. |
| [11] | LIU Ruolu, TANG Haibo, HE Feifei, LUO Fengying, WANG Jinge, YANG Na, LI Hongwei, ZHANG Ruiming. Recent research and prospect of liquid organic hydrogen carries technology [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1731-1741. |
| [12] | WANG Hongyan, MA Ziran, LI Ge, MA Jing, ZHAO Chunlin, ZHOU Jiali, WANG Lei, PENG Shengpan. Research progress in synergistic catalytic elimination of multiple pollutants in flue gas of coal combustion coupled with renewable fuels [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1783-1795. |
| [13] | CHEN Jiayi, GAO Weitao, YIN Yanan, WANG Cheng, OUYANG Hongwu, MAO Zongqiang. Preparation of PEMFC catalysts by electrodeposition [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1796-1809. |
| [14] | WU Chenhe, LIU Yumin, YANG Xinmin, CUI Jiwei, JIANG Shaokun, YE Jinhua, LIU Lequan. Particulate photocatalysts for light-driven overall water splitting [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1810-1822. |
| [15] | LIU Yurong, WANG Xingbao, LI Wenying. Regulation of catalyst acid sites and its effect on the deep hydrogenation performance of anthracene [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1832-1839. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||