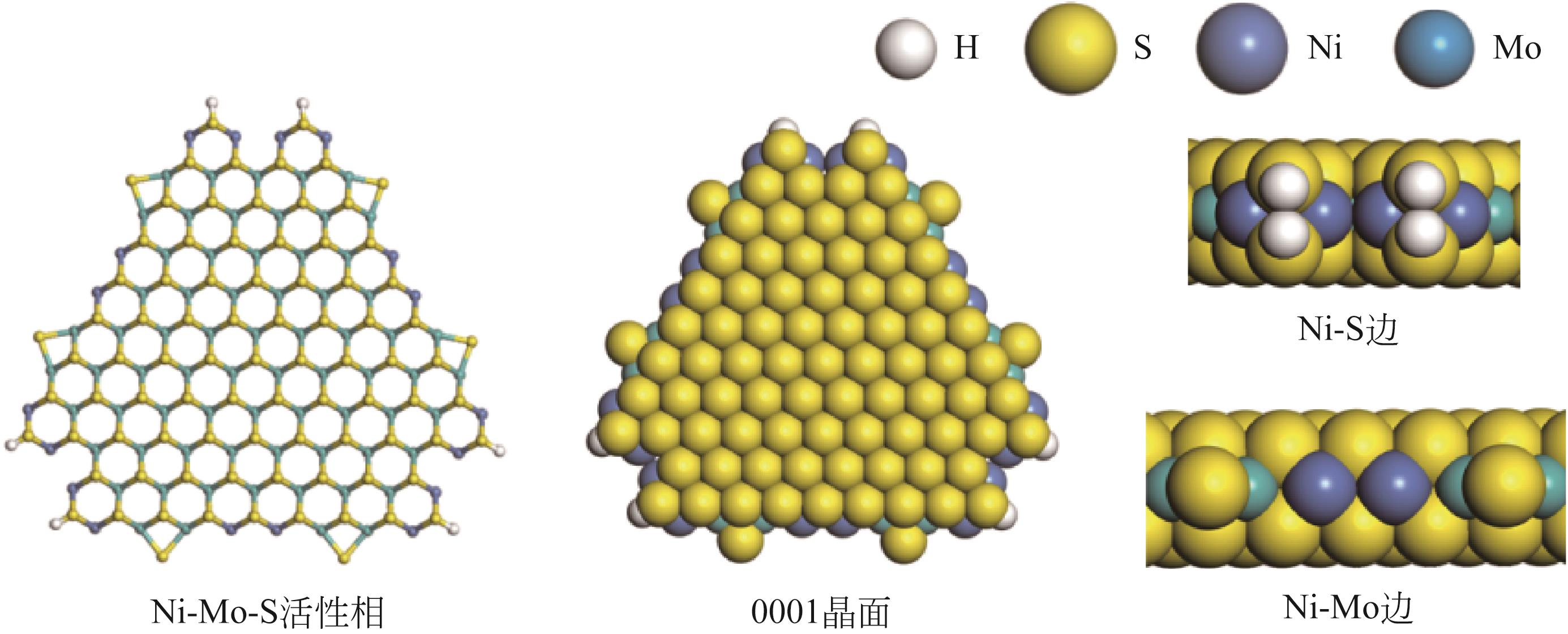
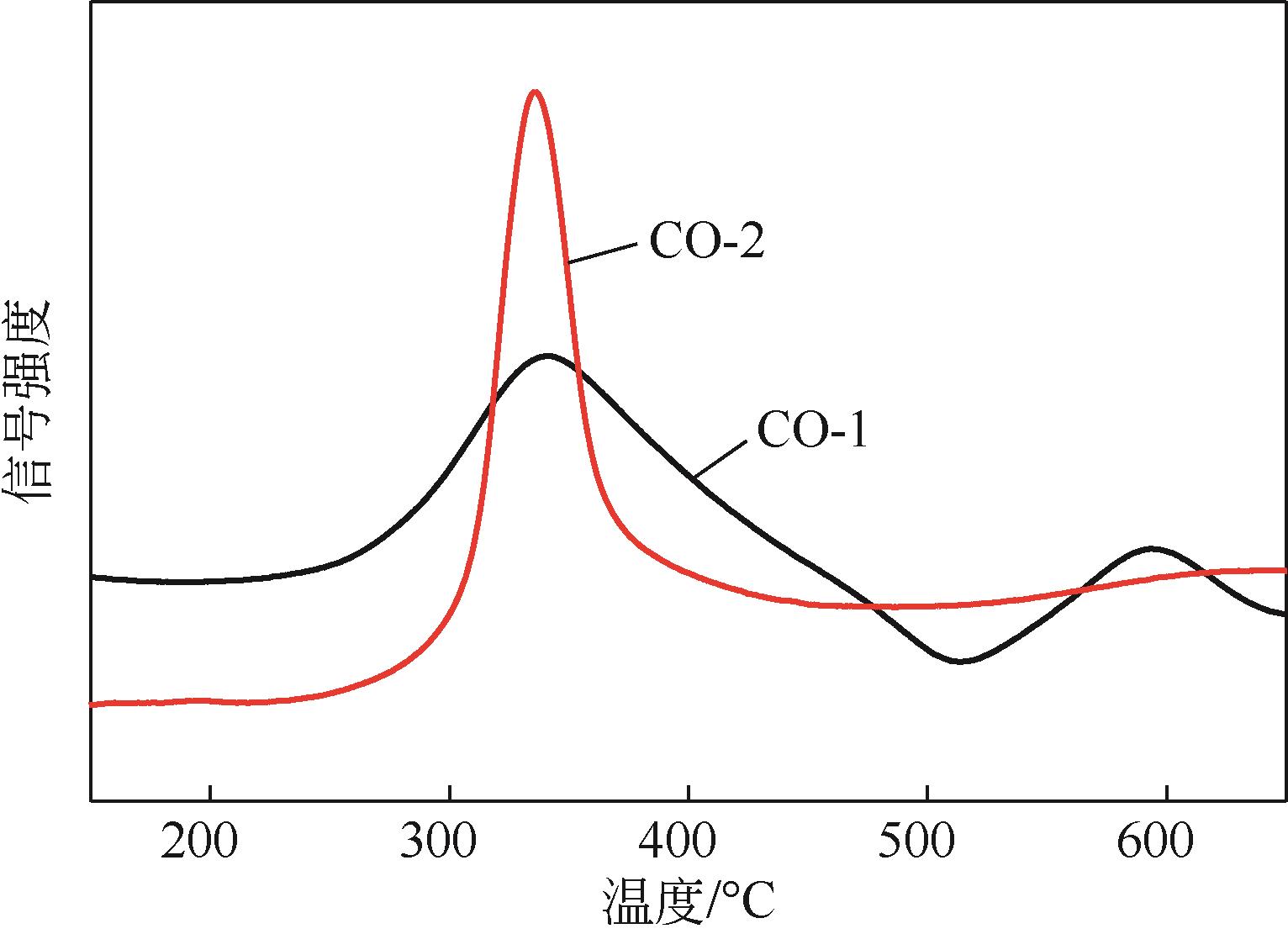
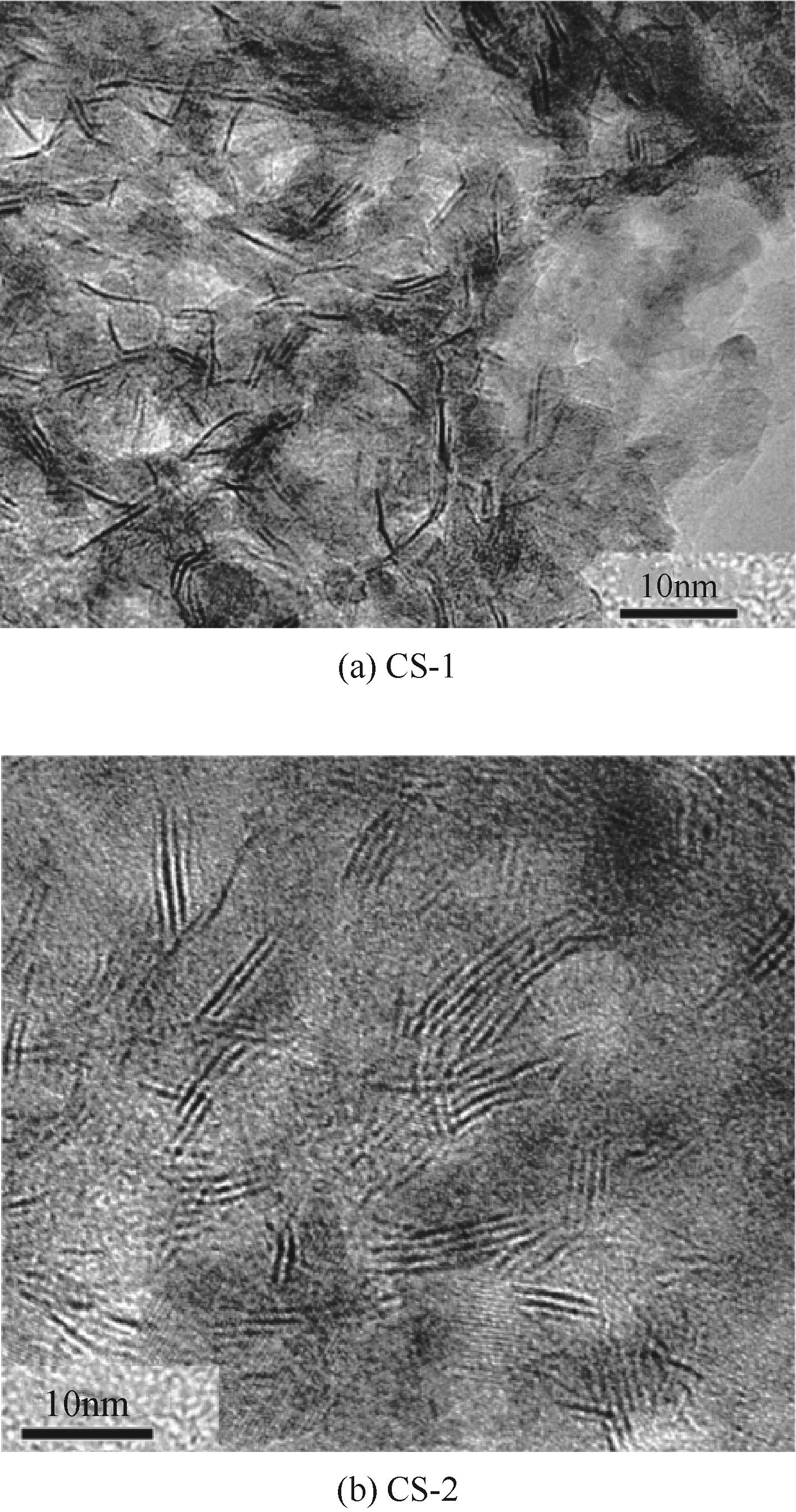
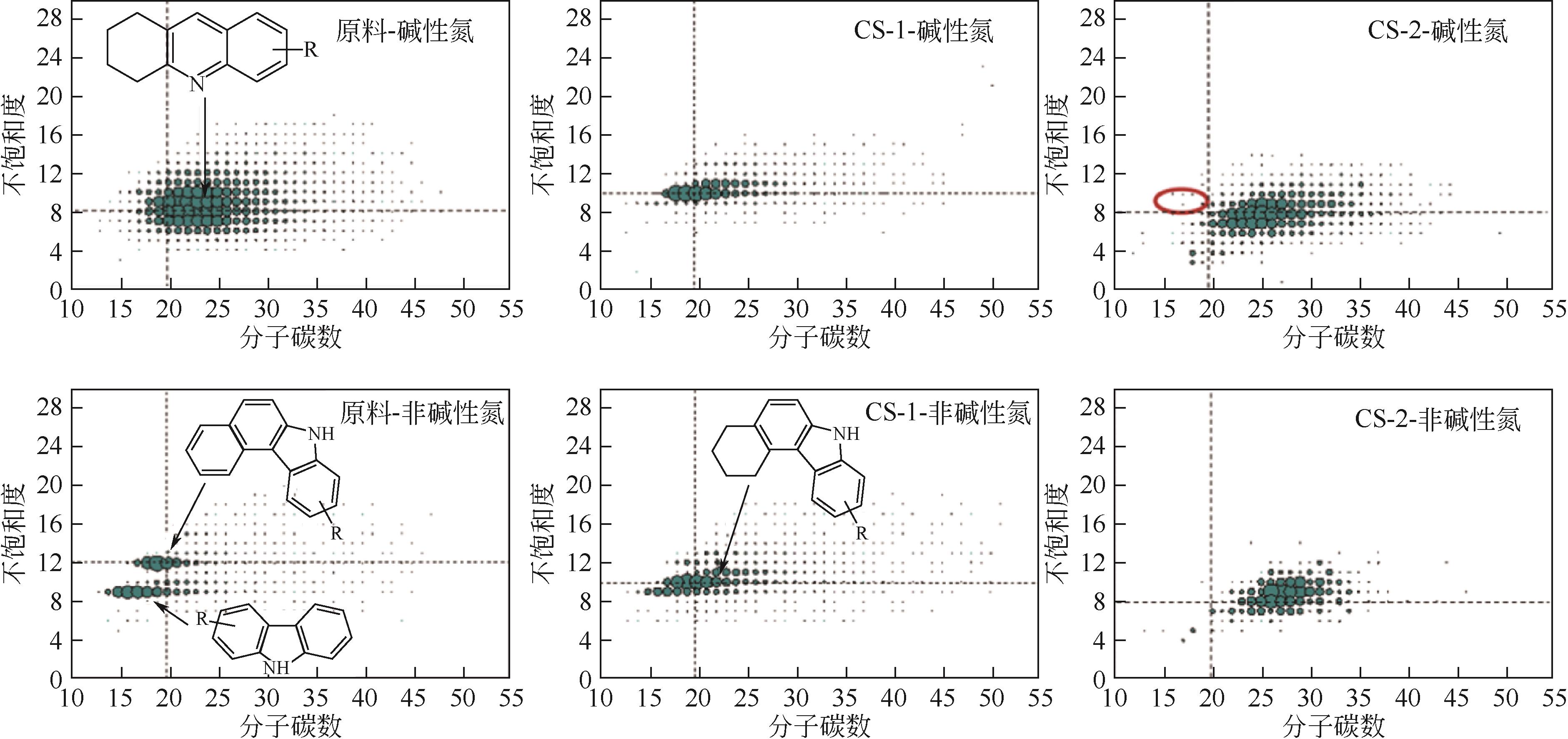
Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (5): 2436-2448.DOI: 10.16085/j.issn.1000-6613.2023-2068
• Clean and efficient utilization of fossil energy • Previous Articles
Design of heavy oil hydrodenitrogenation catalysts based on hydrogenation performance determined by structure of nitrogen compounds
DING Sijia1( ), JIANG Shujiao1, YANG Zhanlin1(
), JIANG Shujiao1, YANG Zhanlin1( ), PENG Shaozhong1, JIANG Qianmin2
), PENG Shaozhong1, JIANG Qianmin2
- 1.Sinopec (Dalian) Petrochemical Research Institute, Dalian 116045, Liaoning, China
2.College of Chemical Engineering and Environment, China University of Petroleum, Beijing 102249, China
-
Received:2023-11-28Revised:2024-03-14Online:2024-06-15Published:2024-05-15 -
Contact:YANG Zhanlin
基于氮化物结构与加氢行为关系设计重油加氢脱氮催化剂
丁思佳1( ), 蒋淑娇1, 杨占林1(
), 蒋淑娇1, 杨占林1( ), 彭绍忠1, 蒋乾民2
), 彭绍忠1, 蒋乾民2
- 1.中石化(大连)石油化工研究院,辽宁 大连 116045
2.中国石油大学(北京)化学工程与环境学院,北京 102249
-
通讯作者:杨占林 -
作者简介:丁思佳(1987—),男,博士,副研究员,研究方向为催化加氢理论和加氢精制催化剂研发。E-mail:dingsijia.fshy@sinopec.com。
CLC Number:
Cite this article
DING Sijia, JIANG Shujiao, YANG Zhanlin, PENG Shaozhong, JIANG Qianmin. Design of heavy oil hydrodenitrogenation catalysts based on hydrogenation performance determined by structure of nitrogen compounds[J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2436-2448.
丁思佳, 蒋淑娇, 杨占林, 彭绍忠, 蒋乾民. 基于氮化物结构与加氢行为关系设计重油加氢脱氮催化剂[J]. 化工进展, 2024, 43(5): 2436-2448.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-2068
| 计算项目/方法 | 参数 |
|---|---|
| 电子赝势 | 超平滑(ultrasoft)电子赝势 |
| 能量截断 | 10.0eV |
| 电子自洽收敛阶(SCF) | 1.0×10-6 |
| 体系能量收敛阶 | 2.0×10-5Hartree (Ha) |
| 体系残余内应力 | 0.05Ha/Å |
| 色散力矫正方法 | Grimme 06[ |
| 独立交换参数s6 | 1.00 |
| 阻尼衰减参数d | 20.0 |
| 计算项目/方法 | 参数 |
|---|---|
| 电子赝势 | 超平滑(ultrasoft)电子赝势 |
| 能量截断 | 10.0eV |
| 电子自洽收敛阶(SCF) | 1.0×10-6 |
| 体系能量收敛阶 | 2.0×10-5Hartree (Ha) |
| 体系残余内应力 | 0.05Ha/Å |
| 色散力矫正方法 | Grimme 06[ |
| 独立交换参数s6 | 1.00 |
| 阻尼衰减参数d | 20.0 |
| 原料 | 50%馏程温度 /℃ | 95%馏程温度 /℃ | 密度 /g·cm-3 | 硫含量 /μg·g-1 | 氮含量 /μg·g-1 | 链烷烃 质量分数/% | 环烷烃 质量分数/% | 单环芳烃 质量分数/% | 二环及以上芳烃 质量分数/% | 总环芳烃 质量分数/% |
|---|---|---|---|---|---|---|---|---|---|---|
| 常三线柴油 | 309.9 | 360.7 | 855.6 | 10170 | 352 | 40.8 | 28.6 | 15.1 | 14.3 | 29.4 |
| 减二线蜡油 | 445.3 | 525.3 | 906.5 | 14220 | 1094 | 25.4 | 27.9 | 17.3 | 29.5 | 46.8 |
| 减三线蜡油 | 464.3 | 576.9 | 926.3 | 18960 | 1566 | 34.0 | 13.7 | 19.2 | 31.2 | 50.4 |
| 原料 | 50%馏程温度 /℃ | 95%馏程温度 /℃ | 密度 /g·cm-3 | 硫含量 /μg·g-1 | 氮含量 /μg·g-1 | 链烷烃 质量分数/% | 环烷烃 质量分数/% | 单环芳烃 质量分数/% | 二环及以上芳烃 质量分数/% | 总环芳烃 质量分数/% |
|---|---|---|---|---|---|---|---|---|---|---|
| 常三线柴油 | 309.9 | 360.7 | 855.6 | 10170 | 352 | 40.8 | 28.6 | 15.1 | 14.3 | 29.4 |
| 减二线蜡油 | 445.3 | 525.3 | 906.5 | 14220 | 1094 | 25.4 | 27.9 | 17.3 | 29.5 | 46.8 |
| 减三线蜡油 | 464.3 | 576.9 | 926.3 | 18960 | 1566 | 34.0 | 13.7 | 19.2 | 31.2 | 50.4 |
| 碱性氮化物 | 氮原子 电荷/e | 孤对电子 轨道形貌 | 轨道能级 /eV | π-电子 轨道形貌 | 轨道能级 /eV | 偶极矩μ /10-30·C·m | 极化率 /10-40·C·m2·V-1 | 各向异性 /10-40·C·m2·V-1 |
|---|---|---|---|---|---|---|---|---|
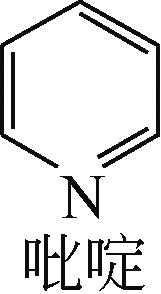 | -0.205 | 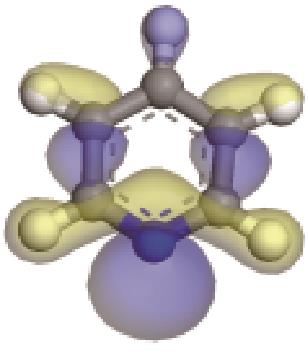 | -6.016 | 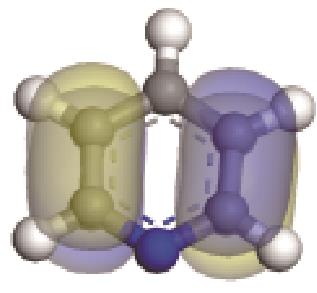 | -6.68 | 5.318 | 13.69 | 10.95 |
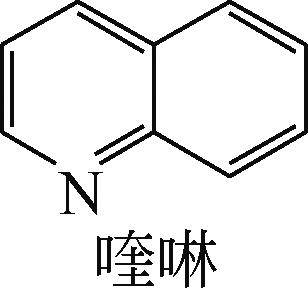 | -0.202 | 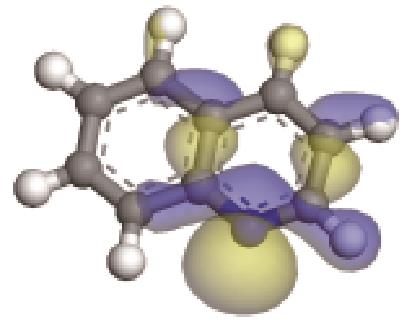 | -6.040 | 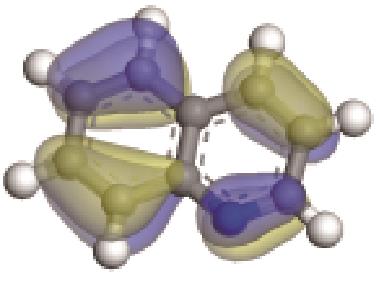 | -5.944 | 4.454 | 25.56 | 23.91 |
 | -0.206 | 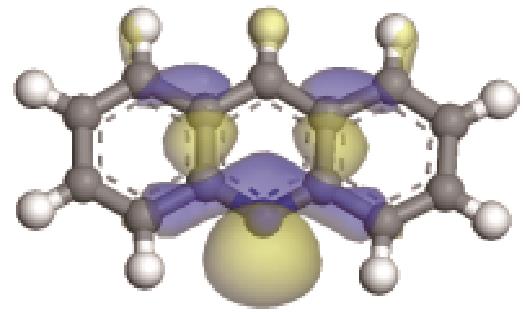 | -6.039 | 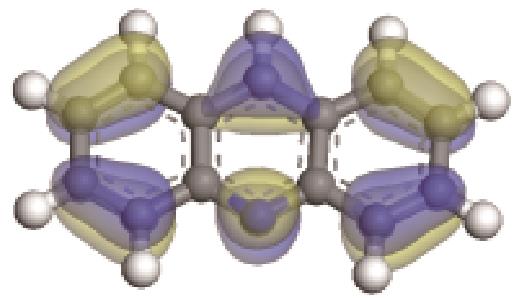 | -5.409 | 2.904 | 40.54 | 42.16 |
 | -0.206 | 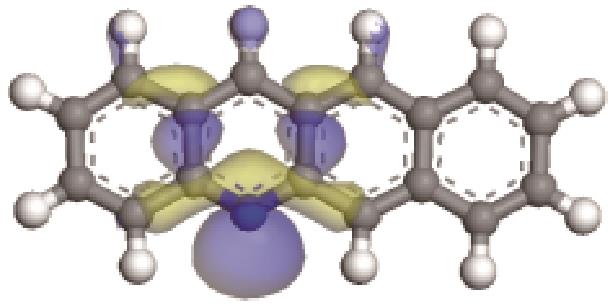 | -6.030 | 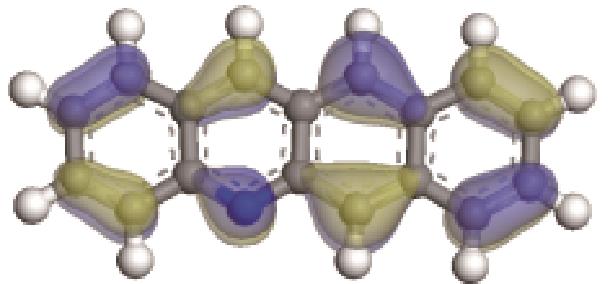 | -4.987 | 2.962 | 58.65 | 68.32 |
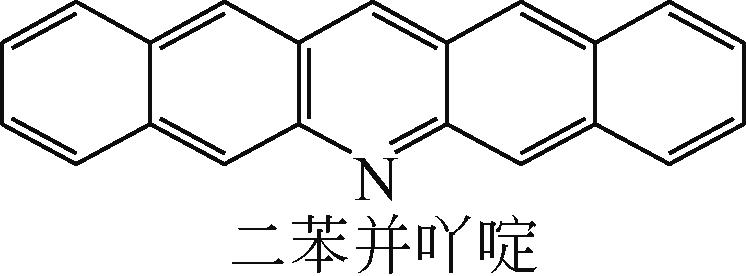 | -0.207 | 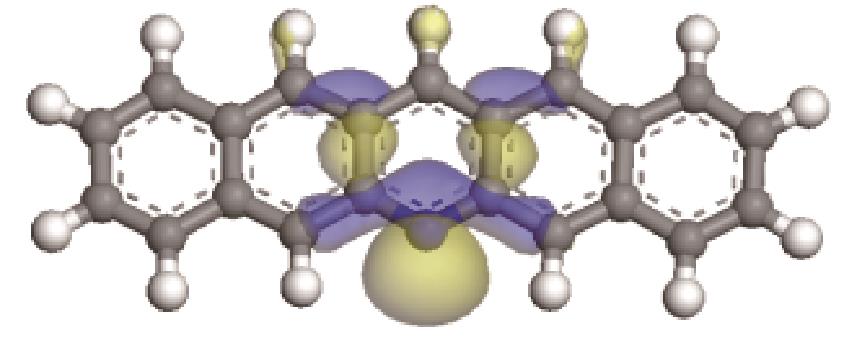 | -6.016 | 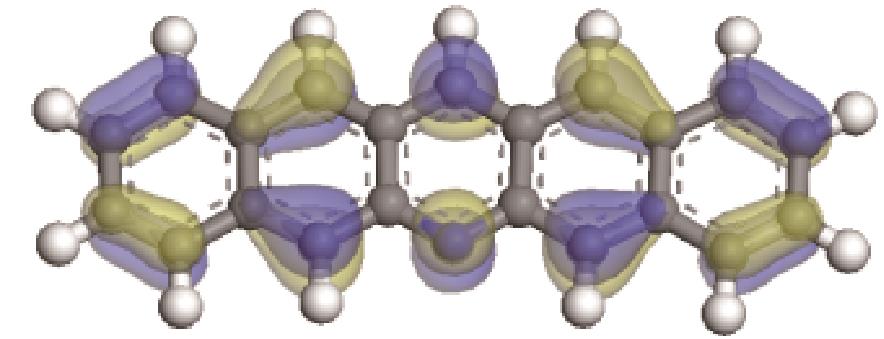 | -4.74 | 2.634 | 77.26 | 91.11 |
| 碱性氮化物 | 氮原子 电荷/e | 孤对电子 轨道形貌 | 轨道能级 /eV | π-电子 轨道形貌 | 轨道能级 /eV | 偶极矩μ /10-30·C·m | 极化率 /10-40·C·m2·V-1 | 各向异性 /10-40·C·m2·V-1 |
|---|---|---|---|---|---|---|---|---|
 | -0.205 |  | -6.016 |  | -6.68 | 5.318 | 13.69 | 10.95 |
 | -0.202 |  | -6.040 |  | -5.944 | 4.454 | 25.56 | 23.91 |
 | -0.206 |  | -6.039 |  | -5.409 | 2.904 | 40.54 | 42.16 |
 | -0.206 |  | -6.030 |  | -4.987 | 2.962 | 58.65 | 68.32 |
 | -0.207 |  | -6.016 |  | -4.74 | 2.634 | 77.26 | 91.11 |
| 非碱性氮化物 | 氮原子Mulliken Charge /e | π-电子轨道形貌 | 轨道能级 /eV | 偶极矩μ /10-30·C·m | 极化率 /10-40·C·m2·V-1 | 各向异性 /10-40·C·m2·V-1 |
|---|---|---|---|---|---|---|
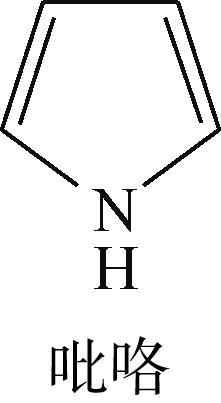 | -0.150 | 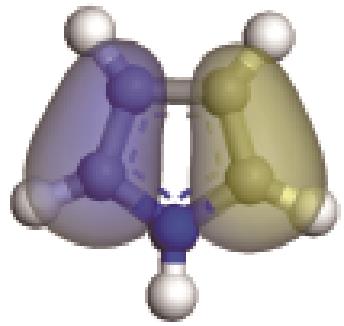 | -5.081 | 6.278 | 11.10 | 8.26 |
 | -0.196 | 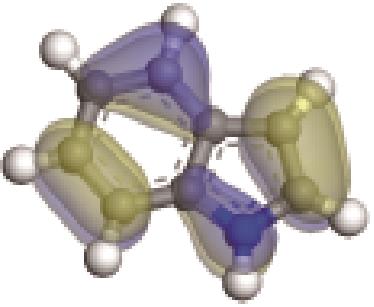 | -5.065 | 7.285 | 22.26 | 20.01 |
 | -0.229 | 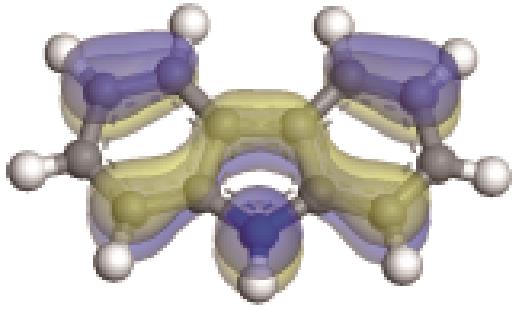 | -5.129 | 5.326 | 35.18 | 33.94 |
 | -0.233 | 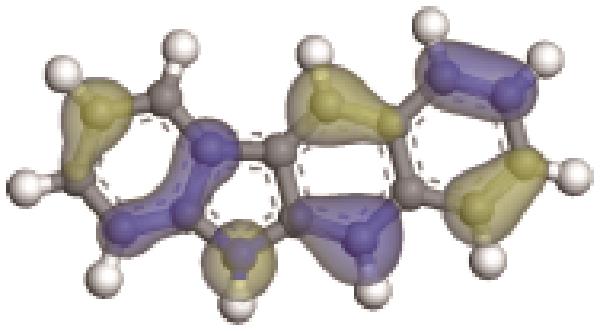 | -4.287 | 5.830 | 51.26 | 54.52 |
 | -0.235 | 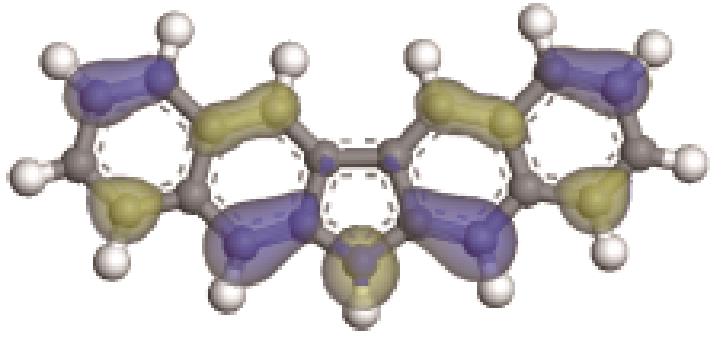 | -4.816 | 4.905 | 69.60 | 82.71 |
| 非碱性氮化物 | 氮原子Mulliken Charge /e | π-电子轨道形貌 | 轨道能级 /eV | 偶极矩μ /10-30·C·m | 极化率 /10-40·C·m2·V-1 | 各向异性 /10-40·C·m2·V-1 |
|---|---|---|---|---|---|---|
 | -0.150 |  | -5.081 | 6.278 | 11.10 | 8.26 |
 | -0.196 |  | -5.065 | 7.285 | 22.26 | 20.01 |
 | -0.229 |  | -5.129 | 5.326 | 35.18 | 33.94 |
 | -0.233 |  | -4.287 | 5.830 | 51.26 | 54.52 |
 | -0.235 |  | -4.816 | 4.905 | 69.60 | 82.71 |
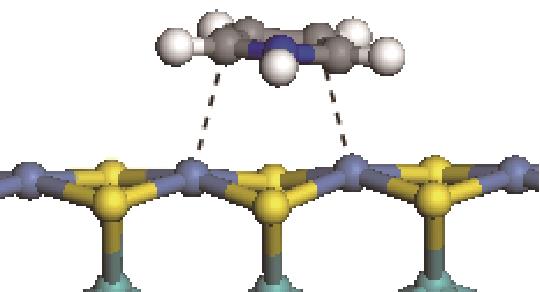
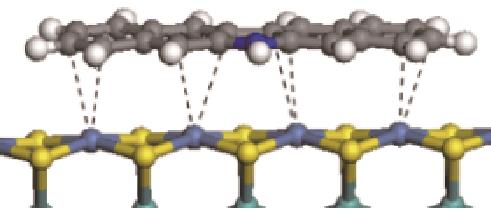
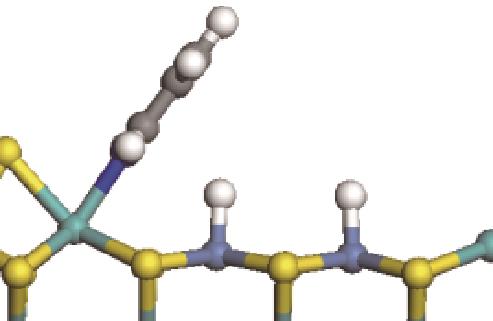
| 碱性氮化物 | 吸附方式 | 吸附形貌 | 结合能/kJ·mol-1 | 氮化物电荷转移量/e |
|---|---|---|---|---|
 | N-Ni | 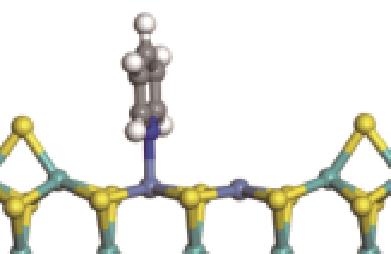 | -100.69 | +0.252 |
| N-Mo | 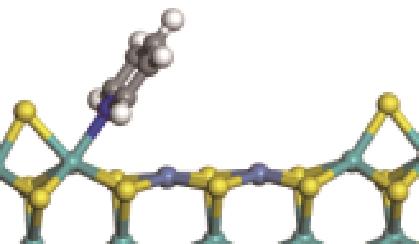 | -117.07 | +0.258 | |
| π-Ni | 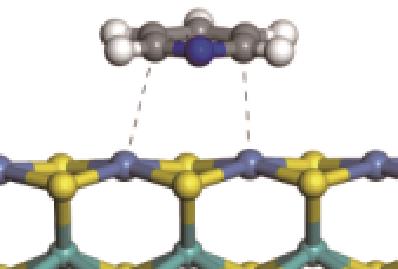 | -48.72 | +0.018 | |
 | N-Ni | 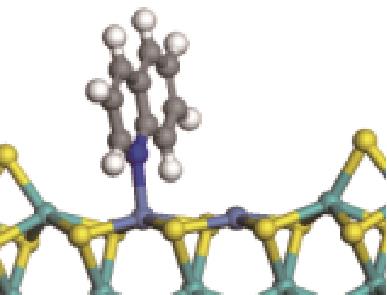 | -118.51 | +0.262 |
| N-Mo | 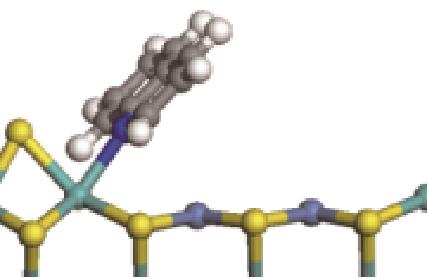 | -145.78 | +0.293 | |
| π-M | 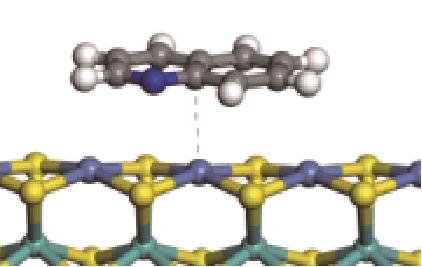 | -92.42 | +0.023 | |
 | N-Ni | 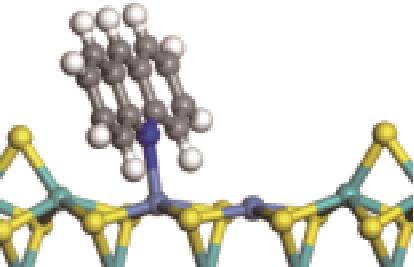 | -112.80 | +0.227 |
| N-Mo | 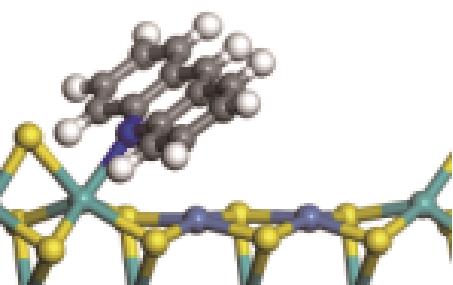 | -154.10 | +0.311 | |
| π-M |  | -112.84 | +0.026 | |
 | N-Ni | 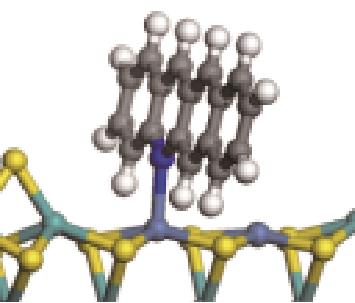 | -122.20 | +0.262 |
| N-Mo |  | -171.91 | +0.326 | |
| π-M | 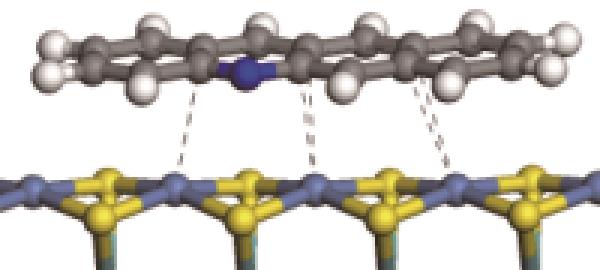 | -135.30 | +0.028 | |
 | N-Ni | 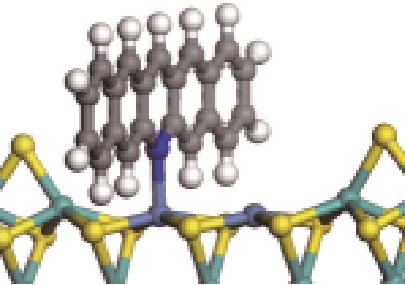 | -124.20 | +0.309 |
| N-Mo | 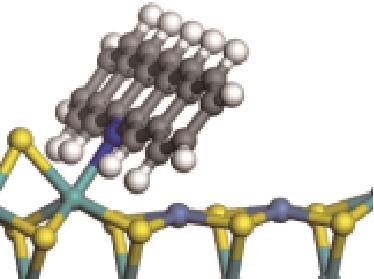 | -172.32 | +0.313 | |
| π-M | 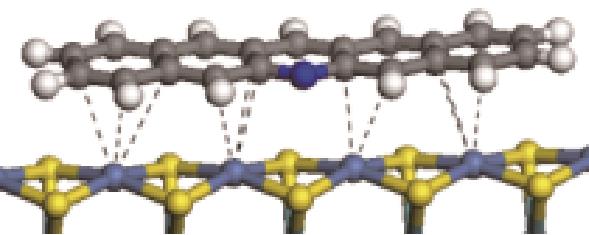 | -164.39 | +0.085 |
| 碱性氮化物 | 吸附方式 | 吸附形貌 | 结合能/kJ·mol-1 | 氮化物电荷转移量/e |
|---|---|---|---|---|
 | N-Ni |  | -100.69 | +0.252 |
| N-Mo |  | -117.07 | +0.258 | |
| π-Ni |  | -48.72 | +0.018 | |
 | N-Ni |  | -118.51 | +0.262 |
| N-Mo |  | -145.78 | +0.293 | |
| π-M |  | -92.42 | +0.023 | |
 | N-Ni |  | -112.80 | +0.227 |
| N-Mo |  | -154.10 | +0.311 | |
| π-M |  | -112.84 | +0.026 | |
 | N-Ni |  | -122.20 | +0.262 |
| N-Mo |  | -171.91 | +0.326 | |
| π-M |  | -135.30 | +0.028 | |
 | N-Ni |  | -124.20 | +0.309 |
| N-Mo |  | -172.32 | +0.313 | |
| π-M |  | -164.39 | +0.085 |
| 非碱性氮化物 | 吸附形貌 | 结合能/kJ·mol-1 | 氮化物电荷转移量/e |
|---|---|---|---|
 |  | -82.68 | +0.032 |
 | 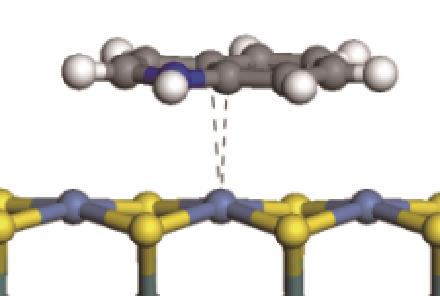 | -81.14 | +0.058 |
 | 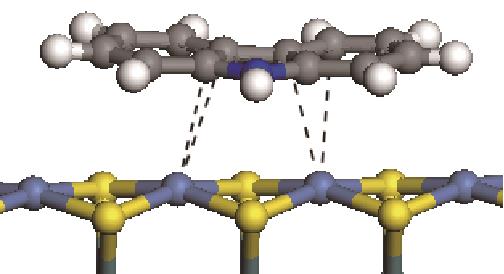 | -114.26 | +0.084 |
 | 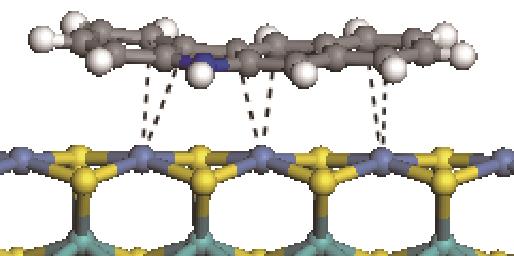 | -121.35 | +0.102 |
 |  | -186.60 | +0.136 |
| 非碱性氮化物 | 吸附形貌 | 结合能/kJ·mol-1 | 氮化物电荷转移量/e |
|---|---|---|---|
 |  | -82.68 | +0.032 |
 |  | -81.14 | +0.058 |
 |  | -114.26 | +0.084 |
 |  | -121.35 | +0.102 |
 |  | -186.60 | +0.136 |
| 加氢前状态 | 加氢过渡态 | 加氢后状态 | 反应能/kJ·mol-1 | 活化能/kJ·mol-1 |
|---|---|---|---|---|
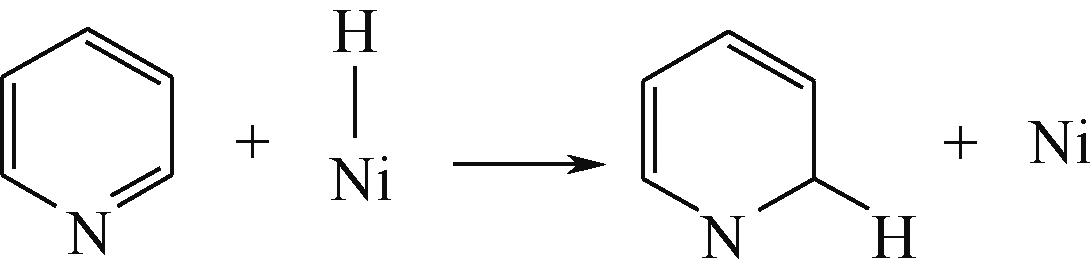 | ||||
 | 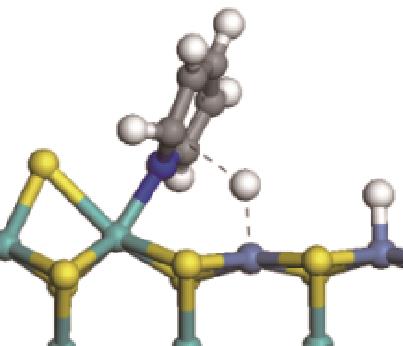 | 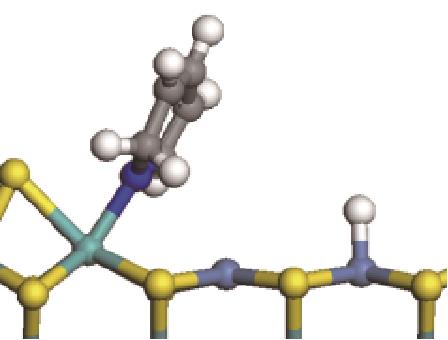 | -11.82 | +79.51 |
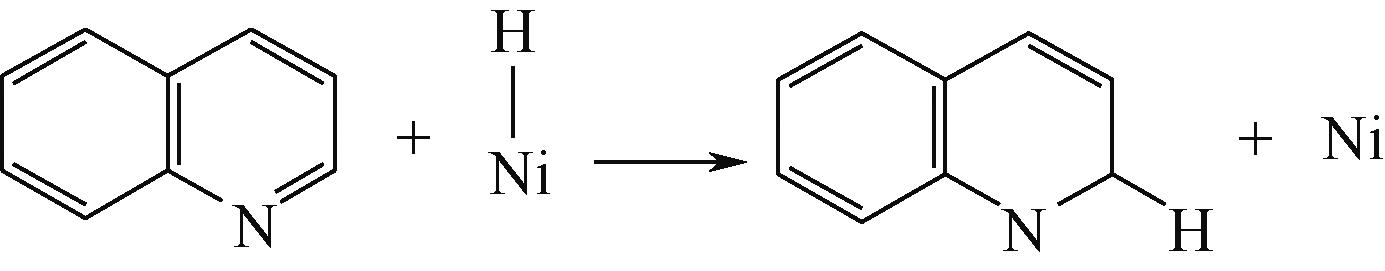 | ||||
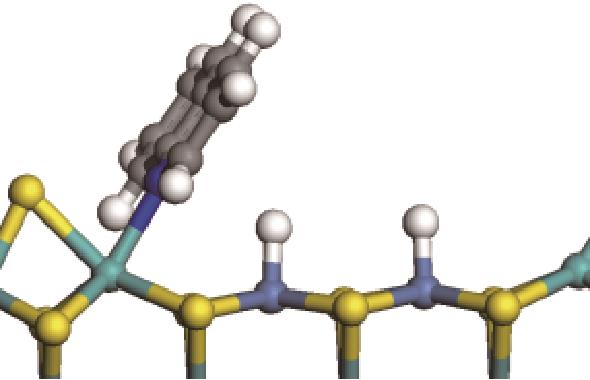 | 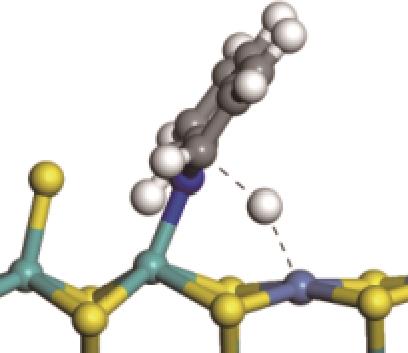 | 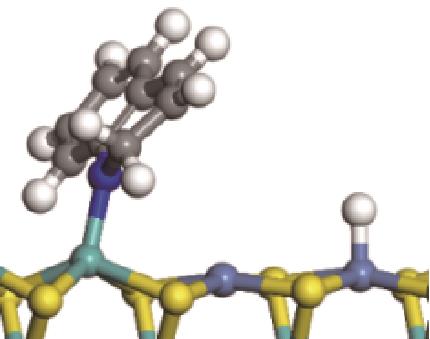 | -18.10 | +86.94 |
 | ||||
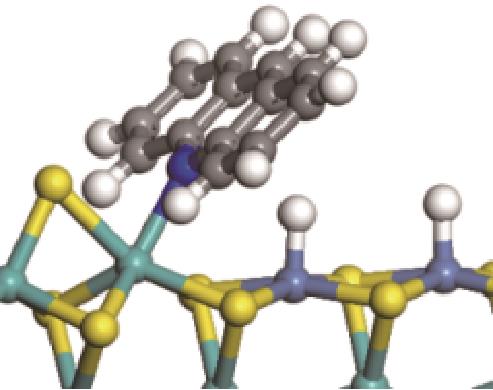 | 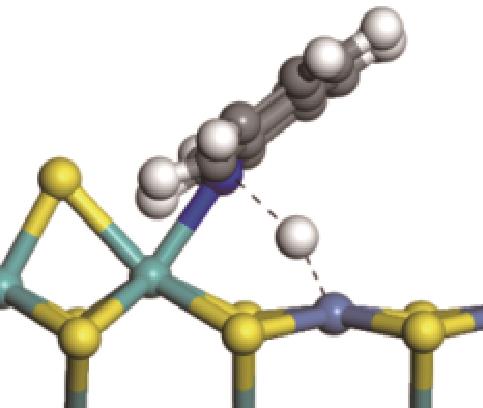 |  | +59.87 | +146.88 |
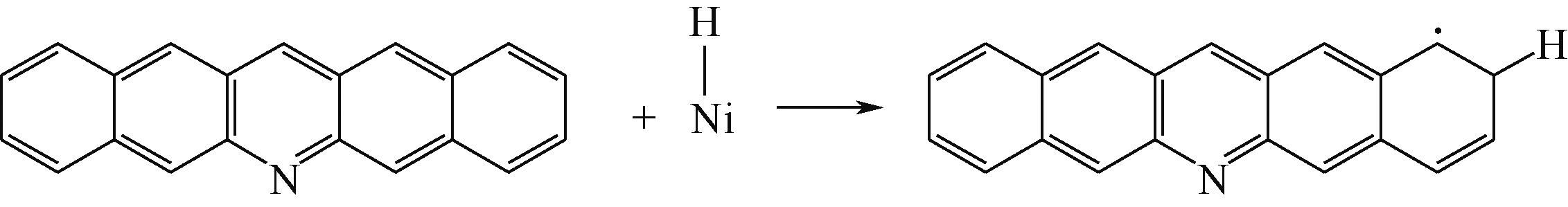 | ||||
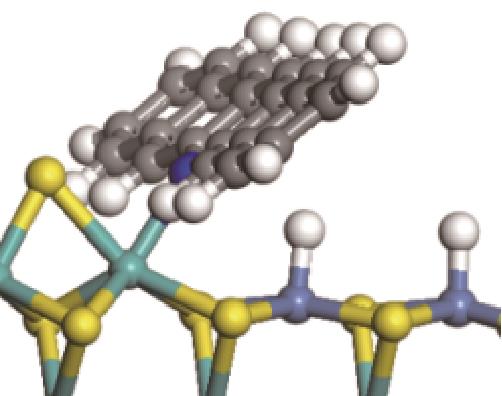 | 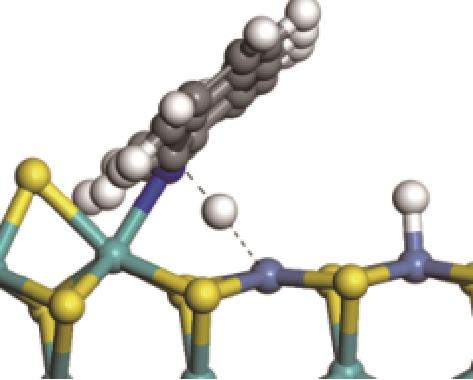 | 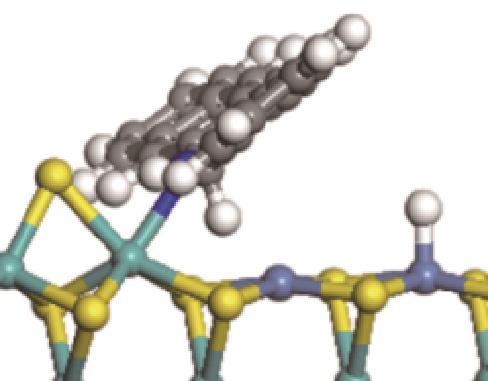 | +45.04 | +139.99 |
| 加氢前状态 | 加氢过渡态 | 加氢后状态 | 反应能/kJ·mol-1 | 活化能/kJ·mol-1 |
|---|---|---|---|---|
 | ||||
 |  |  | -11.82 | +79.51 |
 | ||||
 |  |  | -18.10 | +86.94 |
 | ||||
 |  |  | +59.87 | +146.88 |
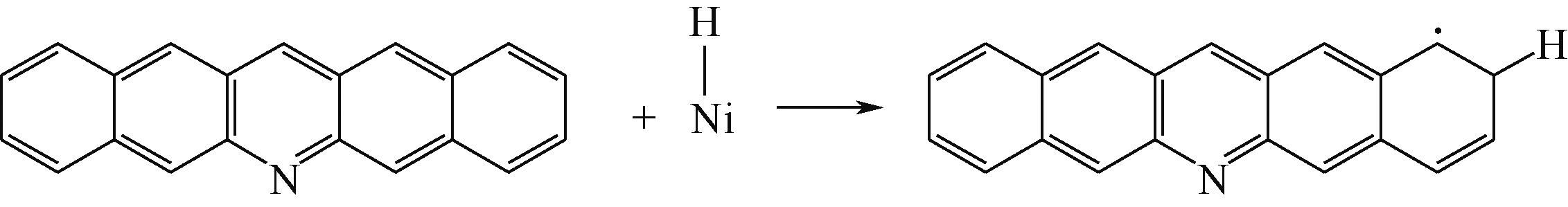 | ||||
 |  |  | +45.04 | +139.99 |
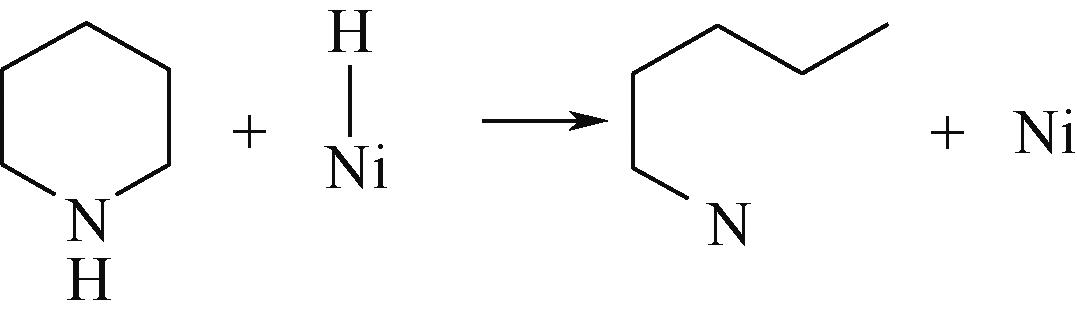
| 氢解前状态 | 加氢过渡态 | 氢解后状态 | 反应能/kJ·mol-1 | 活化能/kJ·mol-1 |
|---|---|---|---|---|
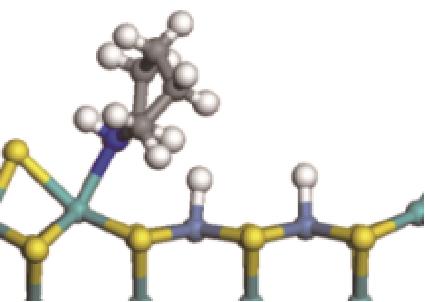
 | ||||
 | 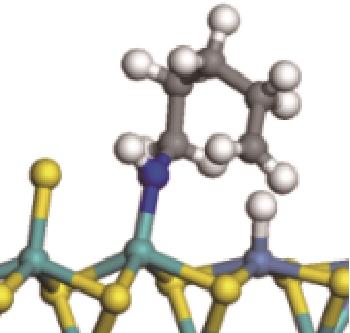 | 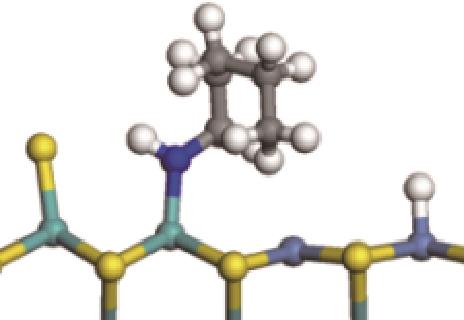 | -118.64 | +197.10 |
 | ||||
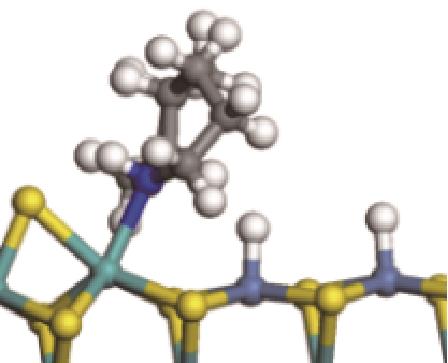 | 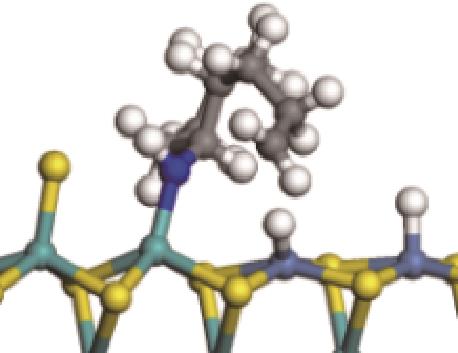 |  | -144.93 | +223.89 |
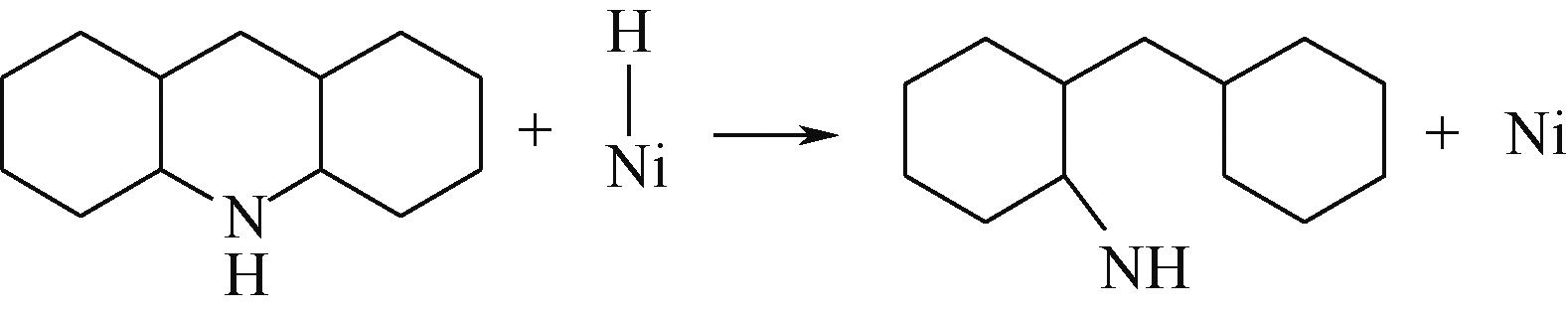 | ||||
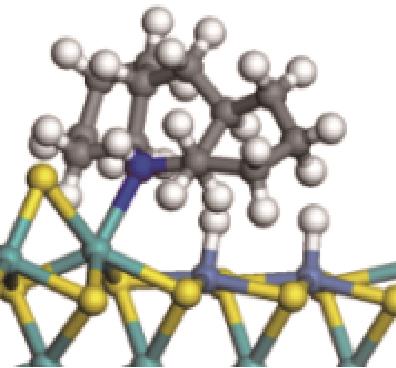 | 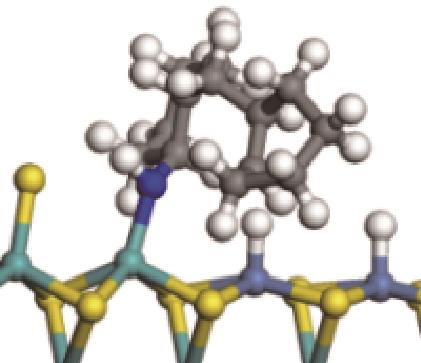 | 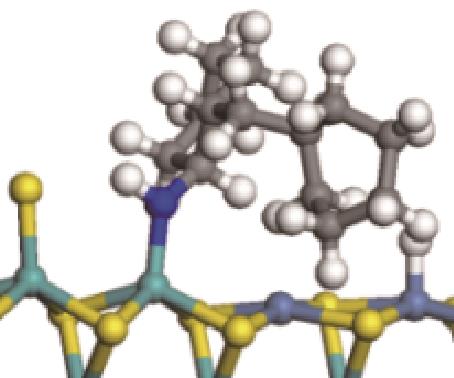 | -99.14 | +206.77 |
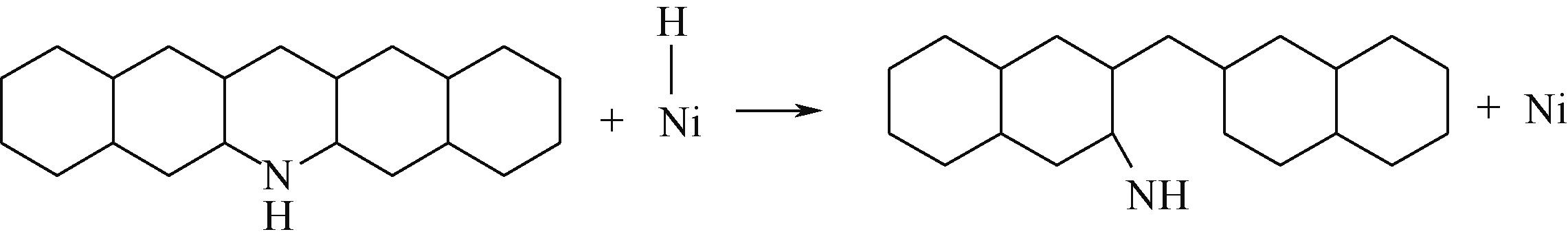 | ||||
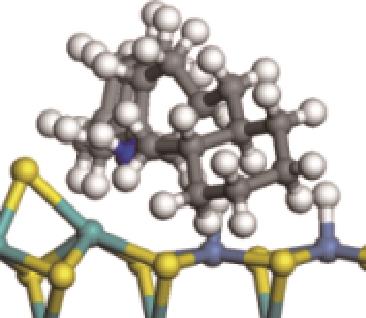 | 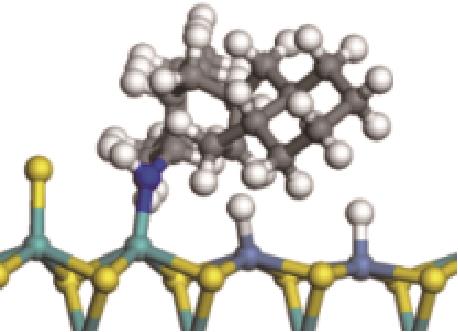 | 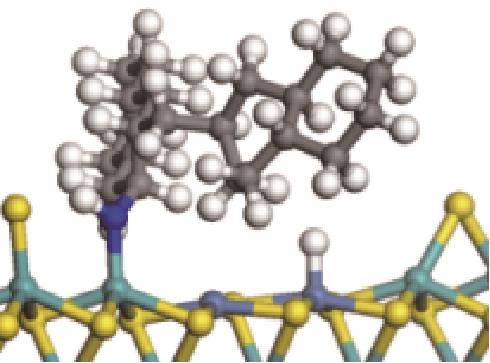 | -84.72 | +223.82 |
| 氢解前状态 | 加氢过渡态 | 氢解后状态 | 反应能/kJ·mol-1 | 活化能/kJ·mol-1 |
|---|---|---|---|---|
 | ||||
 |  |  | -118.64 | +197.10 |
 | ||||
 |  |  | -144.93 | +223.89 |
 | ||||
 |  |  | -99.14 | +206.77 |
 | ||||
 |  |  | -84.72 | +223.82 |
| 催化剂 | 活性相平均堆垛层数 | 活性相平均长度/nm |
|---|---|---|
| CS-1 | 1.3 | 6.2 |
| CS-2 | 2.9 | 7.9 |
| 催化剂 | 活性相平均堆垛层数 | 活性相平均长度/nm |
|---|---|---|
| CS-1 | 1.3 | 6.2 |
| CS-2 | 2.9 | 7.9 |
| 催化剂 | 反应物 | 反应温度 /℃ | 反应压力 /MPa | 液时空速 /mL·mL-1·h-1 | 转化率 /% |
|---|---|---|---|---|---|
| CS-1 | 1-甲基萘 | 300 | 5.0 | 4.0 | 81.3 |
| 二苯并噻吩 | 320 | 4.0 | 4.0 | 94.0 | |
| CS-2 | 1-甲基萘 | 300 | 5.0 | 4.0 | 95.3 |
| 二苯并噻吩 | 320 | 4.0 | 4.0 | 75.7 |
| 催化剂 | 反应物 | 反应温度 /℃ | 反应压力 /MPa | 液时空速 /mL·mL-1·h-1 | 转化率 /% |
|---|---|---|---|---|---|
| CS-1 | 1-甲基萘 | 300 | 5.0 | 4.0 | 81.3 |
| 二苯并噻吩 | 320 | 4.0 | 4.0 | 94.0 | |
| CS-2 | 1-甲基萘 | 300 | 5.0 | 4.0 | 95.3 |
| 二苯并噻吩 | 320 | 4.0 | 4.0 | 75.7 |
| 原料 | 催化剂 | 评价条件 | 产物性质 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
反应压力 /MPa | 反应温度 /℃ | 液时空速 /mL·mL-1·h-1 | 氢油比 /mL·mL-1 | 氮含量 /μg·g-1 | 单环芳烃 质量分数/% | 双环及以上芳烃 质量分数/% | 总芳烃 质量分数/% | |||
| 常三线柴油 | CS-1 | 6.0 | 350 | 2.0 | 500∶1 | 10.3 | 15.8 | 4.4 | 20.2 | |
| CS-2 | 19.6 | 14.7 | 3.5 | 18.2 | ||||||
| 减二线蜡油 | CS-1 | 14.5 | 365 | 1.0 | 1000∶1 | 5.9 | 22.8 | 4.5 | 27.3 | |
| CS-2 | 6.3 | 20.2 | 3.6 | 23.8 | ||||||
| 减三线蜡油 | CS-1 | 16.5 | 380 | 1.0 | 1200∶1 | 11.3 | 20.2 | 3.9 | 24.1 | |
| CS-2 | 3.8 | 14.8 | 3.2 | 18.0 | ||||||
| 原料 | 催化剂 | 评价条件 | 产物性质 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
反应压力 /MPa | 反应温度 /℃ | 液时空速 /mL·mL-1·h-1 | 氢油比 /mL·mL-1 | 氮含量 /μg·g-1 | 单环芳烃 质量分数/% | 双环及以上芳烃 质量分数/% | 总芳烃 质量分数/% | |||
| 常三线柴油 | CS-1 | 6.0 | 350 | 2.0 | 500∶1 | 10.3 | 15.8 | 4.4 | 20.2 | |
| CS-2 | 19.6 | 14.7 | 3.5 | 18.2 | ||||||
| 减二线蜡油 | CS-1 | 14.5 | 365 | 1.0 | 1000∶1 | 5.9 | 22.8 | 4.5 | 27.3 | |
| CS-2 | 6.3 | 20.2 | 3.6 | 23.8 | ||||||
| 减三线蜡油 | CS-1 | 16.5 | 380 | 1.0 | 1200∶1 | 11.3 | 20.2 | 3.9 | 24.1 | |
| CS-2 | 3.8 | 14.8 | 3.2 | 18.0 | ||||||
| 油品名称 | 氮化物类型 | 单芳环氮化物质量分数/% | 双芳环氮化物质量分数/% | 三芳环氮化物质量分数/% | 四芳环及以上氮化物质量分数/% |
|---|---|---|---|---|---|
| 原料 | 碱氮 | 26.3 | 36.8 | 30.7 | 6.2 |
| 非碱氮 | 0.8 | 38.4 | 46.7 | 14.1 | |
| CS-1加氢产物 | 碱氮 | 0.2 | 6.4 | 86.5 | 6.9 |
| 非碱氮 | 0.9 | 6.3 | 81.3 | 11.5 | |
| CS-2加氢产物 | 碱氮 | 0.4 | 34.1 | 54.8 | 10.7 |
| 非碱氮 | 0.1 | 12.7 | 71.9 | 6.3 |
| 油品名称 | 氮化物类型 | 单芳环氮化物质量分数/% | 双芳环氮化物质量分数/% | 三芳环氮化物质量分数/% | 四芳环及以上氮化物质量分数/% |
|---|---|---|---|---|---|
| 原料 | 碱氮 | 26.3 | 36.8 | 30.7 | 6.2 |
| 非碱氮 | 0.8 | 38.4 | 46.7 | 14.1 | |
| CS-1加氢产物 | 碱氮 | 0.2 | 6.4 | 86.5 | 6.9 |
| 非碱氮 | 0.9 | 6.3 | 81.3 | 11.5 | |
| CS-2加氢产物 | 碱氮 | 0.4 | 34.1 | 54.8 | 10.7 |
| 非碱氮 | 0.1 | 12.7 | 71.9 | 6.3 |
| 1 | 王福江, 张毓莹, 龙湘云, 等. 催化裂化柴油馏分加氢精制提高十六烷值研究[J]. 石油炼制与化工, 2013, 44(10): 27-31. |
| WANG Fujiang, ZHANG Yuying, LONG Xiangyun, et al. Study on cetane number improvement of lco by hydrotreating[J]. Petroleum Processing and Petrochemicals, 2013, 44(10): 27-31. | |
| 2 | 柴永明, 相春娥, 孔会清, 等. 馏分油浆态床加氢处理研究 Ⅰ: 催化剂制备方法[J]. 燃料化学学报, 2008, 36(6): 720-725. |
| CHAI Yongming, XIANG Chune, KONG Huiqing, et al. A study on slurry-bed hydrotreating process of distillate oilⅠ: Catalyst preparation[J]. Journal of Fuel Chemistry and Technology, 2008, 36(6): 720-725. | |
| 3 | 王继锋, 梁相程, 温德荣, 等. 重质馏分油加氢精制催化剂的研制、生产和应用[J]. 石油化工高等学校学报, 2001, 14(1): 42-47. |
| WANG Jifeng, LIANG Xiangcheng, WEN Derong, et al. The research, manufacture and application of heavy distillate hydrorefining catalyst[J]. Journal of Petrochemical Universities, 2001, 14(1): 42-47. | |
| 4 | 高玉兰, 曹凤兰, 周勇. FH-5A加氢精制催化剂的研制[J]. 石油炼制与化工, 2002, 33(1): 18-21. |
| GAO Yulan, CAO Fenglan, ZHOU Yong. Preparation of hydrorefining catalyst FH-5A[J]. Petroleum Processing and Petrochemicals, 2002, 33(1): 18-21. | |
| 5 | 刘文勇, 田然, 陈世安, 等. 新型重质馏分油加氢精制催化剂的研制[J]. 化工进展, 2009, 28(3): 401-405. |
| LIU Wenyong, TIAN Ran, CHEN Shian, et al. Study on new catalysts of hydrorefining for VGO[J]. Chemical Industry and Engineering Progress, 2009, 28(3): 401-405. | |
| 6 | 朱全力, 赵旭涛, 赵振兴, 等. 加氢脱硫催化剂与反应机理的研究进展[J]. 分子催化, 2006, 20(4): 372-383. |
| ZHU Quanli, ZHAO Xutao, ZHAO Zhenxing, et al. Research progress of hydrodesulfurization catalyst and reaction mechanism[J]. Journal of Molecular Catalysis, 2006, 20(4): 372-383. | |
| 7 | 刘大鹏, 李永丹. 加氢脱氮催化研究的新进展[J]. 化学进展, 2006, 18(4): 417-428. |
| LIU Dapeng, LI Yongdan. Advances in catalytic hydrodenitrogenation[J]. Progress in Chemistry, 2006, 18(4): 417-428. | |
| 8 | 杨骏, 陈满英, 任杰. Mo、W对Ni/Al2O3催化剂加氢脱氧性能的影响[J]. 化工进展, 2005, 24(12): 1386-1389. |
| YANG Jun, CHEN Manying, REN Jie. Effect of Mo, W addition on performance of Ni/Al2O3 catalyst for hydrodeoxygenation[J]. Chemical Industry and Engineering Progress, 2005, 24(12): 1386-1389. | |
| 9 | 张孔远, 燕京, 吕才山. 重油加氢脱金属催化剂的性能及沉积金属的分布研究[J]. 石油炼制与化工, 2004, 35(8): 30-33. |
| ZHANG Kongyuan, YAN Jing, Caishan LYU. The performance of heavy oilhydrodemetallization catalystand its deposited metal distribution[J]. Petroleum Processing and Petrochemicals, 2004, 35(8): 30-33. | |
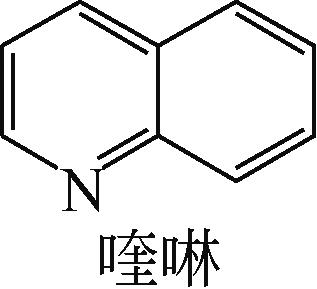
| 10 | 相春娥, 柴永明, 柳云骐, 等. NiMoS/γ-Al2O3上二苯并噻吩加氢脱硫和喹啉加氢脱氮反应的相互影响[J]. 催化学报, 2008, 29(7): 595-601. |
| XIANG Chune, CHAI Yongming, LIU Yunqi, et al. Mutual influence of hydrodesulfurization of dibenzothiophene and hydrodenitrogenation of quinoline over NiMoS/γ-Al2O3 catalyst[J]. Chinese Journal of Catalysis, 2008, 29 (7): 595-601. | |
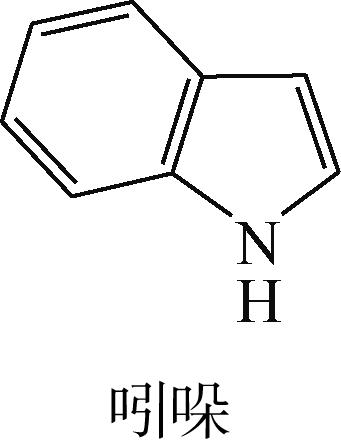
| 11 | 张奇, 刘春艳, 梁长海, 等. NiWS/γ-Al2O3催化剂上吲哚加氢脱氮反应: H2S和喹啉的影响[J]. 燃料化学学报, 2011, 39(5): 361-366. |
| ZHANG Qi, LIU Chunyan, LIANG Changhai, et al. Hydrodenitrogenation of indole over NiWS/γ-Al2O3 catalyst: Effects of H2S and quinoline[J]. Journal of Fuel Chemistry and Technology, 2011, 39(5): 361-366. | |
| 12 | 相春娥, 柴永明, 柳云骐, 等. 二苯并噻吩对吲哚加氢脱氮反应的影响[C]//第十一届全国青年催化学术会议论文集(下). 青岛, 2007: 276-277. |
| XIANG Chun’e, CHAI Yongming, LIU Yunqi, et al. Effect of dibenzothiophene on the denitrification reaction of indole hydrogenation[C]// Proceedings of the 11th National Youth Catalytic Academic Conference (Part Ⅱ). Qingdao, 2007: 276-277. | |
| 13 | ADAMSKI G, DYREK K, KOTARBA A, et al. Kinetic model of indole HDN over molybdenum carbide: Influence of potassium on early and late denitrogenation pathways[J]. Catalysis Today, 2004, 90(1/2): 115-119. |
| 14 | 李贵贤, 曹彦伟, 李梦晨, 等. 煤焦油加氢脱氮反应网络及催化剂研究进展[J]. 化工进展, 2015, 34(5): 1283-1290. |
| LI Guixian, CAO Yanwei, LI Mengchen, et al. Research progress in hydrodenitrogenation reaction network and its catalysts for coal-tar[J]. Chemical Industry and Engineering Progress, 2015, 34(5): 1283-1290. | |
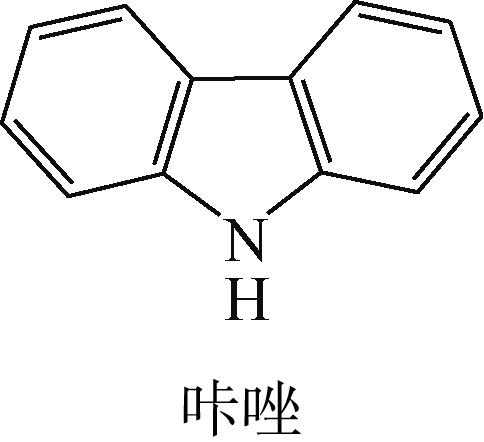
| 15 | 韩姝娜, 周同娜, 柴永明, 等. 存在4, 6-DMDBT时喹啉、吲哚和咔唑在Ni-Mo加氢催化剂上加氢脱氮自抑制作用[J]. 石油学报(石油加工), 2010, 26(3): 351-356. |
| HAN Shuna, ZHOU Tongna, CHAI Yongming, et al. Self-inhibition for HDN of quinoline, indole and carbazole over Ni-Mo catalyst in the presence of 4,6-DMDBT[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2010, 26(3): 351-356. | |
| 16 | 彭全铸, 赵琰. 重质馏分油性质及其加氢处理[J]. 工业催化, 1997, 5(2): 18-27. |
| PENG Quanzhu, ZHAO Yan. Properties of heavy distillates and their hydrotreatment[J]. Gongye Cuihua (Industrial Catalysis), 1997, 5(2): 18-27. | |
| 17 | 王宝俊, 张玉贵, 谢克昌. 量子化学计算在煤的结构与反应性研究中的应用[J]. 化工学报, 2003, 54(4): 477-488. |
| WANG Baojun, ZHANG Yugui, XIE Kechang. Application of quantum chemistry calculation to investigation on coal structure and reactivity[J]. Journal of Chemical Industry and Engineering (China), 2003, 54(4): 477-488. | |
| 18 | 付立海, 李秀梅, 刘博, 等. 两种铜配位聚合物的晶体结构和量子化学计算[J]. 无机化学学报, 2022, 38(11): 2249-2258. |
| FU Lihai, LI Xiumei, LIU Bo, et al. Two copper coordination polymers: Crystal structure and quantum chemistry calculation[J]. Chinese Journal of Inorganic Chemistry, 2022, 38(11): 2249-2258. | |
| 19 | 丁思佳, 彭绍忠, 闫作杰, 等. 量子化学计算在催化加氢研究中的应用[J]. 当代化工, 2021, 50(4): 938-943. |
| DING Sijia, PENG Shaozhong, YAN Zuojie, et al. Application of quantum chemical calculation in catalytic hydrogenation[J]. Contemporary Chemical Industry, 2021, 50(4): 938-943. | |
| 20 | DING Sijia, JIANG Shujiao, ZHOU Yasong, et al. Catalytic characteristics of active corner sites in CoMoS nanostructure hydrodesulfurization—A mechanism study based on DFT calculations[J]. Journal of Catalysis, 2017, 345: 24-38. |
| 21 | DING Sijia, ZHOU Yasong, WEI Qiang, et al. Substituent effects of 4,6-DMDBT on direct hydrodesulfurization routes catalyzed by Ni-Mo-S active nanocluster—A theoretical study[J]. Catalysis Today, 2018, 305: 28-39. |
| 22 | DING Sijia, LI Anqi, JIANG Shujiao, et al. Niobium modification effects on hydrodesulfurization of 4,6-DMDBT catalyzed on Ni-Mo-S active sites: A combination of experiments and theoretical study[J]. Fuel, 2019, 237: 429-441. |
| 23 | DING Sijia, JIANG Shujiao, WANG Jifeng, et al. Effects of the Ni-Mo ratio on olefin selective hydrogenation catalyzed on Ni-Mo-S active sites: A theoretical study by DFT calculation[J]. Fuel, 2020, 277: 118136. |
| 24 | HUMBERT S, IZZET G, RAYBAUD P. Competitive adsorption of nitrogen and sulphur compounds on a multisite model of NiMoS catalyst: A theoretical study[J]. Journal of Catalysis, 2016, 333: 78-93. |
| 25 | INADA Yasuji, ORITA Hideo. Efficiency of numerical basis sets for predicting the binding energies of hydrogen bonded complexes: Evidence of small basis set superposition error compared to Gaussian basis sets[J]. Journal of Computational Chemistry, 2008, 29(2): 225-232. |
| 26 | DELLEY B, ELLIS D E. Efficient and accurate expansion methods for molecules in local density models[J]. The Journal of Chemical Physics, 1982, 76(4): 1949-1960. |
| 27 | CHIGO ANOTA Ernesto, COCOLETZI Gregorio H. GGA-based analysis of the metformin adsorption on BN nanotubes[J]. Physica E: Low-Dimensional Systems and Nanostructures, 2014, 56: 134-140. |
| 28 | GRIMME Stefan. Semiempirical GGA-type density functional constructed with a long-range dispersion correction[J]. Journal of Computational Chemistry, 2006, 27(15): 1787-1799. |
| 29 | GRIMME Stefan. Density functional theory with London dispersion corrections[J]. WIREs Computational Molecular Science, 2011, 1(2): 211-228. |
| 30 | GRIMME Stefan, ANTONY Jens, EHRLICH Stephan, et al. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu[J]. The Journal of Chemical Physics, 2010, 132(15): 154104. |
| [1] | WU Da, JIANG Shujiao, WEI Qiang, YUAN Shenghua, YANG Gang, ZHANG Cheng. Research progress on efficient utilization technology of residue in energy transition [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2343-2353. |
| [2] | GUI Xin, CHEN Huiyong, BAI Boyang, JIA Yongliang, MA Xiaoxun. Catalytic hydrogenation of pyrene over Mo-doped NiC/Al-MCM-41 [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2386-2395. |
| [3] | WANG Bing, WANG Lei, HUANG Xinru, YUAN Hongpeng, LAI Xiaojuan, LI Peng. Synthesis and performance test of an acid and alkali resistant high strength resin [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1992-2000. |
| [4] | LIU Ruolu, TANG Haibo, HE Feifei, LUO Fengying, WANG Jinge, YANG Na, LI Hongwei, ZHANG Ruiming. Recent research and prospect of liquid organic hydrogen carries technology [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1731-1741. |
| [5] | WANG Hongyan, MA Ziran, LI Ge, MA Jing, ZHAO Chunlin, ZHOU Jiali, WANG Lei, PENG Shengpan. Research progress in synergistic catalytic elimination of multiple pollutants in flue gas of coal combustion coupled with renewable fuels [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1783-1795. |
| [6] | CHEN Jiayi, GAO Weitao, YIN Yanan, WANG Cheng, OUYANG Hongwu, MAO Zongqiang. Preparation of PEMFC catalysts by electrodeposition [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1796-1809. |
| [7] | WU Chenhe, LIU Yumin, YANG Xinmin, CUI Jiwei, JIANG Shaokun, YE Jinhua, LIU Lequan. Particulate photocatalysts for light-driven overall water splitting [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1810-1822. |
| [8] | LIU Yurong, WANG Xingbao, LI Wenying. Regulation of catalyst acid sites and its effect on the deep hydrogenation performance of anthracene [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1832-1839. |
| [9] | GUO Xiaodong, MAO Yujiao, LIU Xiangyang, QIU Li, YU Feng, YAN Xiaoliang. Effect of oxygen vacancies in Ni/Sm2O3-CeO2/Al2O3 catalyst on CO2 methanation at low temperature [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1840-1850. |
| [10] | GAO Fei, LIU Zhisong, PAN Keke, LIU Minmin, DAI Bin, DAN Jianming, YU Feng. Vermiculite-supported FeCe bimetallic catalyst for selective catalytic reduction of NO with CO [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1851-1862. |
| [11] | LIU Fangwang, HAN Yi, ZHANG Jiajia, BU Honghong, WANG Xingpeng, YU Chuanfeng, LIU Mengshuai. Research advance of heterogeneous catalytic system for the coupling between CO2 and epoxide into propylene carbonate [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1252-1265. |
| [12] |
ZHANG Pengfei, YAN Zhangyan, REN Liang, ZHAGN Kui, LIANG Jialin, ZHAO Guangle, ZHANG Fanbin, HU Zhihai.
Research progress in the catalytic hydrodealkylation of C |
| [13] | GU Xingpeng, MA Hongqin, LIU Jiahao. Modification of Rainey nickel with phosphorus quantum dots and its catalytic hydrodesulfurization performances [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1293-1301. |
| [14] | ZHANG Shuming, LIU Huazhang. Optimization of Fe1-x O ammonia synthesis catalyst by BP neural network model [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1302-1308. |
| [15] | XIAO Yaoxin, ZHANG Jun, SHAN Rui, YUAN Haoran, CHEN Yong. Catalytic hydrogenation of furfuryl alcohol into pentanediol over Pt/CaO materials [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1318-1327. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||