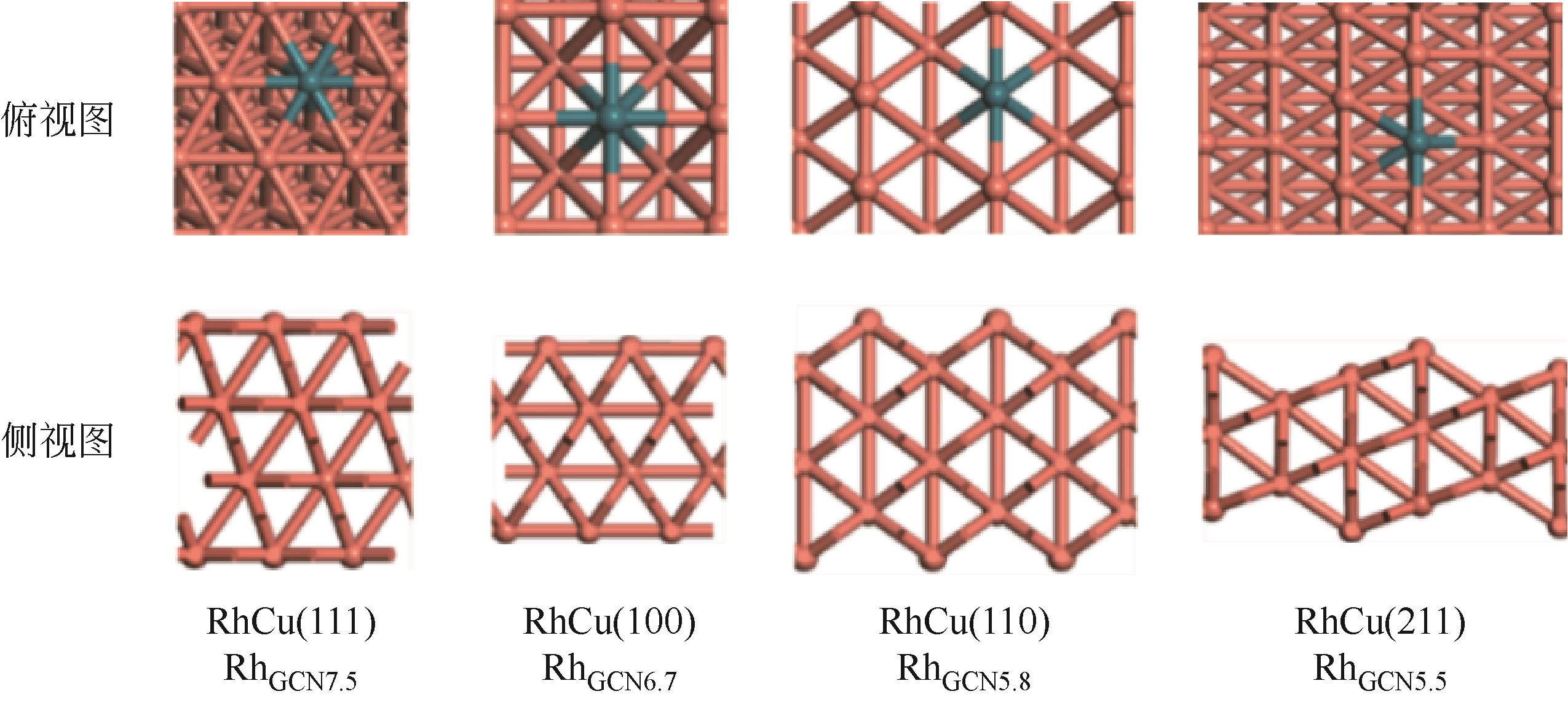
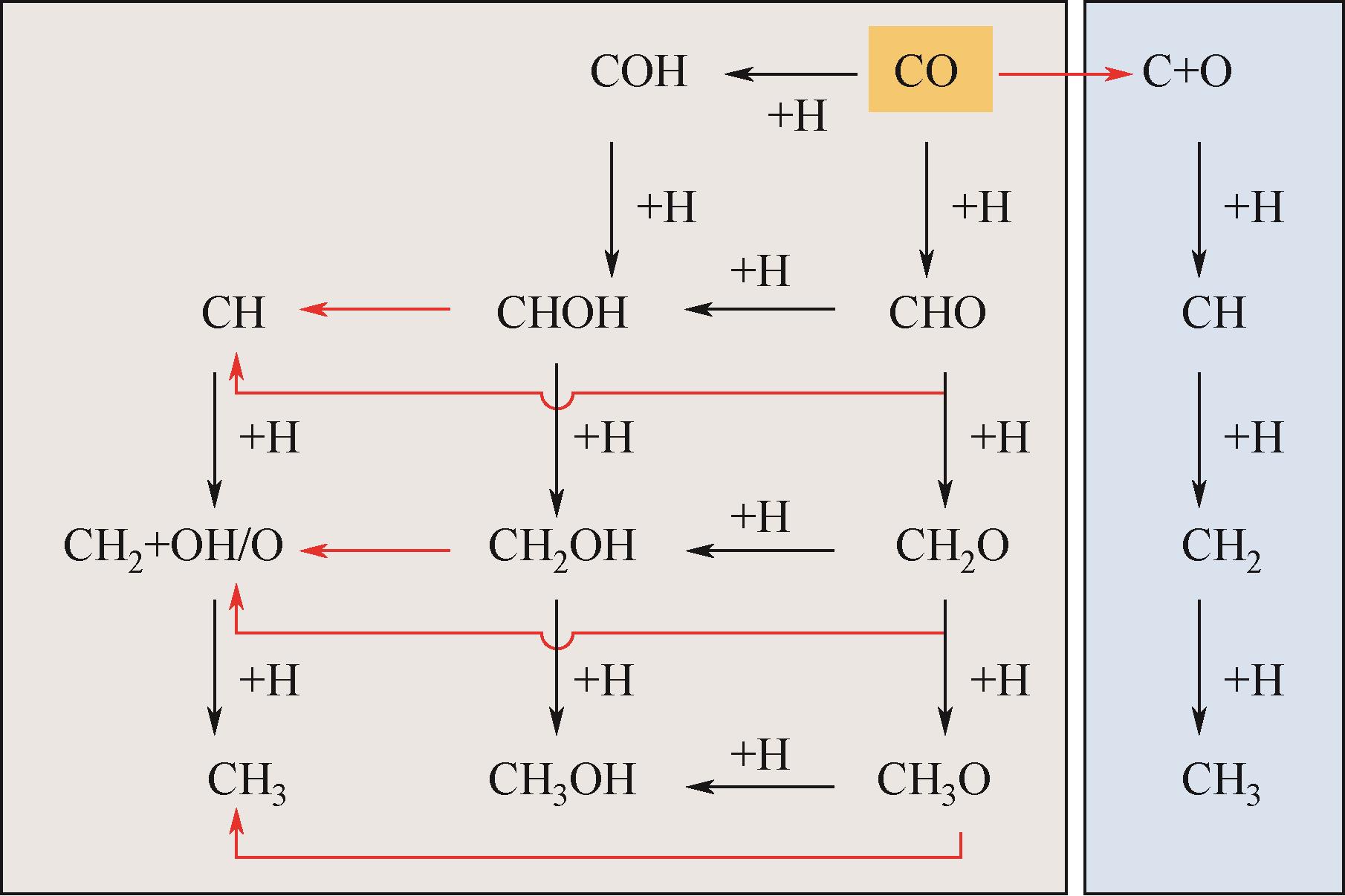
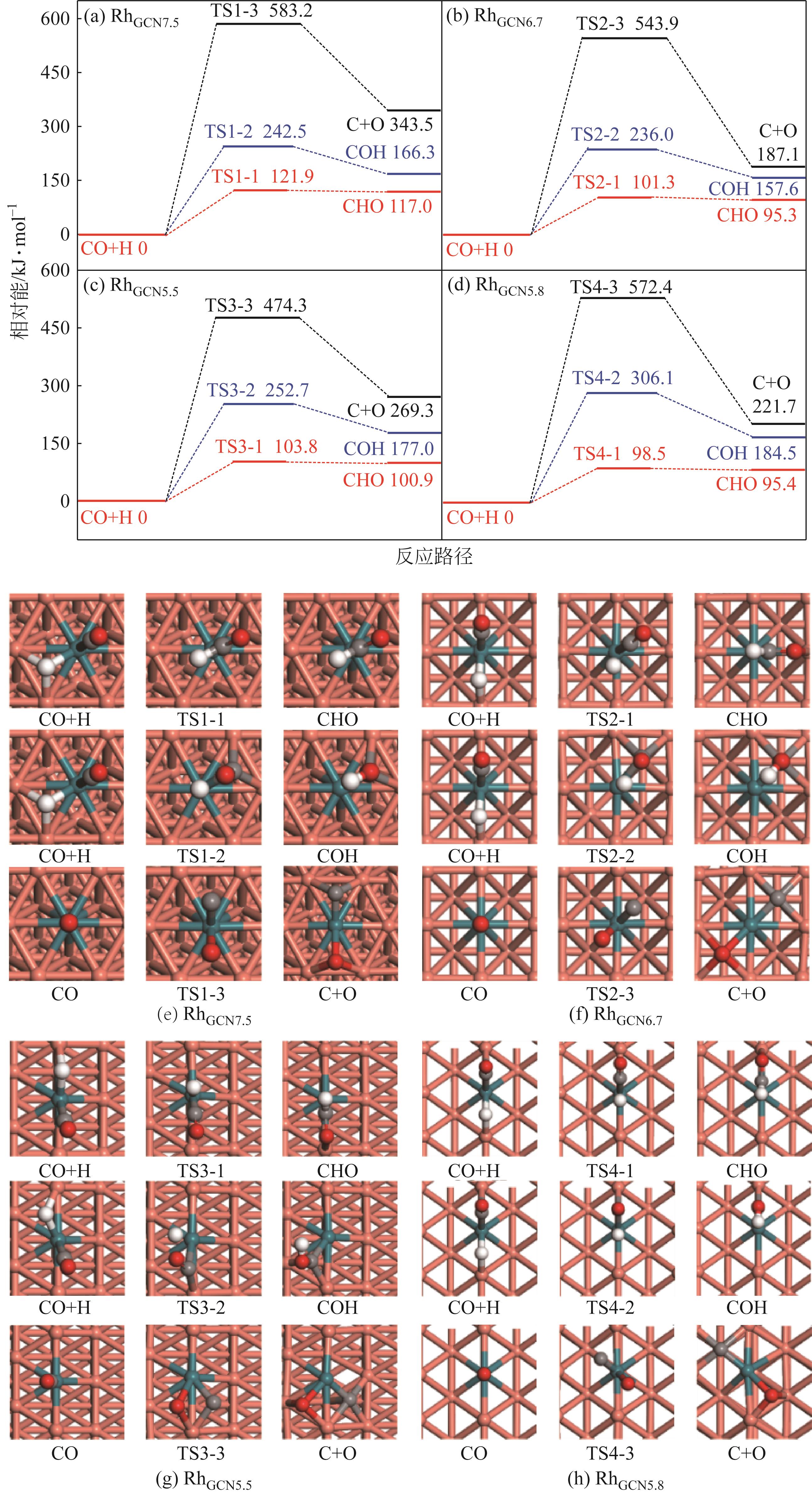
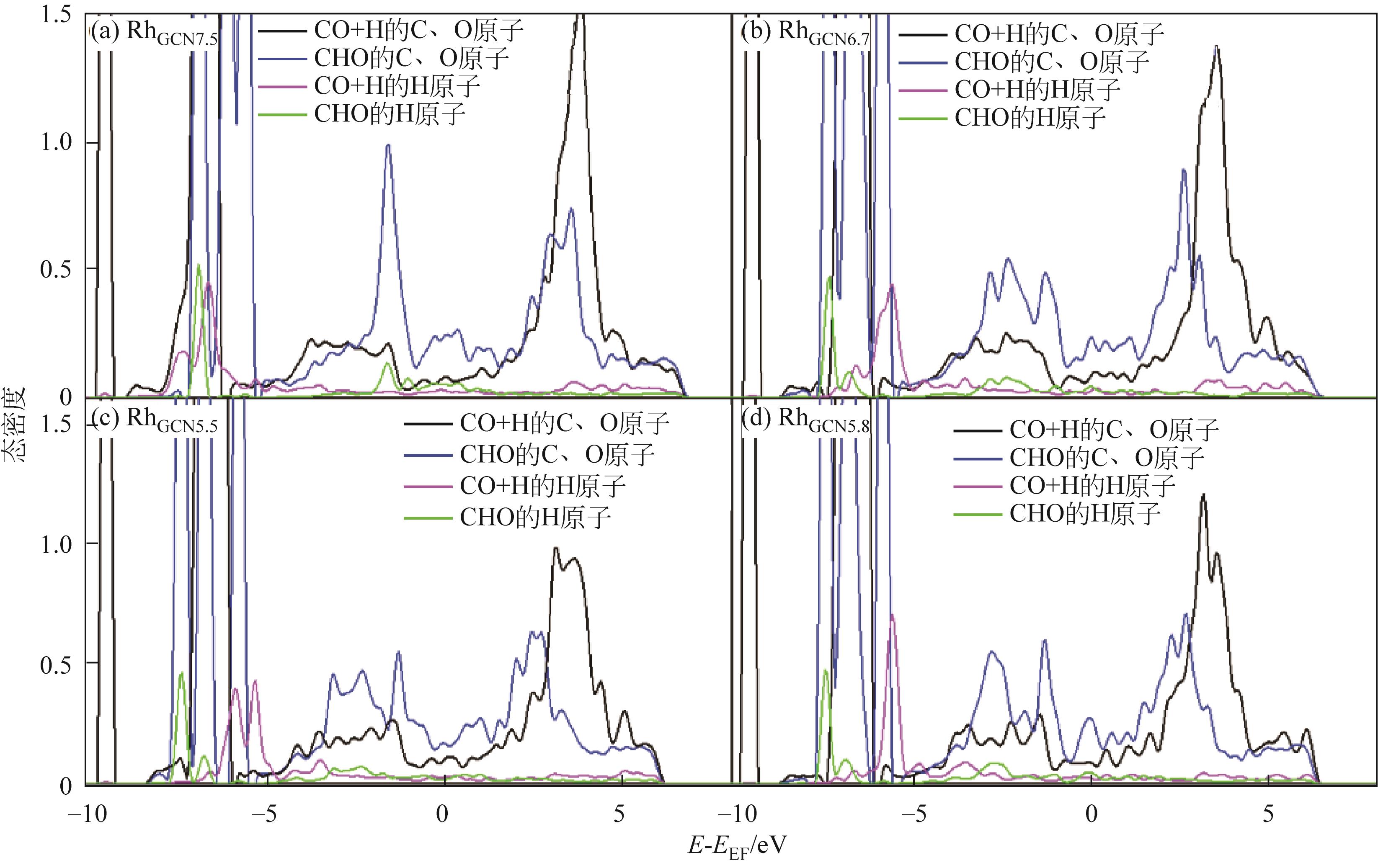
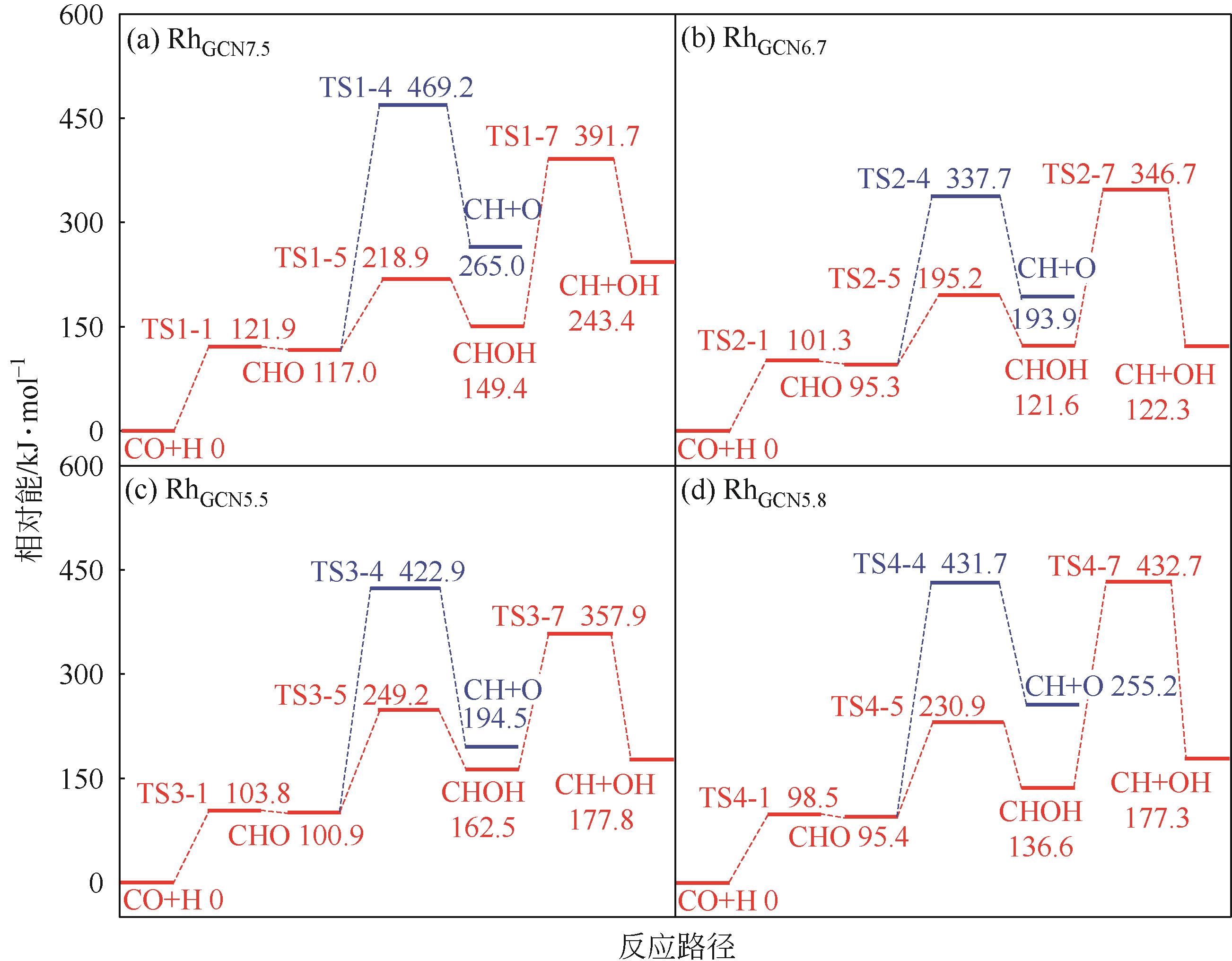
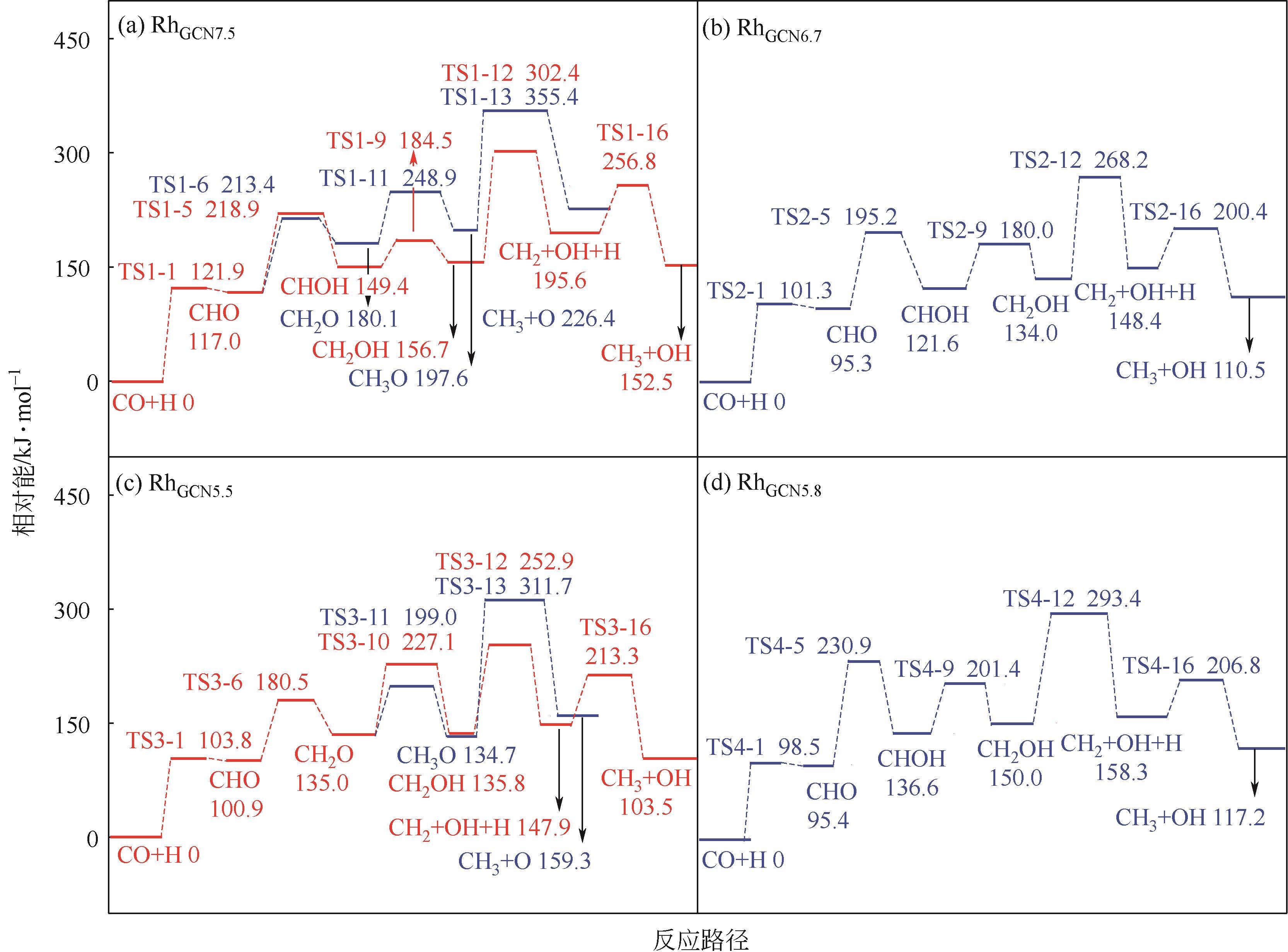
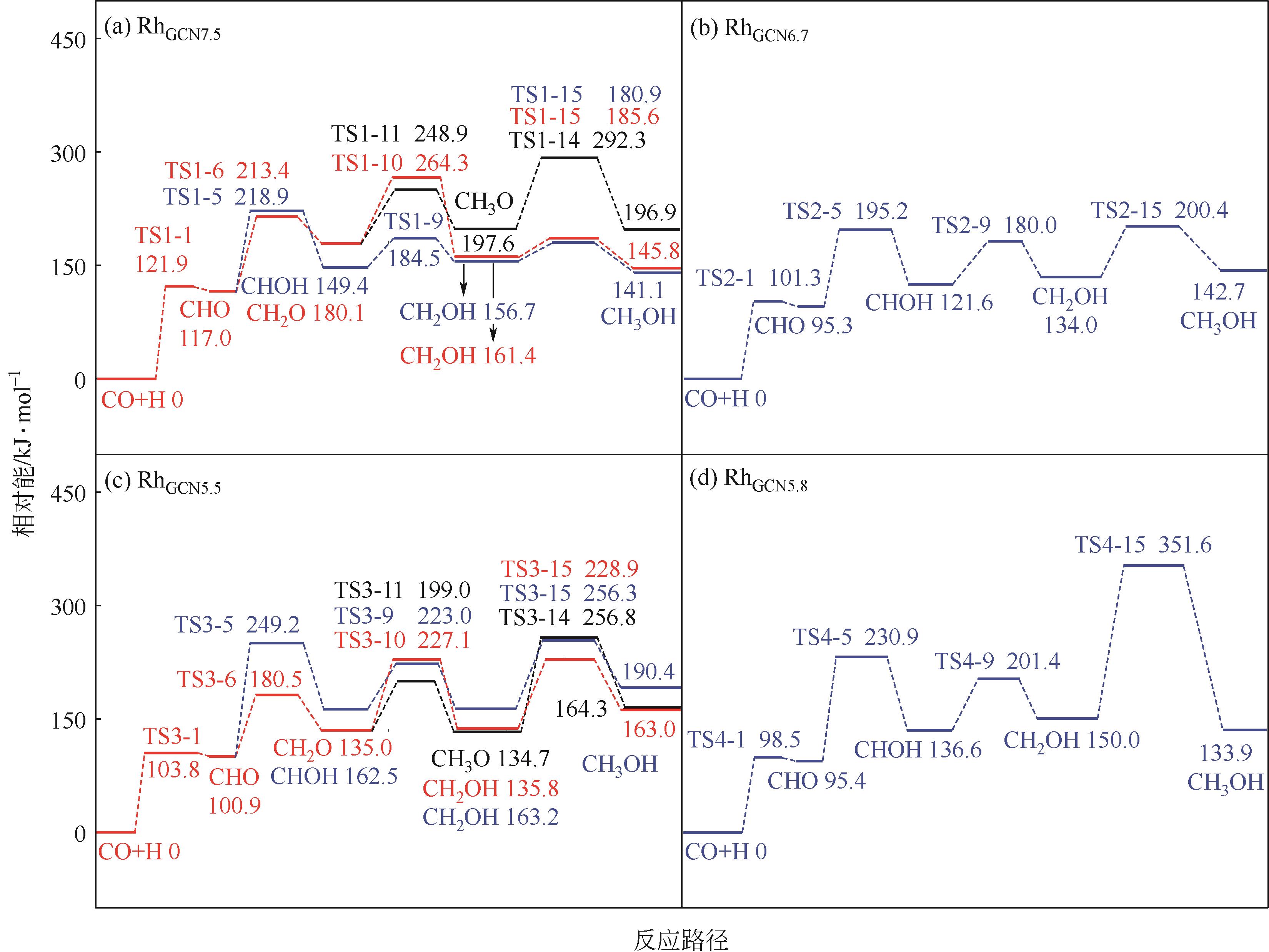
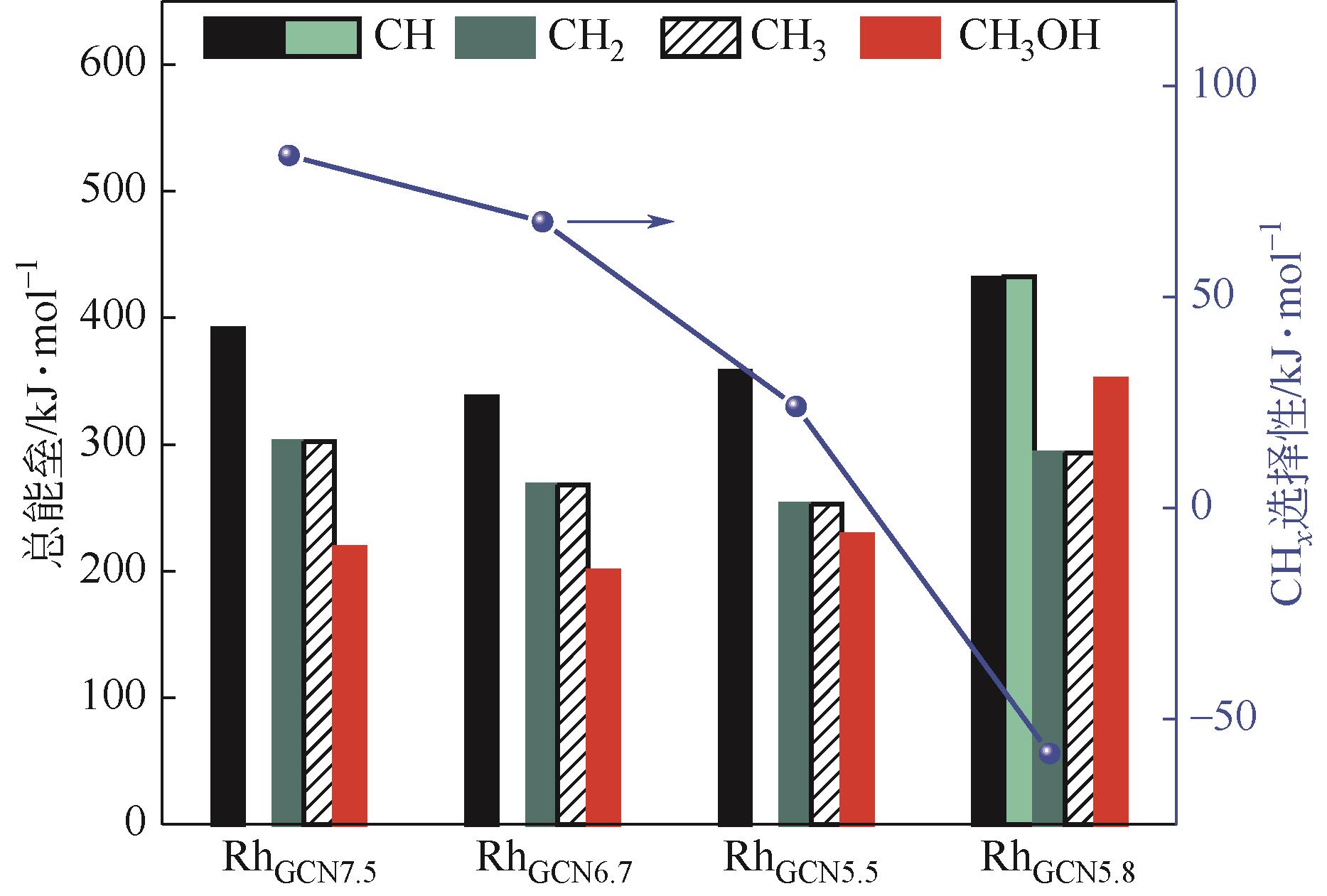
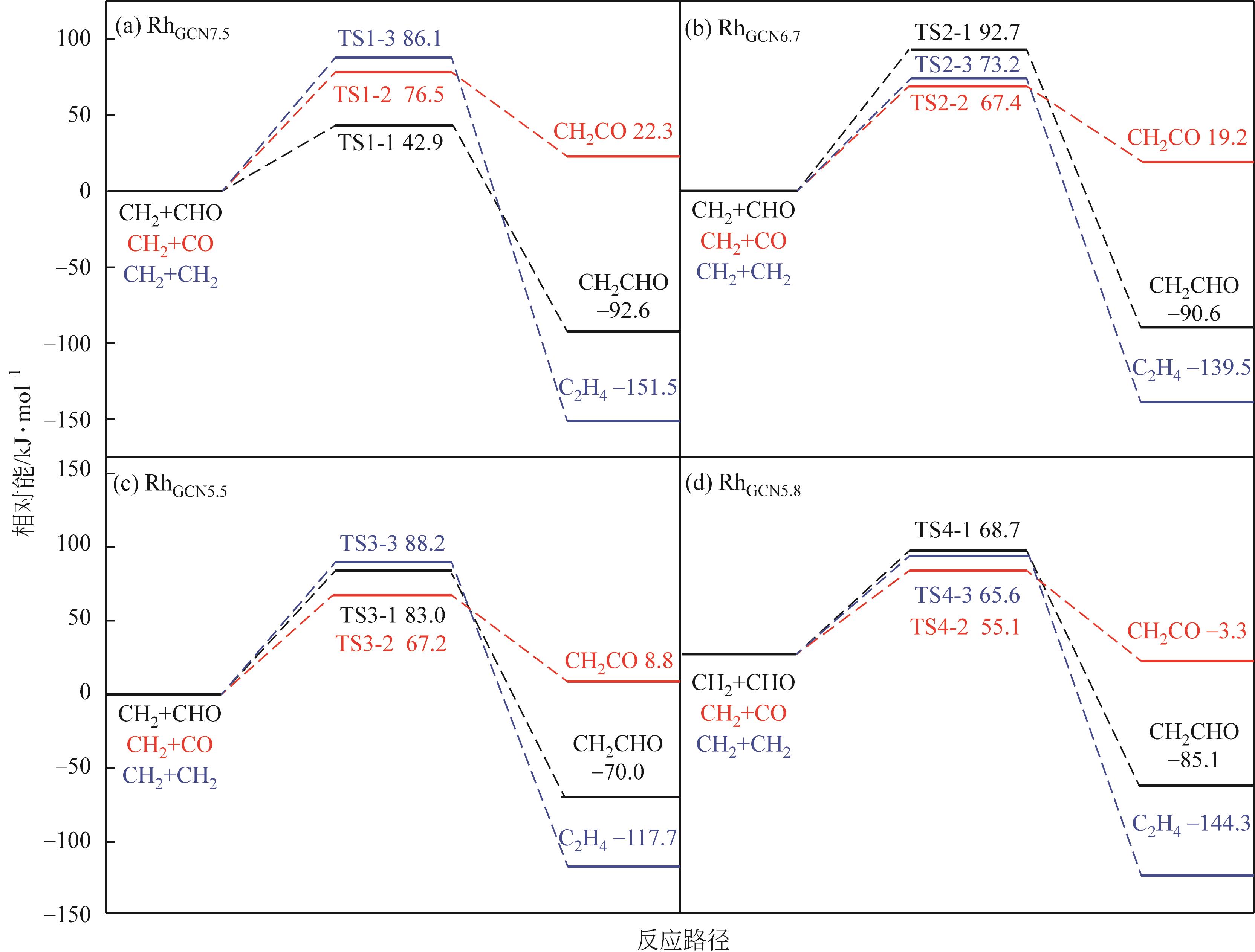
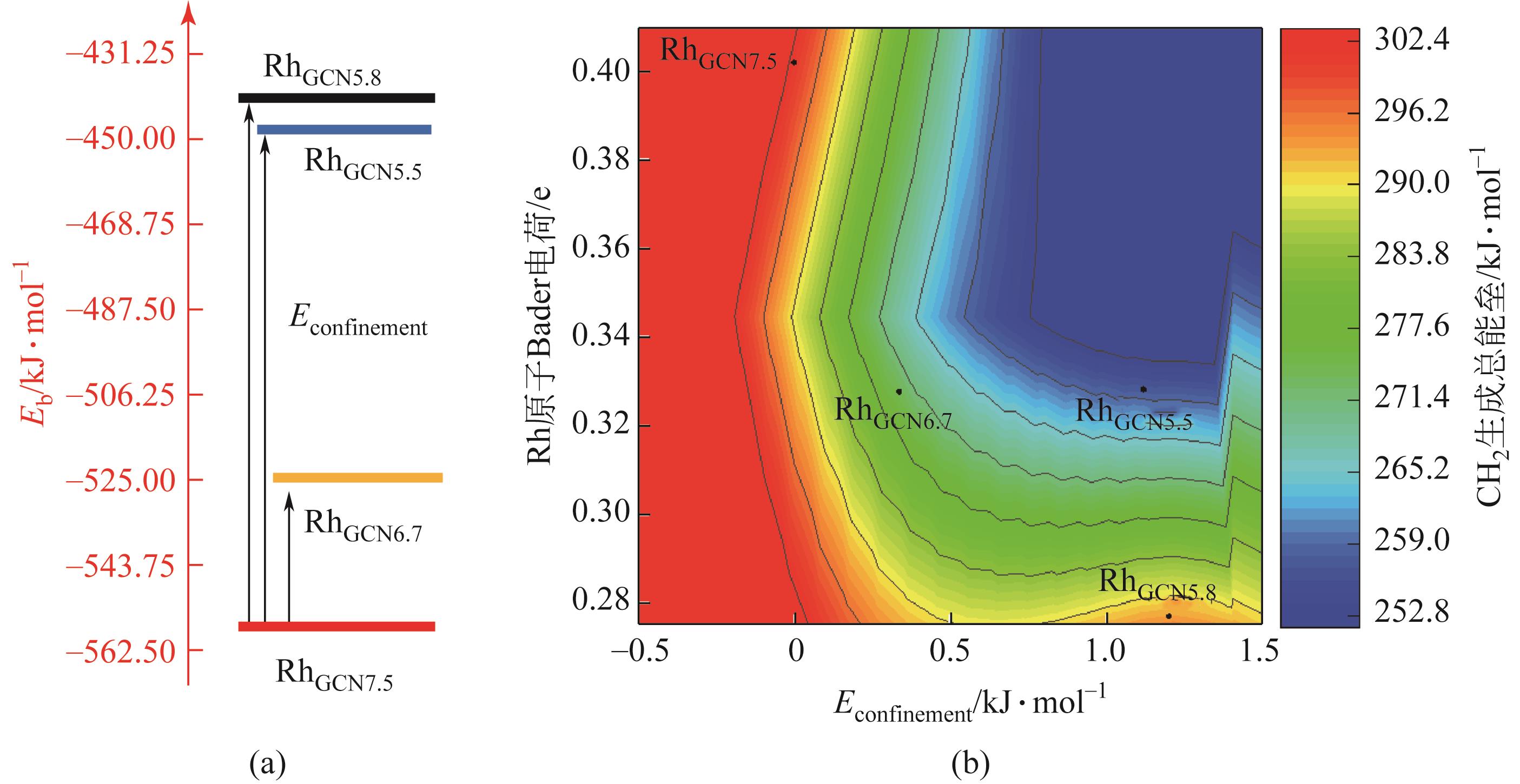
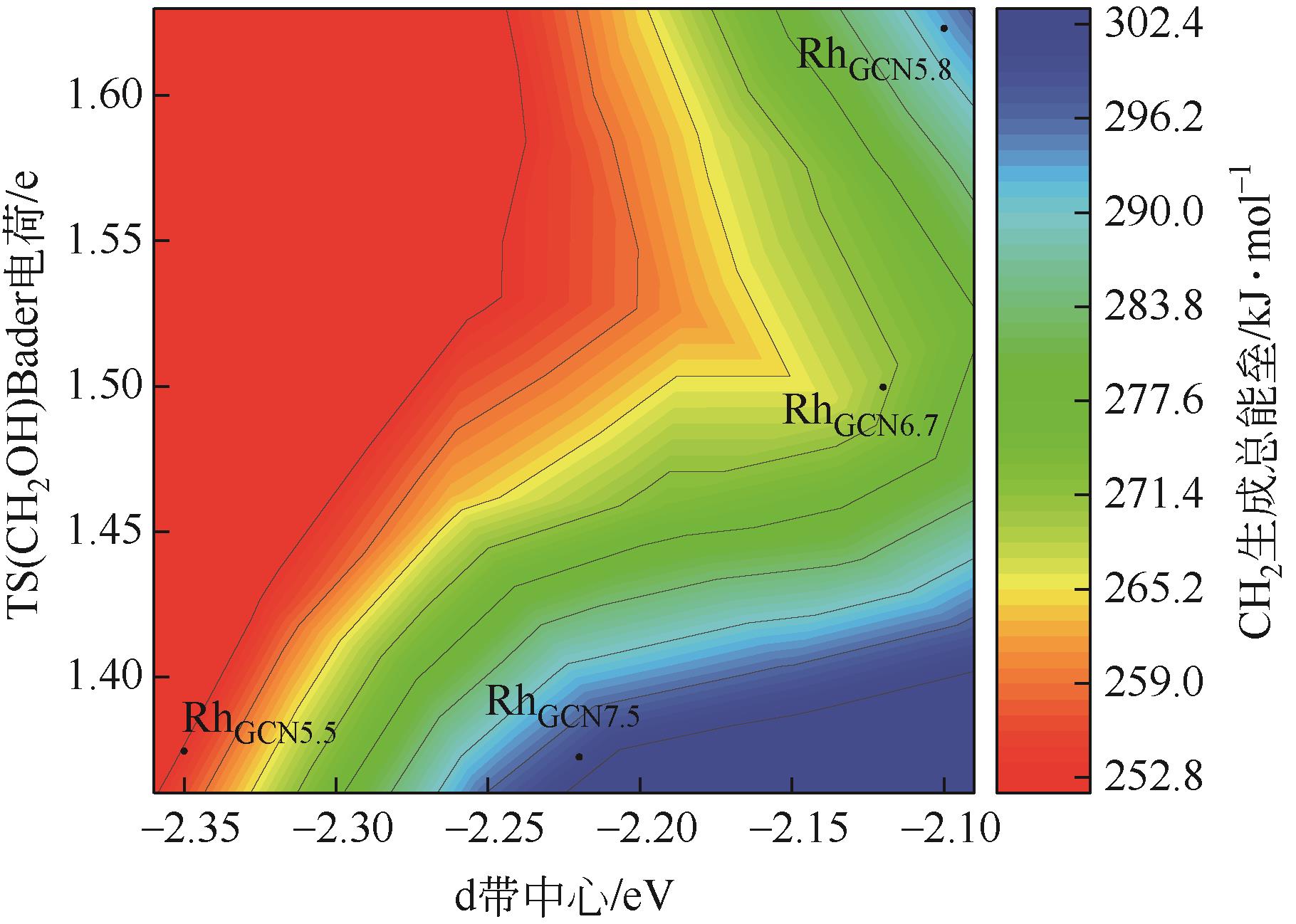
Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (5): 2684-2695.DOI: 10.16085/j.issn.1000-6613.2023-2179
• Catalysis and material technology • Previous Articles
Confined environment of RhCu catalyst to regulate the reaction performance for synthesis gas conversion to CH x
LI Na( ), ZHAO Wantong, LING Lixia, WANG Baojun, ZHANG Riguang(
), ZHAO Wantong, LING Lixia, WANG Baojun, ZHANG Riguang( )
)
- State Key Laboratory of Clean and Efficient Coal Utilization, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
-
Received:2023-12-11Revised:2024-04-22Online:2024-06-15Published:2024-05-15 -
Contact:ZHANG Riguang
RhCu催化剂中限域环境调控合成气转化生成CH x 反应性能
- 太原理工大学省部共建煤基能源清洁高效利用国家重点实验室,山西 太原 030024
-
通讯作者:章日光 -
作者简介:李娜(1999—),女,博士研究生,研究方向为多相催化基础理论。E-mail:lina0731@link.tyut.edu.cn。 -
基金资助:国家重点研发计划(2021YFA1502804);山西省杰出青年科学基金(202103021221005)
CLC Number:
Cite this article
LI Na, ZHAO Wantong, LING Lixia, WANG Baojun, ZHANG Riguang. Confined environment of RhCu catalyst to regulate the reaction performance for synthesis gas conversion to CH x[J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2684-2695.
李娜, 赵婉彤, 凌丽霞, 王宝俊, 章日光. RhCu催化剂中限域环境调控合成气转化生成CH x 反应性能[J]. 化工进展, 2024, 43(5): 2684-2695.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-2179
| 1 | ZHANG Riguang, ZHANG Jin, JIANG Zhao, et al. The cost-effective Cu-based catalysts for the efficient removal of acetylene from ethylene: The effects of Cu valence state, surface structure and surface alloying on the selectivity and activity[J]. Chemical Engineering Journal, 2018, 351: 732-746. |
| 2 | AO Min, PHAM Gia Hung, SUNARSO Jaka, et al. Active centers of catalysts for higher alcohol synthesis from syngas: A review[J]. ACS Catalysis, 2018, 8(8): 7025-7050. |
| 3 | SUN X C, ZHANG R G, WANG B J. Insights into the preference of CH x (x=1—3) formation from CO hydrogenation on Cu(111) surface[J]. Applied Surface Science, 2013, 265: 720-730. |
| 4 | CHOI YongMan, LIU Ping. Mechanism of ethanol synthesis from syngas on Rh(111)[J]. Journal of the American Chemical Society, 2009, 131(36): 13054-13061. |
| 5 | WANG Jiancheng, LIU Zhixue, ZHANG Riguang, et al. Ethanol synthesis from syngas on the stepped Rh(211) surface: Effect of surface structure and composition[J]. Journal of Physical Chemistry C, 2014, 118: 22691-22701. |
| 6 | ZHAO Ning, XU Run, WEI Wei, et al. Cu/Mn/ZrO2 catalyst for alcohol synthesis by Fischer-Tropsch modified elements[J]. Reaction Kinetics and Catalysis Letters, 2002, 75(2): 297-304. |
| 7 | XU Run, WEI Wei, LI Wenhuai, et al. Fe modified CuMnZrO2 catalysts for higher alcohols synthesis from syngas: Effect of calcination temperature[J]. Journal of Molecular Catalysis A: Chemical, 2005, 234(1/2): 75-83. |
| 8 | YAN Huan, ZHAO Xiaoxu, GUO Na, et al. Atomic engineering of high-density isolated Co atoms on graphene with proximal-atom controlled reaction selectivity[J]. Nature Communications, 2018, 9: 3197. |
| 9 | Maria FLYTZANI-STEPHANOPOULOS. Gold atoms stabilized on various supports catalyze the water-gas shift reaction[J]. Accounts of Chemical Research, 2014, 47(3): 783-792. |
| 10 | HE Xiaohui, HE Qian, DENG Yuchen, et al. A versatile route to fabricate single atom catalysts with high chemoselectivity and regioselectivity in hydrogenation[J]. Nature Communications, 2019, 10: 3663. |
| 11 | LI Xinle, Tian Wei GOH, LI Lei, et al. Controlling catalytic properties of Pd nanoclusters through their chemical environment at the atomic level using isoreticular metal-organic frameworks[J]. ACS Catalysis, 2016, 6(6): 3461-3468. |
| 12 | PAN Yuan, CHEN Yinjuan, WU Konglin, et al. Regulating the coordination structure of single-atom Fe-N x C y catalytic sites for benzene oxidation[J]. Nature Communications, 2019, 10: 4290. |
| 13 | LI Siwei, XU Yao, CHEN Yifu, et al. Tuning the selectivity of catalytic carbon dioxide hydrogenation over iridium/cerium oxide catalysts with a strong metal-support interaction[J]. Angewandte Chemie (International Ed in English), 2017, 56(36): 10761-10765. |
| 14 | FU Qiang, LUO Yi. Active sites of Pd-doped flat and stepped Cu(111) surfaces for H2 dissociation in heterogeneous catalytic hydrogenation[J]. ACS Catalysis, 2013, 3(6): 1245-1252. |
| 15 | QI Yamin, WANG Baojun, FAN Maohong, et al. C2H2 semi-hydrogenation on the metal M (M=Cu, Ag, Au) alloyed single-atom Pd catalysts: Effects of Pd coordination number and environment on the catalytic performance[J]. Chemical Engineering Science, 2021, 243: 116786. |
| 16 | SOLYMOSI F, CSERÉNYI J. Enhanced formation of ethane in the conversion of methane over Cu-Rh/SiO2 [J]. Catalysis Letters, 1995, 34(3): 343-350. |
| 17 | ZHAO Yonghui, YANG Mingmei, SUN Dapeng, et al. Rh-decorated Cu alloy catalyst for improved C2 oxygenate formation from syngas[J]. The Journal of Physical Chemistry C, 2011, 115(37): 18247-18256. |
| 18 | BAO Zhenghong, XIAO Kang, QI Xingzhen, et al. Higher alcohol synthesis over Cu-Fe composite oxides with high selectivity to C2+ OH[J]. Journal of Energy Chemistry, 2013, 22(1): 107-113. |
| 19 | WANG Baojun, GUO Weisheng, ZHANG Riguang, et al. C2 oxygenates formation from syngas over the Cu-rich and Rh-rich surfaces of Rh-Cu bimetallic catalysts: Probing into the effects of the surface structure and composition on the catalytic performance[J]. The Journal of Physical Chemistry C, 2019, 123(32): 19528-19539. |
| 20 | ZHANG Riguang, WANG Guiru, WANG Baojun. Insights into the mechanism of ethanol formation from syngas on Cu and an expanded prediction of improved Cu-based catalyst[J]. Journal of Catalysis, 2013, 305: 238-255. |
| 21 | LI Zhiyuan, LI Na, WANG Nan, et al. Mechanism investigations on water gas shift reaction over Cu(111), Cu(100), and Cu(211) surfaces[J]. ACS Omega, 2022, 7(4): 3514-3521. |
| 22 | CAO Kun, Füchsel Gernot, KLEYN Aart W, et al. Hydrogen adsorption and desorption from Cu(111) and Cu(211)[J]. Physical Chemistry Chemical Physics: PCCP, 2018, 20(35): 22477-22488. |
| 23 | DELLEY B. An all-electron numerical method for solving the local density functional for polyatomic molecules[J]. The Journal of Chemical Physics, 1990, 92(1): 508-517. |
| 24 | DELLEY B. From molecules to solids with the DMol3 approach[J]. The Journal of Chemical Physics, 2000, 113(18): 7756-7764. |
| 25 | PERDEW J P, CHEVARY J A, VOSKO S H, et al. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation[J]. Physical Review B, Condensed Matter, 1992, 46(11): 6671-6687. |
| 26 | HAMMER Bjørk, HANSEN Lars Bruno, NØRSKOV Jens K. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals[J]. \prb, 1999, 59(11): 7413-7421. |
| 27 | DOLG Michael, WEDIG Ulrich, STOLL Hermann, et al. Energy-adjusted ab initio pseudopotentials for the first row transition elements[J]. The Journal of Chemical Physics, 1987, 86: 866-872. |
| 28 | TIAN Dongxu, ZHANG Hualei, ZHAO Jijun. Structure and structural evolution of Ag n (n=3—22) clusters using a genetic algorithm and density functional theory method[J]. Solid State Communications, 2007, 144(3/4): 174-179. |
| 29 | HALGREN Thomas A, LIPSCOMB William N. The synchronous-transit method for determining reaction pathways and locating molecular transition states[J]. Chemical Physics Letters, 1977, 49(2): 225-232. |
| 30 | MEDFORD Andrew J, LAUSCHE Adam C, Frank ABILD-PEDERSEN, et al. Activity and selectivity trends in synthesis gas conversion to higher alcohols[J]. Topics in Catalysis, 2014, 57(1): 135-142. |
| 31 | CALLE-VALLEJO F, MARTÍNEZ J I, GARCÍA-LASTRA J M, et al. Fast prediction of adsorption properties for platinum nanocatalysts with generalized coordination numbers[J]. Angewandte Chemie International Edition, 2014, 53(32): 8316-8319. |
| 32 | ZHAO Zhonglong, CHEN Zhengzheng, ZHANG Xu, et al. Generalized surface coordination number as an activity descriptor for CO2 reduction on Cu surfaces[J]. The Journal of Physical Chemistry C, 2016, 120(49): 28125-28130. |
| 33 | VERGA Lucas G, MENDES Paulo C D, OCAMPO-RESTREPO Vivianne K, et al. Exploring the adsorption site coordination as a strategy to tune copper catalysts for CO2 electro-reduction[J]. Catalysis Science & Technology, 2022, 12(3): 869-879. |
| 34 | WEI Cong, ZHANG Riguang, LING Lixia, et al. Syngas-to-C2 oxygenates on Cu-based catalyst: Quantitative insight into the balancing effect of active Cu δ +(0≤δ≤1) sites[J]. Chemical Engineering Science, 2020, 224: 115785. |
| 35 | ZHANG Riguang, WEI Cong, LI Debao, et al. The new role of surface adsorbed CH (x=1—3) intermediates as a co-adsorbed promoter in self-promoting syngas conversion to form CH intermediates and C2 oxygenates on the Rh-doped Cu catalyst[J]. Journal of Catalysis, 2019, 377: 1-12. |
| 36 | CHEN Dachang, CHEN Zhiwen, LU Zhuole, et al. Computational screening of homo and hetero transition metal dimer catalysts for reduction of CO2 to C2 products with high activity and low limiting potential[J]. Journal of Materials Chemistry A, 2020, 8(40): 21241-21254. |
| 37 | NING Yanxiao, WEI Mingming, YU Liang, et al. Nature of interface confinement effect in oxide/metal catalysts[J]. The Journal of Physical Chemistry C, 2015, 119(49): 27556-27561. |
| 38 | HAMMER B, NØRSKOV J K. Electronic factors determining the reactivity of metal surfaces[J]. Surface Science, 1995, 343(3): 211-220. |
| [1] | WANG Xinyu, WANG Chao, ZHANG Mengjuan, LIU Fangzheng, LI Hanyang, WANG Zhenglin, JIA Xin, SONG Xingfei, XU Guangwen, HAN Zhennan. Process stability verification of the two-stage fluidized bed gasification for pine particles to produce clean gas [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2576-2586. |
| [2] | WANG Jiarui, LIU Dawei, DENG Yao, XU Jin, MA Xiaoxun, XU Long. Research progress of oxygen carriers in chemical looping reforming reaction of methane [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2235-2253. |
| [3] | CHENG Haolin, NIAN Yao, HAN You. Progress in the mechanism of CH4 and CO2co-conversion reactions [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 60-75. |
| [4] | YANG Xiazhen, PENG Yifan, LIU Huazhang, HUO Chao. Regulation of active phase of fused iron catalyst and its catalytic performance of Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 310-318. |
| [5] | LU Yang, ZHOU Jinsong, ZHOU Qixin, WANG Tang, LIU Zhuang, LI Bohao, ZHOU Lingtao. Leaching mechanism of Hg-absorption products on CeO2/TiO2 sorbentsin syngas [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3875-3883. |
| [6] | RUAN Peng, YANG Runnong, LIN Zirong, SUN Yongming. Advances in catalysts for catalytic partial oxidation of methane to syngas [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1832-1846. |
| [7] | TIAN Yuan, LOU Shujie, MENG Shanru, YAN Jingru, XIAO Haicheng. Recent progress of Co-based catalysts for higher alcohols synthesis form syngas [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1869-1876. |
| [8] | HU Peng, ZHAO Dan, JI Hongbing. Temperature-controlled biomimetic induced-fit-identification for boosting syngas purification [J]. Chemical Industry and Engineering Progress, 2023, 42(12): 6133-6135. |
| [9] | MI Zehao, HUA Er. Theoretical analysis of CO2 absorption by polyamines-TFSA type protic ionic liquids [J]. Chemical Industry and Engineering Progress, 2023, 42(11): 6015-6030. |
| [10] | LI Wanqi, YANG Fengjuan, JIA Dechen, JIANG Weihong, GU Yang. Biological utilization and conversion of syngas [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 73-85. |
| [11] | DENG Shaobi, BIAN Zhoufeng. Application of core-shell structure catalyst in dry reforming of methane [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 247-254. |
| [12] | ZHANG Dazhou, LU Wenxin, SHANG Kuanxiang, HU Yuan, ZHU Fan, ZHANG Zongfei. Reaction network analysis of dimethyl oxalate hydrogenation to methyl glycolate and recent progress in the heterogeneous catalysts [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 204-214. |
| [13] | SHI Xuan, YANG Dongyuan, HU Haobin, WANG Jiaofei, ZHANG Zhuangzhuang, HE Jianxun, DAI Chengyi, MA Xiaoxun. One-step preparation of toluene/xylene from benzene and syngas over ZnAlCrO x &HZSM-5 bifunctional catalyst [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 247-259. |
| [14] | CAO Zhengkai, MI Xiaobin, WU Ziming, SUN Shike, CAO Junfeng, PENG Deqiang, LIANG Xiangcheng. Pressure drop analysis and application optimization of the unit for removing dust in coal syngas purification [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 15-21. |
| [15] | HU Wende, WANG Yangdong, WANG Chuanming. Research progress on the direct catalytic conversion of syngas to light olefins [J]. Chemical Industry and Engineering Progress, 2022, 41(9): 4754-4766. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||