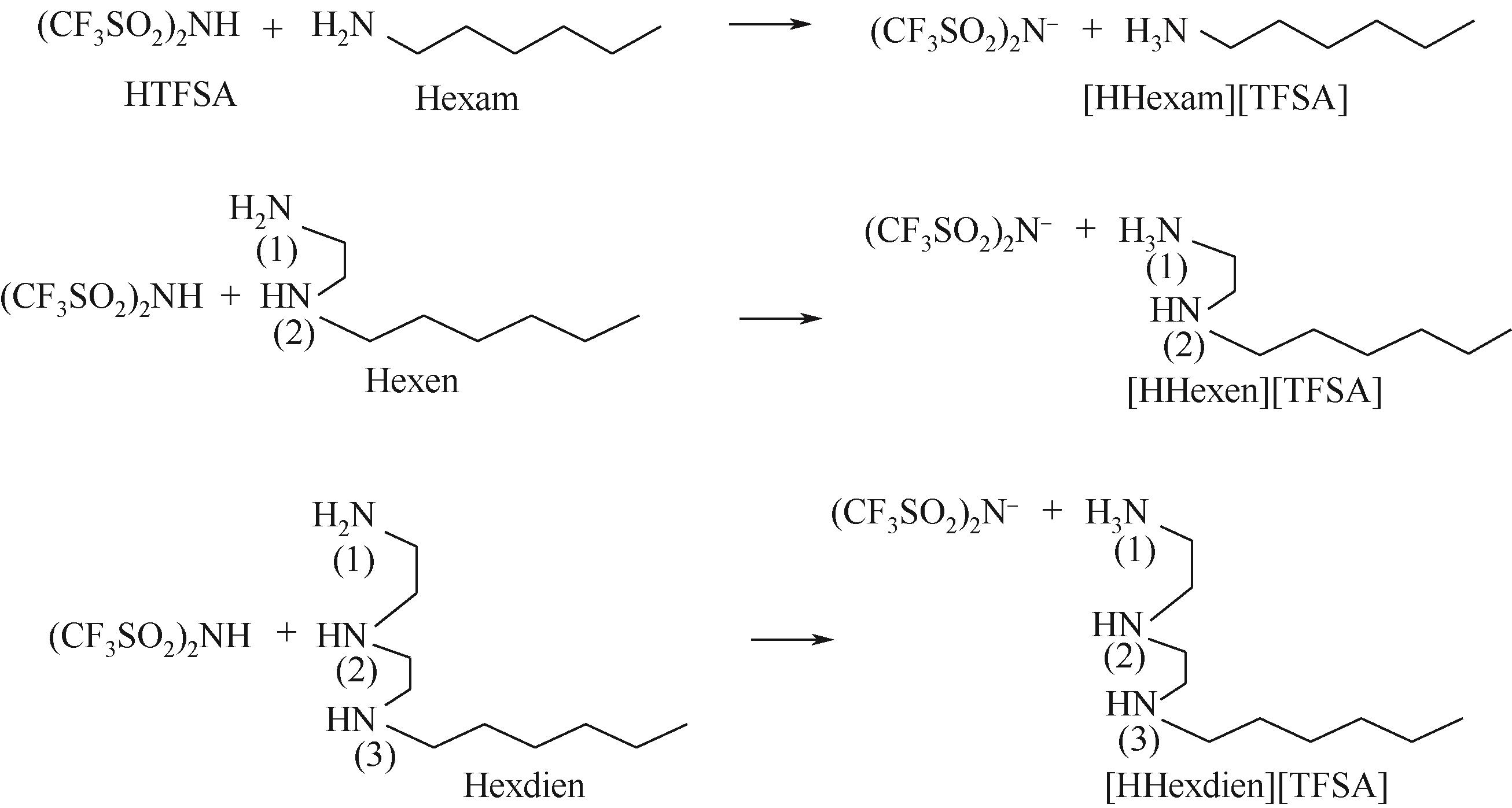
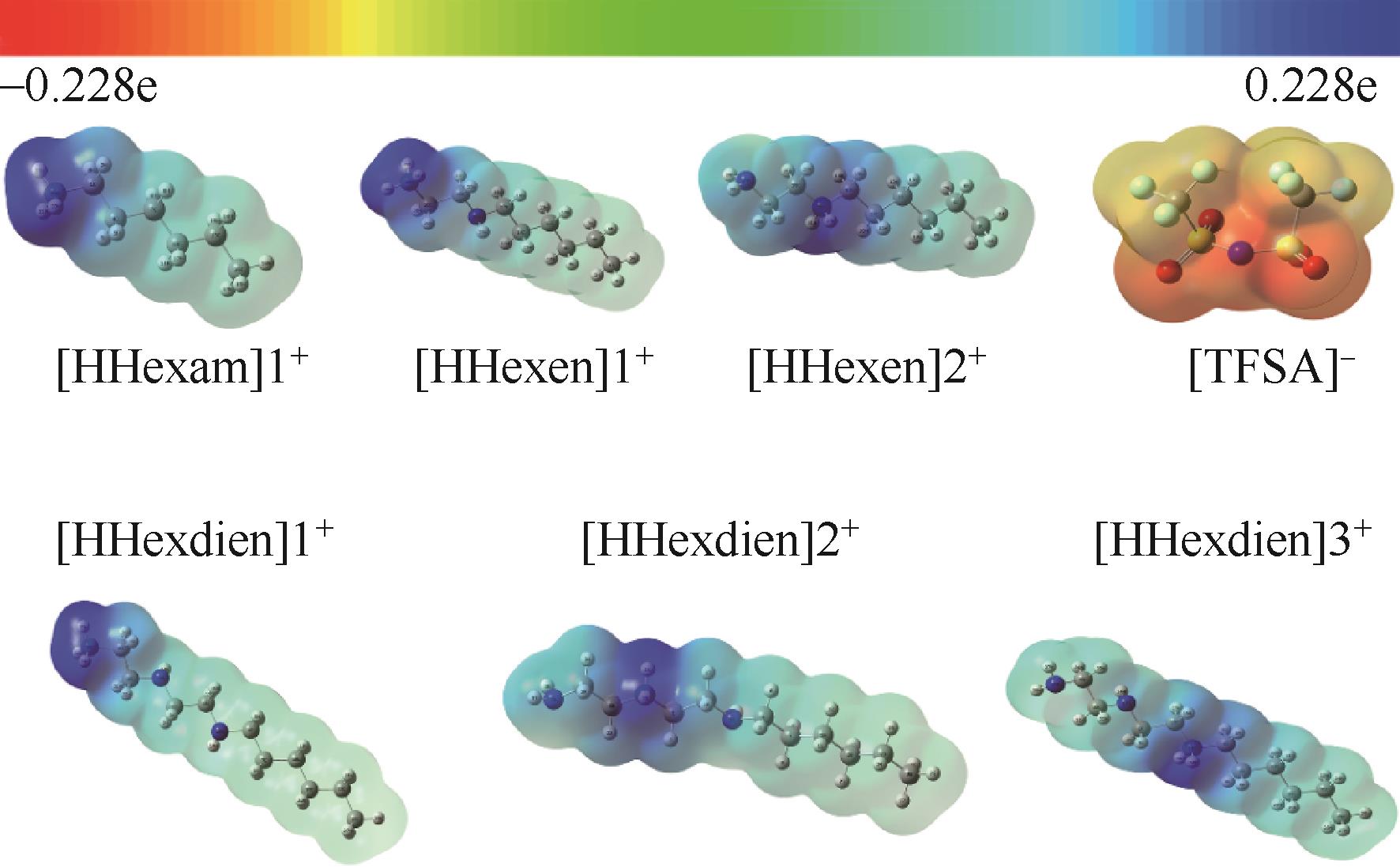
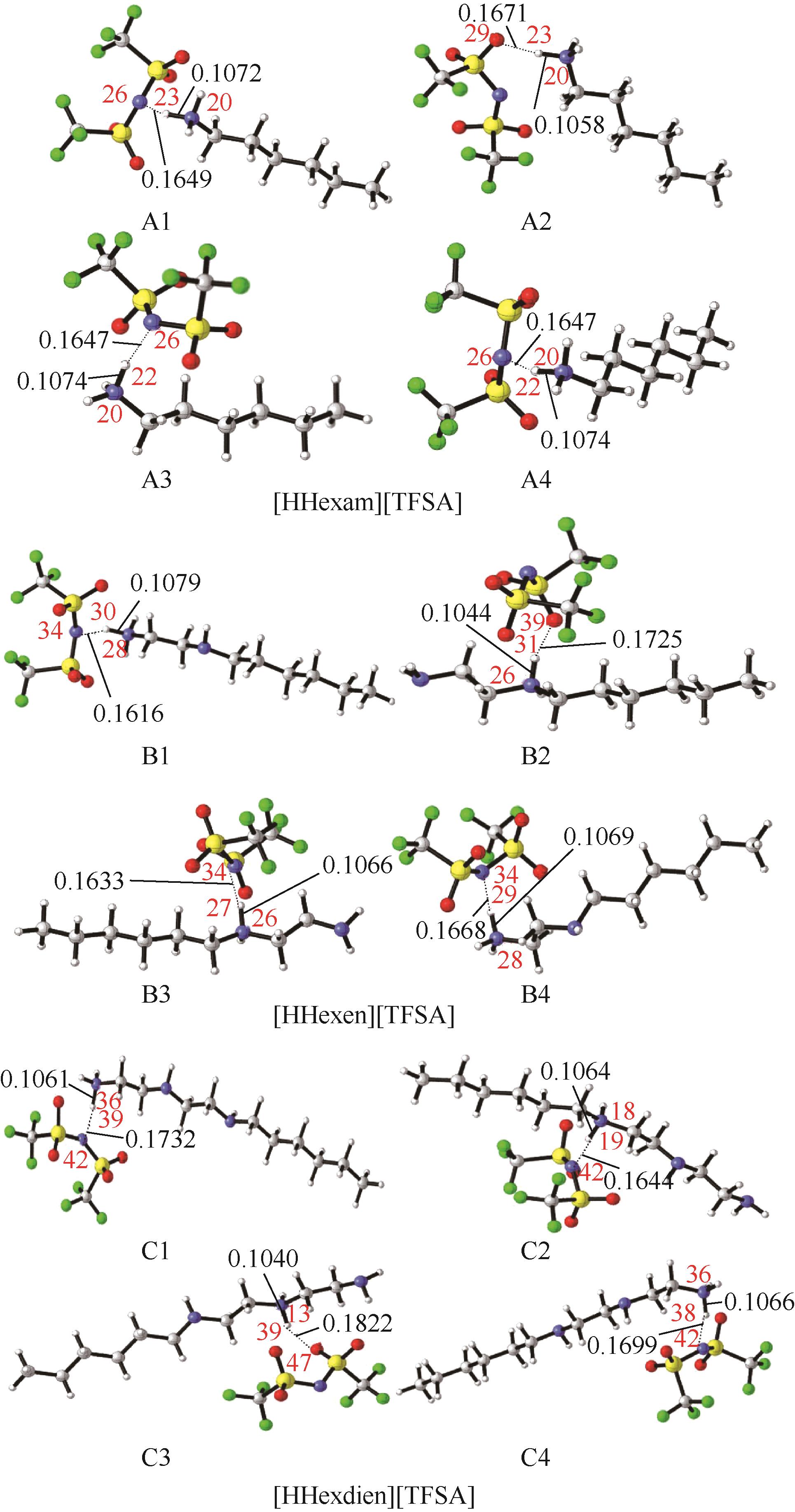
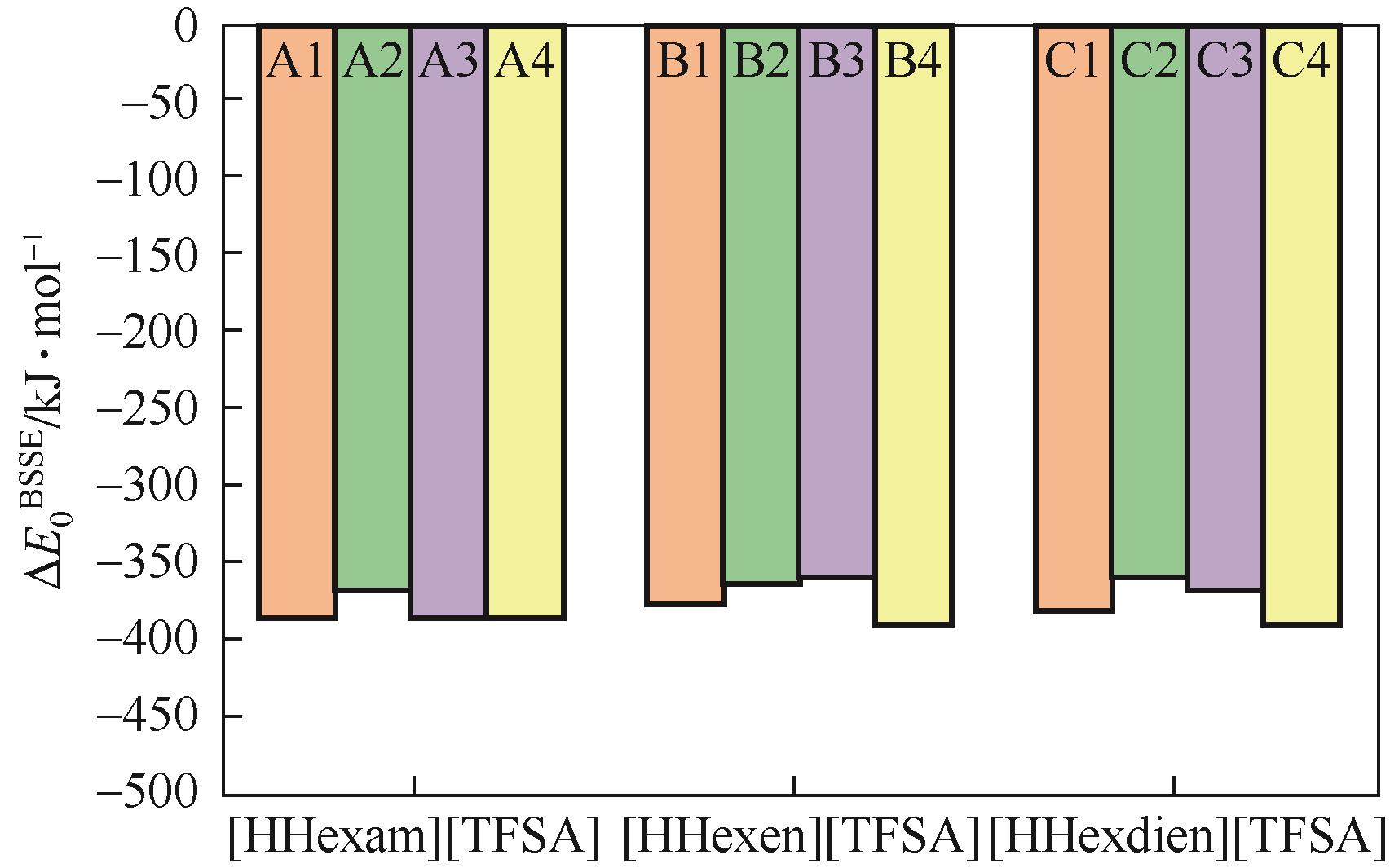
Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (11): 6015-6030.DOI: 10.16085/j.issn.1000-6613.2022-2319
• Resources and environmental engineering • Previous Articles
Theoretical analysis of CO2 absorption by polyamines-TFSA type protic ionic liquids
- 1.Chemical Science and Engineering College, North Minzu University, Yinchuan 750021, Ningxia, China
2.Key Laboratory of Chemical Engineering and Technology, State Ethnic Affairs Commission, Yinchuan 750021, Ningxia, China
3.Ningxia Key Labaratory of Solar Chemical Conversion Technology, Yinchuan 750021, Ningxia, China
-
Received:2022-12-15Revised:2023-03-07Online:2023-12-15Published:2023-11-20 -
Contact:HUA Er
多元胺-TFSA型质子化离子液体吸收CO2的理论分析
- 1.北方民族大学化学与化学工程学院,银川 宁夏 750021
2.国家民委化工技术基础重点实验室
1.宁夏 银川 750021,宁夏太阳能化学转化技术重点实验室,宁夏 银川 750021
-
通讯作者:花儿 -
作者简介:米泽豪(1998—),男,硕士研究生,研究方向为离子液体。E-mail:875501689@qq.com。 -
基金资助:宁夏高等学校研究项目(NGY2020063)
CLC Number:
Cite this article
MI Zehao, HUA Er. Theoretical analysis of CO2 absorption by polyamines-TFSA type protic ionic liquids[J]. Chemical Industry and Engineering Progress, 2023, 42(11): 6015-6030.
米泽豪, 花儿. 多元胺-TFSA型质子化离子液体吸收CO2的理论分析[J]. 化工进展, 2023, 42(11): 6015-6030.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-2319
| 构型 | 化学键 | 振动频率ν/cm-1 | 频率变化值Δν/cm-1 |
|---|---|---|---|
| CO2 | 1C—2O | 1422 | — |
| 1C—3O | 1422 | — | |
| [HHexam][TFSA] | 20N—21H | 3450 | — |
| 20N—22H | 2591 | — | |
| 20N—23H | 3563 | — | |
| A4-1CO2 | 20N—21H | 3410 | 40 |
| 20N—22H | 2709 | -118 | |
| A4-2CO2 | 20N—21H | 3430 | 20 |
| 20N—22H | 2825 | -234 | |
| 20N—23H | 3532 | 31 | |
| A4-3CO2 | 20N—21H | 3421 | 29 |
| 20N—22H | 2985 | -394 | |
| 20N—23H | 3522 | 41 | |
| [HHexen][TFSA] | 26N—27H | 3561 | — |
| 28N—30H | 3555 | — | |
| 28N—31H | 3429 | — | |
| 28N—29H | 2652 | — | |
| B4-1CO2 | 28N—31H | 3424 | 5 |
| 28N—29H | 2760 | -108 | |
| B4-2CO2 | 28N—30H | 3533 | 22 |
| 28N—29H | 2870 | -218 | |
| B4-3CO2 | 28N—30H | 3534 | 21 |
| 28N—29H | 2980 | -328 | |
| B4-4CO2 | 28N—30H | 3530 | 25 |
| 28N—29H | 3035 | -383 | |
| [HHexdien][TFSA] | 36N—37H | 3450 | — |
| 18N—19H | 3527 | — | |
| 13N—14H | 3511 | — | |
| 36N—39H | 3450 | — | |
| 36N—38H | 2706 | — | |
| C4-1CO2 | 36N—39H | 3414 | 36 |
| 36N—38H | 2852 | -147 | |
| C4-2CO2 | 36N—39H | 3417 | 33 |
| 36N—38H | 2916 | -211 | |
| C4-3CO2 | 36N—39H | 3421 | 29 |
| 36N—38H | 2932 | -226 | |
| C4-4CO2 | 36N—39H | 3432 | 18 |
| 36N—38H | 3060 | -354 | |
| C4-5CO2 | 36N—39H | 3436 | 14 |
| 36N—38H | 3206 | -500 |
| 构型 | 化学键 | 振动频率ν/cm-1 | 频率变化值Δν/cm-1 |
|---|---|---|---|
| CO2 | 1C—2O | 1422 | — |
| 1C—3O | 1422 | — | |
| [HHexam][TFSA] | 20N—21H | 3450 | — |
| 20N—22H | 2591 | — | |
| 20N—23H | 3563 | — | |
| A4-1CO2 | 20N—21H | 3410 | 40 |
| 20N—22H | 2709 | -118 | |
| A4-2CO2 | 20N—21H | 3430 | 20 |
| 20N—22H | 2825 | -234 | |
| 20N—23H | 3532 | 31 | |
| A4-3CO2 | 20N—21H | 3421 | 29 |
| 20N—22H | 2985 | -394 | |
| 20N—23H | 3522 | 41 | |
| [HHexen][TFSA] | 26N—27H | 3561 | — |
| 28N—30H | 3555 | — | |
| 28N—31H | 3429 | — | |
| 28N—29H | 2652 | — | |
| B4-1CO2 | 28N—31H | 3424 | 5 |
| 28N—29H | 2760 | -108 | |
| B4-2CO2 | 28N—30H | 3533 | 22 |
| 28N—29H | 2870 | -218 | |
| B4-3CO2 | 28N—30H | 3534 | 21 |
| 28N—29H | 2980 | -328 | |
| B4-4CO2 | 28N—30H | 3530 | 25 |
| 28N—29H | 3035 | -383 | |
| [HHexdien][TFSA] | 36N—37H | 3450 | — |
| 18N—19H | 3527 | — | |
| 13N—14H | 3511 | — | |
| 36N—39H | 3450 | — | |
| 36N—38H | 2706 | — | |
| C4-1CO2 | 36N—39H | 3414 | 36 |
| 36N—38H | 2852 | -147 | |
| C4-2CO2 | 36N—39H | 3417 | 33 |
| 36N—38H | 2916 | -211 | |
| C4-3CO2 | 36N—39H | 3421 | 29 |
| 36N—38H | 2932 | -226 | |
| C4-4CO2 | 36N—39H | 3432 | 18 |
| 36N—38H | 3060 | -354 | |
| C4-5CO2 | 36N—39H | 3436 | 14 |
| 36N—38H | 3206 | -500 |
| 构型 | 化学键 | 电荷分布值(电荷变化值)e | ||||
|---|---|---|---|---|---|---|
| 20N | 21H | 22H | 23H | O | ||
| CO2 | C—O | -0.518 | ||||
| A4 | 20N—21H | -0.737 | 0.441 | |||
| 20N—22H | 0.485 | |||||
| 20N—23H | 0.413 | |||||
| A4-1CO2 | 20N—21H···40O | -0.741 (-0.004) | 0.450 (+0.009) | -0.598 (-0.080) | ||
| A4-2CO2 | 20N—21H···43O | -0.743 (-0.006) | 0.445 (+0.004) | -0.596 (-0.078) | ||
| 20N—23H···40O | 0.430 (+0.017) | -0.593 (-0.075) | ||||
| A4-3CO2 | 20N—21H···40O | -0.741 (-0.004) | 0.449 (+0.008) | -0.592 (-0.074) | ||
| 20N—23H···47O | 0.436 (+0.023) | -0.571 (-0.053) | ||||
| 构型 | 化学键 | 电荷分布值(电荷变化值)e | ||||
|---|---|---|---|---|---|---|
| 20N | 21H | 22H | 23H | O | ||
| CO2 | C—O | -0.518 | ||||
| A4 | 20N—21H | -0.737 | 0.441 | |||
| 20N—22H | 0.485 | |||||
| 20N—23H | 0.413 | |||||
| A4-1CO2 | 20N—21H···40O | -0.741 (-0.004) | 0.450 (+0.009) | -0.598 (-0.080) | ||
| A4-2CO2 | 20N—21H···43O | -0.743 (-0.006) | 0.445 (+0.004) | -0.596 (-0.078) | ||
| 20N—23H···40O | 0.430 (+0.017) | -0.593 (-0.075) | ||||
| A4-3CO2 | 20N—21H···40O | -0.741 (-0.004) | 0.449 (+0.008) | -0.592 (-0.074) | ||
| 20N—23H···47O | 0.436 (+0.023) | -0.571 (-0.053) | ||||
| 构型 | 化学键 | 电荷分布值(电荷变化值)e | |||||
|---|---|---|---|---|---|---|---|
| 26N | 28N | 27H | 30H | 31H | O | ||
| CO2 | C—O | -0.518 | |||||
| B4 | 26N—27H | -0.689 | 0.357 | ||||
| 28N—29H | -0.740 | ||||||
| 28N—30H | 0.415 | ||||||
| 28N—31H | 0.441 | ||||||
| B4-1CO2 | 28N—31H···49O | -0.744 (-0.004) | 0.449 (+0.008) | -0.599 (-0.081) | |||
| B4-2CO2 | 28N—30H···49O | -0.746 (-0.006) | 0.436 (+0.021) | -0.596 (-0.078) | |||
| 28N—31H···52O | -0.746 (-0.006) | 0.441 | -0.600 (-0.082) | ||||
| B4-3CO2 | 28N—30H···49O | -0.745 (-0.005) | 0.440 (+0.025) | -0.608 (-0.090) | |||
| 28N—30H···55O | -0.745 (-0.005) | 0.440 (+0.025) | -0.570 (-0.052) | ||||
| 28N—31H···51O | -0.745 (-0.005) | 0.443 (+0.028) | -0.596 (-0.078) | ||||
| B4-4CO2 | 28N—30H···49O | -0.743 (-0.003) | 0.440 (+0.025) | -0.607 -0.089) | |||
| 28N—30H···55O | -0.743 (-0.003) | 0.440 (+0.025) | -0.572 (-0.054) | ||||
| 28N—31H···51O | -0.743 (-0.003) | 0.444 (+0.003) | -0.595 (-0.077) | ||||
| 构型 | 化学键 | 电荷分布值(电荷变化值)e | |||||
|---|---|---|---|---|---|---|---|
| 26N | 28N | 27H | 30H | 31H | O | ||
| CO2 | C—O | -0.518 | |||||
| B4 | 26N—27H | -0.689 | 0.357 | ||||
| 28N—29H | -0.740 | ||||||
| 28N—30H | 0.415 | ||||||
| 28N—31H | 0.441 | ||||||
| B4-1CO2 | 28N—31H···49O | -0.744 (-0.004) | 0.449 (+0.008) | -0.599 (-0.081) | |||
| B4-2CO2 | 28N—30H···49O | -0.746 (-0.006) | 0.436 (+0.021) | -0.596 (-0.078) | |||
| 28N—31H···52O | -0.746 (-0.006) | 0.441 | -0.600 (-0.082) | ||||
| B4-3CO2 | 28N—30H···49O | -0.745 (-0.005) | 0.440 (+0.025) | -0.608 (-0.090) | |||
| 28N—30H···55O | -0.745 (-0.005) | 0.440 (+0.025) | -0.570 (-0.052) | ||||
| 28N—31H···51O | -0.745 (-0.005) | 0.443 (+0.028) | -0.596 (-0.078) | ||||
| B4-4CO2 | 28N—30H···49O | -0.743 (-0.003) | 0.440 (+0.025) | -0.607 -0.089) | |||
| 28N—30H···55O | -0.743 (-0.003) | 0.440 (+0.025) | -0.572 (-0.054) | ||||
| 28N—31H···51O | -0.743 (-0.003) | 0.444 (+0.003) | -0.595 (-0.077) | ||||
| 构型 | 化学键 | 电荷分布值(电荷变化值)e | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 13N | 18N | 36N | 14H | 19H | 37H | 39H | O | ||
| CO2 | C—O | -0.518 | |||||||
| C4 | 13N—14H | -0.689 | 0.364 | ||||||
| 18N—19H | -0.682 | 0.342 | |||||||
| 36N—37H | -0.738 | 0.415 | |||||||
| 36N—39H | 0.444 | ||||||||
| C4-1CO2 | 36N—39H···56O | -0.742 (-0.004) | 0.455 (+0.011) | -0.600 (-0.082) | |||||
| C4-2CO2 | 18N—19H···57O | -0.688 (-0.006) | 0.358 (+0.016) | -0.557 (-0.039) | |||||
| 36N—39H···59O | -0.739 (-0.001) | 0.454 (+0.010) | -0.601 (-0.083) | ||||||
| C4-3CO2 | 18N—19H···57O | -0.683 (-0.001) | 0.351 (+0.009) | -0.559 (-0.041) | |||||
| 13N—14H···63O | -0.686 | 0.369 (+0.005) | -0.549 (-0.031) | ||||||
| 36N—39H···59O | -0.738 | 0.450 (+0.006) | -0.601 (-0.083) | ||||||
| C4-4CO2 | 18N—19H···57O | -0.683 (-0.001) | 0.350 (+0.008) | -0.526 (-0.008) | |||||
| 36N—39H···59O | -0.735 (+0.003) | 0.450 (+0.006) | -0.597 (-0.079) | ||||||
| 36N—37H···63O | -0.735 (+0.003) | 0.432 (+0.017) | -0.590 (-0.072) | ||||||
| 13N—14H···66O | -0.688 (+0.001) | 0.365 (+0.001) | -0.568 (-0.050) | ||||||
| C4-5CO2 | 18N—19H···57O | -0.682 | 0.351 (+0.009) | -0.557 (-0.039) | |||||
| 36N—39H···59O | -0.737 (+0.001) | 0.450 (+0.006) | -0.612 (-0.094) | ||||||
| 13N—14H···66O | -0.687 (+0.002) | 0.370 (+0.006) | -0.547 (-0.029) | ||||||
| 36N—39H···63O | -0.737 (+0.001) | 0.450 (+0.006) | -0.573 (-0.055) | ||||||
| 构型 | 化学键 | 电荷分布值(电荷变化值)e | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 13N | 18N | 36N | 14H | 19H | 37H | 39H | O | ||
| CO2 | C—O | -0.518 | |||||||
| C4 | 13N—14H | -0.689 | 0.364 | ||||||
| 18N—19H | -0.682 | 0.342 | |||||||
| 36N—37H | -0.738 | 0.415 | |||||||
| 36N—39H | 0.444 | ||||||||
| C4-1CO2 | 36N—39H···56O | -0.742 (-0.004) | 0.455 (+0.011) | -0.600 (-0.082) | |||||
| C4-2CO2 | 18N—19H···57O | -0.688 (-0.006) | 0.358 (+0.016) | -0.557 (-0.039) | |||||
| 36N—39H···59O | -0.739 (-0.001) | 0.454 (+0.010) | -0.601 (-0.083) | ||||||
| C4-3CO2 | 18N—19H···57O | -0.683 (-0.001) | 0.351 (+0.009) | -0.559 (-0.041) | |||||
| 13N—14H···63O | -0.686 | 0.369 (+0.005) | -0.549 (-0.031) | ||||||
| 36N—39H···59O | -0.738 | 0.450 (+0.006) | -0.601 (-0.083) | ||||||
| C4-4CO2 | 18N—19H···57O | -0.683 (-0.001) | 0.350 (+0.008) | -0.526 (-0.008) | |||||
| 36N—39H···59O | -0.735 (+0.003) | 0.450 (+0.006) | -0.597 (-0.079) | ||||||
| 36N—37H···63O | -0.735 (+0.003) | 0.432 (+0.017) | -0.590 (-0.072) | ||||||
| 13N—14H···66O | -0.688 (+0.001) | 0.365 (+0.001) | -0.568 (-0.050) | ||||||
| C4-5CO2 | 18N—19H···57O | -0.682 | 0.351 (+0.009) | -0.557 (-0.039) | |||||
| 36N—39H···59O | -0.737 (+0.001) | 0.450 (+0.006) | -0.612 (-0.094) | ||||||
| 13N—14H···66O | -0.687 (+0.002) | 0.370 (+0.006) | -0.547 (-0.029) | ||||||
| 36N—39H···63O | -0.737 (+0.001) | 0.450 (+0.006) | -0.573 (-0.055) | ||||||
| 构型 | CO2数量n | 电荷转移 | 二阶微扰能E(2)/kJ·mol-1 | 二阶微扰能总值E(2) sum/kJ·mol-1 |
|---|---|---|---|---|
| A4 | 1CO2 | LP(O40)→BD*(N20-H21) | 29 | 29 |
| 2CO2 | LP(O43)→BD*(N20-H21) | 28 | 34 | |
| LP(O40)→BD*(N20-H23) | 4 | |||
| LP(O40)→BD*(N20-H21) | 1 | |||
| 3CO2 | LP(O40)→BD*(N20-H21) | 26 | 43 | |
| LP(O47)→BD*(N20-H23) | 13 | |||
| B4 | 1CO2 | LP(O49)→BD*(N28-H31) | 29 | 29 |
| 2CO2 | LP(O49)→BD*(N28-H30) | 9 | 35 | |
| LP(O52)→BD*(N28-H31) | 25 | |||
| 3CO2 | LP(O49)→BD*(N28-H30) | 2 | 42 | |
| LP(O51)→BD*(N28-H31) | 27 | |||
| LP(O55)→BD*(N28-H30) | 13 | |||
| 4CO2 | LP(O51)→BD*(N28-H31) | 22 | 40 | |
| LP(O55)→BD*(N28-H30) | 15 | |||
| C4 | 1CO2 | LP(O56)→BD*(N36-H39) | 27 | 27 |
| 2CO2 | LP(O59)→BD*(N36-H39) | 29 | 33 | |
| 3CO2 | LP(O59)→BD*(N36-H39) | 31 | 37 | |
| LP(O63)→BD*(N13-H14) | 4 | |||
| 4CO2 | LP(O59)→BD*(N36-H39) | 28 | 36 | |
| LP(O57)→BD*(N18-H19) | 3 | |||
| LP(O63)→BD*(N36-H37) | 2 | |||
| 5CO2 | LP(O59)→BD*(N36-H39) | 20 | 29 | |
| LP(O63)→BD*(N36-H39) | 2 | |||
| LP(O66)→BD*(N13-H14) | 4 |
| 构型 | CO2数量n | 电荷转移 | 二阶微扰能E(2)/kJ·mol-1 | 二阶微扰能总值E(2) sum/kJ·mol-1 |
|---|---|---|---|---|
| A4 | 1CO2 | LP(O40)→BD*(N20-H21) | 29 | 29 |
| 2CO2 | LP(O43)→BD*(N20-H21) | 28 | 34 | |
| LP(O40)→BD*(N20-H23) | 4 | |||
| LP(O40)→BD*(N20-H21) | 1 | |||
| 3CO2 | LP(O40)→BD*(N20-H21) | 26 | 43 | |
| LP(O47)→BD*(N20-H23) | 13 | |||
| B4 | 1CO2 | LP(O49)→BD*(N28-H31) | 29 | 29 |
| 2CO2 | LP(O49)→BD*(N28-H30) | 9 | 35 | |
| LP(O52)→BD*(N28-H31) | 25 | |||
| 3CO2 | LP(O49)→BD*(N28-H30) | 2 | 42 | |
| LP(O51)→BD*(N28-H31) | 27 | |||
| LP(O55)→BD*(N28-H30) | 13 | |||
| 4CO2 | LP(O51)→BD*(N28-H31) | 22 | 40 | |
| LP(O55)→BD*(N28-H30) | 15 | |||
| C4 | 1CO2 | LP(O56)→BD*(N36-H39) | 27 | 27 |
| 2CO2 | LP(O59)→BD*(N36-H39) | 29 | 33 | |
| 3CO2 | LP(O59)→BD*(N36-H39) | 31 | 37 | |
| LP(O63)→BD*(N13-H14) | 4 | |||
| 4CO2 | LP(O59)→BD*(N36-H39) | 28 | 36 | |
| LP(O57)→BD*(N18-H19) | 3 | |||
| LP(O63)→BD*(N36-H37) | 2 | |||
| 5CO2 | LP(O59)→BD*(N36-H39) | 20 | 29 | |
| LP(O63)→BD*(N36-H39) | 2 | |||
| LP(O66)→BD*(N13-H14) | 4 |
| 构型 | CO2数量n | 键临界点BCPs | 拉普拉斯值∇2ρc | 电子密度ρc | 电子密度总值ρc(sum) | 氢键能EHB/kJ·mol-1 | 氢键能总值EHB(sum)/kJ·mol-1 |
|---|---|---|---|---|---|---|---|
| A4 | 1CO2 | 40O···20N—21H | 0.0809 | 0.0190 | 0.0190 | 18 | 18 |
| 2CO2 | 43O···20N—21H | 0.0807 | 0.0189 | 0.0327 | 18 | 31 | |
| 40O···20N—23H | 0.0593 | 0.0138 | 13 | ||||
| 3CO2 | 40O···20N—21H | 0.0756 | 0.0179 | 0.0331 | 17 | 31 | |
| 47O···20N—23H | 0.0626 | 0.0152 | 14 | ||||
| B4 | 1CO2 | 49O···28N—31H | 0.0806 | 0.0189 | 0.0189 | 18 | 18 |
| 2CO2 | 49O···28N—30H | 0.0695 | 0.0159 | 0.0347 | 15 | 33 | |
| 52O···28N—31H | 0.0813 | 0.0188 | 18 | ||||
| 3CO2 | 49O···28N—30H | 0.0468 | 0.0112 | 0.0443 | 10 | 41 | |
| 55O···28N—30H | 0.0597 | 0.0146 | 13 | ||||
| 51O···28N—31H | 0.0794 | 0.0185 | 18 | ||||
| 4CO2 | 49O···28N—30H | 0.0459 | 0.0108 | 0.0430 | 10 | 39 | |
| 51O···28N—31H | 0.0704 | 0.0168 | 15 | ||||
| 55O···28N—30H | 0.0635 | 0.0154 | 14 | ||||
| C4 | 1CO2 | 56O···36N—39H | 0.0831 | 0.0195 | 0.0195 | 19 | 19 |
| 2CO2 | 59O···36N—39H | 0.0837 | 0.0196 | 0.0297 | 19 | 28 | |
| 57O···18N—19H | 0.0373 | 0.0100 | 9 | ||||
| 3CO2 | 57O···18N—19H | 0.0292 | 0.0077 | 0.0375 | 7 | 35 | |
| 63O···13N—14H | 0.0350 | 0.0097 | 8 | ||||
| 59O···36N—39H | 0.0867 | 0.0201 | 20 | ||||
| 4CO2 | 57O···18N—19H | 0.0341 | 0.0096 | 0.0476 | 9 | 44 | |
| 59O···36N—39H | 0.0799 | 0.0187 | 18 | ||||
| 63O···36N—37H | 0.0570 | 0.0132 | 12 | ||||
| 66O···13N—14H | 0.0210 | 0.0060 | 5 | ||||
| 5CO2 | 57O···18N—19H | 0.0333 | 0.0088 | 0.0442 | 8 | 41 | |
| 59O···36N—39H | 0.0625 | 0.0150 | 14 | ||||
| 66O···13N—14H | 0.0316 | 0.0089 | 8 | ||||
| 63O···36N—39H | 0.0475 | 0.0115 | 11 |
| 构型 | CO2数量n | 键临界点BCPs | 拉普拉斯值∇2ρc | 电子密度ρc | 电子密度总值ρc(sum) | 氢键能EHB/kJ·mol-1 | 氢键能总值EHB(sum)/kJ·mol-1 |
|---|---|---|---|---|---|---|---|
| A4 | 1CO2 | 40O···20N—21H | 0.0809 | 0.0190 | 0.0190 | 18 | 18 |
| 2CO2 | 43O···20N—21H | 0.0807 | 0.0189 | 0.0327 | 18 | 31 | |
| 40O···20N—23H | 0.0593 | 0.0138 | 13 | ||||
| 3CO2 | 40O···20N—21H | 0.0756 | 0.0179 | 0.0331 | 17 | 31 | |
| 47O···20N—23H | 0.0626 | 0.0152 | 14 | ||||
| B4 | 1CO2 | 49O···28N—31H | 0.0806 | 0.0189 | 0.0189 | 18 | 18 |
| 2CO2 | 49O···28N—30H | 0.0695 | 0.0159 | 0.0347 | 15 | 33 | |
| 52O···28N—31H | 0.0813 | 0.0188 | 18 | ||||
| 3CO2 | 49O···28N—30H | 0.0468 | 0.0112 | 0.0443 | 10 | 41 | |
| 55O···28N—30H | 0.0597 | 0.0146 | 13 | ||||
| 51O···28N—31H | 0.0794 | 0.0185 | 18 | ||||
| 4CO2 | 49O···28N—30H | 0.0459 | 0.0108 | 0.0430 | 10 | 39 | |
| 51O···28N—31H | 0.0704 | 0.0168 | 15 | ||||
| 55O···28N—30H | 0.0635 | 0.0154 | 14 | ||||
| C4 | 1CO2 | 56O···36N—39H | 0.0831 | 0.0195 | 0.0195 | 19 | 19 |
| 2CO2 | 59O···36N—39H | 0.0837 | 0.0196 | 0.0297 | 19 | 28 | |
| 57O···18N—19H | 0.0373 | 0.0100 | 9 | ||||
| 3CO2 | 57O···18N—19H | 0.0292 | 0.0077 | 0.0375 | 7 | 35 | |
| 63O···13N—14H | 0.0350 | 0.0097 | 8 | ||||
| 59O···36N—39H | 0.0867 | 0.0201 | 20 | ||||
| 4CO2 | 57O···18N—19H | 0.0341 | 0.0096 | 0.0476 | 9 | 44 | |
| 59O···36N—39H | 0.0799 | 0.0187 | 18 | ||||
| 63O···36N—37H | 0.0570 | 0.0132 | 12 | ||||
| 66O···13N—14H | 0.0210 | 0.0060 | 5 | ||||
| 5CO2 | 57O···18N—19H | 0.0333 | 0.0088 | 0.0442 | 8 | 41 | |
| 59O···36N—39H | 0.0625 | 0.0150 | 14 | ||||
| 66O···13N—14H | 0.0316 | 0.0089 | 8 | ||||
| 63O···36N—39H | 0.0475 | 0.0115 | 11 |
| 1 | 雷婷, 喻树楠, 周昶安, 等. 吸附法碳捕集固体胺吸附剂成型技术研究进展[J]. 化工进展, 2022, 41(12): 6213-6225. |
| LEI Ting, YU Shunan, ZHOU Chang'an, et al. Research progress on the shaping technology of solid amine adsorbents for CO2 capture by adsorption method[J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6213-6225. | |
| 2 | 周红军, 周颖, 徐春明. 中国碳中和目标下CO2转化的思考与实践[J]. 化工进展, 2022, 41(6): 3381-3385. |
| ZHOU Hongjun, ZHOU Ying, XU Chunming. Exploration of the CO2 conversion under China’s carbon neutrality goal[J]. Chemical Industry and Engineering Progress, 2022, 41(6): 3381-3385. | |
| 3 | 黄晟, 王静宇, 郭沛, 等. 碳中和目标下能源结构优化的近期策略与远期展望[J]. 化工进展, 2022, 41(11): 5695-5708. |
| HUANG Sheng, WANG Jingyu, GUO Pei, et al. Short-term strategy and long-term prospect of energy structure optimization under carbon neutrality target[J]. Chemical Industry and Engineering Progress, 2022, 41(11): 5695-5708. | |
| 4 | EGOROVA Ksenia S, GORDEEV Evgeniy G, ANANIKOV Valentine P. Biological activity of ionic liquids and their application in pharmaceutics and medicine[J]. Chemical Reviews, 2017, 117(10): 7132-7189. |
| 5 | KALIKIN Nikolai N, KOLESNIKOV Andrei L, BUDKOV Yury A. Polymerized ionic liquids on charged electrodes: New prospects for electrochemistry[J]. Current Opinion in Electrochemistry, 2022, 36: 101134. |
| 6 | SOMMER Julia, BROMBERGER Birgit, ROBBEN Christian, et al. Liquid-liquid extraction of viral particles with ionic liquids[J]. Separation and Purification Technology, 2021, 254: 117591. |
| 7 | ZENG Shaojuan, ZHANG Xiangping, BAI Lu, et al. Ionic-liquid-based CO2 capture systems: structure, interaction and process[J]. Chemical Reviews, 2017, 117(14): 9625-9673. |
| 8 | NOORANI Narmin, MEHRDAD Abbas, ZAREI DIZNAB Rana. Thermodynamic study on carbon dioxide absorption in vinyl imidazolium-amino acid ionic liquids[J]. Fluid Phase Equilibria, 2022, 557: 113433. |
| 9 | TU Zhuoheng, SHI Mingzhen, ZHANG Xiaomin, et al. Selective membrane separation of CO2 using novel epichlorohydrin-amine-based crosslinked protic ionic liquids: Crosslinking mechanism and enhanced salting-out effect[J]. Journal of CO2 Utilization, 2021, 46: 101473. |
| 10 | SHUKLA Shashi Kant, KHOKARALE Santosh G, Thai Q BUI, et al. Ionic liquids: Potential materials for carbon dioxide capture and utilization[J]. Frontiers in Materials, 2019, 6: 42. |
| 11 | KUMAR Pradeep, VARYANI Manish, KHATRI Praveen K, et al. Post combustion capture and conversion of carbon dioxide using histidine derived ionic liquid at ambient conditions[J]. Journal of Industrial and Engineering Chemistry, 2017, 49: 152-157. |
| 12 | MA Jing, ZHU Mingxuan, YANG Xueqing, et al. Different cation-anion interaction mechanisms of diamino protic ionic liquids: A density functional theory study[J]. Chemical Physics Letters, 2021, 774: 138615. |
| 13 | PATIL Kunal R, BARGE Seema S, BHOSALE Babasaheb D, et al. Influence of protic ionic liquids on hydration of glycine based peptides[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2022, 265: 120378. |
| 14 | KONDRATENKO Yulia A, ANTUGANOV Dmitrii O, KADNIKOVA Olga Yu, et al. Synthesis, crystal structure and properties of tris(2-hydroxypropyl)ammonium based protic ionic liquids and protic molten salts[J]. Journal of Molecular Liquids, 2021, 324: 114717. |
| 15 | CAO Bobo, DU Jiuyao, LIU Shuangyue, et al. Carbon dioxide capture by amino-functionalized ionic liquids: DFT based theoretical analysis substantiated by FT-IR investigation[J]. RSC Advances, 2016, 6(13): 10462-10470. |
| 16 | NOORANI Narmin, MEHRDAD Abbas. CO2 solubility in some amino acid-based ionic liquids: Measurement, correlation and DFT studies[J]. Fluid Phase Equilibria, 2020, 517: 112591. |
| 17 | ZHANG Jinrui, LV Naixia, CHAO Yanhong, et al. The interaction nature between hollow silica-based porous ionic liquids and CO2: A DFT study[J]. Journal of Molecular Graphics and Modelling, 2020, 100: 107694. |
| 18 | KUMAR Madhu Deepan, SUNNY Shilpa, JACCOB Madhavan. Conversion of CO2 to cyclic carbonates by metal-ethylenediamine complexes in ionic liquid: A DFT mechanistic study[J]. Journal of CO2 Utilization, 2022, 57: 101872. |
| 19 | MA Jing, WANG Yutong, YANG Xueqing, et al. DFT study on the chemical absorption mechanism of CO2 in diamino protic ionic liquids[J]. The Journal of Physical Chemistry B, 2021, 125(5): 1416-1428. |
| 20 | Hua ER, XU Yu, ZHAO Hong. Properties of mono-protic ionic liquids composed of hexylammonium and hexylethylenediaminium cations with trifluoroacetate and bis (trifluoromethylsulfonyl) imide anions[J]. Journal of Molecular Liquids, 2019, 276: 379-384. |
| 21 | HUA Er, MASAYASU Iida. Protonation properties, Applications and effects: Properties of protic ionic liquids comprising N-alkyl polyamines (Chapter 3) [M]. Nova Science Publishers, 2019, 237-247. |
| 22 | 徐宇, 花儿. 烷基乙二胺-CF3CO2型质子化离子液体的分子间氢键作用[J]. 高等学校化学学报, 2018, 39(9):1954-1960. |
| XU Yu, HUA Er. Hydrogen bonding study on protic ionic liquids composed of N-alkylethylenediaminum cations with trifluoroacetic anion[J]. Chemical Journal of Chinese Universities, 2018, 39(9): 1954-1960. | |
| 23 | XU Yu, HUA Er, HAGHANI Abdolhossein. Structure and hydrogen bonding of mono-protic ionic liquids composed of N-alkylethylenediaminium cations and trifluoromethanesulfonate anion[J]. Materials Today Communications, 2021, 28: 102633. |
| 24 | TORKZADEH Mehrangiz, MOOSAVI Majid. DFT and COSMO-RS studies on dicationic ionic liquids (DILs) as potential candidates for CO2 capture: the effects of alkyl side chain length and symmetry in cations[J]. RSC Advances, 2022, 12(54): 35418-35435. |
| 25 | PARR Robert G. Density functional theory of atoms and molecules[C]//FUKUI K, PULLMAN B. Horizons of Quantum Chemistry: Proceedings of the Third International Congress of Quantum Chemistry Held at Kyoto. Japan: Springer Netherlands, 1980: 5-15. |
| 26 | ZHAO Yan, TRUHLAR Donald G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals[J]. Theoretical Chemistry Accounts, 2008, 120(1): 215-241. |
| 27 | BOYS S F, BERNARDI F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors[J]. Molecular Physics, 2002, 100(1): 65-73. |
| 28 | Renqing LÜ, WU Chongchong, LIN Jin, et al. Theoretical study on interactions between trifluoromethanesulfonate(triflate) based ionic liquid and thiophene[J]. Journal of Molecular Liquids, 2017, 237: 289-294. |
| 29 | ANBU V, VIJAYALAKSHMI K A, KARUNATHAN R, et al. Explosives properties of high energetic trinitrophenyl nitramide molecules: A DFT and AIM analysis[J]. Arabian Journal of Chemistry, 2019, 12(5): 621-632. |
| 30 | HUNT Patricia A, ASHWORTH Claire R, MATTHEWS Richard P. Hydrogen bonding in ionic liquids[J]. Chemical Society Reviews, 2015, 44(5): 1257-1288. |
| 31 | DOMAGALA Małgorzata, JABLONSKI Mirosław, DUBIS Alina T, et al. Testing of exchange-correlation functionals of DFT for a reliable description of the electron density distribution in organic molecules[J]. International Journal of Molecular Sciences, 2022, 23(23): 14719. |
| 32 | Julia CONTRERAS-GARCÍA, JOHNSON Erin R, KEINAN Shahar, et al. NCIPLOT: A program for plotting non-covalent interaction regions[J]. Journal of Chemical Theory and Computation, 2011, 7(3): 625-632. |
| 33 | 姜焱龙, 张妮, 李淡然, 等. 基于COSMO-RS方法筛选离子液体用于焦油脱除[J]. 化工学报, 2022, 73(4): 1704-1713. |
| JIANG Yanlong, ZHANG Ni, LI Danran, et al. Selected ionic liquids by COSMO-RS method for tar removal[J]. CIESC Journal, 2022, 73(4): 1704-1713. | |
| 34 | 刘向阳. 气体在离子液体中溶解度的实验测量与理论计算[D]. 西安: 西安交通大学, 2017. |
| LIU Xiangyang. Measurement and calculation of gas solubility in ionic liquid[D]. Xi'an: XI'an Jiaotong University, 2017. | |
| 35 | HUSSAIN Shahid, DONG Haifeng, ZENG Shaojuan, et al. Investigation uncovered the impact of anions on CO2 absorption by low viscous ether functionalized pyridinium ionic liquids[J]. Journal of Molecular Liquids, 2021, 336: 116362. |
| 36 | PINTO J, FABRE E, MURSHED S S. Evaluation of stability, viscosity and electrical conductivity of [C2mim][DCA] based ionanocolloids[J]. Heat and Mass Transfer, 2022: 1-7. |
| 37 | YANG Fuxin, WANG Bangju, JIAO Yanhao, et al. Density and viscosity of three ionic liquids with 2,2,2-trifluoroethanol[J]. The Journal of Chemical Thermodynamics, 2023, 181: 107038. |
| 38 | ZHANG Xiaosong, PAN Jiawei, WANG Liang, et al. COSMO-based solvent selection and Aspen plus process simulation for tar absorptive removal[J]. Applied Energy, 2019, 251: 113314. |
| 39 | 吴进, 张承龙, 王瑞雪, 等. 离子液体吸收废印刷线路板热拆解过程中挥发性有机物——基于COSMO-RS模型[J]. 中国环境科学, 2020, 40(5): 1946-1952. |
| WU Jin, ZHANG Chenglong, WANG Ruixue, et al. Absorbing volatile organic compounds discharged during thermal dismantling of waste printed circuit boards using ionic liquids—Based on COSMO-RS model[J]. China Environmental Science, 2020, 40(5): 1946-1952. | |
| 40 | LIU Yanrong, YU Hang, SUN Yunhao, et al. Screening deep eutectic solvents for CO2 capture with COSMO-RS[J]. Frontiers in Chemistry, 2020, 8: 82. |
| [1] | YANG Hanyue, KONG Lingzhen, CHEN Jiaqing, SUN Huan, SONG Jiakai, WANG Sicheng, KONG Biao. Decarbonization performance of downflow tubular gas-liquid contactor of microbubble-type [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 197-204. |
| [2] | ZHENG Qian, GUAN Xiushuai, JIN Shanbiao, ZHANG Changming, ZHANG Xiaochao. Photothermal catalysis synthesis of DMC from CO2 and methanol over Ce0.25Zr0.75O2 solid solution [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 319-327. |
| [3] | SUN Yuyu, CAI Xinlei, TANG Jihai, HUANG Jingjing, HUANG Yiping, LIU Jie. Optimization and energy-saving of a reactive distillation process for the synthesis of methyl methacrylate [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 56-63. |
| [4] | WANG Yaogang, HAN Zishan, GAO Jiachen, WANG Xinyu, LI Siqi, YANG Quanhong, WENG Zhe. Strategies for regulating product selectivity of copper-based catalysts in electrochemical CO2 reduction [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4043-4057. |
| [5] | LIU Yi, FANG Qiang, ZHONG Dazhong, ZHAO Qiang, LI Jinping. Cu facets regulation of Ag/Cu coupled catalysts for electrocatalytic reduction of carbon dioxide [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4136-4142. |
| [6] | HUANG Yufei, LI Ziyi, HUANG Yangqiang, JIN Bo, LUO Xiao, LIANG Zhiwu. Research progress on catalysts for photocatalytic CO2 and CH4 reforming [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4247-4263. |
| [7] | LOU Baohui, WU Xianhao, ZHANG Chi, CHEN Zhen, FENG Xiangdong. Advances in nanofluid for CO2 absorption and separation [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3802-3815. |
| [8] | LYU Chao, ZHANG Xiwen, JIN Lijian, YANG Linjun. Efficient capture of CO2 by a new biphasic solvent-ionic liquid system [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3226-3232. |
| [9] | WANG Keju, ZHAO Cheng, HU Xiaomei, YUN Junge, WEI Ninghan, JIANG Xueying, ZOU Yun, CHEN Zhihang. Research progress of low temperature catalytic oxidation of VOCs by metal oxides [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2402-2412. |
| [10] | MA Yuan, XIAO Qingyue, YUE Junrong, CUI Yanbin, LIU Jiao, XU Guangwen. CO xco-methanation over a Ni-based catalyst supported on CeO2-Al2O3 composite [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2421-2428. |
| [11] | HE Zhiyong, GUO Tianfo, WANG Jinli, LYU Feng. Progress of CO2/epoxide copolymerization catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1847-1859. |
| [12] | FU Le, YANG Yang, XU Wenqing, GENG Zanbu, ZHU Tingyu, HAO Runlong. Research progress in CO2 capture technology using novel biphasic organic amine absorbent [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2068-2080. |
| [13] | CHEN Chongming, ZENG Siming, LUO Xiaona, SONG Guosheng, HAN Zhongge, YU Jinxing, SUN Nannan. Preparation and performance of carbon supported potassium-based CO2 adsorbent derived from hyper-cross linked polymers [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1540-1550. |
| [14] | WANG Qiuhua, WU Jiashuai, ZHANG Weifeng. Research progress of alkaline industrial solid wastes mineralization for carbon dioxide sequestration [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1572-1582. |
| [15] | WANG Xiaoyue, ZHANG Weimin, YAO Zhengyang, GUO Xiaohong, LI Congming. Research progress of reverse water gas shift reaction [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1583-1594. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||