Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (5): 2402-2412.DOI: 10.16085/j.issn.1000-6613.2022-1312
• Industrial catalysis • Previous Articles Next Articles
Research progress of low temperature catalytic oxidation of VOCs by metal oxides
WANG Keju1,2( ), ZHAO Cheng2, HU Xiaomei2, YUN Junge2,3, WEI Ninghan1,2, JIANG Xueying1,2, ZOU Yun1(
), ZHAO Cheng2, HU Xiaomei2, YUN Junge2,3, WEI Ninghan1,2, JIANG Xueying1,2, ZOU Yun1( ), CHEN Zhihang2(
), CHEN Zhihang2( )
)
- 1.College of Chemistry and Chemical Engineering, Guangxi University, Nanning 530004, Guangxi, China
2.Guangdong Province Engineering Laboratory for Air Pollution Control, Guangdong Key Lab of Water & Air Pollution Control, South China Institute of Environmental Sciences, Ministry of Ecology and Environment, Guangzhou 510655, Guangdong, China
3.College of Resources and Environment, Xiangtan University, Xiangtan 411105, Hunan, China
-
Received:2022-07-12Revised:2022-10-23Online:2023-06-02Published:2023-05-10 -
Contact:ZOU Yun, CHEN Zhihang
金属氧化物低温催化氧化VOCs的研究进展
王科菊1,2( ), 赵成2, 胡晓玫2, 云军阁2,3, 魏凝涵1,2, 姜雪迎1,2, 邹昀1(
), 赵成2, 胡晓玫2, 云军阁2,3, 魏凝涵1,2, 姜雪迎1,2, 邹昀1( ), 陈志航2(
), 陈志航2( )
)
- 1.广西大学化学化工学院,广西 南宁 530004
2.生态环境部华南环境科学研究所,广东省水与大气污染防治重点 实验室,广东省大气污染控制工程实验室,广东 广州 510655
3.湘潭大学资源与环境学院,湖南 湘潭 411105
-
通讯作者:邹昀,陈志航 -
作者简介:王科菊(1997—),男,硕士研究生,研究方向为大气污染控制。E-mail:351924900@qq.com。 -
基金资助:国家自然科学基金(21978174);广州市科技计划(201904020038)
CLC Number:
Cite this article
WANG Keju, ZHAO Cheng, HU Xiaomei, YUN Junge, WEI Ninghan, JIANG Xueying, ZOU Yun, CHEN Zhihang. Research progress of low temperature catalytic oxidation of VOCs by metal oxides[J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2402-2412.
王科菊, 赵成, 胡晓玫, 云军阁, 魏凝涵, 姜雪迎, 邹昀, 陈志航. 金属氧化物低温催化氧化VOCs的研究进展[J]. 化工进展, 2023, 42(5): 2402-2412.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-1312
| 催化剂 | 污染物 | 浓度/μL·L-1 | 空速/mL∙g-1∙h-1 | T90/℃ | Oads/Olatt | 参考文献 |
|---|---|---|---|---|---|---|
| MnO2 | 甲苯 | 1000 | 40000 | 228 | 0.78 | [ |
| Co-MnO2 | 甲苯 | 1000 | 40000 | 227 | 0.86 | [ |
| Ce-MnO2 | 甲苯 | 1000 | 40000 | 234 | 0.57 | [ |
| Cu-MnO2 | 甲苯 | 1000 | 40000 | 219 | 1.07 | [ |
| Co3O4 | 甲苯 | 1000 | 78000 | 249 | 0.88 | [ |
| CuCo2O4 | 甲苯 | 1000 | 78000 | 221 | 1.12 | [ |
| NiCo2O4 | 甲苯 | 1000 | 78000 | 234 | 1.07 | [ |
| ZnCo2O4 | 甲苯 | 1000 | 78000 | 279 | 0.80 | [ |
| 催化剂 | 污染物 | 浓度/μL·L-1 | 空速/mL∙g-1∙h-1 | T90/℃ | Oads/Olatt | 参考文献 |
|---|---|---|---|---|---|---|
| MnO2 | 甲苯 | 1000 | 40000 | 228 | 0.78 | [ |
| Co-MnO2 | 甲苯 | 1000 | 40000 | 227 | 0.86 | [ |
| Ce-MnO2 | 甲苯 | 1000 | 40000 | 234 | 0.57 | [ |
| Cu-MnO2 | 甲苯 | 1000 | 40000 | 219 | 1.07 | [ |
| Co3O4 | 甲苯 | 1000 | 78000 | 249 | 0.88 | [ |
| CuCo2O4 | 甲苯 | 1000 | 78000 | 221 | 1.12 | [ |
| NiCo2O4 | 甲苯 | 1000 | 78000 | 234 | 1.07 | [ |
| ZnCo2O4 | 甲苯 | 1000 | 78000 | 279 | 0.80 | [ |
| 催化剂 | 金属比例 | 污染物 | 浓度 /μL·L-1 | 空速 /mL∙g-1∙h-1 | T90/℃ | 参考 文献 |
|---|---|---|---|---|---|---|
| CuMnO x | Cu/Mn=0∶1 | 甲苯 | 500 | 22500 | 229 | [ |
| CuMnO x | Cu/Mn=0.06∶1 | 甲苯 | 500 | 22500 | 226 | [ |
| CuMnO x | Cu/Mn=0.08∶1 | 甲苯 | 500 | 22500 | 225 | [ |
| CuMnO x | Cu/Mn=0.10∶1 | 甲苯 | 500 | 22500 | 216 | [ |
| CuMnO x | Cu/Mn=0.12∶1 | 甲苯 | 500 | 22500 | 223 | [ |
| MnCoO x | Mn/Co=0∶1 | 甲苯 | 1000 | 40000 | 246 | [ |
| MnCoO x | Mn/Co=0.2∶0.8 | 甲苯 | 1000 | 40000 | 227 | [ |
| MnCoO x | Mn/Co=0.3∶0.7 | 甲苯 | 1000 | 40000 | 220 | [ |
| MnCoO x | Mn/Co=0.4∶0.6 | 甲苯 | 1000 | 40000 | 215 | [ |
| MnCoO x | Mn/Co=0.5∶0.5 | 甲苯 | 1000 | 40000 | 219 | [ |
| FeMnO x | Fe/Mn=0∶1 | 氯苯 | 600 | 20000① | 310 | [ |
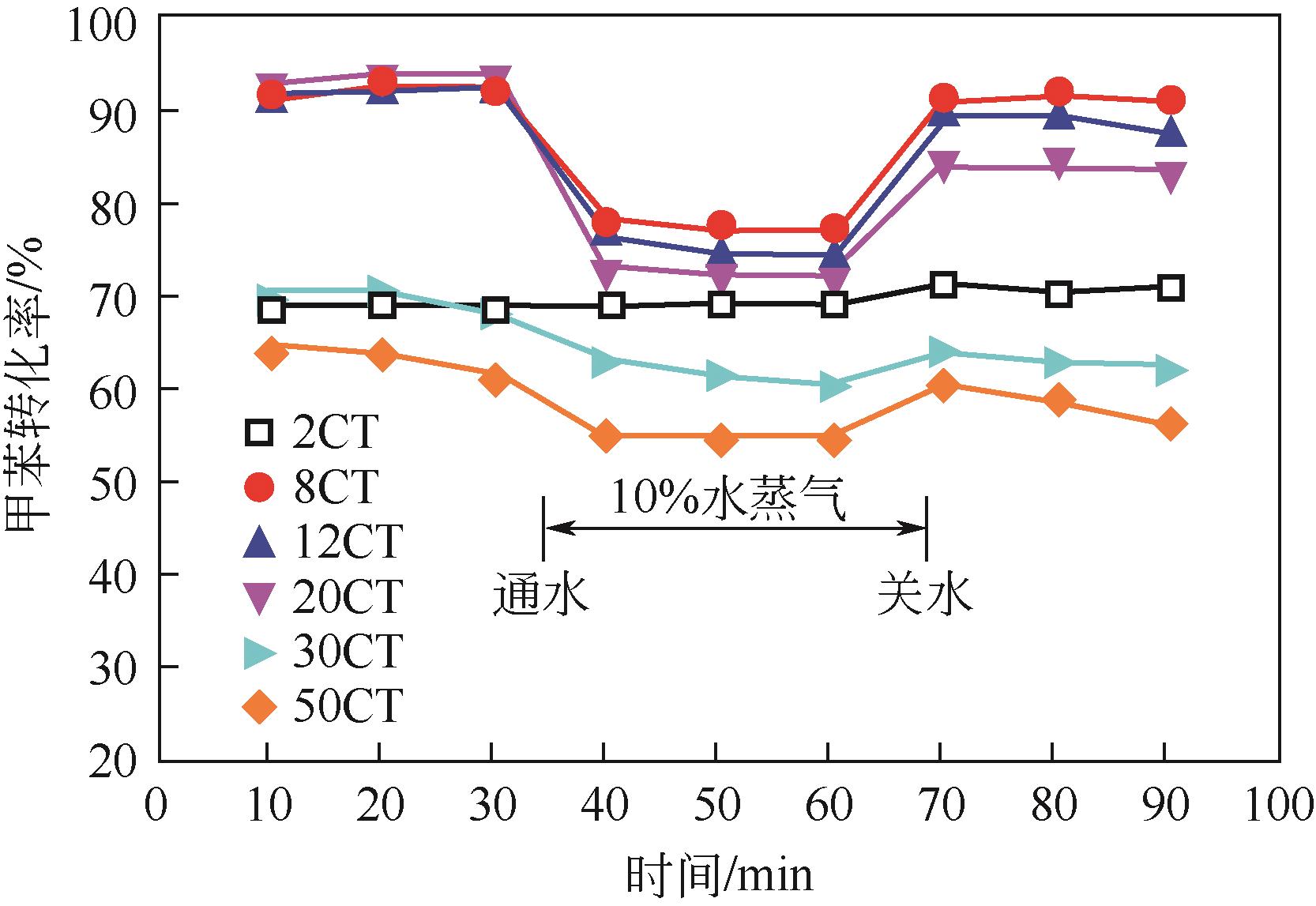
| FeMnO x | Fe/Mn=1∶8 | 氯苯 | 600 | 20000① | 340 | [ |
| FeMnO x | Fe/Mn=1∶1 | 氯苯 | 600 | 20000① | 197 | [ |
| FeMnO x | Fe/Mn=2∶1 | 氯苯 | 600 | 20000① | 295 | [ |
| FeMnO x | Fe/Mn=1∶0 | 氯苯 | 600 | 20000① | >400 | [ |
| 催化剂 | 金属比例 | 污染物 | 浓度 /μL·L-1 | 空速 /mL∙g-1∙h-1 | T90/℃ | 参考 文献 |
|---|---|---|---|---|---|---|
| CuMnO x | Cu/Mn=0∶1 | 甲苯 | 500 | 22500 | 229 | [ |
| CuMnO x | Cu/Mn=0.06∶1 | 甲苯 | 500 | 22500 | 226 | [ |
| CuMnO x | Cu/Mn=0.08∶1 | 甲苯 | 500 | 22500 | 225 | [ |
| CuMnO x | Cu/Mn=0.10∶1 | 甲苯 | 500 | 22500 | 216 | [ |
| CuMnO x | Cu/Mn=0.12∶1 | 甲苯 | 500 | 22500 | 223 | [ |
| MnCoO x | Mn/Co=0∶1 | 甲苯 | 1000 | 40000 | 246 | [ |
| MnCoO x | Mn/Co=0.2∶0.8 | 甲苯 | 1000 | 40000 | 227 | [ |
| MnCoO x | Mn/Co=0.3∶0.7 | 甲苯 | 1000 | 40000 | 220 | [ |
| MnCoO x | Mn/Co=0.4∶0.6 | 甲苯 | 1000 | 40000 | 215 | [ |
| MnCoO x | Mn/Co=0.5∶0.5 | 甲苯 | 1000 | 40000 | 219 | [ |
| FeMnO x | Fe/Mn=0∶1 | 氯苯 | 600 | 20000① | 310 | [ |
| FeMnO x | Fe/Mn=1∶8 | 氯苯 | 600 | 20000① | 340 | [ |
| FeMnO x | Fe/Mn=1∶1 | 氯苯 | 600 | 20000① | 197 | [ |
| FeMnO x | Fe/Mn=2∶1 | 氯苯 | 600 | 20000① | 295 | [ |
| FeMnO x | Fe/Mn=1∶0 | 氯苯 | 600 | 20000① | >400 | [ |
| 催化剂 | 焙烧温度 /℃ | 污染物 | 浓度 /μL·L-1 | 空速 /mL∙g-1∙h-1 | T90/℃ | ID/I2g | 参考 文献 |
|---|---|---|---|---|---|---|---|
| Co3O4 | 300 | 甲苯 | 1000 | 40000 | 240 | — | [ |
| Co3O4 | 350 | 甲苯 | 1000 | 40000 | 251 | — | [ |
| Co3O4 | 400 | 甲苯 | 1000 | 40000 | 260 | — | [ |
| Fe1Mn4 | 300 | 甲苯 | 500 | 100000 | — | 0.93 | [ |
| Fe1Mn4 | 400 | 甲苯 | 500 | 100000 | — | 0.91 | [ |
| Fe1Mn4 | 500 | 甲苯 | 500 | 100000 | — | 0.87 | [ |
| Cu1Co2Fe1O x | 400 | 甲苯 | 800 | 60000 | 238 | — | [ |
| Cu1Co2Fe1O x | 500 | 甲苯 | 800 | 60000 | 246 | — | [ |
| Cu1Co2Fe1O x | 600 | 甲苯 | 800 | 60000 | 289 | — | [ |
| Cu1Co2Fe1O x | 700 | 甲苯 | 800 | 60000 | 322 | — | [ |
| 催化剂 | 焙烧温度 /℃ | 污染物 | 浓度 /μL·L-1 | 空速 /mL∙g-1∙h-1 | T90/℃ | ID/I2g | 参考 文献 |
|---|---|---|---|---|---|---|---|
| Co3O4 | 300 | 甲苯 | 1000 | 40000 | 240 | — | [ |
| Co3O4 | 350 | 甲苯 | 1000 | 40000 | 251 | — | [ |
| Co3O4 | 400 | 甲苯 | 1000 | 40000 | 260 | — | [ |
| Fe1Mn4 | 300 | 甲苯 | 500 | 100000 | — | 0.93 | [ |
| Fe1Mn4 | 400 | 甲苯 | 500 | 100000 | — | 0.91 | [ |
| Fe1Mn4 | 500 | 甲苯 | 500 | 100000 | — | 0.87 | [ |
| Cu1Co2Fe1O x | 400 | 甲苯 | 800 | 60000 | 238 | — | [ |
| Cu1Co2Fe1O x | 500 | 甲苯 | 800 | 60000 | 246 | — | [ |
| Cu1Co2Fe1O x | 600 | 甲苯 | 800 | 60000 | 289 | — | [ |
| Cu1Co2Fe1O x | 700 | 甲苯 | 800 | 60000 | 322 | — | [ |
| 催化剂 | 制备方法 | 污染物 | 浓度 /μL·L-1 | 空速 /mL∙g-1∙h-1 | T90 /℃ | 参考文献 |
|---|---|---|---|---|---|---|
| CeO2-M | 原位热解法 | 甲苯 | 1000 | 20000 | 223 | [ |
| CeO2-P | 沉淀法 | 甲苯 | 1000 | 20000 | 280 | [ |
| CeMn-IM | 浸渍法 | 甲苯 | 500 | 60000① | 261 | [ |
| CeMn-CP | 共沉淀法 | 甲苯 | 500 | 60000① | 259 | [ |
| CeMn-SG | 溶胶凝胶法 | 甲苯 | 500 | 60000① | 249 | [ |
| CeMn-HT | 水热法 | 甲苯 | 500 | 60000① | 246 | [ |
| MnCu0.5 | 水热氧化还原法 | 甲苯 | 1000 | 40000 | 210 | [ |
| MnCu0.75-H2O2 | 氧化还原沉淀法 | 甲苯 | 1000 | 40000 | 236 | [ |
| MnCu0.75-P | 共沉淀法 | 甲苯 | 1000 | 40000 | 240 | [ |
| Cu0.4Ce0.6-DR | 双氧化还原法 | 甲苯 | 500 | 50000 | 约246 | [ |
| Cu0.4Ce0.6-C | 共沉淀法 | 甲苯 | 500 | 50000 | 约270 | [ |
| 催化剂 | 制备方法 | 污染物 | 浓度 /μL·L-1 | 空速 /mL∙g-1∙h-1 | T90 /℃ | 参考文献 |
|---|---|---|---|---|---|---|
| CeO2-M | 原位热解法 | 甲苯 | 1000 | 20000 | 223 | [ |
| CeO2-P | 沉淀法 | 甲苯 | 1000 | 20000 | 280 | [ |
| CeMn-IM | 浸渍法 | 甲苯 | 500 | 60000① | 261 | [ |
| CeMn-CP | 共沉淀法 | 甲苯 | 500 | 60000① | 259 | [ |
| CeMn-SG | 溶胶凝胶法 | 甲苯 | 500 | 60000① | 249 | [ |
| CeMn-HT | 水热法 | 甲苯 | 500 | 60000① | 246 | [ |
| MnCu0.5 | 水热氧化还原法 | 甲苯 | 1000 | 40000 | 210 | [ |
| MnCu0.75-H2O2 | 氧化还原沉淀法 | 甲苯 | 1000 | 40000 | 236 | [ |
| MnCu0.75-P | 共沉淀法 | 甲苯 | 1000 | 40000 | 240 | [ |
| Cu0.4Ce0.6-DR | 双氧化还原法 | 甲苯 | 500 | 50000 | 约246 | [ |
| Cu0.4Ce0.6-C | 共沉淀法 | 甲苯 | 500 | 50000 | 约270 | [ |
| 催化剂 | 载体 | 污染物 | 浓度/μL·L-1 | 空速/mL∙g-1∙h-1 | T90/℃ | 参考文献 |
|---|---|---|---|---|---|---|
| MnO x /HZ-5 | 纳米中空HZSM-5 | 甲苯 | 1000 | 15000 | 255 | [ |
| MnO x /MZ-5 | 微米HZSM-5 | 甲苯 | 1000 | 15000 | 282 | [ |
| Co3O4 | — | 丙烷 | — | 80000 | >400 | [ |
| Co3O4/ZSM-5 | ZSM-5 | 丙烷 | — | 80000 | 360 | [ |
| Co3O4/Silicalite-1 | Silicalite-1 | 二氯甲烷 | 1000 | 30000 | 420 | [ |
| Co3O4/ZSM-5 | ZSM-5 | 二氯甲烷 | 1000 | 30000 | 370 | [ |
| Co3O4/TS-1 | TS-1 | 二氯甲烷 | 1000 | 30000 | 406 | [ |
| Mn/Ce-Zr(C-T-1) | 界面CeO2-ZrO2 | 甲苯 | 1000 | 30000 | 254 | [ |
| Mn/Ce-Zr-ss | 简单CeO2-ZrO2 | 甲苯 | 1000 | 30000 | 288 | [ |
| Co3O4/TiO2 | TiO2 | 甲苯 | 1000 | 60000 | 377 | [ |
| Co3O4/YSZ | YSZ | 甲苯 | 1000 | 60000 | 280 | [ |
| Co3O4 | — | 甲苯 | 1000 | 60000 | 312 | [ |
| 催化剂 | 载体 | 污染物 | 浓度/μL·L-1 | 空速/mL∙g-1∙h-1 | T90/℃ | 参考文献 |
|---|---|---|---|---|---|---|
| MnO x /HZ-5 | 纳米中空HZSM-5 | 甲苯 | 1000 | 15000 | 255 | [ |
| MnO x /MZ-5 | 微米HZSM-5 | 甲苯 | 1000 | 15000 | 282 | [ |
| Co3O4 | — | 丙烷 | — | 80000 | >400 | [ |
| Co3O4/ZSM-5 | ZSM-5 | 丙烷 | — | 80000 | 360 | [ |
| Co3O4/Silicalite-1 | Silicalite-1 | 二氯甲烷 | 1000 | 30000 | 420 | [ |
| Co3O4/ZSM-5 | ZSM-5 | 二氯甲烷 | 1000 | 30000 | 370 | [ |
| Co3O4/TS-1 | TS-1 | 二氯甲烷 | 1000 | 30000 | 406 | [ |
| Mn/Ce-Zr(C-T-1) | 界面CeO2-ZrO2 | 甲苯 | 1000 | 30000 | 254 | [ |
| Mn/Ce-Zr-ss | 简单CeO2-ZrO2 | 甲苯 | 1000 | 30000 | 288 | [ |
| Co3O4/TiO2 | TiO2 | 甲苯 | 1000 | 60000 | 377 | [ |
| Co3O4/YSZ | YSZ | 甲苯 | 1000 | 60000 | 280 | [ |
| Co3O4 | — | 甲苯 | 1000 | 60000 | 312 | [ |
| 催化剂 | 形貌 | 污染物 | 浓度 /μL·L-1 | 空速 /mL∙g-1∙h-1 | T90 /℃ | 参考文献 |
|---|---|---|---|---|---|---|
| CeO2-S | 球形 | 苯乙烯 | 600 | 15000 | 184 | [ |
| CeO2-R | 棒状 | 苯乙烯 | 600 | 15000 | 219 | [ |
| CeO2-O | 八面体 | 苯乙烯 | 600 | 15000 | 221 | [ |
| CeO2-C | 立方体 | 苯乙烯 | 600 | 15000 | >350 | [ |
| Co3O4-R | 棒状 | 邻二甲苯 | 100 | 120000 | 270 | [ |
| Co3O4-S | 球形 | 邻二甲苯 | 100 | 120000 | 295 | [ |
| MnO2-PS | 多孔纳米片 | 丙烷 | 2000 | 30000 | 235 | [ |
| MnO2-R | 棒状 | 丙烷 | 2000 | 30000 | 295 | [ |
| MnO2-B | 块状 | 丙烷 | 2000 | 30000 | 318 | [ |
| Ce1Mn2 | 核壳结构 | 甲苯 | 1000 | 60000 | 245 | [ |
| CeO2 | — | 甲苯 | 1000 | 60000 | 315 | [ |
| MnO2 | — | 甲苯 | 1000 | 60000 | 290 | [ |
| CeO2@Co3O4 | 核壳结构 | 甲苯 | 2000 | 20000 | 225 | [ |
| CeO2 | — | 甲苯 | 2000 | 20000 | >300 | [ |
| Co3O4 | — | 甲苯 | 2000 | 20000 | 285 | [ |
| 催化剂 | 形貌 | 污染物 | 浓度 /μL·L-1 | 空速 /mL∙g-1∙h-1 | T90 /℃ | 参考文献 |
|---|---|---|---|---|---|---|
| CeO2-S | 球形 | 苯乙烯 | 600 | 15000 | 184 | [ |
| CeO2-R | 棒状 | 苯乙烯 | 600 | 15000 | 219 | [ |
| CeO2-O | 八面体 | 苯乙烯 | 600 | 15000 | 221 | [ |
| CeO2-C | 立方体 | 苯乙烯 | 600 | 15000 | >350 | [ |
| Co3O4-R | 棒状 | 邻二甲苯 | 100 | 120000 | 270 | [ |
| Co3O4-S | 球形 | 邻二甲苯 | 100 | 120000 | 295 | [ |
| MnO2-PS | 多孔纳米片 | 丙烷 | 2000 | 30000 | 235 | [ |
| MnO2-R | 棒状 | 丙烷 | 2000 | 30000 | 295 | [ |
| MnO2-B | 块状 | 丙烷 | 2000 | 30000 | 318 | [ |
| Ce1Mn2 | 核壳结构 | 甲苯 | 1000 | 60000 | 245 | [ |
| CeO2 | — | 甲苯 | 1000 | 60000 | 315 | [ |
| MnO2 | — | 甲苯 | 1000 | 60000 | 290 | [ |
| CeO2@Co3O4 | 核壳结构 | 甲苯 | 2000 | 20000 | 225 | [ |
| CeO2 | — | 甲苯 | 2000 | 20000 | >300 | [ |
| Co3O4 | — | 甲苯 | 2000 | 20000 | 285 | [ |
| 催化剂 | 甲苯浓度/μL·L-1 | NO x 浓度/μL·L-1 | 空速/mL∙g-1∙h-1 | 甲苯转化温度(未引入NO x )/℃ | 甲苯转化温度(引入NO x )/℃ | 参考文献 |
|---|---|---|---|---|---|---|
| Mn/CeO2 | 100 | 500 | 10000 | T80=215 | T 80=226 | [ |
| Mn/TiO2 | 100 | 500 | 10000 | T 80=239 | T 80=247 | [ |
| Mn2Cu1Al1O x | 800 | 100 | 60000① | T 100=250 | T 94=250 | [ |
| MnO x -CeO2 | 50 | 500 | 60000 | T 47=150 | T 26=150 | [ |
| CuCeAl x | 1000 | 600 | 50000① | T 90≈300 | T 90≈250 | [ |
| 催化剂 | 甲苯浓度/μL·L-1 | NO x 浓度/μL·L-1 | 空速/mL∙g-1∙h-1 | 甲苯转化温度(未引入NO x )/℃ | 甲苯转化温度(引入NO x )/℃ | 参考文献 |
|---|---|---|---|---|---|---|
| Mn/CeO2 | 100 | 500 | 10000 | T80=215 | T 80=226 | [ |
| Mn/TiO2 | 100 | 500 | 10000 | T 80=239 | T 80=247 | [ |
| Mn2Cu1Al1O x | 800 | 100 | 60000① | T 100=250 | T 94=250 | [ |
| MnO x -CeO2 | 50 | 500 | 60000 | T 47=150 | T 26=150 | [ |
| CuCeAl x | 1000 | 600 | 50000① | T 90≈300 | T 90≈250 | [ |
| 催化剂 | (Oα/O)/% | ΔOα/% | (V5+/V)/% | (SO |
|---|---|---|---|---|
| V-Cu/ZSM-5 | 48.86 | — | 72.25 | — |
| Cu/ZSM-5 | 38.95 | — | — | — |
| V-Cu/ZSM-5-使用后 | 53.17 | 4.31 | 63.32 | 52.36 |
| Cu/ZSM-5-使用后 | 58.89 | 19.94 | — | 64.55 |
| 催化剂 | (Oα/O)/% | ΔOα/% | (V5+/V)/% | (SO |
|---|---|---|---|---|
| V-Cu/ZSM-5 | 48.86 | — | 72.25 | — |
| Cu/ZSM-5 | 38.95 | — | — | — |
| V-Cu/ZSM-5-使用后 | 53.17 | 4.31 | 63.32 | 52.36 |
| Cu/ZSM-5-使用后 | 58.89 | 19.94 | — | 64.55 |
| 1 | YE Z, J-M GIRAUDON, NUNS N, et al. Influence of the preparation method on the activity of copper-manganese oxides for toluene total oxidation[J]. Applied Catalysis B: Environmental, 2018, 223: 154-166. |
| 2 | PARK E J, SEO H O, KIM Y D. Influence of humidity on the removal of volatile organic compounds using solid surfaces[J]. Catalysis Today, 2017, 295: 3-13. |
| 3 | WU P, JIN X J, QIU Y C, et al. Recent progress of thermocatalytic and photo/thermocatalytic oxidation for VOCs purification over manganese-based oxide catalysts[J]. Environmental Science & Technology, 2021, 55(8): 4268-4286. |
| 4 | LIU L Z, SUN J T, DING J D, et al. Highly active Mn3- x Fe x O4 spinel with defects for toluene mineralization: Insights into regulation of the oxygen vacancy and active metals[J]. Inorganic Chemistry, 2019, 58(19): 13241-13249. |
| 5 | HE C, CHENG J, ZHANG X, et al. Recent advances in the catalytic oxidation of volatile organic compounds: A review based on pollutant sorts and sources[J]. Chemical Reviews, 2019, 119(7): 4471-4568. |
| 6 | JIN J, LI P, CHUN D H, et al. Defect dominated hierarchical Ti-metal-organic frameworks via a linker competitive coordination strategy for toluene removal[J]. Advanced Functional Materials, 2021, 31: 2102511. |
| 7 | LI S J, DANG X Q, YU X, et al. High energy efficient degradation of toluene using a novel double dielectric barrier discharge reactor[J]. Journal of Hazardous Materials, 2020, 400: 123259. |
| 8 | BELLUOMO I, BOSHIER P R, MYRIDAKIS A, et al. Selected ion flow tube mass spectrometry for targeted analysis of volatile organic compounds in human breath[J]. Nature Protocols, 2021, 16: 3419-3438. |
| 9 | DU X Y, LI C T, ZHANG J, et al. Highly efficient simultaneous removal of HCHO and elemental mercury over Mn-Co oxides promoted Zr-AC samples[J]. Journal of Hazardous Materials, 2021, 408: 124830. |
| 10 | SHU Y J, JI J, XU Y, et al. Promotional role of Mn doping on catalytic oxidation of VOCs over mesoporous TiO2 under vacuum ultraviolet (VUV) irradiation[J]. Applied Catalysis B: Environmental, 2018, 220: 78-87. |
| 11 | LEE J E, OK Y S, TSANG D C W, et al. Recent advances in volatile organic compounds abatement by catalysis and catalytic hybrid processes: A critical review[J]. Science of The Total Environment, 2020, 719: 137405. |
| 12 | WENG X L, MENG Q J, LIU J J, et al. Catalytic oxidation of chlorinated organics over lanthanide perovskites: Effects of phosphoric acid etching and water vapor on chlorine desorption behavior[J]. Environmental Science & Technology, 2019, 53(2): 884-893. |
| 13 | 周远松, 高凤雨, 唐晓龙, 等. 金属氧化物催化CO还原NO的研究进展[J]. 化工进展, 2019, 38(11): 4941-4948. |
| ZHOU Y S, GAO F Y, TANG X L, et al. Research progress on NO reduction by CO over metal oxide catalysts[J]. Chemical Industry and Engineering Progress, 2019, 38(11): 4941-4948. | |
| 14 | LUO M M, CHENG Y, PENG X Z, et al. Copper modified manganese oxide with tunnel structure as efficient catalyst for low-temperature catalytic combustion of toluene[J]. Chemical Engineering Journal, 2019, 369: 758-765. |
| 15 | WANG H P, LU Y Y, HAN Y X, et al. Enhanced catalytic toluene oxidation by interaction between copper oxide and manganese oxide in Cu-O-Mn/γ-Al2O3 catalysts[J]. Applied Surface Science, 2017, 420: 260-266. |
| 16 | YANG Q L, WANG D, WANG C Z, et al. Promotion effect of Ga-Co spinel derived from layered double hydroxides for toluene oxidation[J]. ChemCatChem, 2018, 10: 4838-4843. |
| 17 | SUN H, YU X L, YANG X Q, et al. Au/Rod-like MnO2 catalyst via thermal decomposition of manganite precursor for the catalytic oxidation of toluene[J]. Catalysis Today, 2019, 332: 153-159. |
| 18 | GENTY E, SIFFERT S, COUSIN R. Investigation of reaction mechanism and kinetic modelling for the toluene total oxidation in presence of CoAlCe catalyst[J]. Catalysis Today, 2019, 333: 28-35. |
| 19 | LONG R, MAO K K, GONG M, et al. Tunable oxygen activation for catalytic organic oxidation: Schottky junction versus plasmonic effects[J]. Angewandte Chemie, 2014, 53(12): 3205-3209. |
| 20 | DONG C, QU Z P, QIN Y, et al. Revealing the highly catalytic performance of spinel CoMn2O4 for toluene oxidation: Involvement and replenishment of oxygen species using in situ designed-TP techniques[J]. ACS Catalysis, 2019, 9(8): 6698-6710. |
| 21 | WANG H L, LUO S T, ZHANG M S, et al. Roles of oxygen vacancy and O x - in oxidation reactions over CeO2 and Ag/CeO2 nanorod model catalysts[J]. Journal of Catalysis, 2018, 368: 365-378. |
| 22 | LI Z Q, REN F, CHEN Z C, et al. Improved NO x emissions and combustion characteristics for a retrofitted down-fired 300-MWe utility boiler[J]. Environmental Science & Technology, 2010, 44(10): 3926-3931. |
| 23 | WANG D, CHEN Q Z, ZHANG X, et al. Multipollutant control (MPC) of flue gas from stationary sources using SCR technology: A critical review[J]. Environmental Science & Technology, 2021, 55(5): 2743-2766. |
| 24 | SUN S R, LIU W B, GUAN W S, et al. Effects of air pollution control devices on volatile organic compounds reduction in coal-fired power plants[J]. Science of the Total Environment, 2021,782: 146828. |
| 25 | LIU J, WANG T, CHENG J, et al. Distribution of organic compounds in coal-fired power plant emissions[J]. Energy & Fuels, 2019, 33(6): 5430-5437. |
| 26 | PENG K, HOU Y Q, ZHANG Y Z, et al. Engineering oxygen vacancies in metal-doped MnO2 nanospheres for boosting the low-temperature toluene oxidation[J]. Fuel, 2022, 314: 123123. |
| 27 | LI K G, LI T, DAI Y H, et al. Highly active urchin-like MCo2O4 (M=Co, Cu, Ni or Zn) spinel for toluene catalytic combustion[J]. Fuel, 2022, 318: 123648. |
| 28 | ZHOU X Y, LAI X X, LIN T, et al. Preparation of a monolith MnO x -CeO2/La-Al2O3 catalyst and its properties for catalytic oxidation of toluene[J]. New Journal of Chemistry, 2018, 20(42): 16875-16885. |
| 29 | ZHANG Y Z, ZENG Z Q, LI Y F, et al. Effect of the A-site cation over spinel AMn2O4 (A=Cu2+, Ni2+, Zn2+) for toluene combustion: Enhancement of the synergy and the oxygen activation ability[J]. Fuel, 2021, 288: 119700. |
| 30 | DONG C, QU Z P, JIANG X, et al. Tuning oxygen vacancy concentration of MnO2 through metal doping for improved toluene oxidation[J]. Journal of Hazardous Materials, 2020, 391: 122181. |
| 31 | ZHANG W L, LI M Y, WANG X T, et al. Boosting catalytic toluene combustion over Mn doped Co3O4 spinel catalysts: Improved mobility of surface oxygen due to formation of Mn-O-Co bonds[J]. Applied Surface Science, 2022, 590: 153140. |
| 32 | SU Z A, YANG W H, WANG C Z, et al. Roles of oxygen vacancies in the bulk and surface of CeO2 for toluene catalytic combustion[J]. Environmental Science & Technology, 2020, 54(19): 12684-12692. |
| 33 | WANG Y, WANG G, DENG W, et al. Study on the structure-activity relationship of Fe-Mn oxide catalysts for chlorobenzene catalytic combustion[J]. Chemical Engineering Journal, 2020, 395: 125172. |
| 34 | SHEN Y J, DENG J, IMPENG S, et al. Boosting toluene combustion by engineering Co-O strength in cobalt oxide catalysts[J]. Environmental Science & Technology, 2020, 54(16): 10342-10350. |
| 35 | WANG Y, WU J, WANG G, et al. Oxygen vacancy engineering in Fe doped akhtenskite-type MnO2 for low-temperature toluene oxidation[J]. Applied Catalysis B: Environmental, 2021, 285: 119873. |
| 36 | LI Z, YAN Q H, JIANG Q, et al. Oxygen vacancy mediated CuyCo3- y Fe1O x mixed oxide as highly active and stable toluene oxidation catalyst by multiple phase interfaces formation and metal doping effect[J]. Applied Catalysis B: Environmental, 2020, 269: 118827. |
| 37 | ZHANG P F, LU H F, ZHOU Y, et al. Mesoporous MnCeO x solid solutions for low temperature and selective oxidation of hydrocarbons[J]. Nature Communications, 2015, 6(1): 1-10. |
| 38 | CHEN X, CHEN X, YU E Q, et al. In situ pyrolysis of Ce-MOF to prepare CeO2 catalyst with obviously improved catalytic performance for toluene combustion[J]. Chemical Engineering Journal, 2018, 344: 469-479. |
| 39 | ZHANG X J, ZHAO J G, SONG Z X, et al. The catalytic oxidation performance of toluene over the Ce-Mn-O x catalysts: Effect of synthetic routes[J]. Journal of Colloid and Interface Science, 2020, 562: 170-181. |
| 40 | LI J R, ZHANG W P, LI C, et al. Efficient catalytic degradation of toluene at a readily prepared Mn-Cu catalyst: Catalytic performance and reaction pathway[J]. Journal of Colloid and Interface Science, 2021, 591: 396-408. |
| 41 | ZENG Y Q, K-G HAW, WANG Z G, et al. Double redox process to synthesize CuO-CeO2 catalysts with strong Cu-Ce interaction for efficient toluene oxidation[J]. Journal of Hazardous Materials, 2021, 404: 124088. |
| 42 | ZHANG C H, WANG C, HUANG H, et al. Insights into the size and structural effects of zeolitic supports on gaseous toluene oxidation over MnO x /HZSM-5 catalysts[J]. Applied Surface Science, 2019, 486: 108-120. |
| 43 | LIU C F, HE L C, WANG X F, et al. Tailoring Co3O4 active species to promote propane combustion over Co3O4/ZSM-5 catalyst[J]. Molecular Catalysis, 2022, 524: 112297. |
| 44 | ZHANG L, HUANG S S, DENG W, et al. Dichloromethane catalytic combustion over Co3O4 catalysts supported on MFI type zeolites[J]. Microporous and Mesoporous Materials, 2021, 312: 110599. |
| 45 | ZHOU C X, ZHANG H L, ZHANG Z, et al. Improved reactivity for toluene oxidation on MnO x /CeO2-ZrO2 catalyst by the synthesis of cubic-tetragonal interfaces[J]. Applied Surface Science, 2021, 539: 148188. |
| 46 | CHAI G T, DU D, WANG C, et al. Spinel Co3O4 oxides-support synergistic effect on catalytic oxidation of toluene[J]. Applied Catalysis A: General, 2021, 614: 118044. |
| 47 | ZHANG Y, LU J C, ZHANG L M, et al. Investigation into the catalytic roles of oxygen vacancies during gaseous styrene degradation process via CeO2 catalysts with four different morphologies[J]. Applied Catalysis B: Environmental, 2022, 309: 121249. |
| 48 | MA Y, WANG L, MA J Z, et al. Investigation into the enhanced catalytic oxidation of o‑xylene over MOF-derived Co3O4 with different shapes: The role of surface twofold-coordinate lattice oxygen (O2f)[J]. ACS Catalysis, 2021, 11(11): 6614-6625. |
| 49 | WU S P, LIU H M, HUANG Z, et al. O-vacancy-rich porous MnO2 nanosheets as highly efficient catalysts for propane catalytic oxidation[J]. Applied Catalysis B: Environmental, 2022, 312: 121387. |
| 50 | LUO Y J, LIN D F, ZHENG Y B, et al. MnO2 nanoparticles encapsuled in spheres of Ce-Mn solid solution: Efficient catalyst and good water tolerance for low-temperature toluene oxidation[J]. Applied Surface Science, 2020, 504: 144481. |
| 51 | FANG W, CHEN J H, ZHOU X Y, et al. Zeolitic imidazolate framework-67-derived CeO2@Co3O4 core-shell microspheres with enhanced catalytic activity toward toluene oxidation[J]. Industrial & Engineering Chemistry Research, 2020, 59(22): 10328-10337. |
| 52 | KAWI S, TE M. MCM-48 supported chromium catalyst for trichloroethylene oxidation[J]. Catalysis Today, 1998, 44(1/2/3/4): 101-109. |
| 53 | CHOYA A, DE RIVAS B, GONZÁLEZ-VELASCO J R, et al. Oxidation of lean methane over cobalt catalysts supported on ceria/alumina[J]. Applied Catalysis A: General, 2020, 591: 117381. |
| 54 | ZHANG W D, DESCORME C, VALVERDE J L, et al. Cu-Co mixed oxide catalysts for the total oxidation of toluene and propane[J]. Catalysis Today, 2022, 384/385/386: 238-245. |
| 55 | MENG L Q, ZHAO H B. Low-temperature complete removal of toluene over highly active nanoparticles CuO-TiO2 synthesized via flame spray pyrolysis[J]. Applied Catalysis B: Environmental, 2020, 264: 118427. |
| 56 | LEISTNER K, OLSSON L. Deactivation of Cu/SAPO-34 during low-temperature NH3-SCR[J]. Applied Catalysis B: Environmental, 2015, 165: 192-199. |
| 57 | ZHANG X D, BI F K, ZHU Z Q, et al. The promoting effect of H2O on rod-like MnCeO x derived from MOFs for toluene oxidation: A combined experimental and theoretical investigation[J]. Applied Catalysis B: Environmental, 2021, 297: 120393. |
| 58 | PAN H, CHEN Z H, MA M D, et al. Mutual inhibition mechanism of simultaneous catalytic removal of NO x and toluene on Mn-based catalysts[J]. Journal of Colloid and Interface Science, 2022, 607: 1189-1200. |
| 59 | LI Z, GAO Y S, WANG Q. The influencing mechanism of NH3 and NO x addition on the catalytic oxidation of toluene over Mn2Cu1Al1O x catalyst[J]. Journal of Cleaner Production, 2022, 348: 131152. |
| 60 | YE L M, LU P, PENG Y, et al. Impact of NO x and NH3 addition on toluene oxidation over MnO x -CeO2 catalyst[J]. Journal of Hazardous Materials, 2021, 416: 125939. |
| 61 | ZHAO L K, HUANG Y, ZHANG J F, et al. Al2O3-modified CuO-CeO2 catalyst for simultaneous removal of NO and toluene at wide temperature range[J]. Chemical Engineering Journal, 2020, 397: 125419. |
| 62 | LI J H, XIAO G F, GUO Z Y, et al. ZSM-5-supported V-Cu bimetallic oxide catalyst for remarkable catalytic oxidation of toluene in coal-fired flue gas[J]. Chemical Engineering Journal, 2021, 419: 129675. |
| 63 | WANG Z, XIE K Y, ZHENG J, et al. Studies of sulfur poisoning process via ammonium sulfate on MnO2/γ-Al2O3 catalyst for catalytic combustion of toluene[J]. Applied Catalysis B: Environmental, 2021, 298: 120595. |
| 64 | XIAO G F, GUO Z Y, LI J H, et al. Insights into the effect of flue gas on synergistic elimination of toluene and NO x over V2O5-MoO3(WO3)/TiO2 catalysts[J]. Chemical Engineering Journal, 2022, 435: 134914. |
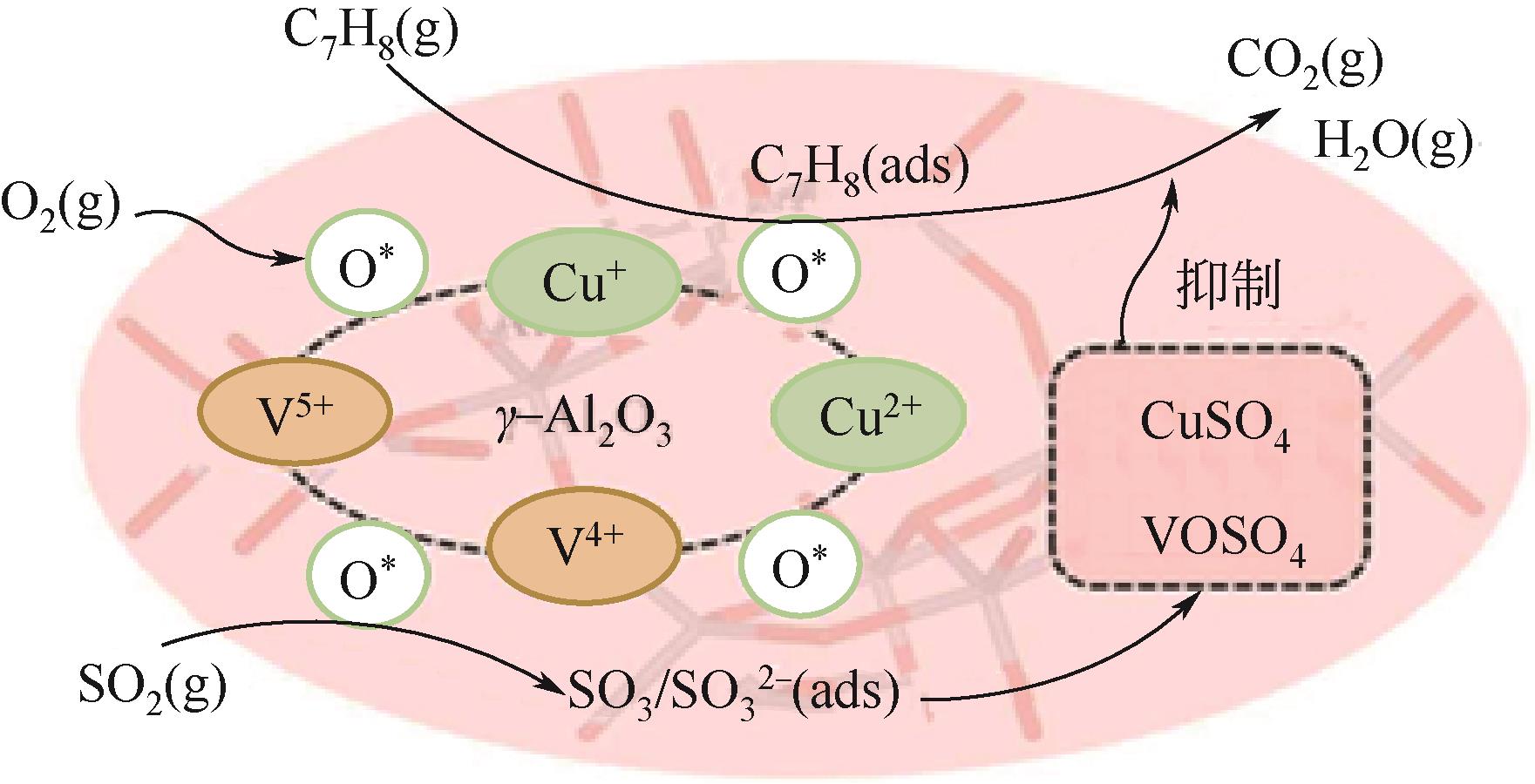
| 65 | LYU Y, XU J Y, CAO Q Q, et al. Highly efficient removal of toluene over Cu-V oxides modified γ-Al2O3 in the presence of SO2 [J]. Journal of Hazardous Materials, 2022, 436: 129041. |
| 66 | YU X H, WANG L Y, CHEN M Z, et al. Enhanced activity and sulfur resistance for soot combustion on three-dimensionally ordered macroporous-mesoporous Mn x Ce1- x O δ /SiO2 catalysts[J]. Applied Catalysis B: Environmental, 2019, 254: 246-259. |
| [1] | YANG Hanyue, KONG Lingzhen, CHEN Jiaqing, SUN Huan, SONG Jiakai, WANG Sicheng, KONG Biao. Decarbonization performance of downflow tubular gas-liquid contactor of microbubble-type [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 197-204. |
| [2] | ZHENG Qian, GUAN Xiushuai, JIN Shanbiao, ZHANG Changming, ZHANG Xiaochao. Photothermal catalysis synthesis of DMC from CO2 and methanol over Ce0.25Zr0.75O2 solid solution [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 319-327. |
| [3] | ZHANG Jie, BAI Zhongbo, FENG Baoxin, PENG Xiaolin, REN Weiwei, ZHANG Jingli, LIU Eryong. Effect of PEG and its compound additives on post-treatment of electrolytic copper foils [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 374-381. |
| [4] | WANG Lele, YANG Wanrong, YAO Yan, LIU Tao, HE Chuan, LIU Xiao, SU Sheng, KONG Fanhai, ZHU Canghai, XIANG Jun. Influence of spent SCR catalyst blending on the characteristics and deNO x performance for new SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 489-497. |
| [5] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [6] | SUN Yuyu, CAI Xinlei, TANG Jihai, HUANG Jingjing, HUANG Yiping, LIU Jie. Optimization and energy-saving of a reactive distillation process for the synthesis of methyl methacrylate [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 56-63. |
| [7] | XU Maoyu, TAO Shuai, QI Cong, LIANG Lin. Start-up and temperature fluctuation of loop heat pipe with flat disk evaporator [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4531-4537. |
| [8] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [9] | LIN Xiaopeng, XIAO Youhua, GUAN Yichen, LU Xiaodong, ZONG Wenjie, FU Shenyuan. Recent progress of flexible electrodes for ion polymer-metal composites (IPMC) [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4770-4782. |
| [10] | TAN Jihuai, YU Min, ZHANG Tongtong, HUANG Nengkun, WANG Ziwen, ZHU Xinbao. Manufacturing of tannin polypropoxy ether carboxylates as efficient and improved migration resistance plasticizers for PVC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4847-4855. |
| [11] | ZHU Chuanqiang, RU Jinbo, SUN Tingting, XIE Xingwang, LI Changming, GAO Shiqiu. Characteristics of selective non-catalytic reduction of NO x with solid polymer denitration agent [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4939-4946. |
| [12] | SHI Tianxi, SHI Yonghui, WU Xinying, ZHANG Yihao, QIN Zhe, ZHAO Chunxia, LU Da. Effects of Fe2+ on the performance of Anammox EGSB reactor [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5003-5010. |
| [13] | MAO Shanjun, WANG Zhe, WANG Yong. Group recognition hydrogenation: From concept to application [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3917-3922. |
| [14] | WANG Baoying, WANG Huangying, YAN Junying, WANG Yaoming, XU Tongwen. Research progress of polymer inclusion membrane in metal separation and recovery [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3990-4004. |
| [15] | XIANG Yang, HUANG Xun, WEI Zidong. Recent progresses in the activity and selectivity improvement of electrocatalytic organic synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4005-4014. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||