Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (3): 1540-1550.DOI: 10.16085/j.issn.1000-6613.2022-2305
• Materials science and technology • Previous Articles Next Articles
Preparation and performance of carbon supported potassium-based CO2 adsorbent derived from hyper-cross linked polymers
CHEN Chongming1( ), ZENG Siming1, LUO Xiaona2, SONG Guosheng1, HAN Zhongge1, YU Jinxing3(
), ZENG Siming1, LUO Xiaona2, SONG Guosheng1, HAN Zhongge1, YU Jinxing3( ), SUN Nannan2
), SUN Nannan2
- 1.State Grid Hebei Electric Power Research Institute, Shijiazhuang 050021, Hebei, China
2.Shanghai Advanced Research Institute, Chinese Academy of Sciences, Shanghai 201210, China
3.State Grid Hebei Energy Technology Service Co. , Ltd. , Shijiazhuang 050021, Hebei, China
-
Received:2022-12-11Revised:2023-02-09Online:2023-04-10Published:2023-03-15 -
Contact:YU Jinxing
基于超交联聚合物前体的碳载钾基CO2吸附剂制备和性能
陈崇明1( ), 曾四鸣1, 罗小娜2, 宋国升1, 韩忠阁1, 郁金星3(
), 曾四鸣1, 罗小娜2, 宋国升1, 韩忠阁1, 郁金星3( ), 孙楠楠2
), 孙楠楠2
- 1.国网河北省电力有限公司电力科学研究院,河北 石家庄 050021
2.中国科学院上海高等研究院,上海 201210
3.国网河北能源技术服务有限公司,河北 石家庄 050021
-
通讯作者:郁金星 -
作者简介:陈崇明(1983—),男,高级工程师,研究方向为烟气污染物治理技术。E-mail:dyy_chencm@163.com。 -
基金资助:国网河北能源技术服务有限公司自主项目(TSS2021-06)
CLC Number:
Cite this article
CHEN Chongming, ZENG Siming, LUO Xiaona, SONG Guosheng, HAN Zhongge, YU Jinxing, SUN Nannan. Preparation and performance of carbon supported potassium-based CO2 adsorbent derived from hyper-cross linked polymers[J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1540-1550.
陈崇明, 曾四鸣, 罗小娜, 宋国升, 韩忠阁, 郁金星, 孙楠楠. 基于超交联聚合物前体的碳载钾基CO2吸附剂制备和性能[J]. 化工进展, 2023, 42(3): 1540-1550.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-2305
| 样品 | 比表面积/m2·g-1 | 微孔比表面积/m2·g-1 | 总孔容/cm3·g-1 | 微孔孔容/cm3·g-1 | 平均孔径/nm | 钾质量分数/% |
|---|---|---|---|---|---|---|
| HCP | 1186 | 268 | 1.634 | 0.123 | 0.63 | — |
| HCP-Ox | 519 | 243 | 0.391 | 0.102 | 0.61 | — |
| HCP-K | 8 | 7 | 0.016 | 0.003 | 0.96 | 6.54 |
| HCP-K650 | 16 | 23 | 0.012 | 0.008 | 0.96 | 14.42 |
| HCP-K750 | 70 | 68 | 0.033 | 0.026 | 0.60 | 18.22 |
| HCP-K850 | 46 | 41 | 0.030 | 0.016 | 0.73 | 21.31 |
| 样品 | 比表面积/m2·g-1 | 微孔比表面积/m2·g-1 | 总孔容/cm3·g-1 | 微孔孔容/cm3·g-1 | 平均孔径/nm | 钾质量分数/% |
|---|---|---|---|---|---|---|
| HCP | 1186 | 268 | 1.634 | 0.123 | 0.63 | — |
| HCP-Ox | 519 | 243 | 0.391 | 0.102 | 0.61 | — |
| HCP-K | 8 | 7 | 0.016 | 0.003 | 0.96 | 6.54 |
| HCP-K650 | 16 | 23 | 0.012 | 0.008 | 0.96 | 14.42 |
| HCP-K750 | 70 | 68 | 0.033 | 0.026 | 0.60 | 18.22 |
| HCP-K850 | 46 | 41 | 0.030 | 0.016 | 0.73 | 21.31 |
| 样品 | 40℃下CO2的吸附量/mmol·g-1 | |
|---|---|---|
| 体积分数15% CO2/N2 | 纯CO2 | |
| HCP | 0.83 | 3.33 |
| HCP-Ox | 0.92 | 3.59 |
| HCP-K | 1.13 | 3.82 |
| HCP-K650 | 6.14 | 13.03 |
| HCP-K750 | 8.89 | 13.14 |
| HCP-K850 | 7.38 | 12.60 |
| 样品 | 40℃下CO2的吸附量/mmol·g-1 | |
|---|---|---|
| 体积分数15% CO2/N2 | 纯CO2 | |
| HCP | 0.83 | 3.33 |
| HCP-Ox | 0.92 | 3.59 |
| HCP-K | 1.13 | 3.82 |
| HCP-K650 | 6.14 | 13.03 |
| HCP-K750 | 8.89 | 13.14 |
| HCP-K850 | 7.38 | 12.60 |
| 样品 | 温度/℃ | CO2分压/bar | 吸附量/mmol·g-1 | 测试方法 | 参考文献 |
|---|---|---|---|---|---|
| SNS-20 | 50 | 0.15 | 1.15 | 吸附等温线 | [ |
| SAC | 40 | 0.15 | 0.85 | 吸附等温线 | [ |
| p-2-973-1.5 | 50 | 0.15 | 1.20 | 吸附等温线 | [ |
| C@MF-700 | 50 | 0.15 | 1.30 | 吸附等温线 | [ |
| NC-1-500 | 35 | 0.15 | 1.10 | 热重分析 | [ |
| RN-450-3 | 25 | 0.10 | 0.94 | 穿透曲线 | [ |
| PGC-K | 27 | 0.17 | 1.10 | 穿透曲线 | [ |
| ClCTF-1-650 | 25 | 0.10 | 0.68 | 穿透曲线 | [ |
| NGC-650-4 | 25 | 0.10 | 0.84 | 穿透曲线 | [ |
| 3D-MPCFW-11-18 | 45 | 0.15 | 1.05 | 吸附等温线 | [ |
| OTS-1-550 | 25 | 0.20 | 1.51 | 穿透曲线 | [ |
| CTS-NaNH2(1:1)-700 | 40 | 0.15 | 0.60 | 吸附等温线 | [ |
| KNC-1-800 | 40 | 0.15 | 1.12 | 吸附等温线 | [ |
| ACDES 9 | 45 | 0.10 | 0.49 | 穿透曲线 | [ |
| PSK-2-650 | 40 | 0.15 | 1.01 | 穿透曲线 | [ |
| HCP-K750 | 40 | 0.15 | 2.02 | 热重分析 | 本文 |
| HCP-K750 | 40 | 0.15 | 1.63 | 穿透曲线 | 本文 |
| 样品 | 温度/℃ | CO2分压/bar | 吸附量/mmol·g-1 | 测试方法 | 参考文献 |
|---|---|---|---|---|---|
| SNS-20 | 50 | 0.15 | 1.15 | 吸附等温线 | [ |
| SAC | 40 | 0.15 | 0.85 | 吸附等温线 | [ |
| p-2-973-1.5 | 50 | 0.15 | 1.20 | 吸附等温线 | [ |
| C@MF-700 | 50 | 0.15 | 1.30 | 吸附等温线 | [ |
| NC-1-500 | 35 | 0.15 | 1.10 | 热重分析 | [ |
| RN-450-3 | 25 | 0.10 | 0.94 | 穿透曲线 | [ |
| PGC-K | 27 | 0.17 | 1.10 | 穿透曲线 | [ |
| ClCTF-1-650 | 25 | 0.10 | 0.68 | 穿透曲线 | [ |
| NGC-650-4 | 25 | 0.10 | 0.84 | 穿透曲线 | [ |
| 3D-MPCFW-11-18 | 45 | 0.15 | 1.05 | 吸附等温线 | [ |
| OTS-1-550 | 25 | 0.20 | 1.51 | 穿透曲线 | [ |
| CTS-NaNH2(1:1)-700 | 40 | 0.15 | 0.60 | 吸附等温线 | [ |
| KNC-1-800 | 40 | 0.15 | 1.12 | 吸附等温线 | [ |
| ACDES 9 | 45 | 0.10 | 0.49 | 穿透曲线 | [ |
| PSK-2-650 | 40 | 0.15 | 1.01 | 穿透曲线 | [ |
| HCP-K750 | 40 | 0.15 | 2.02 | 热重分析 | 本文 |
| HCP-K750 | 40 | 0.15 | 1.63 | 穿透曲线 | 本文 |
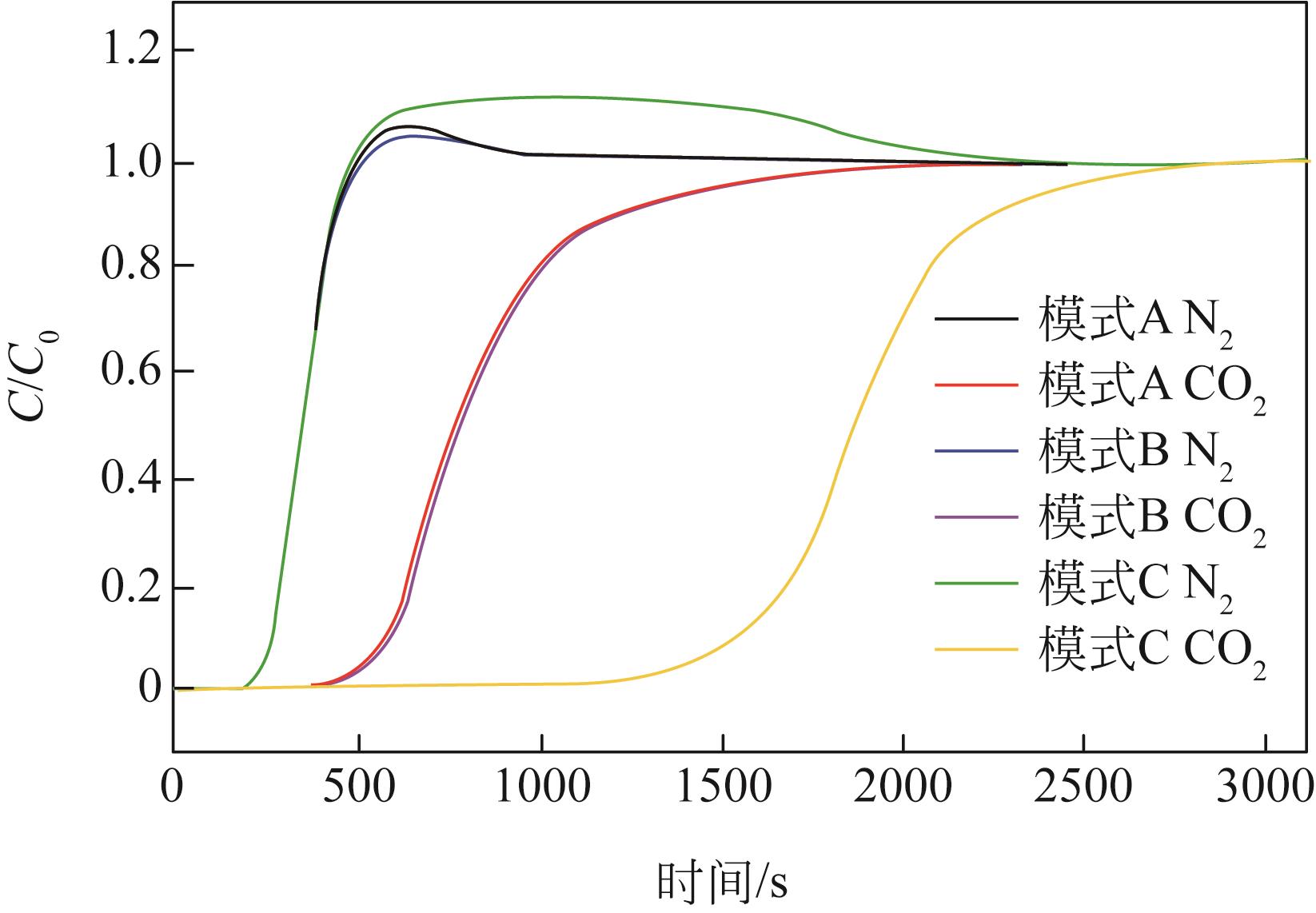
| 模式 | 烟气状态 | 是否预处理 | 循环数 | 进气流速 /mL·min-1 | 吸附量 /mmol·g-1 |
|---|---|---|---|---|---|
| 模式A | 干燥烟气 | 否 | 1 | 100 | 0.37 |
| 2 | 100 | 0.38 | |||
| 3 | 50 | 0.48 | |||
| 模式B | 潮湿烟气 | 否 | 4 | 100 | 0.37 |
| 5 | 100 | 0.53 | |||
| 6 | 100 | 0.56 | |||
| 模式C | 潮湿烟气 | 是 | 7 | 100 | 1.53 |
| 8 | 100 | 1.56 | |||
| 9 | 100 | 1.57 | |||
| 10 | 100 | 1.60 | |||
| 11 | 100 | 1.63 |
| 模式 | 烟气状态 | 是否预处理 | 循环数 | 进气流速 /mL·min-1 | 吸附量 /mmol·g-1 |
|---|---|---|---|---|---|
| 模式A | 干燥烟气 | 否 | 1 | 100 | 0.37 |
| 2 | 100 | 0.38 | |||
| 3 | 50 | 0.48 | |||
| 模式B | 潮湿烟气 | 否 | 4 | 100 | 0.37 |
| 5 | 100 | 0.53 | |||
| 6 | 100 | 0.56 | |||
| 模式C | 潮湿烟气 | 是 | 7 | 100 | 1.53 |
| 8 | 100 | 1.56 | |||
| 9 | 100 | 1.57 | |||
| 10 | 100 | 1.60 | |||
| 11 | 100 | 1.63 |
| 22 | DAWSON Robert, COOPER Andrew I, ADAMS Dave J. Nanoporous organic polymer networks[J]. Progress in Polymer Science, 2012, 37(4): 530-563. |
| 23 | 谭良骁, 谭必恩. 超交联微孔聚合物研究进展[J]. 化学学报, 2015, 73(6): 530-540. |
| TAN Liangxiao, TAN Bien. Research progress in hypercrosslinked microporous organic polymers[J]. Acta Chimica Sinica, 2015, 73(6): 530-540. | |
| 24 | JIA Zhifang, WANG Kewei, TAN Bien, et al. Hollow hyper-cross-linked nanospheres with acid and base sites as efficient and water-stable catalysts for one-pot tandem reactions[J]. ACS Catalysis, 2017, 7(5): 3693-3702. |
| 25 | KARIMI Mohsen, SILVA José A C, GONÇALVES Carmem N d P, et al. CO2 capture in chemically and thermally modified activated carbons using breakthrough measurements: Experimental and modeling study[J]. Industrial & Engineering Chemistry Research, 2018, 57(32): 11154-11166. |
| 26 | WOODWARD Robert T, STEVENS Lee A, Dawson Robert, et al. Swellable, water- and acid-tolerant polymer sponges for chemoselective carbon dioxide capture[J]. Journal of the American Chemical Society, 2014, 136(25): 9028-9035. |
| 27 | LI Buyi, GONG Ruini, WANG Wei, et al. A new strategy to microporous polymers: Knitting rigid aromatic building blocks by external cross-linker[J]. Macromolecules, 2011, 44(8): 2410-2414. |
| 28 | SHENG Yujie, CHEN Qibin, MAHURIN Shannon M, et al. Fibers with hyper-crosslinked functional porous frameworks[J]. Macromolecular Rapid Communications, 2018, 39(8): e1700767. |
| 29 | TSYURUPA M P, BORISOV Yu A, BLINNIKOVA Z K, et al. On the origin of absorbance band around 1700cm-1 in FTIR spectra of hypercrosslinked polystyrene[J]. Protection of Metals and Physical Chemistry of Surfaces, 2014, 50(1): 59-63. |
| 30 | 朴海燕, 任秀丽, 孟龙月, 等. ZnCl2活化制备N掺杂多孔碳材料及其CO2吸附性能研究[J]. 材料导报, 2015, 29(S1): 310-312. |
| PIAO Haiyan, REN Xiuli, MENG Longyue, et al. Preparation of N-doped porous carbons by ZnCl2 activation and their performance for carbon dioxide adsorption[J]. Materials Review, 2015, 29(S1): 310-312. | |
| 31 | THOMMES Matthias, KANEKO Katsumi, NEIMARK Alexander V, et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report)[J]. Pure and Applied Chemistry, 2015, 87(9/10): 1051-1069. |
| 32 | LUDWINOWICZ Jowita, JARONIEC Mietek. Potassium salt-assisted synthesis of highly microporous carbon spheres for CO2 adsorption[J]. Carbon, 2015, 82: 297-303. |
| 33 | CUI Xili, YANG Qiwei, XIONG Yijun, et al. Preparation of ordered N-doped mesoporous carbon materials via a polymer-ionic liquid assembly[J]. Chemical Communications, 2017, 53(36): 4915-4918. |
| 34 | KIM Yun Kon, KIM Gi Mihn, LEE Jae W. Highly porous N-doped carbons impregnated with sodium for efficient CO2 capture[J]. Journal of Materials Chemistry A, 2015, 3(20): 10919-10927. |
| 35 | LI Li, WANG Xuefei, ZHONG Junjun, et al. Nitrogen-enriched porous polyacrylonitrile-based carbon fibers for CO2 capture[J]. Industrial & Engineering Chemistry Research, 2018, 57(34): 11608-11616. |
| 36 | PAN Ying, ZHAO Yuxin, MU Shanjun, et al. Cation exchanged MOF-derived nitrogen-doped porous carbons for CO2 capture and supercapacitor electrode materials[J]. Journal of Materials Chemistry A, 2017, 5(20): 9544-9552. |
| 37 | WANG Chaohai, KIM Jeonghun, TANG Jing, et al. New strategies for novel MOF-derived carbon materials based on nanoarchitectures[J]. Chem., 2020, 6(1): 19-40. |
| 38 | WANG Baodeng, ZHU Chenming, ZHANG Zhongzheng, et al. Facile, low-cost, and sustainable preparation of hierarchical porous carbons from ion exchange resin: An improved potassium activation strategy[J]. Fuel, 2016, 179: 274-280. |
| 39 | Young-Jung HEO, PARK Soo-Jin. H2O2/steam activation as an eco-friendly and efficient top-down approach to enhancing porosity on carbonaceous materials: The effect of inevitable oxygen functionalities on CO2 capture[J]. Green Chemistry, 2018, 20(22): 5224-5234. |
| 40 | DENG Shubo, WEI Haoran, CHEN Tao, et al. Superior CO2 adsorption on pine nut shell-derived activated carbons and the effective micropores at different temperatures[J]. Chemical Engineering Journal, 2014, 253: 46-54. |
| 41 | LIU Lei, XIE Zhenghu, DENG Qingfang, et al. One-pot carbonization enrichment of nitrogen in microporous carbon spheres for efficient CO2 capture[J]. Journal of Materials Chemistry A, 2017, 5(1): 418-425. |
| 42 | REN Xiaomin, LI He, CHEN Jian, et al. N-doped porous carbons with exceptionally high CO2 selectivity for CO2 capture[J]. Carbon, 2017, 114: 473-481. |
| 43 | WANG Liwei, RAO Linli, XIA Binbin, et al. Highly efficient CO2 adsorption by nitrogen-doped porous carbons synthesized with low-temperature sodium amide activation[J]. Carbon, 2018, 130: 31-40. |
| 44 | GUO Liping, HU Qingtao, ZHANG Peng, et al. Polyacrylonitrile-derived sponge-like micro/macroporous carbon for selective CO2 separation[J]. Chemistry, 2018, 24(33): 8369-8374. |
| 45 | YAO Ke xin, CHEN Yanli, LU Yue, et al. Ultramicroporous carbon with extremely narrow pore distribution and very high nitrogen doping for efficient methane mixture gases upgrading[J]. Carbon, 2017, 122: 258-265. |
| 46 | YUE Limin, RAO Linli, WANG Linlin, et al. Efficient CO2 adsorption on nitrogen-doped porous carbons derived from d-glucose[J]. Energy & Fuels, 2018, 32(6): 6955-6963. |
| 47 | LI Yao, WANG Xin, CAO Minhua. Three-dimensional porous carbon frameworks derived from mangosteen peel waste as promising materials for CO2 capture and supercapacitors[J]. Journal of CO2 Utilization, 2018, 27: 204-216. |
| 48 | ZHANG Yan, ZHANG Peixin, YU Weikang, et al. Facile and controllable preparation of ultramicroporous biomass-derived carbons and application on selective adsorption of gas-mixtures[J]. Industrial & Engineering Chemistry Research, 2018, 57(42): 14191-14201. |
| 49 | YANG Chunliang, ZHAO Tianxiang, PAN Hongyan, et al. Facile preparation of N-doped porous carbon from chitosan and NaNH2 for CO2 adsorption and conversion[J]. Chemical Engineering Journal, 2022, 432: 134347. |
| 50 | LIU Baogen, SHI Rui, MA Xiancheng, et al. High yield nitrogen-doped carbon monolith with rich ultramicropores prepared by in situ activation for high performance of selective CO2 capture[J]. Carbon, 2021, 181: 270-279. |
| 51 | HUSSIN Farihahusnah, AROUA Mohamed Kheireddine, YUSOFF Rozita. Adsorption of CO2 on palm shell based activated carbon modified by deep eutectic solvent: Breakthrough adsorption study[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105333. |
| 52 | ZHANG Yan, WEI Ziqi, LIU Xing, et al. Synthesis of palm sheath derived-porous carbon for selective CO2 adsorption[J]. RSC Advances, 2022, 12(14): 8592-8599. |
| 53 | MEIS Niels N A H, FREY Anne Mette, BITTER Johannes H, et al. Carbon nanofiber-supported K2CO3 as an efficient low-temperature regenerable CO2 sorbent for post-combustion capture[J]. Industrial & Engineering Chemistry Research, 2013, 52(36): 12812-12818. |
| 54 | DUTCHER Bryce, FAN Maohong, LEONARD Brian. Use of multifunctional nanoporous TiO(OH)2 for catalytic NaHCO3 decomposition-eventually for Na2CO3/NaHCO3 based CO2 separation technology[J]. Separation and Purification Technology, 2011, 80(2): 364-374. |
| 55 | GUO Yafei, ZHAO Chuanwen, LI Changhai. Thermogravimetric analysis of carbonation behaviors of several potassium-based sorbents in low concentration CO2 [J]. Journal of Thermal Analysis and Calorimetry, 2015, 119(1): 441-451. |
| 56 | LEE Soo Chool, CHOI Bo Yun, Chong Kul RYU, et al. The effect of water on the activation and the CO2 capture capacities of alkali metal-based sorbents[J]. Korean Journal of Chemical Engineering, 2006, 23(3): 374-379. |
| 1 | 黄晶. 中国碳捕集利用与封存技术评估报告[M]. 北京: 科学出版社, 2021. |
| HUANG Jing. National assessment report on development of carbon capture utilization and strorage technology in China[M]. Beijing: Science Press, 2021. | |
| 2 | 张贤, 李凯, 马乔, 等. 碳中和目标下CCUS技术发展定位与展望[J]. 中国人口·资源与环境, 2021, 31(9): 29-33. |
| ZHANG Xian, LI Kai, MA Qiao, et al. Orientation and prospect of CCUS development under carbon neutrality target[J]. China Population, Resources and Environment, 2021, 31(9): 29-33. | |
| 3 | Global CCS Institute. Global status of CCS 2022[R]. 2022. |
| 4 | Mai BUI, ADJIMAN Claire S, BARDOW André, et al. Carbon capture and storage (CCS): The way forward[J]. Energy & Environmental Science, 2018, 11(5): 1062-1176. |
| 5 | CHAI Slyvester Yew Wang, Lock Hei NGU, Bing Shen HOW. Review of carbon capture absorbents for CO2 utilization[J]. Greenhouse Gases: Science and Technology, 2022, 12(3): 394-427. |
| 6 | 郭真良, 卞晓律, 杜宇搏, 等. 集成二氧化碳捕集与甲烷化转化研究进展[J]. 燃料化学学报, 2023, 51(3): 293-303. |
| GUO Zhenliang, BIAN Xiaolv, DU Yubo, et al. Recent advances in integrated carbon dioxide capture and methanation technology[J]. Journal of Fuel Chemistry and Technology, 2023, 51(3): 293-303. | |
| 7 | KARIMI Mohsen, SHIRZAD Mohammad, SILVA José A C, et al. Biomass/Biochar carbon materials for CO2 capture and sequestration by cyclic adsorption processes: A review and prospects for future directions[J]. Journal of CO2 Utilization, 2022, 57: 101890. |
| 8 | KUMAR Santosh, SRIVASTAVA Rohit, Joonseok KOH. Utilization of zeolites as CO2 capturing agents: Advances and future perspectives[J]. Journal of CO2 Utilization, 2020, 41: 101251. |
| 9 | 符乐, 杨阳, 徐文青, 等. 新型相变有机胺吸收捕集CO2技术研究进展[J/OL]. 化工进展, . |
| FU Le, YANG Yang, XU Wenqing, et al. Research progress in CO2 capture technology using novel biphasicorganic amine absorbent[J/OL]. Chemical Industry and Engineering Progress, . | |
| 10 | 耿一琪, 郭彦霞, 樊飙, 等. CaO基吸附剂捕集CO2及其抗烧结改性研究进展[J]. 燃料化学学报, 2021, 49(7): 998-1013. |
| GENG Yiqi, GUO Yanxia, FAN Biao, et al. Research progress of calcium-based adsorbents for CO2 capture and anti-sintering modification[J]. Journal of Fuel Chemistry and Technology, 2021, 49(7): 998-1013. | |
| 11 | ZHAO Chuanwen, CHEN Xiaoping, ANTHONY Edward J, et al. Capturing CO2 in flue gas from fossil fuel-fired power plants using dry regenerable alkali metal-based sorbent[J]. Progress in Energy and Combustion Science, 2013, 39(6): 515-534. |
| 12 | 鲁雪婷, 蒲彦锋, 李磊, 等. 氨基修饰的金属有机框架Cu3(BTC)2的制备及其CO2吸附性能研究[J]. 燃料化学学报, 2019, 47(3): 338-343. |
| LU Xueting, PU Yanfeng, LI Lei, et al. Preparation of metal-organic frameworks Cu3(BTC)2 with amino-functionalization for CO2 adsorption[J]. Journal of Fuel Chemistry and Technology, 2019, 47(3): 338-343. | |
| 13 | OZDEMIR John, MOSLEH Imann, ABOLHASSANI Mojtaba, et al. Covalent organic frameworks for the capture, fixation, or reduction of CO2 [J]. Frontiers in Energy Research, 2019, 7: 77. |
| 14 | ZHAO Chuanwen, GUO Yafei, LI Changhai, et al. Removal of low concentration CO2 at ambient temperature using several potassium-based sorbents[J]. Applied Energy, 2014, 124: 241-247. |
| 15 | LEE Soo Chool, CHOI Bo Yun, LEE Tae Jin, et al. CO2 absorption and regeneration of alkali metal-based solid sorbents[J]. Catalysis Today, 2006, 111(3/4): 385-390. |
| 16 | LEE Soo Chool, KWON Yong Mok, CHAE Ho Jin, et al. Improving regeneration properties of potassium-based alumina sorbents for carbon dioxide capture from flue gas[J]. Fuel, 2013, 104: 882-885. |
| 17 | LI Lei, ZHANG Bingsheng, WANG Feng, et al. Study of the novel KMgAl sorbents for CO2 capture[J]. Energy & Fuels, 2013, 27(9): 5388-5396. |
| 18 | LUO Hongchao, CHIOYAMA Hideyuki, Stephan THÜRMER, et al. Kinetics and structural changes in CO2 Capture of K2CO3 under a moist condition[J]. Energy & Fuels, 2015, 29(7): 4472-4478. |
| 19 | GUO Baihe, WANG Yanlin, GUO Jingnan, et al. Experiment and kinetic model study on modified potassium-based CO2 adsorbent[J]. Chemical Engineering Journal, 2020, 399: 125849. |
| 20 | WANG Peng, SUN Jian, GUO Yafei, et al. Structurally improved, urea-templated, K2CO3-based sorbent pellets for CO2 capture[J]. Chemical Engineering Journal, 2019, 374: 20-28. |
| 21 | YI Chang-Keun, Sung-Ho JO, SEO Yongwon, et al. Continuous operation of the potassium-based dry sorbent CO2 capture process with two fluidized-bed reactors[J]. International Journal of Greenhouse Gas Control, 2007, 1(1): 31-36. |
| 57 | SHIGEMOTO Naoya, YANAGIHARA Tetsu, SUGIYAMA Shigeru, et al. Material balance and energy consumption for CO2 recovery from moist flue gas employing K2CO3-on-activated carbon and its evaluation for practical adaptation[J]. Energy & Fuels, 2006, 20(2): 721-726. |
| 58 | ZHAO Chuanwen, GUO Yafei, LI Changhai, et al. Carbonation behavior of K2CO3/AC in low reaction temperature and CO2 concentration[J]. Chemical Engineering Journal, 2014, 254: 524-530. |
| [1] | ZHENG Qian, GUAN Xiushuai, JIN Shanbiao, ZHANG Changming, ZHANG Xiaochao. Photothermal catalysis synthesis of DMC from CO2 and methanol over Ce0.25Zr0.75O2 solid solution [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 319-327. |
| [2] | DAI Huantao, CAO Lingyu, YOU Xinxiu, XU Haoliang, WANG Tao, XIANG Wei, ZHANG Xueyang. Adsorption properties of CO2 on pomelo peel biochar impregnated by lignin [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 356-363. |
| [3] | CHEN Chongming, CHEN Qiu, GONG Yunqian, CHE Kai, YU Jinxing, SUN Nannan. Research progresses on zeolite-based CO2 adsorbents [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 411-419. |
| [4] | ZHANG Jie, WANG Fangfang, XIA Zhonglin, ZHAO Guangjin, MA Shuangchen. Current SF6 emission, emission reduction and future prospects under “carbon peaking and carbon neutrality” [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 447-460. |
| [5] | SUN Yuyu, CAI Xinlei, TANG Jihai, HUANG Jingjing, HUANG Yiping, LIU Jie. Optimization and energy-saving of a reactive distillation process for the synthesis of methyl methacrylate [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 56-63. |
| [6] | YANG Hanyue, KONG Lingzhen, CHEN Jiaqing, SUN Huan, SONG Jiakai, WANG Sicheng, KONG Biao. Decarbonization performance of downflow tubular gas-liquid contactor of microbubble-type [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 197-204. |
| [7] | WANG Shengyan, DENG Shuai, ZHAO Ruikai. Research progress on carbon dioxide capture technology based on electric swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 233-245. |
| [8] | YANG Ying, HOU Haojie, HUANG Rui, CUI Yu, WANG Bing, LIU Jian, BAO Weiren, CHANG Liping, WANG Jiancheng, HAN Lina. Coal tar phenol-based carbon nanosphere prepared by Stöber method for adsorption of CO2 [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5011-5018. |
| [9] | WANG Yaogang, HAN Zishan, GAO Jiachen, WANG Xinyu, LI Siqi, YANG Quanhong, WENG Zhe. Strategies for regulating product selectivity of copper-based catalysts in electrochemical CO2 reduction [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4043-4057. |
| [10] | LIU Yi, FANG Qiang, ZHONG Dazhong, ZHAO Qiang, LI Jinping. Cu facets regulation of Ag/Cu coupled catalysts for electrocatalytic reduction of carbon dioxide [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4136-4142. |
| [11] | HUANG Yufei, LI Ziyi, HUANG Yangqiang, JIN Bo, LUO Xiao, LIANG Zhiwu. Research progress on catalysts for photocatalytic CO2 and CH4 reforming [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4247-4263. |
| [12] | LOU Baohui, WU Xianhao, ZHANG Chi, CHEN Zhen, FENG Xiangdong. Advances in nanofluid for CO2 absorption and separation [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3802-3815. |
| [13] | BAI Yadi, DENG Shuai, ZHAO Ruikai, ZHAO Li, YANG Yingxia. Exploration on standardized test scheme and experimental performance of temperature swing adsorption carbon capture unit [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3834-3846. |
| [14] | GU Shiya, DONG Yachao, LIU Linlin, ZHANG Lei, ZHUANG Yu, DU Jian. Design and optimization of pipeline system for carbon capture considering intermediate nodes [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2799-2808. |
| [15] | ZHU Yajing, XU Yan, JIAN Meipeng, LI Haiyan, WANG Chongchen. Progress of metal-organic frameworks for uranium extraction from seawater [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3029-3048. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||