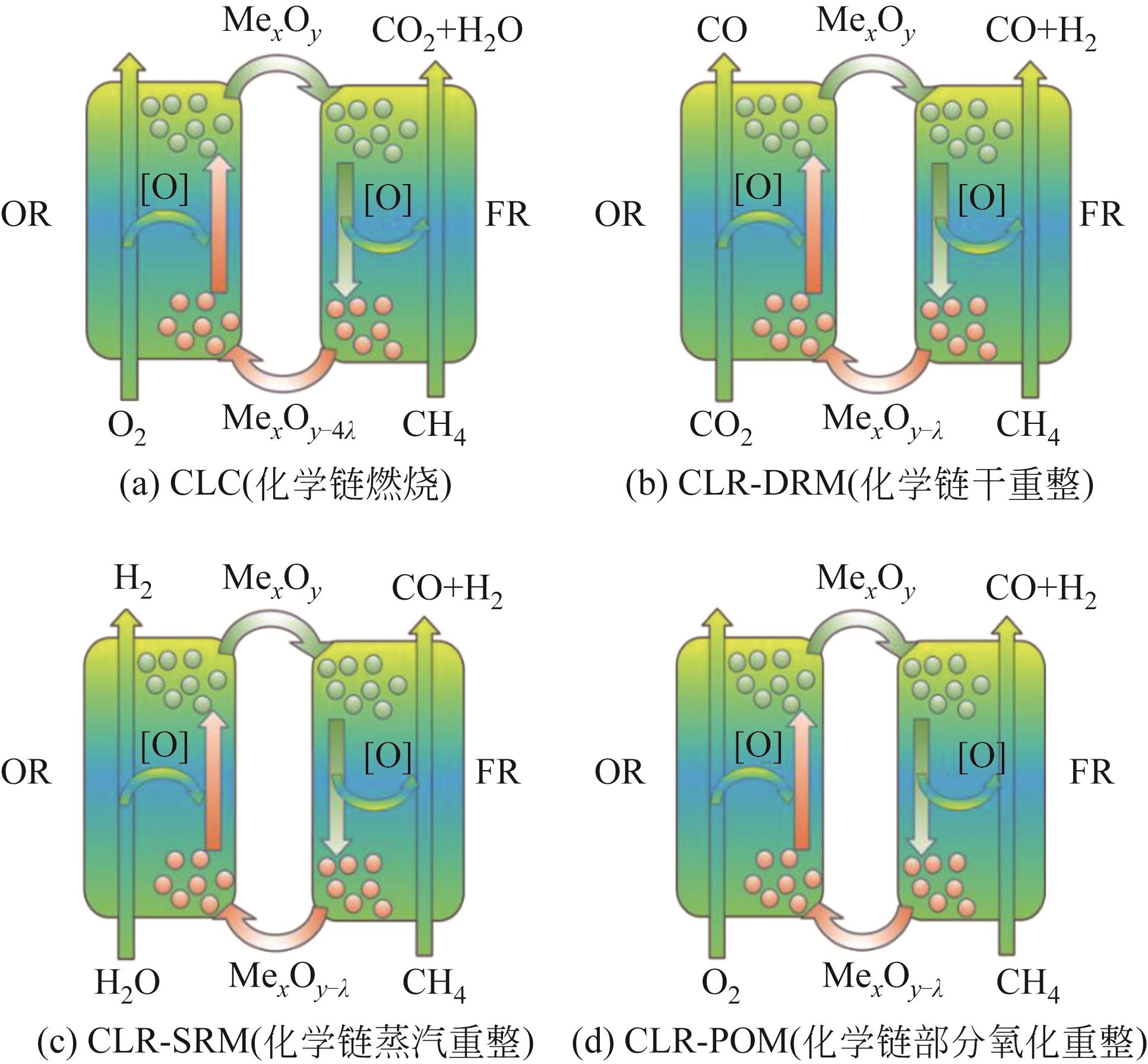
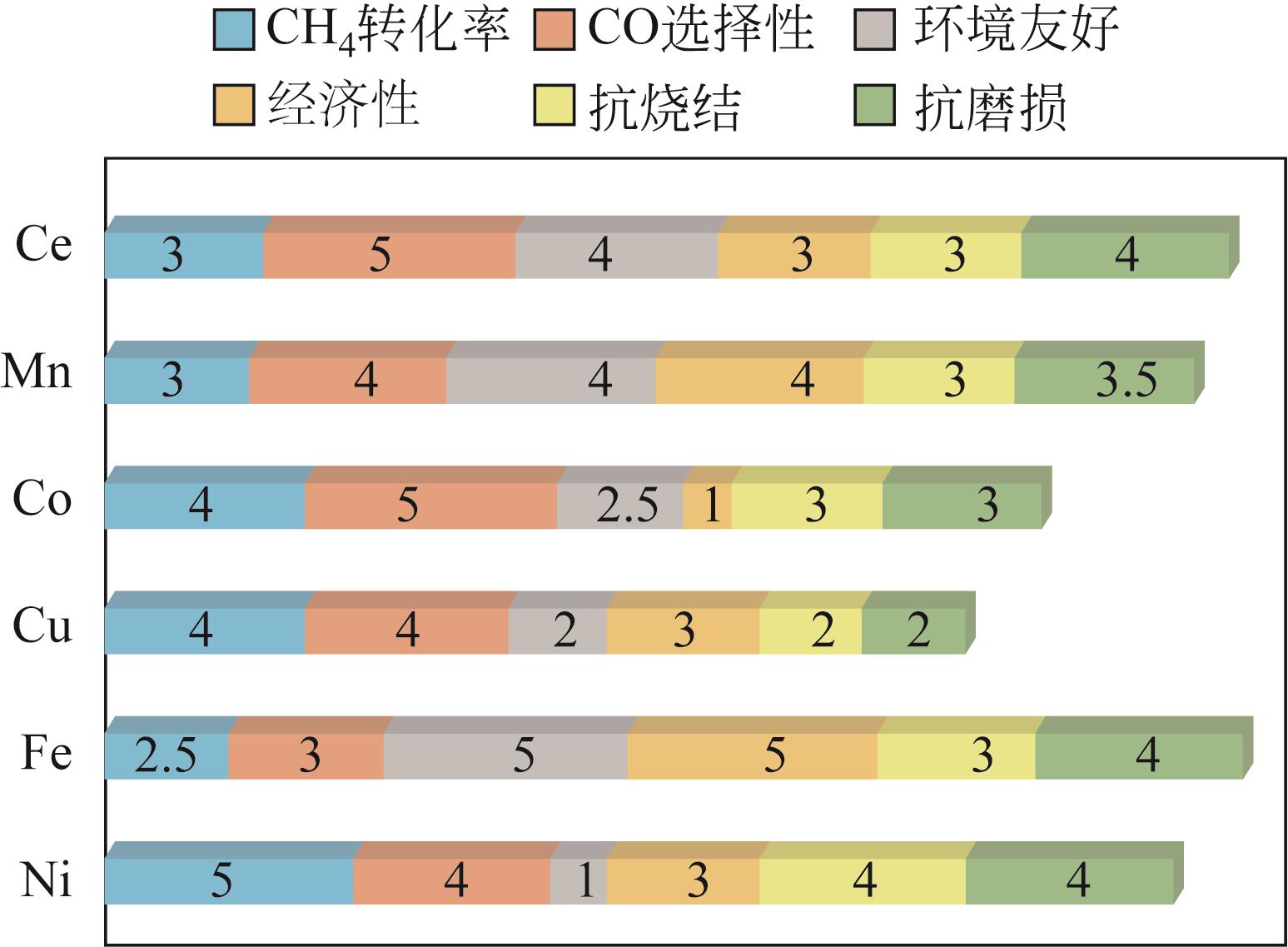
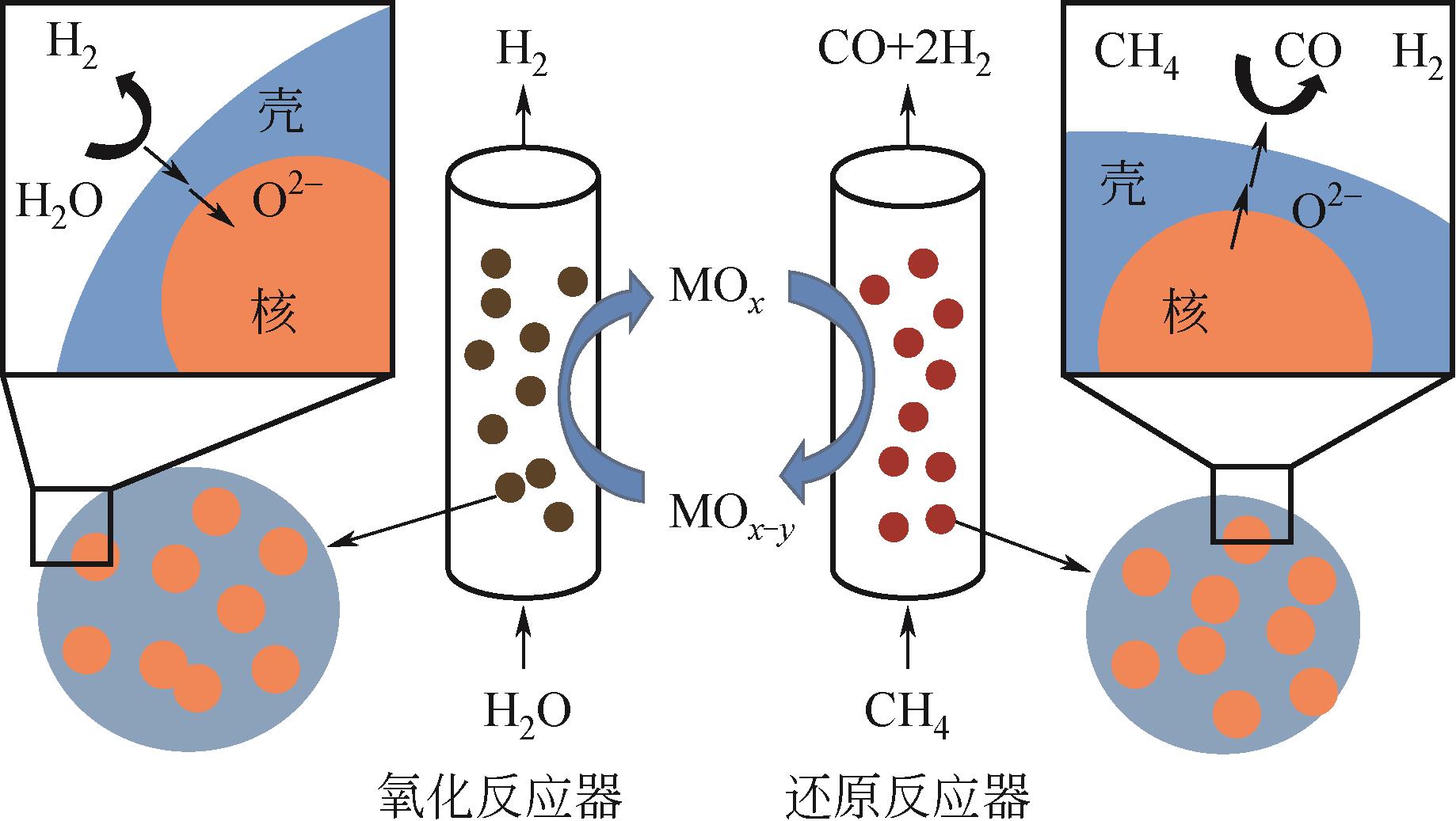
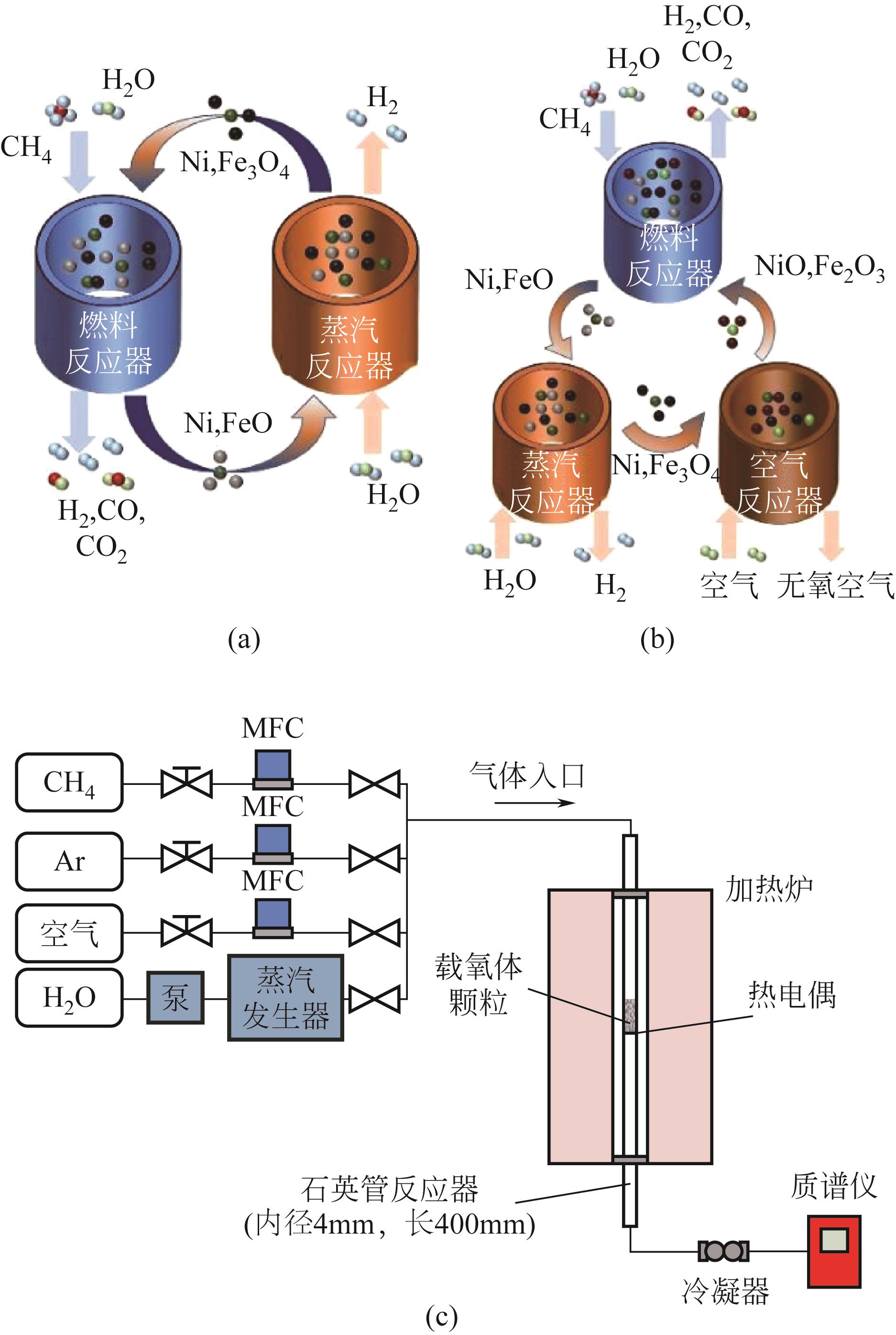
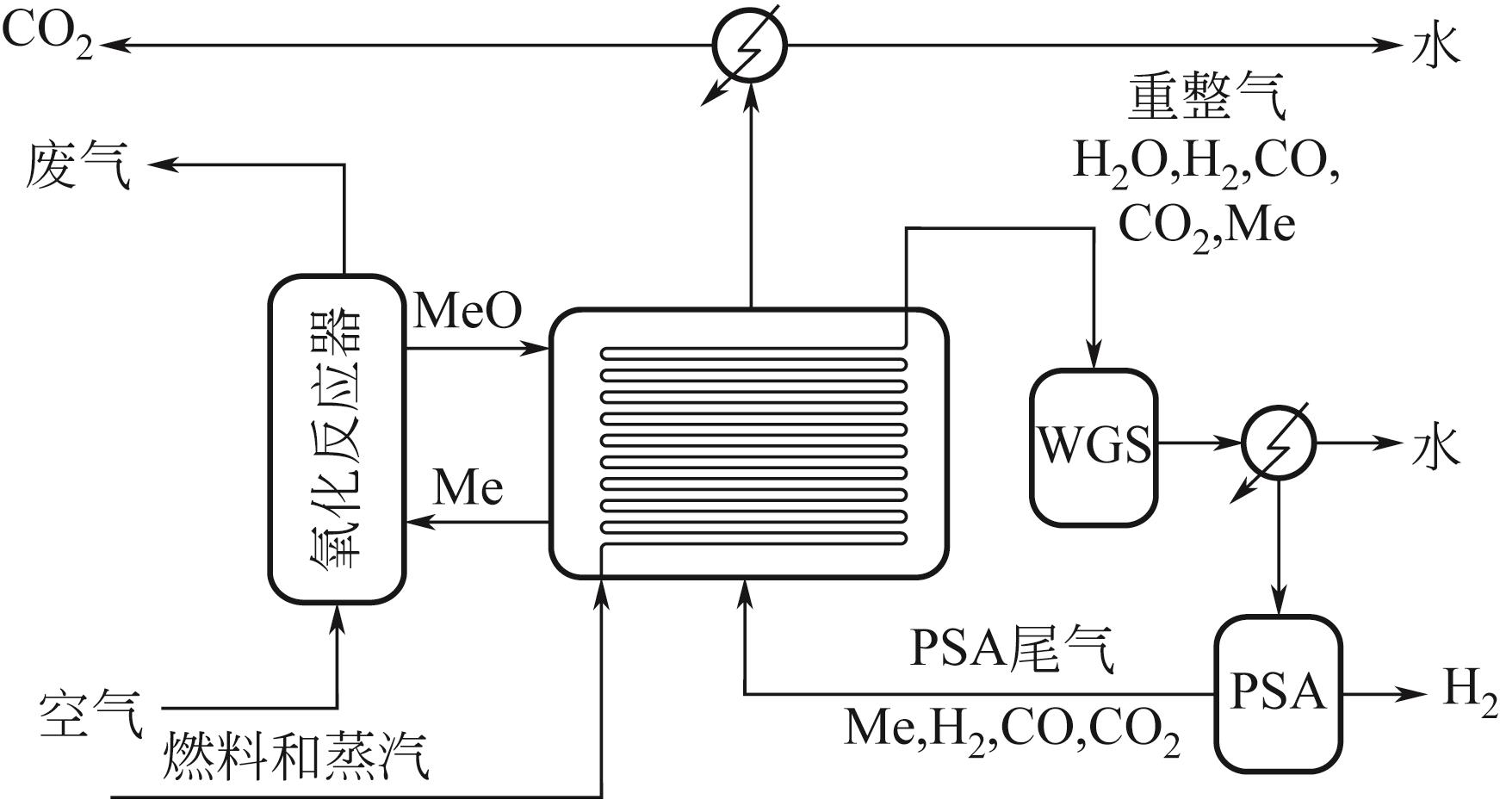
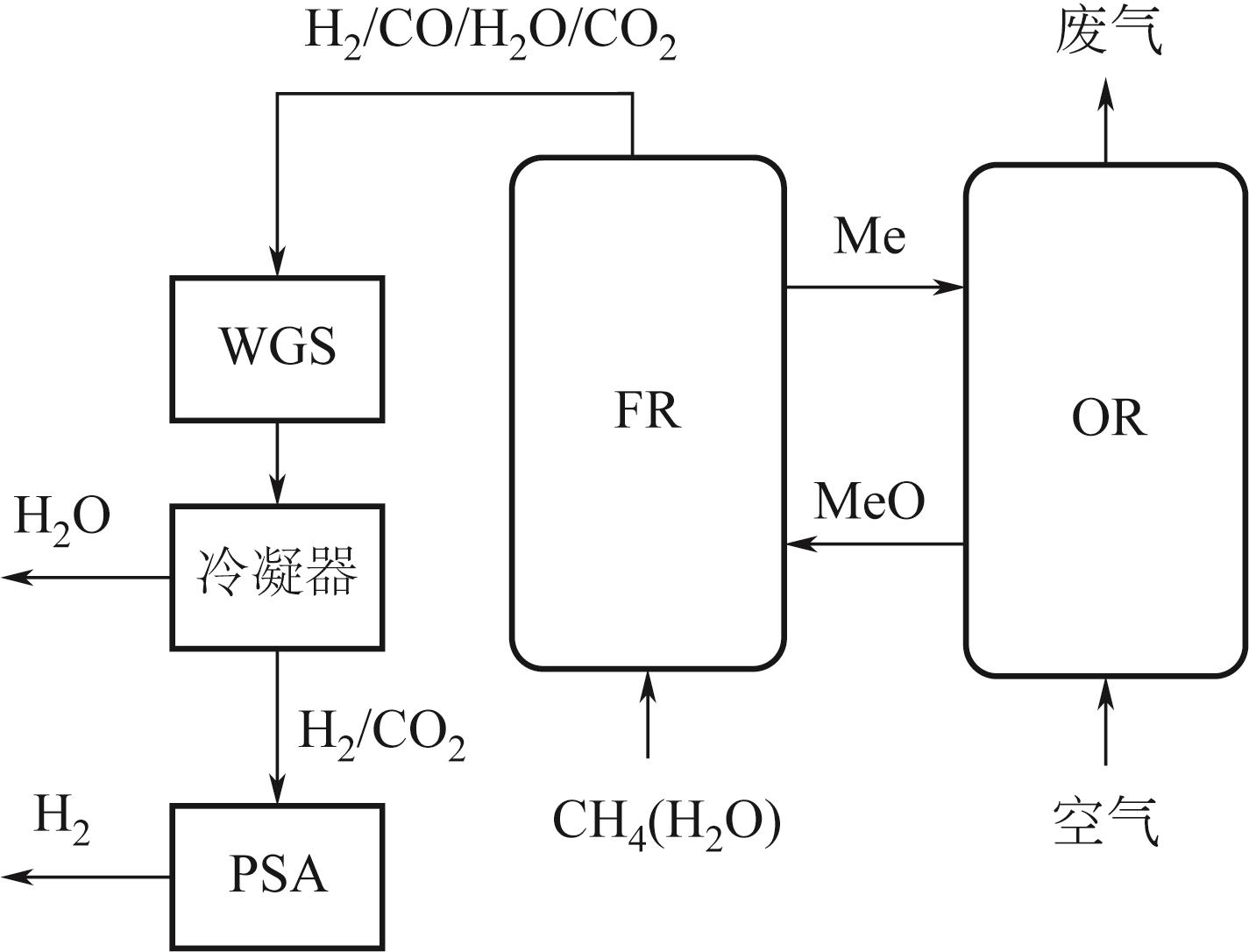
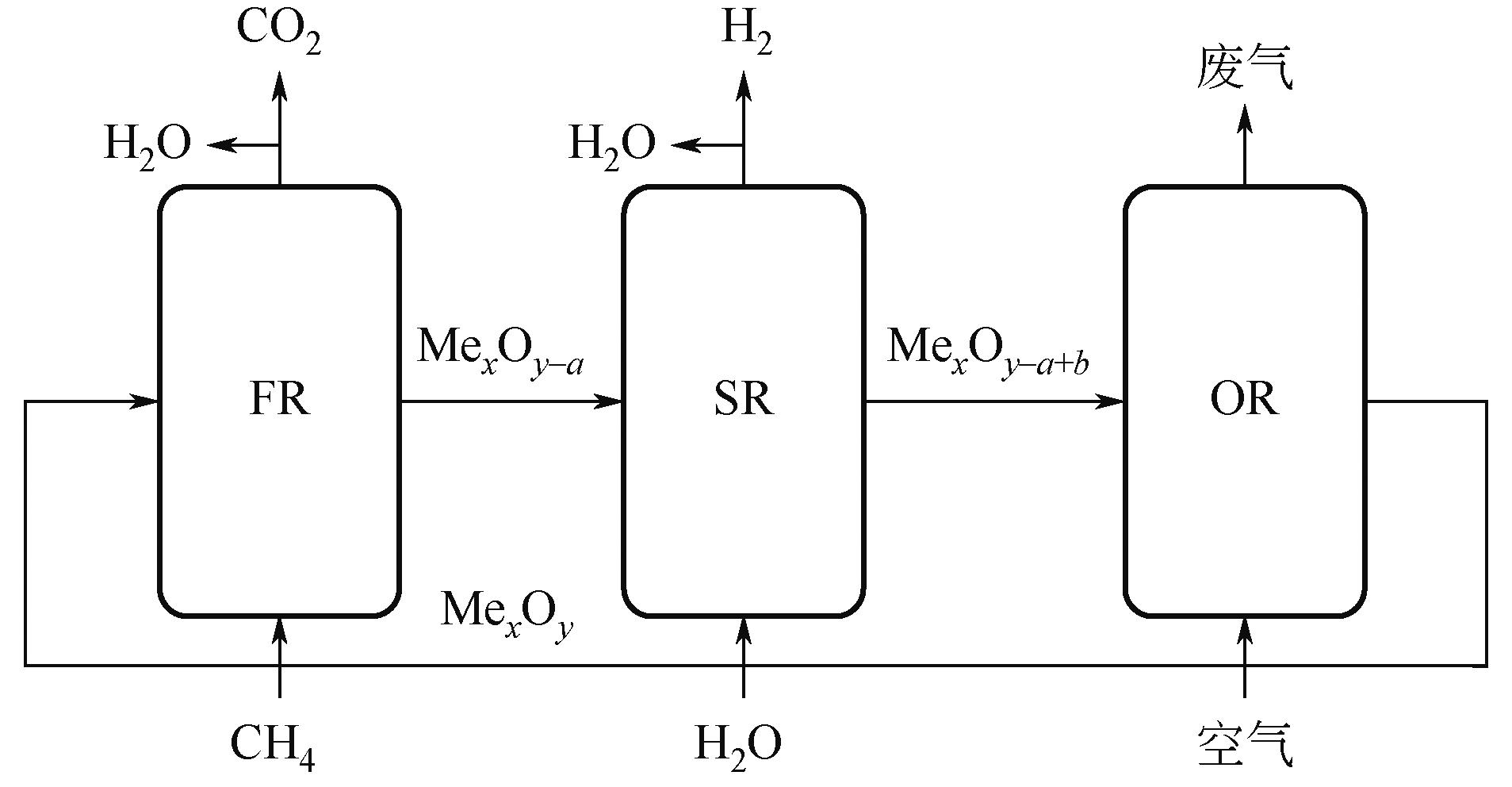
Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (5): 2235-2253.DOI: 10.16085/j.issn.1000-6613.2023-2104
• Clean and efficient utilization of fossil energy • Previous Articles
Research progress of oxygen carriers in chemical looping reforming reaction of methane
WANG Jiarui( ), LIU Dawei(
), LIU Dawei( ), DENG Yao, XU Jin, MA Xiaoxun, XU Long(
), DENG Yao, XU Jin, MA Xiaoxun, XU Long( )
)
- School of Chemical Engineering, Northwest University, International Science & Technology Cooperation Base of MOST for Clean Utilization of Hydrocarbon Resources, Chemical Engineering Research Center of the Ministry of Education for Advanced Use Technology of Shanbei Energy, Shaanxi Research Center of Engineering Technology for Clean Coal Conversion, Collaborative Innovation Center for Development of Energy and Chemical Industry in Northern Shaanxi, Xi’an 710127, Shaanxi, China
-
Received:2023-11-30Revised:2024-03-05Online:2024-06-15Published:2024-05-15 -
Contact:LIU Dawei, XU Long
载氧体在甲烷化学链重整反应中的研究进展
王嘉锐( ), 刘大伟(
), 刘大伟( ), 邓耀, 徐瑾, 马晓迅, 徐龙(
), 邓耀, 徐瑾, 马晓迅, 徐龙( )
)
- 西北大学化工学院,碳氢资源清洁利用国际科技合作基地,陕北能源先进化工利用技术教育部工程研究中心,陕西省洁净煤转化工程技术研究中心,陕北能源化工产业发展协同创新中心,陕西 西安 710127
-
通讯作者:刘大伟,徐龙 -
作者简介:王嘉锐(1999—),男,硕士研究生,研究方向为化学链重整。E-mail:18392596723@163.com。 -
基金资助:陕西省创新能力支撑计划(2024RS-CXTD-53);陕西省重点研发计划(2022QCY-LL-69);西安市科技计划(22GXFW0132);国家自然科学基金(22008197);咸阳市科技计划(2021ZDYF-NY-0017);榆林市科技计划(CXY-2021-129)
CLC Number:
Cite this article
WANG Jiarui, LIU Dawei, DENG Yao, XU Jin, MA Xiaoxun, XU Long. Research progress of oxygen carriers in chemical looping reforming reaction of methane[J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2235-2253.
王嘉锐, 刘大伟, 邓耀, 徐瑾, 马晓迅, 徐龙. 载氧体在甲烷化学链重整反应中的研究进展[J]. 化工进展, 2024, 43(5): 2235-2253.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-2104
| 金属 | 主要氧化物形式 | 氧转移能力排序 | 熔点/℃ |
|---|---|---|---|
| Ni | NiO | ― | NiO:1987;Ni:1453 |
| Fe | FeO、Fe2O3和Fe3O4 | FeO>Fe3O4>Fe2O3 | FeO:1370;Fe3O4:1565;Fe2O3:1594 |
| Cu | CuO和Cu2O | CuO>Cu2O | CuO:1446;Cu2O:1232 |
| Co | CoO和Co3O4 | Co3O4>CoO | Co3O4:895;CoO:1935 |
| Mn | MnO、Mn3O4、Mn2O3和MnO2 | MnO>MnO2>Mn3O4>Mn2O3 | MnO:1650;MnO2:535;Mn3O4:1567;Mn2O3:1080 |
| Ce | CeO2和Ce2O3 | CeO2>Ce2O3 | CeO2:1950;Ce2O3:2177 |
| 金属 | 主要氧化物形式 | 氧转移能力排序 | 熔点/℃ |
|---|---|---|---|
| Ni | NiO | ― | NiO:1987;Ni:1453 |
| Fe | FeO、Fe2O3和Fe3O4 | FeO>Fe3O4>Fe2O3 | FeO:1370;Fe3O4:1565;Fe2O3:1594 |
| Cu | CuO和Cu2O | CuO>Cu2O | CuO:1446;Cu2O:1232 |
| Co | CoO和Co3O4 | Co3O4>CoO | Co3O4:895;CoO:1935 |
| Mn | MnO、Mn3O4、Mn2O3和MnO2 | MnO>MnO2>Mn3O4>Mn2O3 | MnO:1650;MnO2:535;Mn3O4:1567;Mn2O3:1080 |
| Ce | CeO2和Ce2O3 | CeO2>Ce2O3 | CeO2:1950;Ce2O3:2177 |
| 载氧体 | 合成方法 | 实验条件 | 用途 | 参考文献 |
|---|---|---|---|---|
| La0.9Sr0.1FeO3/YSZ | 球磨法 | 反应温度850℃ | 甲烷化学链干重整,固定床反应器 | [ |
| Ce-Fe-Zr-O/MgO | 机械混合 | 反应温度800℃,原料气为5%(体积分数)CH4/N2 | 甲烷化学链蒸汽重整,固定床反应器 | [ |
| 5NiO-RM | 浸渍法 | 反应温度900℃,CO选择性可达94.1% | 甲烷化学链蒸汽重整,固定床反应器 | [ |
| Cu-Al2O3 | 浸渍法 | 反应温度950℃,CH4转化率为96% | 甲烷自热化学链重整,流化床反应器 | [ |
| CeZr0.5GdO4 | 溶胶凝胶法 | 反应温度800~850℃,CH4转化率和(H2+CO)的摩尔比的均值在90%左右 | 甲烷化学链部分氧化重整,固定床反应器 | [ |
| Mg0.1(Cu0.3Ni0.3Mn0.4)0.9Fe2O4 | 共沉淀法 | 反应温度500~750℃ | 甲烷化学链蒸汽重整,固定床反应器 | [ |
| Mg改性的Fe2O3/Al2O3 | 共沉淀法 | 反应温度900℃,在15次循环后,CO选择性和CH4转化率分别保持在96%和82% | 甲烷化学链蒸汽重整,流化床反应器 | [ |
| 核壳型Fe2O3/MgO | 水热沉淀法 | 反应温度800℃ | 甲烷化学链干重整,流化床反应器 | [ |
| Ce9Co1O δ -10CS | 溶液燃烧法 | 反应温度700℃ | 甲烷化学链干重整,固定床反应器 | [ |
| 载氧体 | 合成方法 | 实验条件 | 用途 | 参考文献 |
|---|---|---|---|---|
| La0.9Sr0.1FeO3/YSZ | 球磨法 | 反应温度850℃ | 甲烷化学链干重整,固定床反应器 | [ |
| Ce-Fe-Zr-O/MgO | 机械混合 | 反应温度800℃,原料气为5%(体积分数)CH4/N2 | 甲烷化学链蒸汽重整,固定床反应器 | [ |
| 5NiO-RM | 浸渍法 | 反应温度900℃,CO选择性可达94.1% | 甲烷化学链蒸汽重整,固定床反应器 | [ |
| Cu-Al2O3 | 浸渍法 | 反应温度950℃,CH4转化率为96% | 甲烷自热化学链重整,流化床反应器 | [ |
| CeZr0.5GdO4 | 溶胶凝胶法 | 反应温度800~850℃,CH4转化率和(H2+CO)的摩尔比的均值在90%左右 | 甲烷化学链部分氧化重整,固定床反应器 | [ |
| Mg0.1(Cu0.3Ni0.3Mn0.4)0.9Fe2O4 | 共沉淀法 | 反应温度500~750℃ | 甲烷化学链蒸汽重整,固定床反应器 | [ |
| Mg改性的Fe2O3/Al2O3 | 共沉淀法 | 反应温度900℃,在15次循环后,CO选择性和CH4转化率分别保持在96%和82% | 甲烷化学链蒸汽重整,流化床反应器 | [ |
| 核壳型Fe2O3/MgO | 水热沉淀法 | 反应温度800℃ | 甲烷化学链干重整,流化床反应器 | [ |
| Ce9Co1O δ -10CS | 溶液燃烧法 | 反应温度700℃ | 甲烷化学链干重整,固定床反应器 | [ |
| 合成方法 | 优点 | 缺点 | 参考文献 |
|---|---|---|---|
| 球磨法 | 简单的机械混合,适合大规模生产,过程简单易于控制,目标产物的产率高 | 物理方法的混合导致材料的均匀性较差,产物易出现团聚现象等 | [ |
| 共沉淀法 | 将原料配成混合溶液,加入氨水搅拌沉淀得到样品。该方法所需实验设备简单,载氧体颗粒小且活性高,容易在实验室制备得到 | 该方法的缺点在于组分之间容易出现偏析,造成分布不均 | [ |
| 水热沉淀法 | 将固体原料在水溶液中混合并在高压釜上加热,随后过滤分离高压釜得到的沉淀并干燥。该方法的组分间混合均匀,得到的样品颗粒小且活性好 | 步骤较为烦琐,需较多的热量输入 | [ |
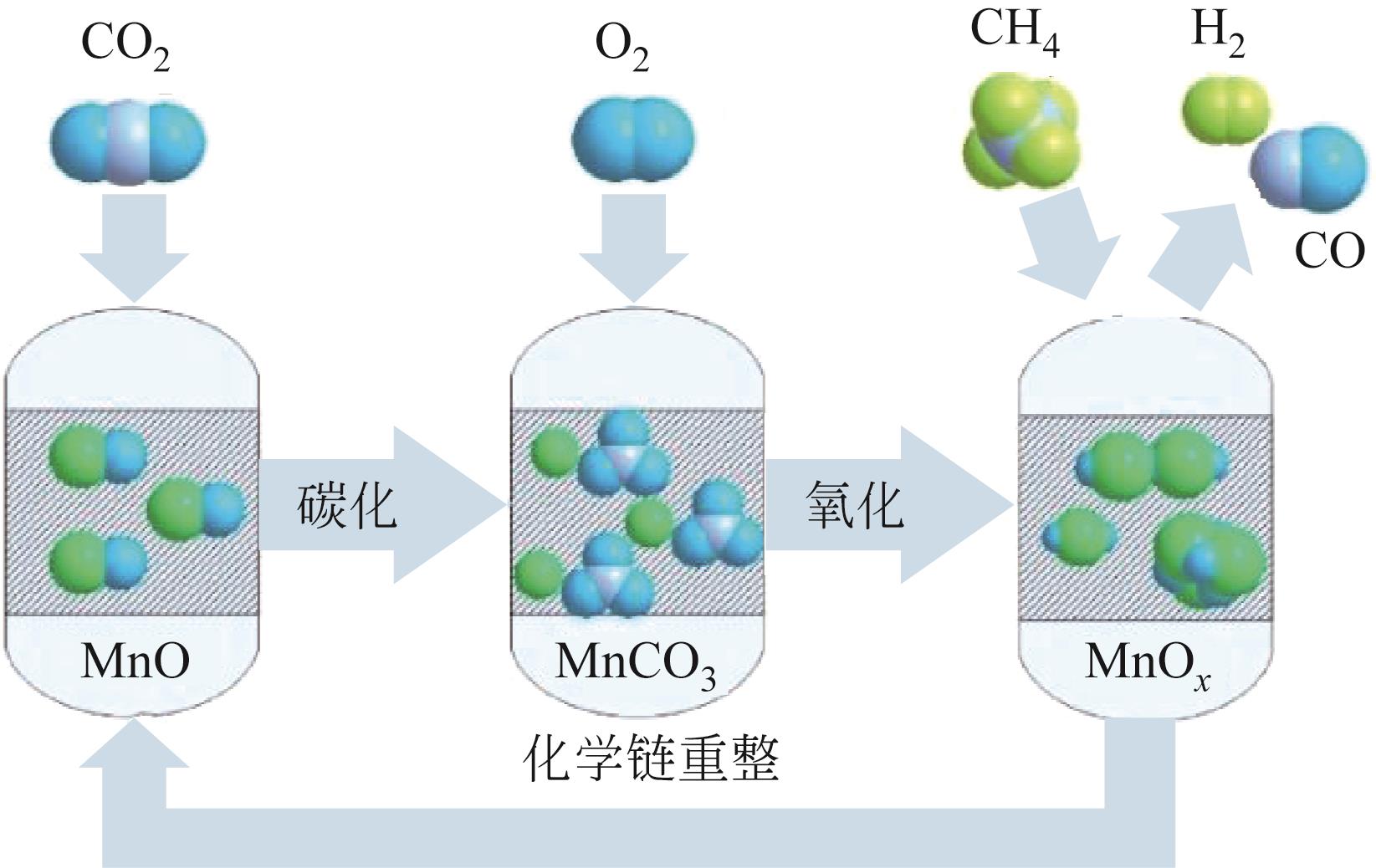
| 浸渍法 | 浸渍法常用于活性物质在载体上的负载过程。它的优点是制备工艺简单,操作方便 | 两相的直接混合过程导致其负载的活性组分的量较少且分布不均 | [ |
| 溶液燃烧法 | 通常在含有金属硝酸盐和燃料的水溶液中发生一系列自持续的还原-氧化反应,将初始反应溶液预热至150∼200℃,水分被蒸干且反应物被燃料迅速点燃,从而形成纳米级的粉末材料。它的优点是反应时间短,生成大量气态产物,抑制了颗粒的生长,有利于合成具有高比表面积的纳米晶体 | 缺乏对粉末形态的控制会产生粉末团聚等问题,此外不完全燃烧也会产生残留有机杂质(来源于燃料,如柠檬酸和甘氨酸等) | [ |
| 溶胶凝胶法 | 溶胶凝胶法需要将原料的水溶液加入胶凝剂中,经水解缩合形成溶胶,最后通过缩聚陈化反应得到凝胶。该方法成本低且操作简单,所得到的样品纯度高且热处理温度较低等 | 由于所用到的胶凝剂如金属醇盐等成本高且有毒害,难以大规模商业化应用 | [ |
| 合成方法 | 优点 | 缺点 | 参考文献 |
|---|---|---|---|
| 球磨法 | 简单的机械混合,适合大规模生产,过程简单易于控制,目标产物的产率高 | 物理方法的混合导致材料的均匀性较差,产物易出现团聚现象等 | [ |
| 共沉淀法 | 将原料配成混合溶液,加入氨水搅拌沉淀得到样品。该方法所需实验设备简单,载氧体颗粒小且活性高,容易在实验室制备得到 | 该方法的缺点在于组分之间容易出现偏析,造成分布不均 | [ |
| 水热沉淀法 | 将固体原料在水溶液中混合并在高压釜上加热,随后过滤分离高压釜得到的沉淀并干燥。该方法的组分间混合均匀,得到的样品颗粒小且活性好 | 步骤较为烦琐,需较多的热量输入 | [ |
| 浸渍法 | 浸渍法常用于活性物质在载体上的负载过程。它的优点是制备工艺简单,操作方便 | 两相的直接混合过程导致其负载的活性组分的量较少且分布不均 | [ |
| 溶液燃烧法 | 通常在含有金属硝酸盐和燃料的水溶液中发生一系列自持续的还原-氧化反应,将初始反应溶液预热至150∼200℃,水分被蒸干且反应物被燃料迅速点燃,从而形成纳米级的粉末材料。它的优点是反应时间短,生成大量气态产物,抑制了颗粒的生长,有利于合成具有高比表面积的纳米晶体 | 缺乏对粉末形态的控制会产生粉末团聚等问题,此外不完全燃烧也会产生残留有机杂质(来源于燃料,如柠檬酸和甘氨酸等) | [ |
| 溶胶凝胶法 | 溶胶凝胶法需要将原料的水溶液加入胶凝剂中,经水解缩合形成溶胶,最后通过缩聚陈化反应得到凝胶。该方法成本低且操作简单,所得到的样品纯度高且热处理温度较低等 | 由于所用到的胶凝剂如金属醇盐等成本高且有毒害,难以大规模商业化应用 | [ |
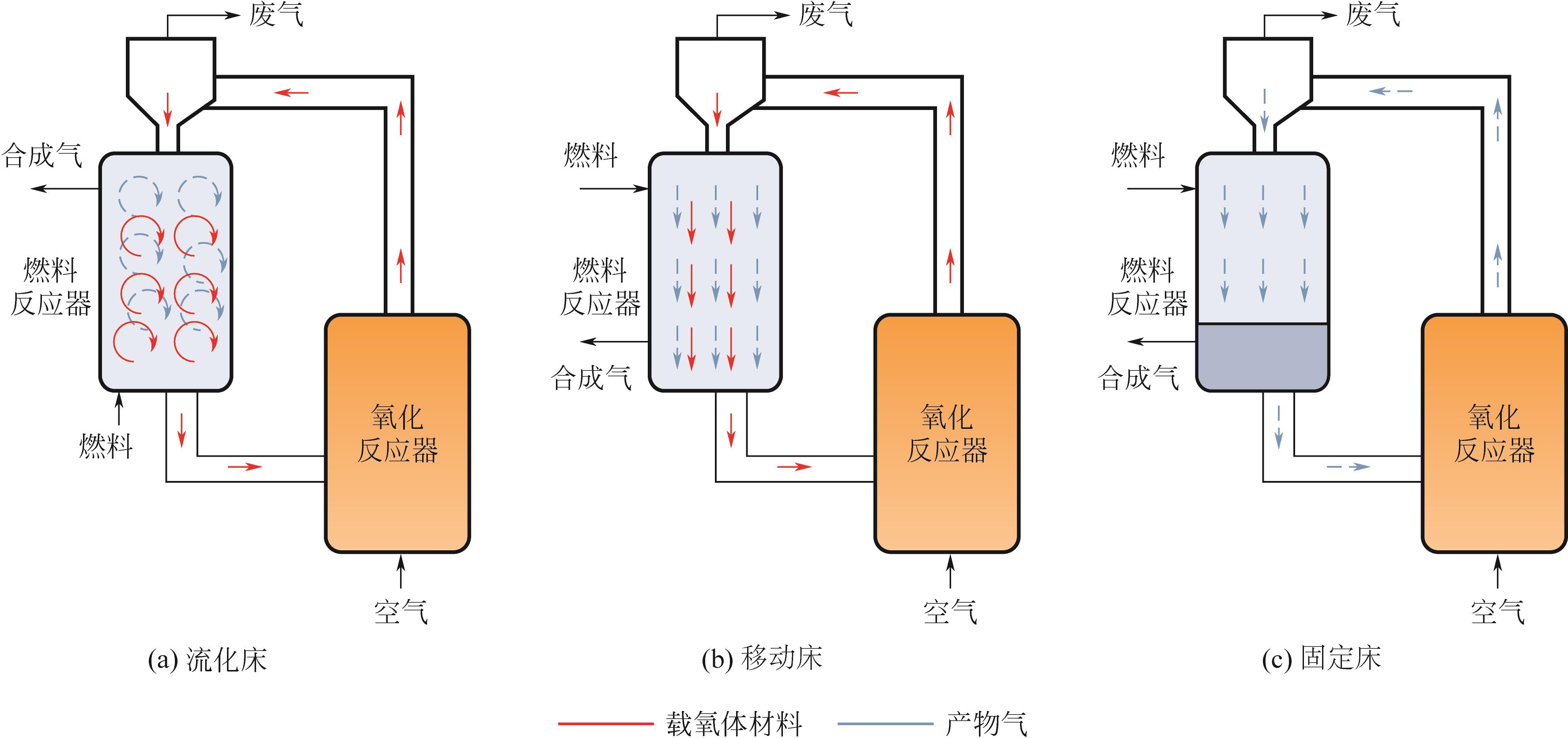
| 反应器类型 | 气固接触模式 | 优点 | 缺点 |
|---|---|---|---|
| 流化床 | 固体颗粒在气体中悬浮 | 连续的气固流动,传热和传质效率高,热点形成和固体团聚的风险低 | 颗粒磨损率高,流态化的高气速带来能量消耗和损失,且需要热量和反应条件的综合考虑进行设计 |
| 移动床 | 将固体从顶部连续加入床层,积累后在底部卸出,进行循环使用 | 连续的气固流动,可调节的固体停留时间,可实现的反应物的热力学转化极限 | 由于固体流动、固体团聚、床层温度变化等因素,需要限制气速以匹配固体流动的速率 |
| 固定床 | 直接填充床层的模式,固体静止不动和气体反应 | 易于生产、操作和放大,运营成本低,颗粒磨损率低 | 传质和传热存在限制、床层易出现热点导致固体团聚,固体材料再生困难 |
| 反应器类型 | 气固接触模式 | 优点 | 缺点 |
|---|---|---|---|
| 流化床 | 固体颗粒在气体中悬浮 | 连续的气固流动,传热和传质效率高,热点形成和固体团聚的风险低 | 颗粒磨损率高,流态化的高气速带来能量消耗和损失,且需要热量和反应条件的综合考虑进行设计 |
| 移动床 | 将固体从顶部连续加入床层,积累后在底部卸出,进行循环使用 | 连续的气固流动,可调节的固体停留时间,可实现的反应物的热力学转化极限 | 由于固体流动、固体团聚、床层温度变化等因素,需要限制气速以匹配固体流动的速率 |
| 固定床 | 直接填充床层的模式,固体静止不动和气体反应 | 易于生产、操作和放大,运营成本低,颗粒磨损率低 | 传质和传热存在限制、床层易出现热点导致固体团聚,固体材料再生困难 |
| 1 | SUN Zhenkun, LU Dennis Y, SYMONDS Robert T, et al. Chemical looping reforming of CH4 in the presence of CO2 using ilmenite ore and NiO-modified ilmenite ore oxygen carriers[J]. Chemical Engineering Journal, 2020, 401: 123481. |
| 2 | SHAH Vedant, CHENG Zhuo, BASER Deven S, et al. Highly selective production of syngas from chemical looping reforming of methane with CO2 utilization on MgO-supported calcium ferrite redox materials[J]. Applied Energy, 2021, 282: 116111. |
| 3 | 苏小平, 王力, 杨武, 等. 甲烷化学链重整制合成气的研究进展[J]. 地下水, 2017, 39(4): 198-202. |
| SU Xiaoping, WANG Li, YANG Wu, et al. Research progress of methane chemical chain reforming to synthesis gas[J]. Ground Water, 2017, 39(4): 198-202. | |
| 4 | HE Jiameng, YANG Qian, SONG Zhengping, et al. Improving the carbon resistance of iron-based oxygen carrier for hydrogen production via chemical looping steam methane reforming: A review[J]. Fuel, 2023, 351: 128864. |
| 5 | 吴兴亮, 吕凌辉, 马清祥, 等. 甲烷二氧化碳重整镍基催化剂的研究进展[J]. 洁净煤技术, 2021, 27(3): 129-137. |
| WU Xingliang, Linghui LYU, MA Qingxiang, et al. Research progress of nickel-based catalysts for carbon dioxide reforming of methane[J]. Clean Coal Technology, 2021, 27(3): 129-137. | |
| 6 | LI Danyang, XU Ruidong, GU Zhenhua, et al. Chemical-looping conversion of methane: A review[J]. Energy Technology, 2020, 8(8): 1900925. |
| 7 | 胡弼文, 刘庆, 蔡雨阳, 等. 非常规天然气相关理论技术及前景[C] // 第33届全国天然气学术年会, 南宁, 2023: 778-787. |
| Hu B, Liu Q, Cai Y, et al. Theory, technology and prospect of unconventional natural gas [C] // The 33rd National Natural Gas Academic Annual Conference, Nanning, 2023: 778-787. | |
| 8 | LI Di, ROHANI Vandad, FABRY Frédéric, et al. Direct conversion of CO2 and CH4 into liquid chemicals by plasma-catalysis[J]. Applied Catalysis B: Environmental, 2020, 261: 118228. |
| 9 | ZHU Zengzan, GUO Wenyi, ZHANG Ying, et al. Research progress on methane conversion coupling photocatalysis and thermocatalysis[J]. Carbon Energy, 2021, 3(4): 519-540. |
| 10 | SASTRE Daniel, SERRANO David P, PIZARRO Patricia, et al. Chemical insights on the activity of La1- x Sr x FeO3 perovskites for chemical looping reforming of methane coupled with CO2-splitting[J]. Journal of CO2 Utilization, 2019, 31: 16-26. |
| 11 | ABDULLAH Bawadi, GHANI Nur Azeanni ABD, Dai-Viet N VO. Recent advances in dry reforming of methane over Ni-based catalysts[J]. Journal of Cleaner Production, 2017, 162: 170-185. |
| 12 | LIU Changjun, YE Jingyun, JIANG Jiaojun, et al. Progresses in the preparation of coke resistant Ni-based catalyst for steam and CO2 reforming of methane[J]. ChemCatChem, 2011, 3(3): 529-541. |
| 13 | HUA Xiuning, WANG Wei. Chemical looping combustion: A new low-dioxin energy conversion technology[J]. Journal of Environmental Sciences (China), 2015, 32: 135-145. |
| 14 | Sanaz DANESHMAND-JAHROMI, SEDGHKERDAR Mohammad Hashem, MAHINPEY Nader. A review of chemical looping combustion technology: Fundamentals, and development of natural, industrial waste, and synthetic oxygen carriers[J]. Fuel, 2023, 341: 127626. |
| 15 | JACOBSON Mark Z. Review of solutions to global warming, air pollution, and energy security[J]. Energy & Environmental Science, 2009, 2(2): 148-173. |
| 16 | CEBRUCEAN Dumitru, CEBRUCEAN Viorica, IONEL Ioana. CO2 capture and storage from fossil fuel power plants[J]. Energy Procedia, 2014, 63: 18-26. |
| 17 | BUELENS Lukas C, GALVITA Vladimir V, POELMAN Hilde, et al. Super-dry reforming of methane intensifies CO2 utilization via Le Chatelier’s principle[J]. Science, 2016, 354(6311): 449-452. |
| 18 | ZHU Lin, HE Yangdong, LI Luling, et al. Tech-economic assessment of second-generation CCS: Chemical looping combustion[J]. Energy, 2018, 144: 915-927. |
| 19 | ADANEZ Juan, ABAD Alberto, Francisco GARCIA-LABIANO, et al. Progress in chemical-looping combustion and reforming technologies[J]. Progress in Energy and Combustion Science, 2012, 38(2): 215-282. |
| 20 | HU Jiawei, GALVITA Vladimir, POELMAN Hilde, et al. Advanced chemical looping materials for CO2 utilization: A review[J]. Materials, 2018, 11(7): 1187. |
| 21 | BHAVSAR Saurabh, NAJERA Michelle, SOLUNKE Rahul, et al. Chemical looping: To combustion and beyond[J]. Catalysis Today, 2014, 228: 96-105. |
| 22 | DENG Guixian, LI Kongzhai, ZHANG Guifang, et al. Enhanced performance of red mud-based oxygen carriers by CuO for chemical looping combustion of methane[J]. Applied Energy, 2019, 253: 113534. |
| 23 | SIRIWARDANE Ranjani, TIAN Hanjing, SIMONYI Thomas, et al. Synergetic effects of mixed copper-iron oxides oxygen carriers in chemical looping combustion[J]. Fuel, 2013, 108: 319-333. |
| 24 | GALVITA Vladimir V, POELMAN Hilde, DETAVERNIER Christophe, et al. Catalyst-assisted chemical looping for CO2 conversion to CO[J]. Applied Catalysis B: Environmental, 2015, 164: 184-191. |
| 25 | MOGHTADERI Behdad. Review of the recent chemical looping process developments for novel energy and fuel applications[J]. Energy & Fuels, 2012, 26(1): 15-40. |
| 26 | PLOU J, DURÁN P, HERGUIDO J, et al. Purified hydrogen from synthetic biogas by joint methane dry reforming and steam-iron process: Behaviour of metallic oxides and coke formation[J]. Fuel, 2014, 118: 100-106. |
| 27 | ZHU Xing, LI Kongzhai, WEI Yonggang, et al. Chemical-looping steam methane reforming over a CeO2-Fe2O3 oxygen carrier: Evolution of its structure and reducibility[J]. Energy & Fuels, 2014, 28(2): 754-760. |
| 28 | HOLLADAY J D, HU J, KING D L, et al. An overview of hydrogen production technologies[J]. Catalysis Today, 2009, 139(4): 244-260. |
| 29 | GALVITA Vladimir, SUNDMACHER Kai. Hydrogen production from methane by steam reforming in a periodically operated two-layer catalytic reactor[J]. Applied Catalysis A: General, 2005, 289(2): 121-127. |
| 30 | HE Fang, WEI Yonggang, LI Haibin, et al. Synthesis gas generation by chemical-looping reforming using Ce-based oxygen carriers modified with Fe, Cu, and Mn oxides[J]. Energy & Fuels, 2009, 23(4): 2095-2102. |
| 31 | LI Danyang, LI Kongzhai, XU Ruidong, et al. Enhanced CH4 and CO oxidation over Ce1– x Fe x O2– δ hybrid catalysts by tuning the lattice distortion and the state of surface iron species[J]. ACS Applied Materials & Interfaces, 2019, 11(21): 19227-19241. |
| 32 | DE VOS Yoran, JACOBS Marijke, VAN DER VOORT Pascal, et al. Development of stable oxygen carrier materials for chemical looping processes—A review[J]. Catalysts, 2020, 10(8): 926. |
| 33 | WENZEL Marcus, DHARANIPRAGADA NVR Aditya, GALVITA Vladimir V, et al. CO production from CO2 via reverse water-gas shift reaction performed in a chemical looping mode: Kinetics on modified iron oxide[J]. Journal of CO2 Utilization, 2016, 17: 60-68. |
| 34 | SONG Tao, SHEN Laihong. Review of reactor for chemical looping combustion of solid fuels[J]. International Journal of Greenhouse Gas Control, 2018, 76: 92-110. |
| 35 | HOSSAIN Mohammad M, DE LASA Hugo I. Chemical-looping combustion (CLC) for inherent CO2 separations—A review[J]. Chemical Engineering Science, 2008, 63(18): 4433-4451. |
| 36 | FAN Liang-Shih, ZENG Liang, WANG William, et al. Chemical looping processes for CO2 capture and carbonaceous fuel conversion—Prospect and opportunity[J]. Energy & Environmental Science, 2012, 5(6): 7254-7280. |
| 37 | ZHOU Zhihao, SUN Zhenkun, DUAN L. Chemical looping: A flexible platform technology for CH4 conversion coupled with CO2 utilization[J]. Current Opinion in Green and Sustainable Chemistry, 2023, 39: 100721. |
| 38 | HU Jiawei, GALVITA V, POELMAN H, et al. Pressure-induced deactivation of core-shell nanomaterials for catalyst-assisted chemical looping[J]. Applied Catalysis B: Environmental, 2019, 247: 86-99. |
| 39 | HAFIZI A, RAHIMPOUR M R, HASSANAJILI S. Calcium promoted Fe/Al2O3 oxygen carrier for hydrogen production via cyclic chemical looping steam methane reforming process[J]. International Journal of Hydrogen Energy, 2015, 40(46): 16159-16168. |
| 40 | HUANG Weichen, KUO Yulin, SU Yuming, et al. A facile method for sodium-modified Fe2O3/Al2O3 oxygen carrier by an air atmospheric pressure plasma jet for chemical looping combustion process[J]. Chemical Engineering Journal, 2017, 316: 15-23. |
| 41 | YIN Xianglei, WANG Shen, SUN Rong, et al. A Ce-Fe oxygen carrier with a core-shell structure for chemical looping steam methane reforming[J]. Industrial & Engineering Chemistry Research, 2020, 59: 9775-9786. |
| 42 | Zhou Z, Li L, Liu X, et al. Accelerated syngas generation from chemical looping CH4 reforming by using reduced ilmenite ore as catalyst[J]. Fuel Processing Technology, 2022, 232: 107270. |
| 43 | HAFIZI A, RAHIMPOUR M R, HASSANAJILI S. Hydrogen production via chemical looping steam methane reforming process: Effect of cerium and calcium promoters on the performance of Fe2O3/Al2O3 oxygen carrier[J]. Applied Energy, 2016, 165: 685-694. |
| 44 | YUAN Kai, WANG Yuhao, LI Kongzhai, et al. LaFe0.8Co0.15Cu0.05O3 supported on silicalite-1 as a durable oxygen carrier for chemical looping reforming of CH4 coupled with CO2 reduction[J]. ACS Applied Materials & Interfaces, 2022, 14(34): 39004-39013. |
| 45 | ZHU Yanyan, SUN Xueyan, Liu Weiwei, et al. Microstructure and reactivity evolution of LaFeAl oxygen carrier for syngas production via chemical looping CH4/CO2 reforming[J]. International Journal of Hydrogen Energy, 2017, 42(52): 30509-30524. |
| 46 | ZHAO Xiao, ZHOU Hui, SIKARWAR Vineet Singh, et al. Biomass-based chemical looping technologies: The good, the bad and the future[J]. Energy & Environmental Science, 2017, 10(9): 1885-1910. |
| 47 | AHMAD SALAM Farooqi, MOHAMMAD Yusuf, ASMAWATI Mohd Zabidi Noor, et al. A comprehensive review on improving the production of rich-hydrogen via combined steam and CO2 reforming of methane over Ni-based catalysts[J]. International Journal of Hydrogen Energy, 2021, 46(60): 31024-31040. |
| 48 | Hou K, Hughes R. The kinetics of methane steam reforming over a Ni/α-Al2O catalyst[J]. Chemical Engineering Journal, 2001, 82(1): 311-328. |
| 49 | KAWI Sibudjing, KATHIRASER Yasotha, NI Jun, et al. Progress in synthesis of highly active and stable nickel-based catalysts for carbon dioxide reforming of methane[J]. ChemSusChem, 2015, 8(21): 3556-3575. |
| 50 | GAO Xingyuan, LI Jinyu, ZHENG Mudi, et al. Recent progress in anti-coking Ni catalysts for thermo-catalytic conversion of greenhouse gases[J]. Process Safety and Environmental Protection, 2021, 156: 598-616. |
| 51 | GAYÁN P, DE DIEGO L F, GARCÍA-LABIANO F, et al. Effect of support on reactivity and selectivity of Ni-based oxygen carriers for chemical-looping combustion[J]. Fuel, 2008, 87(12): 2641-2650. |
| 52 | Ma S, Cheng F, Meng J, et al. Ni-enhanced red mud oxygen carrier for chemical looping steam methane reforming[J]. Fuel Processing Technology, 2022, 230: 107204. |
| 53 | MESHKSAR M, DANESHMAND-JAHROMI S, RAHIMPOUR M R. Synthesis and characterization of cerium promoted Ni/SBA-16 oxygen carrier in cyclic chemical looping steam methane reforming[J]. Journal of the Taiwan Institute of Chemical Engineers, 2017, 76: 73-82. |
| 54 | FENG Y, WANG N, GUO X. Influence mechanism of supports on the reactivity of Ni-based oxygen carriers for chemical looping reforming: A DFT study[J]. Fuel, 2018, 229: 88-94. |
| 55 | LIU Rui, ZHANG Xianhua, LIU Tao, et al. Dynamic oxygen migration and reaction over ceria-supported nickel oxides in chemical looping partial oxidation of methane[J]. Applied Catalysis B: Environmental, 2023, 328: 122478. |
| 56 | 孙艳茹. 铁基载氧体颗粒结构设计及其化学链甲烷干重整性能研究[D]. 北京: 中国科学院大学(中国科学院过程工程研究所), 2021. |
| SUN Yanru. Particle structure design of iron-based oxygen carrier toward to chemical looping dry reforming[D].Beijing: Institute of Process Engineering, Chinese Academy of Sciences, 2021. | |
| 57 | GUAN Yu, LIU Yinhe, LIN Xiaolong, et al. Research progress and perspectives of solid fuels chemical looping reaction with Fe-based oxygen carriers[J]. Energy & Fuels, 2022, 36(23): 13956-13984. |
| 58 | MORE Amey, BHAVSAR Saurabh, VESER Prof Götz. Iron-nickel alloys for carbon dioxide activation by chemical looping dry reforming of methane[J]. Energy Technology, 2016, 4(10): 1147-1157. |
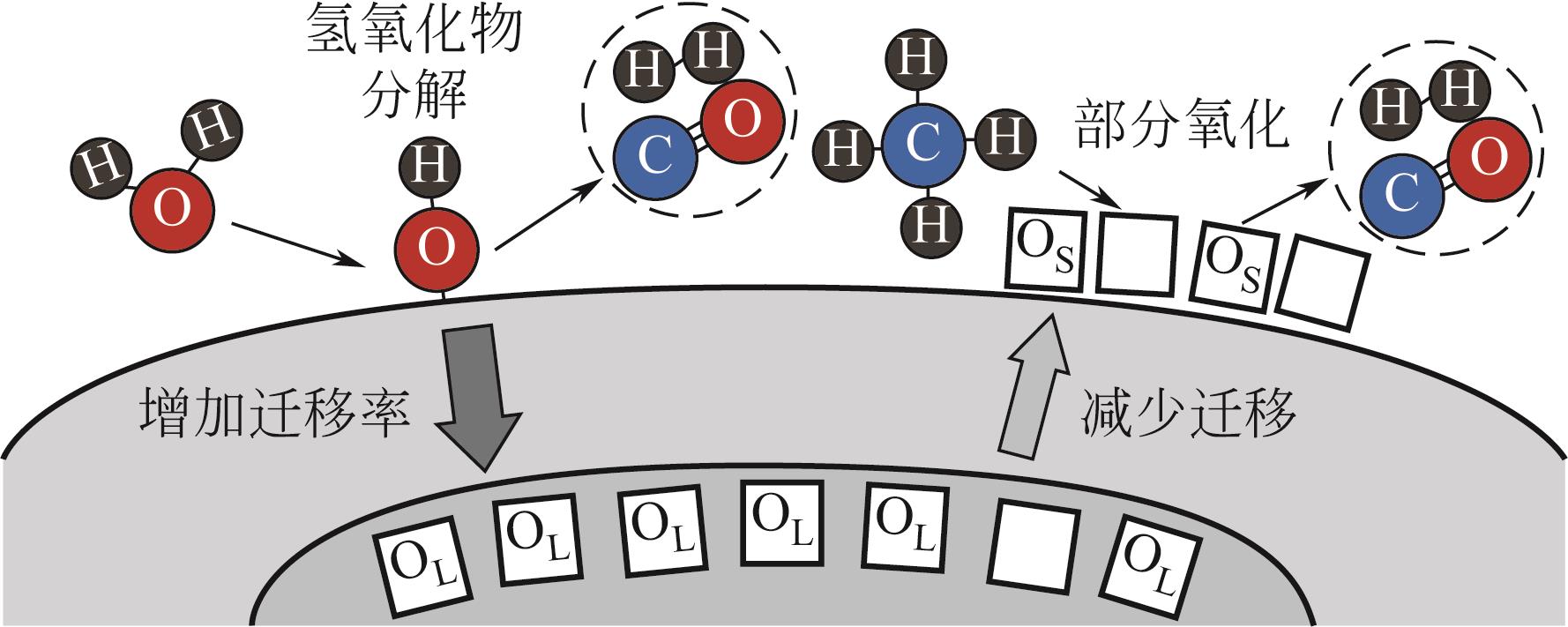
| 59 | ZHANG Huixin, YU Xiyang, SU Xue, et al. Dopant-Enhanced harmonization of α-Fe2O3 oxygen migration and surface catalytic reactions during chemical looping reforming of methane[J]. Chemical Engineering Journal, 2024, 481: 148446. |
| 60 | DE VOS Yoran, JACOBS Marijke, VAN DRIESSCHE Isabel, et al. Processing and characterization of Fe-based oxygen carriers for chemical looping for hydrogen production[J]. International Journal of Greenhouse Gas Control, 2018, 70: 12-21. |
| 61 | LI Yang, LIU Mingkai, ZHANG Jinrui, et al. Mid-temperature chemical looping methane reforming for hydrogen production via iron-based oxygen carrier particles[J]. Fuel Processing Technology, 2024, 253: 108026. |
| 62 | 王志美. 制备参数对铜基载氧体性能影响的实验研究[D]. 沈阳: 东北大学, 2013. |
| WANG Zhimei. The experiment study of synthesis parameters on the Cu-based oxygen carriers[D].Shenyang: Northeastern University, 2013. | |
| 63 | NEAL Luke, SHAFIEFARHOOD Arya, LI Fanxing. Effect of core and shell compositions on MeO x @La y Sr1- y FeO3 core-shell redox catalysts for chemical looping reforming of methane[J]. Applied Energy, 2015, 157: 391-398. |
| 64 | CABELLO A, MENDIARA T, ABAD A, et al. Production of hydrogen by chemical looping reforming of methane and biogas using a reactive and durable Cu-based oxygen carrier[J]. Fuel, 2022, 322: 124250. |
| 65 | NADGOUDA Sourabh G, GUO Mengqing, TONG Andrew, et al. High purity syngas and hydrogen coproduction using copper-iron oxygen carriers in chemical looping reforming process[J]. Applied Energy, 2019, 235: 1415-1426. |
| 66 | JIN Hongguang, OKAMOTO Toshihiro, ISHIDA Masaru. Development of a novel chemical-looping combustion: Synthesis of a looping material with a double metal oxide of CoO-NiO[J]. Energy & Fuels, 1998, 12(6): 1272-1277. |
| 67 | ALALWAN Hayder A, CWIERTNY David M, GRASSIAN Vicki H. Co3O4 nanoparticles as oxygen carriers for chemical looping combustion: A materials characterization approach to understanding oxygen carrier performance[J]. Chemical Engineering Journal, 2017, 319: 279-287. |
| 68 | 曾良鹏, 黄樊, 祝星, 等. 铈基与钴基Co3O4-CeO2氧载体上甲烷化学链转化特性: 产物选择性控制[J]. 高等学校化学学报, 2017, 38(1): 115-125. |
| ZENG Liangpeng, HUANG Fan, ZHU Xing, et al. Chemical looping conversion of methane over CeO2-based and Co3O4-based Co3O4-CeO2 oxygen carriers: Controlling of product selectivity[J]. Chemical Journal of Chinese Universities, 2017, 38(1): 115-125. | |
| 69 | Tian H, Simonyi T, Poston J, et al. Effect of hydrogen sulfide on chemical looping combustion of coal-derived synthesis gas over bentonite-supported metal-oxide oxygen carriers[J]. Industrial & Engineering Chemistry Research, 2009, 48(18): 8418-8430. |
| 70 | ADÁNEZ J, DE DIEGO L F, GARCÍA LABIANO F, et al. Selection of oxygen carriers for chemical-looping combustion[J]. Energy & Fuels, 2004, 18(2): 371-377. |
| 71 | ZHAO Yunlei, JIN Bo, YAO Wenxing, et al. Thermodynamic simulation and experimental investigation of manganese oxide (MnO x ) for integrated CO2 capture and conversion via chemical looping route[J]. Fuel, 2023, 344: 127997. |
| 72 | CAMPBELL Charles T, PEDEN Charles H F. Oxygen vacancies and catalysis on ceria surfaces[J]. Science, 2005, 309(5735): 713-714. |
| 73 | Zheng Y, Zhu X, Wang H, et al. Characteristic of macroporous CeO2-ZrO2 oxygen carrier for chemical-looping steam methane reforming[J]. Journal of Rare Earths, 2014, 32(9): 842-848. |
| 74 | LI Ruiming, ZHANG Juping, SHI Jian, et al. Regulation of metal-support interface of Ni/CeO2 catalyst and the performance of low temperature chemical looping dry reforming of methane[J]. Journal of Fuel Chemistry and Technology, 2022, 50(11): 1458-1470. |
| 75 | ZHENG Yane, LI Kongzhai, WANG Hua, et al. Enhanced activity of CeO2-ZrO2 solid solutions for chemical-looping reforming of methane via tuning the macroporous structure[J]. Energy & Fuels, 2016, 30(1): 638-647. |
| 76 | ZHANG Weixiang, ZHANG Lina, PEI S, et al. Rational design and reduction kinetics of efficient Ce-Co oxygen carriers for chemical looping reforming of methane[J]. Fuel, 2023, 345: 128208. |
| 77 | MICCIO F, LANDI E, NATALI Murri A, et al. Fluidized bed reforming of methane by chemical looping with cerium oxide oxygen carriers[J]. Chemical Engineering Research and Design, 2023, 191: 568-577. |
| 78 | ZHAO Kun, HE Fang, HUANG Zhen, et al. CaO/MgO modified perovskite type oxides for chemical-looping steam reforming of methane[J]. Journal of Fuel Chemistry and Technology, 2016, 44(6): 680-688. |
| 79 | SHI R, WATERHOUSE G I N, ZHANG T. Recent progress in photocatalytic CO2 reduction over perovskite oxides[J]. Solar RRL, 2017, 1(11): 1700126. |
| 80 | FARHANG Yaghoub, Ehsan TAHERI-NASSAJ, REZAEI Mehran. Improvement of CO oxidation and CH4 combustion by Pd and Pt partial substitution on LaMn0.5Cu0.5O3 perovskite[J]. Langmuir, 2023, 39(44): 15465-15473. |
| 81 | Hou X, Ren J, Li F, et al. Research progress of perovskite materials as catalysts[J]. IOP Conference Series: Earth and Environmental Science, 2019, 295(3): 032020. |
| 82 | RONG Yaoguang, HU Yue, MEI Anyi, et al. Challenges for commercializing perovskite solar cells[J]. Science, 2018, 361(6408): eaat8235. |
| 83 | DING Haoran, XU Yongqing, LUO Cong, et al. A novel composite perovskite-based material for chemical-looping steam methane reforming to hydrogen and syngas[J]. Energy Conversion and Management, 2018, 171: 12-19. |
| 84 | CHANG Wenxi, GAO Yuming, HE Jiahui, et al. Asymmetric coordination activated lattice oxygen in perovskite ferrites for selective anaerobic oxidation of methane[J]. Journal of Materials Chemistry A, 2023, 11(9): 4651-4660. |
| 85 | XIA Xue, CHANG Wenxi, CHENG Shuwen, et al. Oxygen activity tuning via FeO6 octahedral tilting in perovskite ferrites for chemical looping dry reforming of methane[J]. ACS Catalysis, 2022, 12(12): 7326-7335. |
| 86 | LEE Minbeom, Hyun Suk LIM, KIM Yikyeom, et al. Enhancement of highly-concentrated hydrogen productivity in chemical looping steam methane reforming using Fe-substituted LaCoO3 [J]. Energy Conversion and Management, 2020, 207: 112507. |
| 87 | CAO Dingshan, LUO Cong, WU Fan, et al. Screening loaded perovskite oxygen carriers for chemical looping steam methane reforming[J]. Journal of Environmental Chemical Engineering, 2022, 10(2): 107315. |
| 88 | YIN Xianglei, WANG Shen, WANG Baoyi, et al. Perovskite-type LaMn1- x B x O3+ δ (B=Fe, CO and Ni) as oxygen carriers for chemical looping steam methane reforming[J]. Chemical Engineering Journal, 2021, 422: 128751. |
| 89 | GALINSKY N, MISHRA A, ZHANG J, et al. Ca1- x A x MnO3 (A=Sr and Ba) perovskite based oxygen carriers for chemical looping with oxygen uncoupling (CLOU)[J]. Applied Energy, 2015, 157: 358-367. |
| 90 | 沈阳, 赵坤, 何方, 等. 三维有序大孔钙钛矿型氧化物LaFe0.7Co0.3O3的合成及甲烷化学链水蒸气重整性能[J]. 燃料化学学报, 2016, 44(10): 1168-1176. |
| SHEN Yang, ZHAO Kun, HE Fang, et al. Synthesis of three-dimensionally ordered macroporous LaFe0.7Co0.3O3 perovskites and their performance for chemical-looping steam reforming of methane[J]. Journal of Fuel Chemistry and Technology, 2016, 44(10): 1168-1176. | |
| 91 | Li M, Zhao K, Zhao Z, et al. Enhanced hydrogen-rich syngas generation in chemical looping methane reforming using an interstitial doped La1.6Sr0.4FeCoO6 [J]. International Journal of Hydrogen Energy, 2019, 44(21): 10250-10264. |
| 92 | YANG Liuqing, ZHAO Zirui, CUI Chen, et al. Effect of nickel and cobalt doping on the redox performance of SrFeO3- δ toward chemical looping dry reforming of methane[J]. Energy & Fuels, 2023, 37(16): 12045-12057. |
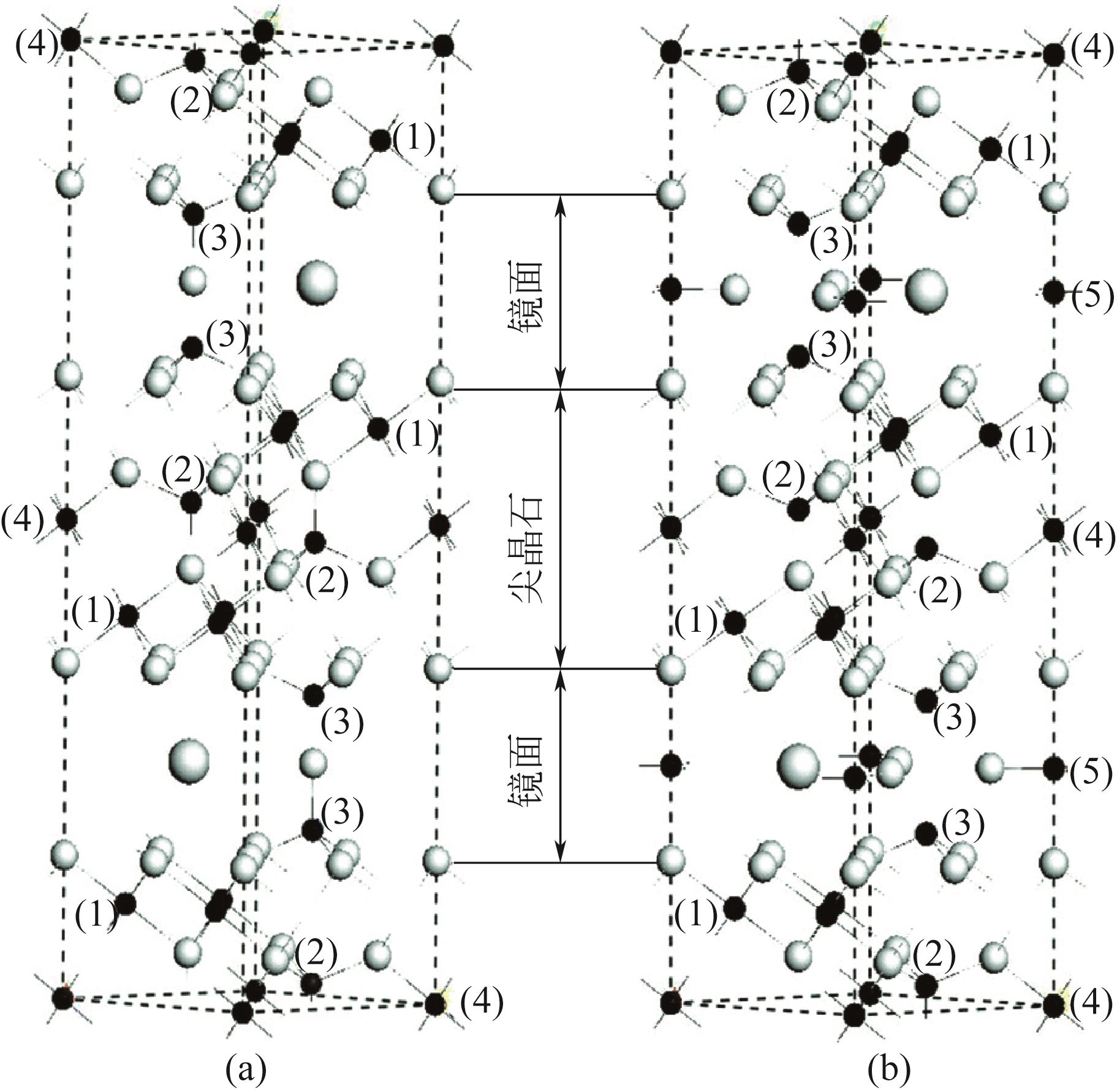
| 93 | TIAN M, WANG X D, ZHANG T. Hexaaluminates: A review of the structure, synthesis and catalytic performance[J]. Catalysis Science & Technology, 2016, 6(7): 1984-2004. |
| 94 | YANG Qian, CHEN Lihua, JIN Nannan, et al. Boosted carbon resistance of ceria-hexaaluminate by in situ formed CeFe x Al1– x O3 as oxygen pool for chemical looping dry reforming of methane[J]. Applied Catalysis B: Environmental, 2023, 330: 122636. |
| 95 | 靳南南, 张立, 朱燕燕, 等. 甲烷化学链重整制合成气用氧载体的研究进展[J]. 天然气化工(C1化学与化工), 2019, 44(3): 106-116. |
| JIN Nannan, ZHANG Li, ZHU Yanyan, et al. Research progress in oxygen carriers for syngas production via chemical looping reforming of methane[J]. Natural Gas Chemical Industry, 2019, 44(3): 106-116. | |
| 96 | ZHU Yanyan, WANG Xiaodong, WANG Aiqin, et al. Identification of the chemical state of Fe in barium hexaaluminate using Rietveld refinement and 57Fe Mössbauer spectroscopy[J]. Journal of Catalysis, 2011, 283(2): 149-160. |
| 97 | ZHU Yanyan, LIU Ruilin, SUN Xueyan, et al. Metal modified hexaaluminates for syngas generation and CO2 utilization via chemical looping[J]. International Journal of Hydrogen Energy, 2019, 44(21): 10218-10231. |
| 98 | Zhu Y, Jin N, Liu R, et al. Bimetallic BaFe2MAl9O19 (M = Mn, Ni, and Co) hexaaluminates as oxygen carriers for chemical looping dry reforming of methane[J]. Applied Energy, 2020, 258: 114070. |
| 99 | 刘永卓. 化学链燃烧过程钙基载氧体的研究[D]. 青岛: 青岛科技大学, 2010. |
| LIU Yongzhuo. Study on the Ca-based oxygen carriers for the chemical looping combustion[D].Qingdao: Qingdao University of Science & Technology, 2010. | |
| 100 | DANIEL Sastre, ÁLVAREZ Galván Consuelo, PATRICIA Pizarro, et al. Enhanced performance of CH4 dry reforming over La0.9Sr0.1FeO3/YSZ under chemical looping conditions[J]. Fuel, 2022, 309: 122122. |
| 101 | LONG Yanhui, LI Kongzhai, GU Z, et al. Ce-Fe-Zr-O/MgO coated monolithic oxygen carriers for chemical looping reforming of methane to co-produce syngas and H2 [J]. Chemical Engineering Journal, 2020, 388: 124190. |
| 102 | HESSAMODIN Nourbakhsh, YASIN Khani, AKBAR Zamaniyan, et al. Hydrogen and syngas production through dynamic chemical looping reforming-decomposition of methane[J]. International Journal of Hydrogen Energy, 2022, 47(17): 9835-9852. |
| 103 | NAZARI Mousa, SOLTANIEH Mohammad, HEYDARINASAB Amir, et al. Synthesis of a new self-supported Mg y (Cu x Ni0.6- x Mn0.4)1- y Fe2O4 oxygen carrier for chemical looping steam methane reforming process[J]. International Journal of Hydrogen Energy, 2021, 46(37): 19397-19420. |
| 104 | HU Jun, LI Haobo, CHEN Shiyi, et al. Enhanced Fe2O3/Al2O3 oxygen carriers for chemical looping steam reforming of methane with different Mg ratios[J]. Industrial & Engineering Chemistry Research, 2022, 61(2): 1022-1031. |
| 105 | SUN Yanru, LI Jun, LI Hongzhong. Core-shell-like Fe2O3/MgO oxygen carriers matched with fluidized bed reactor for chemical looping reforming[J]. Chemical Engineering Journal, 2022, 431: 134173. |
| 106 | KUO Yulin, HSU Weu-Mau, CHIU Ping-Chin, et al. Assessment of redox behavior of nickel ferrite as oxygen carriers for chemical looping process[J]. Ceramics International, 2013, 39(5): 5459-5465. |
| 107 | BOUKHA Z, JIMÉNEZ-GONZÁLEZ C, RIVAS B, et al. Synthesis, characterisation and performance evaluation of spinel-derived Ni/Al2O3 catalysts for various methane reforming reactions[J]. Applied Catalysis B-environmental, 2014, 158: 190-201. |
| 108 | MINHAS Rashid, KHOJA Asif Hussain, NAEEM Nida, et al. Thermal steam methane reforming over bimetal-loaded hemp-derived activated carbon-based catalyst for hydrogen production[J]. Research on Chemical Intermediates, 2023, 49(7): 3181-3203. |
| 109 | Mukasyan A S, Epstein P, Dinka P. Solution combustion synthesis of nanomaterials[J]. Proceedings of the Combustion Institute, 2007, 31(2): 1789-1795. |
| 110 | 刘黎明. 煤基化学链燃烧技术的氧载体研究[D]. 武汉: 华中科技大学, 2007. |
| LIU Liming. Study on oxygen carriers for chemical looping combustion of coal[D].Wuhan: Huazhong University of Science and Technology, 2007. | |
| 111 | YIKYEOM Kim, SEOK Kim Hyeon, DOHYUNG Kang, et al. Enhanced redox performance of LaFeO3 perovskite through in situ exsolution of iridium nanoparticles for chemical looping steam methane reforming[J]. Chemical Engineering Journal, 2023, 468: 143662. |
| 112 | SAYYED Sheraj Z, VAIDYA Prakash D. Chemical looping-steam reforming of biogas and methane over lanthanum-based perovskite for improved production of syngas and hydrogen[J]. Energy & Fuels, 2023, 37(23): 19082-19091. |
| 113 | TANG Mingchen, XU Long, FAN Maohong. Progress in oxygen carrier development of methane-based chemical-looping reforming: A review[J]. Applied Energy, 2015, 151: 143-156. |
| 114 | Jochen STRÖHLE, ORTH Matthias, EPPLE Bernd. Design and operation of a 1MWth chemical looping plant[J]. Applied Energy, 2014, 113: 1490-1495. |
| 115 | MARX Falko, DIERINGER Paul, Jochen STRÖHLE, et al. Design of a 1MWth pilot plant for chemical looping gasification of biogenic residues[J]. Energies, 2021, 14(9): 2581. |
| 116 | HOSSEINI Seyyed Yaghoob, KHOSRAVI-NIKOU Mohammad Reza, SHARIATI Ahmad. Production of hydrogen and syngas using chemical looping technology via cerium-iron mixed oxides[J]. Chemical Engineering and Processing-Process Intensification, 2019, 139: 23-33. |
| 117 | WANG Yajing, ZHENG Yane, WANG Yuhao, et al. Syngas production modified by oxygen vacancies over CeO2-ZrO2-CuO oxygen carrier via chemical looping reforming of methane[J]. Applied Surface Science, 2019, 481: 151-160. |
| 118 | PARK Cody, HSIEH Tien-Lin, POTTIMURTHY Yaswanth, et al. Design and operations of a 15kWth subpilot unit for the methane-to-syngas chemical looping process with CO2 utilization[J]. Industrial & Engineering Chemistry Research, 2020, 59(15): 6886-6899. |
| 119 | ZENG Liang, LUO Siwei, SRIDHAR Deepak, et al. Chemical looping processes: Particle characterization, ionic diffusion-reaction mechanism and reactor engineering[J]. Reviews in Chemical Engineering, 2012, 28(1): 1-42. |
| 120 | JOSHI Anuj, SHAH Vedant, MOHAPATRA Pinak, et al. Chemical looping—A perspective on the next-gen technology for efficient fossil fuel utilization[J]. Advances in Applied Energy, 2021, 3: 100044. |
| 121 | ORTIZ M, DE DIEGO L F, ABAD A, et al. Hydrogen production by auto-thermal chemical-looping reforming in a pressurized fluidized bed reactor using Ni-based oxygen carriers[J]. International Journal of Hydrogen Energy, 2010, 35(1): 151-160. |
| 122 | LYNGFELT Anders, MOLDENHAUER Patrick, BIERMANN Max, et al. Operational experiences of chemical-looping combustion with 18 manganese ores in a 300W unit[J]. International Journal of Greenhouse Gas Control, 2023, 127: 103937. |
| 123 | Tobias PRÖLL, KOLBITSCH Philipp, Johannes BOLHÀR-NORDENKAMPF, et al. A novel dual circulating fluidized bed system for chemical looping processes[J]. AIChE Journal, 2009, 55(12): 3255-3266. |
| 124 | ZACHARIAS Robert, VISENTIN Simone, BOCK Sebastian, et al. High-pressure hydrogen production with inherent sequestration of a pure carbon dioxide stream via fixed bed chemical looping[J]. International Journal of Hydrogen Energy, 2019, 44(16): 7943-7957. |
| 125 | BOCK Sebastian, ZACHARIAS Robert, HACKER Viktor. Co-production of pure hydrogen, carbon dioxide and nitrogen in a 10kW fixed-bed chemical looping system[J]. Sustainable Energy & Fuels, 2020, 4(3): 1417-1426. |
| 126 | HUA Xiuning, ZHU Jie, WU Xiaoshuang, et al. Packed bed chemical looping platform: Design and operation of 30kWth pilot unit[J]. Procedia Environmental Sciences, 2016, 31: 81-90. |
| 127 | Magnus RYDÉN, LYNGFELT Anders. Using steam reforming to produce hydrogen with carbon dioxide capture by chemical-looping combustion[J]. International Journal of Hydrogen Energy, 2006, 31(10): 1271-1283. |
| 128 | GARCÍA-DÍEZ E, GARCÍA-LABIANO F, DE DIEGO L F, et al. Optimization of hydrogen production with CO2 capture by autothermal chemical-looping reforming using different bioethanol purities[J]. Applied Energy, 2016, 169: 491-498. |
| 129 | HE Zirui, DE WILDE Juray. Numerical simulation of commercial scale autothermal chemical looping reforming and bi-reforming for syngas production[J]. Chemical Engineering Journal, 2021, 417: 128088. |
| 130 | KHAN Mohammed N, SHAMIM Tariq. Thermodynamic screening of suitable oxygen carriers for a three reactor chemical looping reforming system[J]. International Journal of Hydrogen Energy, 2017, 42(24): 15745-15760. |
| 131 | KHAN Mohammed N, SHAMIM Tariq. Investigation of hydrogen generation in a three reactor chemical looping reforming process[J]. Applied Energy, 2016, 162: 1186-1194. |
| 132 | MA Shiwei, LI Meng, WANG Genbao, et al. Effects of Zr doping on Fe2O3/CeO2 oxygen carrier in chemical looping hydrogen generation[J]. Chemical Engineering Journal, 2018, 346: 712-725. |
| 133 | CHAVDA Akash, MEHTA Pranav, HARICHANDAN Atal. Numerical analysis of multiphase flow in chemical looping reforming process for hydrogen production and CO2 capture[J]. Experimental and Computational Multiphase Flow, 2022, 4(4): 360-376. |
| 134 | ZHENG Teng, LI Mengjun, MEI Daofeng, et al. Effect of H2S presence on chemical looping reforming (CLR) of biogas with a firebrick supported NiO oxygen carrier[J]. Fuel Processing Technology, 2022, 226: 107088. |
| 135 | STOPPACHER B, BOCK S, MALLI K, et al. The influence of hydrogen sulfide contaminations on hydrogen production in chemical looping processes[J]. Fuel, 2022, 307: 121677. |
| [1] | WANG Yanhong, JIANG Lei, XUE Shuai, LI Hongwei, JIA Yuting. Analysis on heat transfer characteristics of supercritical methane in precooling channels [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1690-1699. |
| [2] | LIU Ruolu, TANG Haibo, HE Feifei, LUO Fengying, WANG Jinge, YANG Na, LI Hongwei, ZHANG Ruiming. Recent research and prospect of liquid organic hydrogen carries technology [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1731-1741. |
| [3] | QIAN Zhiguang, WANG Shixue, ZHU Yu, YUE Like. Start-up characteristics of high-temperature proton exchange membrane fuel cell stacks based on flat heat pipes [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1754-1763. |
| [4] | WU Chenhe, LIU Yumin, YANG Xinmin, CUI Jiwei, JIANG Shaokun, YE Jinhua, LIU Lequan. Particulate photocatalysts for light-driven overall water splitting [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1810-1822. |
| [5] | LIU Yurong, WANG Xingbao, LI Wenying. Regulation of catalyst acid sites and its effect on the deep hydrogenation performance of anthracene [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1832-1839. |
| [6] | GUO Xiaodong, MAO Yujiao, LIU Xiangyang, QIU Li, YU Feng, YAN Xiaoliang. Effect of oxygen vacancies in Ni/Sm2O3-CeO2/Al2O3 catalyst on CO2 methanation at low temperature [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1840-1850. |
| [7] | WANG Xudong, LIU Dunyu, XU Kailong, LIU Qiuqi, FAN Yunpei, JIN Jing. Impacts of CeO2 oxygen carriers on the conversion of mercury in chemical looping combustion of coal [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 2191-2200. |
| [8] | ZHANG Pengfei, CHEN Weipeng, XIAO Zhuonan, LYU Qinggang, ZHANG Shunfeng, ZHANG Zifeng. Red brick doping modified Baiyun Obo iron ore concentrate oxygen carrier performance [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 2226-2234. |
| [9] | XIAO Yaoxin, ZHANG Jun, SHAN Rui, YUAN Haoran, CHEN Yong. Catalytic hydrogenation of furfuryl alcohol into pentanediol over Pt/CaO materials [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1318-1327. |
| [10] | LI Weijie, KANG Jincan, ZHANG Chuanming, LIN Lina, LI Changxin, ZHU Hongping. Selective hydrogenation of methyl 3-hydroxypropionate over zirconium-modified Cu/SiO2 catalysts [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1328-1341. |
| [11] | LI Kairui, GAO Zhaohua, LIU Tiantian, LI Jing, WEI Haisheng. Tuning the catalytic performance of Rh/FePO4 catalyst by reduction temperature for quinoline selective hydrogenation [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1342-1349. |
| [12] | HUANG Sheng, YANG Zhenli, LI Zhenyu. Analysis of optimization path of developing China's hydrogen industry [J]. Chemical Industry and Engineering Progress, 2024, 43(2): 882-893. |
| [13] | SU Mengjun, LIU Jian, XIN Jing, CHEN Yufei, ZHANG Haihong, HAN Longnian, ZHU Yuanbao, LI Hongbao. Progress in the application of gas-liquid mixing intensification in fixed-bed hydrogenation [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 100-110. |
| [14] | GAI Hongwei, ZHANG Chenjun, QU Jingying, SUN Huailu, TUO Yongxiao, WANG Bin, JIN Xu, ZHANG Xi, FENG Xiang, CHEN De. Research progress on catalytic dehydrogenation process intensification for liquid organic hydride carrier hydrogen storage [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 164-185. |
| [15] | WANG Lihua, CAI Suhang, JIANG Wentao, LUO Qian, LUO Yong, CHEN Jianfeng. Research progress of micro and nano scale gas-liquid mass transfer to intensify catalytic hydrogenation of oil products [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 19-33. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||