Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (4): 1832-1846.DOI: 10.16085/j.issn.1000-6613.2022-1109
• Industrial catalysis • Previous Articles Next Articles
Advances in catalysts for catalytic partial oxidation of methane to syngas
RUAN Peng1( ), YANG Runnong1,2(
), YANG Runnong1,2( ), LIN Zirong1, SUN Yongming2
), LIN Zirong1, SUN Yongming2
- 1.Guangdong Foran Technology Company Limited, Foshan 528000, Guangdong, China
2.Guangzhou Institute of Energy Conversion, Chinese Academy of Science, Guangzhou 510640, Guangdong, China
-
Received:2022-06-13Revised:2022-08-22Online:2023-05-08Published:2023-04-25 -
Contact:YANG Runnong
甲烷催化部分氧化制合成气催化剂的研究进展
- 1.广东佛燃科技有限公司,广东 佛山 528000
2.中国科学院广州能源研究所,广东 广州 510640
-
通讯作者:杨润农 -
作者简介:阮鹏(1980—),男,高级工程师,研究方向为新型燃气技术的应用。E-mail:ruanpeng@fsgas.com。 -
基金资助:佛山市社会领域科技攻关专项(2120001008444)
CLC Number:
Cite this article
RUAN Peng, YANG Runnong, LIN Zirong, SUN Yongming. Advances in catalysts for catalytic partial oxidation of methane to syngas[J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1832-1846.
阮鹏, 杨润农, 林梓荣, 孙永明. 甲烷催化部分氧化制合成气催化剂的研究进展[J]. 化工进展, 2023, 42(4): 1832-1846.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-1109
| 甲烷转化途径 | 目标产物 | 优势 | 劣势 |
|---|---|---|---|
| 直接转化 | 烯烃、烷烃、醇类、醛类、 芳香烃等 | 工艺简单,运输成本低 | 高温、高压条件下反应,反应转化率低,产物选择性低 |
| 间接转化 | 合成气(CO+H2) | 工艺相对成熟,能源利用率高 | 工艺较复杂、生产成本较高 |
| 水蒸气重整 | 工艺成熟 | 强吸热反应、高能耗,对催化剂和反应设备要求高、投资成本高,产物氢碳比较高 | |
| 二氧化碳重整 | 以常见的温室气体CO2为原料、反应绿色环保 | 强吸热反应、高能耗,产物氢碳比较低,催化剂易积炭 | |
催化部分氧化 (CPOM) | 弱放热反应、能耗低,产物氢碳比理想,反应迅速 | 工艺尚不成熟,存在安全隐患 | |
| 自热重整 | 自供热、能耗低 | 控制复杂,对反应设备要求高 |
| 甲烷转化途径 | 目标产物 | 优势 | 劣势 |
|---|---|---|---|
| 直接转化 | 烯烃、烷烃、醇类、醛类、 芳香烃等 | 工艺简单,运输成本低 | 高温、高压条件下反应,反应转化率低,产物选择性低 |
| 间接转化 | 合成气(CO+H2) | 工艺相对成熟,能源利用率高 | 工艺较复杂、生产成本较高 |
| 水蒸气重整 | 工艺成熟 | 强吸热反应、高能耗,对催化剂和反应设备要求高、投资成本高,产物氢碳比较高 | |
| 二氧化碳重整 | 以常见的温室气体CO2为原料、反应绿色环保 | 强吸热反应、高能耗,产物氢碳比较低,催化剂易积炭 | |
催化部分氧化 (CPOM) | 弱放热反应、能耗低,产物氢碳比理想,反应迅速 | 工艺尚不成熟,存在安全隐患 | |
| 自热重整 | 自供热、能耗低 | 控制复杂,对反应设备要求高 |
| 催化剂组成 | 反应条件 | 不同CH4转化率下的温度/℃ | 不同CO选择性下的温度/℃ | 不同H2选择性下的温度/℃ | 参考文献 | |||
|---|---|---|---|---|---|---|---|---|
| T50 | T90 | T50 | T90 | T50 | T90 | |||
| Ni(7.7)/Al2O3 | C/O比例为1.0 WHSV=157500mL/(g·h) | 600 | — | 600 | 800 | <600 | 780 | [ |
| Ni(2.8)Co(2.6)/Al2O3 | 755 | — | 763 | — | 765 | — | ||
| Co(6.8)/Al2O3 | — | — | — | — | — | — | ||
| Ni(2.5)/La-CeOx | C/O比例为1.0 | <500 | 625 | <550 | 700 | * | * | [ |
| Ni(5)/La-CeOx | <500 | 625 | <550 | — | * | * | ||
| Ni(10)/La-CeOx | 618 | 645 | 630 | — | * | * | ||
| Ni/MgO | C/O比例为1.0 WHSV=520000mL/(g·h) | 503 | 625 | 503 | 510 | * | * | [ |
| La-Ni@SiO2 | C/O比例为1.0 WHSV=72000mL/(g·h) | <650 | 738 | <650 | 712 | <650 | 730 | [ |
| Rh(0.5)/Al2O3 | C/O比例为1.0 GHSV=6000h-1 | 525 | 765 | 535 | 730 | <500 | 560 | [ |
| Rh(1)/γ-Al2O3 | C/O比例为1.0 WHSV=48000mL/(g·h) | 478 | 765 | 588 | 800 | — | — | [ |
| Rh(1)/γ-Al2O3-W | 470 | 765 | 580 | 800 | — | — | ||
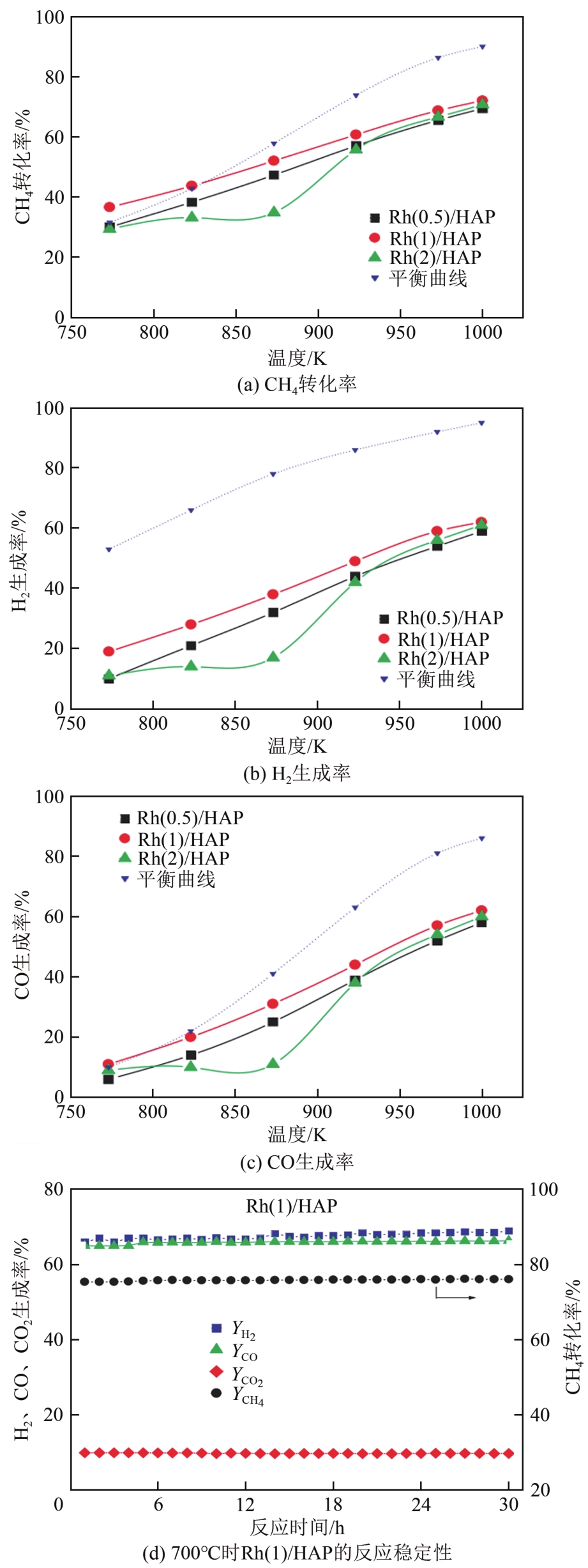
| Rh(0.5)/HAP | C/O比例为1.0 GHSV=192000h-1 | 613 | — | 685 | — | 680 | — | [ |
| Rh(1)/HAP | 587 | — | 667 | — | 662 | — | ||
| Rh(2)/HAP | 637 | — | 677 | — | 677 | — | ||
| Pt(0.5)-Ru(0.5)/CeZrO x -Al2O3 | C/O比例为1.0 GHSV=53000h-1 | 565 | 785 | 670 | — | 550 | 610 | [ |
| Pt(0.5)-Ru(0.5)/CeZrO x -Al2O3 | C/O比例为0.75 GHSV=26000h-1 | 570 | 780 | 640 | — | 578 | 595 | [ |
| La(0.5)Ca(0.5)Co(1)O3-δ | C/O比例为1.0 GHSV=12200h-1 | 800 | 860 | 765 | 853 | 760 | 850 | [ |
| LaNiO3 | C/O比例为1.0 WHSV=216000 mL/(g·h) | 350 | — | 525 | — | 430 | — | [ |
| 有序介孔LaNiO3 | <300 | 740 | 490 | — | <300 | — | ||
| 催化剂组成 | 反应条件 | 不同CH4转化率下的温度/℃ | 不同CO选择性下的温度/℃ | 不同H2选择性下的温度/℃ | 参考文献 | |||
|---|---|---|---|---|---|---|---|---|
| T50 | T90 | T50 | T90 | T50 | T90 | |||
| Ni(7.7)/Al2O3 | C/O比例为1.0 WHSV=157500mL/(g·h) | 600 | — | 600 | 800 | <600 | 780 | [ |
| Ni(2.8)Co(2.6)/Al2O3 | 755 | — | 763 | — | 765 | — | ||
| Co(6.8)/Al2O3 | — | — | — | — | — | — | ||
| Ni(2.5)/La-CeOx | C/O比例为1.0 | <500 | 625 | <550 | 700 | * | * | [ |
| Ni(5)/La-CeOx | <500 | 625 | <550 | — | * | * | ||
| Ni(10)/La-CeOx | 618 | 645 | 630 | — | * | * | ||
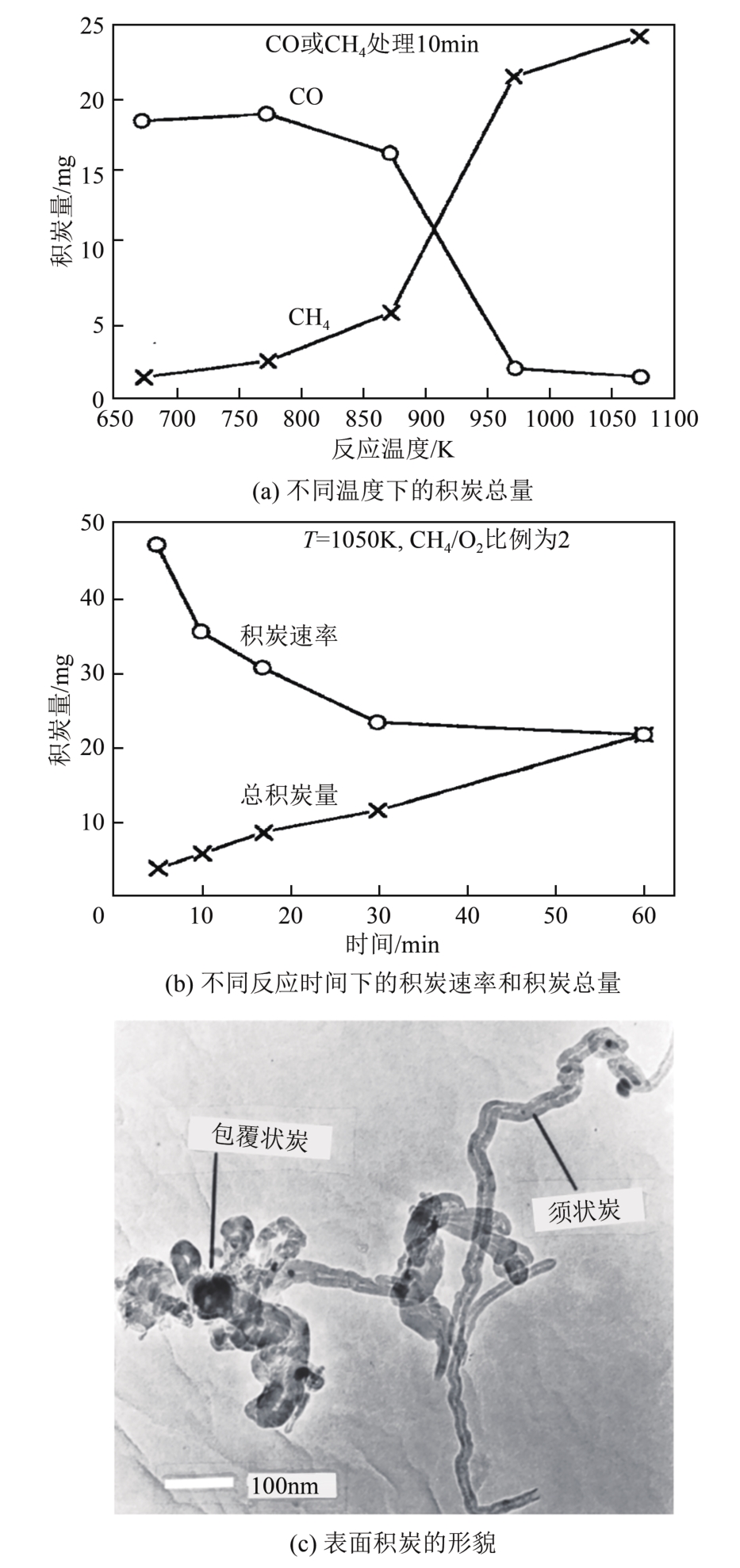
| Ni/MgO | C/O比例为1.0 WHSV=520000mL/(g·h) | 503 | 625 | 503 | 510 | * | * | [ |
| La-Ni@SiO2 | C/O比例为1.0 WHSV=72000mL/(g·h) | <650 | 738 | <650 | 712 | <650 | 730 | [ |
| Rh(0.5)/Al2O3 | C/O比例为1.0 GHSV=6000h-1 | 525 | 765 | 535 | 730 | <500 | 560 | [ |
| Rh(1)/γ-Al2O3 | C/O比例为1.0 WHSV=48000mL/(g·h) | 478 | 765 | 588 | 800 | — | — | [ |
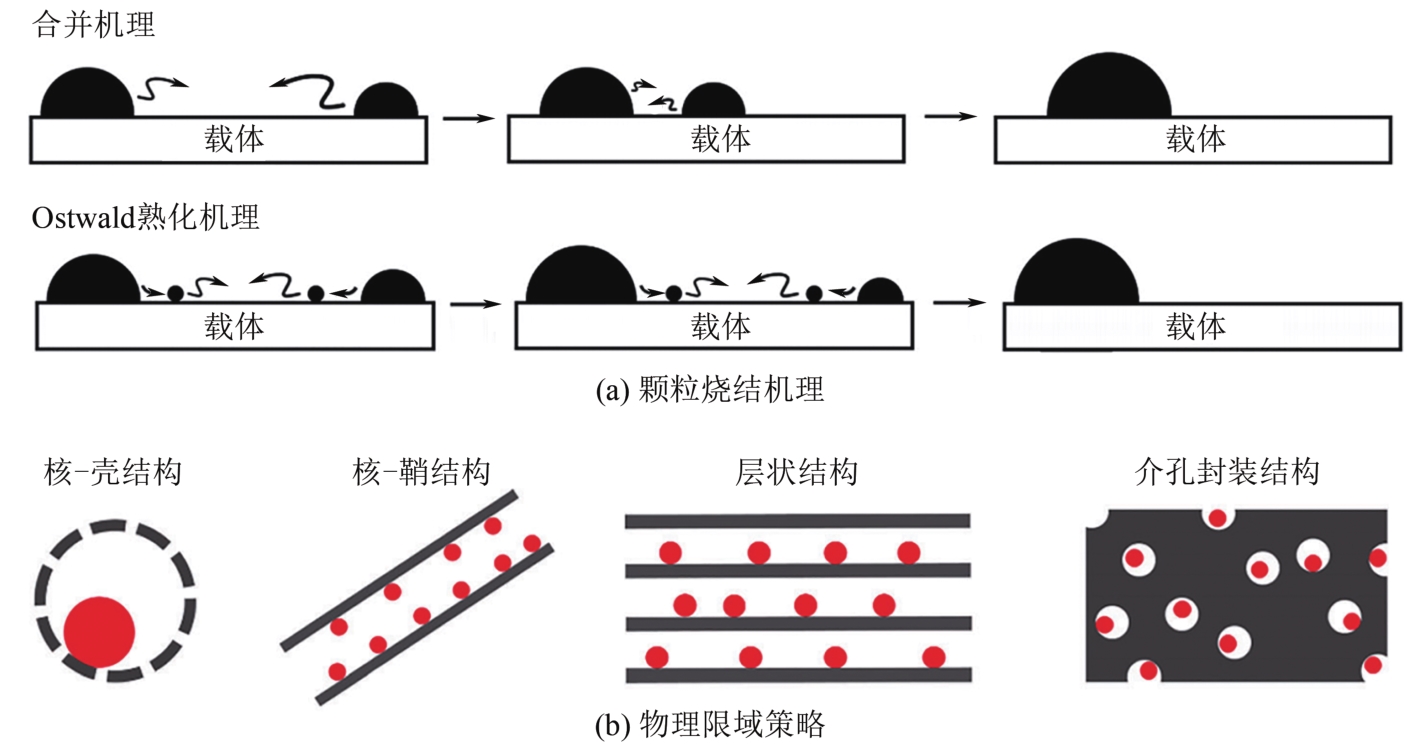
| Rh(1)/γ-Al2O3-W | 470 | 765 | 580 | 800 | — | — | ||
| Rh(0.5)/HAP | C/O比例为1.0 GHSV=192000h-1 | 613 | — | 685 | — | 680 | — | [ |
| Rh(1)/HAP | 587 | — | 667 | — | 662 | — | ||
| Rh(2)/HAP | 637 | — | 677 | — | 677 | — | ||
| Pt(0.5)-Ru(0.5)/CeZrO x -Al2O3 | C/O比例为1.0 GHSV=53000h-1 | 565 | 785 | 670 | — | 550 | 610 | [ |
| Pt(0.5)-Ru(0.5)/CeZrO x -Al2O3 | C/O比例为0.75 GHSV=26000h-1 | 570 | 780 | 640 | — | 578 | 595 | [ |
| La(0.5)Ca(0.5)Co(1)O3-δ | C/O比例为1.0 GHSV=12200h-1 | 800 | 860 | 765 | 853 | 760 | 850 | [ |
| LaNiO3 | C/O比例为1.0 WHSV=216000 mL/(g·h) | 350 | — | 525 | — | 430 | — | [ |
| 有序介孔LaNiO3 | <300 | 740 | 490 | — | <300 | — | ||
| 1 | 吴玉玺, 韩婷婷, 解子恒, 等. 直接碳固体氧化物燃料电池研究进展: 碳燃料和逆向Boudouard反应催化剂[J]. 储能科学与技术, 2021, 10(6): 1977-1986. |
| WU Yuxi, HAN Tingting, XIE Ziheng, et al. Recent progress in direct carbon solid oxide fuel cells: carbon fuels and reverse Boudouard reaction catalysts[J]. Energy Storage Science and Technology, 2021, 10(6): 1977-1986. | |
| 2 | 宋乃建, 郭明媛, 南皓雄, 等. 过渡金属基催化剂用于氧析出反应的研究进展[J]. 储能科学与技术, 2021, 10(6): 1906-1917. |
| SONG Naijian, GUO Mingyuan, Haoxiong NAN, et al. Recent advances in transition metal-based catalysts for oxygen evolution reaction[J]. Energy Storage Science and Technology, 2021, 10(6): 1906-1917. | |
| 3 | HE Feng, LI Fanxing. Perovskite promoted iron oxide for hybrid water-splitting and syngas generation with exceptional conversion[J]. Energy & Environmental Science, 2015, 8(2): 535-539. |
| 4 | SZIMA S, CORMOS A M, CORMOS C C. Flexible hydrogen and power co-generation based on dry methane reforming with carbon capture[C]//28th European Symposium on Computer Aided Proless Engineering, Part B, Amsterdam: Elsevier, 2018: 1281-1286. |
| 5 | 刘蕊. 双金属氧化物载氧体在化学链甲烷部分氧化中的反应机理研究[D]. 天津: 天津大学, 2020. |
| LIU Rui. Reaction mechanism of bimetal oxide oxygen carriers for chemical looping partial oxidation of methane[D]. Tianjin: Tianjin University, 2020. | |
| 6 | 张翔宇, 李振花. 甲烷部分氧化制合成气催化剂的研究进展[J]. 化工进展, 2002, 21(12): 903-907. |
| ZHANG Xiangyu, LI Zhenhua. Progress in catalyst of partial oxidation of methane to syngas[J]. Chemical Industry and Engineering Progress, 2002, 21(12): 903-907. | |
| 7 | 余长林, 周晓春. 甲烷催化部分氧化制合成气研究新进展[J]. 天然气化工, 2011, 36(5): 67-72. |
| YU Changlin, ZHOU Xiaochun. Research progress in preparation of syngas by catalytic partial oxidation of methane[J]. Natural Gas Chemical Industry, 2011, 36(5): 67-72. | |
| 8 | DIPU A L, OHBUCHI S, NISHIKAWA Y, et al. Direct nonoxidative conversion of methane to higher hydrocarbons over silica-supported nickel phosphide catalyst[J]. ACS Catalysis, 2020, 10(1): 375-379. |
| 9 | ABDELSAYED V, SHEKHAWAT D, SMITH M, et al. Microwave-assisted conversion of low rank coal under methane environment[J]. Energy & Fuels, 2019, 33(2): 905-915. |
| 10 | LI Duanxing, BASLYMAN W S, SIRITANARATKUL B, et al. Oxidative-coupling-assisted methane aromatization: A simulation study[J]. Industrial & Engineering Chemistry Research, 2019, 58(51): 22884-22892. |
| 11 | 徐锋, 李凡, 朱丽华, 等. 甲烷直接催化氧化制甲醇催化剂及反应机理[J]. 化工进展, 2019, 38(10): 4564-4573. |
| XU Feng, LI Fan, ZHU Lihua, et al. Catalyst and reaction mechanism for direct catalytic oxidation of methane to methanol[J]. Chemical Industry and Engineering Progress, 2019, 38(10): 4564-4573. | |
| 12 | BAI Shuxing, LIU Fangfang, HUANG Bolong, et al. High-efficiency direct methane conversion to oxygenates on a cerium dioxide nanowires supported rhodium single-atom catalyst[J]. Nature Communications, 2020, 11: 954. |
| 13 | 于彦存. 甲烷部分氧化制合成气Ni-Ce x Zr1- x O2催化剂的研究[D]. 天津: 天津大学, 2007. |
| YU Yancun. Study on partial oxidation of methane to syngas over Ni-Ce x Zr1- x O2 catalyst[D]. Tianjin: Tianjin University, 2007. | |
| 14 | LIU Bing, LI Wenping, XU Yuebing, et al. Insight into the intrinsic active site for selective production of light olefins in cobalt-catalyzed Fischer-Tropsch synthesis[J]. ACS Catalysis, 2019, 9(8): 7073-7089. |
| 15 | 田丰源, 周明扬, 汪维, 等. 基于固体氧化物燃料电池的甲烷电化学部分氧化[J]. 陶瓷学报, 2021, 42(3): 406-413. |
| TIAN Fengyuan, ZHOU Mingyang, WANG Wei, et al. Electrochemical partial oxidation of methane through solid oxide fuel cell[J]. Journal of Ceramics, 2021, 42(3): 406-413. | |
| 16 | ZHANG Haotian, SUN Zhuxing, HU Yun hang. Steam reforming of methane: Current states of catalyst design and process upgrading[J]. Renewable and Sustainable Energy Reviews, 2021, 149: 111330. |
| 17 | FAN L Y, MOKHOV A, SAADABADI S A, et al. Methane steam reforming reaction in solid oxide fuel cells: Influence of electrochemical reaction and anode thickness[J]. Journal of Power Sources, 2021, 507: 230276. |
| 18 | 杨杰, 常辉, 隋志军, 等. 化学链催化甲烷氧化反应研究进展[J]. 化工进展, 2021, 40(4): 1928-1947. |
| YANG Jie, CHANG Hui, SUI Zhijun, et al. Advances in chemical looping methane oxidation[J]. Chemical Industry and Engineering Progress, 2021, 40(4): 1928-1947. | |
| 19 | NIU Juntian, GUO Fan, RAN Jingyu, et al. Methane dry (CO2) reforming to syngas (H2/CO) in catalytic process: From experimental study and DFT calculations[J]. International Journal of Hydrogen Energy, 2020, 45(55): 30267-30287. |
| 20 | HAMBALI H U, JALIL A A, ABDULRASHEED A A, et al. Zeolite and clay based catalysts for CO2 reforming of methane to syngas: A review[J]. International Journal of Hydrogen Energy, 2022, 47(72): 30759-30787. |
| 21 | 阮勇哲, 卢遥, 王胜平. 甲烷干重整Ni基催化剂失活及抑制失活研究进展[J]. 化工进展, 2018, 37(10): 3850-3857. |
| RUAN Yongzhe, LU Yao, WANG Shengping. Progress in deactivation and anti-deactivation of nickel-based catalysts for methane dry reforming[J]. Chemical Industry and Engineering Progress, 2018, 37(10): 3850-3857. | |
| 22 | 王奕然, 曾令志, 娄舒洁, 等. 天然气制氢技术研究进展[J]. 石化技术与应用, 2019, 37(5): 361-366. |
| WANG Yiran, ZENG Lingzhi, LOU Shujie, et al. Review of hydrogen production from natural gas[J]. Petrochemical Technology & Application, 2019, 37(5): 361-366. | |
| 23 | 王芬. 甲烷合成与转化的抗积碳、抗烧结催化剂研究[D]. 厦门: 厦门大学, 2019. |
| WANG Fen. Study on coke and sintering resistant catalysts for methane synthesis and conversion[D]. Xiamen: Xiamen University, 2019. | |
| 24 | 龚思琦. 催化部分氧化-固体氧化物燃料电池系统特性研究[D]. 北京: 清华大学, 2019. |
| GONG Siqi. Research on characteristics of catalytic partial oxidation-solid oxide fuel cell systems[D]. Beijing: Tsinghua University, 2019. | |
| 25 | FAN Dongjie, GAO Yi, LIU Fangsheng, et al. Autothermal reforming of methane over an integrated solid oxide fuel cell reactor for power and syngas co-generation[J]. Journal of Power Sources, 2021, 513: 230536. |
| 26 | CHERIF A, NEBBALI R, SHEFFIELD J W, et al. Numerical investigation of hydrogen production via autothermal reforming of steam and methane over Ni/Al2O3 and Pt/Al2O3 patterned catalytic layers[J]. International Journal of Hydrogen Energy, 2021, 46(75): 37521-37532. |
| 27 | LIANDER H. The utilisation of natural gases for the ammonia process[J]. Transactions of the Faraday Society, 1929, 25: 462-472. |
| 28 | PRETTRE M, EICHNER C, PERRIN M. The catalytic oxidation of methane to carbon monoxide and hydrogen[J]. Transactions of the Faraday Society, 1946, 42: 335-339. |
| 29 | 罗春容. SiO2和Al2O3负载的Rh、Ru、Ir催化剂上甲烷部分氧化制合成气反应机理研究[D]. 厦门: 厦门大学, 2008. |
| LUO Chunrong. Mechanistic study of the partial oxidation of methane to synthesis gas over SiO2- and Al2O3-supported Rh, Ru and Ir catalysts[D]. Xiamen: Xiamen University, 2008. | |
| 30 | SMITH M W, SHEKHAWAT D. Fuel Cells: Technologies for fuel processing. Chapters 5—Catalytic partial oxidation[M]. Amsterdam: Elsevier, 2011: 73-128. |
| 31 | HU Y H, RUCKENSTEIN E. Transient kinetic studies of partial oxidation of CH4 [J]. Journal of Catalysis, 1996, 158(1): 260-266. |
| 32 | HICKMAN D A, SCHMIDT L D. Production of syngas by direct catalytic oxidation of methane[J]. Science, 1993, 259(5093): 343-346. |
| 33 | HICKMAN D A, SCHMIDT L D. Synthesis gas formation by direct oxidation of methane over Pt monoliths[J]. Journal of Catalysis, 1992, 138(1): 267-282. |
| 34 | VERMEIREN W J M, BLOMSMA E, JACOBS P A. Catalytic and thermodynamic approach of the oxyreforming reaction of methane[J]. Catalysis Today, 1992, 13(2/3): 427-436. |
| 35 | WENG Weizheng, CHEN Mingshu, YAN Qiangu, et al. Mechanistic study of partial oxidation of methane to synthesis gas over supported rhodium and ruthenium catalysts using in situ time-resolved FTIR spectroscopy[J]. Catalysis Today, 2000, 63(2/3/4): 317-326. |
| 36 | HOU Zhaoyin, GAO Jing, GUO Jianzhong, et al. Deactivation of Ni catalysts during methane autothermal reforming with CO2 and O2 in a fluidized-bed reactor[J]. Journal of Catalysis, 2007, 250(2): 331-341. |
| 37 | JALALI R, NEMATOLLAHI B, REZAEI M, et al. Mesoporous nanostructured Ni/MgAl2O4 catalysts: Highly active and stable catalysts for syngas production in combined dry reforming and partial oxidation[J]. International Journal of Hydrogen Energy, 2019, 44(21): 10427-10442. |
| 38 | 余林, 袁书华, 田久英, 等. 甲烷部分氧化制合成气载体及助剂对Ni系催化剂活性的影响[J]. 催化学报, 2001, 22(4): 383-386. |
| YU Lin, YUAN Shuhua, TIAN Jiuying, et al. Partial oxidation of methane to syngas: Effects of support and promoter on catalytic performance of Ni/Al2O3 catalyst[J]. Chinese Journal of Catalysis, 2001, 22(4): 383-386. | |
| 39 | OSMAN A I, MEUDAL J, LAFFIR F, et al. Enhanced catalytic activity of Ni on η-Al2O3 and ZSM-5 on addition of ceria zirconia for the partial oxidation of methane[J]. Applied Catalysis B: Environmental, 2017, 212: 68-79. |
| 40 | KONDRATENKO V A, BERGER-KARIN C, KONDRATENKO E V. Partial oxidation of methane to syngas over γ-Al2O3-supported Rh nanoparticles: Kinetic and mechanistic origins of size effect on selectivity and activity[J]. ACS Catalysis, 2014, 4(9): 3136-3144. |
| 41 | LANZA R, CANU P, JÄRÅS S G. Methane partial oxidation over Pt-Ru catalyst: An investigation on the mechanism[J]. Applied Catalysis A: General, 2010, 375(1): 92-100. |
| 42 | SCARABELLO A, NOGARE D D, CANU P, et al. Partial oxidation of methane on Rh/ZrO2 and Rh/Ce-ZrO2 on monoliths: Catalyst restructuring at reaction conditions[J]. Applied Catalysis B: Environmental, 2015, 174/175: 308-322. |
| 43 | LANZA R, CANU P, JÄRÅS S G. Partial oxidation of methane over Pt-Ru bimetallic catalyst for syngas production[J]. Applied Catalysis A: General, 2008, 348(2): 221-228. |
| 44 | XU Xia, LANE A M. Water-treated Rh/γ-Al2O3 catalyst for methane partial oxidation[J]. Applied Petrochemical Research, 2016, 6(2): 163-166. |
| 45 | BOUKHA Z, GIL-CALVO M, DE RIVAS B, et al. Behaviour of Rh supported on hydroxyapatite catalysts in partial oxidation and steam reforming of methane: On the role of the speciation of the Rh particles[J]. Applied Catalysis A: General, 2018, 556: 191-203. |
| 46 | RABE S, TRUONG T B, VOGEL F. Low temperature catalytic partial oxidation of methane for gas-to-liquids applications[J]. Applied Catalysis A: General, 2005, 292: 177-188. |
| 47 | JAVED A H, SHAHZAD N, BUTT F A, et al. Synthesis of bimetallic Co-Ni/ZnO nanoprisms (ZnO-NPr) for hydrogen-rich syngas production via partial oxidation of methane[J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106887. |
| 48 | CINAR T, ALTINCEKIC T G. Synthesis and investigation of bimetallic Ni-Co/Al2O3 nanocatalysts using the polyol process[J]. Particulate Science and Technology, 2016, 34(6): 725-735. |
| 49 | FAKEEHA A H, ARAFAT Y, IBRAHIM A A, et al. Highly selective syngas/H2 production via partial oxidation of CH4 using (Ni, Co and Ni-Co)/ZrO2-Al2O3 catalysts: Influence of calcination temperature[J]. Processes, 2019, 7(3): 141. |
| 50 | ZAGAYNOV I V, LOKTEV A S, MUKHIN I E, et al. Influence of the Ni/Co ratio in bimetallic NiCo catalysts on methane conversion into synthesis gas[J]. Mendeleev Communications, 2017, 27(5): 509-511. |
| 51 | CHEEPHAT C, DAORATTANACHAI P, LAOSIRIPOJANA N. Effects of Re additional and Co-fed water reactants on bimetallic Ni-Fe based catalysts for catalytic partial oxidation of methane[J]. Journal of Sustainable Energy & Environment, 2017, 8: 59-63. |
| 52 | ZHU Huaiyu, WANG Wei, RAN Ran, et al. Iron incorporated Ni-ZrO2 catalysts for electric power generation from methane[J]. International Journal of Hydrogen Energy, 2012, 37(12): 9801-9808. |
| 53 | BAWORNRUTTANABOONYA K, LAOSIRIPOJANA N, MUJUMDAR A S, et al. Catalytic partial oxidation of CH4 over bimetallic Ni-Re/Al2O3: Kinetic determination for application in microreactor [J]. AIChE Journal, 2018, 64(5): 1691-1701. |
| 54 | CIMINO S, LISI L, RUSSO G, et al. Effect of partial substitution of Rh catalysts with Pt or Pd during the partial oxidation of methane in the presence of sulphur[J]. Catalysis Today, 2010, 154(3/4): 283-292. |
| 55 | TOMISHIGE K, KANAZAWA S, SATO M, et al. Catalyst design of Pt-modified Ni/Al2O3 catalyst with flat temperature profile in methane reforming with CO2 and O2 [J]. Catalysis Letters, 2002, 84: 69-74. |
| 56 | PAULETTO G, LIBRETTO N, BOFFITO D C, et al. Ni/CeO2 promoted Ru and Pt supported on FeCrAl gauze for cycling methane catalytic partial oxidation-CPOX[J]. Applied Catalysis B: Environmental, 2021, 286: 119849. |
| 57 | LI L C, DOSTAGIR N H, SHROTRI A, et al. Partial oxidation of methane to syngas via formate intermediate found for a ruthenium-rhenium bimetallic catalyst[J]. ACS Catalysis, 2021, 11(7): 3782-3789. |
| 58 | BRACKMANN R, PEREZ C A, SCHMAL M. LaCoO3 perovskite on ceramic monoliths-Pre and post reaction analyzes of the partial oxidation of methane[J]. International Journal of Hydrogen Energy, 2014, 39(26): 13991-14007. |
| 59 | BASHAN V, UST Y. Perovskite catalysts for methane combustion: Applications, design, effects for reactivity and partial oxidation[J]. International Journal of Energy Research, 2019, 43(14): 7755-7789. |
| 60 | CIHLAR J J, VRBA R, CASTKOVA K, et al. Effect of transition metal on stability and activity of La-Ca-M-(Al)-O (M = Co, Cr, Fe and Mn) perovskite oxides during partial oxidation of methane[J]. International Journal of Hydrogen Energy, 2017, 42(31): 19920-19934. |
| 61 | SANTOS M, NETO R C, NORONHA F B, et al. Perovskite as catalyst precursors in the partial oxidation of methane: the effect of cobalt, nickel and pretreatment[J]. Catalysis Today, 2018, 299: 229-241. |
| 62 | ZHU T L, FLYTZANI-STEPHANOPOULOS M. Catalytic partial oxidation of methane to synthesis gas over Ni-CeO2 [J]. Applied Catalysis A: General, 2001, 208(1/2): 403-417. |
| 63 | CHOUDHARY V R, MAMMAN A S, SANSARE S D. Selective oxidation of methane to CO and H2 over Ni/MgO at low temperatures[J]. Angewandte Chemie International Edition in English, 1992, 31(9): 1189-1190. |
| 64 | LI Lei, YAO Yao, SUN Bo, et al. Highly active and stable lanthanum-doped core-shell-structured Ni@SiO2 catalysts for the partial oxidation of methane to syngas[J]. ChemCatChem, 2013, 5(12): 3781-3787. |
| 65 | 龚思琦, 曾洪瑜, 史翊翔, 等. 基于甲烷催化部分氧化的SOFC性能研究[J]. 燃烧科学与技术, 2019, 25(1): 60-65. |
| GONG Siqi, ZENG Hongyu, SHI Yixiang, et al. Study of performance of SOFC based on catalytic partial oxidation of methane[J]. Journal of Combustion Science and Technology, 2019, 25(1): 60-65. | |
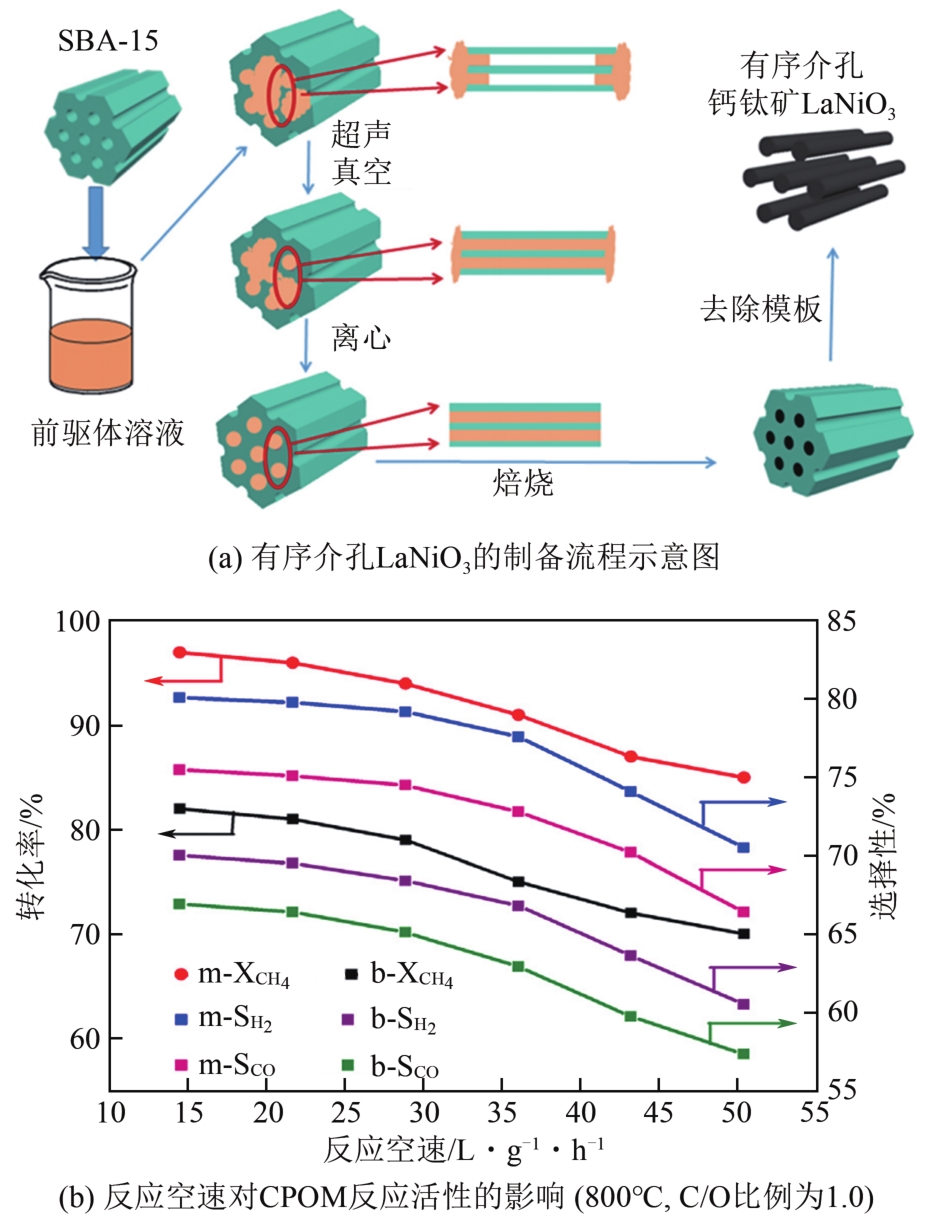
| 66 | DUAN Qianlin, WANG Junwen, DING Chuanmin, et al. Partial oxidation of methane over Ni based catalyst derived from order mesoporous LaNiO3 perovskite prepared by modified nanocasting method[J]. Fuel, 2017, 193: 112-118. |
| 67 | OSMAN A I. Catalytic hydrogen production from methane partial oxidation: Mechanism and kinetic study[J]. Chemical Engineering & Technology, 2020, 43(4): 641-648. |
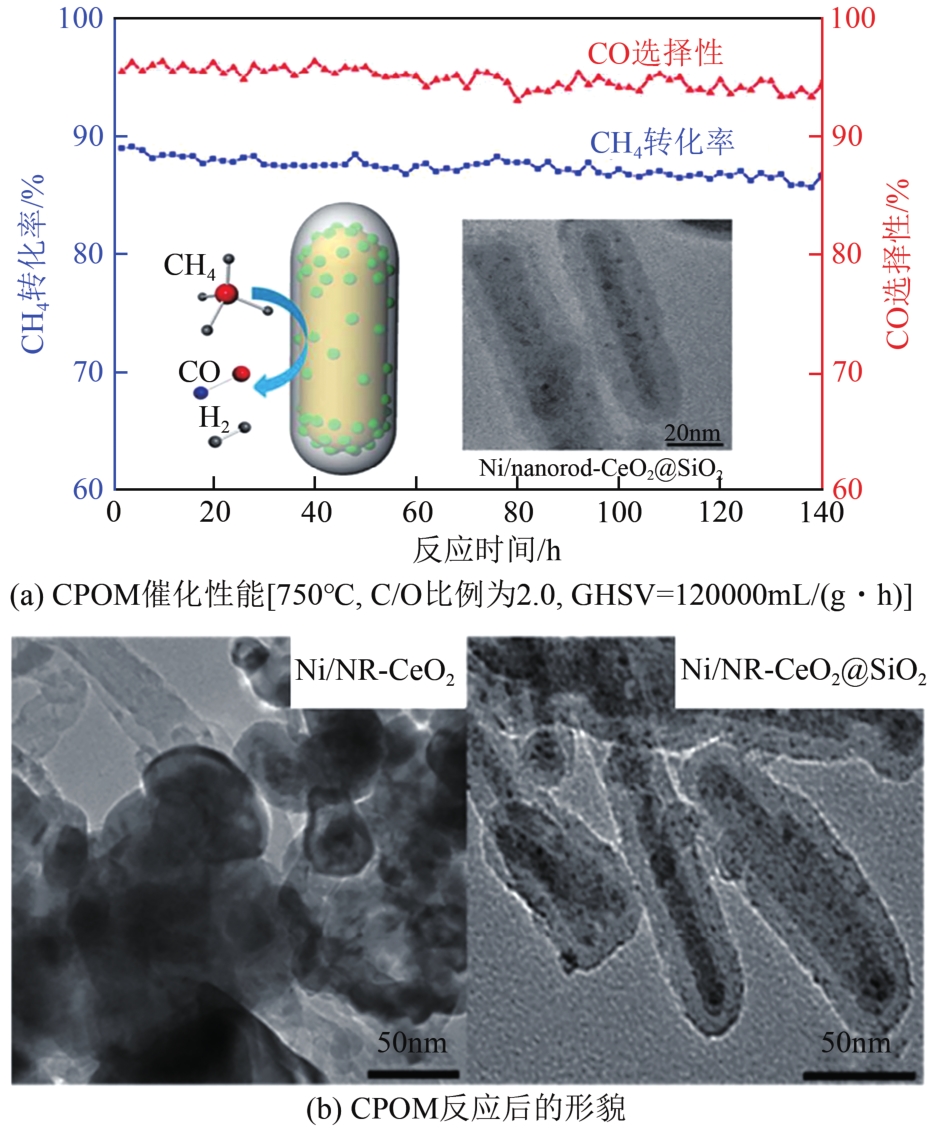
| 68 | GUO Songsong, WANG Junwen, DING Chuanmin, et al. Confining Ni nanoparticles in honeycomb-like silica for coking and sintering resistant partial oxidation of methane[J]. International Journal of Hydrogen Energy, 2018, 43(13): 6603-6613. |
| 69 | STOTZ H, MAIER L, DEUTSCHMANN O. Methane oxidation over palladium: On the mechanism in fuel-rich mixtures at high temperatures[J]. Topics in Catalysis, 2017, 60(1/2): 83-109. |
| 70 | JAWORSKI Z, ZAKRZEWSKA B, PIANKO-OPRYCH P. On thermodynamic equilibrium of carbon deposition from gaseous C—H—O mixtures: Updating for nanotubes[J]. Reviews in Chemical Engineering, 2017, 33(3): 217-235. |
| 71 | JAWORSKI Z, PIANKO-OPRYCH P. On nanotube carbon deposition at equilibrium in catalytic partial oxidation of selected hydrocarbon fuels[J]. International Journal of Hydrogen Energy, 2017, 42(27): 16920-16931. |
| 72 | CLARIDGE J B, GREEN M L H, TSANG S C, et al. A study of carbon deposition on catalysts during the partial oxidation of methane to synthesis gas[J]. Catalysis Letters, 1993, 22(4): 299-305. |
| 73 | TANG S, LIN J, TAN K L. Partial oxidation of methane to syngas over Ni/MgO, Ni/CaO and Ni/CeO2 [J]. Catalysis Letters, 1998, 51: 169-175. |
| 74 | GAO Jing, HOU Zhaoyin, GUO Jianzhong, et al. Catalytic conversion of methane and CO2 to synthesis gas over a La2O3-modified SiO2 supported Ni catalyst in fluidized-bed reactor[J]. Catalysis Today, 2008, 131(1/2/3/4): 278-284. |
| 75 | NICHELE V, SIGNORETTO M, PINNA F, et al. Ni/ZrO2 catalysts in ethanol steam reforming: Inhibition of coke formation by CaO-doping[J]. Applied Catalysis B: Environmental, 2014, 150/151: 12-20. |
| 76 | DAI Yunqian, LU Ping, CAO Zhenming, et al. The physical chemistry and materials science behind sinter-resistant catalysts[J]. Chemical Society Reviews, 2018, 47(12): 4314-4331. |
| 77 | 薛亚楠. 高度分散Pt催化剂的制备及用于POM催化性能的研究[D]. 太原: 太原理工大学, 2019. |
| XUE Yanan. Study on the preparation of highly dispersed Pt catalysts and performance for partial oxidation of methane[D]. Taiyuan: Taiyuan University of Technology, 2019. | |
| 78 | ZHU Shaohong, LIAN Xinyi, FAN Tingting, et al. Thermally stable core-shell Ni/nanorod-CeO2@SiO2 catalyst for partial oxidation of methane at high temperatures[J]. Nanoscale, 2018, 10(29): 14031-14038. |
| [1] | ZHANG Mingyan, LIU Yan, ZHANG Xueting, LIU Yake, LI Congju, ZHANG Xiuling. Research progress of non-noble metal bifunctional catalysts in zinc-air batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 276-286. |
| [2] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [3] | XIE Luyao, CHEN Songzhe, WANG Laijun, ZHANG Ping. Platinum-based catalysts for SO2 depolarized electrolysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 299-309. |
| [4] | YANG Xiazhen, PENG Yifan, LIU Huazhang, HUO Chao. Regulation of active phase of fused iron catalyst and its catalytic performance of Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 310-318. |
| [5] | WANG Lele, YANG Wanrong, YAO Yan, LIU Tao, HE Chuan, LIU Xiao, SU Sheng, KONG Fanhai, ZHU Canghai, XIANG Jun. Influence of spent SCR catalyst blending on the characteristics and deNO x performance for new SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 489-497. |
| [6] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [7] | LAI Shini, JIANG Lixia, LI Jun, HUANG Hongyu, KOBAYASHI Noriyuki. Research progress of ammonia blended fossil fuel [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4603-4615. |
| [8] | CHENG Tao, CUI Ruili, SONG Junnan, ZHANG Tianqi, ZHANG Yunhe, LIANG Shijie, PU Shi. Analysis of impurity deposition and pressure drop increase mechanisms in residue hydrotreating unit [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4616-4627. |
| [9] | WANG Peng, SHI Huibing, ZHAO Deming, FENG Baolin, CHEN Qian, YANG Da. Recent advances on transition metal catalyzed carbonylation of chlorinated compounds [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4649-4666. |
| [10] | ZHANG Qi, ZHAO Hong, RONG Junfeng. Research progress of anti-toxicity electrocatalysts for oxygen reduction reaction in PEMFC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4677-4691. |
| [11] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| [12] | WANG Weitao, BAO Tingyu, JIANG Xulu, HE Zhenhong, WANG Kuan, YANG Yang, LIU Zhaotie. Oxidation of benzene to phenol over aldehyde-ketone resin based metal-free catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4706-4715. |
| [13] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [14] | XIANG Yang, HUANG Xun, WEI Zidong. Recent progresses in the activity and selectivity improvement of electrocatalytic organic synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4005-4014. |
| [15] | WANG Yaogang, HAN Zishan, GAO Jiachen, WANG Xinyu, LI Siqi, YANG Quanhong, WENG Zhe. Strategies for regulating product selectivity of copper-based catalysts in electrochemical CO2 reduction [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4043-4057. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||