Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (S1): 287-298.DOI: 10.16085/j.issn.1000-6613.2023-0837
• Industrial catalysis • Previous Articles Next Articles
Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol
SHI Yongxing1( ), LIN Gang2(
), LIN Gang2( ), SUN Xiaohang2, JIANG Weigeng1, QIAO Dawei1, YAN Binhang2(
), SUN Xiaohang2, JIANG Weigeng1, QIAO Dawei1, YAN Binhang2( )
)
- 1.Jiangsu Huadian Jurong Power Station Co. , Ltd. , Zhenjiang 212000, Jiangsu, China
2.Department of Chemical Engineering, Tsinghua University, Beijing 100084, China
-
Received:2023-05-19Revised:2023-07-29Online:2023-11-30Published:2023-10-25 -
Contact:YAN Binhang
二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展
时永兴1( ), 林刚2(
), 林刚2( ), 孙晓航2, 蒋韦庚1, 乔大伟1, 颜彬航2(
), 孙晓航2, 蒋韦庚1, 乔大伟1, 颜彬航2( )
)
- 1.江苏华电句容发电有限公司,江苏 镇江 212413
2.清华大学化学工程系,北京 100084
-
通讯作者:颜彬航 -
作者简介:时永兴(1972—),男,学士,工程师。E-mail:syx_jrhd@126.com
林刚(1996—),男,硕士研究生,研究方向为二氧化碳催化转化。E-mail:15092354940@163.com。 -
基金资助:国家自然科学基金(21808120)
CLC Number:
Cite this article
SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol[J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298.
时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-0837
| 时间 | 单位/机构 | 规模 | 技术特点 |
|---|---|---|---|
| 2009年 | 三井化学公司 | — | |
| 2009年 | 日本三菱重工 | 100t/a | Cu/ZnO/Al2O3,9MPa,247℃ |
| 2010年 | 德国鲁齐 | — | Cu/ZnO/Al2O3,5~8MPa,220~270℃ |
| 2013年 | 冰岛碳循环公司 | 中试及示范,1000~4000t/a | 地热CO2-发电, 托普索技术 |
| 2016年 | 中国科学院山西煤炭化学研究所 | 单管试验 | Cu/ZnO/Al2O3 |
| 2016年 | 中国科学院上海高等研究院/上海华谊集团 | — | 工业单管试验成功 |
| 2018年 | 德国克莱恩 | — | Cu/ZnO/Al2O3,MegaMax |
| 2019年 | 中国石油大庆油田 | 实验室放大 | Cu/ZnO/Al2O3,大连瑞克技术 |
| 2020年 | 中国科学院大连化学物理研究所 | 中试及示范,1200t/a | 光伏-电解水制氢,ZnZrO x |
| 2020年 | 中国科学院上海高等研究院 | 工业侧线,5000t/a | 富碳天然气,Cu/ZnO/Al2O3 |
| 时间 | 单位/机构 | 规模 | 技术特点 |
|---|---|---|---|
| 2009年 | 三井化学公司 | — | |
| 2009年 | 日本三菱重工 | 100t/a | Cu/ZnO/Al2O3,9MPa,247℃ |
| 2010年 | 德国鲁齐 | — | Cu/ZnO/Al2O3,5~8MPa,220~270℃ |
| 2013年 | 冰岛碳循环公司 | 中试及示范,1000~4000t/a | 地热CO2-发电, 托普索技术 |
| 2016年 | 中国科学院山西煤炭化学研究所 | 单管试验 | Cu/ZnO/Al2O3 |
| 2016年 | 中国科学院上海高等研究院/上海华谊集团 | — | 工业单管试验成功 |
| 2018年 | 德国克莱恩 | — | Cu/ZnO/Al2O3,MegaMax |
| 2019年 | 中国石油大庆油田 | 实验室放大 | Cu/ZnO/Al2O3,大连瑞克技术 |
| 2020年 | 中国科学院大连化学物理研究所 | 中试及示范,1200t/a | 光伏-电解水制氢,ZnZrO x |
| 2020年 | 中国科学院上海高等研究院 | 工业侧线,5000t/a | 富碳天然气,Cu/ZnO/Al2O3 |
| 活性位点 | 催化剂 | 温度 /℃ | 压力 /MPa | 转化率/% | 甲醇选择性/% |
|---|---|---|---|---|---|
| Cu0 | Cu/ZnO/ZrO2[ | 200 | — | 15.5 | 42.1 |
| Cu/ZnO/Al2O3[ | 200 | — | 15.27 | 40.47 | |
| Cu/ZnO/TiO2[ | 200 | — | 10.57 | 53.89 | |
| Cu/ZnO/Y2O3[ | 200 | — | 10.46 | 49.21 | |
| Cu/t-ZrO2[ | 300 | 8 | 13.96 | 92.62 | |
| Cu/m-ZrO2[ | 300 | 8 | 9.28 | 79.87 | |
| Cu/am-ZrO2[ | 300 | 8 | 11.3 | 74.69 | |
| Cu/ZnO/Al2O3/SiO2[ | 210 | 3.4 | 11.7 | 48.0 | |
| Cu/ZnO-In2O3[ | 280 | 2 | 6.3 | 84.5 | |
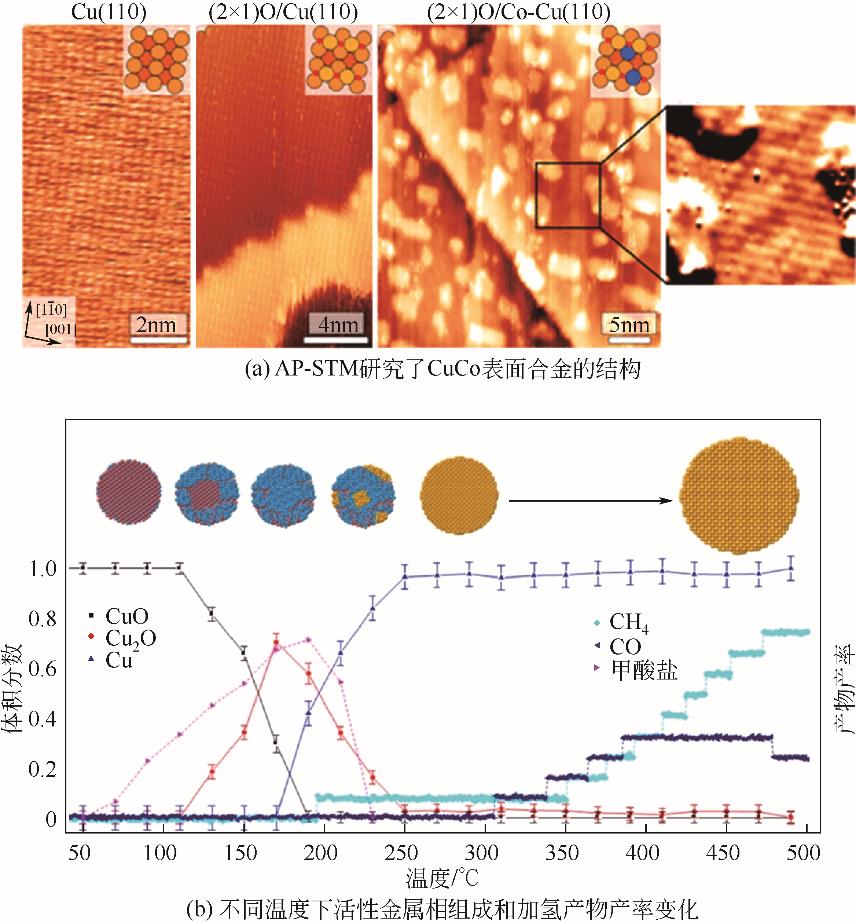
| Cu+ | K-Cu x O/ Cu (111)[ | 220 | 5 | — | 64.14 |
| Cu/ZnO/Al2O3[ | 200 | 2 | 5.19 | 67.5 | |
| Cu/SiO2[ | 320 | 3 | 28.0 | 21.3 | |
| YBa2Cu3O7[ | 240 | 3 | 3.4 | 34.8 | |
| Cu/SiO2[ | 190 | 3 | 5.0 | 79.3 | |
| Cu δ+ | CuO/ZrO2[ | 240 | 2 | 2.4 | 49.4 |
| Cu/ZrO2[ | 240 | 1.5 | 0.36 | 43.08 | |
| Pd-Cu/ZrO2[ | 240 | 1.5 | 1.72 | 53.83 | |
| Cu/ZrO2[ | 220 | 3 | 6.8 | 64.4 | |
Cu-ZnO/ Al2O3-ZrO2[ | 240 | 5 | 14.8 | 75.8 | |
Cu/ZnO 界面 | CuZnAuAl[ | 270 | 5 | 30.0 | 98.8 |
| 活性位点 | 催化剂 | 温度 /℃ | 压力 /MPa | 转化率/% | 甲醇选择性/% |
|---|---|---|---|---|---|
| Cu0 | Cu/ZnO/ZrO2[ | 200 | — | 15.5 | 42.1 |
| Cu/ZnO/Al2O3[ | 200 | — | 15.27 | 40.47 | |
| Cu/ZnO/TiO2[ | 200 | — | 10.57 | 53.89 | |
| Cu/ZnO/Y2O3[ | 200 | — | 10.46 | 49.21 | |
| Cu/t-ZrO2[ | 300 | 8 | 13.96 | 92.62 | |
| Cu/m-ZrO2[ | 300 | 8 | 9.28 | 79.87 | |
| Cu/am-ZrO2[ | 300 | 8 | 11.3 | 74.69 | |
| Cu/ZnO/Al2O3/SiO2[ | 210 | 3.4 | 11.7 | 48.0 | |
| Cu/ZnO-In2O3[ | 280 | 2 | 6.3 | 84.5 | |
| Cu+ | K-Cu x O/ Cu (111)[ | 220 | 5 | — | 64.14 |
| Cu/ZnO/Al2O3[ | 200 | 2 | 5.19 | 67.5 | |
| Cu/SiO2[ | 320 | 3 | 28.0 | 21.3 | |
| YBa2Cu3O7[ | 240 | 3 | 3.4 | 34.8 | |
| Cu/SiO2[ | 190 | 3 | 5.0 | 79.3 | |
| Cu δ+ | CuO/ZrO2[ | 240 | 2 | 2.4 | 49.4 |
| Cu/ZrO2[ | 240 | 1.5 | 0.36 | 43.08 | |
| Pd-Cu/ZrO2[ | 240 | 1.5 | 1.72 | 53.83 | |
| Cu/ZrO2[ | 220 | 3 | 6.8 | 64.4 | |
Cu-ZnO/ Al2O3-ZrO2[ | 240 | 5 | 14.8 | 75.8 | |
Cu/ZnO 界面 | CuZnAuAl[ | 270 | 5 | 30.0 | 98.8 |
| 原位表征技术 | 类型 | 原理 | 工作条件 | 探测深度 | 用于识别活性位点的 结构信息 | 局限性 |
|---|---|---|---|---|---|---|
| 扫描隧道显微镜 | — | 隧道效应 | 大气、真空 | 1~2原子层 | 结构和缺陷 | (1)表面微粒之间的沟槽不能准确探测 (2)受限于材料的导电性 |
| X射线衍射 | 散射光谱 | Bragg衍射 | 超高真空至30MPa 77~2273K | 体相 | 相结构和 缺陷位置 | 不适用于结晶度低的催化剂 |
| 拉曼光谱 | 散射光谱 | Raman散射 | 超高真空至50MPa 77~1237K | 约10nm | 相结构 | (1)拉曼散射效应的低频导致的微弱信号强度 (2)激光可能会加热样品并扰动分析区域 |
| 红外光谱 | 吸收光谱 | 共振吸收 | 超高真空至3MPa 77~1237K | 表面 | 吸附中间体 /化学结构 | (1)不适用于水溶液 (2)由于高温下红外区域中的样品发射,无法在高温下用傅里叶变换红外光谱进行原位研究 |
| X射线光电子能谱 | 激发光谱 | 光电效应 | 超高真空至3kPa 80~1200K | <2nm | 电子结构 | 不适用于具有压敏表面结构的催化剂 |
| X射线吸收光谱 | 吸收光谱 | 共振吸收 | 超高真空至30MPa 80~1773K | 体相 | 电子结构和局部协调环境 | (1)相对不适用于含有有机分子的轻元素 (2)内部工作通常成本高昂,需要基础设施和辐射源 |
| 原位表征技术 | 类型 | 原理 | 工作条件 | 探测深度 | 用于识别活性位点的 结构信息 | 局限性 |
|---|---|---|---|---|---|---|
| 扫描隧道显微镜 | — | 隧道效应 | 大气、真空 | 1~2原子层 | 结构和缺陷 | (1)表面微粒之间的沟槽不能准确探测 (2)受限于材料的导电性 |
| X射线衍射 | 散射光谱 | Bragg衍射 | 超高真空至30MPa 77~2273K | 体相 | 相结构和 缺陷位置 | 不适用于结晶度低的催化剂 |
| 拉曼光谱 | 散射光谱 | Raman散射 | 超高真空至50MPa 77~1237K | 约10nm | 相结构 | (1)拉曼散射效应的低频导致的微弱信号强度 (2)激光可能会加热样品并扰动分析区域 |
| 红外光谱 | 吸收光谱 | 共振吸收 | 超高真空至3MPa 77~1237K | 表面 | 吸附中间体 /化学结构 | (1)不适用于水溶液 (2)由于高温下红外区域中的样品发射,无法在高温下用傅里叶变换红外光谱进行原位研究 |
| X射线光电子能谱 | 激发光谱 | 光电效应 | 超高真空至3kPa 80~1200K | <2nm | 电子结构 | 不适用于具有压敏表面结构的催化剂 |
| X射线吸收光谱 | 吸收光谱 | 共振吸收 | 超高真空至30MPa 80~1773K | 体相 | 电子结构和局部协调环境 | (1)相对不适用于含有有机分子的轻元素 (2)内部工作通常成本高昂,需要基础设施和辐射源 |
| 1 | NOA A. Carbon dioxide now more than 50% higher than pre-industrial levels[R]. 2022. https://www.noaa.gov/news-release/carbon-dioxide-now-more-than-50-higher-than-pre-industrial-levels. |
| 2 | WANG Y, WINTER L R, CHEN J G, et al. CO2 hydrogenation over heterogeneous catalysts at atmospheric pressure: From electronic properties to product selectivity[J]. Green Chemistry, 2021, 23(1): 249-267. |
| 3 | ZHANG Zhihe, YU Zihang, FENG Kai, et al. Eu3+ doping-promoted Ni-CeO2 interaction for efficient low-temperature CO2 methanation[J]. Applied Catalysis B: Environmental, 2022, 317: 121800. |
| 4 | WANG Yaning, FENG Kai, TIAN Jiaming, et al. Atomically dispersed Zn-stabilized Ni δ + enabling tunable selectivity for CO2 hydrogenation[J]. ChemSusChem, 2022, 15(7): e202102439. |
| 5 | RIPLINGER C, SAMPSON M D, RITZMANN A M, et al. Mechanistic contrasts between manganese and rhenium bipyridine electrocatalysts for the reduction of carbon dioxide[J]. Journal of the American Chemical Society, 2014, 136(46): 16285-16298. |
| 6 | ZHANG Xing, WU Zishan, ZHANG Xiao, et al. Highly selective and active CO2 reduction electrocatalysts based on cobalt phthalocyanine/carbon nanotube hybrid structures[J]. Nature Communications, 2017, 8: 14675. |
| 7 | ZHOU Yan, LIU Shengtang, GU Yuming, et al. In(Ⅲ) metal-organic framework incorporated with enzyme-mimicking nickel bis(dithiolene) ligand for highly selective CO2 electroreduction[J]. Journal of the American Chemical Society, 2021, 143(35): 14071-14076. |
| 8 | LIN Gang, ZHANG Yuanyuan, HUA Yutao, et al. Bioinspired metalation of the metal-organic framework MIL-125-NH2 for photocatalytic NADH regeneration and gas-liquid-solid three-phase enzymatic CO2 reduction[J]. Angewandte Chemie International Edition, 2022, 61(31): e202206283. |
| 9 | HUANG Huining, SHI Run, LI Zhengwen, et al. Triphase photocatalytic CO2 reduction over silver-decorated titanium oxide at a gas-water boundary[J]. Angewandte Chemie International Edition, 2022, 61(17): e202200802. |
| 10 | WU H, KONG X Y, WEN X, et al. Metal-organic frameworks decorated cuprous oxide nanowires for long-lived charges applied in selective photocatalytic CO2 reduction to CH4 [J]. Angewandte Chemie International Edition, 2021, 60(15): 8455-8459. |
| 11 | GOEPPERT A, CZAUN M, JONES J P, et al. Recycling of carbon dioxide to methanol and derived products-closing the loop[J]. Chemical Society Reviews, 2014, 43(23): 7995-8048. |
| 12 | 史建公, 刘志坚, 刘春生. 二氧化碳加氢制备甲醇技术进展[J]. 中外能源, 2018, 23(9): 56-70. |
| SHI Jiangong, LIU Zhijian, LIU Chunsheng. Advances in technologies for CO2 hydrogenation to methanol[J]. Sino-Global Energy, 2018, 23(9): 56-70. | |
| 13 | IEA. Putting CO2 to use creating value from emissions[R]. 2019. https://www.iea.org/reports/putting-CO2-to-use. |
| 14 | 中国石化有机原料科技情报中心站. 山西煤炭化学研究所CO2加氢制甲醇技术取得进展[J]. 石油炼制与化工, 2016, 47(9): 45. |
| Sinopec Organic Raw Materials Science and Technology Information Center. Shanxi Institute of Coal Chemistry makes progress in CO2 hydrogenation to methanol technology[J]. Petroleum Processing and Petrochemicals, 2016, 47(9): 45. | |
| 15 | 李灿. 累计稳定运行超1000h,全球首套液态阳光加氢站示范项目通过考核[J]. 上海节能, 2020, 382(10): 1225. |
| LI Can. The world’s first demonstration project of liquid sunlight hydrogenrefueling station passed the evaluation with a total stable operationof over 1000 h [J]. Shanghai Energy Conservation, 2020, 382(10): 1225. | |
| 16 | Andrea ÁLVAREZ, BANSODE Atul, URAKAWA Atsushi, et al. Challenges in the greener production of formates/formic acid, methanol, and DME by heterogeneously catalyzed CO2 hydrogenation processes[J]. Chemical Reviews, 2017, 117(14): 9804-9838. |
| 17 | ZHONG Jiawei, YANG Xiaofeng, WU Zhilian, et al. State of the art and perspectives in heterogeneous catalysis of CO2 hydrogenation to methanol[J]. Chemical Society Reviews, 2020, 49(5): 1385-1413. |
| 18 | 贾晨喜, 邵敬爱, 白小薇, 等. 二氧化碳加氢制甲醇铜基催化剂性能的研究进展[J]. 化工进展, 2020, 39(9): 3658-3668. |
| JIA Chenxi, SHAO Jinai, BAI Xiaowei, et al. Review on Cu-based catalysts for CO2 hydrogenation to methanol[J]. Chemical Industry and Engineering Progress, 2020, 39(9): 3658-3668. | |
| 19 | NIE Xiaowa, LI Wenhui, JIANG Xiao, et al. Chapter two-recent advances in catalytic CO2 hydrogenation to alcohols and hydrocarbons[J]. Advances in Catalysis, 2019: 121-233. |
| 20 | CALAZA Florencia, STIEHLER Christian, FUJIMORI Yuichi, et al. Carbon dioxide activation and reaction induced by electron transfer at an oxide-metal interface[J]. Angewandte Chemie International Edition, 2015, 54(42): 12484-12487. |
| 21 | LI Yawei, CHAN Siew Hwa, SUN Qiang. Heterogeneous catalytic conversion of CO2: A comprehensive theoretical review[J]. Nanoscale, 2015, 7(19): 8663-8683. |
| 22 | ZHANG Wenbo, WANG Liangbing, LIU Haoyu, et al. Integration of quantum confinement and alloy effect to modulate electronic properties of RhW nanocrystals for improved catalytic performance toward CO2 hydrogenation[J]. Nano Letters, 2017, 17(2): 788-793. |
| 23 | RODRIGUEZ J A, LIU P, STACCHIOLA D J, et al. Hydrogenation of CO2 to methanol: Importance of metal-oxide and metal-carbide interfaces in the activation of CO2 [J]. ACS Catalysis, 2015, 5(11): 6696-6706. |
| 24 | LIAO Fenglin, HUANG Yaqun, GE Junwei, et al. Morphology-dependent interactions of ZnO with Cu nanoparticles at the materials’ interface in selective hydrogenation of CO2 to CH3OH[J]. Angewandte Chemie International Edition, 2011, 50(9): 2162-2165. |
| 25 | LIAO Fenglin, ZENG Ziyan, ELEY Clive, et al. Electronic modulation of a copper/zinc oxide catalyst by a heterojunction for selective hydrogenation of carbon dioxide to methanol[J]. Angewandte Chemie International Edition, 2012, 51(24): 5832-5836. |
| 26 | FROST J C. Junction effect interactions in methanol synthesis catalysts[J]. Nature, 1988, 334(6183): 577-580. |
| 27 | YANG X, KATTEL S, SENANAYAKE S D., et al. Low pressure CO2 hydrogenation to methanol over gold nanoparticles activated on a CeO x /TiO2 interface[J]. Journal of the American Chemical Society, 2015, 137(32): 10104-10107. |
| 28 | LIU Cong, YANG Bing, Eric TYO, et al. Carbon dioxide conversion to methanol over size-selected Cu4 clusters at low pressures[J]. Journal of the American Chemical Society, 2015, 137(27): 8676-8679. |
| 29 | NORSKOV J K, ABILD-PEDERSEN F, STUDT F, et al. Density functional theory in surface chemistry and catalysis[J]. Proceedings of the National Academy of Sciences of The United States of America, 2011, 108(3): 937-943. |
| 30 | ACERBI N, TSANG S C, JONES G, et al. Rationalization of interactions in precious metal/ceria catalysts using the d-band center model[J]. Angewandte Chemie International Edition, 2013, 52(30): 7737-7741. |
| 31 | KHAN M U, WANG L, LIU Z, et al. Pt3Co octapods as superior catalysts of CO2 hydrogenation[J]. Angewandte Chemie International Edition, 2016, 55(33): 9548-9552. |
| 32 | PAN W X, CAO R, ROBERTS D L, et al. Methanol synthesis activity of CuZnO catalysts[J]. Journal of Catalysis, 1988, 114(2): 440-446. |
| 33 | NATESAKHAWAT S, LEKSE J W, BALTRUS J P, et al. Active sites and structure-activity relationships of copper-based catalysts for carbon dioxide hydrogenation to methanol[J]. ACS Catalysis, 2012, 2(8): 1667-1676. |
| 34 | YANG Y, MIMS C A, MEI D H, et al. Mechanistic studies of methanol synthesis over Cu from CO/CO2/H2/H2O mixtures: The source of C in methanol and the role of water[J]. Journal of Catalysis, 2013, 298: 10-17. |
| 35 | OSTROVSKII V E. Mechanisms of methanol synthesis from hydrogen and carbon oxides at Cu-Zn-containing catalysts in the context of some fundamental problems of heterogeneous catalysis[J]. Catalysis Today, 2002, 77(3): 141-160. |
| 36 | CHOI Y, FUTAGAMI K, FUJITANI T, et al. The role of ZnO in Cu/ZnO methanol synthesis catalysts-morphology effect or active site model?[J]. Applied Catalysis A: General, 2001, 208(1/2): 163-167. |
| 37 | CHINCHEN G C, DENNY P J, JENNINGS J R, et al. Synthesis of methanol: Part 1. Catalysts and kinetics[J]. Applied Catalysis, 1988, 36: 1-65. |
| 38 | WAUGH K C. Methanol synthesis[J]. Catalysis Today, 1992, 15(1): 51-75. |
| 39 | 沈百荣, 韩继红, 孙琦, 等. EXAFS研究合成甲醇催化剂Cu/ZnO/M x O y [J]. 高等学校化学学报, 1997, 18(12): 2038-2040. |
| SHEN Bairong, HAN Jihong, SUN Qi, et al. EXAFS studies on Cu/ZnO/M x O y catalysts for methanol synthesis[J]. Chemical Journal of Chinese Universities, 1997, 18(12): 2038-2040. | |
| 40 | 沈百荣, 方志刚, 孙琦, 等. EXAFS研究合成甲醇催化剂Cu/ZnO/Al2O3的制备条件[J]. 复旦学报(自然科学版), 1999, 38(6): 681-684. |
| SHEN Bairong, FANG Zhigang, SUN Qi, et al. EXAFS study on the preparation of Cu/ZnO/Al2O3 catalyst for methanol synthesis[J]. Journal of Fudan University (Natural Science), 1999, 38(6): 681-684. | |
| 41 | MA Z Y, YANG C, WEI W, et al. Catalytic performance of copper supported on zirconia polymorphs for CO hydrogenation[J]. Journal of Molecular Catalysis A: Chemical, 2005, 231(1/2): 75-81. |
| 42 | SZANYI J, Wayne GOODMAN D.. Methanol synthesis on a Cu(100) catalyst[J]. Catalysis Letters, 1991, 10(5): 383-390. |
| 43 | LIAO Wenjie, LIU Ping. Methanol synthesis from CO2 hydrogenation over a potassium-promoted Cu x O/Cu(111) (x≤2) model surface: Rationalizing the potential of potassium in catalysis[J]. ACS Catalysis, 2020, 10(10): 5723-5733. |
| 44 | DASIREDDY V D, LIKOZAR B. The role of copper oxidation state in Cu/ZnO/Al2O3 catalysts in CO2 hydrogenation and methanol productivity[J]. Renewable Energy, 2019, 140: 452-460. |
| 45 | WANG Z Q, XU Z N, PENG S Y, et al. High-performance and long-lived Cu/SiO2 nanocatalyst for CO2 hydrogenation[J]. ACS Catalysis, 2015, 5(7): 4255-4259. |
| 46 | GAO L Z, AU C T. CO2 hydrogenation to methanol on a YBa2Cu3O7 catalyst[J]. Journal of Catalysis, 2000, 189(1): 1-15. |
| 47 | YU Jiafeng, YANG Meng, ZHANG Jixin, et al. Stabilizing Cu+ in Cu/SiO2 catalysts with a Shattuckite-like structure boosts CO2 hydrogenation into methanol[J]. ACS Catalysis, 2020, 10(24): 14694-14706. |
| 48 | KULKARNI G U. EXAFS and XPS investigations of Cu/ZnO catalysts and their interaction with CO and methanol[J]. Topics in Catalysis, 2003, 22(3/4): 183-189. |
| 49 | NAKAMURA J, UCHIJIMA T, KANAI Y, et al. The role of ZnO in Cu/ZnO methanol synthesis catalysts[J]. Catalysis Today, 1996, 28(3): 223-230. |
| 50 | DAI W L, SUN Q, DENG J F, et al. XPS studies of Cu/ZnO/Al2O3 ultra-fine catalysts derived by a novel gel oxalate co-precipitation for methanol synthesis by CO2+H2 [J]. Applied Surface Science, 2001, 177(3): 172-179. |
| 51 | OKAMOTO Yasuaki, FUKINO Kiyotaka, IMANAKA Toshinobu, et al. Surface characterization of copper(Ⅱ) oxide-zinc oxide methanol-synthesis catalysts by X-ray photoelectron spectroscopy. 2. Reduced catalysts[J]. The Journal of Physical Chemistry, 1983, 87(19): 3747-3754. |
| 52 | LIU Jinyao, SHI Jiangliu, HE Dehua, et al. Surface active structure of ultra-fine Cu/ZrO2 catalysts used for the CO2+H2 to methanol reaction[J]. Applied Catalysis A: General, 2001, 218(1/2): 113-119. |
| 53 | HAN Aizhe, DING Jie, ZHONG Qin. Role of single-atom Pd in Cu/ZrO2 catalysts for CO2 hydrogenation to methanol[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2022, 641: 128535. |
| 54 | YU Jiahui, LIU Shuai, MU Xueliang, et al. Cu-ZrO2 catalysts with highly dispersed Cu nanoclusters derived from ZrO2@HKUST-1 composites for the enhanced CO2 hydrogenation to methanol[J]. Chemical Engineering Journal, 2021, 419: 129656. |
| 55 | GUO Qing, LI Shaozhong, LI Jin, et al. Enhanced CO2 hydrogenation to methanol on the mesostructured Cu-ZnO/Al2O3-ZrO2 catalyst[J]. ACS Applied Energy Materials, 2021, 4(8): 8311-8321. |
| 56 | KULD S, CONRADSEN C, MOSES P G, et al. Quantification of zinc atoms in a surface alloy on copper in an industrial-type methanol synthesis catalyst[J]. Angewandte Chemie International Edition, 2014, 53(23): 5941-5945. |
| 57 | FUJITANI T, NAKAMURA J. The chemical modification seen in the Cu/ZnO methanol synthesis catalysts[J]. Applied Catalysis A: General, 2000, 191(1/2): 111-129. |
| 58 | MARTIN Oliver, MONDELLI Cecilia, Daniel CURULLA-FERRé, et al. Zinc-rich copper catalysts promoted by gold for methanol synthesis[J]. ACS Catalysis, 2015, 5(9): 5607-5616. |
| 59 | KATTEL S, RAMIREZ P J, CHEN J G, et al. Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts[J]. Science, 2017, 355(6331): 1296-1299. |
| 60 | KATTEL S, RAMIREZ P J, CHEN J G, et al. Response to comment on “Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts”[J]. Science, 2017, 357(6354): eaan8210. |
| 61 | LUNKENBEIN Thomas, SCHUMANN Julia, BEHRENS Malte, et al. Formation of a ZnO overlayer in industrial Cu/ZnO/Al2O3 catalysts induced by strong metal-support interactions[J]. Angewandte Chemie International Edition, 2015, 54(15): 4544-4548. |
| 62 | BEHRENS Malte, FURCHE Andreas, KASATKIN Igor, et al. The potential of microstructural optimization in metal/oxide catalysts: Higher intrinsic activity of copper by partial embedding of copper nanoparticles[J]. ChemCatChem, 2010, 2(7): 816-818. |
| 63 | 崔晓静, 颜丽红, 王慧芳, 等. CO2加氢制甲醇铜基催化剂:分散度调控选择性[J]. 高校化学工程学报, 2022, 36(4): 562-569. |
| CUI Xiaojing, YAN Lihong, WANG Huifang, et al. Improvement of methanol selectivity in CO2 hydrogenation via tuning Cu dispersion on support[J]. Journal of Chemical Engineering of Chinese Universities, 2022, 36(4): 562-569. | |
| 64 | 李金林, 白瑞洁, 张煜华, 等. Cu/ZnO-In2O3催化剂用于CO2加氢制甲醇的催化性能[J]. 中南民族大学学报(自然科学版), 2022, 41(3): 257-262. |
| LI Jinlin, BAI Ruijie, ZHANG Yuhua, et al. Catalytic performances of Cu/ZnO-In2O3 catalysts for CO2 hydrogenation to methanol[J]. Journal of South-Central Minzu University (Natural Science Edition), 2022, 41(3): 257-262. | |
| 65 | FENG Kai, WANG Yaning, GUO Man, et al. In-situ/operando techniques to identify active sites for thermochemical conversion of CO2 over heterogeneous catalysts[J]. Journal of Energy Chemistry, 2021, 62: 153-171. |
| 66 | ZENG Shanghong, SHAN Shiyao, LU Aolin, et al. Copper-alloy catalysts: Structural characterization and catalytic synergies[J]. Catalysis Science & Technology, 2021, 11(17): 5712-5733. |
| 67 | EREN Baran, TORRES Daniel, KARSLIOGLU Osman, et al. Structure of copper-cobalt surface alloys in equilibrium with carbon monoxide gas[J]. Journal of the American Chemical Society, 2018, 140(21): 6575-6581. |
| 68 | MARCINKOWSKI M D, DARBY M T, LIU J, et al. Pt/Cu single-atom alloys as coke-resistant catalysts for efficient C-H activation[J]. Nature Chemistry, 2018, 10(3): 325-332. |
| 69 | BERSANI M, GUPTA K, MISHRA A K, et al. Combined EXAFS, XRD, DRIFTS, and DFT study of nano copper-based catalysts for CO2 hydrogenation[J]. ACS Catalysis, 2016, 6(9): 5823-5833. |
| 70 | LUM Y, AGER J W. Evidence for product-specific active sites on oxide-derived Cu catalysts for electrochemical CO2 reduction[J]. Nature Catalysis, 2019, 2(1): 86-93. |
| 71 | ZHANG Baohua, GUO Zhihao, ZUO Zhuang, et al. The ensemble effect of nitrogen doping and ultrasmall SnO2 nanocrystals on graphene sheets for efficient electroreduction of carbon dioxide[J]. Applied Catalysis B: Environmental, 2018, 239: 441-449. |
| 72 | LI Fengwang, CHEN Lu, XUE Mianqi, et al. Towards a better Sn: Efficient electrocatalytic reduction of CO2 to formate by Sn/SnS2 derived from SnS2 nanosheets[J]. Nano Energy, 2017, 31: 270-277. |
| 73 | LIU X M, LU G Q, YAN Z F, et al. Recent advances in catalysts for methanol synthesis via hydrogenation of CO and CO2 [J]. Industrial & Engineering Chemistry Research, 2003, 42(25): 6518-6530. |
| 74 | WU Jianbo, SHAN Hao, CHEN Wenlong, et al. In situ environmental TEM in imaging gas and liquid phase chemical reactions for materials research[J]. Advanced Materials, 2016, 28(44): 9686-9712. |
| 75 | MUKUNDAN Vineetha, SHAN Shiyao, ZHONG Chuanjian, et al. Effect of chemical composition on the nanoscale ordering transformations of physical mixtures of Pd and Cu nanoparticles[J]. Journal of Nanomaterials, 2018, 2018: 9087320. |
| 76 | MA L, LUO X, Jeremy KROPF A., et al. Insight into the catalytic mechanism of bimetallic platinum-copper core-shell nanostructures for nonaqueous oxygen evolution reactions[J]. Nano Letters, 2016, 16(1): 781-785. |
| 77 | WU Jinfang, SHAN Shiyao, PETKOV Valeri, et al. Composition-structure-activity relationships for palladium-alloyed nanocatalysts in oxygen reduction reaction: An ex-situ/in-situ high energy X-ray diffraction study[J]. ACS Catalysis, 2015, 5(9): 5317-5327. |
| 78 | MUKUNDAN Vineetha, YIN Jun, JOSEPH Pharrah, et al. Nanoalloying and phase transformations during thermal treatment of physical mixtures of Pd and Cu nanoparticles[J]. Science and Technology of Advanced Materials, 2014, 15(2): 025002. |
| 79 | SHAN Shiyao, PETKOV Valeri, PRASAI Binay, et al. Catalytic activity of bimetallic catalysts highly sensitive to the atomic composition and phase structure at the nanoscale[J]. Nanoscale, 2015, 7(45): 18936-18948. |
| 80 | KAUFFMAN D R, ALFONSO D R, TAFEN D N, et al. Selective electrocatalytic reduction of CO2 into CO at small, thiol-capped Au/Cu nanoparticles[J]. The Journal of Physical Chemistry C, 2018, 122(49): 27991-28000. |
| 81 | CHUNG W C, HSU S Y, PAO C W, et al. Correlation of photocatalytic CO2 conversion and electronic structure of UiO-66 and Cu-UiO-66-NH2 under irradiation studied by in-situ X-ray absorption spectroscopy[J]. Journal of CO2 Utilization, 2022, 60: 101961. |
| 82 | JANGAM A, HONGMANOROM P, HUI W M, et al. CO2 hydrogenation to methanol over partially reduced Cu-SiO2P catalysts: The crucial role of hydroxyls for methanol selectivity[J]. ACS Applied Energy Materials, 2021, 4(11): 12149-12162. |
| 83 | FAN Ruoyu, ZHANG Yange, HU Zhi, et al. Synergistic catalysis of cluster and atomic copper induced by copper-silica interface in transfer-hydrogenation[J]. Nano Research, 2021, 14(12): 4601-4609. |
| 84 | PETER M, FENDT J, PLEINTINGER S, et al. On the interaction of carbon monoxide with ternary Cu/ZnO/Al2O3 catalysts: Modeling of dynamic morphological changes and the influence on elementary step kinetics[J]. Catalysis Science & Technology, 2012, 2(11): 2249-2257. |
| 85 | MARCOS F C, CAVALCANTI F M, PETROLINI D D, et al. Effect of operating parameters on H2/CO2 conversion to methanol over Cu-Zn oxide supported on ZrO2 polymorph catalysts: Characterization and kinetics[J]. Chemical Engineering Journal, 2022, 427: 130947. |
| 86 | WANG Weiwei, QU Zhenping, SONG Lixin, et al. CO2 hydrogenation to methanol over Cu/CeO2 and Cu/ZrO2 catalysts: Tuning methanol selectivity via metal-support interaction[J]. Journal of Energy Chemistry, 2020, 40: 22-30. |
| 87 | BUKHTIYAROVA M V, BULAVCHENKO O A, BUKHTIYAROV A V, et al. Selective hydrogenation of 5-acetoxymethylfurfural over Cu-based catalysts in a flow reactor: Effect of Cu-Al layered double hydroxides synthesis conditions on catalytic properties[J]. Catalysts, 2022, 12(8): 878. |
| 88 | JIN Sen, SHAO Wei, CHEN Shichuan, et al. Ultrathin in-plane heterostructures for efficient CO2 chemical fixation[J]. Angewandte Chemie International Edition, 2022, 61(3): e202113411. |
| 89 | CUI Xiaojing, YAN Wenjun, YANG Huanhuan, et al. Preserving the active Cu-ZnO interface for selective hydrogenation of CO2 to dimethyl ether and methanol[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(7): 2661-2672. |
| 90 | ZHU Jiadong, SU Yaqiong, CHAI Jiachun, et al. Mechanism and nature of active sites for methanol synthesis from CO/CO2 on Cu/CeO2 [J]. ACS Catalysis, 2020, 10(19): 11532-11544. |
| 91 | MARCOS F C, LIN L, BETANCOURT L E, et al. Insights into the methanol synthesis mechanism via CO2 hydrogenation over Cu-ZnO-ZrO2 catalysts: Effects of surfactant/Cu-Zn-Zr molar ratio[J]. Journal of CO2 Utilization, 2020, 41: 101215. |
| 92 | ZHU M, TIAN P, FORD M E, et al. Nature of reactive oxygen intermediates on copper-promoted iron-chromium oxide catalysts during CO2 activation[J]. ACS Catalysis, 2020, 10(14): 7857-7863. |
| 93 | CHEN Aling, YU Xiaojuan, ZHOU Yan, et al. Structure of the catalytically active copper-ceria interfacial perimeter[J]. Nature Catalysis, 2019, 2(4): 334-341. |
| 94 | LI Didi, XU Fang, TANG Xuan, et al. Induced activation of the commercial Cu/ZnO/Al2O3 catalyst for the steam reforming of methanol[J]. Nature Catalysis, 2022, 5(2): 99-108. |
| 95 | LIU Xinyu, LUO Jie, WANG Hengwei, et al. In situ spectroscopic characterization and theoretical calculations identify partially reduced ZnO1- x /Cu interfaces for methanol synthesis from CO2 [J]. Angewandte Chemie International Edition, 2022, 61(23): e202202330. |
| 96 | ZHANG Jingpeng, WANG Yaning, TIAN Jiaming, et al. Cu/LaFeO3 as an efficient and stable catalyst for CO2 reduction: Exploring synergistic effect between Cu and LaFeO3 [J]. AIChE Journal, 2022, 68(6): e17640. |
| 97 | ZHANG Jingpeng, SUN Xiaohang, WU Congyi, et al. Engineering Cu+/CeZrO x interfaces to promote CO2 hydrogenation to methanol[J]. Journal of Energy Chemistry, 2023, 77: 45-53. |
| 98 | FENG Kai, TIAN Jiaming, GUO Man, et al. Experimentally unveiling the origin of tunable selectivity for CO2 hydrogenation over Ni-based catalysts[J]. Applied Catalysis B: Environmental, 2021, 292: 120191. |
| 99 | YAN Binhang, WU Qiyuan, CEN Jiajie, et al. Highly active subnanometer Rh clusters derived from Rh-doped SrTiO3 for CO2 reduction[J]. Applied Catalysis B: Environmental, 2018, 237: 1003-1011. |
| [1] | WANG Jijie, HAN Zhe, CHEN Siyu, TANG Chizhou, SHA Feng, TANG Shan, YAO Tingting, LI Can. Liquid sunshine methanol [J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1309-1317. |
| [2] | LIU Chang, LIU Zhongwen. Perspective on the one-step CO2 hydrogenation to dimethyl ether [J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1115-1120. |
| [3] | ZHENG Renyang. Advances in structure design and alkane isomerization performance of metal-zeolite bifunctional catalyst [J]. Chemical Industry and Engineering Progress, 2021, 40(7): 3785-3790. |
| [4] | WANG Yimeng, LIU Jianjun, ZUO Shengli, LI Kang. Research progress of active sites of MoS2 photoelectrocatalyst: optimization and performance [J]. Chemical Industry and Engineering Progress, 2021, 40(7): 3747-3759. |
| [5] | YANG Panpan, SUN Qi, ZHANG Yulong, HAN Yifan. Research progress of the role of CO2 in methanol synthesis [J]. Chemical Industry and Engineering Progress, 2018, 37(S1): 94-101. |
| [6] | AN Xia, NING Wensheng, LIU Huazhang. Research progress of Cu-Co bimetallic catalysts to the production of higher alcohols from syngas [J]. Chemical Industry and Engineering Progress, 2018, 37(10): 3843-3849. |
| [7] | CHEN Xiong,ZHENG Huayan,YANG Hao,LI Zhong. Influence of CO2 in ageing process on the catalytic property of CuO/ZnO/Al2O3 catalyst [J]. Chemical Industry and Engineering Progree, 2013, 32(11): 2644-2649. |
| [8] | QIN Zhiqiang,GAO Wengui,WANG Hua,HAN Chong,GUO Wei. Research on rare-earth promoter Pr-modified Cu/Zn/ZrO2 catalyst for methanol synthesis [J]. Chemical Industry and Engineering Progree, 2013, 32(04): 820-823. |
| [9] | SUN Zhiwei,ZHANG Li,YAN Yunfei. Numerical analysis on the characteristics of carbon deposition of catalytic partial oxidation of methane under gravity [J]. Chemical Industry and Engineering Progree, 2012, 31(04): 839-843. |
| [10] | GUO Xiaoming,MAO Dongsen,LU Guanzhong,WANG Song. Progress in catalysts for methanol synthesis from CO2 hydrogenation [J]. Chemical Industry and Engineering Progree, 2012, 31(03): 477-488. |
| [11] | BAI Shaofen,LIU Xinmei,YAN Zifeng. Advance in research on reaction mechanism and active sites for methanol synthesis [J]. Chemical Industry and Engineering Progree, 2011, 30(7): 1466-. |
| [12] | GE Hui,LI Xuekuan,QIN Zhangfeng,WANG Jianguo. Recent progress of catalyst development for deep hydrodesulfurization of oil products [J]. Chemical Industry and Engineering Progree, 2008, 27(10): 1490-. |
| [13] | ZHANG Juntao,ZHANG Guoli,LIANG Shengrong,FENG Xiao. Recent developments in heterogeneous catalyst of ethylene oligomerization to α-olefins [J]. Chemical Industry and Engineering Progree, 2008, 27(1): 57-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||