化工进展 ›› 2022, Vol. 41 ›› Issue (1): 227-236.DOI: 10.16085/j.issn.1000-6613.2021-0258
等离子体与Cu-Pd/S-1催化剂协同催化甲烷转化制低碳烯烃
毕文菲( ), 代成义(
), 代成义( ), 李雪梅, 贺建勋, 赵彬然, 马晓迅(
), 李雪梅, 贺建勋, 赵彬然, 马晓迅( )
)
- 西北大学化工学院,国家碳氢资源清洁利用国际科技合作基地,陕北能源先进化工利用技术教育部工程研究 中心,陕西省洁净煤转化工程技术研究中心,陕北能源化工产业发展协同创新中心,陕西 西安 710069
-
收稿日期:2021-02-04修回日期:2021-03-08出版日期:2022-01-05发布日期:2022-01-24 -
通讯作者:代成义,马晓迅 -
作者简介:毕文菲(1995—),女,硕士研究生,研究方向为非热等离子体条件下CH4转化。E-mail:1106380402@qq.com 。
Synergistic catalysis of methane to light olefins by plasma and Cu-Pd/S-1 catalyst
BI Wenfei( ), DAI Chengyi(
), DAI Chengyi( ), LI Xuemei, HE Jianxun, ZHAO Binran, MA Xiaoxun(
), LI Xuemei, HE Jianxun, ZHAO Binran, MA Xiaoxun( )
)
- School of Chemical Engineering, Northwest University; International Science and Technology Cooperation Base of MOST for Clean Utilization of Hydrocarbon Resources; Chemical Engineering Research Center of the Ministry of Education for Advance Use Technology of Shanbei Energy; Shaanxi Research Center of Engineering Technology for Clean Coal Conversion; Collaborative Innovation Center for Shanbei Energy and Chemical Industry Development, Xi’an 710069, Shaanxi, China
-
Received:2021-02-04Revised:2021-03-08Online:2022-01-05Published:2022-01-24 -
Contact:DAI Chengyi,MA Xiaoxun
摘要:
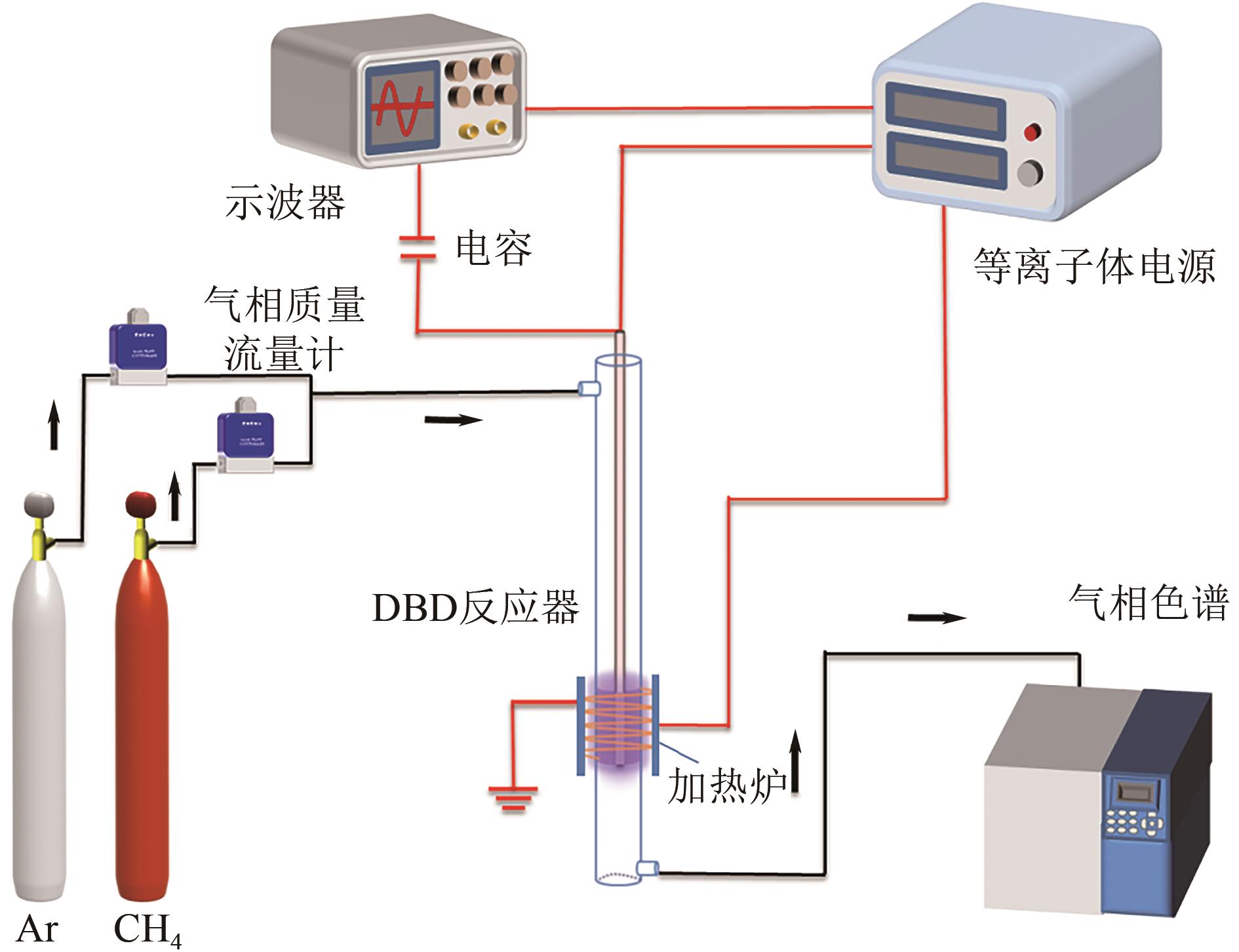
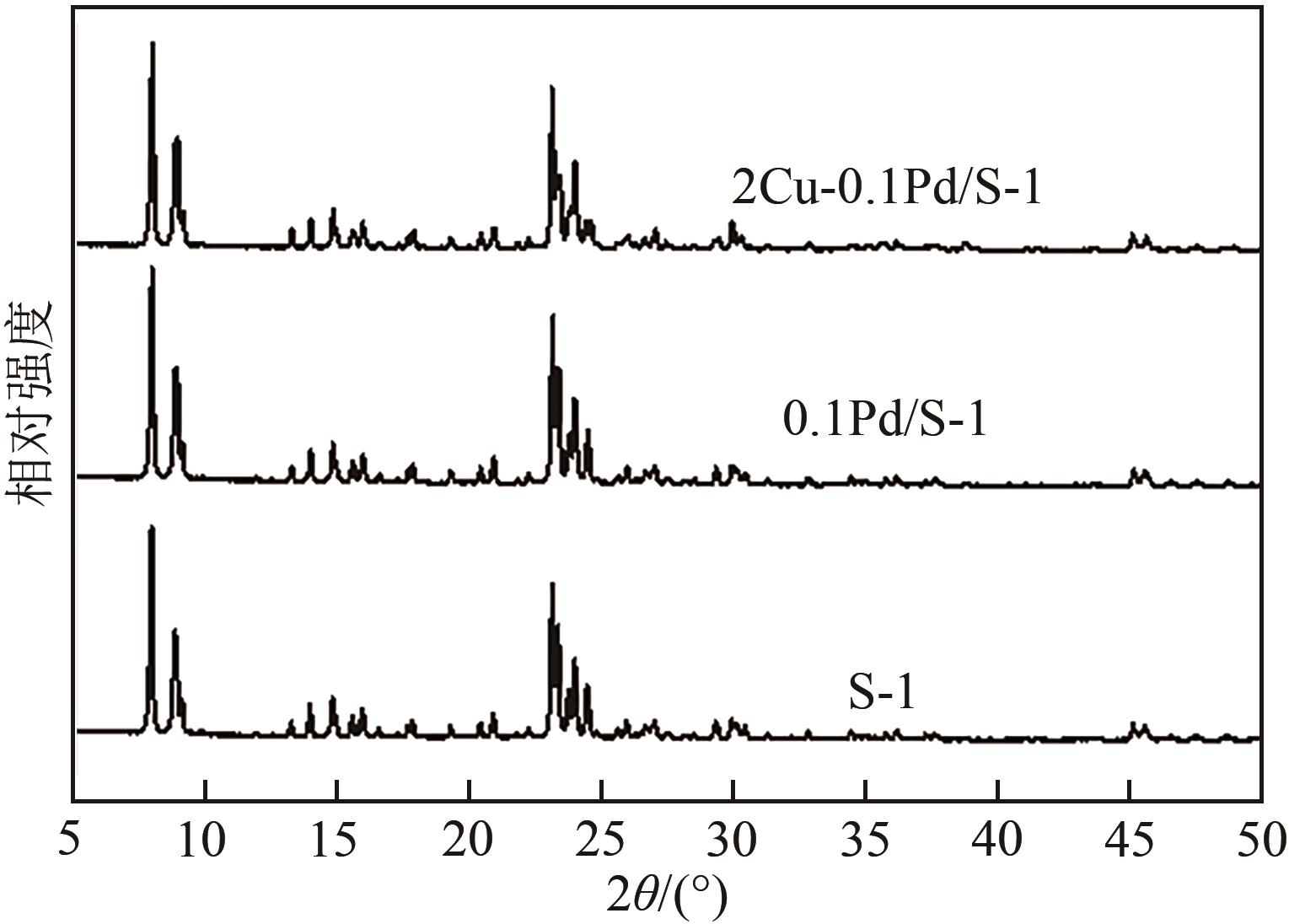
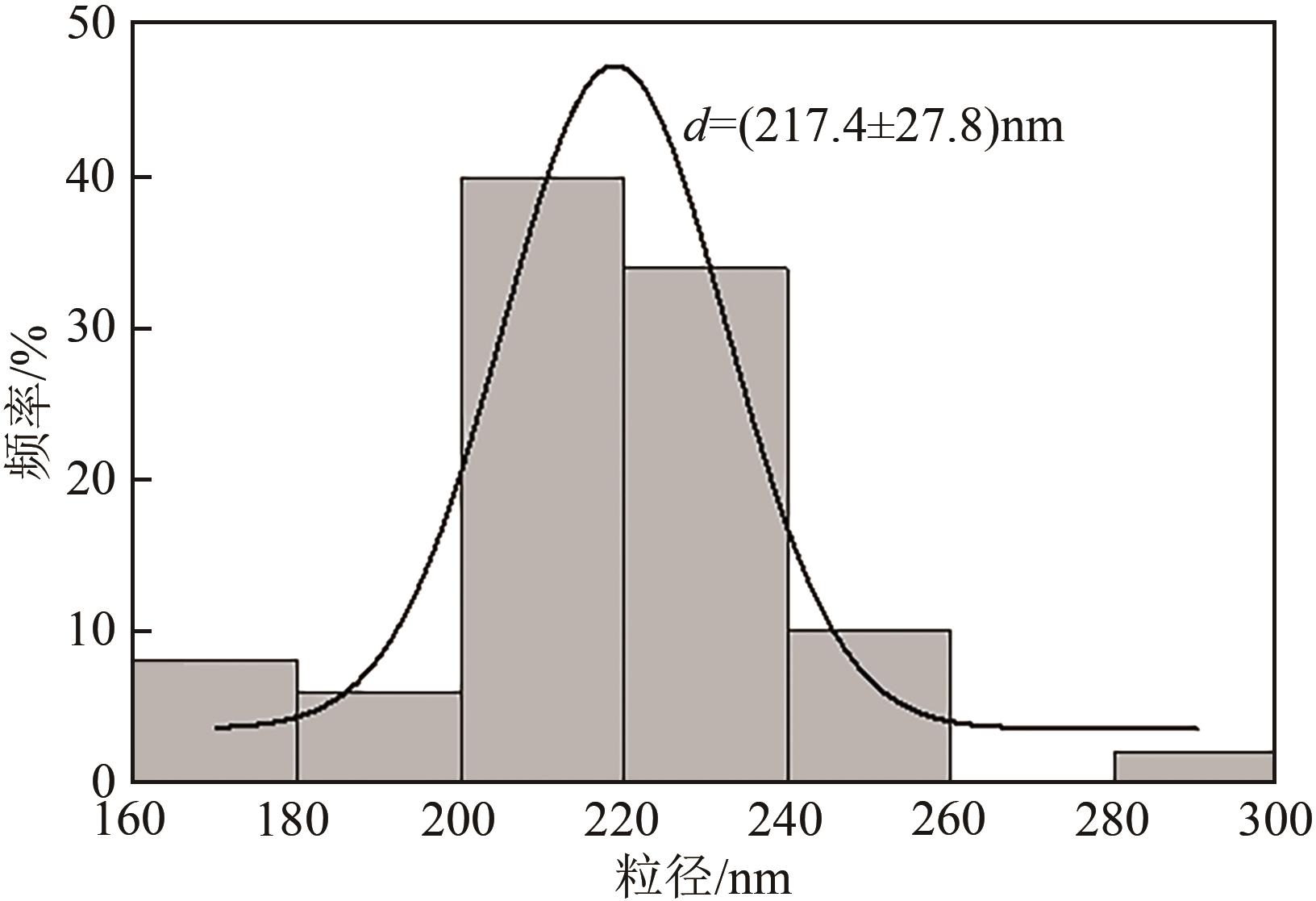
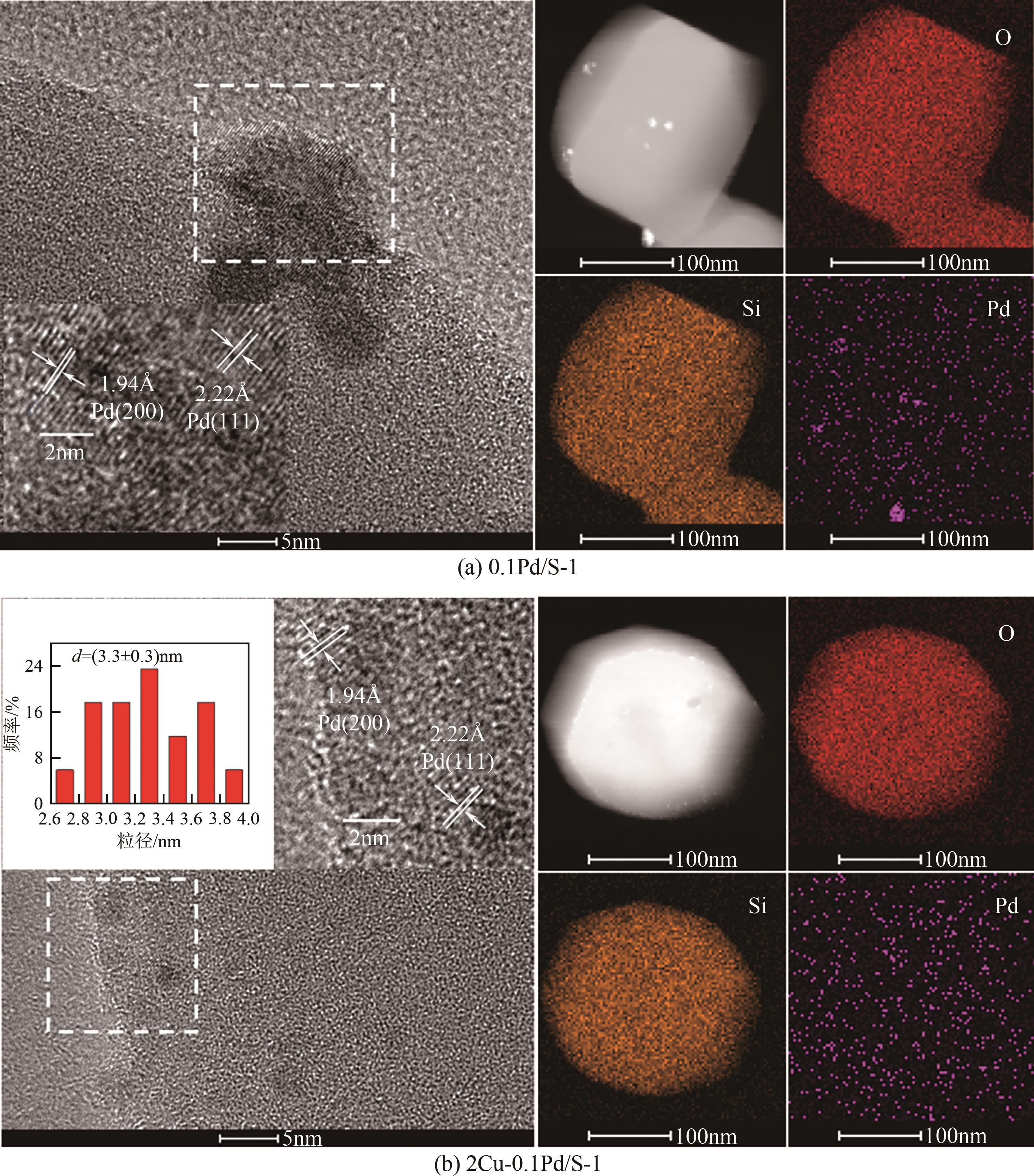
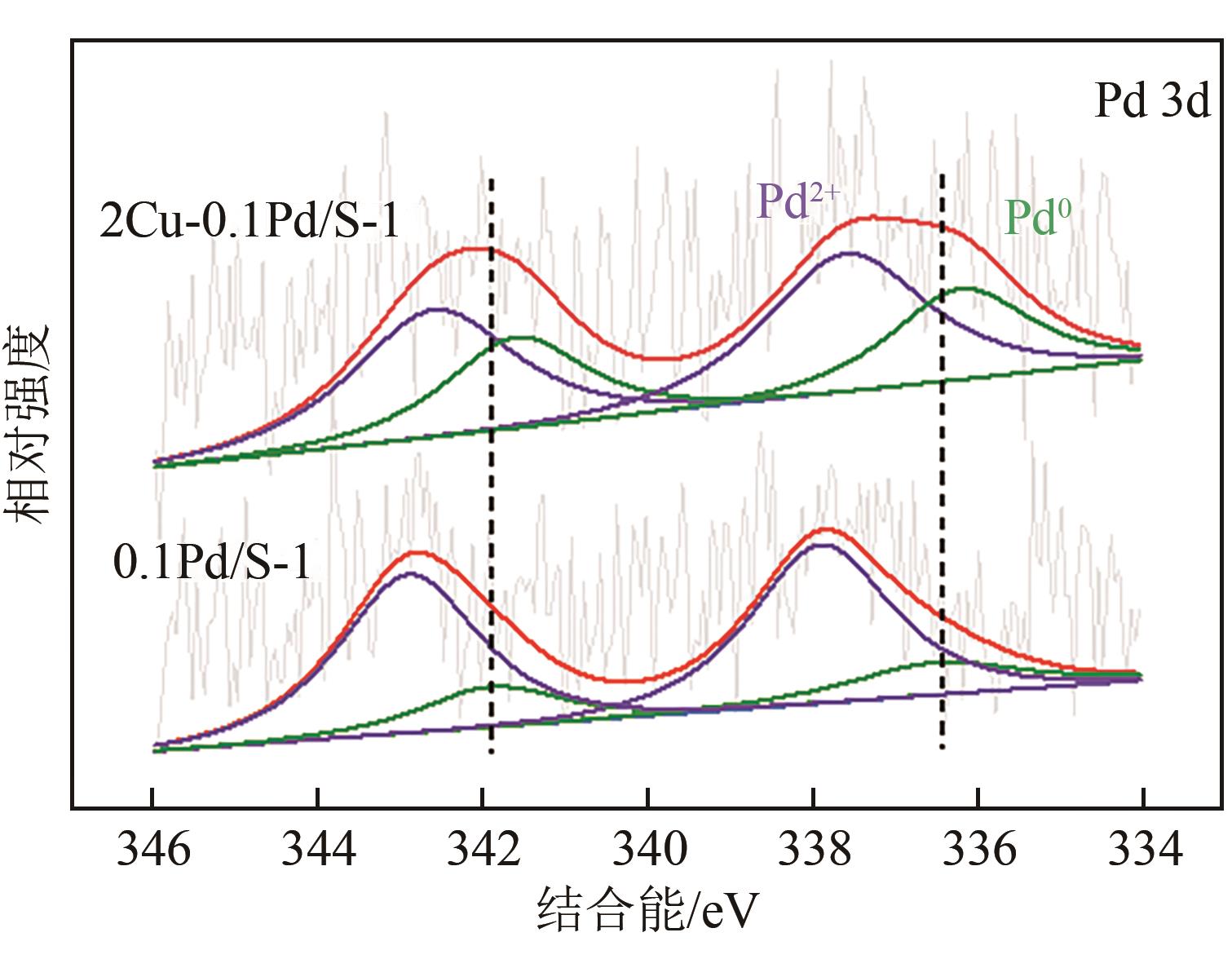
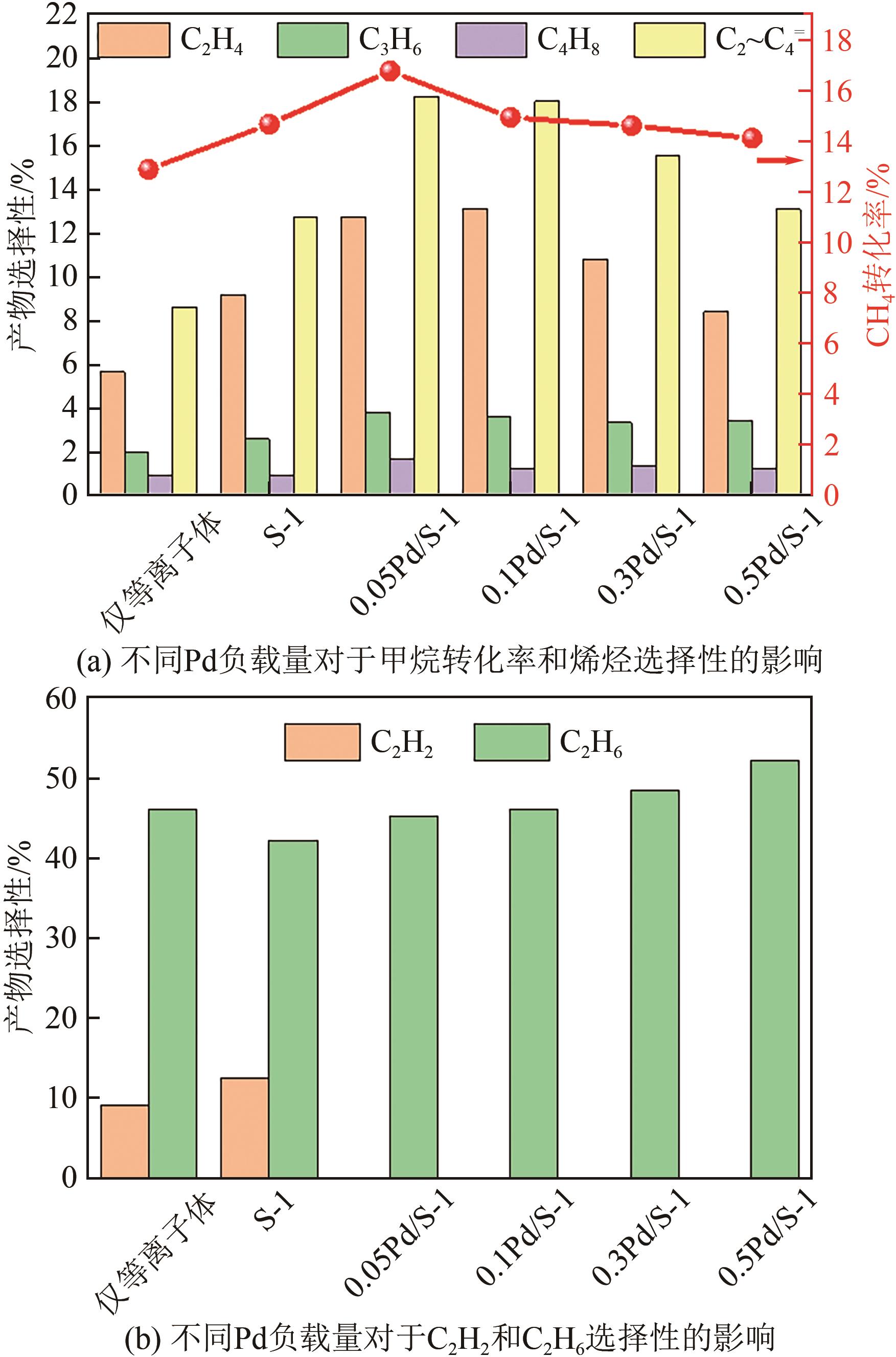
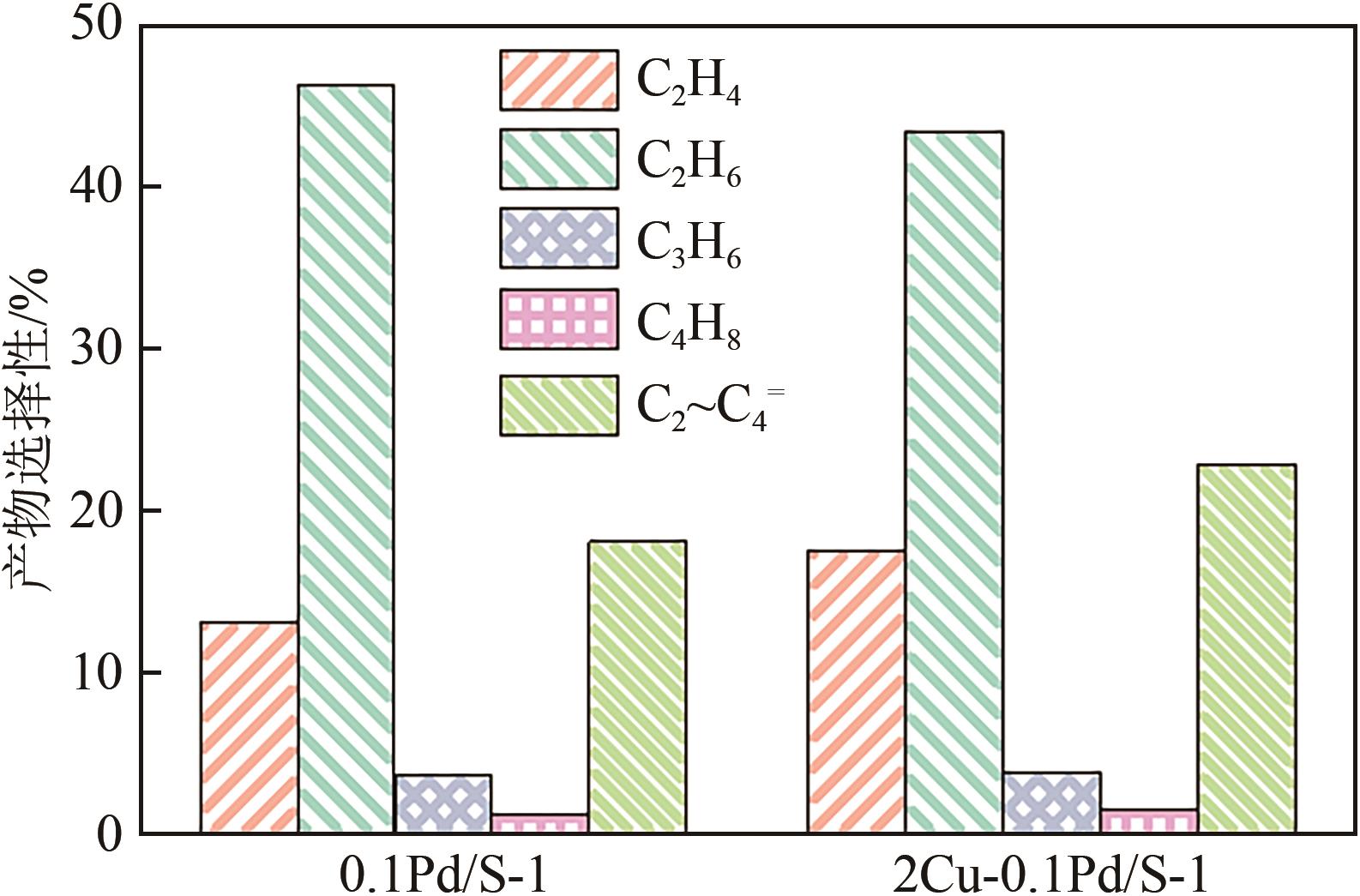
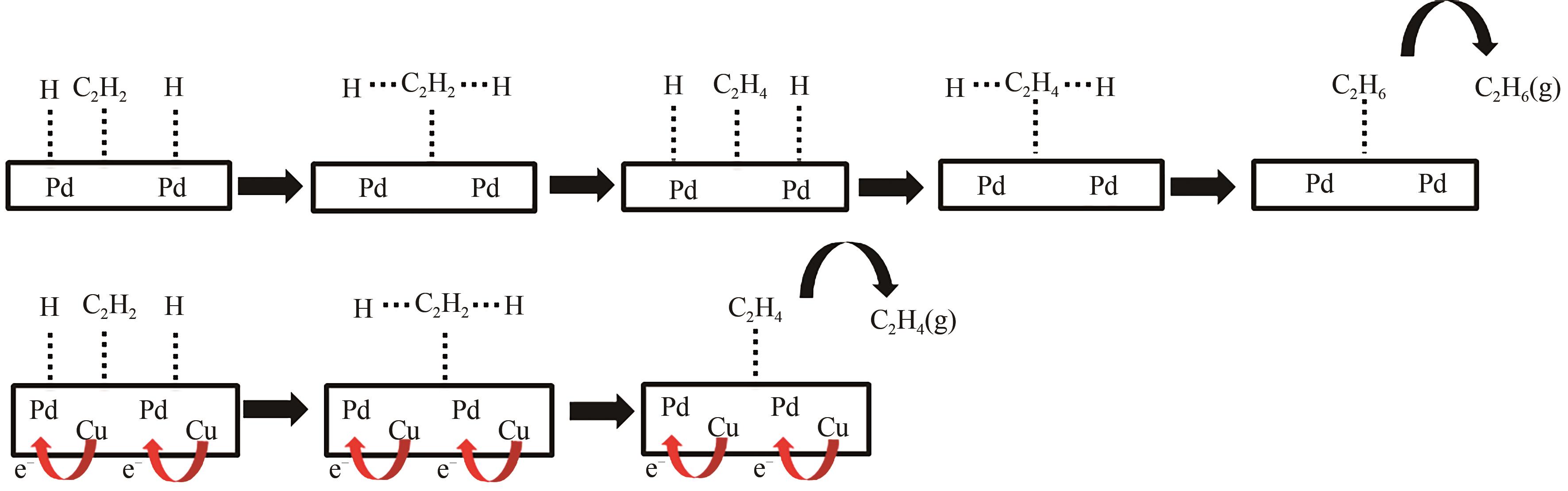
采用等体积浸渍法制备了Pd、Cu-Pd改性的S-1催化剂,利用介质阻挡放电(DBD)等离子体反应器研究了甲烷无氧转化制低碳烯烃(C2~C4=)的性能,重点关注了乙烯的产量。探讨了Ar的添加和特定输入能量(SIE)对甲烷转化率以及产物分布的影响。实验结果表明,等离子体与催化剂协同催化与仅使用等离子体相比性能更优异,使乙烯选择性提高了3.1倍,C2~C4=的选择性提高了2.7倍;与S-1相比,Pd/S-1具有更高的乙烯选择性,这是因为在S-1上负载金属Pd有助于乙炔原位加氢生成乙烯;适宜的Pd负载量有利于提高烯烃选择性,而过高的Pd负载量倾向于不饱和烃的连续加氢,导致了烷烃的生成;与单金属Pd改性相比,Cu-Pd双金属改性抑制了乙烯的进一步加氢,提高了乙烯的选择性。通过扫描电子显微镜(SEM)、透射电子显微镜(TEM)、高倍透射电子显微镜(HRTEM)、X射线粉末衍射(XRD)、X射线光电子能谱(XPS)对催化剂进行了表征分析。结果表明,Cu的加入使自身电子向Pd转移,增加了Pd电子密度;另外,Cu的存在提高了Pd的分散性。以2Cu-0.1Pd/S-1为催化剂时可以得到更优异的反应性能。
中图分类号:
引用本文
毕文菲, 代成义, 李雪梅, 贺建勋, 赵彬然, 马晓迅. 等离子体与Cu-Pd/S-1催化剂协同催化甲烷转化制低碳烯烃[J]. 化工进展, 2022, 41(1): 227-236.
BI Wenfei, DAI Chengyi, LI Xuemei, HE Jianxun, ZHAO Binran, MA Xiaoxun. Synergistic catalysis of methane to light olefins by plasma and Cu-Pd/S-1 catalyst[J]. Chemical Industry and Engineering Progress, 2022, 41(1): 227-236.
| 催化剂 | 元素/% | ||||
|---|---|---|---|---|---|
| O | Si | C | Pd | Cu | |
| 0.1Pd/S-1 | 48.80 | 29.07 | 22.11 | 0.02 | — |
| 2Cu-0.1Pd/S-1 | 51.08 | 28.48 | 19.93 | 0.05 | 0.45 |
表1 不同催化剂的XPS表面元素质量分数
| 催化剂 | 元素/% | ||||
|---|---|---|---|---|---|
| O | Si | C | Pd | Cu | |
| 0.1Pd/S-1 | 48.80 | 29.07 | 22.11 | 0.02 | — |
| 2Cu-0.1Pd/S-1 | 51.08 | 28.48 | 19.93 | 0.05 | 0.45 |
| 1 | WANG X Y, CAO R W, CHEN K, et al. Synthesis gas conversion to lower olefins over ZnCr-SAPO-34 catalysts: role of ZnO-ZnCr2O4 interface[J]. ChemCatChem, 2020, 12(17): 4387-4395. |
| 2 | SHAO J, FU T J, LI Z. The selective and stable synthesis of aromatics from methanol via two-step route using light alkenes as intermediates[J]. Fuel, 2020, 280: 118609. |
| 3 | 屈叶青, 唐鹏武, 陈健.甲烷制低碳烯烃技术简析[J]. 石油化工技术与经济, 2017, 33(4): 52-56. |
| QU Yeqing, TANG Pengwu, CHEN Jian. Technical analysis of light olefins from methane[J]. Technology & Economics in Petrochemicals, 2017, 33(4): 52-56. | |
| 4 | 高佳良, 张根, 程序, 等. 咪唑改性MCM-22分子筛的制备及甲烷无氧芳构化性能[J]. 化工进展, 2019, 38(3): 1387-1395. |
| GAO Jialiang, ZHANG Gen, CHENG Xu, et al. Preparation of imidazole modified MCM-22 molecular sieves and its catalytic performance in methane dehydroaromatization[J]. Chemical Industry and Engineering Progress, 2019, 38(3): 1387-1395. | |
| 5 | 杨涛,鲁伊恒. 甲烷催化转化制高碳烃技术进展[J]. 广东化工, 2017, 44(3): 71-72. |
| YANG Tao, LU Yiheng. Methane conversion higher hydrocarbons progress[J]. Guangdong Chemical Industry, 2017, 44(3): 71-72. | |
| 6 | LIU Y T, DENG D H, BAO X H. Catalysis for selected C1 chemistry[J]. Chem., 2020, 6(10): 2497-2514. |
| 7 | GUO D, LU Y, RUAN Y Z, et al. Effects of extrinsic defects originating from the interfacial reaction of CeO2-x-nickel silicate on catalytic performance in methane dry reforming[J]. Applied Catalysis B: Environmentai, 2020, 277: 119278. |
| 8 | CHEN M Y, SONG Y, LIU B, et al. Experimental investigation of the promotion effect of CO on catalytic behavior of Mo/HZSM-5 catalyst in CH4 dehydroaromatization at 1073 K[J]. Fuel, 2020, 262: 116674. |
| 9 | 刘剑, 汲永钢, 万书宝, 等. 低碳烯烃生产技术新进展[J]. 乙烯工业, 2019, 31(1): 12-15. |
| LIU Jian, JI Yonggang, WAN Shubao, et al. New progress in the production technology of low carbon olefin[J]. Ethylene Industry, 2019, 31(1): 12-15, 5. | |
| 10 | 高思, 倪文骁, 陈士朋, 等. Ni/MgO-Al2O3的制备方法对甲烷联合重整反应的影响[J]. 精细石油化工进展, 2020, 21(1): 46-48. |
| GAO Si, NI Wenxiao, CHEN Shipeng, et al. Effect of Ni/MgO-Al2O3 preparation method on combined reforming reaction of methane[J]. Advances in Fine Petrochemicals, 2020, 21(1): 46-48. | |
| 11 | 孟豪, 林明桂, 牛鹏宇, 等. La2Zr2O7催化剂结构相变对甲烷氧化偶联反应性能的影响[J]. 燃料化学学报, 2020, 48(8): 949-959. |
| MENG Hao, LIN Minggui, NIU Pengyu, et al. Effect of phase transformation of La2Zr2O7 catalysts on catalytic performance for the oxidative coupling of methane[J]. Journal of Fuel Chemistry and Technology, 2020, 48(8): 949-959. | |
| 12 | SHIMIZU T, KITAYAMA Y, KODAMA T. Thermochemical conversion of CH4 to C2-hydrocarbons and H2 over SnO2/Fe3O4/SiO2 in methane-water co-feed system[J]. Energy & Fuels, 2001, 15: 463-469. |
| 13 | FARRELL B L, IGENEGBAI V O, LINIC S. A viewpoint on direct methane conversion to ethane and ethylene using oxidative coupling on solid catalysts[J]. ACS Catalysis, 2016, 6(7): 4340-4346. |
| 14 | GUO X G, FANG G Z, LI G, et al. Direct, nonoxidative conversion of methane to ethylene, aromatics, and hydrogen[J]. Science, 2014, 344(6184): 616-619. |
| 15 | WANG K J, LI X S, ZHU A M. A green process for high-concentration ethylene and hydrogen production from methane in a plasma-followed-by-catalyst reactor[J]. Plasma Science and Technology, 2011, 13(1): 77-81. |
| 16 | SEKINE Y, TANAKA K, MATSUKATA M, et al. Oxidative coupling of methane on Fe-doped La2O3 catalyst[J]. Energy & Fuels, 2009, 23(2): 613-616. |
| 17 | MINEA T, BEKEROM D C M VAN DEN, PEETERS F J J, et al. Non-oxidative methane coupling to C2 hydrocarbons in a microwave plasma reactor[J]. Plasma Processes and Polymers, 2018, 15(11): 1800087. |
| 18 | BARBOUN P, MEHTA P, HERRERA F A, et al. Distinguishing plasma contributions to catalyst performance in plasma-assisted ammonia synthesis[J]. ACS Sustainable Chemistry Engineering, 2019, 7(9): 8621-8630. |
| 19 | 陈焕浩, 范晓雷. 非热等离子体催化转化C1分子及其催化剂研究进展[J].化工进展, 2021, 40(6): 3034-3045. |
| CHEN Huanhao, FAN Xiaolei. Review on non-thermal plasma (NTP) catalytic conversion of C1 molecules and its catalysts[J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3034-3045. | |
| 20 | JIN Z, WANG L, ZUIDEMA E, et al. Hydrophobic zeolite modification for in situ peroxide formation in methane oxidation to methanol[J]. Science, 2020, 367(6474): 193-197. |
| 21 | CHAN S I, LU Y J, NAGABABU P, et al. Efficient oxidation of methane to methanol by dioxygen mediated by tricopper clusters[J]. Angewandte Chemie, 2013, 125(13): 3819-3823. |
| 22 | MAHAMMADUNNISA S, MANOJ KUMAR REDDY P, RAMARAJU B, et al. Catalytic nonthermal plasma reactor for dry reforming of methane[J]. Energy & Fuels, 2013, 27(8): 4441-4447. |
| 23 | ZHANG X L, DAI B, ZHU A M, et al. The simultaneous activation of methane and carbon dioxide to C2 hydrocarbons under pulse corona plasma over La2O3/γ-Al2O3 catalyst[J]. Catalysis Today, 2002, 72(3/4): 223-227. |
| 24 | RUEANGJITT N, SREETHAWONG T, CHAVADEJ S, et al. Non-oxidative reforming of methane in a mini-gliding arc discharge reactor: effects of feed methane concentration, feed flow rate, electrode gap distance, residence time, and catalyst distance[J]. Plasma Chemistry and Plasma Processing, 2011, 31(4): 517-534. |
| 25 | ZHANG J Q, ZHANG J S, YANG Y J, et al. Oxidative coupling and reforming of methane with carbon dioxide using a pulsed microwave plasma under atmospheric pressure[J]. Energy & Fuels, 2003, 17(1): 54-59. |
| 26 | LI X S, ZHU B, SHI C, et al. Carbon dioxide reforming of methane in kilohertz spark-discharge plasma at atmospheric pressure[J]. AIChE Journal, 2011, 57(10): 2854-2860. |
| 27 | ZHANG J Q, YANG Y J, ZHANG J S, et al. Non-oxidative coupling of methane to C2 hydrocarbons under above-atmospheric pressure using pulsed microwave plasma[J]. Energy & Fuels, 2002, 16(3): 687-693. |
| 28 | SENTEK J, KRAWCZYK K, MŁOTEK M, et al. Plasma-catalytic methane conversion with carbon dioxide in dielectric barrier discharges[J]. Applied Catalysis B: Environmental, 2010, 94(1/2): 19-26. |
| 29 | LI X S, ZHU A M, WANG K J, et al. Methane conversion to C2 hydrocarbons and hydrogen in atmospheric non-thermal plasmas generated by different electric discharge techniques[J]. Catalysis Today, 2004, 98(4): 617-624. |
| 30 | KASINATHAN P, PARK S, CHOI W C, et al. Plasma-enhanced methane direct conversion over particle-size adjusted MOx/Al2O3 (M=Ti and Mg) catalysts[J]. Plasma Chemistry and Plasma Processing, 2014, 34(6): 1317-1330. |
| 31 | LÓPEZ N, VARGAS-FUENTES C. Promoters in the hydrogenation of alkynes in mixtures: insights from density functional theory[J]. Chemical Communications, 2012, 48(10): 1379-1391. |
| 32 | PONGTHAWORNSAKUN B, WIMONSUPAKIT N, PANPRANOT P. Preparation of TiO2 supported Au-Pd and Cu-Pd by the combined strong electrostatic adsorption and electroless deposition for selective hydrogenation of acetylene[J]. Journal of Chemical Sciences, 2017, 129(11): 1721-1734. |
| 33 | MCCUE A J, MCRITCHIE C J, SHEPHERD A M, et al. Cu/Al2O3 catalysts modified with Pd for selective acetylene hydrogenation[J]. Journal of Catalysis, 2014, 319: 127-135. |
| 34 | SEGURA Y, LÓPEZ N, PÉREZ-RAMÍREZ J. Origin of the superior hydrogenation selectivity of gold nanoparticles in alkyne+alkene mixtures: triple-versus double-bond activation[J]. Journal of Catalysis, 2007, 247(2): 383-386. |
| 35 | BRIDIER B, LÓPEZ N, PÉREZ-RAMÍREZ J. Molecular understanding of alkyne hydrogenation for the design of selective catalysts[J]. Dalton Transactions, 2010, 39(36): 8412-8419. |
| 36 | WANG S, ZHAO Z J, CHANG X, et al. Activation and spillover of hydrogen on sub-1 nm palladium nanoclusters confined within sodalite zeolite for the semi-hydrogenation of alkynes[J]. Angewandte Chemie International Edition, 2019, 58(23): 7668-7672. |
| 37 | KIM S S, KWON B, KIM J. Plasma catalytic methane conversion over sol-gel derived Ru/TiO2 catalyst in a dielectric-barrier discharge reactor[J]. Catalysis Communications, 2007, 8(12): 2204-2207. |
| 38 | GÓRSKA A, KRAWCZYK K, JODZIS S, et al. Non-oxidative methane coupling using Cu/ZnO/Al2O3 catalyst in DBD[J]. Fuel, 2011, 90(5): 1946-1952. |
| 39 | JO S, LEE D H, KANG W S, et al. Methane activation using noble gases in a dielectric barrier discharge reactor[J]. Physics of Plasmas, 2013, 20(8): 083509. |
| 40 | JO S, KIM T, LEE D H, et al. Effect of the electric conductivity of a catalyst on methane activation in a dielectric barrier discharge reactor[J]. Plasma Chemistry and Plasma Processing, 2014, 34(1): 175-186. |
| 41 | KHALIFEH O, TAGHVAEI H, MOSALLANEJAD A, et al. Extra pure hydrogen production through methane decomposition using nanosecond pulsed plasma and Pt-Re catalyst[J]. Chemical Engineering Journal, 2016, 294, 132-145. |
| 42 | DAI C Y, LI X M, ZHANG A F, et al. Pd and Pd-CuO nanoparticles in hollow silicalite-1 single crystals for enhancing selectivity and activity for the Suzuki-Miyaura reaction[J]. RSC Advances, 2015, 5(50): 40297-40302. |
| 43 | DAI C Y, ZHANG S H, ZHANG A F, et al. Hollow zeolite encapsulated Ni-Pt bimetals for sintering and coking resistant dry reforming of methane[J]. Journal of Materials Chemistry A, 2015, 3(32): 16461-16468. |
| 44 | FOX E B, VELU S, ENGELHARD M H, et al. Characterization of CeO2-supported Cu-Pd bimetallic catalyst for the oxygen-assisted water-gas shift reaction[J]. Journal of Catalysis, 2008, 260(2): 358-370. |
| 45 | CAO Y Q, SUI Z J, ZHU Y A, et al. Selective hydrogenation of acetylene over Pd-In/Al2O3 catalyst: promotional effect of indium and composition-dependent performance[J]. ACS Catalysis, 2017, 7(11): 7835-7846. |
| 46 | 程博贞. 电石乙炔加氢制乙烯Pd基催化剂的研究[D]. 石河子: 石河子大学, 2019. |
| CHENG Bozhen. Study on Pd based catalyst for hydrogenation of calcium carbide acetylene to ethylene[D]. Shihezi: Shihezi University, 2019. | |
| 47 | 杨春雁, 杨卫亚, 凌凤香, 等. 负载型金属催化剂表面金属分散度的测定[J]. 化工进展, 2010, 29(8): 1468-1473, 1501. |
| YANG Chunyan, YANG Weiya, LING Fengxiang, et al. Determination of metal dispersion on supported metal catalyst surface[J]. Chemical Industry and Engineering Progress, 2010, 29(8): 1468-1473, 1501. | |
| 48 | GOUJARD V, TATIBOUËT J M, BATIOT-DUPEYRAT C. Carbon dioxide reforming of methane using a dielectric barrier discharge reactor: effect of helium dilution and kinetic model[J]. Plasma Chemistry and Plasma Processing, 2011, 31(2): 315-325. |
| 49 | XU C, TU X. Plasma-assisted methane conversion in an atmospheric pressure dielectric barrier discharge reactor[J]. Journal of Energy Chemistry, 2013, 22(3): 420-425. |
| 50 | MEI D H, ZHU X B, HE Y L, et al. Plasma-assisted conversion of CO2 in a dielectric barrier discharge reactor: understanding the effect of packing materials[J]. Plasma Sources Science & Technology, 2014, 24(1): 015011. |
| 51 | TAHERASLANI M, GARDENIERS H. Plasma catalytic conversion of CH4 to alkanes, olefins and H2 in a packed bed DBD reactor[J]. Processes, 2020, 8(7): 774. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [6] | 王正坤, 黎四芳. 双子表面活性剂癸炔二醇的绿色合成[J]. 化工进展, 2023, 42(S1): 400-410. |
| [7] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [8] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [9] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [10] | 许友好, 王维, 鲁波娜, 徐惠, 何鸣元. 中国炼油创新技术MIP的开发策略及启示[J]. 化工进展, 2023, 42(9): 4465-4470. |
| [11] | 耿源泽, 周俊虎, 张天佑, 朱晓宇, 杨卫娟. 部分填充床燃烧器中庚烷均相/异相耦合燃烧[J]. 化工进展, 2023, 42(9): 4514-4521. |
| [12] | 赖诗妮, 江丽霞, 李军, 黄宏宇, 小林敬幸. 含碳掺氨燃料的研究进展[J]. 化工进展, 2023, 42(9): 4603-4615. |
| [13] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [14] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [15] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||