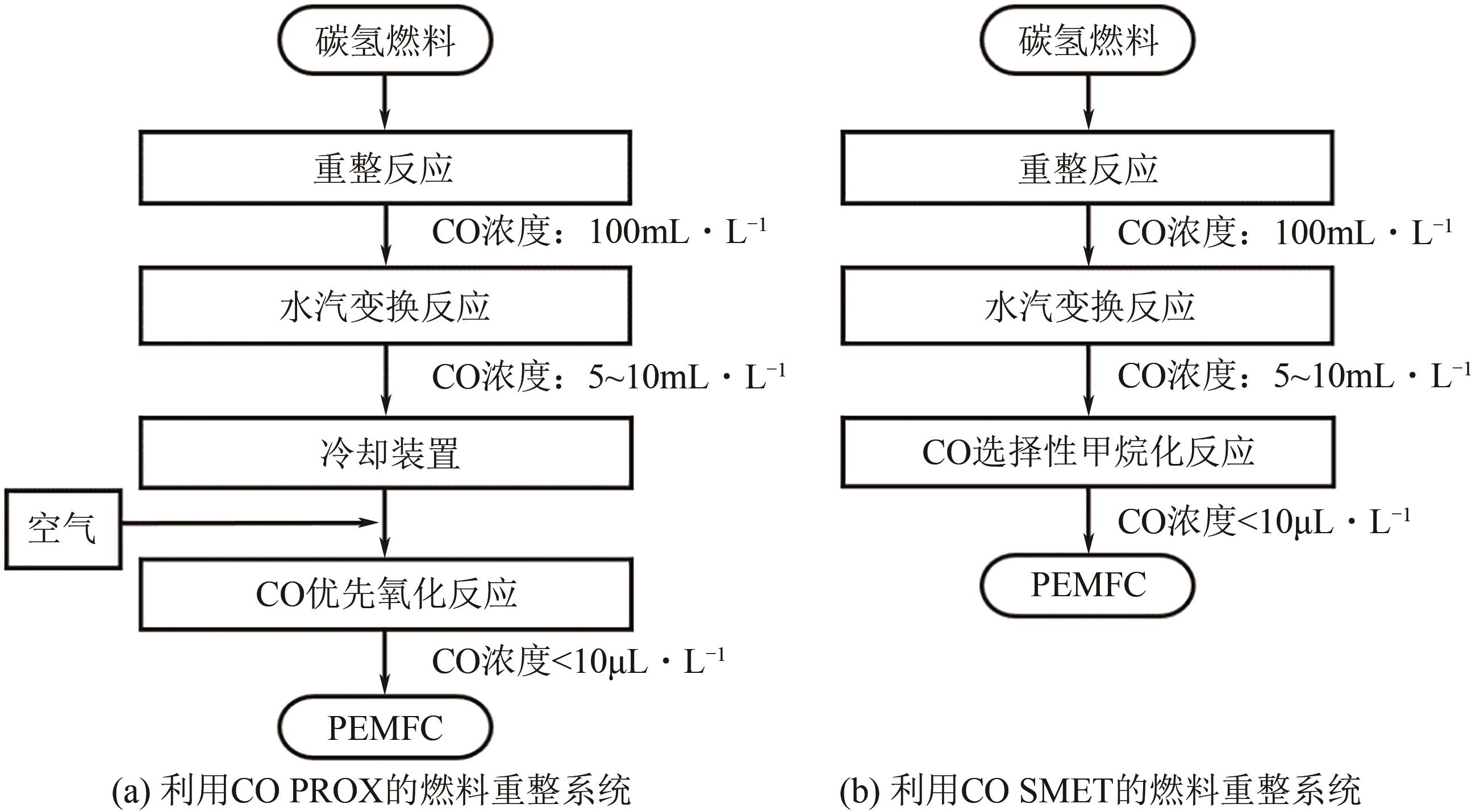
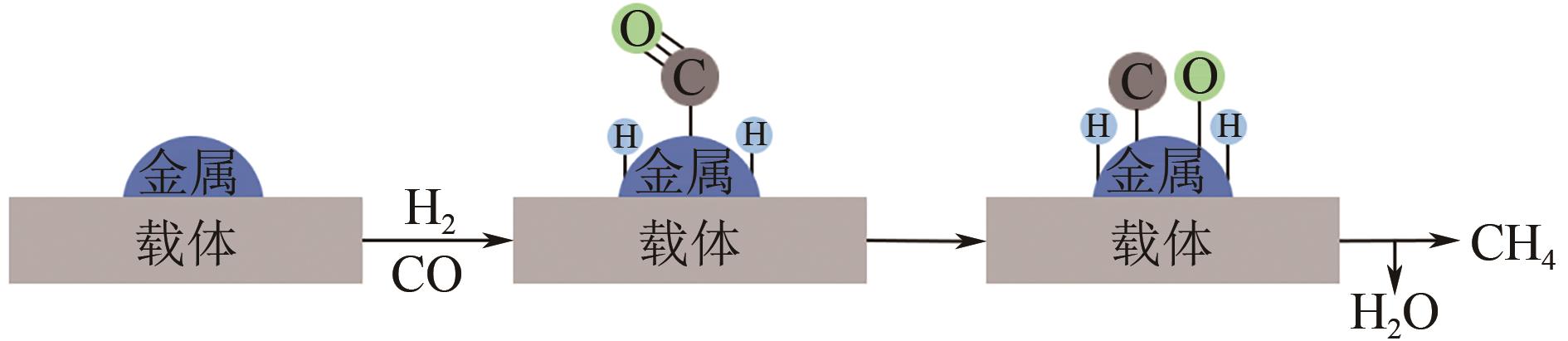
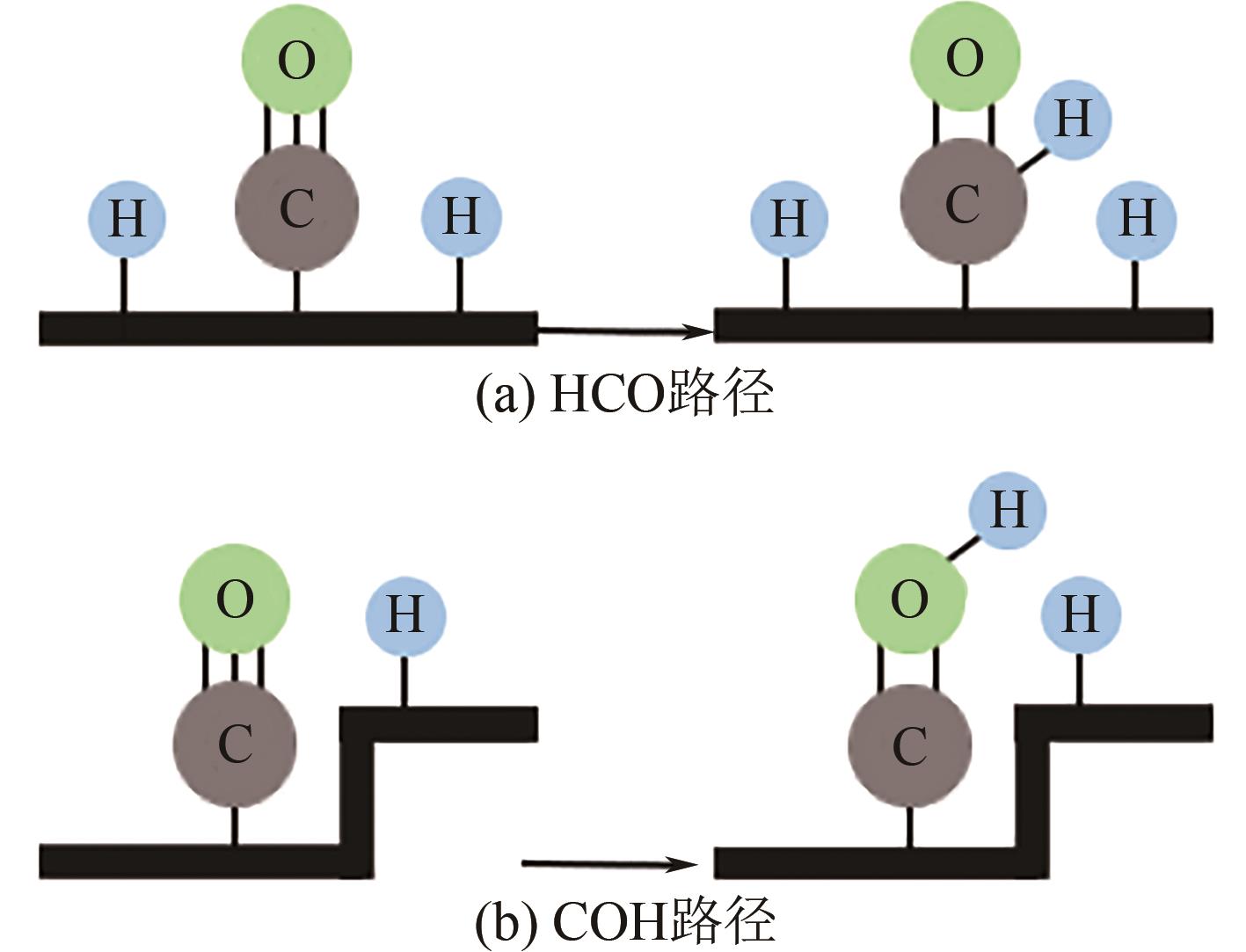
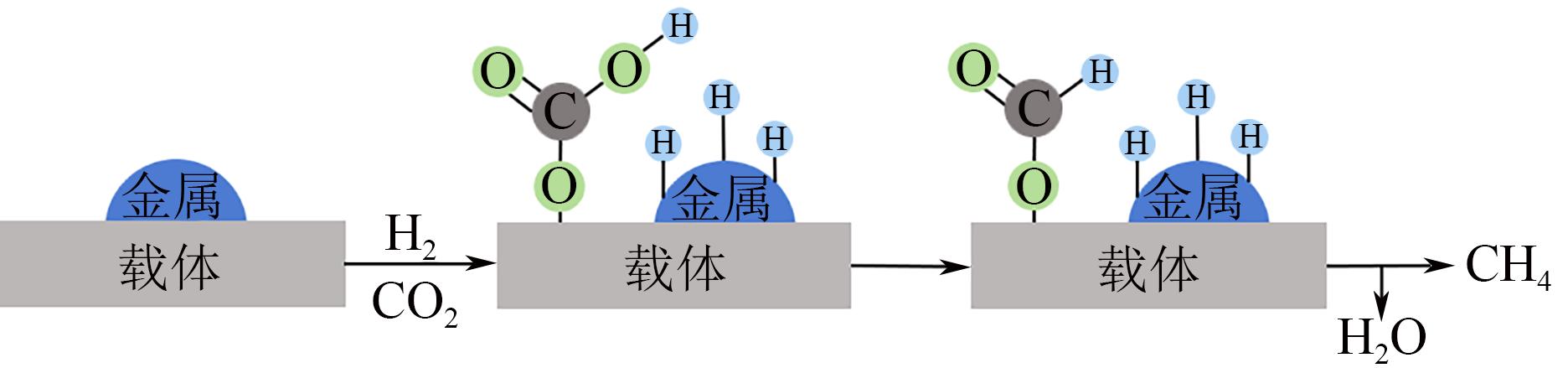
化工进展 ›› 2022, Vol. 41 ›› Issue (1): 120-132.DOI: 10.16085/j.issn.1000-6613.2021-0114
CO选择性甲烷化的研究进展
- 北京科技大学能源与环境工程学院,北京 100083
-
收稿日期:2021-01-18修回日期:2021-03-30出版日期:2022-01-05发布日期:2022-01-24 -
通讯作者:包成 -
作者简介:纪子柯(1997—),男,硕士研究生,研究方向为CO深度去除。E-mail:jzk1597530@163.com 。 -
基金资助:国家自然科学基金面上项目(51976009)
Research progress of selective CO methanation
- School of Energy and Environmental Engineering, University of Science and Technology Beijing, Beijing 100083, China
-
Received:2021-01-18Revised:2021-03-30Online:2022-01-05Published:2022-01-24 -
Contact:BAO Cheng
摘要:
CO选择性甲烷化被认为是适用于低温燃料电池的、最具发展潜力的CO深度去除技术,而该技术大规模应用的关键在于高性能负载型催化剂的开发。本文综述了近些年来CO选择性甲烷化的研究进展,以催化剂的选取和评判标准为起点,着重论述了CO和CO2甲烷化的反应机理、粒径效应以及载体和助剂对催化剂活性和选择性的影响,最后总结了CO选择性甲烷化的研究并对未来的研究方向进行了展望。分析表明,选取合适的活性组分负载量以及载体和助剂可以大幅度提高催化剂的CO甲烷化活性,而通过氯离子改性以及Ru-Ni双金属的制备来控制金属-载体作用界面则是提高催化剂CO甲烷化选择性的关键。指出对甲烷化反应机理的研究和具有长期稳定性催化剂的开发是未来CO选择性甲烷化研究的重点。
中图分类号:
引用本文
纪子柯, 包成. CO选择性甲烷化的研究进展[J]. 化工进展, 2022, 41(1): 120-132.
JI Zike, BAO Cheng. Research progress of selective CO methanation[J]. Chemical Industry and Engineering Progress, 2022, 41(1): 120-132.
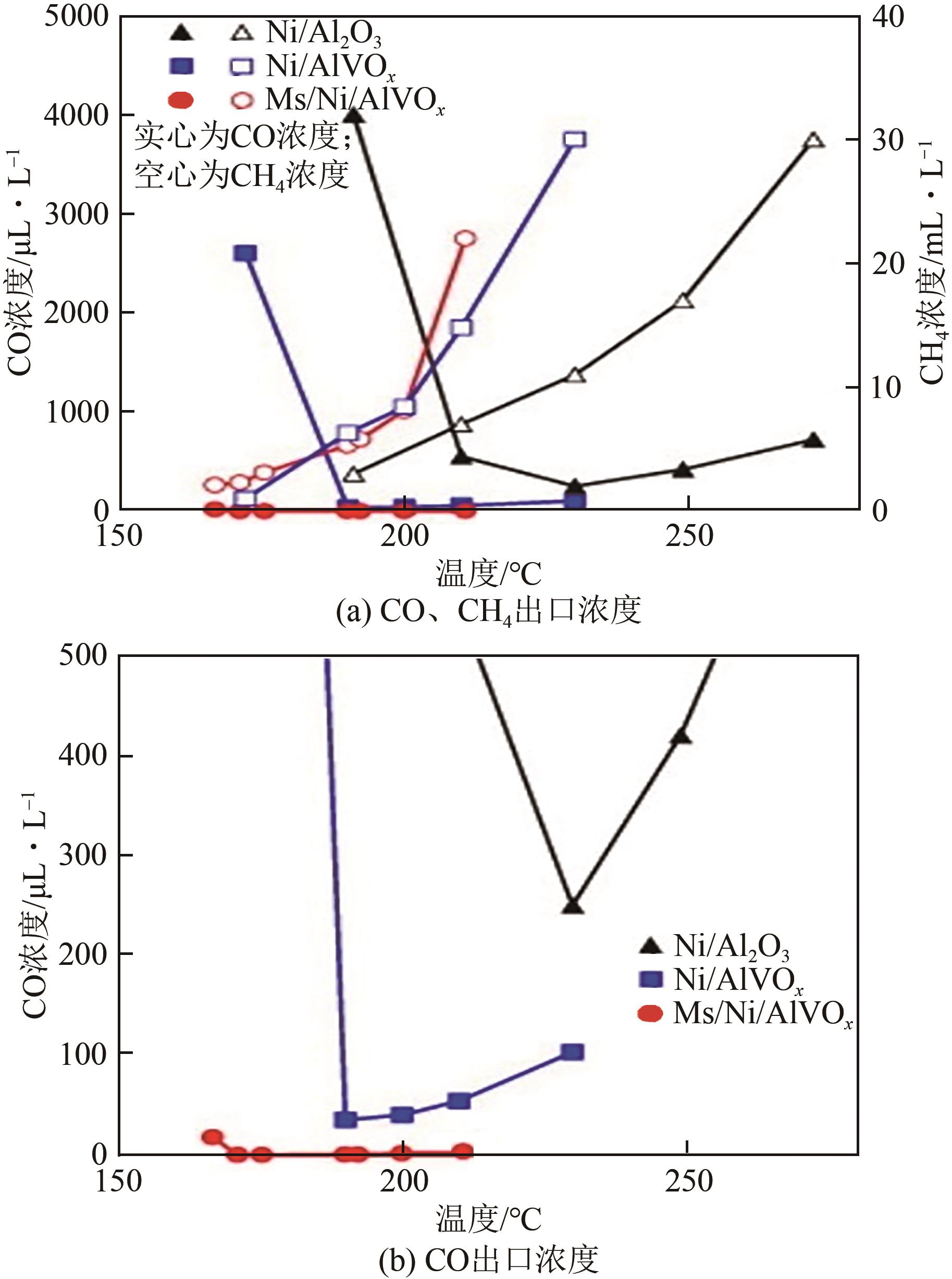
表1 不同催化剂的CO选择性甲烷化性能
| 催化剂 | 反应气体体积分数/% | 质量空速 | 体积空速 | Tmin | Smin(CO) | Tmax | Smax(CO) | 参考文献 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CO | CO2 | H2 | H2O | /cm3·h-1·g-1 | /h-1 | /℃ | /% | /℃ | /% | ||
| 5%Ru/Al2O3 | 0.56 | 21.7 | 42.2 | He | 19800 | 210 | 100 | 244 | 50 | [ | |
| Ni/ZrO2 | 1 | 20 | 79 | 0 | 8500 | 215 | 100 | 350 | 50 | [ | |
| 3%Ru/50%NiAlOx/NF | 1 | 20 | 79 | 0 | 250 | 180 | 100 | 280 | 50 | [ | |
| RuNi/Al2O3-CNTs/NF | 1 | 20 | 79 | 0 | 1400 | 190 | 100 | 250 | 50 | [ | |
| MS/Ni/AlVOx | 0.43 | 17.1 | 67.9 | 14.53 | 2400 | 170 | 100 | 200 | 50 | [ | |
| 0.22TMS/0.039V/Ni | 0.43 | 17.1 | 67.9 | 14.53 | 4800 | 164 | 100 | 198 | 50 | [ | |
| Ni/CeO2(Cl*) | 1 | 20 | 65 | 10 | 29000 | 240 | 90 | 285 | 50 | [ | |
| Ni(Cl0.1)/ZrO2 | 1 | 18 | 70 | N2 | 15000 | 215 | 100 | 295 | 50 | [ | |
| Ru-Ni/TiO2-Al2O3 | 1 | 20 | 50 | He | 2400 | 210 | 95 | 220 | 80 | [ | |
| 1%Ru/MA-40Ni | 0.87 | 17.4 | 68.73 | 13 | 2400 | 190 | 100 | 260 | 50 | [ | |
表1 不同催化剂的CO选择性甲烷化性能
| 催化剂 | 反应气体体积分数/% | 质量空速 | 体积空速 | Tmin | Smin(CO) | Tmax | Smax(CO) | 参考文献 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CO | CO2 | H2 | H2O | /cm3·h-1·g-1 | /h-1 | /℃ | /% | /℃ | /% | ||
| 5%Ru/Al2O3 | 0.56 | 21.7 | 42.2 | He | 19800 | 210 | 100 | 244 | 50 | [ | |
| Ni/ZrO2 | 1 | 20 | 79 | 0 | 8500 | 215 | 100 | 350 | 50 | [ | |
| 3%Ru/50%NiAlOx/NF | 1 | 20 | 79 | 0 | 250 | 180 | 100 | 280 | 50 | [ | |
| RuNi/Al2O3-CNTs/NF | 1 | 20 | 79 | 0 | 1400 | 190 | 100 | 250 | 50 | [ | |
| MS/Ni/AlVOx | 0.43 | 17.1 | 67.9 | 14.53 | 2400 | 170 | 100 | 200 | 50 | [ | |
| 0.22TMS/0.039V/Ni | 0.43 | 17.1 | 67.9 | 14.53 | 4800 | 164 | 100 | 198 | 50 | [ | |
| Ni/CeO2(Cl*) | 1 | 20 | 65 | 10 | 29000 | 240 | 90 | 285 | 50 | [ | |
| Ni(Cl0.1)/ZrO2 | 1 | 18 | 70 | N2 | 15000 | 215 | 100 | 295 | 50 | [ | |
| Ru-Ni/TiO2-Al2O3 | 1 | 20 | 50 | He | 2400 | 210 | 95 | 220 | 80 | [ | |
| 1%Ru/MA-40Ni | 0.87 | 17.4 | 68.73 | 13 | 2400 | 190 | 100 | 260 | 50 | [ | |
表4 CO和CO2甲烷化机理的对比
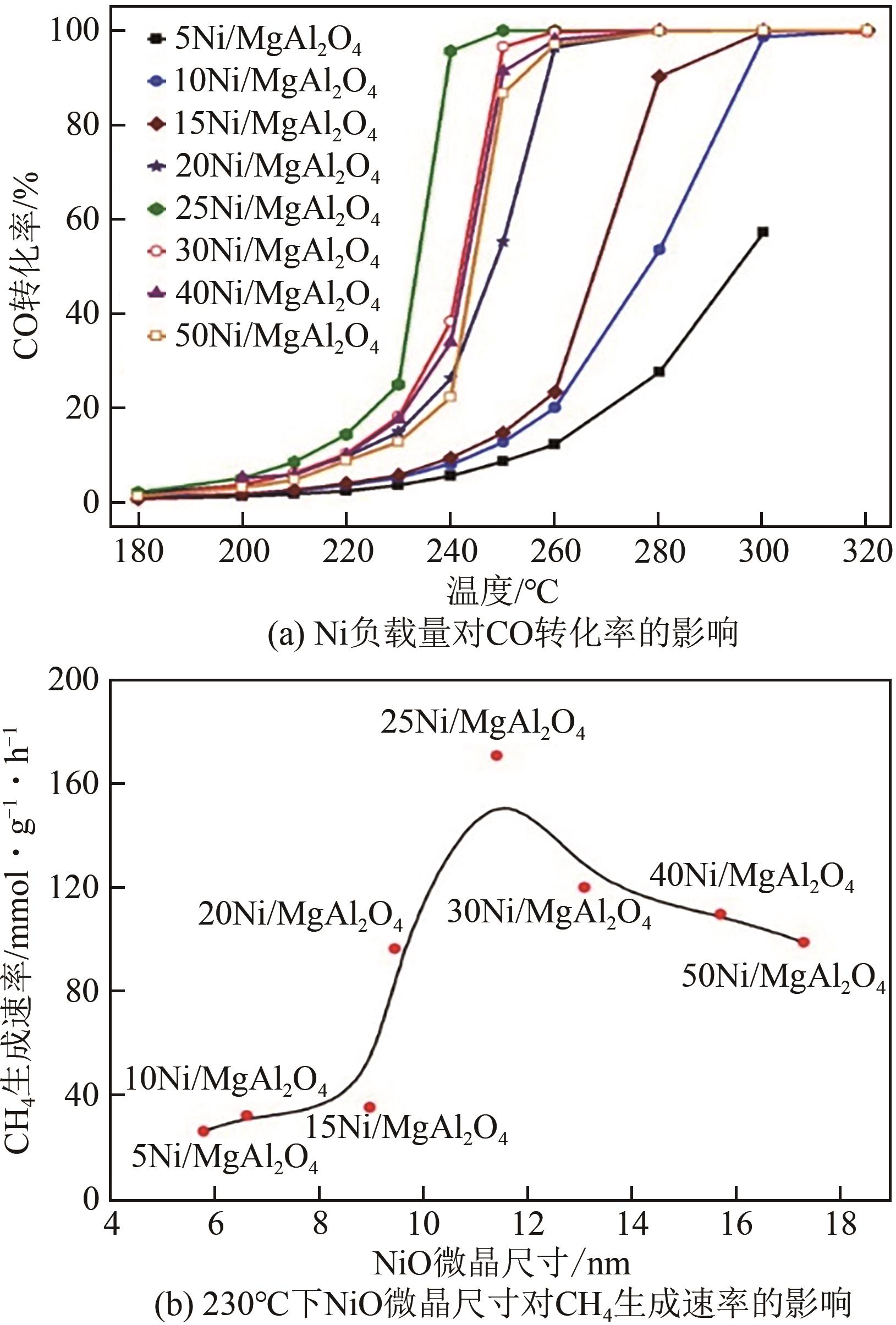
| 反应类型 | 反应方式 | 反应方式判定 | 活性位点位置 | 主要中间体 | 限速步骤 | 影响因素 |
|---|---|---|---|---|---|---|
| CO甲烷化 | 解离 | C-O键断裂是否 有H协助 | 活性金属 | Cad | Cad的加氢 | 温度、表面结构 |
| 缔合 | HCOad | HCOad的生成 | ||||
| COHad | COHad的生成 | |||||
| CO2甲烷化 | 解离 | 是否生成COad中间体 | 活性金属 | COad | COad的解离 | 反应气体组成、制备方法、 活性组分负载量 |
| 缔合 | 金属-载体作用界面 | COad的生成 | ||||
| 金属-载体作用界面 | HCOOad | HCOOad的加氢 |
表4 CO和CO2甲烷化机理的对比
| 反应类型 | 反应方式 | 反应方式判定 | 活性位点位置 | 主要中间体 | 限速步骤 | 影响因素 |
|---|---|---|---|---|---|---|
| CO甲烷化 | 解离 | C-O键断裂是否 有H协助 | 活性金属 | Cad | Cad的加氢 | 温度、表面结构 |
| 缔合 | HCOad | HCOad的生成 | ||||
| COHad | COHad的生成 | |||||
| CO2甲烷化 | 解离 | 是否生成COad中间体 | 活性金属 | COad | COad的解离 | 反应气体组成、制备方法、 活性组分负载量 |
| 缔合 | 金属-载体作用界面 | COad的生成 | ||||
| 金属-载体作用界面 | HCOOad | HCOOad的加氢 |
| 1 | PARK E D, LEE D, LEE H C. Recent progress in selective CO removal in a H2-rich stream[J]. Catalysis Today, 2009, 139(4): 280-290. |
| 2 | SNYTNIKOV P V, ZYRYANOVA M M, SOBYANIN V A. CO-cleanup of hydrogen-rich stream for LT PEM FC feeding: catalysts and their performance in selective CO methanation[J]. Topics in Catalysis, 2016, 59(15): 1394-1412. |
| 3 | VANNICE M A. The catalytic synthesis of hydrocarbons from H2/CO mixtures over the group ⅤⅢ metals[J]. Journal of Catalysis, 1975,37(3):449-461. |
| 4 | TAKENAKA S, SHIMIZU T, OTSUKA K. Complete removal of carbon monoxide in hydrogen-rich gas stream through methanation over supported metal catalysts[J]. International Journal of Hydrogen Energy, 2004, 29(10): 1065-1073. |
| 5 | DJINOVIĆ P, GALLETTI C, SPECCHIA S, et al. Ru-based catalysts for CO selective methanation reaction in H2-rich gases[J]. Catalysis Today, 2011, 164(1): 282-287. |
| 6 | PING D, DONG X F, ZANG Y H, et al. Highly efficient MOF-templated Ni catalyst towards CO selective methanation in hydrogen-rich reformate gases[J]. International Journal of Hydrogen Energy, 2017, 42(23): 15551-15556. |
| 7 | WANG C X, PING D, DONG X F, et al. Construction of Ru/Ni-Al-oxide/Ni-foam monolithic catalyst for deep-removing CO in hydrogen-rich gas via selective methanation[J]. Fuel Processing Technology, 2016, 148: 367-371. |
| 8 | PING D, DONG C J, ZHAO H, et al. A novel hierarchical RuNi/Al2O3-carbon nanotubes/Ni foam catalyst for selective removal of CO in H2-rich fuels[J]. Industrial & Engineering Chemistry Research, 2018, 57(16): 5558-5567. |
| 9 | MIYAO T, SAKURABAYASHI S, SHEN W H, et al. Preparation and catalytic activity of a mesoporous silica-coated Ni-alumina-based catalyst for selective CO methanation[J]. Catalysis Communications, 2015, 58: 93-96. |
| 10 | HAYASHI K, MIYAO T, HIGASHIYAMA K, et al. Catalytic performance of mesoporous silica-covered nickel core-shell particles for selective CO methanation[J]. Applied Catalysis A: General, 2015, 506: 143-150. |
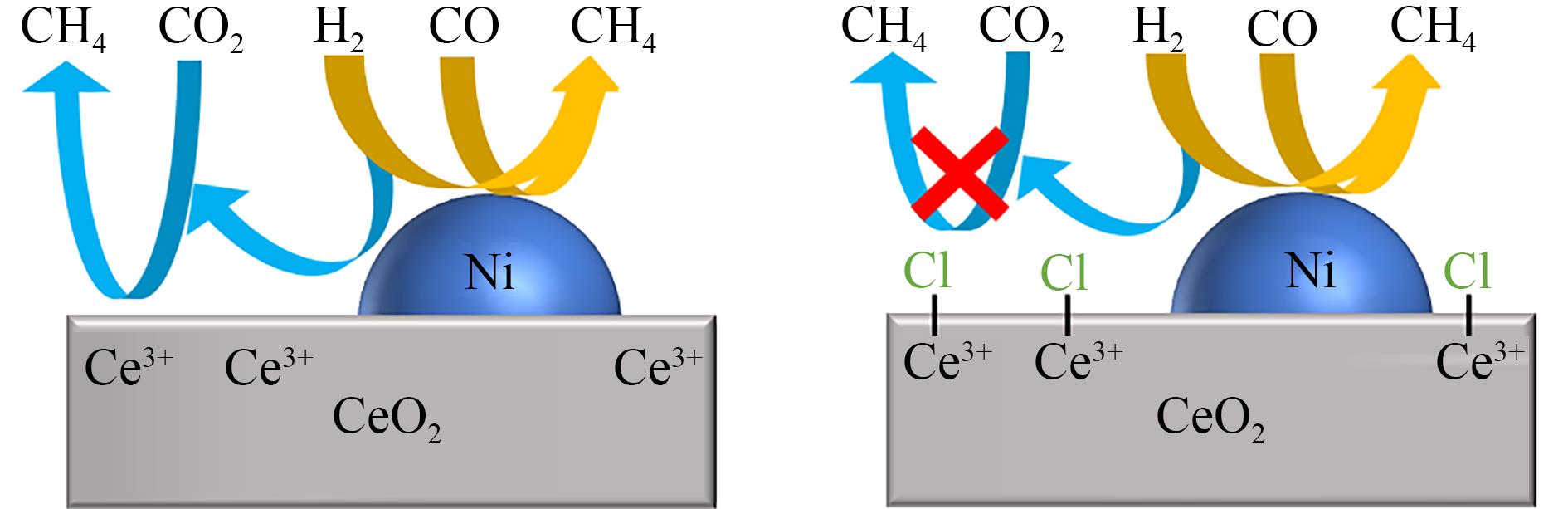
| 11 | KONISHCHEVA M V, POTEMKIN D I, SNYTNIKOV P V, et al. The insights into chlorine doping effect on performance of ceria supported nickel catalysts for selective CO methanation[J]. Applied Catalysis B: Environmental, 2018, 221: 413-421. |
| 12 | GAO Z M, WANG L L, MA H W, et al. Durability of catalytic performance of the chlorine-doped catalyst Ni(Clx)/ZrO2 for selective methanation of CO in H2-rich gas[J]. Applied Catalysis A: General, 2017, 534: 78-84. |
| 13 | DAI X P, LIANG J, MA D, et al. Large-pore mesoporous RuNi-doped TiO2-Al2O3 nanocomposites for highly efficient selective CO methanation in hydrogen-rich reformate gases[J]. Applied Catalysis B: Environmental, 2015, 165: 752-762. |
| 14 | CHEN A H, MIYAO T, HIGASHIYAMA K, et al. High catalytic performance of ruthenium-doped mesoporous nickel-aluminum oxides for selective CO methanation[J]. Angewandte Chemie International Edition, 2010, 49(51): 9895-9898. |
| 15 | LEGRAS B, ORDOMSKY V V, DUJARDIN C, et al. Impact and detailed action of sulfur in syngas on methane synthesis on Ni/γ-Al2O3 catalyst[J]. ACS Catalysis, 2014, 4(8): 2785-2791. |
| 16 | PANAGIOTOPOULOU P, KONDARIDES D I, VERYKIOS X E. Mechanistic study of the selective methanation of CO over Ru/TiO2 catalyst: identification of active surface species and reaction pathways[J]. The Journal of Physical Chemistry C, 2011, 115(4): 1220-1230. |
| 17 | SEHESTED J, DAHL S, JACOBSEN J, et al. Methanation of CO over nickel: mechanism and kinetics at high H2/CO ratios[J]. The Journal of Physical Chemistry B, 2005, 109(6): 2432-2438. |
| 18 | ARAKI M, PONEC V. Methanation of carbon monoxide on nickel and nickel-copper alloys[J]. Journal of Catalysis, 1976, 44(3): 439-448. |
| 19 | TISON Y, NIELSEN K, MOWBRAY D J, et al. Scanning tunneling microscopy evidence for the dissociation of carbon monoxide on ruthenium steps[J]. The Journal of Physical Chemistry C, 2012, 116(27): 14350-14359. |
| 20 | CIOBICA I M, SANTEN R A VAN. Carbon monoxide dissociation on planar and stepped Ru(0001) surfaces[J]. The Journal of Physical Chemistry B, 2003, 107(16): 3808-3812. |
| 21 | FOPPA L, COPÉRET C, COMAS-VIVES A. Increased back-bonding explains step-edge reactivity and particle size effect for CO activation on Ru nanoparticles[J]. Journal of the American Chemical Society, 2016, 138(51): 16655-16668. |
| 22 | VENDELBO S B, JOHANSSON M, MOWBRAY D J, et al. Self blocking of CO dissociation on a stepped ruthenium surface[J]. Topics in Catalysis, 2010, 53(5/6): 357-364. |
| 23 | ECKLE S, ANFANG H G, BEHM R J. Reaction intermediates and side products in the methanation of CO and CO2 over supported Ru catalysts in H2-rich reformate gases[J]. The Journal of Physical Chemistry C, 2011, 115(4): 1361-1367. |
| 24 | WANG Y X, SU Y, ZHU M Y, et al. Mechanism of CO methanation on the Ni4/γ-Al2O3 and Ni3Fe/γ-Al2O3 catalysts: a density functional theory study[J]. International Journal of Hydrogen Energy, 2015, 40(29): 8864-8876. |
| 25 | ZHI C M, WANG Q, WANG B J, et al. Insight into the mechanism of methane synthesis from syngas on a Ni(111) surface: a theoretical study[J]. RSC Advances, 2015, 5(82): 66742-66756. |
| 26 | FAJÍN J L C, GOMES J R B, CORDEIRO M N D S. Mechanistic study of carbon monoxide methanation over pure and rhodium-or ruthenium-doped nickel catalysts[J]. The Journal of Physical Chemistry C, 2015, 119(29): 16537-16551. |
| 27 | ANDERSSON M P, ABILD-PEDERSEN F, REMEDIAKIS I N, et al. Structure sensitivity of the methanation reaction: H2 induced CO dissociation on nickel surfaces[J]. Journal of Catalysis, 2008, 255(1): 6-19. |
| 28 | ZHI C M, ZHANG R G, WANG B J. Comparative studies about CO methanation over Ni(211) and Zr-modified Ni(211) surfaces: qualitative insight into the effect of surface structure and composition[J]. Molecular Catalysis, 2017, 438: 1-14. |
| 29 | FOPPA L, LANNUZZI M, COPÉRET C, et al. CO methanation on ruthenium flat and stepped surfaces: key role of H-transfers and entropy revealed by ab initio molecular dynamics[J]. Journal of Catalysis, 2019, 371: 270-275. |
| 30 | WANG X, HONG Y C, SHI H, et al. Kinetic modeling and transient DRIFTS-MS studies of CO2 methanation over Ru/Al2O3 catalysts[J]. Journal of Catalysis, 2016, 343: 185-195. |
| 31 | FALBO L, VISCONTI C G, LIETTI L, et al. The effect of CO on CO2 methanation over Ru/Al2O3 catalysts: a combined steady-state reactivity and transient DRIFT spectroscopy study[J]. Applied Catalysis B: Environmental, 2019, 256: 117791. |
| 32 | KESAVAN J K, LUISETTO I, TUTI S, et al. Nickel supported on YSZ: the effect of Ni particle size on the catalytic activity for CO2 methanation[J]. Journal of CO2 Utilization, 2018, 23: 200-211. |
| 33 | REN J, GUO H L, YANG J Z, et al. Insights into the mechanisms of CO2 methanation on Ni(111) surfaces by density functional theory[J]. Applied Surface Science, 2015, 351: 504-516. |
| 34 | ALDANA P A U, OCAMPO F, KOBL K, et al. Catalytic CO2 valorization into CH4 on Ni-based ceria-zirconia. Reaction mechanism by operando IR spectroscopy[J]. Catalysis Today, 2013, 215: 201-207. |
| 35 | XU X L, TONG Y Y, HUANG J, et al. Insights into CO2 methanation mechanism on cubic ZrO2 supported Ni catalyst via a combination of experiments and DFT calculations[J]. Fuel, 2021, 283: 118867. |
| 36 | PAN Q S, PENG J X, WANG S, et al. In situ FTIR spectroscopic study of the CO2 methanation mechanism on Ni/Ce0.5Zr0.5O2[J]. Catalysis Science & Technology, 2014, 4(2): 502-509. |
| 37 | SOLIS-GARCIA A, LOUVIER-HERNANDEZ J F, ALMENDAREZ-CAMARILLO A, et al. Participation of surface bicarbonate, formate and methoxy species in the carbon dioxide methanation catalyzed by ZrO2-supported Ni[J]. Applied Catalysis B: Environmental, 2017, 218: 611-620. |
| 38 | RUI Z, RUI N, FAN Z G, et al. Effect of the structure of Ni/TiO2 catalyst on CO2 methanation[J]. International Journal of Hydrogen Energy, 2016, 41(47): 22017-22025. |
| 39 | MIAO B, MA S S K, WANG X, et al. Catalysis mechanisms of CO2 and CO methanation[J]. Catalysis Science & Technology, 2016, 6(12): 4048-4058. |
| 40 | JIA X Y, ZHANG X S, RUI N, et al. Structural effect of Ni/ZrO2 catalyst on CO2 methanation with enhanced activity[J]. Applied Catalysis B: Environmental, 2019, 244: 159-169. |
| 41 | ZHANG Z M, TIAN Y, ZHANG L J, et al. Impacts of nickel loading on properties, catalytic behaviors of Ni/γ-Al2O3 catalysts and the reaction intermediates formed in methanation of CO2[J]. International Journal of Hydrogen Energy, 2019, 44(18): 9291-9306. |
| 42 | TADA S, KIKUCHI R, URASAKI K, et al. Effect of reduction pretreatment and support materials on selective CO methanation over supported Ru catalysts[J]. Applied Catalysis A: General, 2011, 404(1/2): 149-154. |
| 43 | TADA S, KIKUCHI R. Mechanistic study and catalyst development for selective carbon monoxide methanation[J]. Catalysis Science & Technology, 2015, 5(6): 3061-3070. |
| 44 | ECKLE S, AUGUSTIN M, ANFANG H G, et al. Influence of the catalyst loading on the activity and the CO selectivity of supported Ru catalysts in the selective methanation of CO in CO2 containing feed gases[J]. Catalysis Today, 2012, 181(1): 40-51. |
| 45 | PANAGIOTOPOULOU P, KONDARIDES D I, VERYKIOS X E. Selective methanation of CO over supported Ru catalysts[J]. Applied Catalysis B: Environmental, 2009, 88(3/4): 470-478. |
| 46 | ABDEL-MAGEED A M, WIDMANN D, OLESEN S E, et al. Selective CO methanation on highly active Ru/TiO2 catalysts: identifying the physical origin of the observed activation/deactivation and loss in selectivity [J]. ACS Catalysis, 2018, 8(6): 5399-5414. |
| 47 | NEMATOLLAHI B, REZAEI M, LAY E N. Preparation of highly active and stable NiO-CeO2 nanocatalysts for CO selective methanation[J]. International Journal of Hydrogen Energy, 2015, 40(27): 8539-8547. |
| 48 | NEMATOLLAHI B, REZAEI M, AMINI E, et al. Preparation of high surface area Ni/MgAl2O4 nanocatalysts for CO selective methanation[J]. International Journal of Hydrogen Energy, 2017, 43(2): 772-780. |
| 49 | DU J P, GAO J J, GU F N, et al. A strategy to regenerate coked and sintered Ni/Al2O3 catalyst for methanation reaction[J]. International Journal of Hydrogen Energy, 2018, 43(45): 20661-20670. |
| 50 | LIU Y J, GAO J J, LIU Q, et al. Preparation of high-surface-area Ni/α-Al2O3 catalysts for improved CO methanation[J]. RSC Advances, 2015, 5(10): 7539-7546. |
| 51 | ZENG Y, MA H F, ZHANG H T, et al. Highly efficient NiAl2O4-free Ni/γ-Al2O3 catalysts prepared by solution combustion method for CO methanation[J]. Fuel, 2014, 137: 155-163. |
| 52 | 詹吉山, 郭翠梨, 张俊涛, 等. TiO2对Ni/Al2O3催化剂CO甲烷化性能的影响[J]. 燃料化学学报, 2012, 40(5): 589-593. |
| ZHAN J S, GUO C L, ZHANG J T, et al. Effects of TiO2 promoter on the catalytic performance of Ni/Al2O3 in CO methanation[J]. Journal of Fuel Chemistry and Technology, 2012, 40(5): 589-593. | |
| 53 | LE T A, KANG J K, PARK E D. CO and CO2 methanation over Ni/SiC and Ni/SiO2 catalysts[J]. Topics in Catalysis, 2018, 61(15/16/17): 1537-1544. |
| 54 | LE T A, KIM T W, LEE S H, et al. Effects of Na content in Na/Ni/SiO2 and Na/Ni/CeO2 catalysts for CO and CO2 methanation[J]. Catalysis Today, 2017, 303: 159-167. |
| 55 | LAKSHMANAN P, KIM M S, PARK E D. A highly loaded Ni@SiO2 core-shell catalyst for CO methanation[J]. Applied Catalysis A: General, 2016, 513: 98-105. |
| 56 | KOKKA A, RAMANTANI T, PETALA A, et al. Effect of the nature of the support, operating and pretreatment conditions on the catalytic performance of supported Ni catalysts for the selective methanation of CO[J]. Catalysis Today, 2020, 355: 832-843. |
| 57 | XU M, HE S, CHEN H, et al. TiO2-x-modified Ni nanocatalyst with tunable metal-support interaction for water-gas shift reaction[J]. ACS Catalysis, 2017, 7(11): 7600-7609. |
| 58 | CHEN S L, ABDEL-MAGEED A M, GAUCKLER C, et al. Selective CO methanation on isostructural Ru nanocatalysts: the role of support effects[J]. Journal of Catalysis, 2019, 373: 103-115. |
| 59 | ABDEL-MAGEED A M, WIDMANN D, OLESEN S E, et al. Selective CO methanation on Ru/TiO2 catalysts: role and influence of metal-support interactions[J]. ACS Catalysis, 2015, 5(11): 6753-6763. |
| 60 | JIA X Y, RUI N, ZHANG X S, et al. Ni/ZrO2 by dielectric barrier discharge plasma decomposition with improved activity and enhanced coke resistance for CO methanation[J]. Catalysis Today, 2019, 334: 215-222. |
| 61 | SILVA D C D DA, LETICHEVSKY S, BORGES L E P, et al. The Ni/ZrO2 catalyst and the methanation of CO and CO2[J]. International Journal of Hydrogen Energy, 2012, 37(11): 8923-8928. |
| 62 | LE T A, KIM M S, LEE S H, et al. CO and CO2 methanation over supported Ni catalysts[J]. Catalysis Today, 2017, 293/294: 89-96. |
| 63 | KONISHCHEVA M V, POTEMKIN D I, BADMAEV S D, et al. On the mechanism of CO and CO2 methanation over Ni/CeO2 catalysts[J]. Topics in Catalysis, 2016, 59(15/16): 1424-1430. |
| 64 | LIU J, YU J, SU F B, et al. Intercorrelation of structure and performance of Ni-Mg/Al2O3 catalysts prepared with different methods for syngas methanation[J]. Catalysis Science & Technology, 2014, 4(2): 472-481. |
| 65 | 文博, 朱明远, 代斌. Ca2+、Mg2+在MCM-41离子交换制备镍基催化剂对合成气甲烷化的性能分析[J]. 石河子大学学报(自然科学版), 2016, 34(3): 360-366. |
| WEN B, ZHU M Y, DAI B. Preparation of nickel-based catalysts by Ca2+, Mg2+ exchange in MCM-41 for methanation of synthesis gas properties exploration[J]. Journal of Shihezi University (Natural Science), 2016, 34(3): 360-366. | |
| 66 | HAN Y H, QUAN Y H, ZHAO J X, et al. Promotion effect by Mg on the catalytic behavior of MgNi/WO3 in the CO methanation[J]. International Journal of Hydrogen Energy, 2020, 45(55): 29917-29928. |
| 67 | TADA S, KIKUCHI R, TAKAGAKI A, et al. Effect of metal addition to Ru/TiO2 catalyst on selective CO methanation[J]. Catalysis Today, 2014, 232: 16-21. |
| 68 | GONG D D, LI S S, GUO S X, et al. Lanthanum and cerium co-modified Ni/SiO2 catalyst for CO methanation from syngas[J]. Applied Surface Science, 2018, 434: 351-364. |
| 69 | 张旭, 王子宗, 陈建峰. 助剂对煤基合成气甲烷化反应用镍基催化剂的促进作用[J]. 化工进展, 2015, 34(2): 389-396. |
| ZHANG X, WANG Z Z, CHEN J F. Effects of promoters on supported nickel-based syngas methanation catalysts[J]. Chemical Industry and Engineering Progress, 2015, 34(2): 389-396. | |
| 70 | MIYAO T, TANAKA J, SHEN W H, et al. Catalytic activity and durability of a mesoporous silica-coated Ni-alumina-based catalyst for selective CO methanation[J]. Catalysis Today, 2015, 251: 81-87. |
| 71 | 李春启. 新型合成气甲烷化催化剂La2O3-ZrO2-Ni/Al2O3的制备与性能[J]. 化工进展, 2019, 38(6): 2776-2783. |
| LI Chunqi. Preparation of a noval catalyst of La2O3-ZrO2-Ni/Al2O3 and its performance in syngas mechanation[J]. Chemical Industry and Engineering Progress, 2019, 38(6): 2776-2783. | |
| 72 | MIYAO T, SHEN W H, CHEN A H, et al. Mechanistic study of the effect of chlorine on selective CO methanation over Ni alumina-based catalysts[J]. Applied Catalysis A: General, 2014, 486: 187-192. |
| 73 | 高志明, 代倩子, 马宏伟. 添加氯离子对Ni/CeO2催化剂CO选择甲烷化反应催化性能的促进[J]. 北京理工大学学报, 2016, 36(4): 429-434, 440. |
| GAO Z M, DAI Q Z, MA H W. Effect of chlorine doped in Ni/CeO2 catalyst on selective methanation of CO in H2-rich gas[J]. Transactions of Beijing Institute of Technology, 2016, 36(4): 429-434, 440. | |
| 74 | SHIMODA N, SHOJI D, TANI K, et al. Role of trace chlorine in Ni/TiO2 catalyst for CO selective methanation in reformate gas[J]. Applied Catalysis B: Environmental, 2015, 174/175: 486-495. |
| 75 | SHIMODA N, FUJIWARA M, TANI K, et al. Durability of Ni/TiO2 catalyst containing trace chlorine for CO selective methanation[J]. Applied Catalysis A: General, 2018, 557: 7-14. |
| 76 | ZYRYANOVA M M, SNYTNIKOV P V, GULYAEV R V, et al. Performance of Ni/CeO2 catalysts for selective CO methanation in hydrogen-rich gas[J]. Chemical Engineering Journal, 2014, 238: 189-197. |
| 77 | LIU B, YAO N, LI S, et al. Methanation of CO in hydrogen-rich gas on Ni-Ru/SiO2 catalyst: the type of active sites and Ni-Ru synergistic effect[J]. Chemical Engineering Journal, 2016, 304: 476-484. |
| 78 | 吕晓阳, 孟凡会, 李忠. 双金属Ni-Ru催化剂在加氢及制氢反应中的研究进展[J]. 天然气化工(C1化学与化工), 2017, 42(1): 108-113. |
| LYU X Y, MENG F H, LI Z. Recent advance in bimetallic Ni-Ru catalyst for catalytic hydrogenation and hydrogen production reactions[J]. Natural Gas Chemical Industry, 2017, 42(1): 108-113. | |
| 79 | TADA S, KIKUCHI R, TAKAGAKI A, et al. Study of Ru-Ni/TiO2 catalysts for selective CO methanation[J]. Applied Catalysis B: Environmental, 2013, 140/141: 258-264. |
| 80 | TADA S, MINORI D, OTSUKA F, et al. Effect of Ru and Ni ratio on selective CO methanation over Ru-Ni/TiO2[J]. Fuel, 2014, 129: 219-224. |
| 81 | YANG K W, ZHANG M H, YU Y Z. Effect of transition metal-doped Ni(211) for CO dissociation: insights from DFT calculations[J]. Applied Surface Science, 2017, 399: 255-264. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [6] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [7] | 赵景超, 谭明. 表面活性剂对电渗析减量化工业含盐废水的影响[J]. 化工进展, 2023, 42(S1): 529-535. |
| [8] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [9] | 王谨航, 何勇, 史伶俐, 龙臻, 梁德青. 气体水合物阻聚剂研究进展[J]. 化工进展, 2023, 42(9): 4587-4602. |
| [10] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [11] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [12] | 高彦静. 单原子催化技术国际研究态势分析[J]. 化工进展, 2023, 42(9): 4667-4676. |
| [13] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [14] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [15] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||