化工进展 ›› 2021, Vol. 40 ›› Issue (11): 6246-6253.DOI: 10.16085/j.issn.1000-6613.2020-2265
超薄钯铜纳米片组装纳米花的构建及其氧还原性能
刘文栋( ), 张成会, 陈传霞, 倪朋娟, 姜媛媛, 王波, 逯一中(
), 张成会, 陈传霞, 倪朋娟, 姜媛媛, 王波, 逯一中( )
)
- 济南大学材料科学与工程学院,山东 济南 250022
-
收稿日期:2020-11-12修回日期:2021-01-07出版日期:2021-11-05发布日期:2021-11-19 -
通讯作者:逯一中 -
作者简介:刘文栋(1993—),男,硕士研究生,研究方向为电化学。E-mail:17862916099@163.com 。 -
基金资助:国家自然科学基金(21705056);泰山学者青年专家项目(tsqn201812080);山东省省属高校优秀青年基金(ZR2019YQ10)
Engineering ultrathin PdCu nanosheets-composed nanoflowers with high catalytic activity for oxygen reduction reaction
LIU Wendong( ), ZHANG Chenghui, CHEN Chuanxia, NI Pengjuan, JIANG Yuanyuan, WANG Bo, LU Yizhong(
), ZHANG Chenghui, CHEN Chuanxia, NI Pengjuan, JIANG Yuanyuan, WANG Bo, LU Yizhong( )
)
- School of Materials Science and Engineering, University of Jinan, Jinan 250022, Shandong, China
-
Received:2020-11-12Revised:2021-01-07Online:2021-11-05Published:2021-11-19 -
Contact:LU Yizhong
摘要:
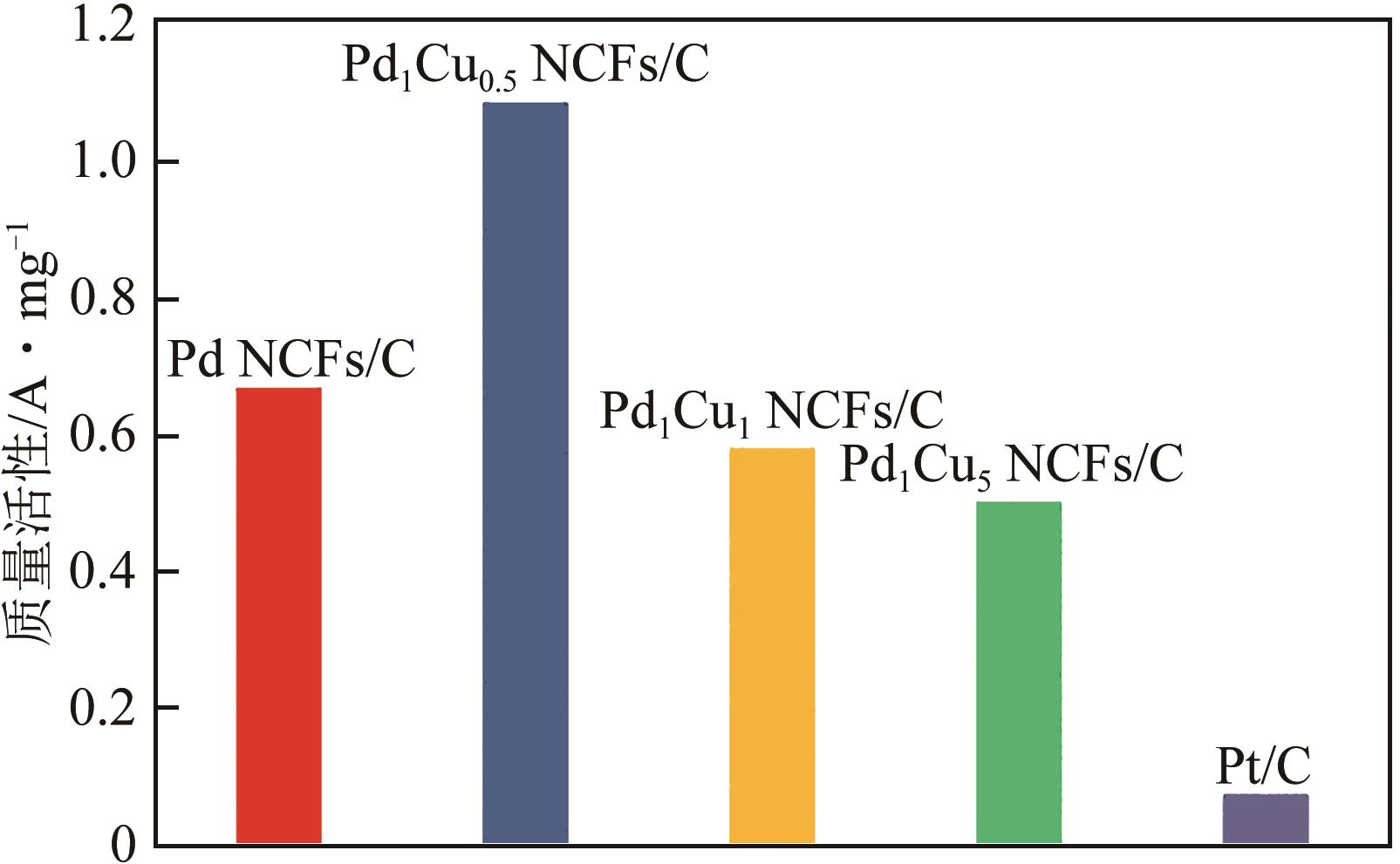
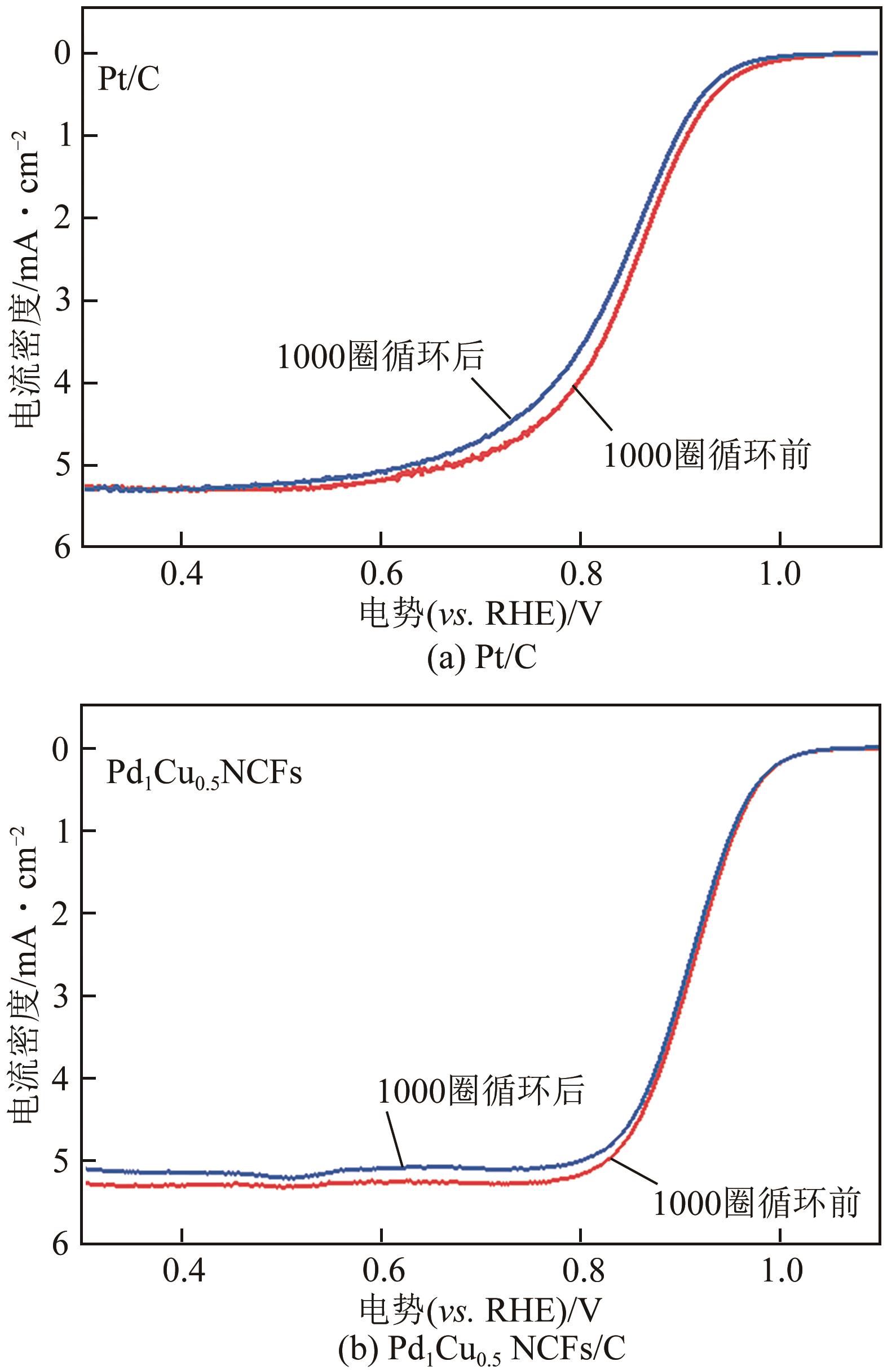
在原子水平上精确调控电催化剂的组成是增强其氧还原(ORR)性能的有效途径。本文采用简单的一步溶剂热还原法制备了由不同PdCu组成的超薄双金属纳米片自组装而成的三维(3D)合金纳米花(Pd1Cux NCFs)。利用透射电子显微镜(TEM)、扫描透射电子显微镜-能谱仪(STEM-EDS)、X射线粉末衍射(XRD)和X射线光电子能谱(XPS)等手段系统表征了Pd1Cux NCFs的形貌、晶体结构及成分。相比于传统二维(2D)纳米材料,Pd1Cux NCFs丰富的快速传质路径、较高的Pd原子利用效率和更强的PdCu双金属间协同作用使得其在碱性介质中表现出增强的ORR性能。另外,本文还研究了Pd、Cu前体用量对ORR性能的影响。结果显示,Pd、Cu前体摩尔比为1∶0.5时,催化剂(Pd1Cu0.5NCFs)在碱性介质中ORR活性和电化学稳定性最佳。其半波电位E1/2(0.937V)远高于商业Pt/C(0.851V);在加速循环1000次扫描后,E1/2几乎没有变化,说明其稳定性优异;0.90V电势下,Pd1Cu0.5 NCFs的质量比活性为1.09A/mg,是商业Pt/C的14.5倍。
中图分类号:
引用本文
刘文栋, 张成会, 陈传霞, 倪朋娟, 姜媛媛, 王波, 逯一中. 超薄钯铜纳米片组装纳米花的构建及其氧还原性能[J]. 化工进展, 2021, 40(11): 6246-6253.
LIU Wendong, ZHANG Chenghui, CHEN Chuanxia, NI Pengjuan, JIANG Yuanyuan, WANG Bo, LU Yizhong. Engineering ultrathin PdCu nanosheets-composed nanoflowers with high catalytic activity for oxygen reduction reaction[J]. Chemical Industry and Engineering Progress, 2021, 40(11): 6246-6253.
| 实验材料与试剂 | 参数 | 商家 |
|---|---|---|
| Na2PdCl4 | 98% | 阿拉丁 |
| CuCl2·2H2O | 99.99% | Sigma-Aldrich |
| W(CO)6 | 97% | Sigma-Aldrich |
| N,N-二甲基甲酰胺(DMF) | 分析纯 | Sigma-Aldrich |
| 乙酸 | 97% | 阿拉丁 |
| Nafion | 5% | 阿拉丁 |
| Vulcan XC-72(导电炭黑) | — | 美国卡博特公司 |
| 无水乙醇 | 分析纯 | Sigma-Aldrich |
| 异丙醇 | 分析纯 | Sigma-Aldrich |
| 商业Pt/C | 20% | Sigma-Aldrich |
| 氢氧化钾 | 分析纯 | 阿拉丁 |
表1 主要实验材料与试剂
| 实验材料与试剂 | 参数 | 商家 |
|---|---|---|
| Na2PdCl4 | 98% | 阿拉丁 |
| CuCl2·2H2O | 99.99% | Sigma-Aldrich |
| W(CO)6 | 97% | Sigma-Aldrich |
| N,N-二甲基甲酰胺(DMF) | 分析纯 | Sigma-Aldrich |
| 乙酸 | 97% | 阿拉丁 |
| Nafion | 5% | 阿拉丁 |
| Vulcan XC-72(导电炭黑) | — | 美国卡博特公司 |
| 无水乙醇 | 分析纯 | Sigma-Aldrich |
| 异丙醇 | 分析纯 | Sigma-Aldrich |
| 商业Pt/C | 20% | Sigma-Aldrich |
| 氢氧化钾 | 分析纯 | 阿拉丁 |
| 催化剂 | 半波电位(E1/2)/V | 质量活性/A·mg-1 | 参考文献 |
|---|---|---|---|
| Pd6Ni | 0.89 | 0.22 | [ |
| B-Pd | — | 0.97 | [ |
| PdMo | 0.95 | 16.37 | [ |
| Pd/W18O49 | 0.875 | 0.216 | [ |
| PdAuCu | — | 1.781 | [ |
| Pd-Cu | 0.90 | 0.59 | [ |
| Pd2FeCo@Pt | 0.880 | 2.5 | [ |
| Pd@PdFe | — | 0.31 | [ |
| Pd1Cu0.5 NCFs | 0.937 | 1.09 | 本工作 |
表2 Pd1Cu0.5 NCFs/C和不同Pd基催化剂在0.1mol/L氢氧化钾溶液中E1/2和质量活性的比较
| 催化剂 | 半波电位(E1/2)/V | 质量活性/A·mg-1 | 参考文献 |
|---|---|---|---|
| Pd6Ni | 0.89 | 0.22 | [ |
| B-Pd | — | 0.97 | [ |
| PdMo | 0.95 | 16.37 | [ |
| Pd/W18O49 | 0.875 | 0.216 | [ |
| PdAuCu | — | 1.781 | [ |
| Pd-Cu | 0.90 | 0.59 | [ |
| Pd2FeCo@Pt | 0.880 | 2.5 | [ |
| Pd@PdFe | — | 0.31 | [ |
| Pd1Cu0.5 NCFs | 0.937 | 1.09 | 本工作 |
| 1 | WINTER Martin, BRODD Ralph J. What are batteries, fuel cells, and supercapacitors?[J]. Chemical Reviews, 2004, 104(10): 4245-4270. |
| 2 | HUANG Hongwen, LI Kan, CHEN Zhao, et al. Achieving remarkable activity and durability toward oxygen reduction reaction based on ultrathin Rh-doped Pt nanowires[J]. Journal of the American Chemical Society, 2017, 139 (24): 8152-8159. |
| 3 | STAMENKOVIC Vojislav R, FOWLER Ben, Bongjin Simon MUN, et al. Improved oxygen reduction activity on Pt3Ni (111) via increased surface site availability[J]. Science, 2007, 315(5811): 493-497. |
| 4 | CHEN Chen, KANG Yijin, HUO Ziyang, et al. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces[J]. Science, 2014, 343(6177): 1339. |
| 5 | GUO Shaojun, ZHANG Xu, ZHU Wenlei, et al. Nanocatalyst superior to Pt for oxygen reduction reactions: the case of core/shell Ag(Au)/CuPd nanoparticles[J]. Journal of the American Chemical Society, 2014, 136(42): 15026-15033. |
| 6 | XIA Bao Yu, YAN Ya, LI Nan, et al. A metal-organic framework-derived bifunctional oxygen electrocatalyst[J]. Nature Energy, 2016, 1(1): 15006. |
| 7 | GAO Dunfeng, ZHOU Hu, WANG Jing, et al. Size-dependent electrocatalytic reduction of CO2 over Pd nanoparticles[J]. Journal of the American Chemical Society, 2015, 137(13): 4288-4291. |
| 8 | GAO Dunfeng, ZHOU Hu, CAI Fan, et al. Switchable CO2 electroreduction via engineering active phases of Pd nanoparticles[J]. Nano Research, 2017, 10(6): 2181-2191. |
| 9 | ANDREADIS George, SONG Shuqin, TSIAKARAS Panagiotis. Direct ethanol fuel cell anode simulation model[J]. Journal of Power Sources, 2006, 157(2): 657-665. |
| 10 | SHAO Minhua, LIU Ping, ZHANG Junliang, et al. Origin of enhanced activity in palladium alloy electrocatalysts for oxygen reduction reaction[J]. The Journal of Physical Chemistry B, 2007, 111(24): 6772-6775. |
| 11 | SHAO Minhua, SASAKI Kotaro, ADZIC Radoslav R. Pd-Fe nanoparticles as electrocatalysts for oxygen reduction[J]. Journal of the American Chemical Society, 2006, 128(11): 3526-3527. |
| 12 | HE Chunyong, TAO Juzhou, SHEN Peikang. Solid synthesis of ultrathin palladium and its alloys’ nanosheets on rGO with high catalytic activity for oxygen reduction reaction[J]. ACS Catalysis, 2018, 8(2): 910-919. |
| 13 | GUNJI Takao, Seung Hyo NOH, ANDO Fuma, et al. Electrocatalytic activity of electrochemically dealloyed PdCu3 intermetallic compound towards oxygen reduction reaction in acidic media[J]. Journal of Materials Chemistry A, 2018, 6(30): 14828-14837. |
| 14 | JIANG Guangming, ZHU Huiyuan, ZHANG Xu, et al. Core/shell face-centered tetragonal FePd/Pd nanoparticles as an efficient non-Pt catalyst for the oxygen reduction reaction[J]. ACS Nano, 2015, 9(11): 11014-11022. |
| 15 | CHEN Luyang, GUO Hai, FUJITA Takeshi, et al. Nanoporous PdNi bimetallic catalyst with enhanced electrocatalytic performances for electro-oxidation and oxygen reduction reactions[J]. Advanced Functional Materials, 2011, 21(22): 4364-4370. |
| 16 | NOVOSELOV K S, GEIM A K, MOROZOV S V, et al. Electric field effect in atomically thin carbon films[J]. Science, 2004, 306(5696): 666. |
| 17 | ZHAO Xiaojing, DAI Lei, QIN Qing, et al. Self-supported 3D PdCu alloy nanosheets as a bifunctional catalyst for electrochemical reforming of ethanol[J]. Small, 2017, 13(12): 1602970. |
| 18 | AN Hongming, ZHAO Zhiliang, ZHANG Lianying, et al. Ir-alloyed ultrathin ternary PdIrCu nanosheet-constructed flower with greatly enhanced catalytic performance toward formic acid electrooxidation[J]. ACS Applied Materials & Interfaces, 2018, 10(48): 41293-41298. |
| 19 | SHANG Hongyuan, XU Hui, LIU Qingyun, et al. PdCu alloy nanosheets-constructed 3D flowers: new highly sensitive materials for H2S detection[J]. Sensors and Actuators B: Chemical, 2019, 289: 260-268. |
| 20 | LU Yizhong, WANG Jiong, PENG Yuecheng, et al. Highly efficient and durable Pd hydride nanocubes embedded in 2D amorphous NiB nanosheets for oxygen reduction reaction[J]. Advanced Energy Materials, 2017, 7(21): 1700919. |
| 21 | ZHAO Zipeng, HUANG Xiaoqing, LI Mufan, et al. Synthesis of stable shape-controlled catalytically active β-palladium hydride[J]. Journal of the American Chemical Society, 2015, 137(50): 15672-15675. |
| 22 | GU Zhulan, XIONG Zhiping, REN Fangfang, et al. Flower-like PdCu catalyst with high electrocatalytic properties for ethylene glycol oxidation[J]. Journal of the Taiwan Institute of Chemical Engineers, 2018, 83: 32-39. |
| 23 | HU Chuangang, ZHAI Xiangquan, ZHAO Yang, et al. Small-sized PdCu nanocapsules on 3D graphene for high-performance ethanol oxidation[J]. Nanoscale, 2014, 6(5): 2768-2775. |
| 24 | XU Hui, YAN Bo, ZHANG Ke, et al. Sub-5nm monodispersed PdCu nanosphere with enhanced catalytic activity towards ethylene glycol electrooxidation[J]. Electrochimica Acta, 2018, 261: 521-529. |
| 25 | ZHANG Qianli, ZHENG Jiening, XU Tianqi, et al. Simple one-pot preparation of Pd-on-Cu nanocrystals supported on reduced graphene oxide for enhanced ethanol electrooxidation[J]. Electrochimica Acta, 2014, 132: 551-560. |
| 26 | MOHANTY Ashok, GARG Niti, JIN Rongchao. A universal approach to the synthesis of noble metal nanodendrites and their catalytic properties[J]. Angewandte Chemie International Edition, 2010, 49(29): 4962-4966. |
| 27 | LU Yizhong, JIANG Yuanyuan, CHEN Wei. Graphene nanosheet-tailored PtPd concave nanocubes with enhanced electrocatalytic activity and durability for methanol oxidation[J]. Nanoscale, 2014, 6(6): 3309-3315. |
| 28 | LU Yizhong, CHEN Wei. PdAg alloy nanowires: facile one-step synthesis and high electrocatalytic activity for formic acid oxidation[J]. ACS Catalysis, 2012, 2(1): 84-90. |
| 29 | TAO Hua Bing, ZHANG Junming, CHEN Jiazang, et al. Revealing energetics of surface oxygen redox from kinetic fingerprint in oxygen electrocatalysis[J]. Journal of the American Chemical Society, 2019, 141(35): 13803-13811. |
| 30 | FENG Yonggang, SHAO Qi, JI Yujin, et al. Surface-modulated palladium-nickel icosahedra as high-performance non-platinum oxygen reduction electrocatalysts[J]. Science Advances, 2018, 4(7): eaap8817. |
| 31 | LI Jun, CHEN Junxiang, WANG Qiang, et al. Controllable increase of boron content in B-Pd interstitial nanoalloy to boost the oxygen reduction activity of palladium[J]. Chemistry of Materials, 2017, 29(23): 10060-10067. |
| 32 | LUO Mingchuan, ZHAO Zhonglong, ZHANG Yelong, et al. PdMo bimetallene for oxygen reduction catalysis[J]. Nature, 2019, 574(7776): 81-85. |
| 33 | LU Yizhong, JIANG Yuanyuan, GAO Xiaohui, et al. Strongly coupled Pd nanotetrahedron/tungsten oxide nanosheet hybrids with enhanced catalytic activity and stability as oxygen reduction electrocatalysts[J]. Journal of the American Chemical Society, 2014, 136(33): 11687-11697. |
| 34 | WANG Gongwei, GUAN Jianxin, XIAO Li, et al. Pd skin on AuCu intermetallic nanoparticles: a highly active electrocatalyst for oxygen reduction reaction in alkaline media[J]. Nano Energy, 2016, 29: 268-274. |
| 35 | PENG Xiong, OMASTA Travis J, ROLLER Justin M, et al. Highly active and durable Pd-Cu catalysts for oxygen reduction in alkaline exchange membrane fuel cells[J]. Frontiers in Energy, 2017, 11(3): 299-309. |
| 36 | XIAO Weiping, LIUTHEVICIENE Cordeiro Marco Aurelio, GONG Mingxing, et al. Optimizing the ORR activity of Pd based nanocatalysts by tuning their strain and particle size[J]. Journal of Materials Chemistry A, 2017, 5(20): 9867-9872. |
| 37 | LI Xu, LI Xingxing, LIU Chunxiao, et al. Atomic-level construction of tensile-strained PdFe alloy surface toward highly efficient oxygen reduction electrocatalysis[J]. Nano Letters, 2020, 20(2): 1403-1409. |
| 38 | YAN Dafeng, LI Yunxiao, HUO Jia, et al. Defect chemistry of nonprecious-metal electrocatalysts for oxygen reactions[J]. Advanced Materials, 2017, 29(48): 1606459. |
| 39 | JIANG Yuanyuan, DONG Kai, YAN Xiaoying, et al. Metal-polydopamine framework-derived (Co)/N-doped carbon hollow nanocubes as efficient oxygen electrocatalysts[J]. Sustainable Energy & Fuels, 2020, 4(7): 3370-3377. |
| 40 | LIANG Yongye, LI Yanguang, WANG Hailiang, et al. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction[J]. Nature Materials, 2011, 10(10): 780-786. |
| 41 | GARSANY Yannick, BATURINA Olga A, SWIDER-LYONS Karen E, et al. Experimental methods for quantifying the activity of platinum electrocat alysts for the oxygen reduction reaction[J]. Analytical Chemistry, 2010, 82 (15): 6321-6328. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 胡喜, 王明珊, 李恩智, 黄思鸣, 陈俊臣, 郭秉淑, 于博, 马志远, 李星. 二硫化钨复合材料制备与储钠性能研究进展[J]. 化工进展, 2023, 42(S1): 344-355. |
| [6] | 张杰, 白忠波, 冯宝鑫, 彭肖林, 任伟伟, 张菁丽, 刘二勇. PEG及其复合添加剂对电解铜箔后处理的影响[J]. 化工进展, 2023, 42(S1): 374-381. |
| [7] | 许家珩, 李永胜, 罗春欢, 苏庆泉. 甲醇水蒸气重整工艺的优化[J]. 化工进展, 2023, 42(S1): 41-46. |
| [8] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [9] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [10] | 陈匡胤, 李蕊兰, 童杨, 沈建华. 质子交换膜燃料电池气体扩散层结构与设计研究进展[J]. 化工进展, 2023, 42(S1): 246-259. |
| [11] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [12] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [13] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [14] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [15] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||