化工进展 ›› 2021, Vol. 40 ›› Issue (9): 4931-4947.DOI: 10.16085/j.issn.1000-6613.2021-0402
燃料电池中铂基电催化剂的设计与合成
李瑞松1,2( ), 刘亚琳2, 田浩2, 王谦2, 饶鹏1,2, 李静1,2, 贾春满1,3, 田新龙1,2(
), 刘亚琳2, 田浩2, 王谦2, 饶鹏1,2, 李静1,2, 贾春满1,3, 田新龙1,2( )
)
- 1.海南大学化学工程与技术学院,海南 海口 570228
2.海南大学南海海洋资源利用国家重点实验室,海南 海口 570228
3.海南省精细化工重点实验室,海南 海口 570228
-
收稿日期:2021-03-01修回日期:2021-04-08出版日期:2021-09-05发布日期:2021-09-13 -
通讯作者:田新龙 -
作者简介:李瑞松(1989—),男,博士研究生,研究方向为燃料电池。E-mail:lrs0809@hainanu.edu.cn 。 -
基金资助:海南省重点研发计划(ZDYF2020037);海南省研究生创新科研课题(Hyb2020-05)
Design and preparation of platinum-based electrocatalysts for fuel cells
LI Ruisong1,2( ), LIU Yalin2, TIAN Hao2, WANG Qian2, RAO Peng1,2, LI Jing1,2, JIA Chunman1,3, TIAN Xinlong1,2(
), LIU Yalin2, TIAN Hao2, WANG Qian2, RAO Peng1,2, LI Jing1,2, JIA Chunman1,3, TIAN Xinlong1,2( )
)
- 1.School of Chemical Engineering and Technology, Hainan University, Haikou 570228, Hainan, China
2.State Key Laboratory of Marine Resource Utilization in South China Sea, Hainan University, Haikou 570228, Hainan, China
3.Hainan Provincial Key Lab of Fine Chemistry, Haikou 570228, Hainan, China
-
Received:2021-03-01Revised:2021-04-08Online:2021-09-05Published:2021-09-13 -
Contact:TIAN Xinlong
摘要:
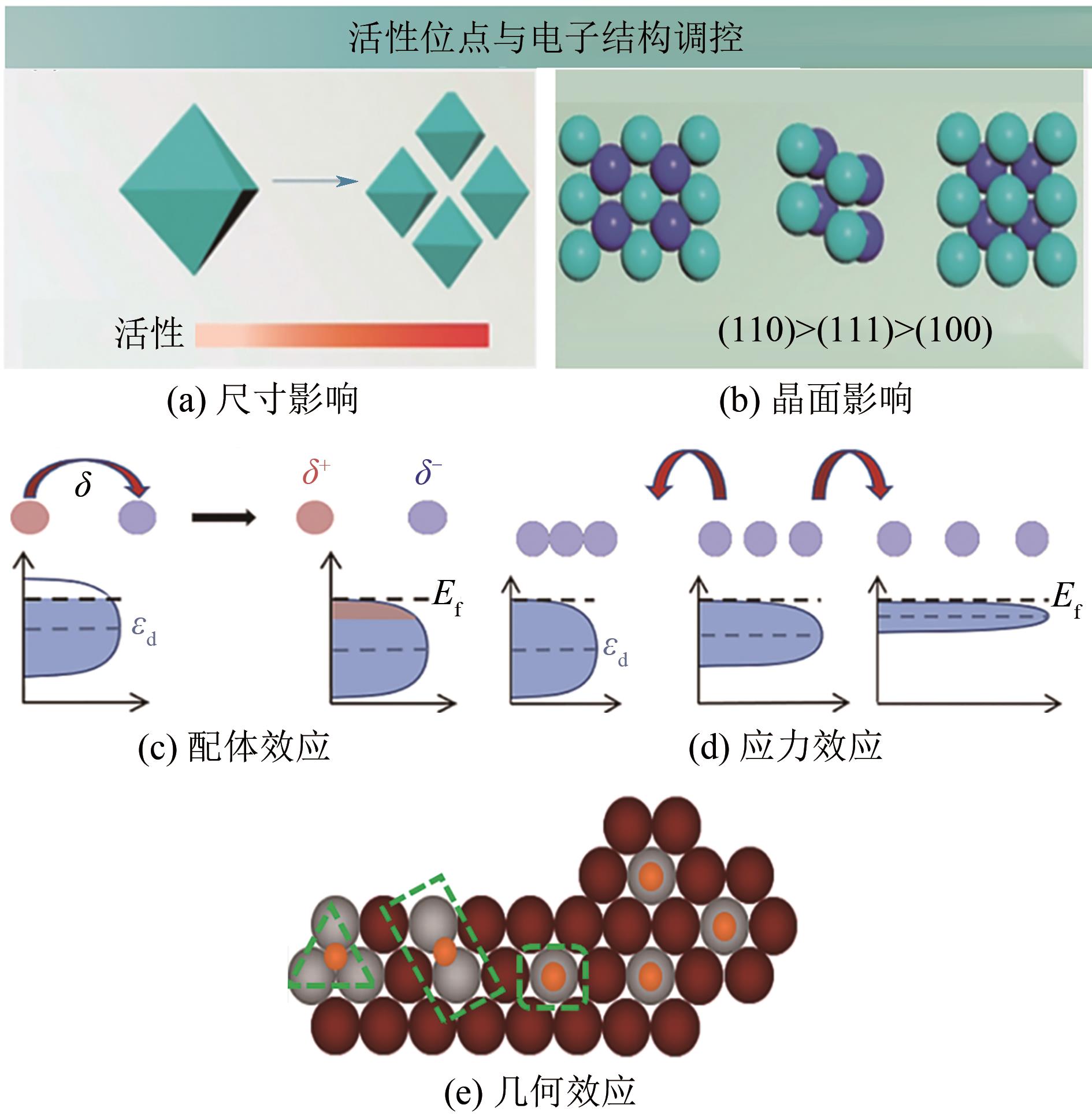
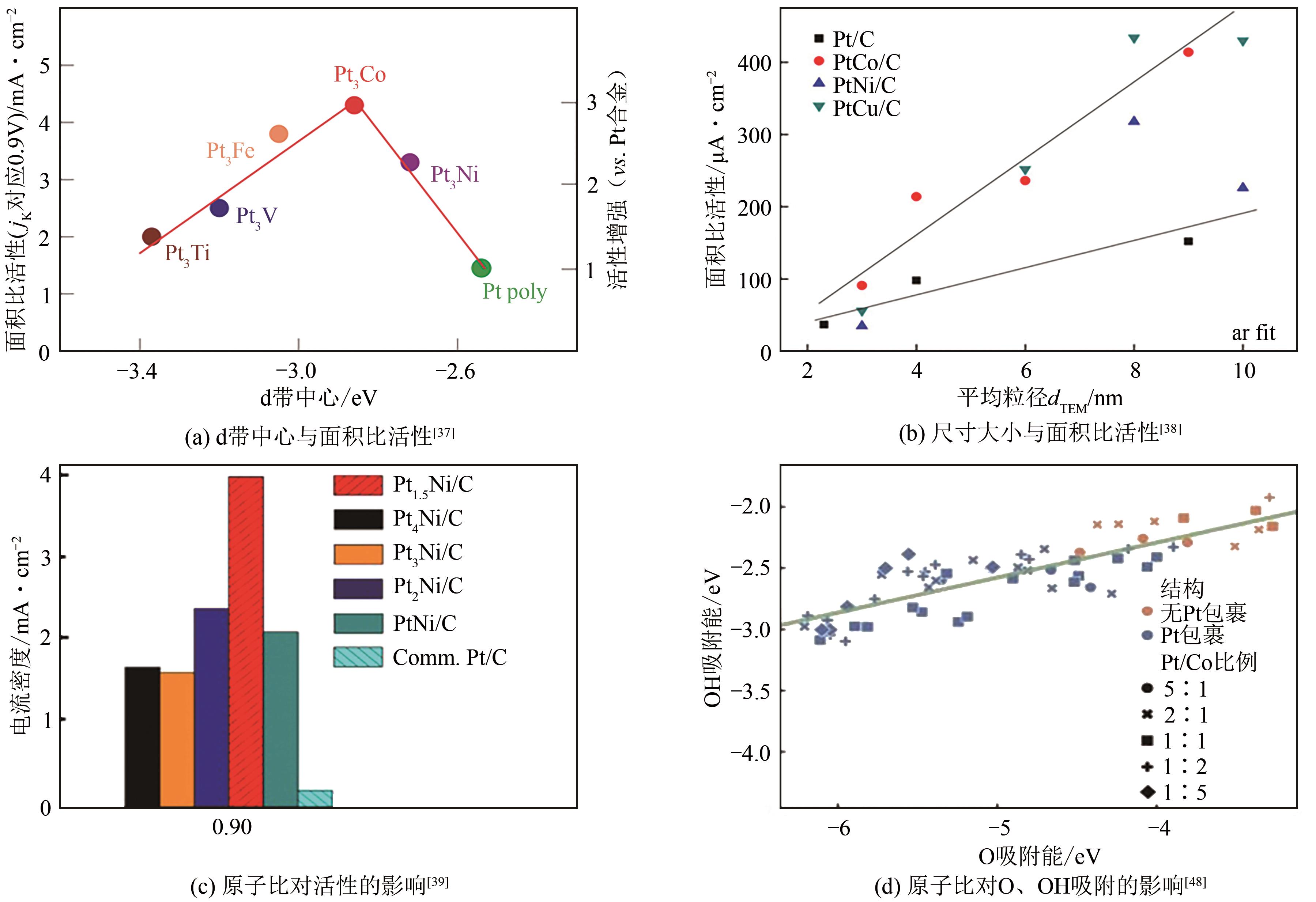
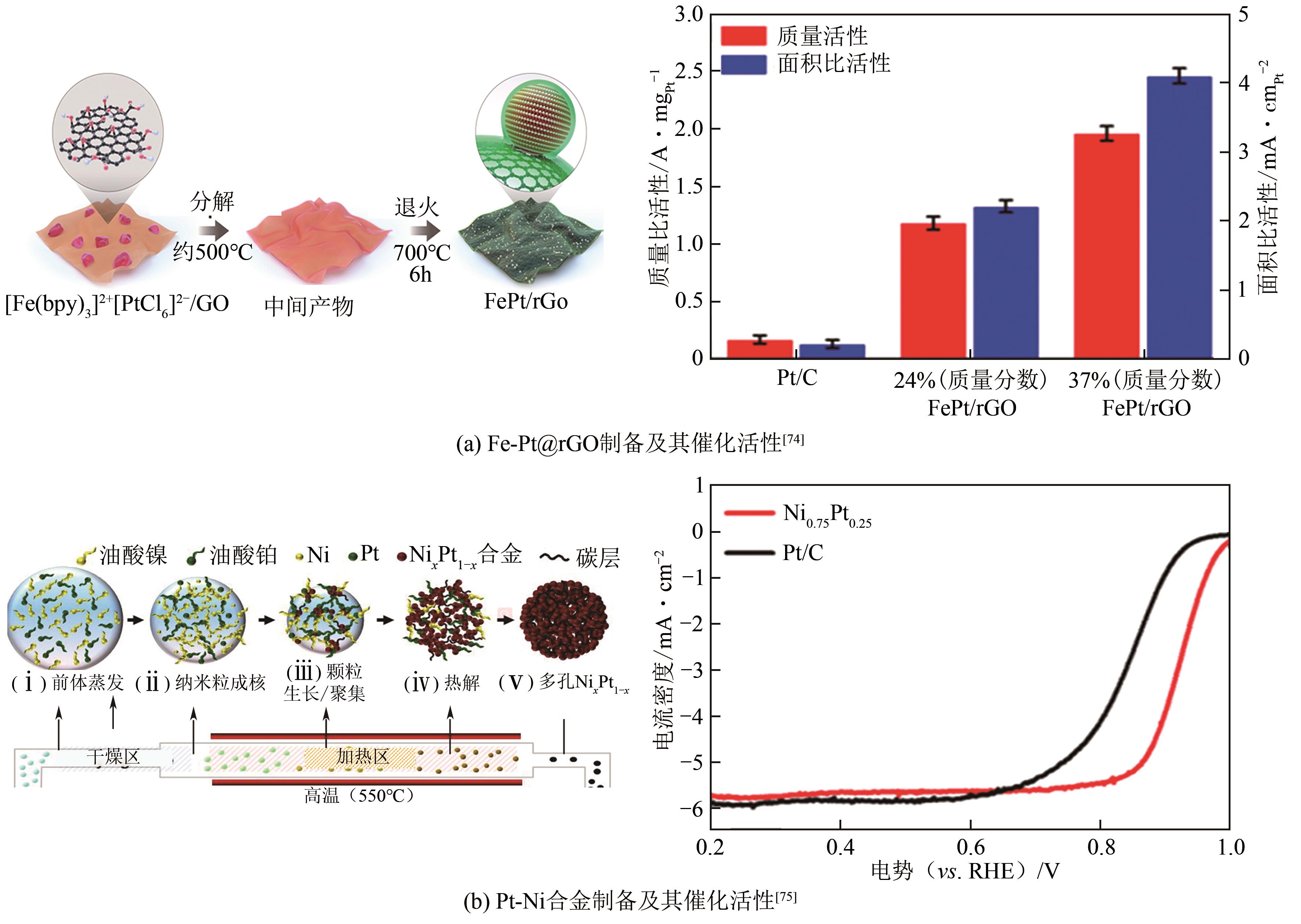
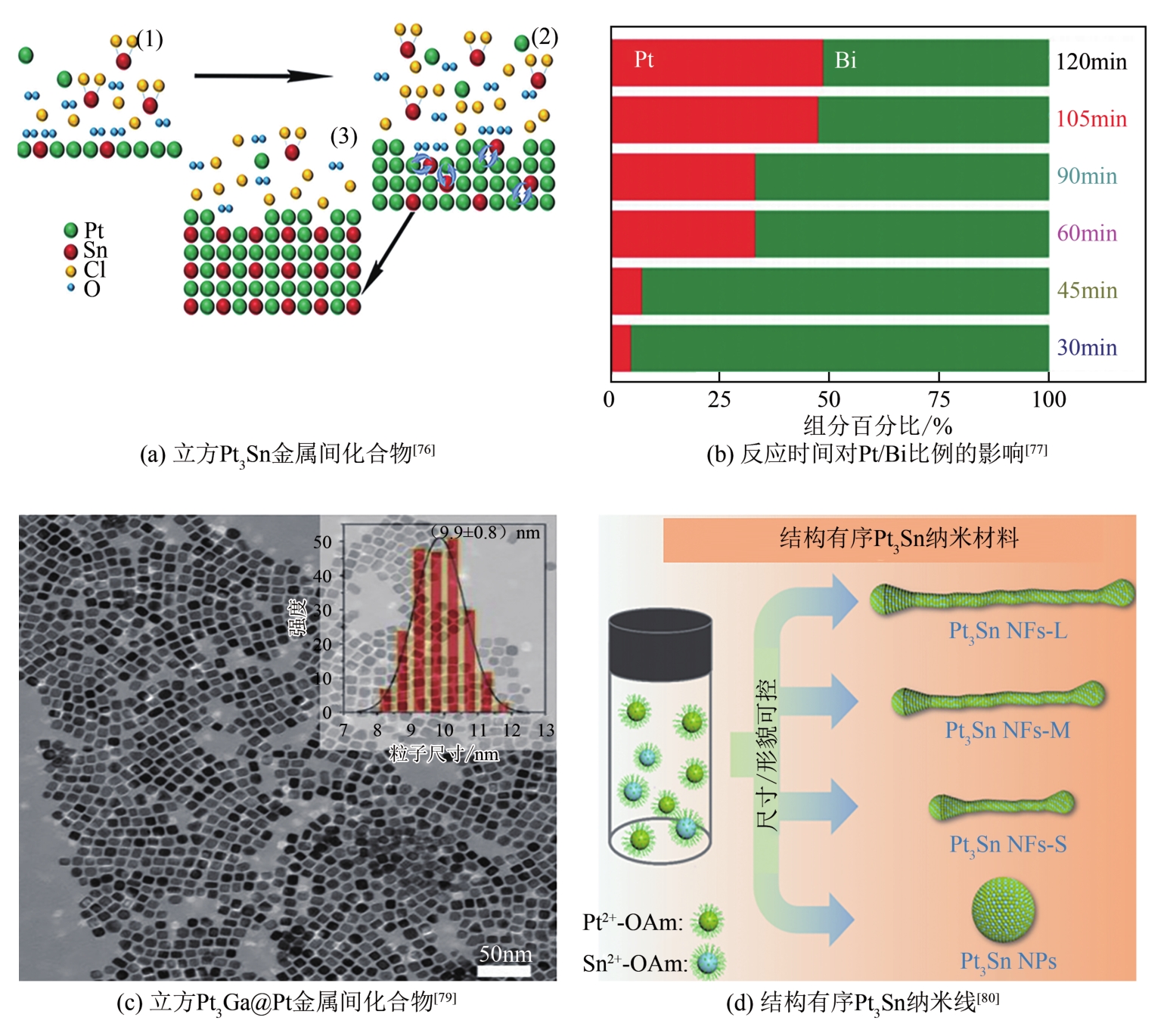
燃料电池具有高能量转化效率、低环境污染等优势,近年来备受关注。然而,阴极氧还原反应和阳极小分子氧化反应成为燃料电池产业化的瓶颈,包括催化剂制备成本高、催化活性低和稳定性差等问题。如何设计高效、稳定的燃料电池催化剂,对于进一步推动燃料电池的应用十分关键,而发展先进的铂(Pt)基电催化剂是最为有效的途径之一。相比于单金属铂纳米晶,铂基无序合金和有序金属间化合物具有独特的物理化学特性,被认为是研究金属电催化剂结构-性能的理想模型。本文综述了高活性、高稳定性的铂基电催化剂的研究现状,首先阐述了铂基电催化剂的催化活性和稳定性的增强机制,着重介绍了铂基合金的调控因素与可控合成,进一步总结了铂基有序金属间化合物的制备策略。最后,对铂基电催化剂的未来发展方向进行了讨论及展望,以期为燃料电池中电催化剂的发展开拓新思路。
中图分类号:
引用本文
李瑞松, 刘亚琳, 田浩, 王谦, 饶鹏, 李静, 贾春满, 田新龙. 燃料电池中铂基电催化剂的设计与合成[J]. 化工进展, 2021, 40(9): 4931-4947.
LI Ruisong, LIU Yalin, TIAN Hao, WANG Qian, RAO Peng, LI Jing, JIA Chunman, TIAN Xinlong. Design and preparation of platinum-based electrocatalysts for fuel cells[J]. Chemical Industry and Engineering Progress, 2021, 40(9): 4931-4947.
| 组成 | 合成方法 | 结构/尺寸 | 催化应用 | 参考文献 |
|---|---|---|---|---|
| Pt4.8Fe | 乙二醇/NaOH体系130℃反应3h | (3.1±1)nm | 氧还原 | [ |
| PtFe | 油胺/葡萄糖体系180℃反应8h | 一维层级 | 甲醇氧化 | [ |
| PtFe/CNT | 瞬态电热法 | 约5nm | 氧还原 | [ |
| Pt3Co | — | 约2.5nm | 氧还原 | [ |
| PtM(Ni、Co、Cu) | H2氛围中350℃反应3h | 约3.0nm | 氧还原 | [ |
| PtCo | 油胺体系240℃反应+HNO3刻蚀 | 纳米框架 | 甲醇氧化 | [ |
| Pt1.5Ni | CO/H2氛围中200℃反应1h | (3.1±1.2)nm | 氧还原 | [ |
| Pt3Ni | 硼氢化物还原+300℃热退火 | 约4.2nm | 氧还原 | [ |
| Pt-Cu | 乙二醇/焦磷酸二氢钠160℃反应6h | 约2.4nm纳米线 | 氧还原 | [ |
| PtCu | N, N-二甲基甲酰胺(DMF)/硼氢化钠/聚乙烯吡咯烷酮(PVP)还原+250℃煅烧2h | 纳米框架 | 甲醇氧化 | [ |
| PtSn | 油胺/油酸130℃反应30min+320℃煅烧 | 超薄片层 | 甲醇、乙醇氧化 | [ |
| Pt3Mn | 甘氨酸/PVP体系200℃反应6h+200℃煅烧2h | 凹面立方体 | 乙二醇氧化 | [ |
表1 近年发展的Pt基合金催化剂的合成方法、结构与应用
| 组成 | 合成方法 | 结构/尺寸 | 催化应用 | 参考文献 |
|---|---|---|---|---|
| Pt4.8Fe | 乙二醇/NaOH体系130℃反应3h | (3.1±1)nm | 氧还原 | [ |
| PtFe | 油胺/葡萄糖体系180℃反应8h | 一维层级 | 甲醇氧化 | [ |
| PtFe/CNT | 瞬态电热法 | 约5nm | 氧还原 | [ |
| Pt3Co | — | 约2.5nm | 氧还原 | [ |
| PtM(Ni、Co、Cu) | H2氛围中350℃反应3h | 约3.0nm | 氧还原 | [ |
| PtCo | 油胺体系240℃反应+HNO3刻蚀 | 纳米框架 | 甲醇氧化 | [ |
| Pt1.5Ni | CO/H2氛围中200℃反应1h | (3.1±1.2)nm | 氧还原 | [ |
| Pt3Ni | 硼氢化物还原+300℃热退火 | 约4.2nm | 氧还原 | [ |
| Pt-Cu | 乙二醇/焦磷酸二氢钠160℃反应6h | 约2.4nm纳米线 | 氧还原 | [ |
| PtCu | N, N-二甲基甲酰胺(DMF)/硼氢化钠/聚乙烯吡咯烷酮(PVP)还原+250℃煅烧2h | 纳米框架 | 甲醇氧化 | [ |
| PtSn | 油胺/油酸130℃反应30min+320℃煅烧 | 超薄片层 | 甲醇、乙醇氧化 | [ |
| Pt3Mn | 甘氨酸/PVP体系200℃反应6h+200℃煅烧2h | 凹面立方体 | 乙二醇氧化 | [ |
| 1 | TIAN X L, LU X F, XIA B Y, et al. Advanced electrocatalysts for the oxygen reduction reaction in energy conversion technologies[J]. Joule, 2020, 4(1): 45-68. |
| 2 | ZHANG L, ZHANG X F, CHEN X L, et al. Facile solvothermal synthesis of Pt71Co29 lamellar nanoflowers as an efficient catalyst for oxygen reduction and methanol oxidation reactions[J]. Journal of Colloid and Interface Science, 2019, 536: 556-562. |
| 3 | ESLAMIBIDGOLI M J, HUANG J, KADYK T, et al. How theory and simulation can drive fuel cell electrocatalysis[J]. Nano Energy, 2016, 29: 334-361. |
| 4 | CASALONGUE H S, KAYA S, VISWANATHAN V, et al. Direct observation of the oxygenated species during oxygen reduction on a platinum fuel cell cathode[J]. Nature Communications, 2013, 4: 2817. |
| 5 | XIAO M L, CHEN Y T, ZHU J B, et al. Climbing the apex of the ORR volcano plot via binuclear site construction: electronic and geometric engineering[J]. Journal of the American Chemical Society, 2019, 141 (44): 17763-17770. |
| 6 | LIU L C, CORMA A. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles[J]. Chemical Reviews, 2018, 118(10): 4981-5079. |
| 7 | ROBINSON J E, LABRADOR N Y, CHEN H, et al. Silicon oxide-encapsulated platinum thin films as highly active electrocatalysts for carbon monoxide and methanol oxidation[J]. ACS Catalysis, 2018, 8 (12): 11423-11434. |
| 8 | BASHYAM R, ZELENAY P. A class of non-precious metal composite catalysts for fuel cells[M]//Materials for Sustainable Energy. London: Macmillan Publishers Ltd., UK, 2010: 247-250. |
| 9 | THOMPSON S T, PAPAGEORGOPOULOS D. Platinum group metal-free catalysts boost cost competitiveness of fuel cell vehicles[J]. Nature Catalysis, 2019, 2(7): 558-561. |
| 10 | BANHAM D, YE S Y. Current status and future development of catalyst materials and catalyst layers for proton exchange membrane fuel cells: an industrial perspective[J]. ACS Energy Letters, 2017, 2(3): 629-638. |
| 11 | YOSHIDA T, KOJIMA K. Toyota MIRAI fuel cell vehicle and progress toward a future hydrogen society[J]. Interface Magazine, 2015, 24(2): 45-49. |
| 12 | YANG L J, SHUI J L, DU L, et al. Carbon-based metal-free ORR electrocatalysts for fuel cells: past, present, and future[J]. Advanced Materials, 2019, 31(13): e1804799. |
| 13 | PEERA S G, MAIYALAGAN T, LIU C, et al. A review on carbon and non-precious metal based cathode catalysts in microbial fuel cells[J]. International Journal of Hydrogen Energy, 2021, 46(4): 3056-3089. |
| 14 | WANG Y, YANG Y, JIA S F, et al. Synergistic Mn-Co catalyst outperforms Pt on high-rate oxygen reduction for alkaline polymer electrolyte fuel cells[J]. Nature Communications, 2019, 10(1): 1506. |
| 15 | CUI C H, GAN L, HEGGEN M, et al. Compositional segregation in shaped Pt alloy nanoparticles and their structural behaviour during electrocatalysis[J]. Nature Materials, 2013, 12(8): 765-771. |
| 16 | MA Z, CANO Z P, YU A P, et al. Enhancing oxygen reduction activity of Pt-based electrocatalysts: from theoretical mechanisms to practical methods[J]. Angewandte Chemie International Edition, 2020, 59(42): 18334-18348. |
| 17 | RÖBNER L, ARMBRÜSTER M. Electrochemical energy conversion on intermetallic compounds: a review[J]. ACS Catalysis, 2019, 9(3): 2018-2062. |
| 18 | NARAYANAN R, EL-SAYED M A. Catalysis with transition metal nanoparticles in colloidal solution: nanoparticle shape dependence and stability[J]. The Journal of Physical Chemistry B, 2005, 109(26): 12663-12676. |
| 19 | SHAO M, PELES A, SHOEMAKER K. Electrocatalysis on platinum nanoparticles: particle size effect on oxygen reduction reaction activity[J]. Nano Letters, 2011, 11(9): 3714-3719. |
| 20 | GAMLER J T L, ASHBERRY H M, SKRABALAK S E, et al. Random alloyed versus intermetallic nanoparticles: a comparison of electrocatalytic performance[J]. Advanced Materials, 2018, 30(40): 1801563. |
| 21 | HUANG L L, ZHENG C Y, SHEN B, et al. High-index-facet metal-alloy nanoparticles as fuel cell electrocatalysts[J]. Advanced Materials, 2020, 32(30): 2002849. |
| 22 | CHOI S I, XIE S F, SHAO M H, et al. Synthesis and characterization of 9nm Pt-Ni octahedra with a record high activity of 3.3A/mgPt for the oxygen reduction reaction[J]. Nano Letters, 2013, 13(7): 3420-3425. |
| 23 | GREELEY J, STEPHENS I E, BONDARENKO A S, et al. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts[J]. Nature Chemistry, 2009, 1(7): 552-556. |
| 24 | LUO M C, GUO S J. Strain-controlled electrocatalysis on multimetallic nanomaterials[J]. Nature Reviews Materials, 2017, 2: 17059. |
| 25 | KARAMAD M, TRIPKOVIC V, ROSSMEISL J. Intermetallic alloys as CO electroreduction catalysts—Role of isolated active sites[J]. ACS Catalysis, 2014, 4(7): 2268-2273. |
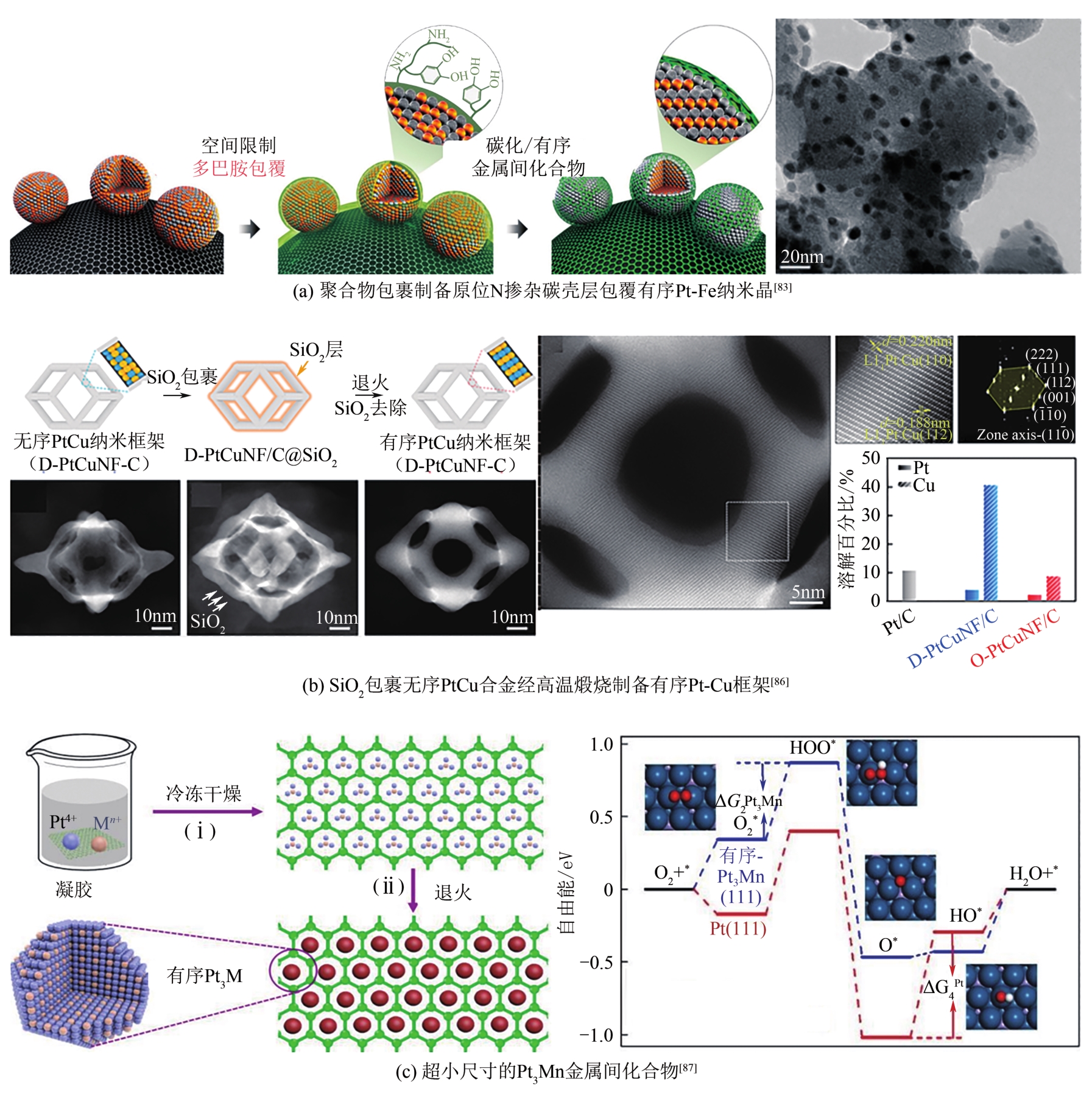
| 26 | LUO S P, TANG M, SHEN P K, et al. Atomic-scale preparation of octopod nanoframes with high-index facets as highly active and stable catalysts[J]. Advanced Materials, 2017, 29(8). DOI: 10.1002/adma.201601687. |
| 27 | SASAKI K, KUTTIYIEL K A, ADZIC R R. Designing high performance Pt monolayer core-shell electrocatalysts for fuel cells[J]. Current Opinion in Electrochemistry, 2020, 21: 368-375. |
| 28 | CHEN S P, LI M F, GAO M Y, et al. High-performance Pt-Co nanoframes for fuel-cell electrocatalysis[J]. Nano Letters, 2020, 20(3): 1974-1979. |
| 29 | YAO W Q, JIANG X J, LI M, et al. Engineering hollow porous platinum-silver double-shelled nanocages for efficient electro-oxidation of methanol[J]. Applied Catalysis B: Environmental, 2021, 282: 119595. |
| 30 | XIAO W P, LEI W, GONG M X, et al. Recent advances of structurally ordered intermetallic nanoparticles for electrocatalysis[J]. ACS Catalysis, 2018, 8(4): 3237-3256. |
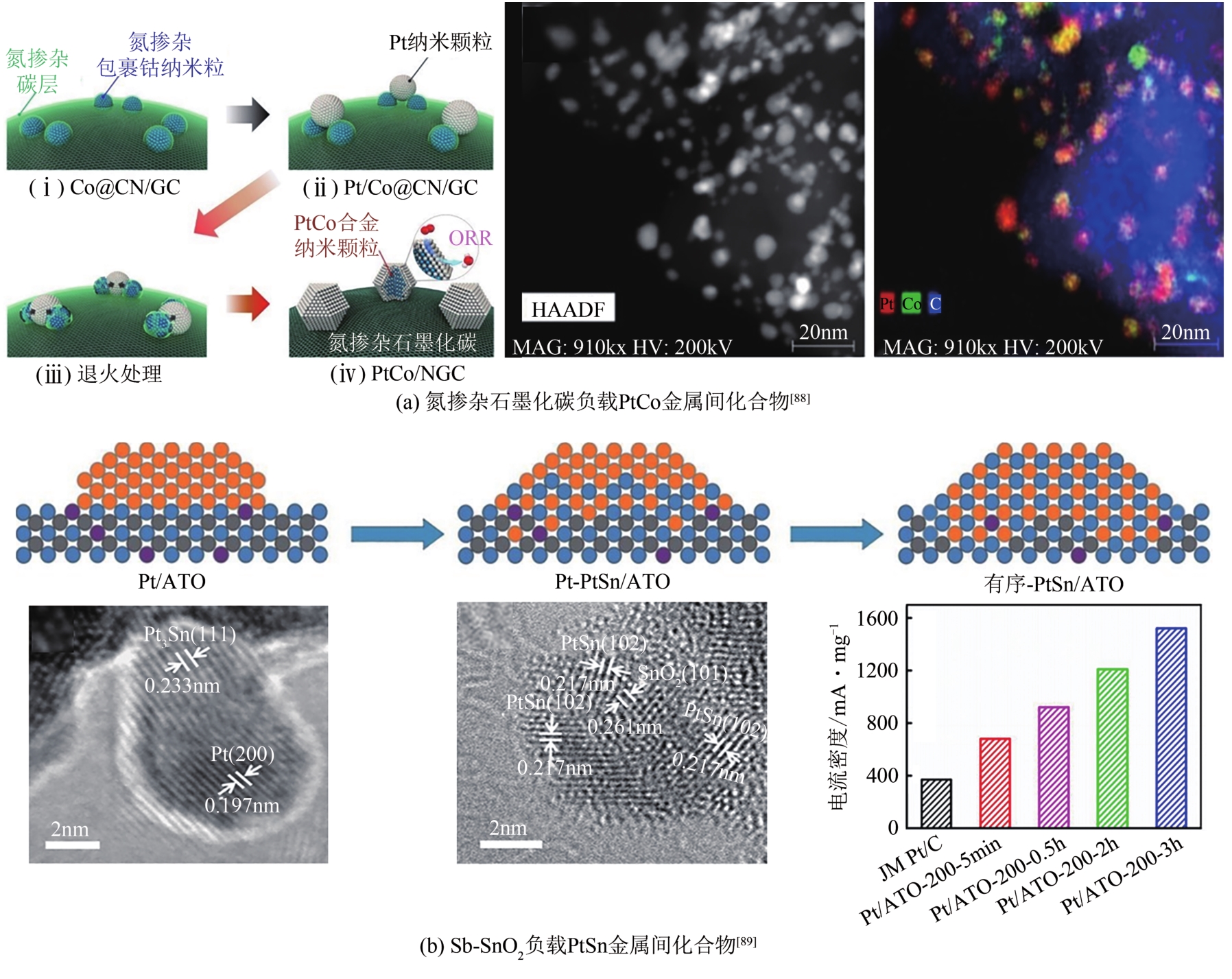
| 31 | PROKOP M, DRAKSELOVA M, BOUZEK K. Review of the experimental study and prediction of Pt-based catalyst degradation during PEM fuel cell operation[J]. Current Opinion in Electrochemistry, 2020, 20: 20-27. |
| 32 | GREELEY J, NØRSKOV J K. Electrochemical dissolution of surface alloys in acids: thermodynamic trends from first-principles calculations[J]. Electrochimica Acta, 2007, 52(19): 5829-5836. |
| 33 | VEJ-HANSEN U G, ROSSMEISL J, STEPHENS I E L, et al. Correlation between diffusion barriers and alloying energy in binary alloys[J]. Physical Chemistry Chemical Physics, 2016, 18(4): 3302-3307. |
| 34 | MAO L C, FU K, JIN J H, et al. PtFe alloy catalyst supported on porous carbon nanofiber with high activity and durability for oxygen reduction reaction[J]. International Journal of Hydrogen Energy, 2019, 44(33): 18083-18092. |
| 35 | WANG L J, TIAN X L, XU Y Y, et al. Engineering one-dimensional and hierarchical PtFe alloy assemblies towards durable methanol electrooxidation[J]. Journal of Materials Chemistry A, 2019, 7(21): 13090-13095. |
| 36 | ZENG S, LYU B, QIAO J, et al. PtFe Alloy nanoparticles confined on carbon nanotube networks as air cathodes for flexible and wearable energy devices[J]. ACS Applied Nano Materials, 2019, 2(12): 7870-7879. |
| 37 | STAMENKOVIC V R, MUN B S, ARENZ M, et al. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces[J]. Nature Materials, 2007, 6(3): 241-247. |
| 38 | JAYASAYEE K, VEEN J A R V, MANIVASAGAM T G, et al. Oxygen reduction reaction (ORR) activity and durability of carbon supported PtM (Co, Ni, Cu) alloys: influence of particle size and non-noble metals[J]. Applied Catalysis B: Environmental, 2012, 111/112: 515-526. |
| 39 | ZHANG C L, HWANG S Y, TROUT A, et al. Solid-state chemistry-enabled scalable production of octahedral Pt-Ni alloy electrocatalyst for oxygen reduction reaction[J]. Journal of the American Chemical Society, 2014, 136 (22): 7805-7808. |
| 40 | JEON T Y, YOO S J, CHO Y H, et al. Effect of de-alloying of Pt-Ni bimetallic nanoparticles on the oxygen reduction reaction[J]. Electrochemistry Communications, 2010, 12(12): 1796-1799. |
| 41 | FANG D H, WAN L K, JIANG Q K, et al. Wavy PtCu alloy nanowire networks with abundant surface defects enhanced oxygen reduction reaction[J]. Nano Research, 2019, 12(11): 2766-2773. |
| 42 | LI X L, ZHOU Y S, DU Y Y, et al. PtCu nanoframes as ultra-high performance electrocatalysts for methanol oxidation[J]. International Journal of Hydrogen Energy, 2019, 44(33): 18050-18057. |
| 43 | CHEN J Y, LIM S C, KUO C H, et al. Sub-1nm PtSn ultrathin sheet as an extraordinary electrocatalyst for methanol and ethanol oxidation reactions[J]. Journal of Colloid and Interface Science, 2019, 545: 54-62. |
| 44 | WANG Y, ZHUO H Y, SUN H, et al. Implanting Mo atoms into surface lattice of Pt3Mn alloys enclosed by high-indexed facets: promoting highly active sites for ethylene glycol oxidation[J]. ACS Catalysis, 2019, 9(1): 442-455. |
| 45 | CORRADINI P G, SANTOS N A, PEREZ J. Pt-Sn-Eu/C catalysts: application of rare earth metals as anodes in direct ethanol fuel cells[J]. Fuel Cells, 2018, 18(1): 73-81. |
| 46 | CHU T K, XIE M, YANG D J, et al. Highly active and durable carbon support Pt-rare earth catalyst for proton exchange membrane fuel cell[J]. International Journal of Hydrogen Energy, 2020, 45(51): 27291-27298. |
| 47 | PEERA S G, LEE T G, SAHU A K. Pt-rare earth metal alloy/metal oxide catalysts for oxygen reduction and alcohol oxidation reactions: an overview[J]. Sustainable Energy & Fuels, 2019, 3(8): 1866-1891. |
| 48 | ZHAO P, QIN X Q, LI H B, et al. New insights into O and OH adsorption on the Pt-Co alloy surface: effects of Pt/Co ratios and structures[J]. Physical Chemistry Chemical Physics, 2020,22(37): 21124-21130. |
| 49 | CAO M N, WU D S, CAO R. Recent advances in the stabilization of platinum electrocatalysts for fuel-cell reactions[J]. ChemCatChem, 2014, 6(1): 26-45. |
| 50 | ZHOU Z Y, TIAN N, HUANG Z Z, et al. Nanoparticle catalysts with high energy surfaces and enhanced activity synthesized by electrochemical method[J]. Faraday Discussions, 2008, 140: 81-92. |
| 51 | DEBE M K. Electrocatalyst approaches and challenges for automotive fuel cells[J]. Nature, 2012, 486(7401): 43-51. |
| 52 | WU J B, QI L, YOU H J, et al. Icosahedral platinum alloy nanocrystals with enhanced electrocatalytic activities[J]. Journal of the American Chemical Society, 2012, 134(29): 11880-11883. |
| 53 | KOBAYASHI S, WAKISAKA M, TRYK D A, et al. Effect of alloy composition and crystal face of Pt-Skin/Pt100-xCox[(111), (100), and (110)] single crystal electrodes on the oxygen reduction reaction activity[J]. The Journal of Physical Chemistry C, 2017, 121(21): 11234-11240. |
| 54 | XU Y C, CUI X Q, WEI S T, et al. Highly active zigzag-like Pt-Zn alloy nanowires with high-index facets for alcohol electrooxidation[J]. Nano Research, 2019, 12(5): 1173-1179. |
| 55 | ZHANG Z C, HUI J F, GUO Z G, et al. Solvothermal synthesis of Pt-Pd alloys with selective shapes and their enhanced electrocatalytic activities[J]. Nanoscale, 2012, 4(8): 2633-2639. |
| 56 | HONG J W, KANG S W, CHOI B S, et al. Controlled synthesis of Pd-Pt alloy hollow nanostructures with enhanced catalytic activities for oxygen reduction[J]. ACS Nano, 2012, 6(3): 2410-2419. |
| 57 | SUI N, WANG K, SHAN X Y, et al. Facile synthesis of hollow dendritic Ag/Pt alloy nanoparticles for enhanced methanol oxidation efficiency[J]. Dalton Transactions, 2017, 46(44): 15541-15548. |
| 58 | WANG Q M, CHEN S G, SHI F, et al. Structural evolution of solid Pt nanoparticles to a hollow PtFe alloy with a Pt-skin surface via space-confined pyrolysis and the nanoscale kirkendall effect[J]. Advanced Materials, 2016, 28(48): 10673-10678. |
| 59 | TIAN X L, ZHAO X, SU Y Q, et al. Engineering bunched Pt-Ni alloy nanocages for efficient oxygen reduction in practical fuel cells[J]. Science, 2019, 366(6467): 850-856. |
| 60 | STRASSER P, KOH S, ANNIYEV T, et al. Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts[J]. Nature Chemistry, 2010, 2(6): 454-460. |
| 61 | LYU X Y, JIA Y, MAO X, et al. Gradient-concentration design of stable core-shell nanostructure for acidic oxygen reduction electrocatalysis[J]. Advanced Materials, 2020, 32(32): 2003493. |
| 62 | LAMER V K, DINEGAR R H. Theory, production and mechanism of formation of monodispersed hydrosols[J]. Journal of the American Chemical Society, 1950, 72(11): 4847-4854. |
| 63 | WU J F, SHAN S Y, CRONK H, et al. Understanding composition-dependent synergy of PtPd alloy nanoparticles in electrocatalytic oxygen reduction reaction[J]. The Journal of Physical Chemistry C, 2017, 121(26): 14128-14136. |
| 64 | QI Y, BIAN T, CHOI S I, et al. Kinetically controlled synthesis of Pt-Cu alloy concave nanocubes with high-index facets for methanol electro-oxidation[J]. Chemical Communications, 2014, 50(5): 560-562. |
| 65 | WANG K L, WANG F, ZHAO Y F, et al. Surface-tailored PtPdCu ultrathin nanowires as advanced electrocatalysts for ethanol oxidation and oxygen reduction reaction in direct ethanol fuel cell[J]. Journal of Energy Chemistry, 2021, 52: 251-261. |
| 66 | WANG Y, JIANG X, FU G T, et al. Cu5Pt dodecahedra with low-Pt content: facile synthesis and outstanding formic acid electrooxidation[J]. ACS Applied Materials & Interfaces, 2019, 11(38): 34869-34877. |
| 67 | LONG X Y, YIN P, LEI T, et al. Methanol electro-oxidation on Cu@Pt/C core-shell catalyst derived from Cu-MOF[J]. Applied Catalysis B: Environmental, 2020, 260: 118187. |
| 68 | ZOU J S, WU M, NING S L, et al. Ru@Pt core-shell nanoparticles: impact of the atomic ordering of the Ru metal core on the electrocatalytic activity of the Pt shell[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(9): 9007-9016. |
| 69 | ALINEZHAD A, BENEDETTI T M, GLOAG L, et al. Controlling Pt crystal defects on the surface of Ni-Pt core-shell nanoparticles for active and stable electrocatalysts for oxygen reduction[J]. ACS Applied Nano Materials, 2020, 3(6): 5995-6000. |
| 70 | WANG J, LI B, YERSAK T, et al. Recent advances in Pt-based octahedral nanocrystals as high performance fuel cell catalysts[J]. Journal of Materials Chemistry A, 2016, 4(30): 11559-11581. |
| 71 | XIAO X Y, JEONG H, SONG J, et al. Facile synthesis of Pd@Pt core-shell nanocubes with low Pt content via direct seed-mediated growth and their enhanced activity for formic acid oxidation[J]. Chemical Communications, 2019, 55(79): 11952-11955. |
| 72 | HAN L, CUI P L, HE H Y, et al. A seed-mediated approach to the morphology-controlled synthesis of bimetallic copper-platinum alloy nanoparticles with enhanced electrocatalytic performance for the methanol oxidation reaction[J]. Journal of Power Sources, 2015, 286: 488-494. |
| 73 | BAO M J, AMIINU I S, PENG T, et al. Surface evolution of PtCu alloy shell over Pd nanocrystals leads to superior hydrogen evolution and oxygen reduction reactions[J]. ACS Energy Letters, 2018, 3(4): 940-945. |
| 74 | YOO T Y, YOO J M, SINHA A K, et al. Direct synthesis of intermetallic platinum-alloy nanoparticles highly loaded on carbon supports for efficient electrocatalysis[J]. Journal of the American Chemical Society, 2020, 142(33): 14190-14200. |
| 75 | SOHN H S, XIAO Q F, SEUBSAI A, et al. Thermally robust porous bimetallic (NixPt1-x) alloy mesocrystals within carbon framework: high-performance catalysts for oxygen reduction and hydrogenation reactions[J]. ACS Applied Materials & Interfaces, 2019, 11(24): 21435-21444. |
| 76 | RONG H P, MAO J J, XIN P Y, et al. Kinetically controlling surface structure to construct defect-rich intermetallic nanocrystals: effective and stable catalysts[J]. Advanced Materials, 2016, 28(13): 2540-2546. |
| 77 | FENG Y G, SHAO Q, LV F, et al. Intermetallic PtBi nanoplates boost oxygen reduction catalysis with superior tolerance over chemical fuels[J]. Advanced Science, 2020, 7(1): 1800178. |
| 78 | YUAN X L, JIANG X J, CAO M H, et al. Intermetallic PtBi core/ultrathin Pt shell nanoplates for efficient and stable methanol and ethanol electro-oxidization[J]. Nano Research, 2019, 12(2): 429-436. |
| 79 | FENG Q C, ZHAO S, HE D S, et al. Strain engineering to enhance the electrooxidation performance of atomic-layer Pt on intermetallic Pt3Ga[J]. Journal of the American Chemical Society, 2018, 140(8): 2773-2776. |
| 80 | ZHU Y M, BU L Z, SHAO Q, et al. Structurally ordered Pt3Sn nanofibers with highlighted antipoisoning property as efficient ethanol oxidation electrocatalysts[J]. ACS Catalysis, 2020, 10(5): 3455-3461. |
| 81 | KIM J, LEE Y, SUN S. Structurally ordered FePt nanoparticles and their enhanced catalysis for oxygen reduction reaction[J]. Journal of the American Chemical Society, 2010, 132(14): 4996-4997. |
| 82 | GONG M X, XIAO D D, DENG Z P, et al. Structure evolution of PtCu nanoframes from disordered to ordered for the oxygen reduction reaction[J]. Applied Catalysis B: Environmental, 2021, 282: 119617. |
| 83 | CHUNG D Y, JUN S W, YOON G, et al. Highly durable and active PtFe nanocatalyst for electrochemical oxygen reduction reaction[J]. Journal of the American Chemical Society, 2015, 137(49): 15478-15485. |
| 84 | ZHAO Y G, WANG C, LIU J J, et al. PDA-assisted formation of ordered intermetallic CoPt3 catalysts with enhanced oxygen reduction activity and stability[J]. Nanoscale, 2018, 10(19): 9038-9043. |
| 85 | HU Y Z, SHEN T, ZHAO X R, et al. Combining structurally ordered intermetallics with N-doped carbon confinement for efficient and anti-poisoning electrocatalysis[J]. Applied Catalysis B: Environmental, 2020, 279: 119370. |
| 86 | KIM H Y, KWON T, HA Y, et al. Intermetallic PtCu nanoframes as efficient oxygen reduction electrocatalysts[J]. Nano Letters, 2020, 20(10): 7413-7421. |
| 87 | ZHANG B T, FU G T, LI Y T, et al. General strategy for synthesis of ordered Pt3 M intermetallics with ultrasmall particle size[J]. Angew. Chem. Int. Ed., 2020, 59(20): 7857-7863. |
| 88 | JUNG W S, LEE W H, OH H S, et al. Highly stable and ordered intermetallic PtCo alloy catalyst supported on graphitized carbon containing Co@CN for oxygen reduction reaction[J]. Journal of Materials Chemistry A, 2020, 8(38): 19833-19842. |
| 89 | CHEN W, LEI Z, ZENG T, et al. Structurally ordered PtSn intermetallic nanoparticles supported on ATO for efficient methanol oxidation reaction[J]. Nanoscale, 2019, 11(42): 19895-19902. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 许家珩, 李永胜, 罗春欢, 苏庆泉. 甲醇水蒸气重整工艺的优化[J]. 化工进展, 2023, 42(S1): 41-46. |
| [6] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [7] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [8] | 赵景超, 谭明. 表面活性剂对电渗析减量化工业含盐废水的影响[J]. 化工进展, 2023, 42(S1): 529-535. |
| [9] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [10] | 陈匡胤, 李蕊兰, 童杨, 沈建华. 质子交换膜燃料电池气体扩散层结构与设计研究进展[J]. 化工进展, 2023, 42(S1): 246-259. |
| [11] | 王谨航, 何勇, 史伶俐, 龙臻, 梁德青. 气体水合物阻聚剂研究进展[J]. 化工进展, 2023, 42(9): 4587-4602. |
| [12] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [13] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [14] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [15] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||