化工进展 ›› 2021, Vol. 40 ›› Issue (7): 4011-4020.DOI: 10.16085/j.issn.1000-6613.2020-1529
低温中和-加压酸浸提取煤矸石中铝铁
- 昆明理工大学化学工程学院,云南 昆明 650500
-
收稿日期:2020-08-03修回日期:2020-11-20出版日期:2021-07-06发布日期:2021-07-19 -
通讯作者:夏举佩 -
作者简介:李浩林(1995—),男,硕士研究生,研究方向为固体废弃物共伴生资源利用。E-mail:lhl951102@qq.com 。 -
基金资助:国家自然科学基金(21566018)
Extraction of aluminum and iron from coal gangue by low temperature neutralization-pressure acid leaching
LI Haolin( ), ZENG Dehui, ZHENG Guangya, XIA Jupei(
), ZENG Dehui, ZHENG Guangya, XIA Jupei( )
)
- Faculty of Chemical Engineering, Kunming University of Science and Technology, Kunming 650500, Yunnan, China
-
Received:2020-08-03Revised:2020-11-20Online:2021-07-06Published:2021-07-19 -
Contact:XIA Jupei
摘要:
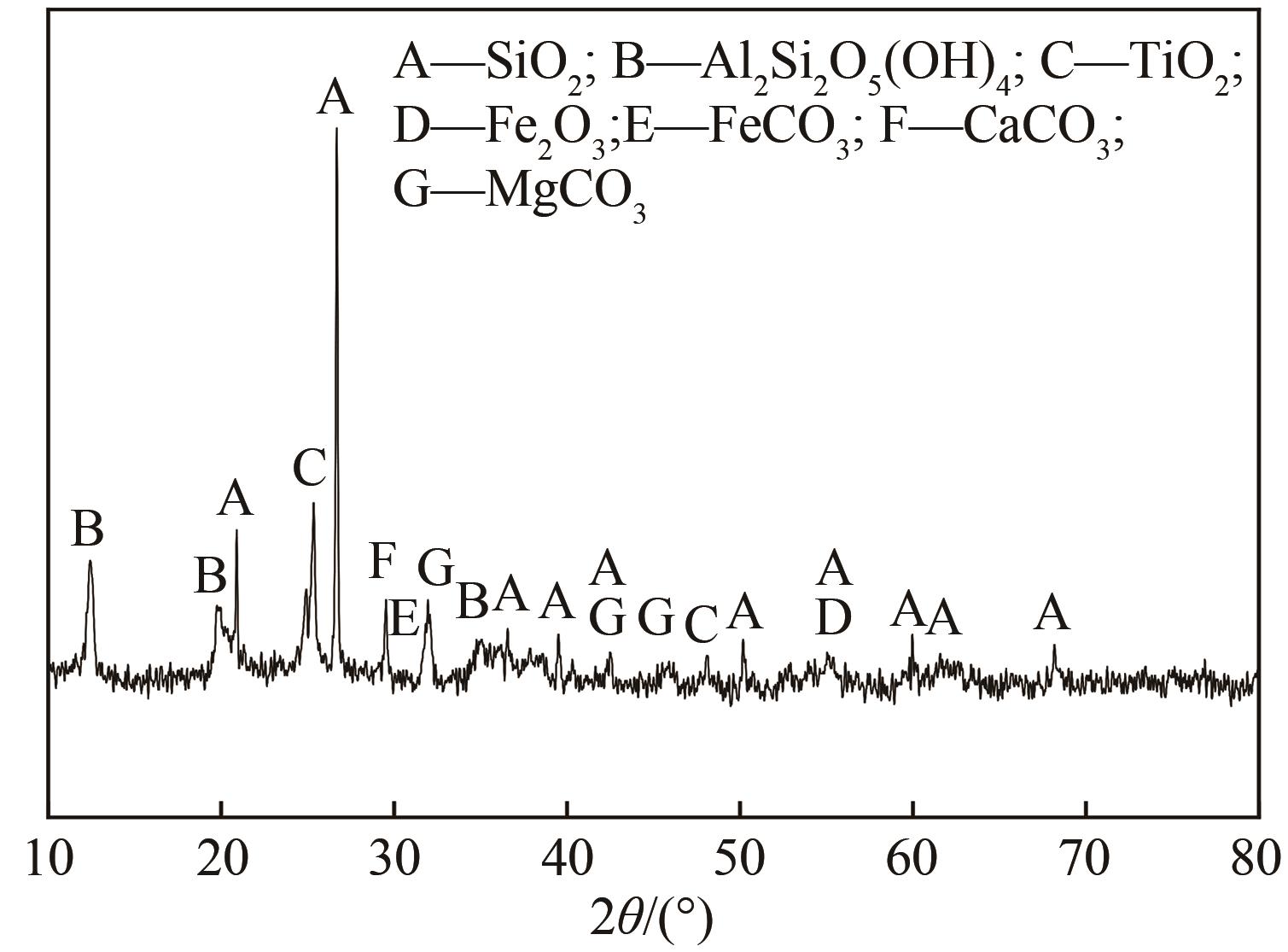
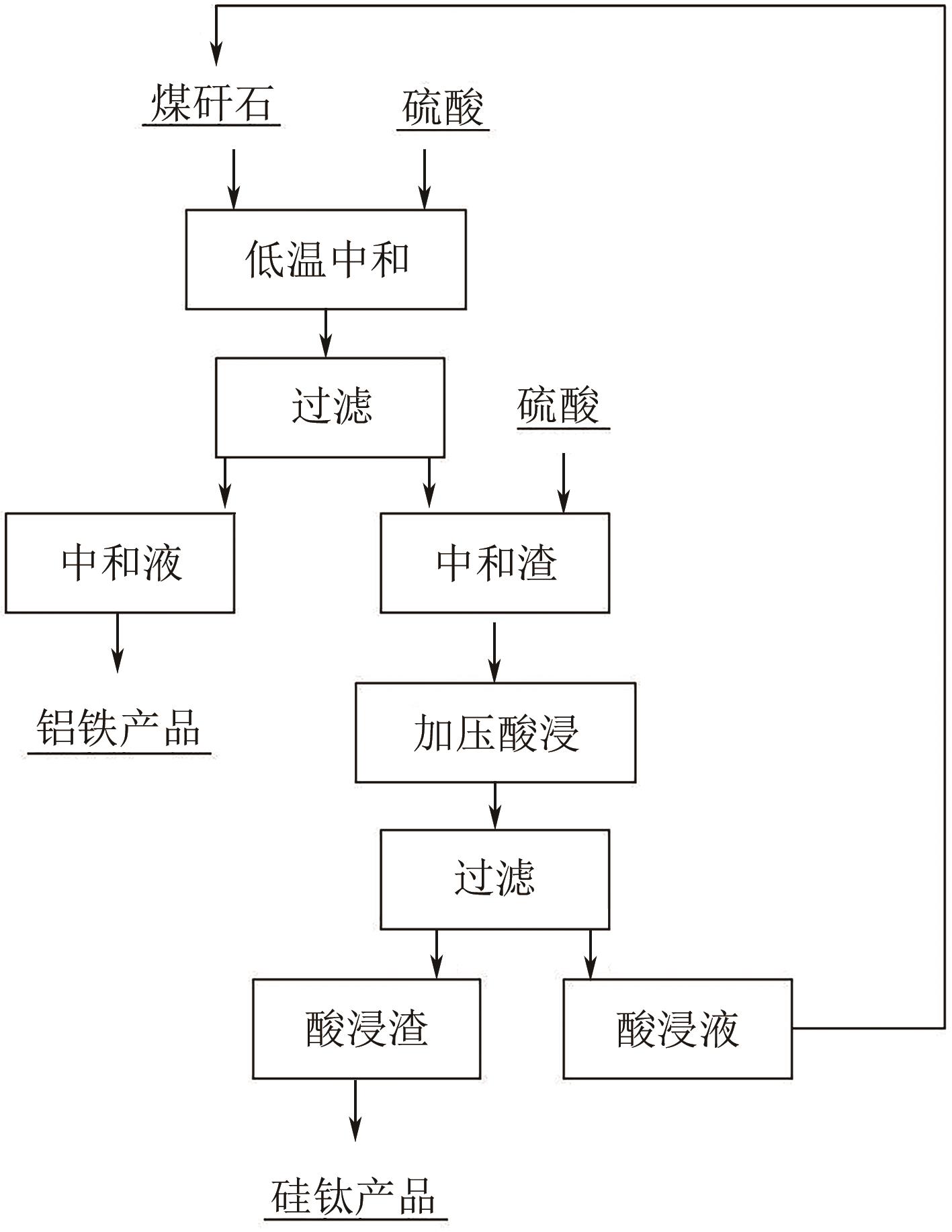
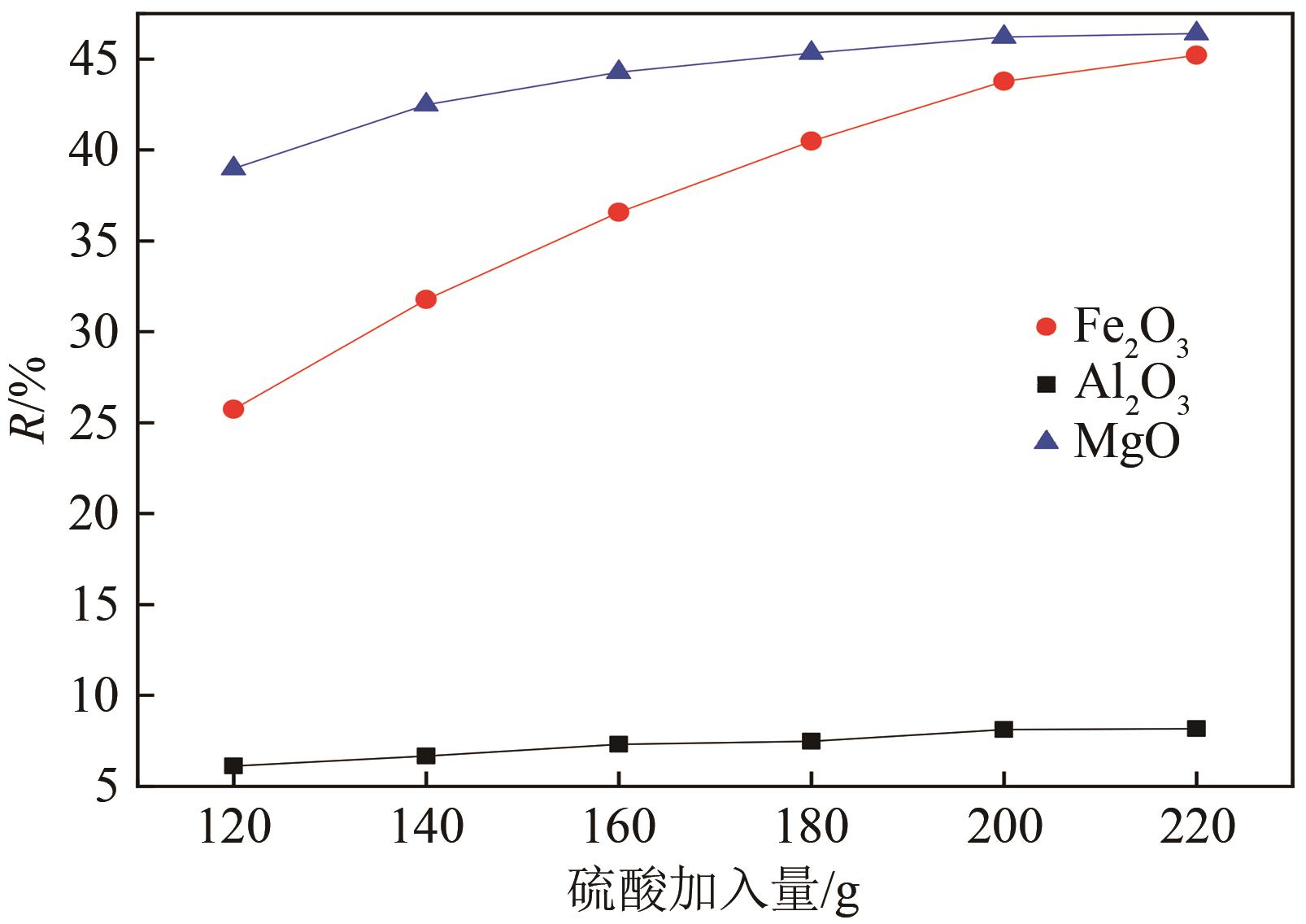
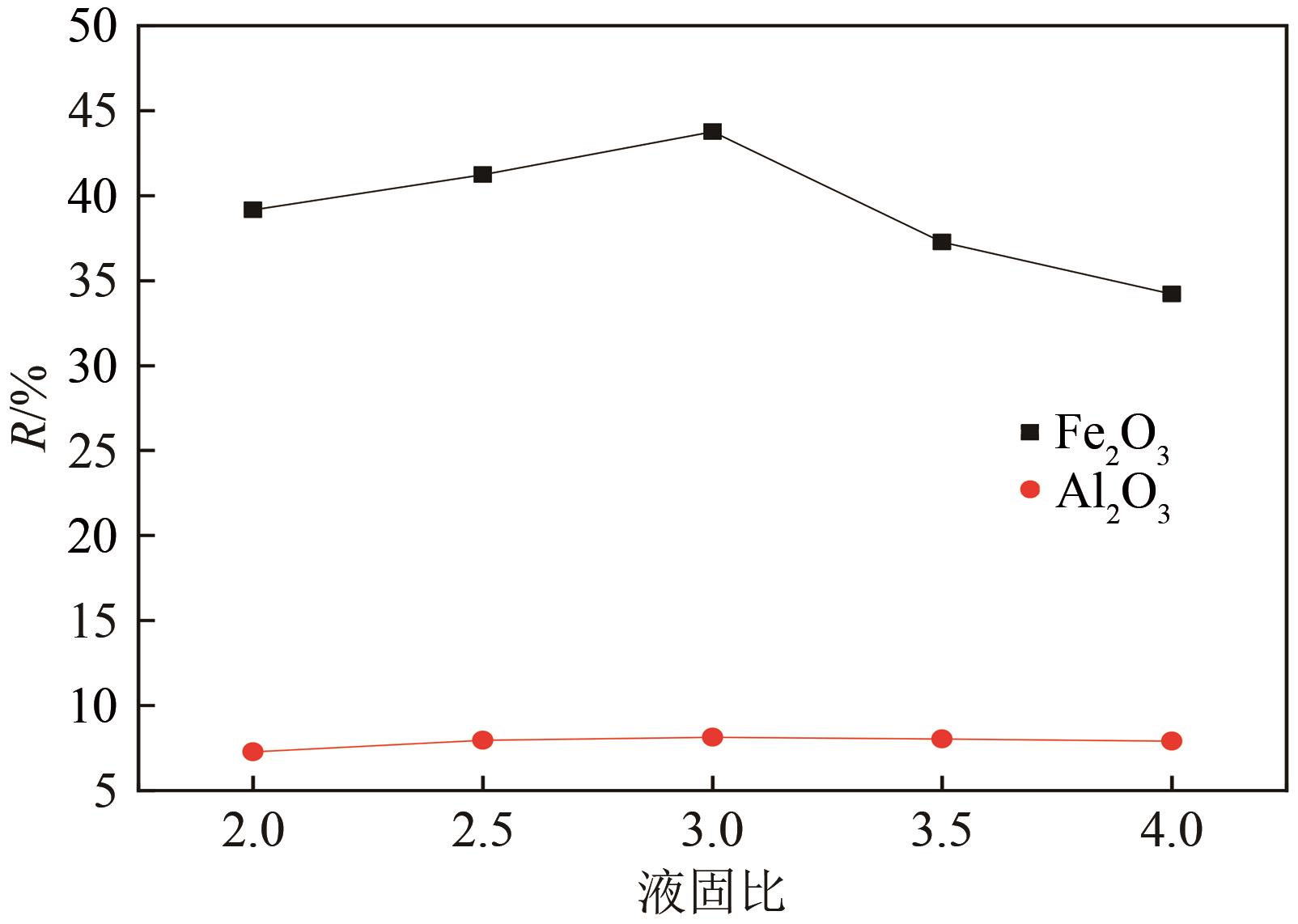
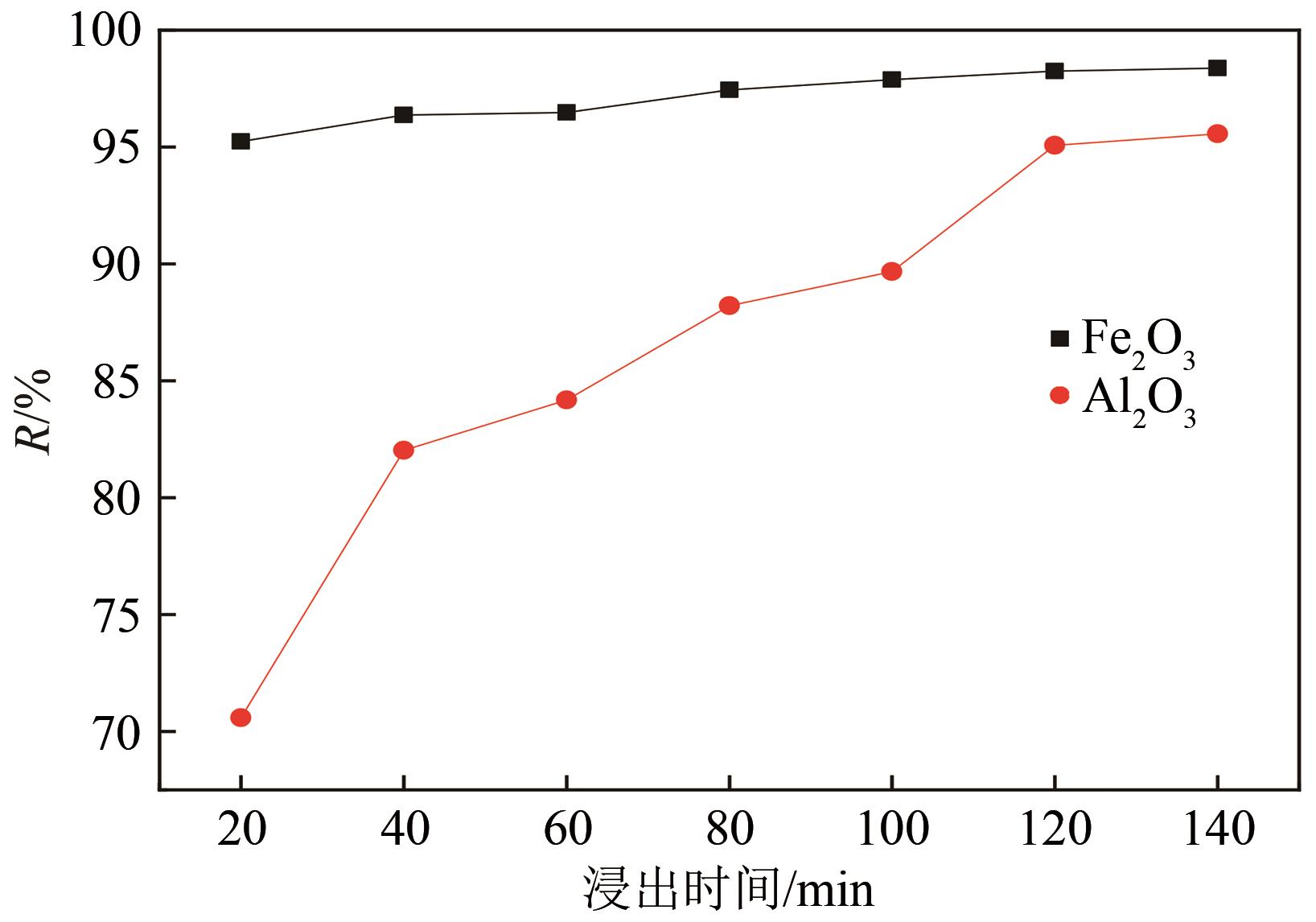
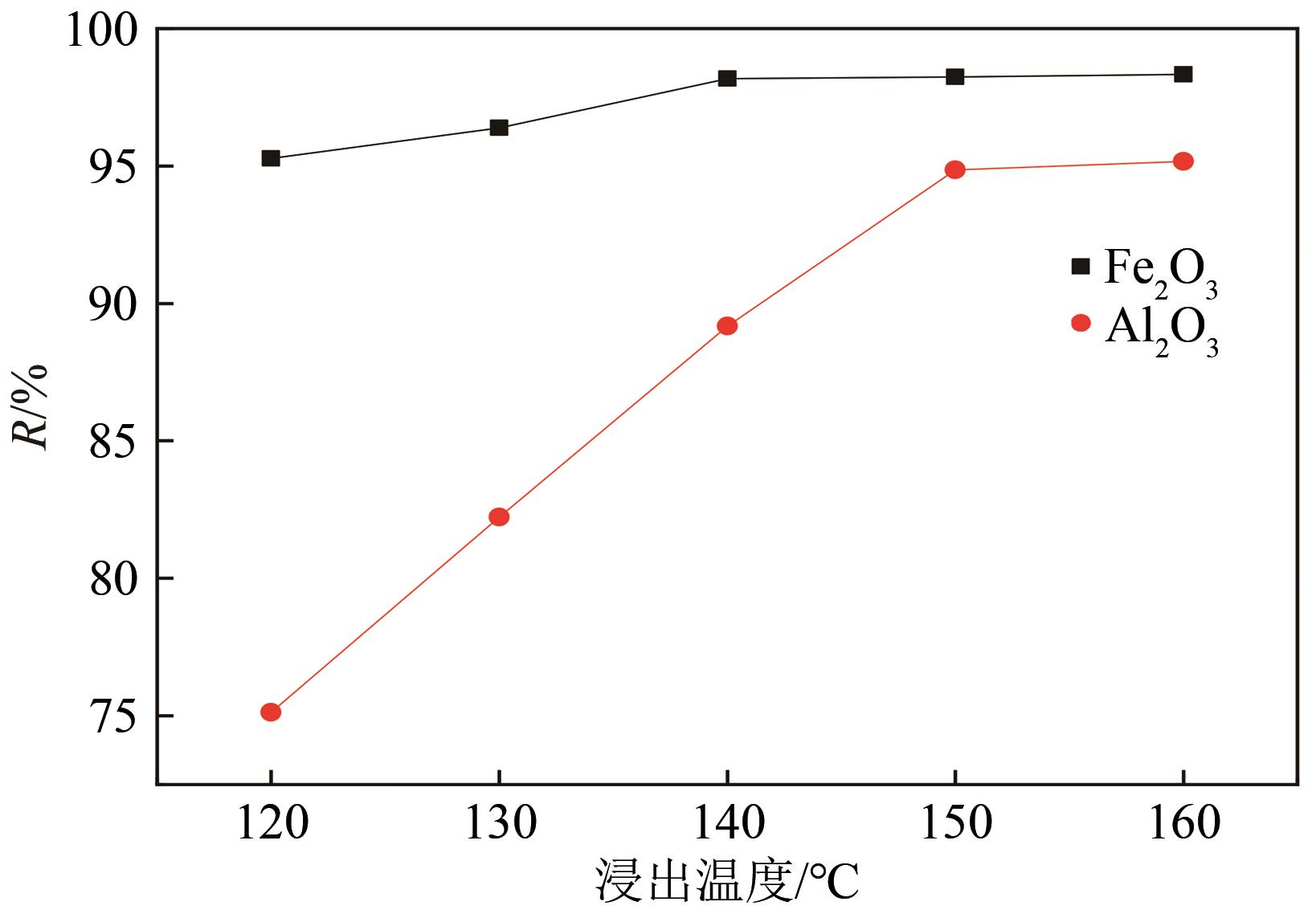
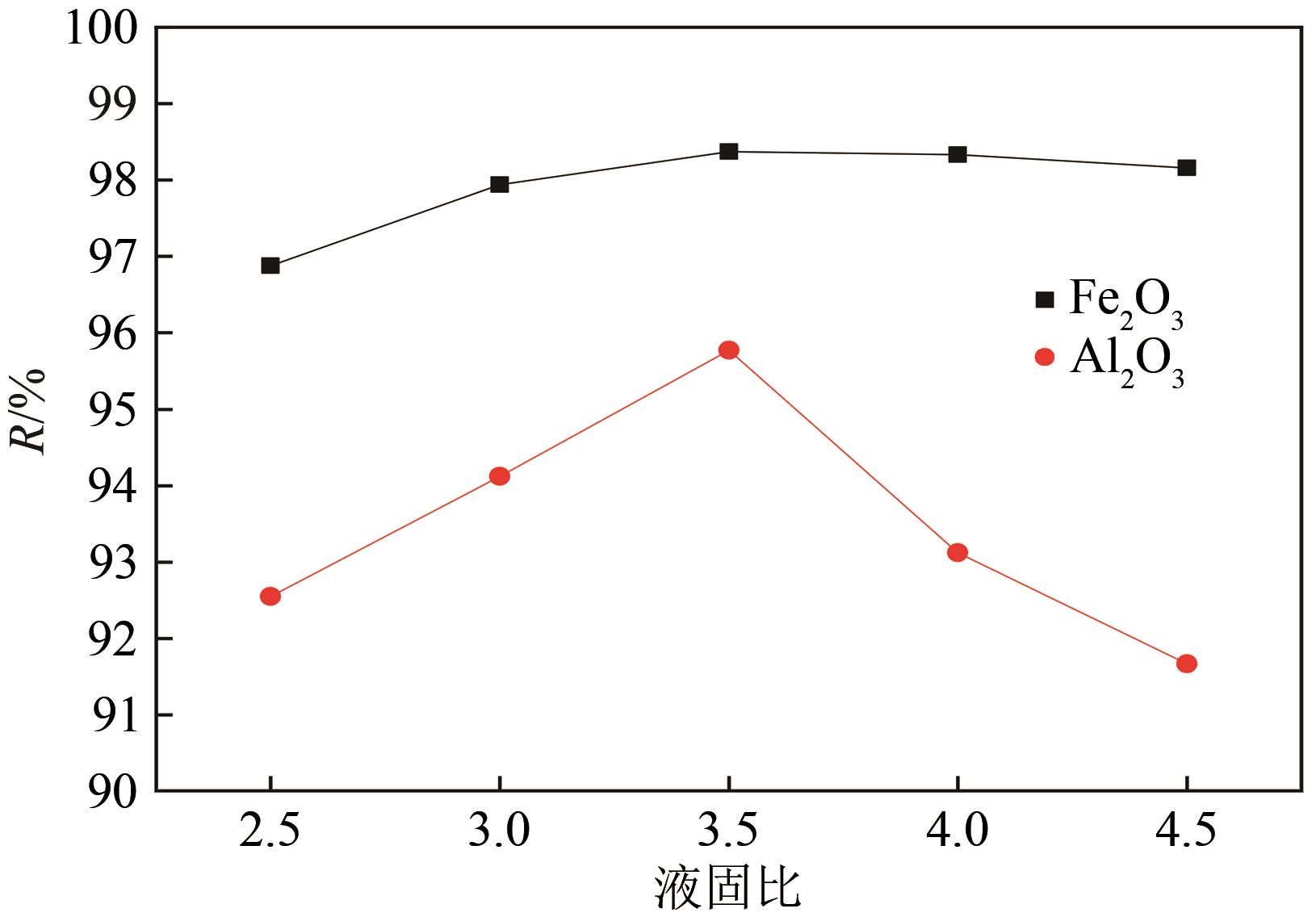
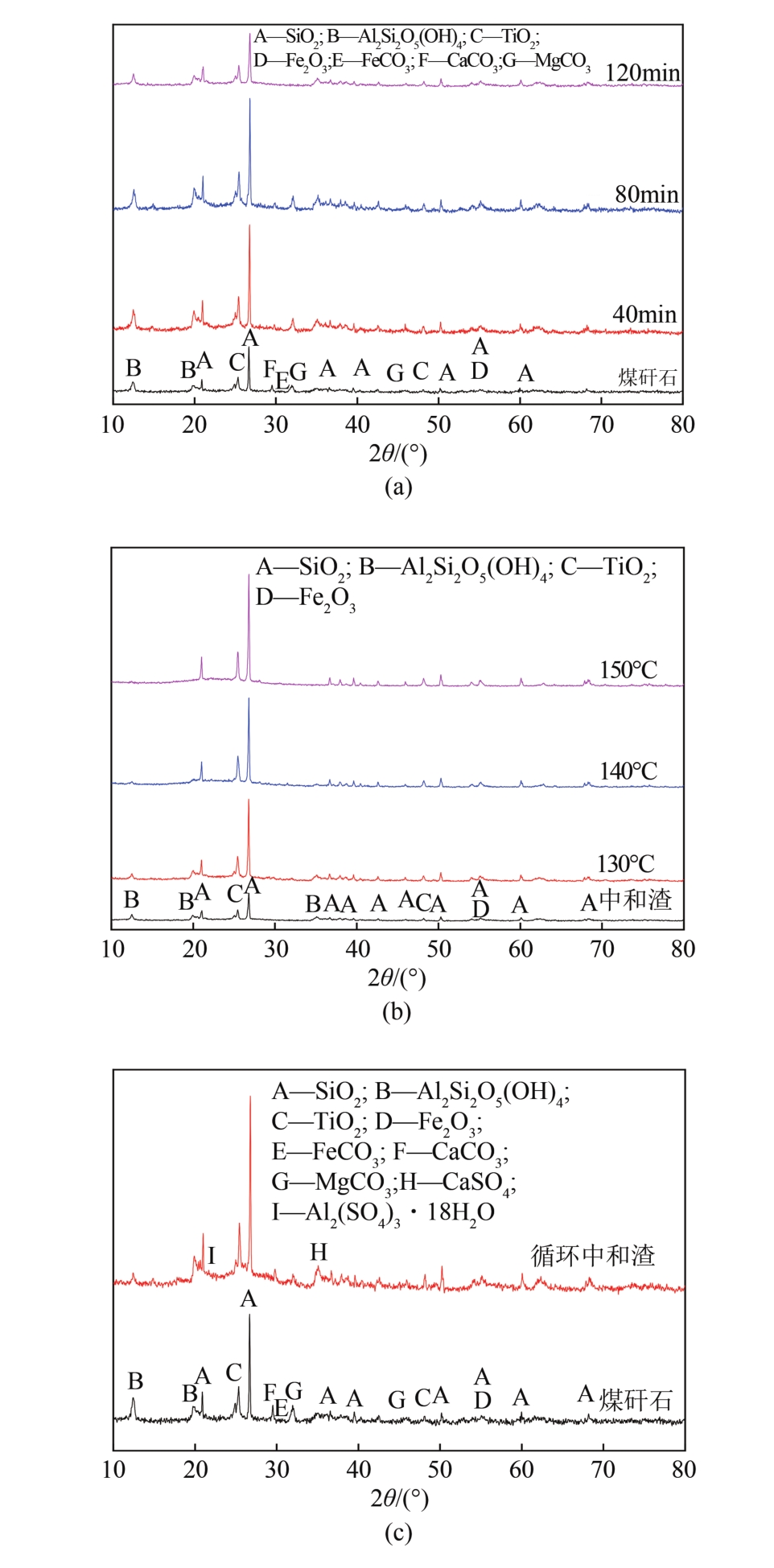
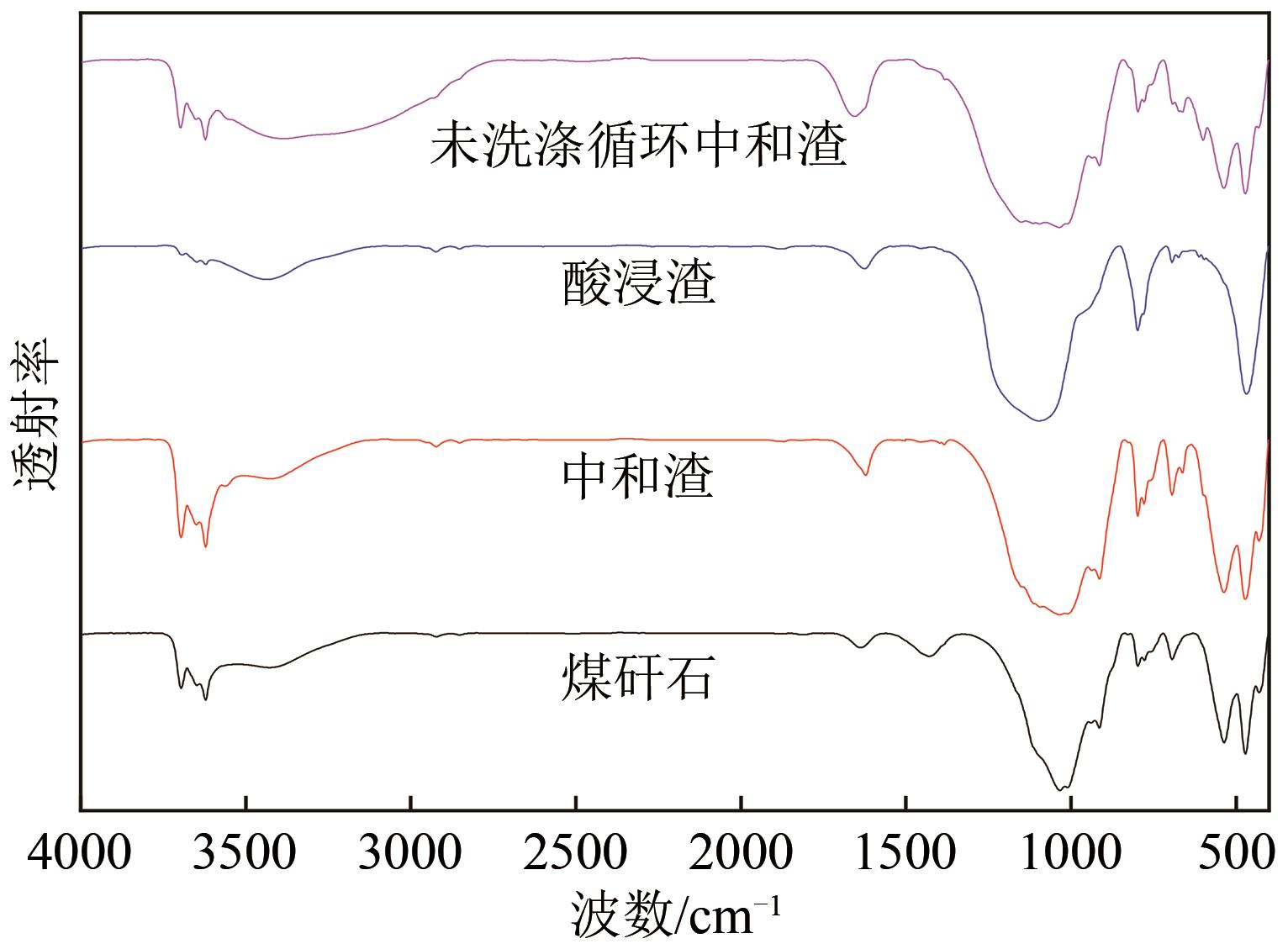
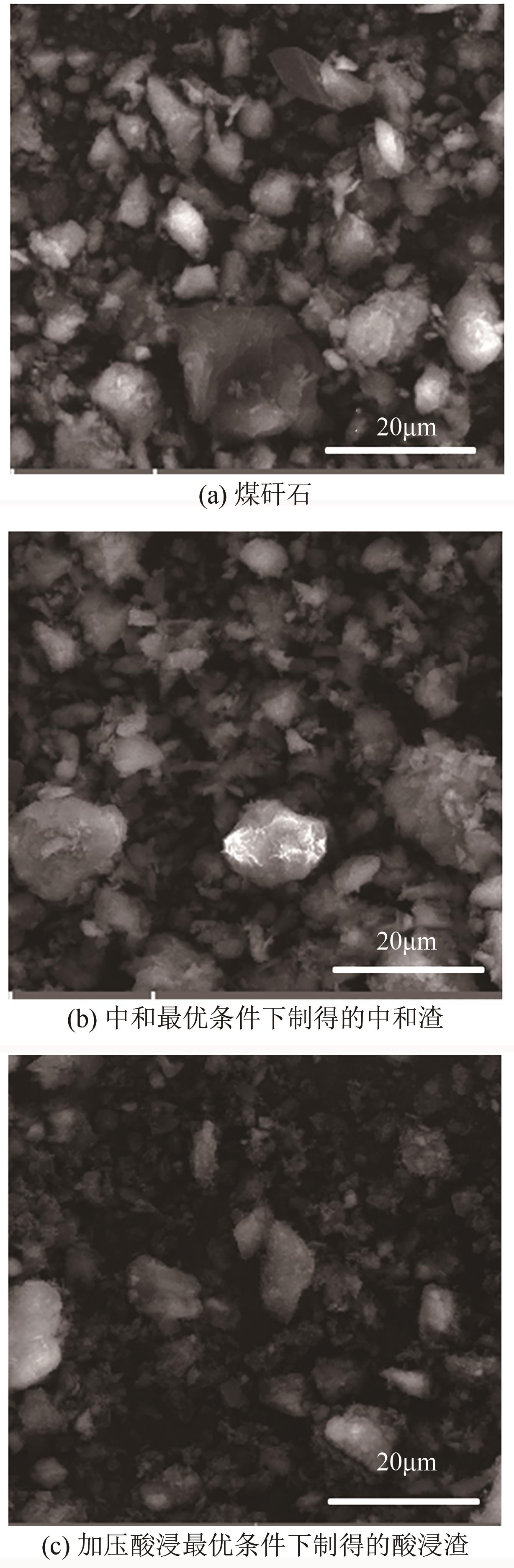
以贵州盘县煤矸石为研究对象,为解决其工业生产提取铝铁时酸耗量大、酸利用率低及后续铝铁产品分离困难等问题,根据其矿物组成特点,本文首次采用低温中和-加压酸浸工艺对铝铁提取进行了详细研究。室温下中和最优工艺条件为20%理论酸耗、浸出时间120min、液固比3∶1(硫酸溶液与固体的质量比,以g/g计);以中和渣为原料,煤矸石理论酸耗为基础,加压酸浸最优工艺条件为浸出时间120min、浸出温度150℃、液固比3.5∶1(硫酸溶液与固体的质量比,以g/g计)。在此条件下,氧化铁浸出率为98.37%,氧化铝浸出率为95.77%,酸浸渣灰分中氧化硅质量分数为90.2%,氧化钛质量分数为9.18%。以最优工艺条件下的酸浸液循环中和新鲜煤矸石,得到的铝铁提取液中氧化铁浓度为57.95g/L,氧化铝浓度为62.20g/L。相比常规酸浸工艺具有酸耗低、酸利用率高等优点。借助X射线衍射仪(XRD)、傅里叶红外光谱仪(FTIR)和扫描电子显微镜(SEM)等分析手段,初步对两步溶出过程进行了机理分析,为煤矸石工业生产提取铝铁提供了新路线和理论支撑。
中图分类号:
引用本文
李浩林, 曾德恢, 郑光亚, 夏举佩. 低温中和-加压酸浸提取煤矸石中铝铁[J]. 化工进展, 2021, 40(7): 4011-4020.
LI Haolin, ZENG Dehui, ZHENG Guangya, XIA Jupei. Extraction of aluminum and iron from coal gangue by low temperature neutralization-pressure acid leaching[J]. Chemical Industry and Engineering Progress, 2021, 40(7): 4011-4020.
| 成分 | 质量分数/% | 成分 | 质量分数/% |
|---|---|---|---|
| SiO2 | 39.41 | MgO | 2.06 |
| TiO2 | 4.43 | K2O | 1.72 |
| Fe2O3 | 12.38 | Na2O | 0.71 |
| Al2O3 | 18.52 | 烧蚀量 | 17.4 |
| CaO | 2.92 |
表1 煤矸石化学组成
| 成分 | 质量分数/% | 成分 | 质量分数/% |
|---|---|---|---|
| SiO2 | 39.41 | MgO | 2.06 |
| TiO2 | 4.43 | K2O | 1.72 |
| Fe2O3 | 12.38 | Na2O | 0.71 |
| Al2O3 | 18.52 | 烧蚀量 | 17.4 |
| CaO | 2.92 |
| 热力学函数 | |||
|---|---|---|---|
| ?H/kJ·mol-1 | -49.13 | -26.74 | -91.84 |
| ?G/kJ·mol-1 | -95.54 | -72.64 | -134.71 |
表2 0℃时煤矸石中碳酸盐与硫酸反应过程热力学分析
| 热力学函数 | |||
|---|---|---|---|
| ?H/kJ·mol-1 | -49.13 | -26.74 | -91.84 |
| ?G/kJ·mol-1 | -95.54 | -72.64 | -134.71 |
| 1 | 武彦辉. 我国煤矸石的处置利用现状及展望[J]. 中国环保产业, 2019(1): 53-55. |
| WU Yanhui. Disposal and utilization of coal gangue and its prospect in China[J]. China Environmental Protection Industry, 2019(1): 53-55. | |
| 2 | 赵双菊, 张彩朗, 杨小姝. 我国煤矸石综合利用现状综述[J]. 建材发展导向, 2012, 10(6): 47-51. |
| ZHAO Shuangju, ZHANG Cailang, YANG Xiaoshu. Summary of comprehensive utilization of coal gangue in China[J]. Development Guide to Building Materials, 2012, 10(6): 47-51. | |
| 3 | 张国权. 探析绿色发展理念下的煤矸石处理与利用[J]. 资源节约与环保, 2019(10): 145. |
| ZHANG Guoquan. Analysis on the treatment and utilization of coal gangue under the concept of green development[J]. Resource Conservation and Environmental Protection, 2019(10): 145. | |
| 4 | 杨越. 我国煤矸石堆存现状及其大宗量综合利用途径[J]. 中国资源综合利用, 2014, 32(6): 18-22. |
| YANG Yue. Coal gangue stacked and its comprehensive utilization[J]. Resources Economization&Environmental Protection, 2014, 32(6): 18-22. | |
| 5 | 贾鲁涛, 吴倩云. 煤矸石特性及其资源化综合利用现状[J]. 煤炭技术, 2019, 38(11): 37-40. |
| JIA Lutao, WU Qianyun. Properties and comprehensive utilization status of coal gangue resource[J]. Coal Technology, 2019, 38(11): 37-40. | |
| 6 | 郭彦霞, 张圆圆, 程芳琴. 煤矸石综合利用的产业化及其展望[J]. 化工学报, 2014, 65(7): 2443-2453. |
| GUO Yanxia, ZHANG Yuanyuan, CHENG Fangqin. Industrial development and prospect about comprehensive utilization of coal gangue[J]. CIESC Journal, 2014, 65(7): 2443-2453. | |
| 7 | 贾敏. 煤矸石综合利用研究进展[J]. 矿产保护与利用, 2019, 39(4): 46-52. |
| JIA Min. The current situation research on comprehensive utilization of coal gangue[J]. Conservation and Utilization of Mineral Resources, 2019, 39(4): 46-52. | |
| 8 | 重庆煤研所煤化室矸石利用研究组. 国内外煤矸石处理和利用概况[J]. 川煤科技, 1975(1): 13-24. |
| Gangue Utilization Research Group of Coal Chemical Chamber of Chongqing Coal Research Institute. General situation of coal gangue treatment and utilization at home and abroad[J]. Sichuan Coal Technology, 1975(1): 13-24. | |
| 9 | LI Jingyu,YOU Lifeng,WEI Yan. The current situation of comprehensive utilization of coal gangue in China[J]. Advanced Materials Research, 2012, 524/525/526/527: 915-918. |
| 10 | ZHAN Xiaoyuan, WANG Fang, SONG Zhenqi, et al. Development model of circular eco-industrial park for comprehensive utilization of coal gangue in coal enterprise[J]. Materials Science Forum, 2014, 787: 71-75. |
| 11 | HAN Lina, REN Weiguo, WANG Bing, et al. Extraction of SiO2 and Al2O3 from coal gangue activated by supercritical water[J]. Fuel, 2019, 253: 1184-1192. |
| 12 | GUO Yanxia, YAN Kezhou, CUI Li, et al. Improved extraction of alumina from coal gangue by surface mechanically grinding modification[J]. Powder Technology, 2016, 302: 33-41. |
| 13 | 金会心, 吴复忠, 朱明燕, 等. 贵州六盘水煤矸石的矿物特性[J]. 过程工程学报, 2014, 14(1): 151-156. |
| JIN Huixin, WU Fuzhong, ZHU Mingyan, et al. Mineral characteristics of coal gangue from Liupanshui in Guizhou province[J]. The Chinese journal of process engineering, 2014, 14(1): 151-156. | |
| 14 | 刘成龙. 煤矸石酸浸提取工艺与机理研究[D]. 昆明: 昆明理工大学, 2015. |
| LIU Chenglong. Study on extraction technology and mechanism of coal gangue acid leaching[D]. Kunming: Kunming University of Science and Technology, 2015. | |
| 15 | 李浩林, 夏举佩, 曾德恢, 等. 加压酸浸煤矸石中氧化铝工艺及动力学研究[J]. 煤炭转化, 2020, 43(2): 89-96. |
| LI Haolin, XIA Jupei, ZENG Dehui, et al. Dynamics analysis and technical of leaching alumina from coal gangue by pressured acid leaching process[J]. Coal Coversion, 2020, 43(2): 89-96. | |
| 16 | 李宛霖, 夏举佩, 郑光亚, 等. 煤矸石酸浸提取多金属过程[J]. 化工科技, 2019, 27(3): 41-45. |
| LI Wanlin, XIA Jupei, ZHENG Guangya, et al. Extraction process of polymetallic from coal gangue by acid leaching[J]. Science & Technology in Chemical Industry, 2019, 27(3): 41-45. | |
| 17 | 郑光亚, 李银, 夏举佩, 等. 煤矸石低温中和过程铁、钙、镁的溶出及动力学[J]. 化工进展, 2019, 38(12): 5594-5602. |
| ZHENG Guangya, LI Yin, XIA Jupei, et al. Dissolution kinetics of iron, calcium and magnesium from coal gangue during low-temperature neutralization[J]. Chemical Industry and Engineering Progress, 2019, 38(12): 5594-5602. | |
| 18 | 谷立轩, 夏举佩, 张召述. 影响酸法提取煤矸石中氧化铝主要因素的试验研究[J]. 安全与环境学报, 2012, 12(2): 88-91. |
| GU Lixuan, XIA Jupei, ZHANG Zhaoshu. Affecting factors on the alumina extracting from the coal gangue with sulfuric acid[J]. Journal of Safety and Environment, 2012, 12(2): 88-91. | |
| 19 | 唐凤翔, 张济宇. 两种高岭土的酸浸反应宏观动力学的比较[J]. 煤炭转化, 2002(2): 91-95. |
| TANG Fengxiang, ZHANG Jiyu. Apparent kinetics comparison between the two kinds of kaolin on acid-leaching reaction [J]. Coal conversion, 2002(2): 91-95. | |
| 20 | 陈甘堂. 化学反应工程[M]. 北京: 化学工业出版社, 2007. |
| CHEN Gantang. Chemical reaction engineering[M].Beijing: Chemical Industry Press, 2007. | |
| 21 | SANGITA S, NAYAK N, PANDA. Extraction of aluminium as aluminium sulphate from thermal power plant fly ashes[J]. Transactions of Nonferrous Metals Society of China, 2017, 27(9): 2082-2089. |
| 22 | SCOTT FOGLER H. 化学反应工程原理[M]. 4版. 北京: 化学工业出版社, 2006. |
| SCOTT FOGLER H. Elements of chemical reaction engineering[M]. 4th ed. Beijing: Chemical Industry Press, 2006. | |
| 23 | 许越. 化学反应动力学[M]. 北京: 化学工业出版社, 2004. |
| XU Yue. Chemical reaction kinetics[M]. Beijing: Chemical Industry Press, 2004. | |
| 24 | 蒋开喜. 加压湿法冶金[M]. 北京: 高等教育出版社, 2016. |
| JIANG Kaixi. Pressure hydrometallurgy[M]. Beijing: Higher Education Press, 2016. | |
| 25 | 汪胜东, 蒋训雄, 范艳青, 等. 低铝硅比铝土矿酸浸试验研究[J]. 有色金属(冶炼部分), 2008(5): 29-31. |
| WANG Shengdong, JIANG Xunxiong, FAN Yanqing, et al. Study on acid leaching of low ratio of Al2O3/SiO2 bauxite[J]. Nonferrous Metals(Extractive Metallurgy), 2008(5):29-31. | |
| 26 | 郑光亚, 陈正杰, 刘成龙, 等. 硫酸铝低温水解产物特性及其动力学研究[J]. 化学研究与应用, 2019, 31(7): 1357-1363. |
| ZHENG Guangya, CHEN Zhengjie, LIU Chenlong, et al. Hydrolytic kinetics and characteristics of aluminum sulfate of hydrolysis products at low temperature[J]. Chemical Research and Application, 2019, 31(7): 1357-1363. | |
| 27 | 李祥, 李凡, 刘元芬, 等. 中药石膏X射线衍射分析及指纹图谱的确定[J]. 世界中西医结合杂志, 2006(2): 91-93. |
| LI Xiang, LI Fan, LIU Yuanfen, et al. X-ray diffraction analysis of Chinese medicine gypsum and determination of fingerprint[J]. Word Journal of Integrated Traditional and Western Medicine, 2006(2): 91-93. | |
| 28 | 翟倩, 刘银, 冉小信, 等. 微波法提取煤矸石中氧化铝的实验研究[J]. 安徽理工大学学报(自然科学版), 2018, 38(4): 41-45. |
| ZHAI Qian, LIU Yin, RAN Xiaoxin, et al. Experimental study on aluminum oxide extraction from coal gangue by microwave [J]. Journal of Anhui University of Science and Technology(Natural Science), 2018, 38(4): 41-45. | |
| 29 | 张鹏, 王雨露, 张文, 等. 聚合硫酸铝钛的制备与结构表征[J]. 化工进展, 2018, 37(7): 2740-2747. |
| ZHANG Peng, WANG Yulu, ZHANG Wen, et al. Synthesis and characterization of an inorganic composite polymer coagulant of poly aluminum titanium sulfate[J]. Chemical Industry and Enginering Progress, 2018, 37(7): 2740-2747. | |
| 30 | XIAO Jin, LI Fachuang, ZHONG Qifan, et al. Separation of aluminum and silica from coal gangue by elevated temperature acid leaching for the preparation of alumina and SiC[J]. Hydrometallurgy, 2015, 155: 118-124. |
| 31 | ASUHA S, TALINTUYA T, HAN T, et al. Selective extraction of aluminum from coal-bearing kaolinite by room-temperature mechanochemical method for the preparation of γ-Al2O3 powder[J]. Powder Technology, 2018, 325: 121-125. |
| 32 | 朱宝忠, 孙运兰, 谢承卫. 不同煅烧温度下贵州兴义煤矸石的光谱学研究[J]. 煤炭学报, 2008(9): 1049-1052. |
| ZHU Baozhong, SUN Yunlan, XIE Chengwei. Spectroscopy research on the Guizhou Xingyi gangue of different calcined temperatures[J].Jorrnal of China Coal Society, 2008(9): 1049-1052. | |
| 33 | 孙晓雪, 孙玉柱, 于建国, 等. 硫酸铝冷却结晶动力学研究[J]. 无机盐工业, 2015, 47(8): 23-25. |
| SUN Xiaoxue, SUN Yuzhu, YU Jianguo, et al. Cooling crystallization kinetics of aluminum sulfate[J]. Inorganic Chemicals Industry, 2015, 47(8): 23-25. |
| [1] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [2] | 王敏, 毛玉红, 陈超, 白丹. 水处理工艺中铝盐水解物的毒性、形态及控制研究进展[J]. 化工进展, 2023, 42(S1): 479-488. |
| [3] | 汪鹏, 张洋, 范兵强, 何登波, 申长帅, 张贺东, 郑诗礼, 邹兴. 高碳铬铁盐酸浸出过程工艺及动力学[J]. 化工进展, 2023, 42(S1): 510-517. |
| [4] | 舒斌, 陈建宏, 熊健, 吴其荣, 喻江涛, 杨平. 碳中和目标下推动绿色甲醇发展的必要性分析[J]. 化工进展, 2023, 42(9): 4471-4478. |
| [5] | 许中硕, 周盼盼, 王宇晖, 黄威, 宋新山. 硫铁矿介导的自养反硝化研究进展[J]. 化工进展, 2023, 42(9): 4863-4871. |
| [6] | 史天茜, 石永辉, 武新颖, 张益豪, 秦哲, 赵春霞, 路达. Fe2+对厌氧氨氧化EGSB反应器运行性能的影响[J]. 化工进展, 2023, 42(9): 5003-5010. |
| [7] | 于志庆, 黄文斌, 王晓晗, 邓开鑫, 魏强, 周亚松, 姜鹏. B掺杂Al2O3@C负载CoMo型加氢脱硫催化剂性能[J]. 化工进展, 2023, 42(7): 3550-3560. |
| [8] | 王帅旗, 王从新, 王学林, 田志坚. 无溶剂快速合成ZSM-12分子筛[J]. 化工进展, 2023, 42(7): 3561-3571. |
| [9] | 龚鹏程, 严群, 陈锦富, 温俊宇, 苏晓洁. 铁酸钴复合碳纳米管活化过硫酸盐降解铬黑T的性能及机理[J]. 化工进展, 2023, 42(7): 3572-3581. |
| [10] | 李海东, 杨远坤, 郭姝姝, 汪本金, 岳婷婷, 傅开彬, 王哲, 何守琴, 姚俊, 谌书. 炭化与焙烧温度对植物基铁碳微电解材料去除As(Ⅲ)性能的影响[J]. 化工进展, 2023, 42(7): 3652-3663. |
| [11] | 徐沛瑶, 陈标奇, KANKALA Ranjith Kumar, 王士斌, 陈爱政. 纳米材料用于铁死亡联合治疗的研究进展[J]. 化工进展, 2023, 42(7): 3684-3694. |
| [12] | 杨竞莹, 施万胜, 黄振兴, 谢利娟, 赵明星, 阮文权. 改性纳米零价铁材料制备的研究进展[J]. 化工进展, 2023, 42(6): 2975-2986. |
| [13] | 吴锋振, 刘志炜, 谢文杰, 游雅婷, 赖柔琼, 陈燕丹, 林冠烽, 卢贝丽. 生物质基铁/氮共掺杂多孔炭的制备及其活化过一硫酸盐催化降解罗丹明B[J]. 化工进展, 2023, 42(6): 3292-3301. |
| [14] | 杨红梅, 高涛, 鱼涛, 屈撑囤, 高家朋. 高铁酸盐处理难降解有机物磺化酚醛树脂[J]. 化工进展, 2023, 42(6): 3302-3308. |
| [15] | 刘含笑, 吴黎明, 林青阳, 周烨, 罗象, 桂志军, 刘小伟, 单思珂, 朱前林, 陆诗建. 碳足迹评估技术及其在重点工业行业的应用[J]. 化工进展, 2023, 42(5): 2201-2218. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||