化工进展 ›› 2021, Vol. 40 ›› Issue (3): 1413-1424.DOI: 10.16085/j.issn.1000-6613.2020-0845
ZnO基光电极的构筑及其光电催化水分解性能研究进展
- 1.兰州城市学院培黎机械工程学院,甘肃 兰州 730070
2.中国科学院兰州化学物理研究所,甘肃 兰州 730000
-
收稿日期:2020-05-18出版日期:2021-03-05发布日期:2021-03-17 -
通讯作者:符淑瑢 -
作者简介:符淑瑢(1990—),女,博士,讲师,研究方向为光电催化。E-mail:shurongfu@126.com 。 -
基金资助:兰州城市学院博士科研启动基金(LZCU-BS2019-44)
Research progress of fabrication of ZnO-based photoanode and photoelectrocatalytic water splitting performances
FU Shurong1( ), ZHANG Qinsheng2, LU Jinzhi2, MA Zhanwei2
), ZHANG Qinsheng2, LU Jinzhi2, MA Zhanwei2
- 1.School of Bailie Mechanical Engineering, Lanzhou City University, Lanzhou 730070, Gansu, China
2.Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, Gansu, China
-
Received:2020-05-18Online:2021-03-05Published:2021-03-17 -
Contact:FU Shurong
摘要:
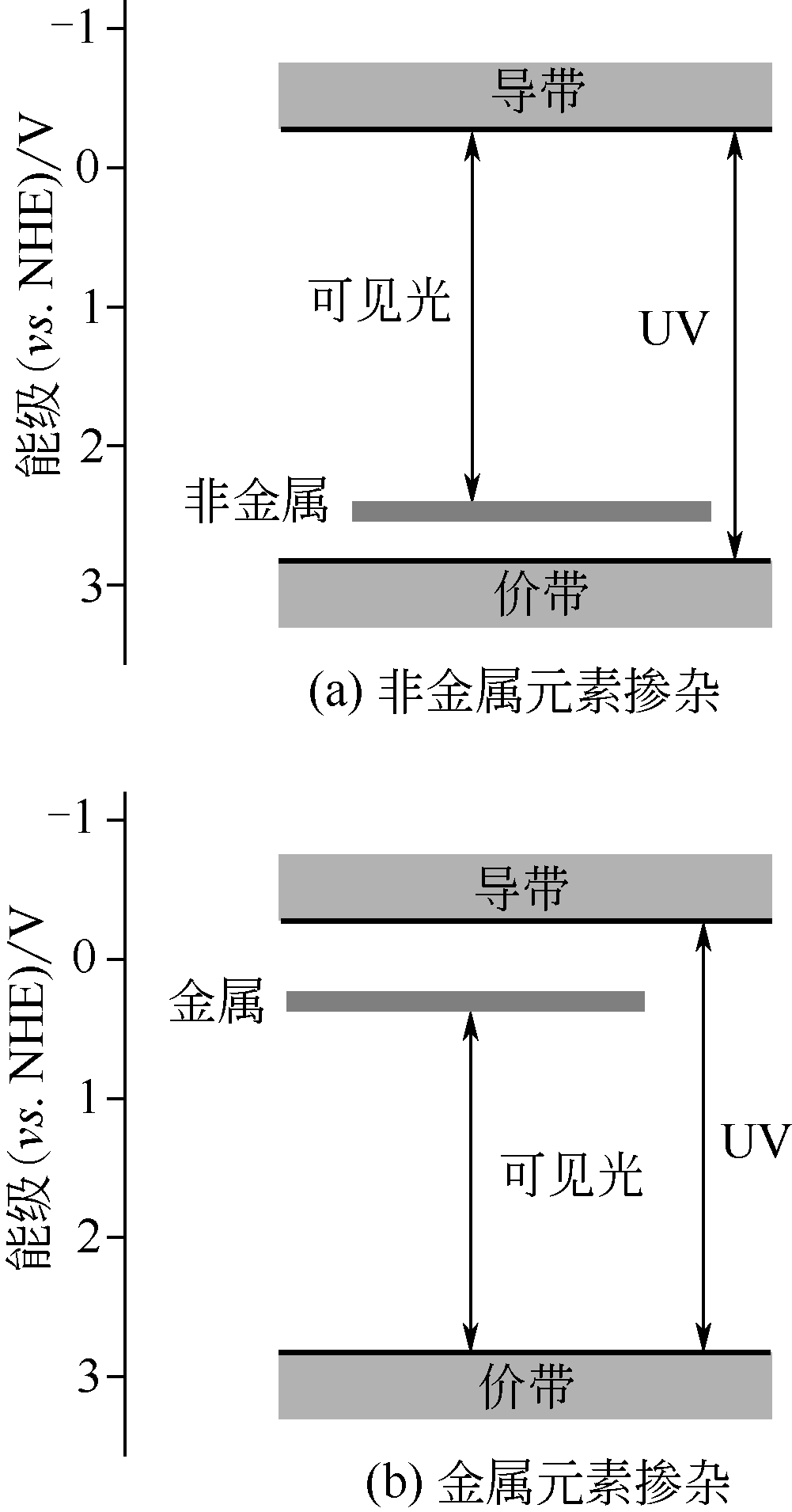
光电催化水分解制取氢气是最理想的制氢技术之一。光电极材料作为光电催化水分解反应系统最核心的部分,决定着太阳能到化学能的转换效率。氧化锌(ZnO)半导体因具有较低的超电势、高的电子迁移速率和价格低廉等优点,引起了广泛关注。然而,ZnO半导体的禁带较宽、电子-空穴易于复合和表面水氧化反应动力学缓慢,阻碍了其高效利用太阳能和实现理论效率。本文从ZnO的微纳结构和表界面修饰两个方面出发,综述了近年来ZnO基光电极的构筑策略及其光电催化性能的研究进展。首先阐述了ZnO的微观形貌和缺陷对光电性质的影响。然后总结了元素掺杂、量子点敏化、贵金属沉积、异质结构造和共催化剂沉积等策略对ZnO基半导体的表界面的构筑及对光电催化性能的影响。最后对未来高效ZnO基半导体光电极研究方向进行了展望,具体包括5个方面:ZnO表面改性;在原子水平构筑复合半导体催化剂的相界面;用廉价双金属或多金属纳米颗粒取代纯贵金属Au、Ag和Pt纳米颗粒;构建高效的电催化剂助剂;在ZnO半导体和助剂界面引入空穴储存层或电子堵塞层。
中图分类号:
引用本文
符淑瑢, 张勤生, 鲁金芝, 马占伟. ZnO基光电极的构筑及其光电催化水分解性能研究进展[J]. 化工进展, 2021, 40(3): 1413-1424.
FU Shurong, ZHANG Qinsheng, LU Jinzhi, MA Zhanwei. Research progress of fabrication of ZnO-based photoanode and photoelectrocatalytic water splitting performances[J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1413-1424.
| 1 | FUJISHIMA A, HONDA K. Electrochemical photolysis of water at a semiconductor electrode[J]. Nature, 1972, 238(5358): 37-38. |
| 2 | GOVATSI K, SEFERLIS A, NEOPHYTIDES S G, al et, Influence of the morphology of ZnO nanowires on the photoelectrochemical water splitting efficiency[J]. International Journal of Hydrogen Energy, 2018, 43(10): 4866-4879. |
| 3 | RANJITH K S, KUMAR R T R. Surfactant free, simple, morphological and defect engineered ZnO nanocatalyst: effective study on sunlight driven and reusable photocatalytic properties[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2016, 329: 35-45. |
| 4 | GONZALEZ V I, LIRA C M. Vertically-aligned nanostructures of ZnO for excitonic solar cells: a review[J]. Energy & Environmental Science, 2009, 2(1): 19-34. |
| 5 | LI X B, NIU F J, SU J Z, et al. Photoelectrochemical performance dependence on geometric surface area of branched ZnO nanowires[J]. ChemElectroChem, 2018, 5(23): 3717-3722. |
| 6 | MARTINSON A B F, MCGARRAH J E, PARPIA M O K, et al. Dynamics of charge transport and recombination in ZnO nanorod array dye-sensitized solar cells[J]. Physical Chemistry Chemical Physics, 2006, 8(40): 4655-4659. |
| 7 | SUN X, LI Q, JIANG J, al et, Morphology-tunable synthesis of ZnO nanoforest and its photoelectrochemical performance[J]. Nanoscale, 2014, 6(15): 8769-8780. |
| 8 | ZHANG X, LIU Y, KANG Z. 3D Branched ZnO nanowire arrays decorated with plasmonic Au nanoparticles for high-performance photoelectrochemical water splitting[J]. ACS Applied Materials & Interfaces, 2014, 6(6): 4480-4489. |
| 9 | KO S H, LEE D, KANG H W, et al. Nanoforest of hydrothermally grown hierarchical ZnO nanowires for a high efficiency dye-sensitized solar cell[J]. Nano Letters, 2011, 11(2): 666-671. |
| 10 | HSU Y K, LIN Y G, CHEN Y C. Polarity-dependent photoelectrochemical activity in ZnO nanostructures for solar water splitting[J]. Electrochemistry Communications, 2011, 13(12): 1383-1386. |
| 11 | CHEN H, WEI Z, YAN K, et al. Epitaxial growth of ZnO nanodisks with large exposed polar facets on nanowire arrays for promoting photoelectrochemical water splitting[J]. Small, 2014, 10(22): 4760-4769. |
| 12 | LIU Q T, LIU D Y, LI J M, et al. The impact of crystal defects towards oxide semiconductor photoanode for photoelectrochemical water splitting[J]. Frontiers of Physics, 2019, 14(5): 53403. |
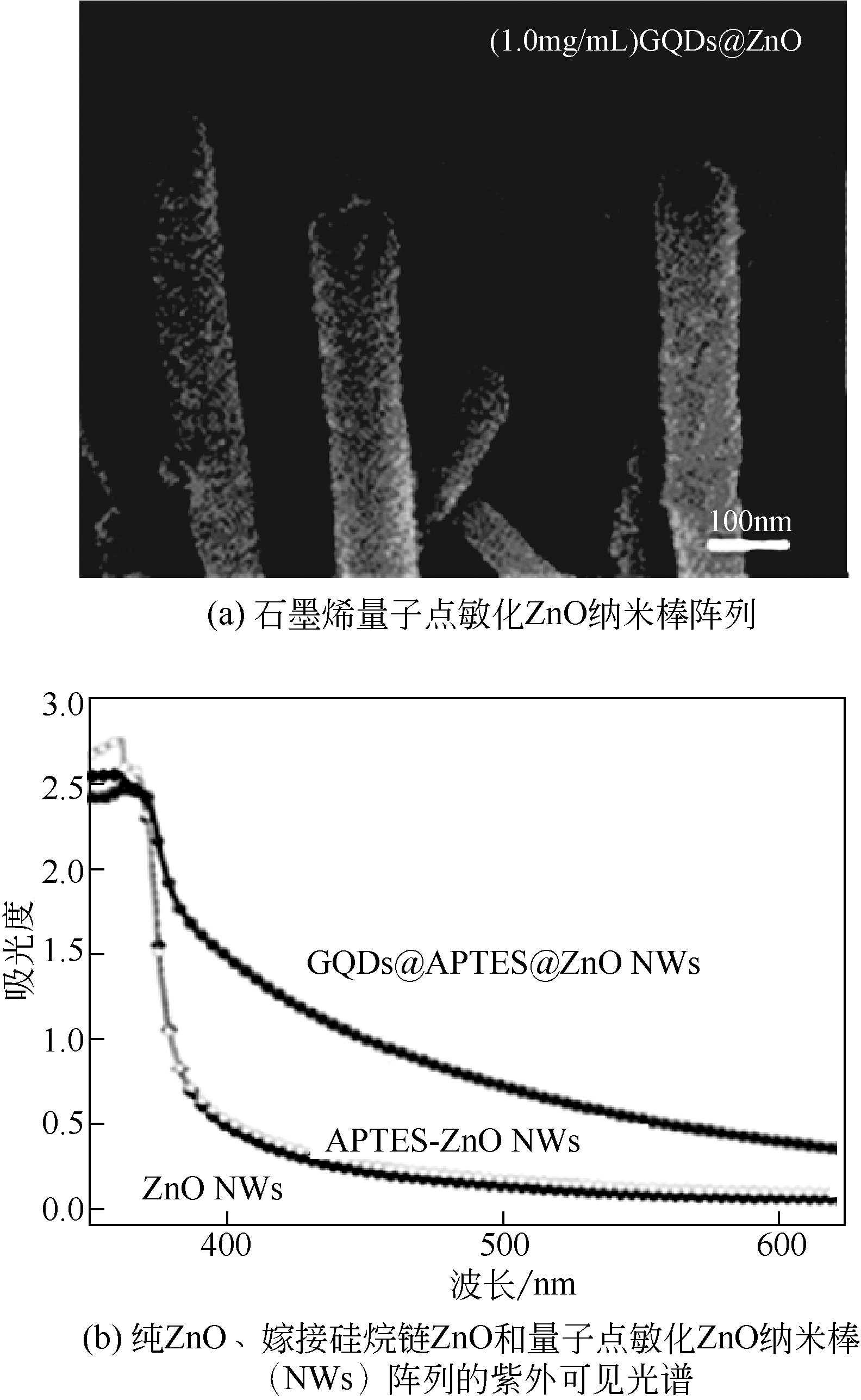
| 13 | FAWZY S M, OMAR M M, ALLAM N K. Photoelectrochemical water splitting by defects in nanostructured multinary transition metal oxides[J]. Solar Energy Materials and Solar Cells, 2019, 194: 184-194. |
| 14 | KRZYWIECKI M, GRZĄDZIEL L, SARFRAZ A, et al. Zinc oxide as a defect-dominated material in thin films for photovoltaic applications-experimental determination of defect levels, quantification of composition, and construction of band diagram[J]. Physical Chemistry Chemical Physics, 2015, 17(15): 10004-10013. |
| 15 | LU X, WANG G, XIE S, et al. Efficient photocatalytic hydrogen evolution over hydrogenated ZnO nanorod arrays[J]. Chemical Communications, 2012, 48(62): 7717-7719. |
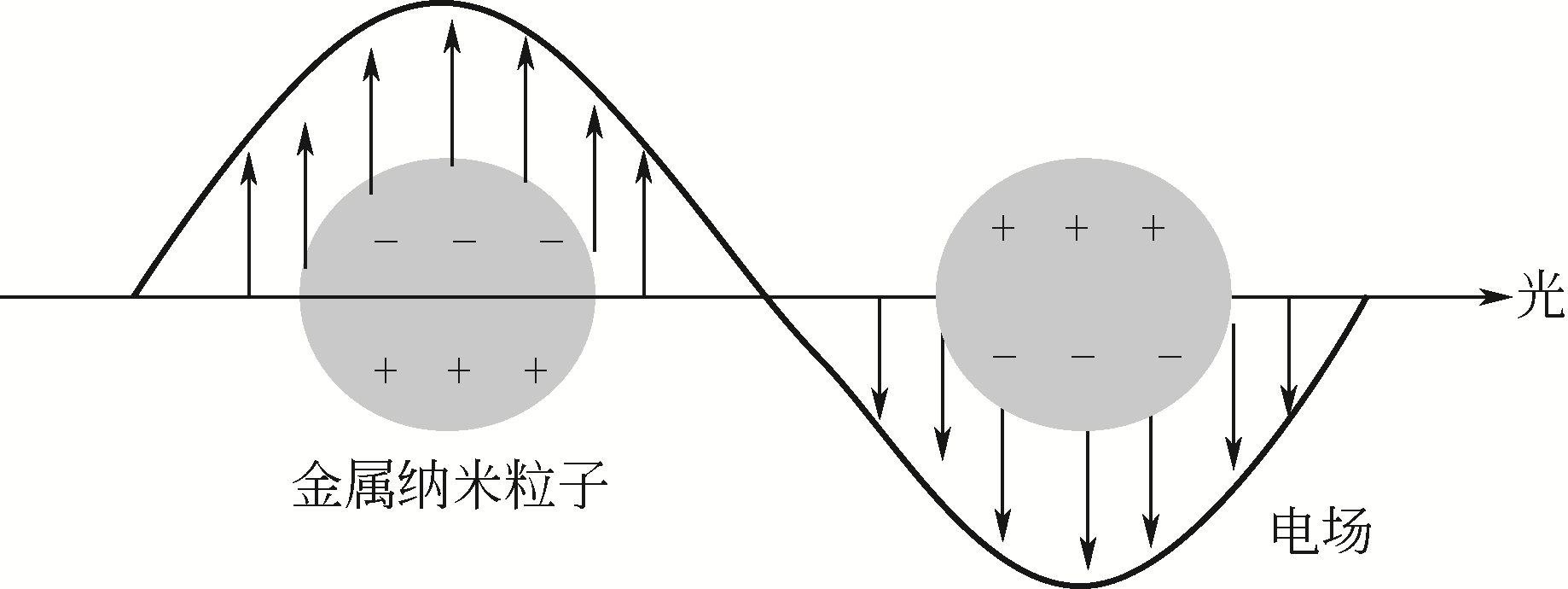
| 16 | LI H, FU Y, LIU H, et al. Defect-controlled ZnO nanorod arrays for enhanced photoelectrochemical performance[J]. Inorganic Chemistry Communications, 2013, 30: 182-186. |
| 17 | COOPER J K, LING Y, LONGO C, et al. Effects of hydrogen treatment and air annealing on ultrafast charge carrier dynamics in ZnO nanowires under in situ photoelectrochemical conditions[J]. The Journal of Physical Chemistry C, 2012, 116(33): 17360-17368. |
| 18 | BABIKIER M, WANG D, WANG J, et al. Fabrication and properties of sulfur (S)-doped ZnO nanorods[J]. Journal of Materials Science: Materials in Electronics, 2014, 25(1): 157-162. |
| 19 | KHAN A, AHMED M I, ADAM A, et al. A novel fabrication methodology for sulfur-doped ZnO nanorods as an active photoanode for improved water oxidation in visible-light regime[J]. Nanotechnology, 2016, 28(5): 055602. |
| 20 | YANG X, WOLCOTT A, WANG G, et al. Nitrogen-doped ZnO nanowire arrays for photoelectrochemical water splitting[J]. Nano Letters, 2009, 9(6): 2331-2336. |
| 21 | WANG M, REN F, ZHOU J, et al. N Doping to ZnO nanorods for photoelectrochemical water splitting under visible light: engineered impurity distribution and terraced band structure[J]. Scientific Reports, 2015, 5(1): 12925. |
| 22 | BHIRUD A P, SATHAYE S D, WAICHAL R P, et al. An eco-friendly, highly stable and efficient nanostructured p-type N-doped ZnO photocatalyst for environmentally benign solar hydrogen production[J]. Green Chemistry, 2012, 14(10): 2790-2798. |
| 23 | 姚凯, 姜宏, 熊春荣. 碳掺杂ZnO薄膜及可见光催化氮合成氨[J]. 化工进展, 2019, 38(2): 913-920. |
| YAO K, JIANG H, XIONG C R. Carbon doped ZnO thin films for visible-light driven conversion of nitrogen to ammonia[J]. Chemical Industry and Engineering Progress, 2019, 38(2): 913-920. | |
| 24 | XIE S, LU X, ZHAI T, et al. Enhanced photoactivity and stability of carbon and nitrogen co-treated ZnO nanorod arrays for photoelectrochemical water splitting[J]. Journal of Materials Chemistry, 2012, 22(28): 14272-14275. |
| 25 | COMMANDEUR D, BROWN G, MCNULTY P, et al. Yttrium-doped ZnO nanorod arrays for increased charge mobility and carrier density for enhanced solar water splitting[J]. The Journal of Physical Chemistry C, 2019, 123(30): 18187-18197. |
| 26 | TYNELL T, KARPPINEN M. Atomic layer deposition of ZnO: a review[J]. Semiconductor Science and Technology, 2014, 29(4): 043001. |
| 27 | LIN Y G, HSU Y K, CHEN Y C, et al. Cobalt-phosphate-assisted photoelectrochemical water oxidation by arrays of molybdenum-doped zinc oxide nanorods[J]. ChemSusChem, 2014, 7(9): 2748-2754. |
| 28 | LI C, REN F, WANG M, et al. V ions implanted ZnO nanorod arrays for photoelectrochemical water splitting under visible light[J]. International Journal of Hydrogen Energy, 2015, 40(3): 1394-1401. |
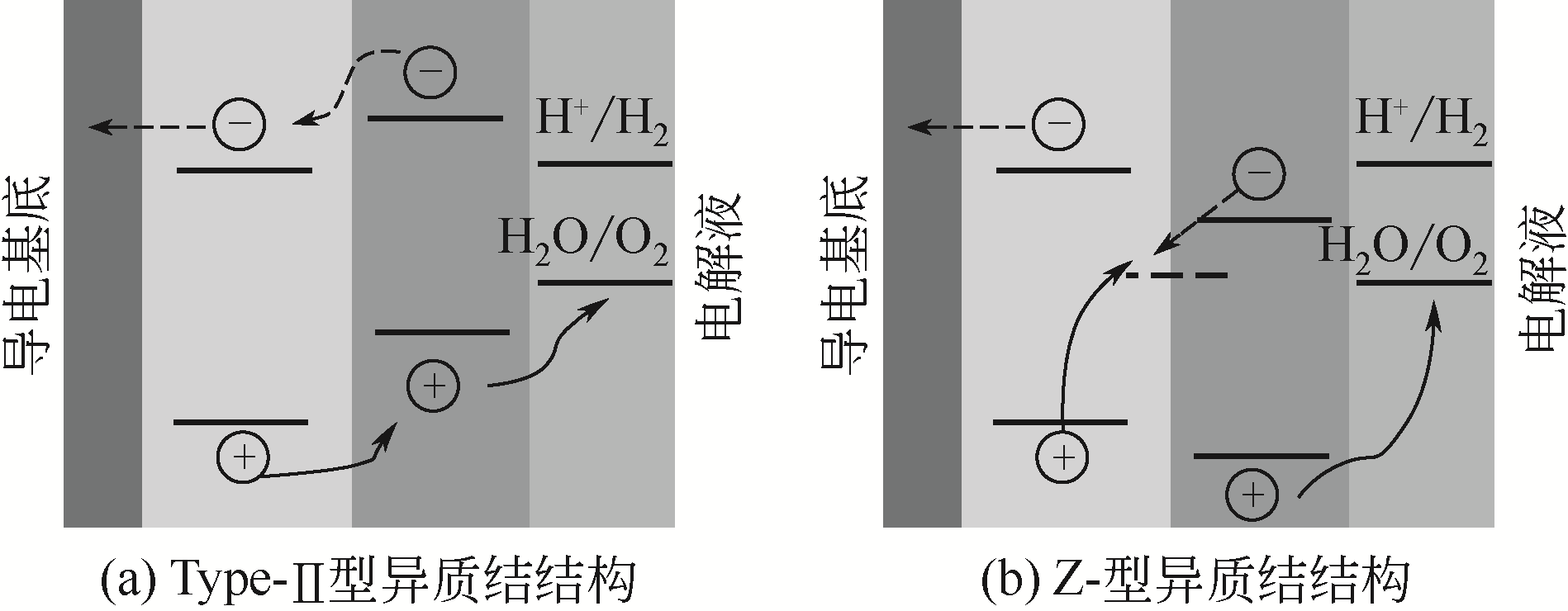
| 29 | LIU Y, KANG Z, ZHANG S, et al. Ferroelectric polarization-enhanced charge separation in a vanadium-doped ZnO photoelectrochemical system[J]. Inorganic Chemistry Frontiers, 2018, 5(7): 1533-1539. |
| 30 | LEE W C, CANCIANI G E, ALWHSHE B O S, et al. Enhanced photoelectrochemical water oxidation by ZnxMyO (M = Ni, Co, K, Na) nanorod arrays[J]. International Journal of Hydrogen Energy, 2016, 41(1): 123-131. |
| 31 | YANG Z, WANG Y, ZHANG D. A novel signal-on photoelectrochemical sensing platform based on biosynthesis of CdS quantum dots sensitizing ZnO nanorod arrays[J]. Sensors and Actuators B: Chemical, 2018, 261: 515-521. |
| 32 | LEE W, MIN S K, DHAS V, et al. Chemical bath deposition of CdS quantum dots on vertically aligned ZnO nanorods for quantum dots-sensitized solar cells[J]. Electrochemistry Communications, 2009, 11(1): 103-106. |
| 33 | JEAN J, CHANG S, BROWN P R, et al. ZnO nanowire arrays for enhanced photocurrent in PbS quantum dot solar cells[J]. Advanced Materials, 2013, 25(20): 2790-2796. |
| 34 | CHEN H M, CHEN C K, CHANG Y C, et al. Quantum dot monolayer sensitized ZnO nanowire-array photoelectrodes: true efficiency for water splitting[J]. Angewandte Chemie: International Edition, 2010, 122(34): 6102-6105. |
| 35 | GUO C X, DONG Y, YANG H B, et al. Graphene quantum dots as a green sensitizer to functionalize ZnO nanowire arrays on F-doped SnO2 glass for enhanced photoelectrochemical water splitting[J]. Advanced Energy Materials, 2013, 3(8): 997-1003. |
| 36 | CHEN C K, SHEN Y P, CHEN H M, et al. Quantum-dot-sensitized nitrogen-doped ZnO for efficient photoelectrochemical water splitting[J]. European Journal of Inorganic Chemistry, 2014, 2014(4): 773-779. |
| 37 | WANG G, YANG X, QIAN F, et al. Double-sided CdS and CdSe quantum dot co-sensitized ZnO nanowire arrays for photoelectrochemical hydrogen generation[J]. Nano Letters, 2010, 10(3): 1088-1092. |
| 38 | ZHOU X, LIU G, YU J, et al. Surface plasmon resonance-mediated photocatalysis by noble metal-based composites under visible light[J]. Journal of Materials Chemistry, 2012, 22(40): 21337-21354. |
| 39 | JIANG R, LI B, FANG C, et al. Metal/semiconductor hybrid nanostructures for plasmon-enhanced applications[J]. Advanced Materials, 2014, 26(31): 5274-5309. |
| 40 | LINIC S, CHRISTOPHER P, INGRAM D B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy[J]. Nature Materials, 2011, 10(12): 911-921. |
| 41 | WEI Y, KE L, KONG J, et al. Enhanced photoelectrochemical water-splitting effect with a bent ZnO nanorod photoanode decorated with Ag nanoparticles[J]. Nanotechnology, 2012, 23(23): 235401. |
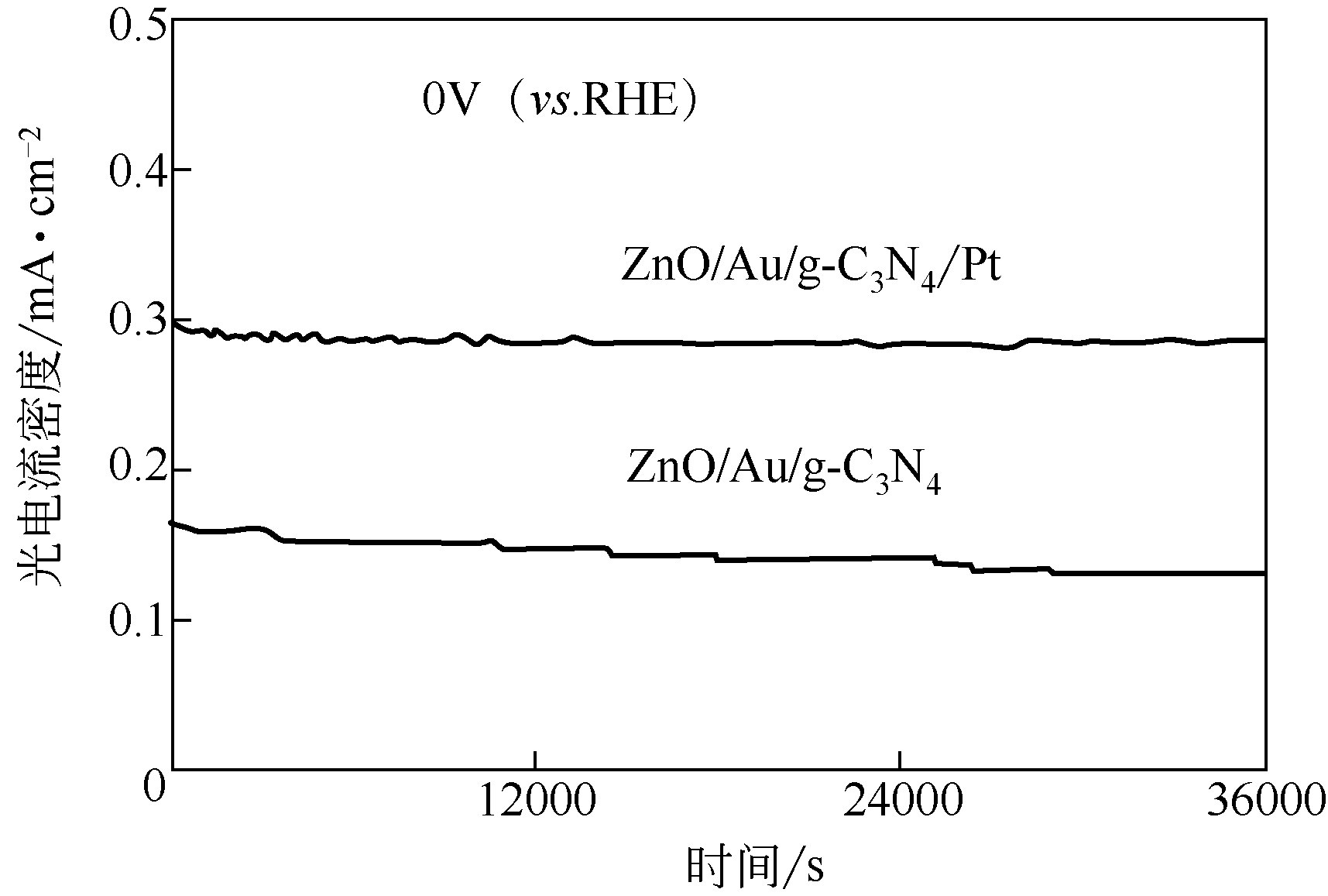
| 42 | WEI Y, KONG J, YANG L, et al. Polydopamine-assisted decoration of ZnO nanorods with Ag nanoparticles: an improved photoelectrochemical anode[J]. Journal of Materials Chemistry A, 2013, 1(16): 5045-5052. |
| 43 | KANG Z, YAN X, WANG Y, et al. Self-powered photoelectrochemical biosensing platform based on Au NPs@ZnO nanorods array[J]. Nano Research, 2016, 9(2): 344-352. |
| 44 | WU M, CHEN W J, SHEN Y H, et al. In situ growth of matchlike ZnO/Au plasmonic heterostructure for enhanced photoelectrochemical water splitting[J]. ACS Applied Materials & Interfaces, 2014, 6(17): 15052-15060. |
| 45 | CHIKATE P R, DAWARE K D, PATIL S S, et al. Effects of Au loading on the enhancement of photoelectrochemical activities of the Au@ZnO nano-heteroarchitecture[J]. New Journal of Chemistry, 2020, 44(14): 5535-5544. |
| 46 | WANG T, JIAO Z, CHEN T, et al. Vertically aligned ZnO nanowire arrays tip-grafted with silver nanoparticles for photoelectrochemical applications[J]. Nanoscale, 2013, 5(16): 7552-7557. |
| 47 | WANG T, JIN B, JIAO Z, et al. Photo-directed growth of Au nanowires on ZnO arrays for enhancing photoelectrochemical performances[J]. Journal of Materials Chemistry A, 2014, 2(37): 15553-15559. |
| 48 | FU S, ZHANG B, HU H, et al. ZnO nanowire arrays decorated with PtO nanowires for efficient solar water splitting[J]. Catalysis Science & Technology, 2018, 8(11): 2789-2793. |
| 49 | TAK Y, HONG S J, LEE J S, et al. Fabrication of ZnO/CdS core/shell nanowire arrays for efficient solar energy conversion[J]. Journal of Materials Chemistry, 2009, 19(33): 5945-5951. |
| 50 | LI Y, LIU Z, WANG Y, et al. ZnO/CuInS2 core/shell heterojunction nanoarray for photoelectrochemical water splitting[J]. International Journal of Hydrogen Energy, 2012, 37(20): 15029-15037. |
| 51 | LIU C, MENG F L, ZHANG L, et al. CuO/ZnO heterojunction nanoarrays for enhanced photoelectrochemical water oxidation[J]. Applied Surface Science, 2019, 469: 276-282. |
| 52 | CAO S, YAN X, KANG Z, et al. Band alignment engineering for improved performance and stability of ZnFe2O4 modified CdS/ZnO nanostructured photoanode for PEC water splitting[J]. Nano Energy, 2016, 24: 25-31. |
| 53 | ZHAO K, YAN X, GU Y, et al. Self-powered photoelectrochemical biosensor based on CdS/RGO/ZnO nanowire array heterostructure[J]. Small, 2016, 12(2): 245-251. |
| 54 | HIEU H N, NGHIA N V, VUONG N M, et al. Omnidirectional Au-embedded ZnO/CdS core/shell nanorods for enhanced photoelectrochemical water-splitting efficiency[J]. Chemical Communications, 2020, 56(28): 3975-3978. |
| 55 | CHEN Y, WANG L, GAO R, et al. Polarization-enhanced direct Z-scheme ZnO-WO3-x nanorod arrays for efficient piezoelectric-photoelectrochemical water splitting[J]. Applied Catalysis B: Environmental, 2019, 259: 118079. |
| 56 | FU S, HU H, FENG C, et al. Epitaxial growth of ZnWO4 hole-storage nanolayers on ZnO photoanodes for efficient solar water splitting[J]. Journal of Materials Chemistry A, 2019, 7(6): 2513-2517. |
| 57 | TAN C, CHEN J, WU X J, et al. Epitaxial growth of hybrid nanostructures[J]. Nature Reviews Materials, 2018, 3(2): 17089. |
| 58 | ZHOU T, WANG J, CHEN S, et al. Bird-nest structured ZnO/TiO2 as a direct Z-scheme photoanode with enhanced light harvesting and carriers kinetics for highly efficient and stable photoelectrochemical water splitting[J]. Applied Catalysis B: Environmental, 2020, 267: 118599. |
| 59 | WANG Q, HISATOMI T, JIA Q, et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%[J]. Nature Materials, 2016, 15(6): 611-615. |
| 60 | YU Z B, XIE Y P, LIU G, et al. Self-assembled CdS/Au/ZnO heterostructure induced by surface polar charges for efficient photocatalytic hydrogen evolution[J]. Journal of Materials Chemistry A, 2013, 1(8): 2773-2776. |
| 61 | YANG X, LI H, ZHANG W, et al. High visible photoelectrochemical activity of Ag nanoparticle-sandwiched CdS/Ag/ZnO nanorods[J]. ACS Applied Materials & Interfaces, 2017, 9(1): 658-667. |
| 62 | GUPTA R, ESWAR N K, MODAK J M, et al. Effect of morphology of zinc oxide in ZnO-CdS-Ag ternary nanocomposite towards photocatalytic inactivation of E. coli under UV and visible light[J]. Chemical Engineering Journal, 2017, 307: 966-980. |
| 63 | LINGAMPALLI S R, GAUTAM U K, RAO C N R. Highly efficient photocatalytic hydrogen generation by solution-processed ZnO/Pt/CdS, ZnO/Pt/Cd1-xZnxS and ZnO/Pt/CdS1-xSex hybrid nanostructures[J]. Energy & Environmental Science, 2013, 6(12): 3589-3594. |
| 64 | KIM Y, SHIN D, CHANG W J, et al. Hybrid Z-scheme using photosystem I and BiVO4 for hydrogen production[J]. Advanced Functional Materials, 2015, 25(16): 2369-2377. |
| 65 | WEN P, SUN Y, LI H, et al. A highly active three-dimensional Z-scheme ZnO/Au/g-C3N4 photocathode for efficient photoelectrochemical water splitting[J]. Applied Catalysis B: Environmental, 2020, 263: 118180. |
| 66 | ZHAN F, YANG Y, LIU W, et al. Facile synthesis of FeOOH quantum dots modified ZnO nanorods films via a metal-solating process[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(6): 7789-7798. |
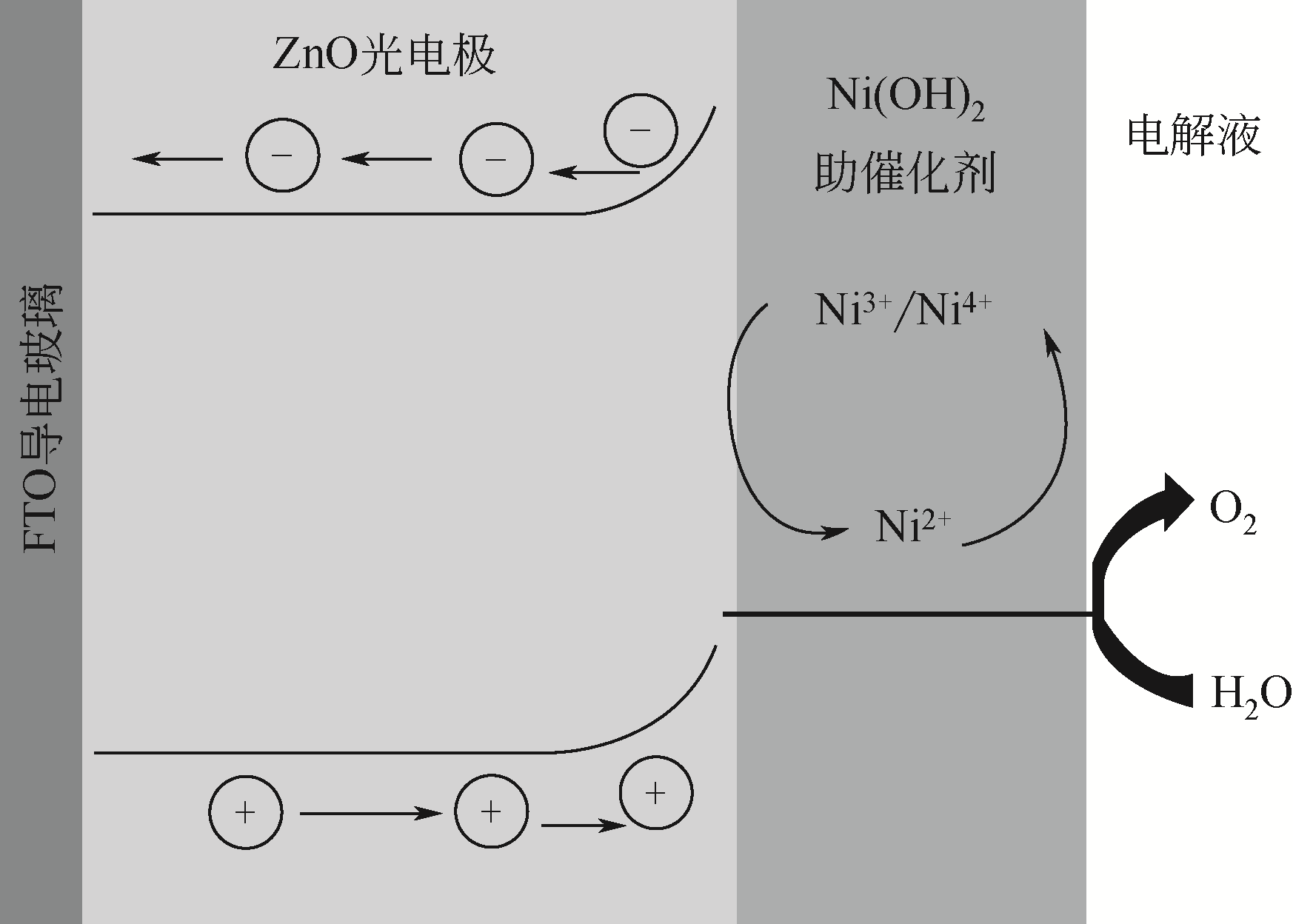
| 67 | MAO Y, YANG H, CHEN J, et al. Significant performance enhancement of ZnO photoanodes from Ni(OH)2 electrocatalyst nanosheets overcoating[J]. Nano Energy, 2014, 6: 10-18. |
| 68 | MAO Y, CHENG Y, WANG J, et al. Amorphous NiO electrocatalyst overcoated ZnO nanorod photoanodes for enhanced photoelectrochemical performance[J]. New Journal of Chemistry, 2016, 40(1): 107-112. |
| 69 | DING C, SHI J, WANG Z, et al. Photoelectrocatalytic water splitting: significance of cocatalysts, electrolyte, and interfaces[J]. ACS Catalysis, 2017, 7(1): 675-688. |
| 70 | SHE H, YUE P, HUANG J, et al. One-step hydrothermal deposition of F:FeOOH onto BiVO4 photoanode for enhanced water oxidation[J]. Chemical Engineering Journal, 2020, 392: 123703. |
| 71 | XU D, XIA T, FAN W, et al. MOF-derived Co3O4 thin film decorated BiVO4 for enhancement of photoelectrochemical water splitting[J]. Applied Surface Science, 2019, 491: 497-504. |
| 72 | LIU C, XU Y, LUO H, et al. Synthesis and photoelectrochemical properties of CoOOH/phosphorus-doped hematite photoanodes for solar water oxidation[J]. Chemical Engineering Journal, 2019, 363: 23-32. |
| 73 | KIM M-W, JOSHI B, SAMUEL E, et al. Electrosprayed MnO2 on ZnO nanorods with atomic layer deposited TiO2 layer for photoelectrocatalytic water splitting[J]. Applied Catalysis B: Environmental, 2020, 271: 118928. |
| 74 | HAYDOUS F, SI W, GUZENKO V A, et al. Improved photoelectrochemical water splitting of CaNbO2N photoanodes by CoPi photodeposition and surface passivation[J]. The Journal of Physical Chemistry C, 2019, 123(2): 1059-1068. |
| 75 | LIU G, SHI J, ZHANG F, et al. A tantalum nitride photoanode modified with a hole-storage layer for highly stable solar water splitting[J]. Angewandte Chemie: International Edition, 2014, 53(28): 7295-7299. |
| 76 | MA Z, THERSLEFF T, GORNE A L, et al. Quaternary core-shell oxynitride nanowire photoanode containing a hole-extraction gradient for photoelectrochemical water oxidation[J]. ACS Applied Materials & Interfaces, 2019, 11(21): 19077-19086. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 胡喜, 王明珊, 李恩智, 黄思鸣, 陈俊臣, 郭秉淑, 于博, 马志远, 李星. 二硫化钨复合材料制备与储钠性能研究进展[J]. 化工进展, 2023, 42(S1): 344-355. |
| [6] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [7] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [8] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [9] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [10] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [11] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [12] | 葛全倩, 徐迈, 梁铣, 王凤武. MOFs材料在光电催化领域应用的研究进展[J]. 化工进展, 2023, 42(9): 4692-4705. |
| [13] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [14] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [15] | 杨斌, 王晓冬, 王燕, 仪桂云, 王铁狼, 时闯, 张战营. 纳米Pt/ZnO异质结构的制备及其气敏性能[J]. 化工进展, 2023, 42(9): 4817-4827. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||