化工进展 ›› 2019, Vol. 38 ›› Issue (11): 5103-5113.DOI: 10.16085/j.issn.1000-6613.2018-2243
吸附强化蒸汽重整制氢中CO2固体吸附剂的研究进展
- 昆明理工大学环境科学与工程学院,云南 昆明 560500
-
收稿日期:2018-11-16出版日期:2019-11-05发布日期:2019-11-05 -
通讯作者:高晓亚 -
作者简介:王云珠(1989—),女,博士研究生,研究方向为醇类水蒸气重整制氢的应用基础。E-mail:wyzkust@163.com 。 -
基金资助:国家自然科学基金(2166060141);昆明理工大学高层次人才平台建设项目(10978172)
Research progress in CO2 solid sorbents for hydrogen production by sorption-enhanced steam reforming: a review
Yunzhu WANG( ),Ziheng PAN,Yi ZHAO,Yongming LUO,Xiaoya GAO(
),Ziheng PAN,Yi ZHAO,Yongming LUO,Xiaoya GAO( )
)
- School of Environmental Science and Engineering,Kunming University of Science and Technology, Kunming 560500, Yunnan,China
-
Received:2018-11-16Online:2019-11-05Published:2019-11-05 -
Contact:Xiaoya GAO
摘要:
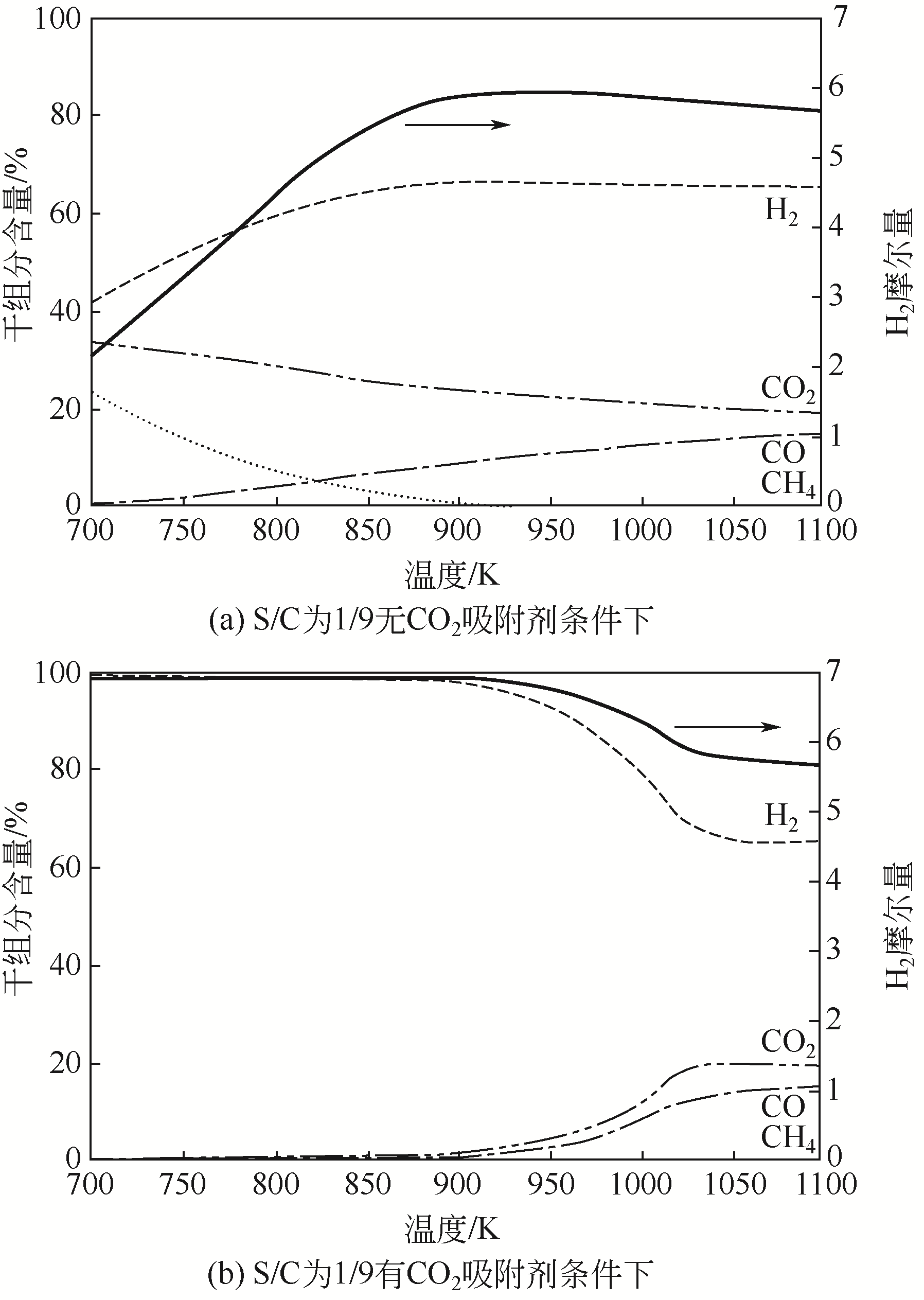
吸附强化蒸汽重整(SESR)制氢技术是集重整反应(H2生产)和选择性分离(CO2吸附)于一体的新型技术。该技术的特点为采用固体吸附剂在高温下对CO2进行原位脱除,以改变反应的正常平衡极限,提高烃类转化率,提高H2产量,减少CO2排放。在整个SESR制氢技术中,吸附剂的选择与反应条件至关重要。本文探讨了CaO、水滑石、Li2ZrO3、Li2SiO3以及双功能吸附剂在SESR制氢过程中的性能,总结了提高这些吸附剂吸附性能的不同方法。确定了固体吸附剂的反应条件,如温度、压力、水蒸气量等因素的影响及相关的反应机理。分析表明,CaO基吸附剂由于其低廉的价格及较高的吸附能力,被认为是最具潜力的吸附剂,然而在SESR制氢过程中,CaO基吸附剂面临着多次循环再生后吸附能力衰减的挑战。集吸附与催化双重功能的吸附催化材料由于可以克服SESR制氢中不同固体催化剂和吸附剂的匹配问题、降低所用固体材料的成本,从而使其在吸附强化蒸汽重整制氢方面具有巨大优势,并成为该领域未来研究的一个重要方向。
中图分类号:
引用本文
王云珠,泮子恒,赵燚,罗永明,高晓亚. 吸附强化蒸汽重整制氢中CO2固体吸附剂的研究进展[J]. 化工进展, 2019, 38(11): 5103-5113.
Yunzhu WANG,Ziheng PAN,Yi ZHAO,Yongming LUO,Xiaoya GAO. Research progress in CO2 solid sorbents for hydrogen production by sorption-enhanced steam reforming: a review[J]. Chemical Industry and Engineering Progress, 2019, 38(11): 5103-5113.
| 稳定吸附剂 | 稳定剂 含量/% | 吸附条件 | 再生条件 | 循环次数 /次 | CO2吸附 /gCO2?g-1 | 参考文献 |
|---|---|---|---|---|---|---|
| Ca12Al14O33 | 7.7 | 650℃,15% CO2,30min | 900℃,100% N2,10min | 30 | 0.600 | [ |
| Ca12Al14O33 | — | 690℃,15% CO2,60min | 950℃,100% N2,15min | 7 | 0.660 | [ |
| Al2O3 | 19 | 750℃,40% CO2,20min | 750℃,100% N2,20min | 30 | 0.360 | [ |
| Ca9Al6O18 | 20 | 650℃,15% CO2,30min | 800℃,100% N2, 10min | 35 | 0.520 | [ |
| Ca3Al2O6 | 34 | 690℃,15% CO2,30min | 800℃,100% N2,5min | 45 | 0.450 | [ |
| CaN40Al1 | — | 690℃,15% CO2,45min | 900℃,100% N2,20min | 60 | 0.400 | [ |
| MgO | 25 | 700℃,20%CO2,10min | 730℃,100% N2,10min | 50 | 0.540 | [ |
| ZrO | 34 | 650℃,15% CO2/N2,30min | 850℃,100% N2,5min | 20 | 0.484 | [ |
| CaZrO3 | 26.2 | 650℃,15% CO2,30min | 750℃,100% Ar,30min | 25 | 0.290 | [ |
| Y2O3 | 20 | 650℃,20% CO2,30min | 850℃,100% N2,5min | 10 | 0.570 | [ |
| TiO2 | 10 | 600℃,20% CO2,10min | 750℃,100% N2,10min | 40 | 0.230 | [ |
| Y2O3,MgO | 40 | 650℃,15% CO2/N2,15min | 900℃,100% N2,8min | 122 | 0.311 | [ |
表1 金属氧化物稳定的CaO基吸附剂及吸附性能
| 稳定吸附剂 | 稳定剂 含量/% | 吸附条件 | 再生条件 | 循环次数 /次 | CO2吸附 /gCO2?g-1 | 参考文献 |
|---|---|---|---|---|---|---|
| Ca12Al14O33 | 7.7 | 650℃,15% CO2,30min | 900℃,100% N2,10min | 30 | 0.600 | [ |
| Ca12Al14O33 | — | 690℃,15% CO2,60min | 950℃,100% N2,15min | 7 | 0.660 | [ |
| Al2O3 | 19 | 750℃,40% CO2,20min | 750℃,100% N2,20min | 30 | 0.360 | [ |
| Ca9Al6O18 | 20 | 650℃,15% CO2,30min | 800℃,100% N2, 10min | 35 | 0.520 | [ |
| Ca3Al2O6 | 34 | 690℃,15% CO2,30min | 800℃,100% N2,5min | 45 | 0.450 | [ |
| CaN40Al1 | — | 690℃,15% CO2,45min | 900℃,100% N2,20min | 60 | 0.400 | [ |
| MgO | 25 | 700℃,20%CO2,10min | 730℃,100% N2,10min | 50 | 0.540 | [ |
| ZrO | 34 | 650℃,15% CO2/N2,30min | 850℃,100% N2,5min | 20 | 0.484 | [ |
| CaZrO3 | 26.2 | 650℃,15% CO2,30min | 750℃,100% Ar,30min | 25 | 0.290 | [ |
| Y2O3 | 20 | 650℃,20% CO2,30min | 850℃,100% N2,5min | 10 | 0.570 | [ |
| TiO2 | 10 | 600℃,20% CO2,10min | 750℃,100% N2,10min | 40 | 0.230 | [ |
| Y2O3,MgO | 40 | 650℃,15% CO2/N2,15min | 900℃,100% N2,8min | 122 | 0.311 | [ |
| 吸附剂 | 吸附温度/℃ | 吸附压力/MPa | 再生温度/℃ | 循环次数/次 | 吸附能力/gCO2·g-1 | 参考文献 |
|---|---|---|---|---|---|---|
| K2CO3-HTlc | 500 | 0.15 | — | 30 | 0.036 | [ |
| K2CO3-HTlc | 200~500 | 0.10 | 500 | 8 | 0.050 | [ |
| HTlc-10Ga | 300 | 0.10 | 300 | 5 | 0.080 | [ |
| cK-HTCGa MW | 300 | 0.31 | 673 | 4 | 0.084 | [ |
| Na-HTlc | 200 | 0.10 | — | — | 0.046 | [ |
表2 类水滑石化合物吸附CO2的吸附性能
| 吸附剂 | 吸附温度/℃ | 吸附压力/MPa | 再生温度/℃ | 循环次数/次 | 吸附能力/gCO2·g-1 | 参考文献 |
|---|---|---|---|---|---|---|
| K2CO3-HTlc | 500 | 0.15 | — | 30 | 0.036 | [ |
| K2CO3-HTlc | 200~500 | 0.10 | 500 | 8 | 0.050 | [ |
| HTlc-10Ga | 300 | 0.10 | 300 | 5 | 0.080 | [ |
| cK-HTCGa MW | 300 | 0.31 | 673 | 4 | 0.084 | [ |
| Na-HTlc | 200 | 0.10 | — | — | 0.046 | [ |
| 催化剂 | 比表面积 /m2·g-1 | 最佳吸附能力 /gCO2·g-1 | 最佳反应温度 /℃ | 氢气选择性 (吸附饱和前)/% | 参考文献 |
|---|---|---|---|---|---|
| CeO2-Ca9Al6O18-CaO/NiO | 15.40 | 0.420 | 550 | 98.0 | [ |
| Ni-CaO-Al2O3 | 29.00 | 0.248 | 500 | 99.4 | [ |
| Ni-CaO-MMT | 58.80 | 0.250 | 600 | 100.0 | [ |
| Ni/(CaO/Al2O3) | 45.23 | 0.042 | 550 | 99.9 | [ |
| K-Ni-Cu-HTlc | 38.00 | 0.030 | 500 | 99.8 | [ |
| Ni/CaO-MgO-Al2O3 | 123.00 | — | 600 | 99.0 | [ |
| Fe/CaO-Ca12Al14O33 | 7.60 | 0.660 | 700 | 98.0 | [ |
表3 吸附增强蒸汽重整制氢的双功能催化剂组成及其性能
| 催化剂 | 比表面积 /m2·g-1 | 最佳吸附能力 /gCO2·g-1 | 最佳反应温度 /℃ | 氢气选择性 (吸附饱和前)/% | 参考文献 |
|---|---|---|---|---|---|
| CeO2-Ca9Al6O18-CaO/NiO | 15.40 | 0.420 | 550 | 98.0 | [ |
| Ni-CaO-Al2O3 | 29.00 | 0.248 | 500 | 99.4 | [ |
| Ni-CaO-MMT | 58.80 | 0.250 | 600 | 100.0 | [ |
| Ni/(CaO/Al2O3) | 45.23 | 0.042 | 550 | 99.9 | [ |
| K-Ni-Cu-HTlc | 38.00 | 0.030 | 500 | 99.8 | [ |
| Ni/CaO-MgO-Al2O3 | 123.00 | — | 600 | 99.0 | [ |
| Fe/CaO-Ca12Al14O33 | 7.60 | 0.660 | 700 | 98.0 | [ |
| 1 | LEE K B, BEAVER M G , CARAM H S , et al . Production of fuel-cell grade hydrogen by thermal swing sorption enhanced reaction concept[J]. International Journal of Hydrogen Energy, 2008, 33: 781-790. |
| 2 | WU Y J , LU P , YU J G , et al . Sorption-enhanced steam reforming of ethanol on NiMgAl multifunctional materials: experimental and numerical investigation[J]. Chemical Engineering Journal, 2013, 231: 36-48. |
| 3 | ILIUTA I , RADFARNIA H R , IIUTA M C . Hydrogen production by sorption-enhanced steam glycerol reforming: sorption kinetics and reactor simulation[J]. AIChE Journal, 2013, 59: 2015-2118. |
| 4 | DOU B L , RICKETT G , DUPONT V , et al . Steam reforming of crude glycerol with in-situ CO2 sorption[J]. Bioresource Technology, 2010, 101: 2436-2442. |
| 5 | DOU B L , DUPONT V , RICKETT G , et al . Hydrogen production by sorption-enhanced steam reforming of glycerol[J]. Bioresource Technology, 2009, 100: 3540-3547. |
| 6 | DOU B L , SONG Y C , WANG C , et al . Hydrogen production from catalytic steam reforming of biodiesel byproduct glycerol: issues and challenges[J]. Renewable and Sustainable Energy Reviews, 2014, 30: 950-960. |
| 7 | DOU B L , WANG C , CHEN H S , et al . Continuous sorption-enhanced steam reforming of glycerol to high-purity hydrogen production[J]. International Journal of Hydrogen Energy, 2013, 38: 11902-11909. |
| 8 | DOU B L , JIANG B , WANG K Q . Sorption enhanced steam reforming of biodiesel by-product glycerol on Ni-CaO-MMT multifunctional catalysts[J]. Chemical Engineering Journal, 2017, 313: 207-216. |
| 9 | MANOVIC V , ANTHONY E J . Carbonation of CaO-based sorbents enhanced by steam addition[J]. Industrial & Engineering Chemistry Research, 2010, 49: 9105-9110. |
| 10 | HARRISON D P . Sorption-enhanced hydrogen production: a review[J]. Industrial & Engineering Chemistry Research, 2008, 47: 6486-6501. |
| 11 | DING Y , ALPAY E . Adsorption-enhanced steam methane reforming[J]. Chemical Engineering Science, 2000, 55: 3929-3940. |
| 12 | LEE K B, BEAVER M G , CARAM H S , et al . Novel thermal-swing sorption-enhanced reaction process concept for hydrogen production by low temperature steam-methane reforming[J]. Industrial & Engineering Chemistry Research, 2007, 46: 5003-5014. |
| 13 | ESSAKI K , MURAMATSU T , KATO M . Effect of equilibrium shift by using lithium silicate pellets in methane steam reforming[J]. International Journal of Hydrogen Energy, 2008, 33: 4555-4559. |
| 14 | DOU B L , WANG C , SONG Y C , et al . Solid sorbents for in-situ CO2 removal during sorption-enhanced steam reforming process: a review[J]. Renewable and Sustainable Energy Reviews, 2016, 53: 536-546. |
| 15 | KIERZKOWSKA A M , PACCIANI R , MULLER C R . CaO-based CO2 sorbents: from fundamentals to the development of new, highly effective materials[J]. ChemSusChem, 2013, 6: 1130-1148. |
| 16 | LIU W Q , AN H , QIN C L , et al . Performance enhancement of calcium oxide sorbents for cyclic CO2 capture-a review[J]. Energy Fuels, 2012, 26: 2751-2767. |
| 17 | BHATTA L K G , SUBRAMANYAM S , CHENGALA M D , et al . Progress in hydrotalcite like compounds and metal-based oxides for CO2 capture: a review[J]. Journal of Cleaner Production, 2015, 103: 171-196. |
| 18 | SUN P , LIM J, GRACE J R . Cyclic CO2 capture by limestone-derived sorbent during prolonged calcination/carbonation cycling[J]. AIChE Journal, 2008, 54: 1668-1677. |
| 19 | LU H , REDDY E P , SMIRNIOTIS P G . Calcium oxide based sorbents for capture of carbon dioxide at high temperatures[J]. Industrial & Engineering Chemistry Research, 2006, 45: 3944-3949. |
| 20 | LU H , SMIRNIOTIS P G , ERNST F O , et al . Nanostructured Ca-based sorbents with high CO2 uptake efficiency[J]. Chemical Engineering Science, 2009, 64: 1936-1943. |
| 21 | LU H , KHAN A , SMIRNIOTIS P G . Relationship between structural properties and CO2 capture performance of CaO-based sorbents obtained from different organometallic precursors[J]. Industrial & Engineering Chemistry Research, 2008, 47: 6216-6220. |
| 22 | LU H , REDDY E , PSMIRNIOTIS P G . Calcium oxide based sorbents for capture of carbon dioxide at high temperatures[J]. Industrial & Engineering Chemistry Research, 2006, 45: 3944-3949. |
| 23 | LIU W Q , LOW N W L, FENG B , et al . Calcium precursors for the production of CaO sorbents for multicycle CO2 capture[J]. Environmental Scienc & Technology, 2010, 44: 841-847. |
| 24 | GRASA G , GONZALEZ B , ALONSO M , et al . Comparison of CaO-based synthetic CO2 sorbents under realistic calcination conditions[J]. Energy Fuels, 2007, 21: 3560-3562. |
| 25 | ABANADES J C , ALVAREZ D . Conversion limits in the reaction of CO2 with lime[J]. Energy Fuels, 2003, 17: 308-315. |
| 26 | FLORIN N H , HARRS A T . Reactivity of CaO derived from nano-sized CaCO3 particles through multiple CO2 capture-and-release cycles[J]. Chemical Engineering Science, 2009, 64: 187-191. |
| 27 | LUO C , ZHENG Y , ZHENG C , et al . Manufacture of calcium-based sorbents for high temperature cyclic CO2 capture via a sol-gel process[J]. International Journal of Greenhouse Gas Control, 2013, 12: 193-199. |
| 28 | LI Z S , CAI N S , HUANG Y Y . Effect of preparation temperature on cyclic CO2 capture and multiple carbonation-calcination cycles for a new Ca-based CO2 sorbent[J]. Industrial & Engineering Chemistry Research, 2006, 45: 1911-1917. |
| 29 | ZHANG M M , PENG Y X , SUN Y Z , et al . Preparation of CaO-Al2O3 sorbent and CO2 capture performance at high temperature[J]. Fuel, 2013, 111: 636-642. |
| 30 | SONG Y Q , JI G Z , ZHAO X , et al . Effects of drying methods on wet chemistry synthesis of Al stabilized CaO sorbents for cyclic CO2 capture[J]. Energy Fuels, 2017, 31: 12521-12529. |
| 31 | LI L Y , KING D L , NIE Z M , et al . Magnesia-stabilized calcium oxide absorbents with improved durability for high temperature CO2 capture[J]. Industrial & Engineering Chemistry Research, 2009, 48: 10604-10613. |
| 32 | LIU W Q , FENG B , WU Y Q , et al . Synthesis of sintering-resistant sorbents for CO2 capture[J]. Environmental Science & Technology, 2010, 44: 3093-3097. |
| 33 | REDDY G K , QUILLIN S , SMIRNIOTIS P . Influence of the synthesis method on the structure and CO2 adsorption properties of Ca/Zr sorbents[J]. Energy Fuels, 2014, 28: 3292-3299. |
| 34 | BRODA M , MULLER C R . Sol-gel-derived, CaO-based, ZrO2-stabilized CO2 sorbents[J]. Fuel, 2014, 127: 94-100. |
| 35 | SOLEIMANISALIM A H , MOHAMMAD H S , DAVOOD K , et al . Pelletizing and coating of synthetic zirconia stabilized calcium based sorbents for application in calcium looping CO2 capture[J]. Industrial & Engineering Chemistry Research, 2017, 56: 5395-5402. |
| 36 | LIU F Q , LI W H , LIU B C , et al . Synthesis, characterization, and high temperature CO2 capture of new CaO based hollow sphere sorbents[J]. Journal of Materials Chemistry A, 2013, 1: 8037-8044. |
| 37 | ZAMBONI I , ZIMMERMANN Y , KIENNEMANN A , et al . Improvement of steam reforming of toluene by CO2 capture using Fe/CaO-Ca12Al14O33 bi-functional materials[J]. International Journal of Hydrogen Energy, 2015, 40: 5297-5304. |
| 38 | KIERZKOWSKA A M , POULIKAKOS L V , BRODA M , et al . Synthesis of calcium-based, Al2O3-stabilized sorbents for CO2 capture using a coprecipitation technique[J]. International Journal of Greenhouse Gas Control, 2013, 15: 48-54. |
| 39 | XU P , XIE M M , CHENG Z M , et al . CO2 capture performance of CaO based sorbents prepared by a sol-gel method[J]. Industrial & Engineering Chemistry Research, 2013, 52: 12161-12169. |
| 40 | ANGELI S D , MARTAVALTZI C S , LEMONIDOU A A . Development of a novelsynthesized Ca-based CO2 sorbent for multicycle operation: parametric study of sorption[J]. Fuel, 2014, 127: 62-69. |
| 41 | LI S , FENG J Q , KOU X C , et al . Al-stabilized double-shelled hollow CaO-based microspheres with superior CO2 adsorption performance[J]. Energy Fuels, 2018, 32: 9692-9700. |
| 42 | LAN P Q , WU S F . Synthesis of a porous nano-CaO/MgO-based CO2 adsorbent[J]. Chem. Eng. Technol., 2014, 37: 580-586. |
| 43 | ANTZARA A N , ARREGI A , HERACLEOUS E , et al . In-depth evaluation of a ZrO2 promoted CaO-based CO2 sorbent in fluidized bed reactor tests[J]. Chemical Engineering Journal, 2018, 333: 697-711. |
| 44 | RADFARNIA H R , ILIUTA M C . Metal oxide-stabilized calcium oxide CO2 sorbent for multicycle operation[J]. Chemical Engineering Journal, 2013, 232: 280-289. |
| 45 | ZHANG X Y , LI Z G , PENG Y , et al . Investigation on a novel CaO-Y2O3 sorbent for efficient CO2 mitigation[J]. Chemical Engineering Journal, 2014, 243: 297-304. |
| 46 | WU S F , WANG L L . Improvement of the stability of a ZrO2-modified Ni-nano CaO sorption complex catalyst for ReSER hydrogen production[J]. International Journal of Hydrogen Energy, 2010, 35: 6518-6524. |
| 47 | HE D L , QIN C L , ZANG Z H , et al . Investigation of Y2O3/M x O y incorporated Ca-based sorbents for efficient and stable CO2 capture at high temperature[J]. Industrial & Engineering Chemistry Research, 2018, 57: 11625-11635. |
| 48 | WU Y T , LIU G C , LIAO Y F . Syngas production by chemical looping gasification of biomass with steam and CaO additive[J]. International Journal of Hydrogen Energy, 2018, 43: 19375-19383. |
| 49 | WANG X D , LI M S , LI S R . Hydrogen production by glycerol steam reforming with/without calcium oxide sorbent: a comparative study of thermodynamic and experimental work[J]. Fuel Processing Technology, 2010, 91: 1812-1818. |
| 50 | HANIF A , DASGUPTA S , DIVEKAR S , et al . A study on high temperature CO2 capture by improved hydrotalcite sorbents[J]. Chemical Engineering Journal, 2014, 236: 91-99. |
| 51 | HALABI M H , COBDEN P D , SCHOUTEN J C , et al . High capacity potassium-promoted hydrotalcite for CO2 capture in H2 production[J]. International Journal of Hydrogen Energy, 2012, 37: 4516-4525. |
| 52 | YAVUZ C T , SHINALL B D , IRETSKII A V , et al . Markedly improved CO2 capture efficiency and stability of gallium substituted hydrotalcites at elevated temperatures[J]. Chemistry of Materials, 2009, 21:3473-3475. |
| 53 | SILVA J M , TRUJILLANO R , RIVES V , et al . High temperature CO2 sorption over modified hydrotalcites[J]. Chemical Engineering Journal, 2017, 325: 25-34. |
| 54 | DEWOOLKAR K D , VAIDYA P D . Tailored hydrotalcite-based hybrid materials for hydrogen production via sorption-enhanced steam reforming of ethanol[J]. International Journal of Hydrogen Energy, 2016, 41: 6094-6106. |
| 55 | LEE C H, LEE K B . Sorption-enhanced water gas shift reaction for high-purity hydrogen production: application of a Na-Mg double salt-based sorbentand the divided section packing concept[J]. Applied Energy, 2017, 205: 316-322. |
| 56 | COSIMO J I , DIEZ V K , XU M , et al . Structure and surface and catalytic properties of Mg-Al basic oxides[J]. Journal of Catalysis, 1998, 178: 499-510. |
| 57 | ZHANG Y X , SU Q Y , WANG Z P , et al . Surface modification of Mg-Al hydrotalcite mixed oxides with potassium[J]. Acta Physico-Chimica Sinca, 2010, 1: 921-926. |
| 58 | HEE J J, CHAN H L , SUJI K , et al . Hydrothermal synthesis of K2CO3-promoted hydrotalcite from hydroxide-form precursors for novel hightemperature CO2 sorbent[J]. ACS Applied Materials & Interfaces, 2014, 6: 6914-6919. |
| 59 | MIGUEL C V , TRUJILLANO R , RIVES V , et al . High temperature CO2 sorption with gallium-substituted and promoted hydrotalcites[J]. Seperation and Purification Technology, 2014, 127: 202-211. |
| 60 | JOEL M , SILVA R , TRUJILLANO, et al . High temperature CO2 sorption over modified hydrotalcites[J]. Chemical Engineering Journal, 2017, 325: 25-34. |
| 61 | KIM S J , LEE K B . Impregnation of hydrotalcite with NaNO3 for enhanced high-temperature CO2 sorption uptake[J]. Chemical Engineering Journal, 2019, 356: 964-972. |
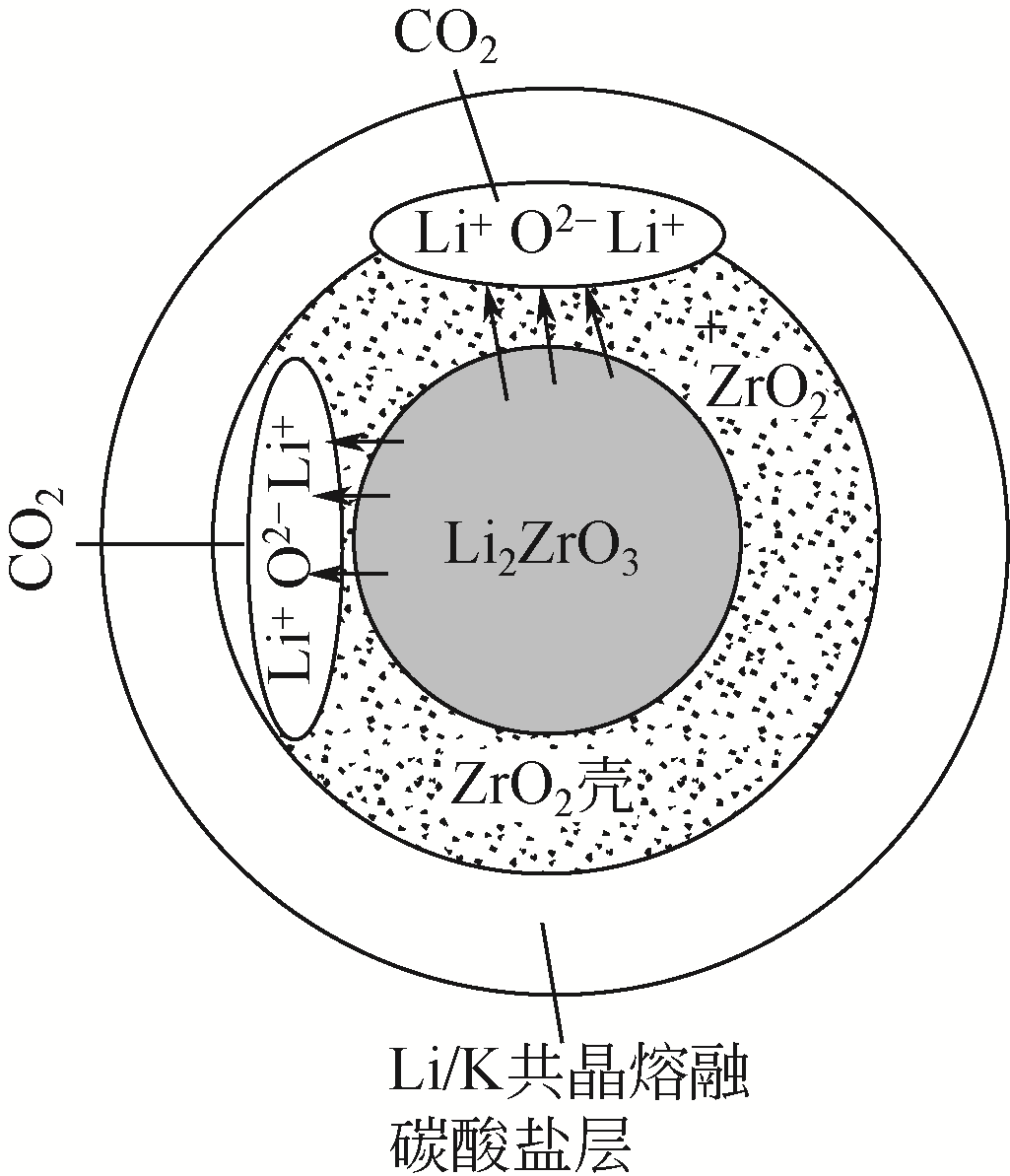
| 62 | NAKAGAWA K , OHASHI T . A reversible change between lithium zirconate and zirconia in molten carbonate[J]. Electrochemistry, 1999, 67: 618-621. |
| 63 | IDA J, XIONG R , LIN Y S . Synthesis and CO2 sorption properties of pure and modified lithium zirconate[J]. Seperation and Purification Technology, 2004, 36: 41-51. |
| 64 | GABRIELE P , MONICA P , MAURIZIA, et al . Experimental and modeling studies on high-temperature capture of CO2 using lithium zirconate based sorbents[J]. Industrial & Engineering Chemistry Research, 2007, 46: 6696-6706. |
| 65 | IDA J, LIN Y S . Mechanism of high-temperature CO2 sorption on lithium zirconate[J]. Environmental Science and Technology, 2003, 37: 1999-2004. |
| 66 | WANG C , CHEN Y , CHENG Z D , et al . Sorption-enhanced steam reforming of glycerol for hydrogen production over a NiO/NiAl2O4 catalyst and Li2ZrO3 based sorbent[J]. Energy Fuels, 2015, 29: 7408-7418. |
| 67 | NAKAGAWA K , OHASHI T . A novel method of CO2 capture from high temperature gases[J]. Journal of the Electrochemical Society, 1998, 145: 1344-1346. |
| 68 | 袁文辉, 梁杰, 李莉 . Li4SiO4的制备表征及CO2吸附与模拟[J] . 化工进展, 2009, 28(s1): 306-309. |
| YUAN W H , LIANG J , LI L . Preparation and characterization of Li4SiO4 and CO2 adsorption and simulation[J]. Chemical Industry and Engineering Progress, 2009, 28(s1): 306-309. | |
| 69 | KATO M , NAKAGAWA K , ESSAKI K , et al . Novel CO2 absorbents using lithium-containing oxide[J]. International Journal of Applied Ceramic Technology, 2005, 2: 467-475. |
| 70 | 王珂,许淘淘,刘跃飞,等 . 硅酸锂的制备及高温吸附二氧化碳的研究进展[J] . 应用化工, 2015, 44 (7): 1326-1330. |
| WANG K , XU T T , LIU Y F , et al . The synthesis routes of Li4SiO4-based sorbents and the development of high-temperature CO2 capture[J]. Applied Chemical Industry, 2015, 44 (7): 1326-1330. | |
| 71 | ZHANG Q , SHEN C , ZHANG S , et al . Steam methane reforming reaction enhanced by a novel K2CO3-doped Li4SiO4 sorbent: investigations on the sorbent and catalyst coupling behaviors and sorbent and catalyst coupling behaviors and sorbent regeneration strategy[J]. International Journal of Hydrogen Energy, 2016, 41: 4831-4842. |
| 72 | DANG C X , WANG H J , YU H . Sorption-enhanced steam reforming of glycerol over Ni-Cu-Ca-Al catalysts for producing fuel-cell grade hydrogen[J]. International Journal of Hydrogen Energy, 2017, 42: 17446-17456. |
| 73 | WU G W , ZHANG C X , LI S R , et al . Sorption enhanced steam reforming of ethanol on Ni-CaO-Al2O3 multifunctional catalysts derived from hydrotalcite-like compounds[J]. Energy & Environmental Science, 2012, 5: 8942-8949. |
| 74 | YANCHESHMEH M S , RADFARNIA H R , ILIUTA M C . Sustainable production of high-purity hydrogen by sorption enhanced steam reforming of glycerol over CeO2 promoted Ca9Al6O18-CaO/NiO bifunctional material[J]. ACS Sustainable Chemical Engineering, 2017, 5: 9774-9786. |
| 75 | DANG C X , YU H , WANG H J , et al . A bi-functional Co-CaO-Ca12Al14O33 catalyst for sorption-enhanced steam reforming of glycerol to high-purity hydrogen[J]. Chemical Engineering Journal, 2016, 286: 329-338. |
| 76 | WANG C , DOU B L , JIANG B , et al . Sorption-enhanced steam reforming of glycerol on Ni-based multifunctional catalysts[J]. International Journal of Hydrogen Energy, 2015, 40: 7037-7044. |
| 77 | CUNHA A F , WU Y J , LI P . Sorption-enhanced steam reforming of ethanol on a novel K-Ni-Cu-hydrotalcite hybrid material[J]. Industrial & Engineering Chemistry Research, 2014, 53: 3842-3853. |
| 78 | CHARISIOU N D , PAPAGERIDIS K N , TZOUNIS L , et al . Ni supported on CaO-MgO-Al2O3 as a highly selective and stable catalyst for H2 production via the glycerol steam reforming reaction[J]. International Journal of Hydrogen Energy, 2019, 44: 256-273. |
| 79 | QI T Y C , YANG Y , WU Y J , et al . Sorption-enhanced methanol steam reforming for hydrogen production by combined copper-based catalysts with hydrotalcites[J]. Chemical Engineering & Processing: Process Intensification, 2018, 127: 72-82. |
| [1] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [2] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [3] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [4] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [5] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [6] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [7] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [8] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [9] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| [10] | 黄玉飞, 李子怡, 黄杨强, 金波, 罗潇, 梁志武. 光催化CO2和CH4重整催化剂研究进展[J]. 化工进展, 2023, 42(8): 4247-4263. |
| [11] | 张亚娟, 徐惠, 胡贝, 史星伟. 化学镀法制备NiCoP/rGO/NF高效电解水析氢催化剂[J]. 化工进展, 2023, 42(8): 4275-4282. |
| [12] | 娄宝辉, 吴贤豪, 张驰, 陈臻, 冯向东. 纳米流体用于二氧化碳吸收分离研究进展[J]. 化工进展, 2023, 42(7): 3802-3815. |
| [13] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| [14] | 张雪伟, 黄亚继, 许月阳, 程好强, 朱志成, 李金壘, 丁雪宇, 王圣, 张荣初. 碱性吸附剂对燃煤烟气中SO3的吸附特性[J]. 化工进展, 2023, 42(7): 3855-3864. |
| [15] | 陆洋, 周劲松, 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛. CeO2/TiO2吸附剂煤气脱汞产物的浸出规律[J]. 化工进展, 2023, 42(7): 3875-3883. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||