化工进展 ›› 2019, Vol. 38 ›› Issue (04): 2046-2055.DOI: 10.16085/j.issn.1000-6613.2018-0740
燃煤电厂烟气MDEA/PZ混合胺法碳捕集工艺模拟分析
- 1. 中国能源建设集团广东省电力设计研究院有限公司,广东 广州 510663
2. 浙江大学能源清洁利用国家重点实验室,浙江 杭州 310027
-
收稿日期:2018-04-11修回日期:2018-05-30出版日期:2019-04-05发布日期:2019-04-05 -
通讯作者:方梦祥 -
作者简介:<named-content content-type="corresp-name">林海周</named-content>(1989—),男,博士后,研究方向为燃煤电厂烟气二氧化碳捕集。E-mail:<email>linhaizhou@gedi.com.cn</email>。|方梦祥,教授,博士生导师,研究方向为煤及生物质燃烧和气化技术、CO2控制技术。E-mail:<email>mxfang@zju.edu.cn</email>。 -
基金资助:广东省自然科学基金博士启动项目(2018A030310692);基金项目:中国能源建设集团广东省电力设计研究院博士后科研项目(EV04531W)
Simulation and analysis of carbon dioxide capture process using MDEA/PZ blend solution in a coal-fired power plant
Haizhou LIN1,2( ),Haizhong LUO1,Aiguo PEI1,Mengxiang FANG2(
),Haizhong LUO1,Aiguo PEI1,Mengxiang FANG2( )
)
- 1. China Energy Engineering Group Guangdong Electric Power Design Institute Co., Ltd., Guangzhou 510663, Guangdong China
2. State Key Laboratory of Clean Energy Utilization, Zhejiang University, Hangzhou 310027, Zhejiang, China
-
Received:2018-04-11Revised:2018-05-30Online:2019-04-05Published:2019-04-05 -
Contact:Mengxiang FANG
摘要:
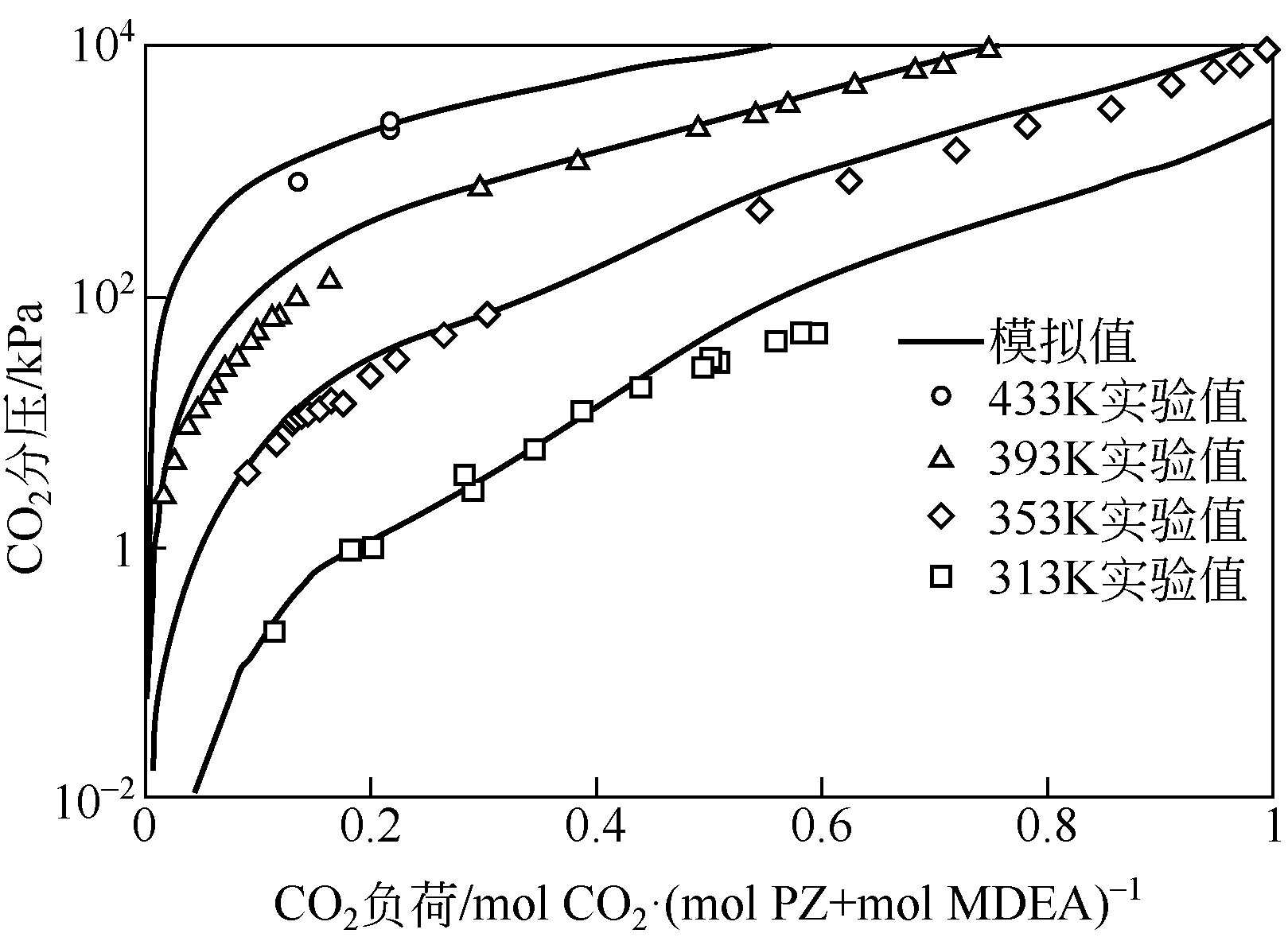
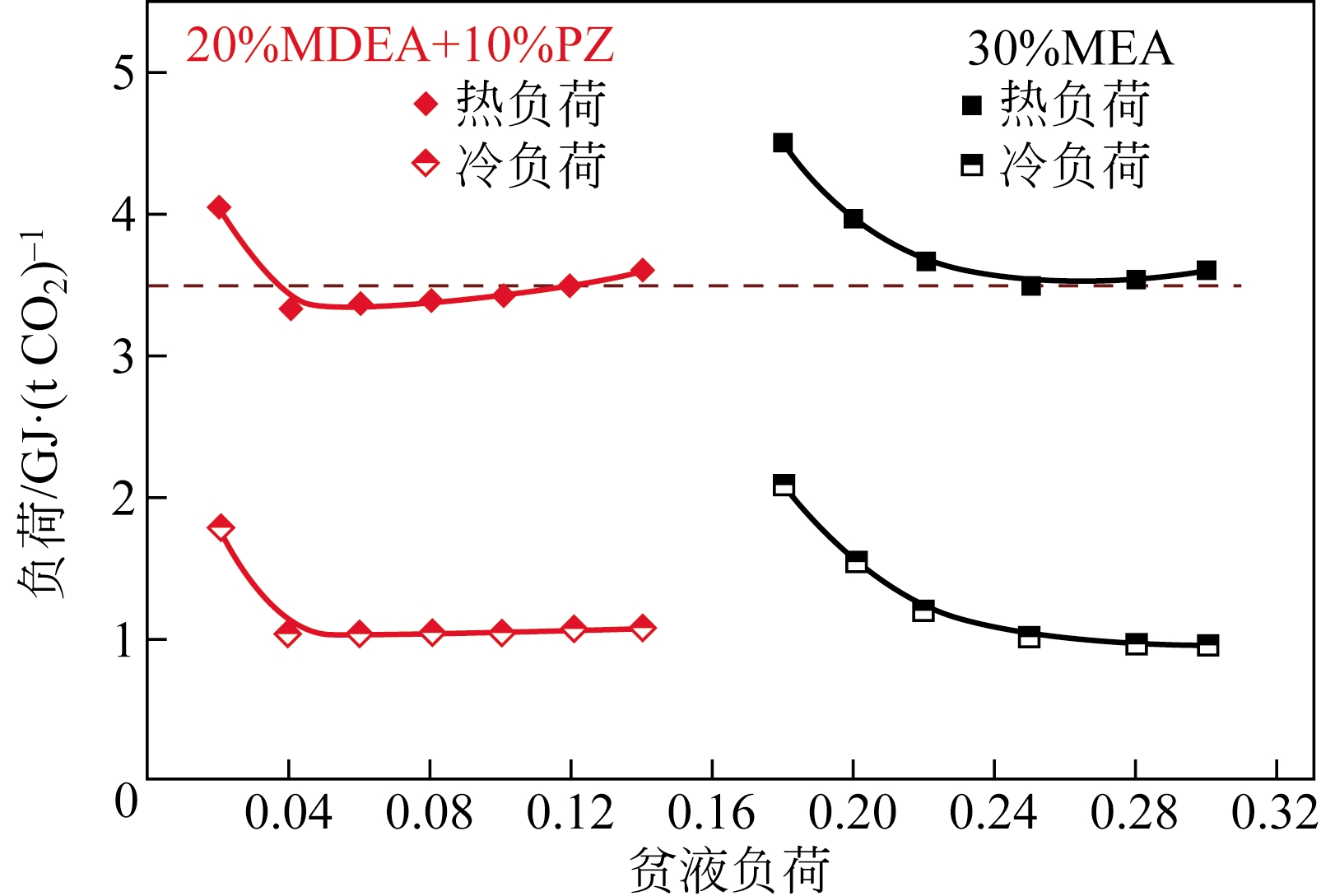
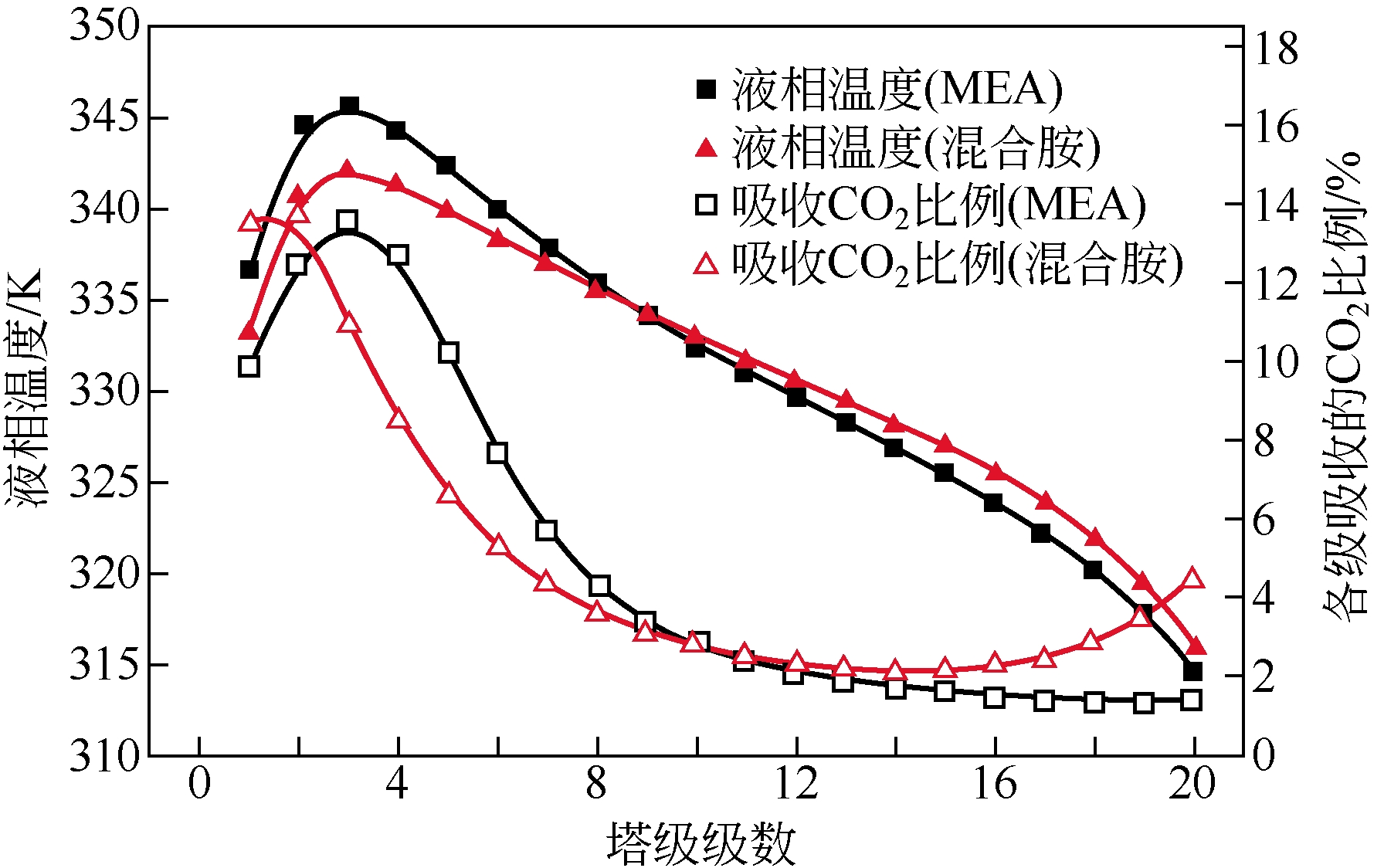
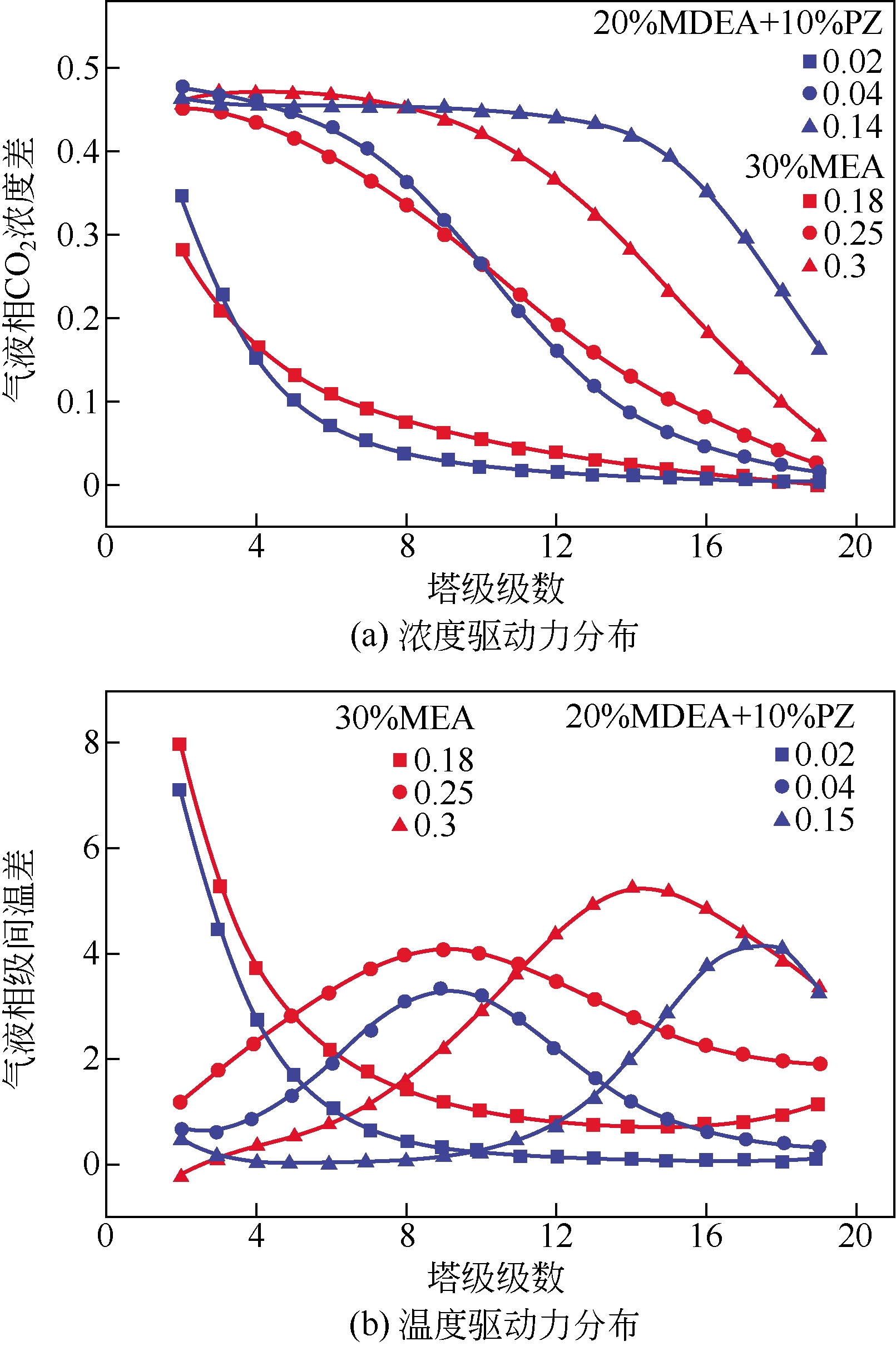
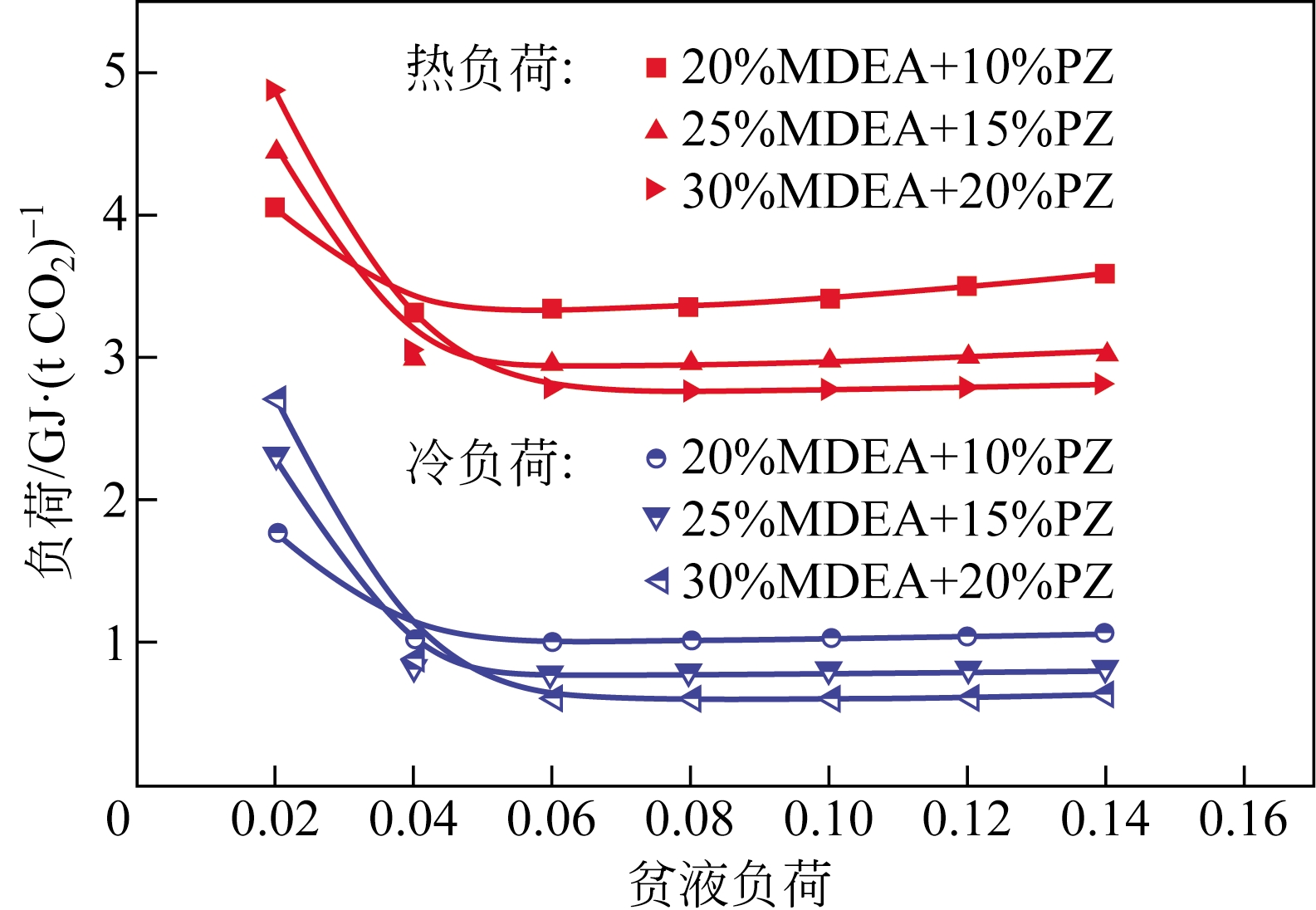
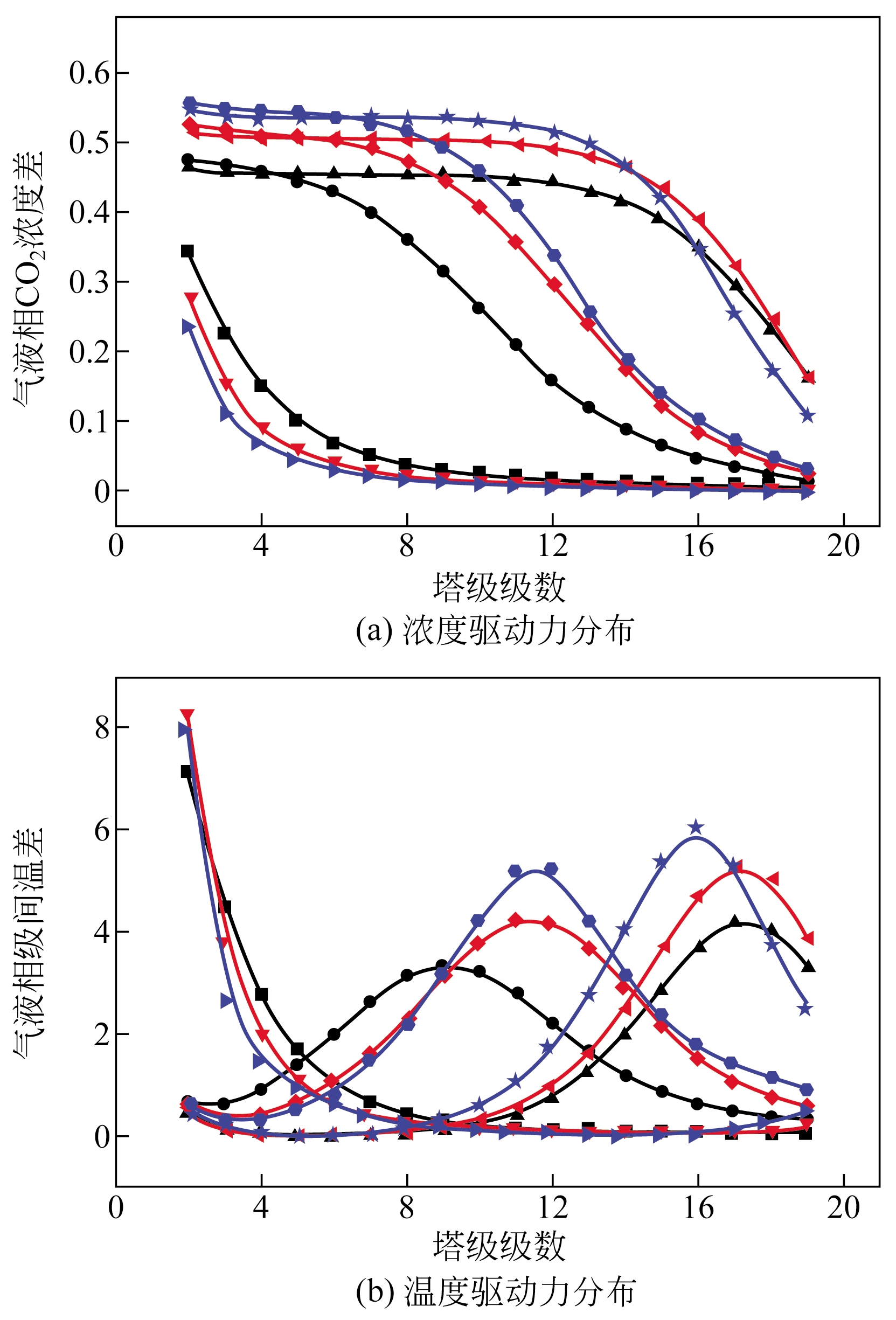
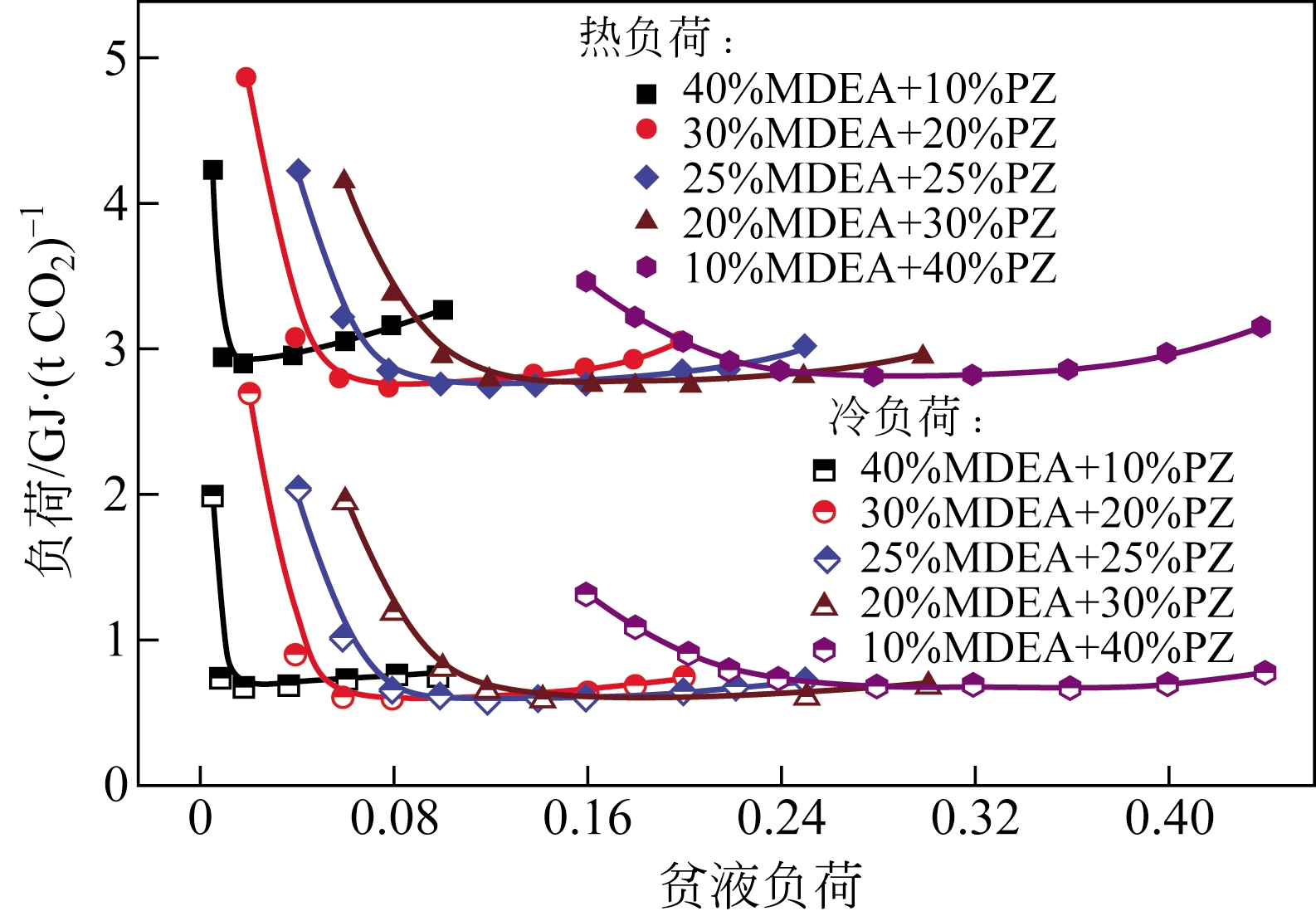
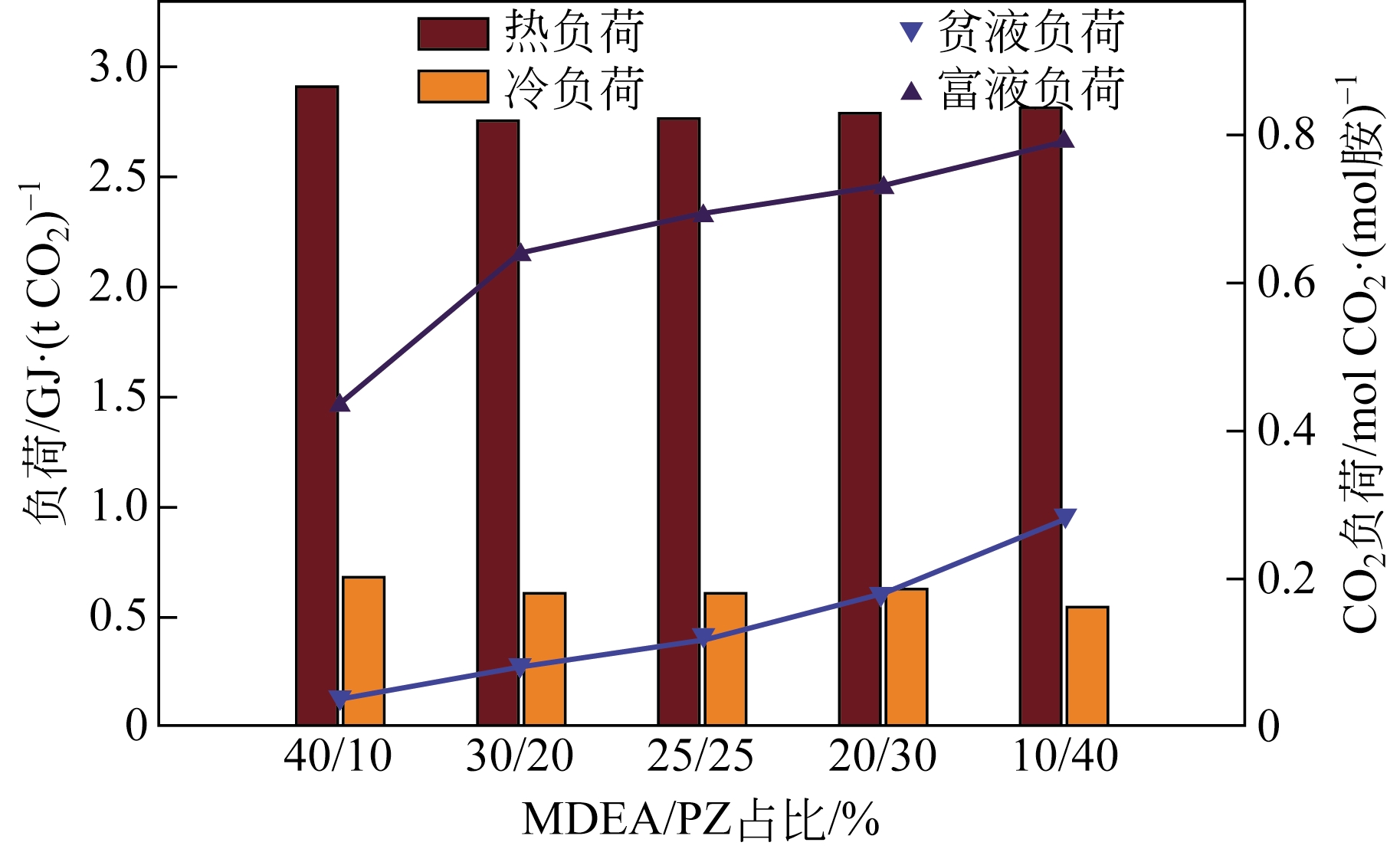
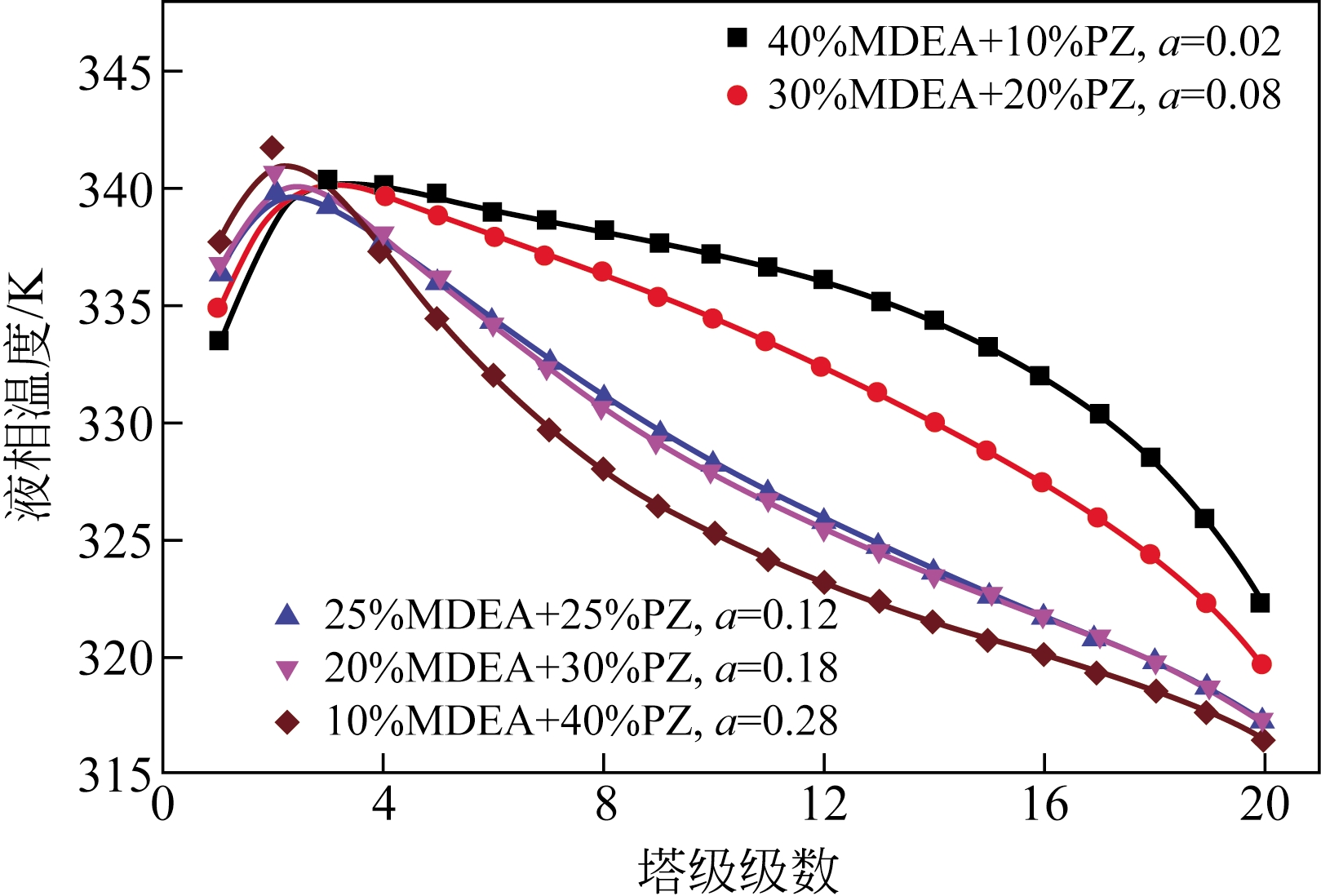
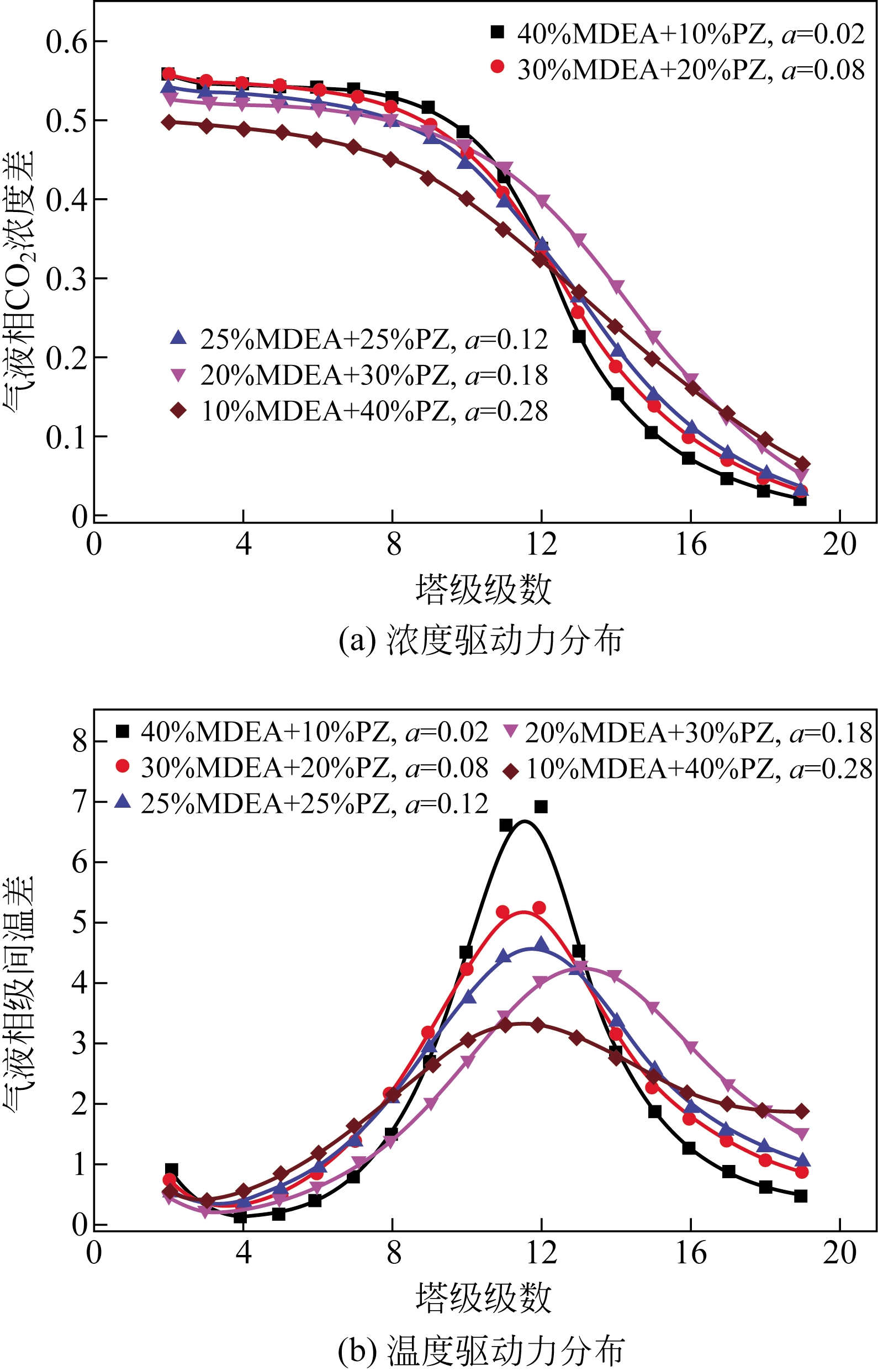
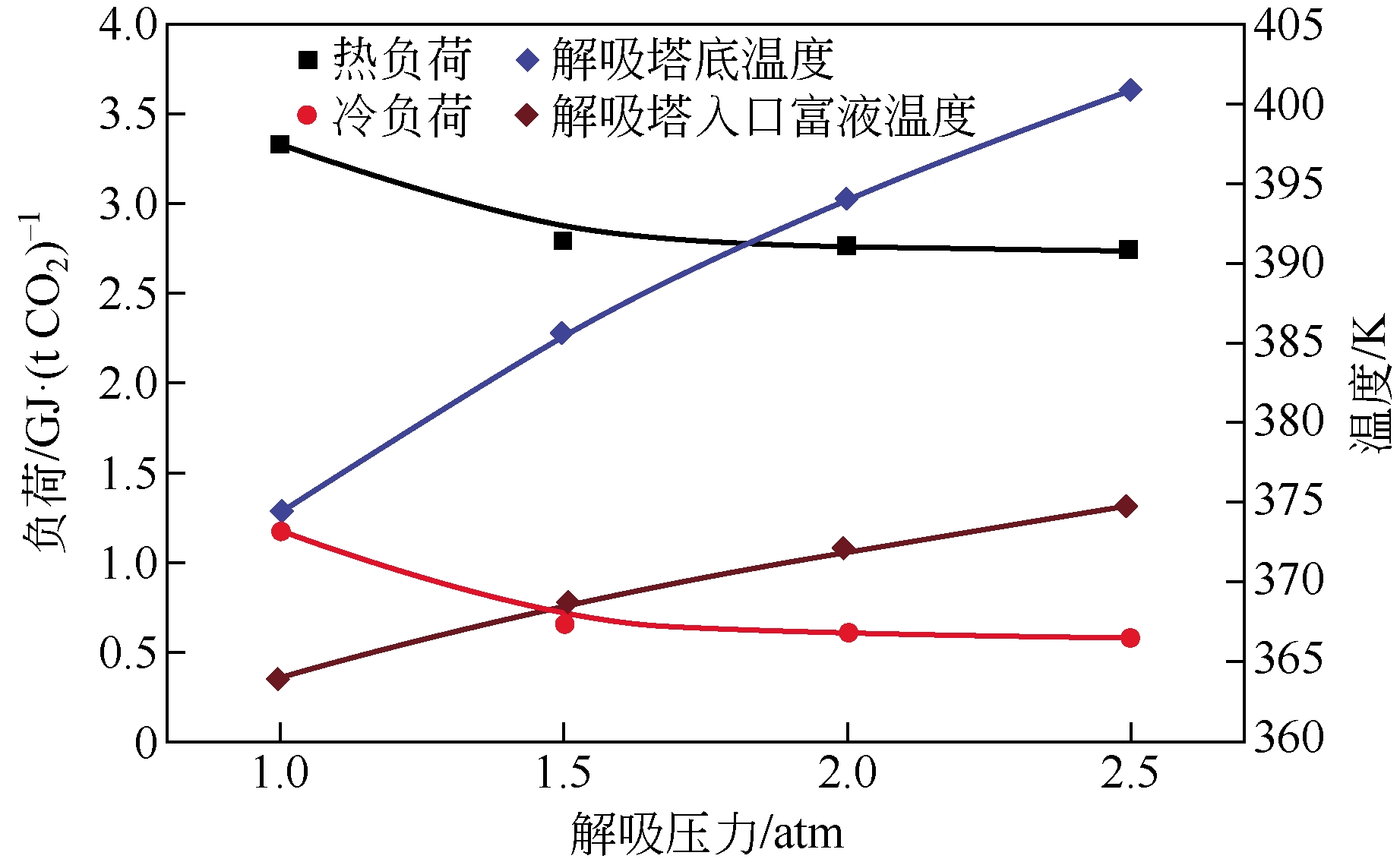
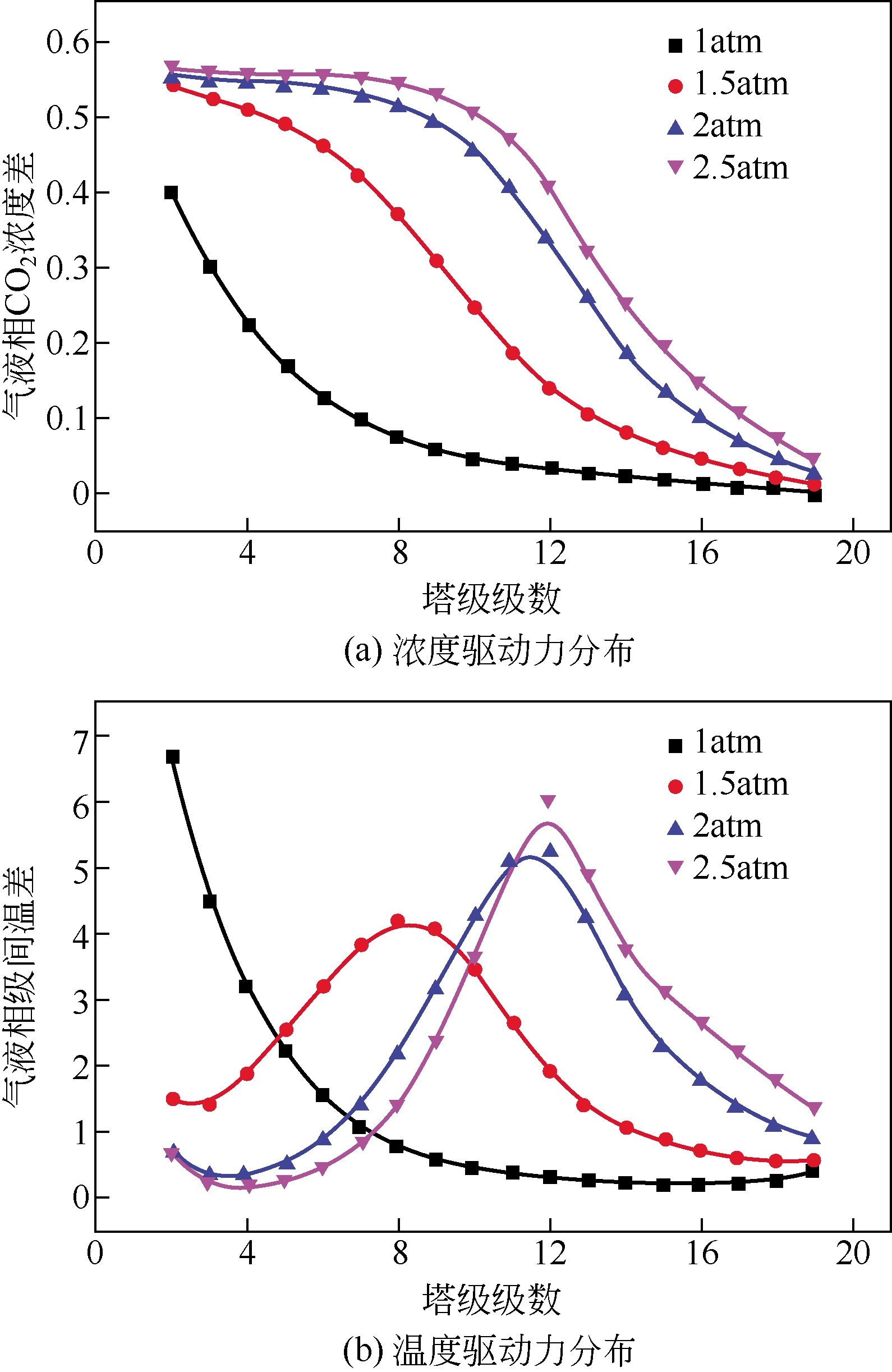
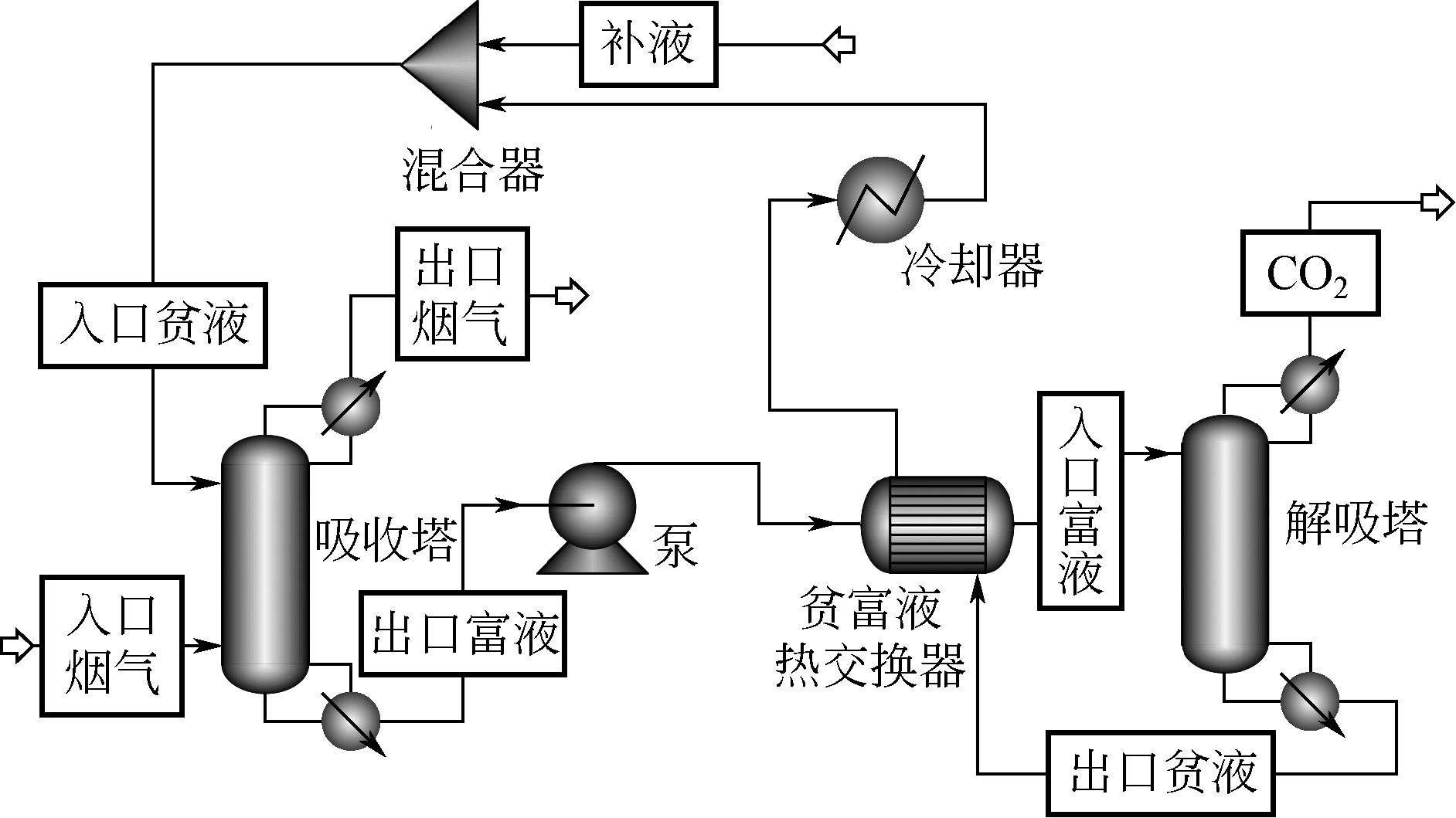
采用混合胺吸收剂替代传统一乙醇胺(MEA)吸收剂是降低有机胺法碳捕集工艺能耗的重要方法。利用Aspen plus软件模拟了以甲基二乙醇胺(MDEA)/哌嗪(PZ)混合胺为吸收剂的燃煤电厂每年百万吨CO2捕集工艺系统,考察了贫液负荷、MDEA/PZ混合胺浓度、MDEA/PZ比例和解吸压力等因素对解吸塔再沸器热负荷和冷凝器冷负荷的影响。通过对这些影响因素下吸收塔内液相温度分布和CO2负荷分布变化揭示了MDEA/PZ对CO2的吸收特性。此外,进一步分析了不同影响因素下解吸塔内气液相CO2浓度驱动力和气液相级间温度驱动力分布特性,发现了强浓度驱动力和低温度驱动力分布更有利于降低再生能耗。研究表明,由30%MDEA和20%PZ组成的混合胺液在贫液负荷为0.08和解吸压力为2.02×105Pa时,再沸器热负荷和塔顶冷凝负荷分别为2.76GJ/tCO2和0.60GJ/tCO2,相比传统MEA吸收剂降低了20.92%和40.0%。
中图分类号:
引用本文
林海周, 罗海中, 裴爱国, 方梦祥. 燃煤电厂烟气MDEA/PZ混合胺法碳捕集工艺模拟分析[J]. 化工进展, 2019, 38(04): 2046-2055.
Haizhou LIN, Haizhong LUO, Aiguo PEI, Mengxiang FANG. Simulation and analysis of carbon dioxide capture process using MDEA/PZ blend solution in a coal-fired power plant[J]. Chemical Industry and Engineering Progress, 2019, 38(04): 2046-2055.
| 序号 | 方程 | 指前因子 | 活化能 /J·mol?1 |
|---|---|---|---|
| 平衡反应 | |||
| (1) | 2H2O H3O++OH? H3O++OH? | — | — |
| (2) | HCO3 ? + H2O CO3 2?+H3O+ CO3 2?+H3O+ | — | — |
| (3) | PZH+ + H2O PZ+H3O+ PZ+H3O+ | — | — |
| (4) | H2O + H+PZCOO? H3O++PZCOO? H3O++PZCOO? | — | — |
| (5) | MDEAH+ + H2O MDEA+H3O+ MDEA+H3O+ | — | — |
| 动力学反应 | |||
| (6) | CO2 + OH?H CO3 ? CO3 ? | 1.33×1017 | 55458.99 |
| (7) | HCO3 ? CO2 + OH? CO2 + OH? | 6.63×1016 | 107393.5 |
| (8) | PZ + CO2 + H2O PZCOO?+H3O+ PZCOO?+H3O+ | 1.70×1010 | 1335.302 |
| (9) | PZCOO? + H3O+ PZ+CO2+H2O PZ+CO2+H2O | 3.40×1023 | 59272.34 |
| (10) | PZCOO? + CO2 + H2O | 1.04×1014 | 33647.52 |
| (11) | PZ(COO)2 2? + H3O+ PZCOO?+CO2+H2O PZCOO?+CO2+H2O | 3.20×1020 | 36383.84 |
| (12) | MDEA + CO2 + H2O MDEAH++HCO3 ? MDEAH++HCO3 ? | 6.85×1010 | 37794.49 |
| (13) | MDEAH+ + HCO3 ? MDEA+CO2+H2O MDEA+CO2+H2O | 6.62×1017 | 92638.15 |
表1 MDEA-PZ-CO2-H2O体系反应方程及参数
| 序号 | 方程 | 指前因子 | 活化能 /J·mol?1 |
|---|---|---|---|
| 平衡反应 | |||
| (1) | 2H2O H3O++OH? H3O++OH? | — | — |
| (2) | HCO3 ? + H2O CO3 2?+H3O+ CO3 2?+H3O+ | — | — |
| (3) | PZH+ + H2O PZ+H3O+ PZ+H3O+ | — | — |
| (4) | H2O + H+PZCOO? H3O++PZCOO? H3O++PZCOO? | — | — |
| (5) | MDEAH+ + H2O MDEA+H3O+ MDEA+H3O+ | — | — |
| 动力学反应 | |||
| (6) | CO2 + OH?H CO3 ? CO3 ? | 1.33×1017 | 55458.99 |
| (7) | HCO3 ? CO2 + OH? CO2 + OH? | 6.63×1016 | 107393.5 |
| (8) | PZ + CO2 + H2O PZCOO?+H3O+ PZCOO?+H3O+ | 1.70×1010 | 1335.302 |
| (9) | PZCOO? + H3O+ PZ+CO2+H2O PZ+CO2+H2O | 3.40×1023 | 59272.34 |
| (10) | PZCOO? + CO2 + H2O | 1.04×1014 | 33647.52 |
| (11) | PZ(COO)2 2? + H3O+ PZCOO?+CO2+H2O PZCOO?+CO2+H2O | 3.20×1020 | 36383.84 |
| (12) | MDEA + CO2 + H2O MDEAH++HCO3 ? MDEAH++HCO3 ? | 6.85×1010 | 37794.49 |
| (13) | MDEAH+ + HCO3 ? MDEA+CO2+H2O MDEA+CO2+H2O | 6.62×1017 | 92638.15 |
| 设备 | 直径/m | 塔高/m | 级数 | 填料 | 操作压力/atm |
|---|---|---|---|---|---|
| 吸收塔 | 16 | 25 | 20 | Mellapak 250Y | 1.0 |
| 解吸塔 | 10 | 8 | 20 | Mellapak 250Y | 2.0 |
表2 吸收塔和解吸塔初始参数
| 设备 | 直径/m | 塔高/m | 级数 | 填料 | 操作压力/atm |
|---|---|---|---|---|---|
| 吸收塔 | 16 | 25 | 20 | Mellapak 250Y | 1.0 |
| 解吸塔 | 10 | 8 | 20 | Mellapak 250Y | 2.0 |
| 1 | 中国电力企业联合会 . 中国煤电清洁发展报告[EB/OL]. [2017-09-22]. . |
| The China Electricity Council . The development of Chinese coal cleaning report [EB/OL]. [2017-09-22]. . | |
| 2 | 英国石油公司 . BP世界能源统计年鉴[EB/OL]. [2017-06-12]. . |
| BP . BP statistical review of world energy [EB/OL]. [2017-06-12]. . | |
| 3 | 国家发展与改革委员会 . 全国碳排放权交易市场建设方案(发电行业)[EB/OL]. [2017-12-18]. |
| The National Development and Reform Commission (NDRC) . The national carbon emissions trading market construction scheme (power generation industry) [EB/OL]. [2017-12-18]. . | |
| 4 | RUBIN E S , MANTRIPRAGADA H , MARKS A , et al . The outlook for improved carbon capture technology [J]. Progress in Energy and Combustion Scienc, 2012, 38(5): 630-671. |
| 5 | ROCHELLE G T . Amine scrubbing for CO2 capture [J]. Science, 2009, 325(5948): 1652-1654. |
| 6 | IDEM R , SUPAP T , SHI H , et al . Practical experience in post-combustion CO2 capture using reactive solvents in large pilot and demonstration plants[J]. International Journal of Greenhouse Gas Control, 2015, 40: 6-25. |
| 7 | LIANG Zhiwu , FU Kaiyun , IDEM R , et al . Review on current advances, future challenges and consideration issues for post-combustion CO2 capture using amine-based absorbents[J]. Chinese Journal of Chemical Engineering 2016, 24(2): 278-288. |
| 8 | LI Kangkang , LEIGH W , FERON P , et al . Systematic study of aqueous monoethanolamine (MEA)-based CO2 capture process: techno-economic assessment of the MEA process and its improvements[J]. Applied Energy, 2016, 165: 648-659. |
| 9 | 方梦祥, 周旭萍, 王涛, 等 . CO2化学吸收剂[J]. 化学进展, 2015, 27(12): 1808-1814. |
| FANG Mengxiang , ZHOU Xuping , WANG Tao , et al . Solvent development in CO2 chemical absorption [J]. Progress in Chemistry, 2015, 27(12): 1808-1814. | |
| 10 | 陈健, 罗伟亮, 李晗 . 有机胺吸收二氧化碳的热力学和动力学研究进展[J]. 化工学报, 2014, 65(1): 12-21. |
| CHEN Jian , LUO Weiliang , LI Han . A review for research on thermodynamics and kinetics of carbon dioxide absorption with organic amines[J]. CIESC Journal, 2014, 65(1): 12-21. | |
| 11 | CLOSMANN F , NGUYEN T , ROCHELLE G T . MDEA/piperazine as a solvent for CO2 capture[J]. Energy Procedia, 2009, 1(1): 1351-1357. |
| 12 | FRAILIE P T . Modeling of carbon dioxide absorption/stripping by aqueous methyldiethanolamine/piperazine[D]. Austin: The University of Texas at Austin, 2014. |
| 13 | SVENSSON H , HULTEBERG C , KARLSSON H T . Heat of absorption of CO2 in aqueous solutions of N-methyldiethanolamine and piperazine[J]. International Journal of Greenhouse Gas Control, 2013, 17: 89-98. |
| 14 | KHAN A A , HALDER G N , SAHA A K . Experimental investigation on efficient carbon dioxide capture using piperazine (PZ) activated aqueous methyldiethanolamine (MDEA) solution in a packed column [J]. International Journal of Greenhouse Gas Control, 2017, 64(s): 163-173. |
| 15 | HUANG Jicai , GONG Maoqiong , DONG Xueqiang , et al . CO2 solubility in aqueous solutions of N-methyldiethanolamine plus piperazine by electrolyte NRTL model[J]. Science China-Chemistry, 2016, 59(3): 360-369. |
| 16 | MOIOLI S , PELLEGRINI L A . Modeling the methyldiethanolamine-piperazine scrubbing system for CO2 removal: thermodynamic analysis [J]. Frontiers of Chemical Science and Engineering, 2016, 10(1): 162-175. |
| 17 | SAMANTA A , BANDYOPADHYAY S S . Absorption of carbon dioxide into piperazine activated aqueous N-methyldiethanolamine[J]. Chemical Engineering Journal, 2011, 171(3): 734-741. |
| 18 | SAIDI M . Rate-based modeling of CO2 absorption into piperazine-activated aqueous N-methyldiethanolamine solution: kinetic and mass transfer analysis[J]. International Journal of Chemical Kinetics, 2017, 49(9): 690-708. |
| 19 | TOBIESEN F A , SVENDSEN H F , MEJDELL T . Modeling of blast furnace CO2 capture using amine absorbents[J]. Industrial & Engineering Chemistry Research, 2007, 46(23): 7811-7819. |
| 20 | MUDHASAKUL S , KU H M, DOUGLAS P L . A simulation model of a CO2 absorption process with methyldiethanolamine solvent and piperazine as an activator[J]. International Journal of Greenhouse Gas Control, 2013, 15(s): 134-141. |
| 21 | ZHAO Bin , LIU Fangzheng , CUI Zheng , et al . Enhancing the energetic efficiency of MDEA/PZ-based CO2 capture technology for a 650 MW power plant: process improvement[J]. Applied Energy, 2017, 185: 362-375. |
| 22 | DUBOIS L , THOMAS D . Comparison of various configurations of the absorption-regeneration process using different solvents for the post-combustion CO2 capture applied to cement plant flue gases[J]. International Journal of Greenhouse Gas Control, 2018, 69: 20-35. |
| 23 | 李晗, 陈健 . 单乙醇胺吸收CO2的热力学模型和过程模拟[J]. 化工学报, 2014, 65(1): 47-54. |
| LI Han , CHEN Jian . Thermodynamic modeling and process simulation for CO2 absorption into aqueous monoethanolamine solution [J]. CIESC Journal, 2014, 65(1): 47-54. | |
| 24 | 张克舫, 刘中良, 王远亚, 等 . 化学吸收法CO2捕集解吸能耗的分析计算[J]. 化工进展, 2013, 32(12): 3008-3014. |
| ZHANG Kefang , LIU Zhongliang , WANG Yuanya , et al . Analysis and calculation of the desorption energy consumption of CO2 capture process by chemical absorption method[J]. Chemical Industry and Engineering Progress, 2013, 32(12): 3008-3014. | |
| 25 | 张亚萍, 刘建周, 季芹芹, 等 . 醇胺法捕集燃煤烟气CO2工艺模拟及优化[J]. 化工进展, 2013, 32(4): 930-935. |
| ZHANG Yaping , LIU Jianzhou , JI Qinqin , et al . Process simulation and optimization of flue gas CO2 capture by the alkanolamine solutions [J]. Chemical Industry and Engineering Progress, 2013, 32(4): 930-935. | |
| 26 | WANG Tao , HE Hui , YU Wei , et al . Process simulations of CO2 desorption in the interaction between the novel direct steam stripping process and solvents[J]. Energy & Fuels, 2017, 31(4): 4255-4262. |
| 27 | Aspen Technology Inc . ENRTL-RK rate-based model of the CO2 capture process by mixed PZ and MDEA using Aspen plus[EB/OL]. [2018-02-10]. . |
| 28 | DAMARTZIS T , PAPADOPOULOS A I , SEFERLIS P . Process flowsheet design optimization for various amine-based solvents in post-combustion CO2 capture plants[J]. Journal of Cleaner Production, 2016, 111: 204-216. |
| 29 | BEK-PEDERSEN E , GANI R . Design and synthesis of distillation systems using a driving-force-based approach[J]. Chemical Engineering & Processing Process Intensification, 2004, 43(3): 251-262. |
| 30 | REZAZADEH F , GALE W F , LIN Y J , et al . Energy performance of advanced reboiled and flash stripper configurations for CO2 capture using monoethanolamine[J]. Industrial & Engineering Chemistry Research, 2016, 55(16): 4622-4631. |
| 31 | LI Kangkang , COUSINS A , YU Hai , et al . Systematic study of aqueous monoethanolamine-based CO2 capture process: model development and process improvement[J]. Energy Science & Engineering, 2016, 4(1): 23-39. |
| 32 | FAN Zhen , LIU Kun , QI Guojie , et al . Aspen modeling for MEA-CO2 loop: dynamic gridding for accurate column profile [J]. International Journal of Greenhouse Gas Control, 2015, 37: 318-324. |
| 33 | YING Jiru , RAETS S , EIMER D . The activator mechanism of piperazine in aqueous methyldiethanolamine solutions[J]. Energy Procedia, 2017, 114: 2078-2087. |
| 34 | ZHANG W , CHEN J , LUO X , et al . Modelling and process analysis of post-combustion carbon capture with the blend of 2-amino-2-methyl-1-propanol and piperazine[J]. International Journal of Greenhouse Gas Control, 2017, 63: 37-46. |
| 35 | KHALILPOUR R . Flexible operation scheduling of a power plant integrated with PCC processes under market dynamics[J]. Industrial & Engineering Chemistry Research, 2014, 53(19): 8132-8146. |
| [1] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [2] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [3] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| [4] | 顾诗亚, 董亚超, 刘琳琳, 张磊, 庄钰, 都健. 考虑中间节点的碳捕集管路系统设计与优化[J]. 化工进展, 2023, 42(6): 2799-2808. |
| [5] | 符乐, 杨阳, 徐文青, 耿錾卜, 朱廷钰, 郝润龙. 新型相变有机胺吸收捕集CO2技术研究进展[J]. 化工进展, 2023, 42(4): 2068-2080. |
| [6] | 夏少波, 段璐, 王建朋, 纪任山. 飞灰含湿量对耦合电袋除尘器性能影响规律[J]. 化工进展, 2023, 42(4): 2101-2108. |
| [7] | 尚玉, 肖满, 崔秋芳, 涂特, 晏水平. CO2捕集工艺中热再生气余热的PVDF/BN-OH平板复合膜回收特性[J]. 化工进展, 2023, 42(3): 1618-1628. |
| [8] | 沈天绪, 沈来宏. 基于3kW塔式串行流化床差异燃料的化学链燃烧解析[J]. 化工进展, 2023, 42(1): 138-147. |
| [9] | 王璐, 张磊, 都健. 机器学习高效筛选用于CO2/N2选择性吸附分离的沸石材料[J]. 化工进展, 2023, 42(1): 148-158. |
| [10] | 王一茹, 宋小三, 水博阳, 王三反. 胺功能化介孔二氧化硅捕集CO2的研究进展[J]. 化工进展, 2022, 41(S1): 536-544. |
| [11] | 周红军, 周颖, 徐春明. 中国碳达峰碳中和目标下炼化一体化新路径与实践[J]. 化工进展, 2022, 41(4): 2226-2230. |
| [12] | 张卫风, 周武, 王秋华. 相变吸收捕集烟气中CO2技术的发展现状[J]. 化工进展, 2022, 41(4): 2090-2101. |
| [13] | 郑鹏, 李蔚玲, 郭亚飞, 孙健, 王瑞林, 赵传文. 鼓泡床中电石渣加速碳酸化分析与响应面优化[J]. 化工进展, 2022, 41(3): 1528-1538. |
| [14] | 孔祥宇, 谢亮, 王延民, 翟尚鹏, 王建国. CO2的捕集及资源化利用[J]. 化工进展, 2022, 41(3): 1187-1198. |
| [15] | 唐思扬, 李星宇, 鲁厚芳, 钟山, 梁斌. 低能耗化学吸收碳捕集技术展望[J]. 化工进展, 2022, 41(3): 1102-1106. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 PZ(COO)2 2? + H3O+
PZ(COO)2 2? + H3O+