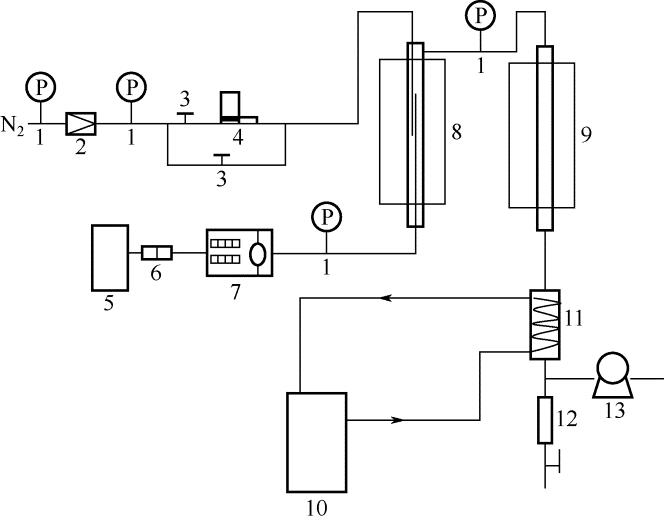
化工进展 ›› 2019, Vol. 38 ›› Issue (04): 1758-1767.DOI: 10.16085/j.issn.1000-6613.2018-1153
介孔结构和助剂Zn对不同晶粒大小ZSM-5催化甲醇制芳烃反应性能的影响
张春梅( ),付廷俊(
),付廷俊( ),邵娟,马哲,王玉杰,马倩,崔丽萍,李忠(
),邵娟,马哲,王玉杰,马倩,崔丽萍,李忠( )
)
- 太原理工大学煤科学与技术教育部和山西省重点实验室,山西 太原 030024
-
收稿日期:2018-06-01修回日期:2018-09-20出版日期:2019-04-05发布日期:2019-04-05 -
通讯作者:付廷俊,李忠 -
作者简介:张春梅(1993—),女,硕士研究生,研究方向为碳一化学。E-mail:<email>1007331442@qq.com</email>。|付廷俊,副教授,硕士生导师,研究方向为碳一化学。E-mail:<email>futingjun@tyut.edu.cn</email>|李忠,教授,博士生导师,研究方向为碳一化学。E-mail:<email>lizhong@tyut.edu.cn</email> -
基金资助:国家重点研发计划(2018YFB0604901);国家自然科学基金(21606160,21878207);山西省自然科学基金(201701D221039,201701D121025)
Effects of mesoporous structure and Zn promoter on methanol to aromatics performance over different crystal sized ZSM-5 catalysts
Chunmei ZHANG( ),Tingjun FU(
),Tingjun FU( ),Juan SHAO,Zhe MA,Yujie WANG,Qian MA,Liping CUI,Zhong LI(
),Juan SHAO,Zhe MA,Yujie WANG,Qian MA,Liping CUI,Zhong LI( )
)
- Key Laboratory of Coal Science and Technology, Ministry of Education and Shanxi Province, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
-
Received:2018-06-01Revised:2018-09-20Online:2019-04-05Published:2019-04-05 -
Contact:Tingjun FU,Zhong LI
摘要:
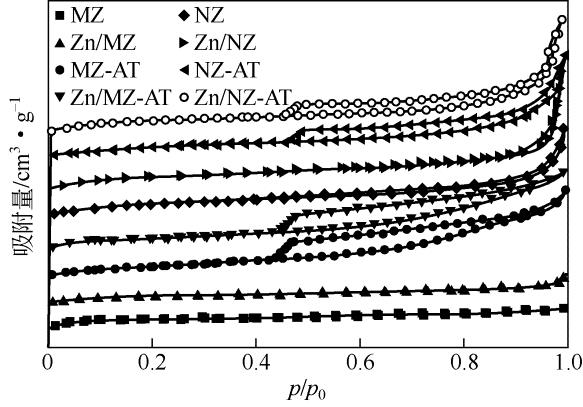
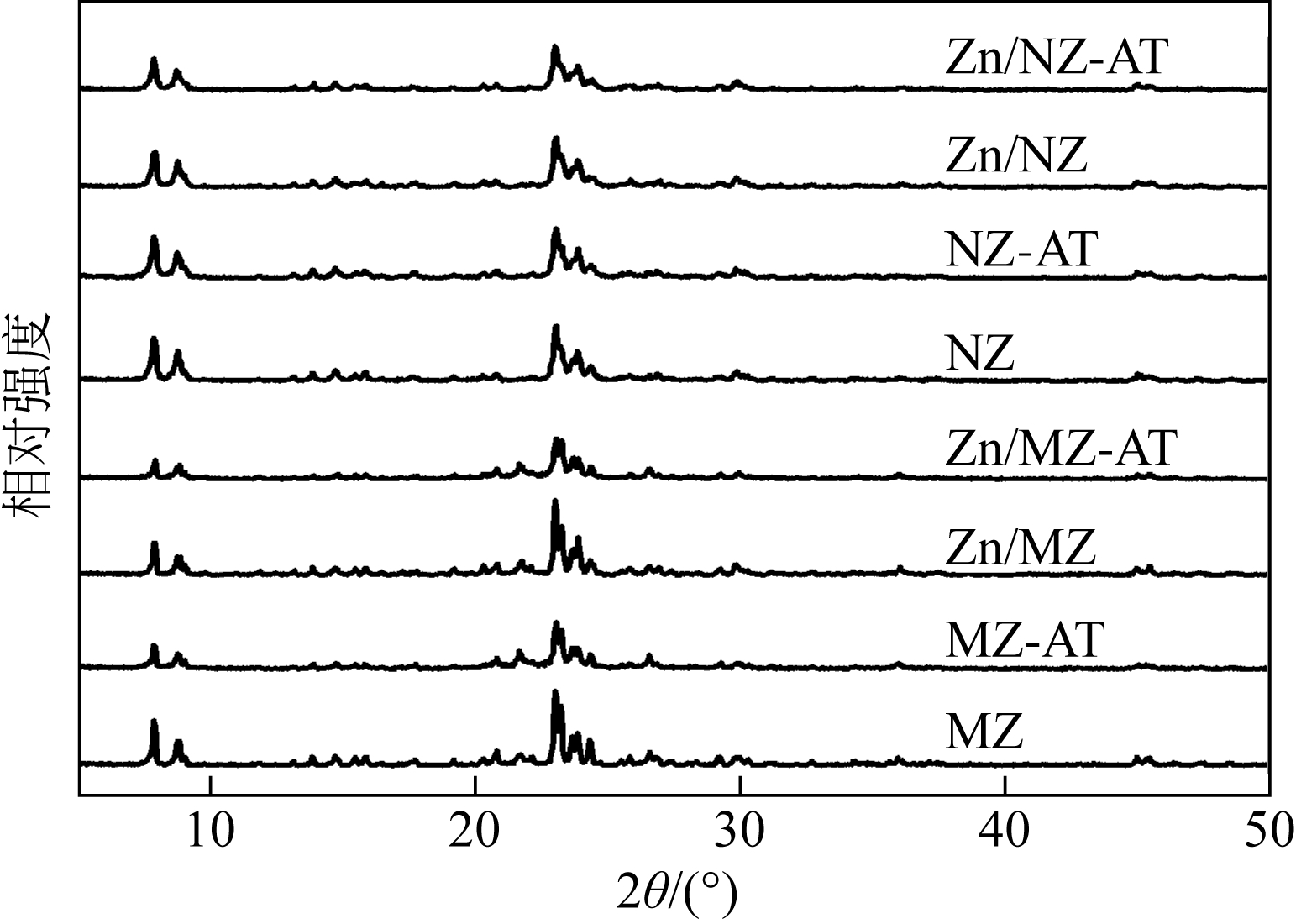
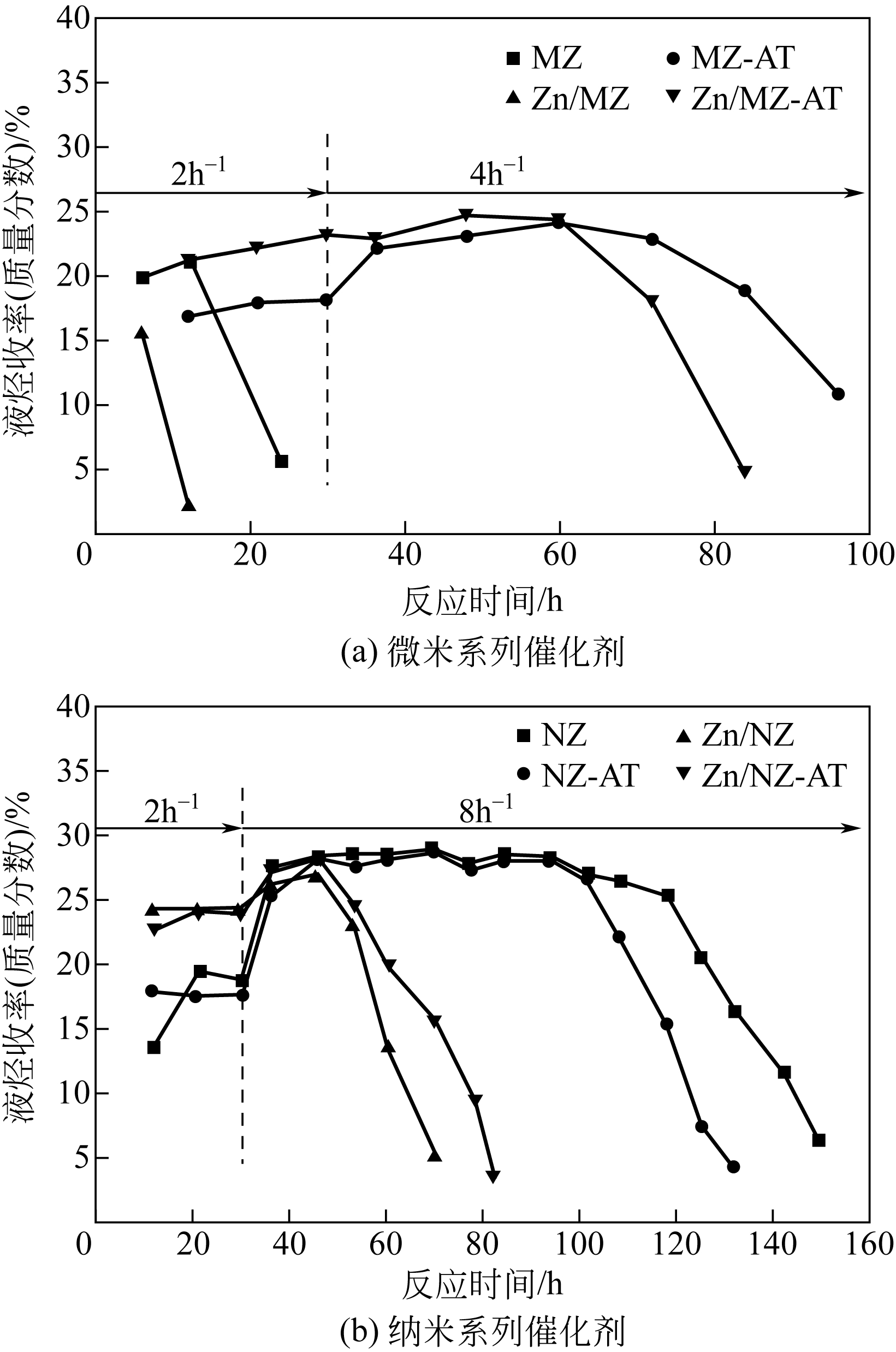
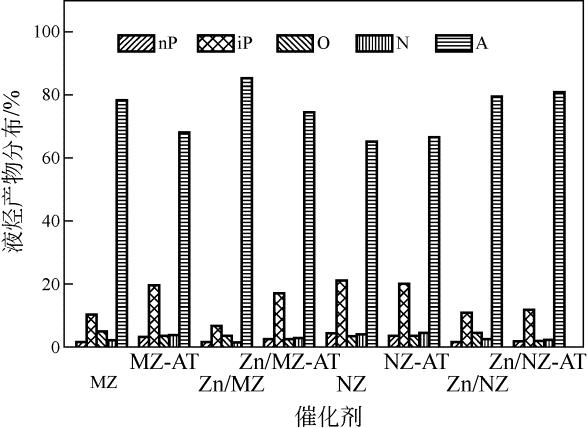
通过碱处理和添加助剂Zn对微米ZSM-5和纳米ZSM-5进行改性,获得具有不同孔结构和酸性质的催化剂。采用氮气吸附、X射线衍射、透射电镜、氨气程序升温脱附(NH3-TPD)和热重(TG)技术对不同催化剂进行表征,结合催化性能评价,考察晶粒尺寸、介孔结构和Zn助剂对其催化甲醇制芳烃(MTA)反应性能的影响。结果表明,碱处理引入介孔后,孔体积均增大,总酸量都降低;微米催化剂外表面积显著增加,但纳米催化剂外表面积却有所下降;负载金属Zn后,比表面积、结晶度和总酸量都降低。在P=0.5MPa、T=430℃、WHSV=2h-1的反应条件下,负载Zn的微米催化剂由于具有较高的酸量,其芳烃与苯、甲苯和二甲苯(BTX)选择性最高,分别为85.11%和66.85%,但是稳定性较差,催化寿命仅为12h。但相较于未改性的纳米ZSM-5原粉来说,碱处理后又负载Zn的催化剂,液烃中芳烃选择性从纳米原粉的65.20%增加到80.82%,BTX选择性从纳米原粉的42.30%提高到49.56%,而在甲醇进样量增加4倍,即WHSV=8h-1时,催化剂仍显示出较好的稳定性,寿命可达84h。可见,在小晶粒ZSM-5上碱处理扩孔并引入Zn助剂可以有效提高甲醇制芳烃反应性能。
中图分类号:
引用本文
张春梅, 付廷俊, 邵娟, 马哲, 王玉杰, 马倩, 崔丽萍, 李忠. 介孔结构和助剂Zn对不同晶粒大小ZSM-5催化甲醇制芳烃反应性能的影响[J]. 化工进展, 2019, 38(04): 1758-1767.
Chunmei ZHANG, Tingjun FU, Juan SHAO, Zhe MA, Yujie WANG, Qian MA, Liping CUI, Zhong LI. Effects of mesoporous structure and Zn promoter on methanol to aromatics performance over different crystal sized ZSM-5 catalysts[J]. Chemical Industry and Engineering Progress, 2019, 38(04): 1758-1767.
| 催化剂 | S BET ① /m2·g-1 | S mic ② /m2·g-1 | S ext ② /m2·g-1 | V total ③ /cm3·g-1 | V mic ② /cm3·g-1 | V meso ④ /cm3·g-1 |
|---|---|---|---|---|---|---|
| MZ | 350 | 302 | 48 | 0.21 | 0.12 | 0.09 |
| MZ-AT | 355 | 176 | 179 | 0.50 | 0.09 | 0.41 |
| Zn/MZ | 318 | 271 | 47 | 0.23 | 0.12 | 0.11 |
| Zn/MZ-AT | 332 | 165 | 167 | 0.45 | 0.08 | 0.37 |
| NZ | 402 | 282 | 120 | 0.51 | 0.13 | 0.38 |
| NZ-AT | 377 | 271 | 106 | 0.60 | 0.13 | 0.47 |
| Zn/NZ | 365 | 239 | 126 | 0.59 | 0.12 | 0.47 |
| Zn/NZ-AT | 349 | 240 | 109 | 0.62 | 0.12 | 0.50 |
表1 不同ZSM-5催化剂的织构性质
| 催化剂 | S BET ① /m2·g-1 | S mic ② /m2·g-1 | S ext ② /m2·g-1 | V total ③ /cm3·g-1 | V mic ② /cm3·g-1 | V meso ④ /cm3·g-1 |
|---|---|---|---|---|---|---|
| MZ | 350 | 302 | 48 | 0.21 | 0.12 | 0.09 |
| MZ-AT | 355 | 176 | 179 | 0.50 | 0.09 | 0.41 |
| Zn/MZ | 318 | 271 | 47 | 0.23 | 0.12 | 0.11 |
| Zn/MZ-AT | 332 | 165 | 167 | 0.45 | 0.08 | 0.37 |
| NZ | 402 | 282 | 120 | 0.51 | 0.13 | 0.38 |
| NZ-AT | 377 | 271 | 106 | 0.60 | 0.13 | 0.47 |
| Zn/NZ | 365 | 239 | 126 | 0.59 | 0.12 | 0.47 |
| Zn/NZ-AT | 349 | 240 | 109 | 0.62 | 0.12 | 0.50 |
| 催化剂 | 结晶度/% | 催化剂 | 结晶度/% |
|---|---|---|---|
| MZ | 100 | NZ | 100 |
| MZ-AT | 90 | NZ-AT | 98 |
| Zn/MZ | 96 | Zn/NZ | 94 |
| Zn/MZ-AT | 86 | Zn/NZ-AT | 86 |
表2 不同ZSM-5催化剂的相对结晶度
| 催化剂 | 结晶度/% | 催化剂 | 结晶度/% |
|---|---|---|---|
| MZ | 100 | NZ | 100 |
| MZ-AT | 90 | NZ-AT | 98 |
| Zn/MZ | 96 | Zn/NZ | 94 |
| Zn/MZ-AT | 86 | Zn/NZ-AT | 86 |
| 催化剂 | 酸量①/mmol·g-1 | |||
|---|---|---|---|---|
| 总量 | 弱酸 | 中强酸 | 强酸 | |
| MZ | 1.21 | 0.48 | 0.26 | 0.47 |
| MZ-AT | 0.91 | 0.41 | 0.28 | 0.22 |
| Zn/MZ | 0.92 | 0.37 | 0.24 | 0.31 |
| Zn/MZ-AT | 0.85 | 0.35 | 0.34 | 0.16 |
| NZ | 0.76 | 0.24 | 0.19 | 0.33 |
| NZ-AT | 0.74 | 0.24 | 0.19 | 0.31 |
| Zn/NZ | 0.66 | 0.25 | 0.22 | 0.19 |
| Zn/NZ-AT | 0.61 | 0.23 | 0.21 | 0.17 |
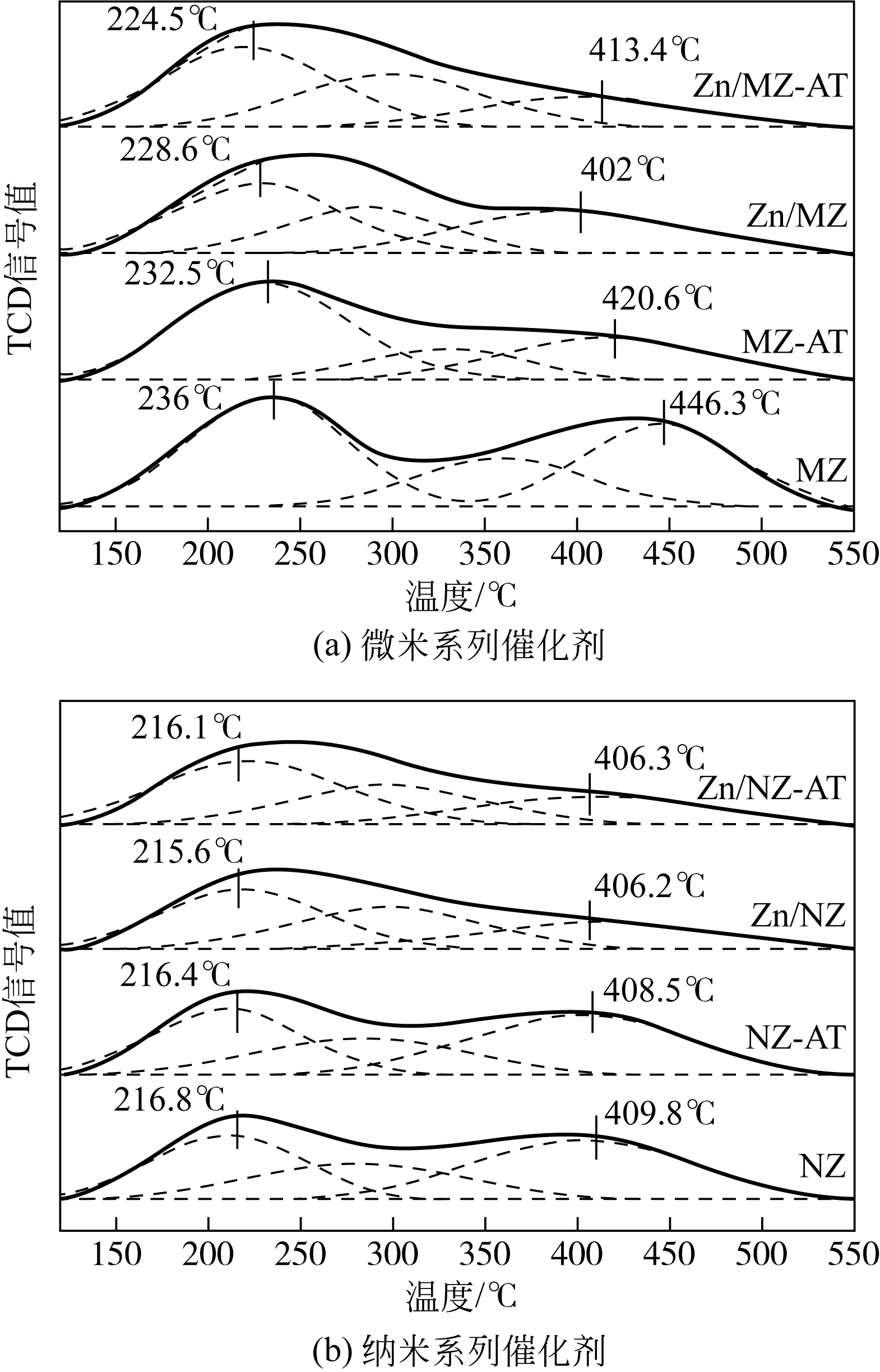
表3 不同ZSM-5催化剂的酸性质
| 催化剂 | 酸量①/mmol·g-1 | |||
|---|---|---|---|---|
| 总量 | 弱酸 | 中强酸 | 强酸 | |
| MZ | 1.21 | 0.48 | 0.26 | 0.47 |
| MZ-AT | 0.91 | 0.41 | 0.28 | 0.22 |
| Zn/MZ | 0.92 | 0.37 | 0.24 | 0.31 |
| Zn/MZ-AT | 0.85 | 0.35 | 0.34 | 0.16 |
| NZ | 0.76 | 0.24 | 0.19 | 0.33 |
| NZ-AT | 0.74 | 0.24 | 0.19 | 0.31 |
| Zn/NZ | 0.66 | 0.25 | 0.22 | 0.19 |
| Zn/NZ-AT | 0.61 | 0.23 | 0.21 | 0.17 |
| 催化剂 | 液烃 | 积炭容量 /g·g-1 cat | 积炭速率 /g·g-1·h-1 | ||
|---|---|---|---|---|---|
| 液烃产能 /g·g-1 cat | 最高液烃收率 /% | 寿命 /h | |||
| MZ | 6.2 | 19.8 | 20 | 0.11 | 5.3×10-3 |
| MZ-AT | 62.8 | 24.0 | 96 | 0.35 | 3.6×10-3 |
| Zn/MZ | 1.9 | 15.6 | 12 | 0.07 | 6.0×10-3 |
| Zn/MZ-AT | 52.0 | 24.3 | 84 | 0.29 | 3.4×10-3 |
| NZ | 237.4 | 28.9 | 149 | 0.32 | 2.2×10-3 |
| NZ-AT | 197.2 | 28.7 | 132 | 0.29 | 2.2×10-3 |
| Zn/NZ | 65.7 | 25.4 | 70 | 0.22 | 3.1×10-3 |
| Zn/NZ-AT | 93.7 | 28.0 | 84 | 0.28 | 3.3×10-3 |
表4 不同ZSM-5催化剂在MTA反应中的催化性能
| 催化剂 | 液烃 | 积炭容量 /g·g-1 cat | 积炭速率 /g·g-1·h-1 | ||
|---|---|---|---|---|---|
| 液烃产能 /g·g-1 cat | 最高液烃收率 /% | 寿命 /h | |||
| MZ | 6.2 | 19.8 | 20 | 0.11 | 5.3×10-3 |
| MZ-AT | 62.8 | 24.0 | 96 | 0.35 | 3.6×10-3 |
| Zn/MZ | 1.9 | 15.6 | 12 | 0.07 | 6.0×10-3 |
| Zn/MZ-AT | 52.0 | 24.3 | 84 | 0.29 | 3.4×10-3 |
| NZ | 237.4 | 28.9 | 149 | 0.32 | 2.2×10-3 |
| NZ-AT | 197.2 | 28.7 | 132 | 0.29 | 2.2×10-3 |
| Zn/NZ | 65.7 | 25.4 | 70 | 0.22 | 3.1×10-3 |
| Zn/NZ-AT | 93.7 | 28.0 | 84 | 0.28 | 3.3×10-3 |
| 催化剂 | S BET ① /m2·g-1 | S mic ② /m2·g-1 | S ext ② /m2·g-1 | V total ③ /cm3·g-1 | V mic ② /cm3·g-1 | V meso ④ /cm3·g-1 |
|---|---|---|---|---|---|---|
| MZ | 14 | 0 | 14 | 0.06 | 0 | 0.06 |
| MZ-AT | 48 | 6 | 42 | 0.14 | 0 | 0.14 |
| Zn/MZ | 4 | 0 | 4 | 0.05 | 0 | 0.05 |
| Zn/MZ-AT | 24 | 0 | 24 | 0.09 | 0 | 0.09 |
| NZ | 189 | 119 | 70 | 0.37 | 0.05 | 0.32 |
| NZ-AT | 199 | 129 | 70 | 0.40 | 0.06 | 0.34 |
| Zn/NZ | 180 | 112 | 68 | 0.37 | 0.05 | 0.32 |
| Zn/NZ-AT | 145 | 79 | 66 | 0.34 | 0.06 | 0.28 |
表5 不同ZSM-5催化剂失活后的织构性质
| 催化剂 | S BET ① /m2·g-1 | S mic ② /m2·g-1 | S ext ② /m2·g-1 | V total ③ /cm3·g-1 | V mic ② /cm3·g-1 | V meso ④ /cm3·g-1 |
|---|---|---|---|---|---|---|
| MZ | 14 | 0 | 14 | 0.06 | 0 | 0.06 |
| MZ-AT | 48 | 6 | 42 | 0.14 | 0 | 0.14 |
| Zn/MZ | 4 | 0 | 4 | 0.05 | 0 | 0.05 |
| Zn/MZ-AT | 24 | 0 | 24 | 0.09 | 0 | 0.09 |
| NZ | 189 | 119 | 70 | 0.37 | 0.05 | 0.32 |
| NZ-AT | 199 | 129 | 70 | 0.40 | 0.06 | 0.34 |
| Zn/NZ | 180 | 112 | 68 | 0.37 | 0.05 | 0.32 |
| Zn/NZ-AT | 145 | 79 | 66 | 0.34 | 0.06 | 0.28 |
| 催化剂 | 苯/% | 甲苯/% | 二甲苯/% | BTX/% |
|---|---|---|---|---|
| MZ | 3.12 | 18.43 | 36.06 | 57.61 |
| MZ-AT | 2.02 | 12.71 | 25.49 | 40.22 |
| Zn/MZ | 3.32 | 25.75 | 37.78 | 66.85 |
| Zn/MZ-AT | 2.01 | 17.81 | 28.81 | 48.63 |
| NZ | 2.66 | 14.68 | 24.96 | 42.30 |
| NZ-AT | 2.46 | 14.25 | 24.30 | 41.01 |
| Zn/NZ | 2.16 | 18.53 | 30.18 | 50.87 |
| Zn/NZ-AT | 2.10 | 18.00 | 29.46 | 49.56 |
表6 不同ZSM-5催化剂液烃中BTX的产物分布(质量分数)
| 催化剂 | 苯/% | 甲苯/% | 二甲苯/% | BTX/% |
|---|---|---|---|---|
| MZ | 3.12 | 18.43 | 36.06 | 57.61 |
| MZ-AT | 2.02 | 12.71 | 25.49 | 40.22 |
| Zn/MZ | 3.32 | 25.75 | 37.78 | 66.85 |
| Zn/MZ-AT | 2.01 | 17.81 | 28.81 | 48.63 |
| NZ | 2.66 | 14.68 | 24.96 | 42.30 |
| NZ-AT | 2.46 | 14.25 | 24.30 | 41.01 |
| Zn/NZ | 2.16 | 18.53 | 30.18 | 50.87 |
| Zn/NZ-AT | 2.10 | 18.00 | 29.46 | 49.56 |
| 1 | 汪哲明, 陈希强, 许烽, 等 . 甲醇制芳烃催化剂研究进展[J]. 化工进展, 2016, 35(5): 1433-1439. |
| WANG Z M , CHEN X Q , XU F , et al . Advance in the research and development of methanol to aromatic catalysts[J]. Chemical Industry and Engineering Progress, 2016, 35(5): 1433-1439. | |
| 2 | 陈庆龄, 杨为民, 滕加伟, 等 . 中国石化煤化工技术最新进展[J]. 催化学报, 2013, 34(1): 217-224. |
| CHEN Q L , YANG W M , TENG J W , et al . Recent advances in coal to chemicals technology developed by Sinopec[J]. Chinese Journal of Catalysis, 2013, 34(1): 217-224. | |
| 3 | ZHANG G Q , TING B , CHEN T F , et al . Conversion of methanol to light aromatics on Zn-modified nano-HZSM-5 zeolite catalysts[J]. Industrial & Engineering Chemistry Research, 2014, 53(39): 14932-14940. |
| 4 | FEI W , WEI Y X , GUO M X . Atomic layer deposition of zinc oxide on HZSM-5 template and its methanol aromatization performance[J]. Catalysis Letters, 2015, 145(3): 860-867. |
| 5 | LIANG T G , CHEN J L , QIN Z F , et al . Conversion of methanol to olefins over H-ZSM-5 zeolite: reaction pathway is related to the framework aluminum siting[J]. ACS Catalysis, 2016, 6(11): 7311-7325. |
| 6 | LOSCH P , PINAR A B , WILLINGER M G , et al . H-ZSM-5 zeolite model crystals: structure-diffusion-activity relationship in methanol-to-olefins catalysis[J]. Journal of Catalysis, 2017, 345: 11-23. |
| 7 | 潘红艳, 田敏, 何志艳, 等 . 甲醇制烯烃用ZSM-5分子筛的研究进展[J]. 化工进展, 2014, 33(10): 2625-2633. |
| PAN H Y , TIAN M , HE Z Y , et al . Advances in research on modified ZSM-5 molecular sieves for conversion of methanol to olefins[J]. Chemical Industry and Engineering Progress, 2014, 33(10): 2625-2633. | |
| 8 | FIROOZI M , BAGHALHA M , ASADI M . The effect of micro and nano particle sizes of H-ZSM-5 on the selectivity of MTP reaction[J]. Catalysis Communications, 2009, 10(12): 1582-1585. |
| 9 | NIU X J , GAO J , WANG K , et al . Influence of crystal size on the catalytic performance of H-ZSM-5 and Zn/H-ZSM-5 in the conversion of methanol to aromatics[J]. Fuel Processing Technology, 2017, 157: 99-107. |
| 10 | SHAO J , FU T J , CHANG J W , et al . Effect of ZSM-5 crystal size on its catalytic properties for conversion of methanol to gasoline[J]. Journal of Fuel Chemistry and Technology, 2017, 45(1): 75-83. |
| 11 | XING L Y , WEI Z H , WEN Z H , et al . Catalytic study for methanol aromatization over hierarchical ZSM-5 zeolite synthesized by kaolin[J]. Petroleum Science & Technology, 2017, 35(24): 2235-2240. |
| 12 | FENG W , GAO X F , DING C M , et al . Effect of weak base modification on ZSM-5 catalyst for methanol to aromatics[J]. Applied Organometallic Chemistry, 2017, 31(6): 3625-3631. |
| 13 | QI R Y , FU T J , WAN W L , et al . Pore fabrication of nano-ZSM-5 zeolite by internal desilication and its influence on the methanol to hydrocarbon reaction[J]. Fuel Processing Technology, 2016, 155: 191-199. |
| 14 | INOUE Y , NAKASHIRO K , ONO Y . Selective conversion of methanol into aromatic hydrocarbons over silver-exchanged ZSM-5 zeolites[J]. Microporous Materials, 1995, 4(5): 379-383. |
| 15 | TIAN T , QIAN W Z , TANG X P , et al . Deactivation of Ag/ZSM-5 catalyst in the aromatization of methanol[J]. Acta Physico-Chimica Sinica, 2010, 26(12): 3305-3309. |
| 16 | LOPEZ-SANCHEZ J A , CONTE M , LANDON P , et al . Reactivity of Ga2O3 clusters on zeolite ZSM-5 for the conversion of methanol to aromatics[J]. Catalysis Letters, 2012, 142(9): 1049-1056. |
| 17 | AL-YASSIR N , AKHTAR M N , OGUNRONBI K , et al . Synthesis of stable H-galloaluminosilicate MFI with hierarchical pore architecture by surfactant-mediated base hydrolysis, and their application in propane aromatization[J]. Journal of Molecular Catalysis A: Chemical, 2012, 360: 1-15. |
| 18 | MENTZEL U V , HOJHOLT K T , HOLM M S , et al . Conversion of methanol to hydrocarbons over conventional and mesoporous H-ZSM-5 and H-Ga-MFI: major differences in deactivation behavior[J]. Applied Catalysis A: General, 2012, 417-418: 290-297. |
| 19 | WANG N , QIAN W , SHEN K , et al . Bayberry-like ZnO/MFI zeolite as high performance methanol-to-aromatics catalyst[J]. Chemical Communications, 2015, 52(10): 2011-2014. |
| 20 | XIN Y B , QI P Y , DUAN X P , et al . Enhanced performance of Zn-Sn/HZMS-5 catalyst for the conversion of methanol to aromatics[J]. Catalysis Letters, 2013, 143(8): 798-806. |
| 21 | COQUEBLIN H , RICHARD A , UZIO D , et al . Effect of the metal promoter on the performances of H-ZSM5 in ethylene aromatization[J]. Catalysis Today, 2016, 289: 62-69. |
| 22 | NI Y M , SUN A M , WU X L , et al . The preparation of nano-sized H[Zn, Al]ZSM-5 zeolite and its application in the aromatization of methanol[J]. Microporous & Mesoporous Materials, 2011, 143(2/3): 435-442. |
| 23 | BI Y , WANG Y L , CHEN X , et al . Methanol aromatization over HZSM-5 catalysts modified with different zinc salts[J]. Chinese Journal of Catalysis, 2014, 35(10): 1740-1751. |
| 24 | NIU X J , GAO J , MIAO Q , et al . Influence of preparation method on the performance of Zn-containing HZSM-5 catalysts in methanol-to-aromatics[J]. Microporous & Mesoporous Materials, 2014, 197: 252-261. |
| 25 | WANG J Y , LI W H , HU J X . Study of methanol to aromatics on ZnHZSM-5 catalyst[J]. Journal of Fuel Chemistry & Technology, 2009, 37(5): 607-612. |
| 26 | ZHOU J , HUA Z L , L Z C, et al . Direct synthetic strategy of mesoporous ZSM-5 zeolites by using conventional block copolymer templates and the improved catalytic properties[J]. ACS Catalysis, 2011, 1(4): 287-291. |
| 27 | Jeongnam KIM , Minkee CHOI , Ryong RYOO . Effect of mesoporosity against the deactivation of MFI zeolite catalyst during the methanol-to-hydrocarbon conversion process[J]. Journal of Catalysis, 2010, 269(1): 219-228. |
| 28 | AHMADPOUR J , TAGHIZADEH M . Catalytic conversion of methanol to propylene over high-silica mesoporous ZSM-5 zeolites prepared by different combinations of mesogenous templates[J]. Journal of Natural Gas Science & Engineering, 2015, 23: 184-194. |
| 29 | WANG Y X , SONG J J , BAXTER N C , et al . Synthesis of hierarchical ZSM-5 zeolites by solid-state crystallization and their catalytic properties[J]. Journal of Catalysis, 2017, 349: 53-65. |
| 30 | GROEN J C , PEFFER L A , MOULIJN J A , et al . Mechanism of hierarchical porosity development in MFI zeolites by desilication: the role of aluminium as a pore-directing agent[J]. Chemistry European Journal, 2005, 11(17): 4983-4994. |
| 31 | FU T J , CHANG J W , SHAO J , et al . Fabrication of a nano-sized ZSM-5 zeolite with intercrystalline mesopores for conversion of methanol to gasoline[J]. Journal of Energy Chemistry, 2017, 26(1): 139-146. |
| 32 | NI Y M , SUN A M , WU X L , et al . Aromatization of methanol over La/Zn/HZSM-5 catalysts[J]. Chinese Journal of Chemical Engineering, 2011, 19(3): 439-445. |
| 33 | WANG X X , ZHANG J F , ZHANG T , et al . Mesoporous ZnZSM-5 zeolites synthesized by onestep desilication and reassembly: a durable catalyst for methanol aromatization[J]. RSC Advances, 2016, 6(28): 23428-23437. |
| 34 | ZHANG J G , QIAN W Z , KONG C Y , et al . Increasing para-xylene selectivity in making aromatics from methanol with a surface-modified Zn/P/ZSM-5 catalyst[J]. ACS Catalysis, 2015, 5(5): 2982-2988. |
| 35 | ROWNAGHI A A , REZAEI F , HEDLUND J . Yield of gasoline-range hydrocarbons as a function of uniform ZSM-5 crystal size[J]. Catalysis Communications, 2011, 14(1): 37-41. |
| 36 | LI J H , TONG K , XI Z W , et al . Highly-efficient conversion of methanol to p-xylene over shape-selective Mg-Zn-Si-HZSM-5 catalyst with fine modification of pore-opening and acidic properties[J]. Catalysis Science & Technology, 2016, 6(13): 4802-4813. |
| 37 | 刘维桥, 雷卫宁, 尚通明, 等 . Zn对HZSM-5分子筛催化剂物化及甲醇芳构化反应性能的影响[J]. 化工进展, 2011, 30(9): 1967-1971. |
| LIU W Q , LEI W N , SHANG T M , et al . Physicochemical and methanol aromatization property of HZSM-5 catalyst promoted by Zn[J]. Chemical Industry and Engineering Progress, 2011, 30(9): 1967-1971. | |
| 38 | YARIPOUR F , SHARIATINIA Z , SAHEBDELFAR S , et al . Effect of boron incorporation on the structure, products selectivities and lifetime of H-ZSM-5 nanocatalyst designed for application in methanol-to-olefins (MTO) reaction[J]. Microporous & Mesoporous Materials, 2015, 203: 41-53. |
| 39 | PINILLA-HERRERO I , BORFECCHIA E , HOLZINGER J , et al . High Zn/Al ratios enhance dehydrogenation vs hydrogen transfer reactions of Zn-ZSM-5 catalytic systems in methanol conversion to aromatics[J]. Journal of Catalysis, 2018, 362: 146-163. |
| [1] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [2] | 许家珩, 李永胜, 罗春欢, 苏庆泉. 甲醇水蒸气重整工艺的优化[J]. 化工进展, 2023, 42(S1): 41-46. |
| [3] | 舒斌, 陈建宏, 熊健, 吴其荣, 喻江涛, 杨平. 碳中和目标下推动绿色甲醇发展的必要性分析[J]. 化工进展, 2023, 42(9): 4471-4478. |
| [4] | 杨静, 李博, 李文军, 刘晓娜, 汤刘元, 刘月, 钱天伟. 焦化污染场地中萘降解菌的分离及降解特性[J]. 化工进展, 2023, 42(8): 4351-4361. |
| [5] | 刘柏成, 李法云, 赵琦慧, 吝美霞. 禾本科植物修复多环芳烃污染土壤研究进展[J]. 化工进展, 2023, 42(7): 3736-3748. |
| [6] | 谭利鹏, 申峻, 王玉高, 刘刚, 徐青柏. 煤沥青和石油沥青共混改性的研究进展[J]. 化工进展, 2023, 42(7): 3749-3759. |
| [7] | 殷鹏镇, 吴芹, 黎汉生. 甲基芳烃液相选择性催化氧化催化剂研究进展[J]. 化工进展, 2023, 42(6): 2916-2943. |
| [8] | 王子健, 柯明, 宋昭峥, 李佳涵, 童燕兵, 孙巾茹. 分子筛催化汽油烷基化降苯技术研究进展[J]. 化工进展, 2023, 42(5): 2371-2389. |
| [9] | 萧垚鑫, 张军, 胡升, 单锐, 袁浩然, 陈勇. 甲醇供氢体系铜锌双金属催化糠醛加氢转化[J]. 化工进展, 2023, 42(3): 1341-1352. |
| [10] | 黄起中, 刘冰, 马红鹏, 吕文杰. 基于新型微通道分离技术的甲醇制烯烃废水处理[J]. 化工进展, 2023, 42(2): 669-676. |
| [11] | 霍文涛, 刘稳, 于强, 安杰, 朱向学, 秦玉才, 李秀杰. MWW分子筛上异丁烯齐聚反应探索[J]. 化工进展, 2023, 42(10): 5205-5212. |
| [12] | 郭晓宇, 李冬晨, 赵炜, 杜朕屹, 李晓良. Au-Pd/MnO2催化剂的制备及其苯甲醇氧化性能[J]. 化工进展, 2023, 42(10): 5223-5231. |
| [13] | 郭峰, 张尚杰, 蒋羽佳, 姜万奎, 信丰学, 章文明, 姜岷. 一碳资源在酵母中的利用与转化[J]. 化工进展, 2023, 42(1): 30-39. |
| [14] | 陈晓云, 郭亚东, 邸璐, 毕冬梅, 李凯凯, 林晓娜. 硼掺杂活性炭催化生物质与塑料共热解制芳烃[J]. 化工进展, 2022, 41(S1): 199-209. |
| [15] | 宋绍彤, 吕忠武, 姜增琨, 李阳, 吴培, 张雅琳, 张然, 陈金洋, 鞠雅娜. ZSM-5/γ-Al2O3质量比对FCC轻汽油异构化/芳构化性能影响[J]. 化工进展, 2022, 41(S1): 229-238. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||