化工进展 ›› 2025, Vol. 44 ›› Issue (2): 971-981.DOI: 10.16085/j.issn.1000-6613.2024-0149
沉淀法合成氧化镁吸附剂及其对氟化物的吸附机理
- 兰州理工大学石油化工学院,甘肃 兰州 730050
-
收稿日期:2024-01-19修回日期:2024-05-21出版日期:2025-02-25发布日期:2025-03-10 -
通讯作者:赵鹬 -
作者简介:赵鹬(1981—),教授,博士生导师,研究方向为多相催化与新材料。E-mail:yzhao@lut.edu.cn。 -
基金资助:甘肃省高等学校产业支撑计划(2020C-06)
Synthesis of magnesium oxide adsorbent through the precipitation method and its adsorption mechanism for fluoride
ZHAO Yu( ), SHI Ling, ZHANG Dongqiang, LI Ning
), SHI Ling, ZHANG Dongqiang, LI Ning
- School of Petrochemical Engineering, Lanzhou University of Science and Technology, Lanzhou 730050, Gansu, China
-
Received:2024-01-19Revised:2024-05-21Online:2025-02-25Published:2025-03-10 -
Contact:ZHAO Yu
摘要:
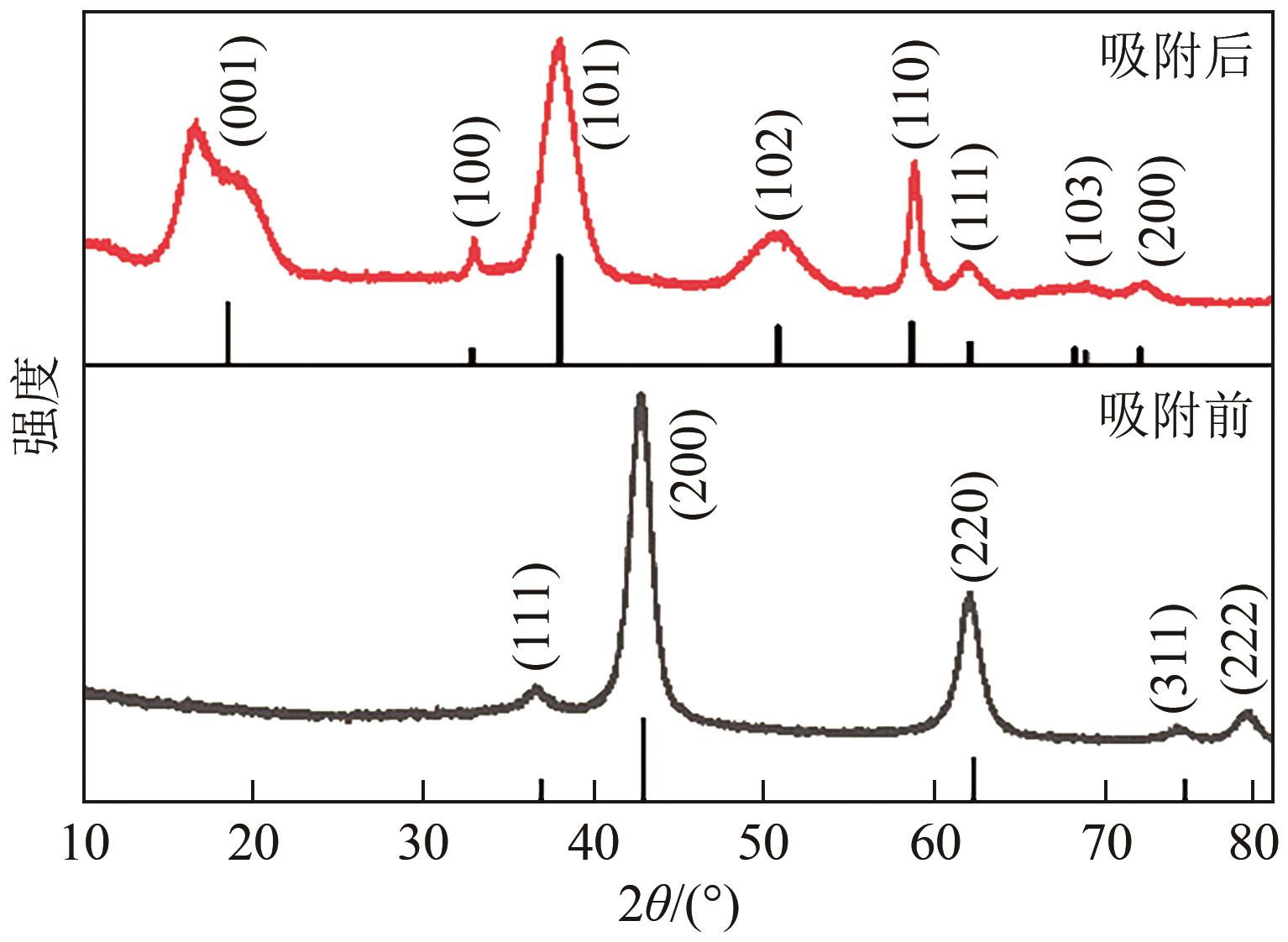
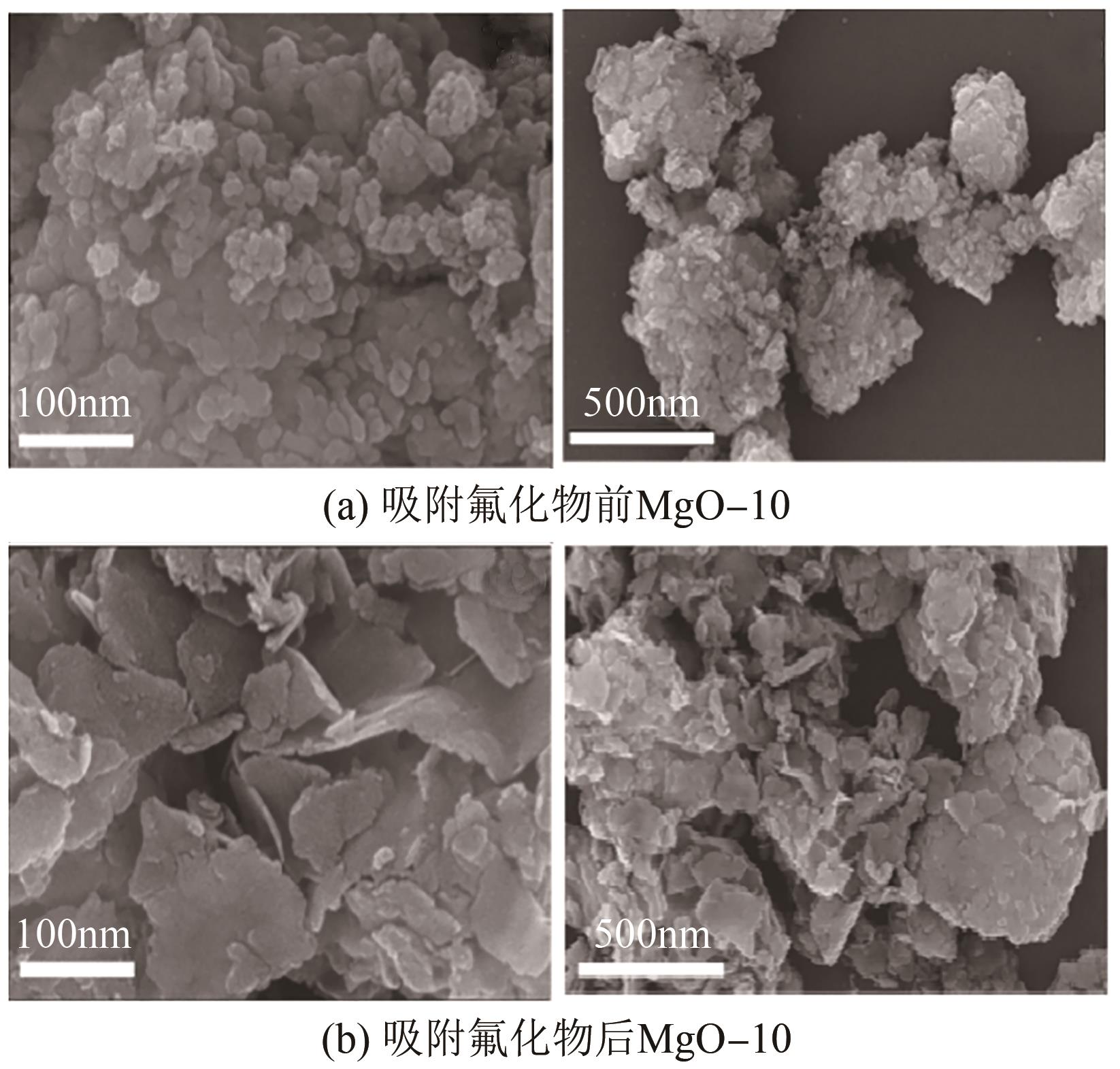
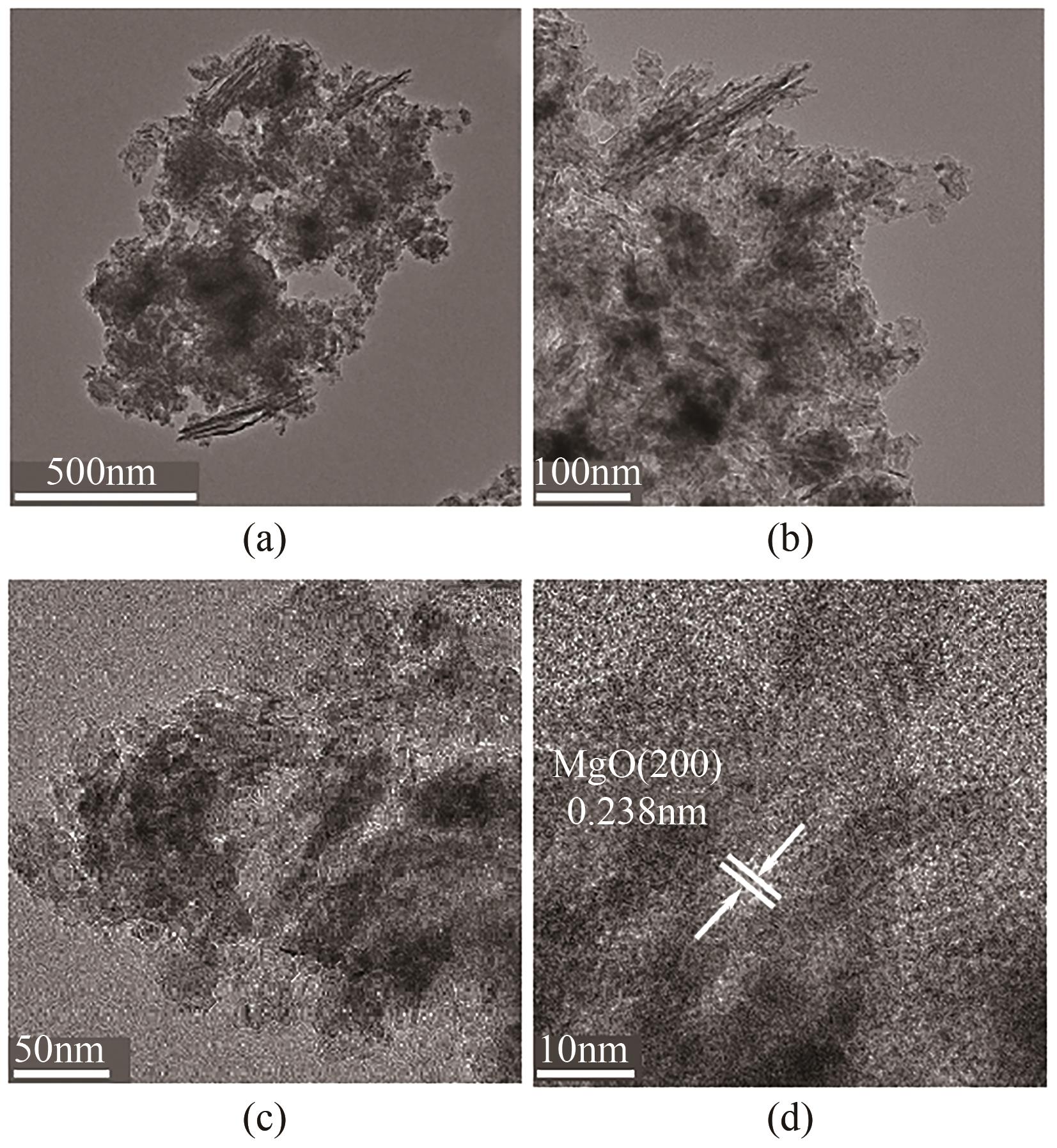
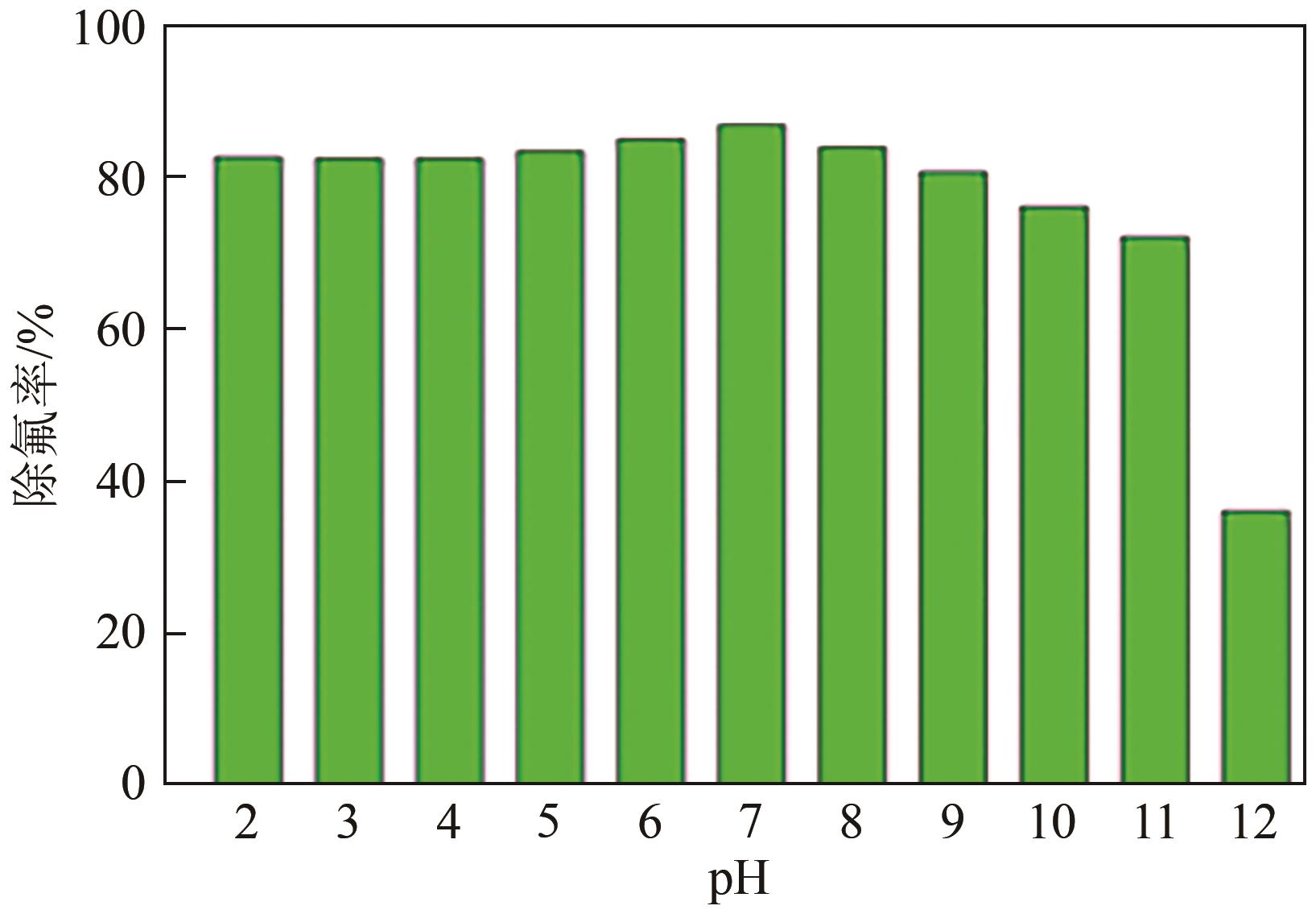
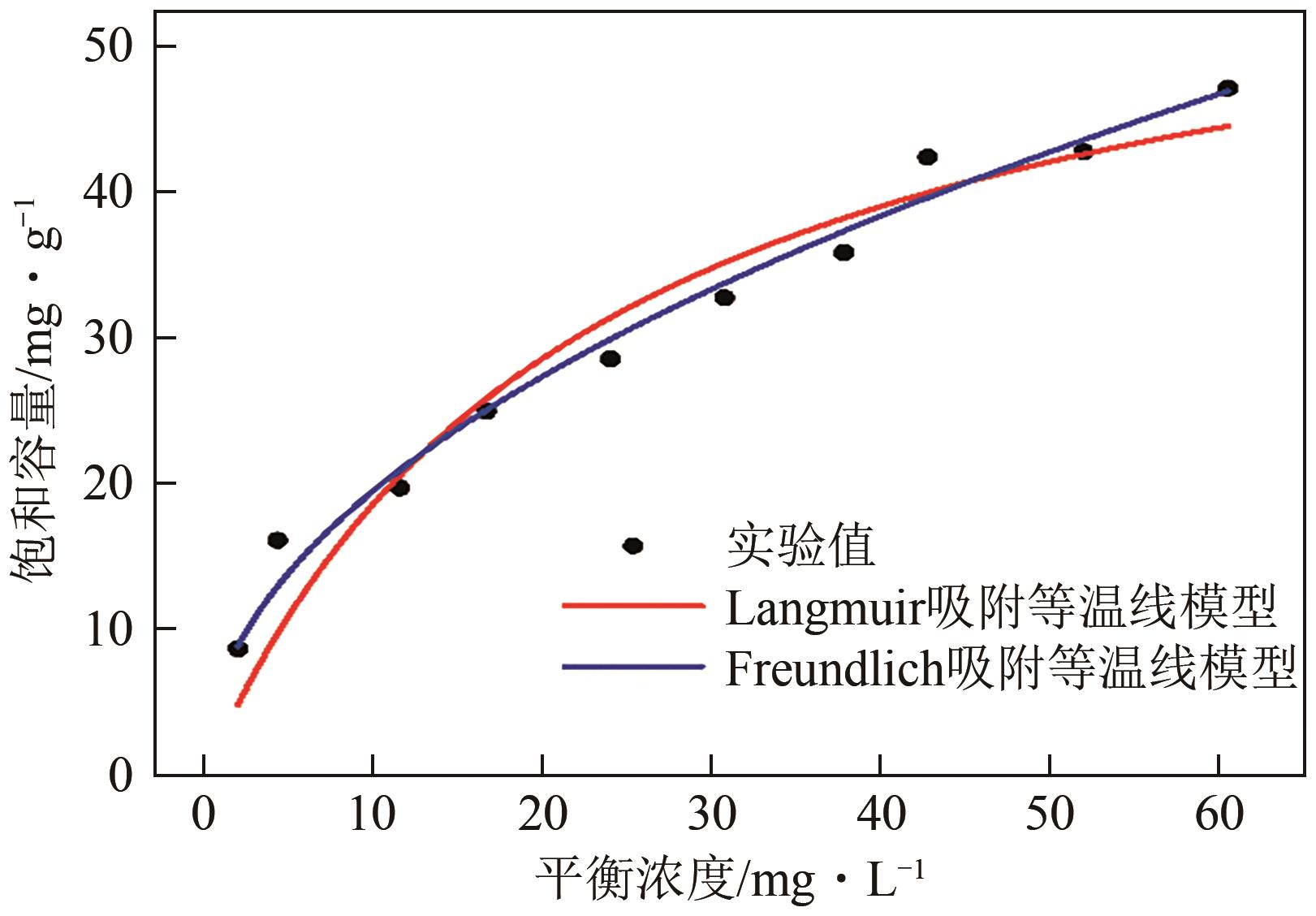
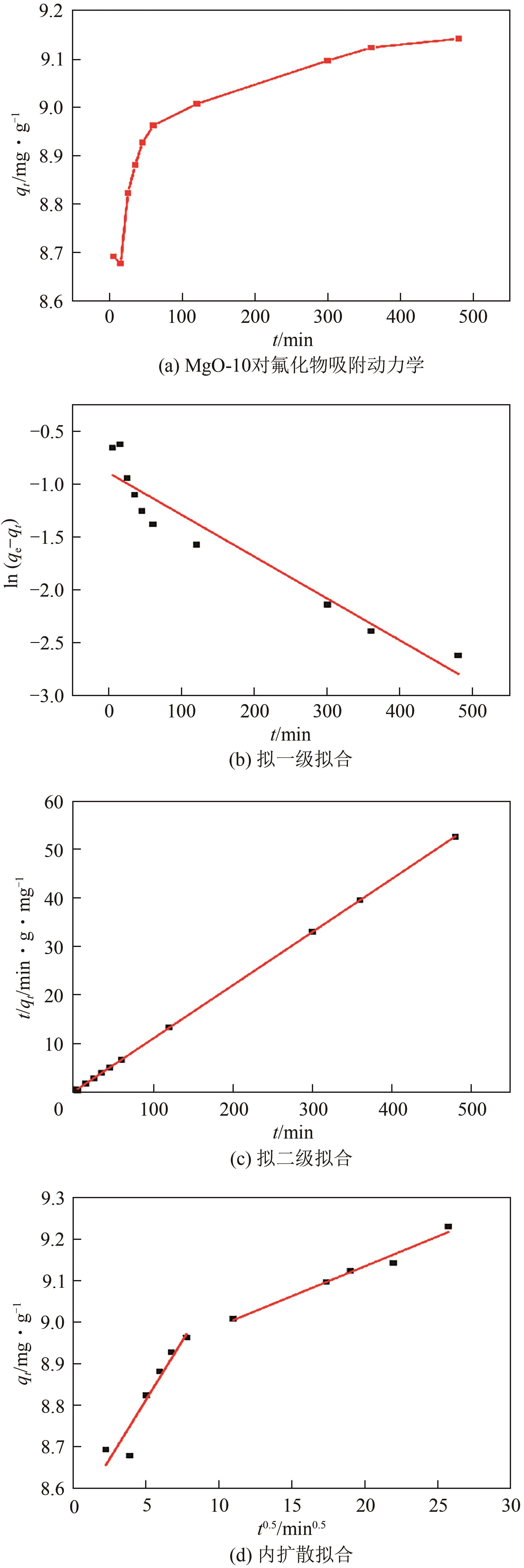
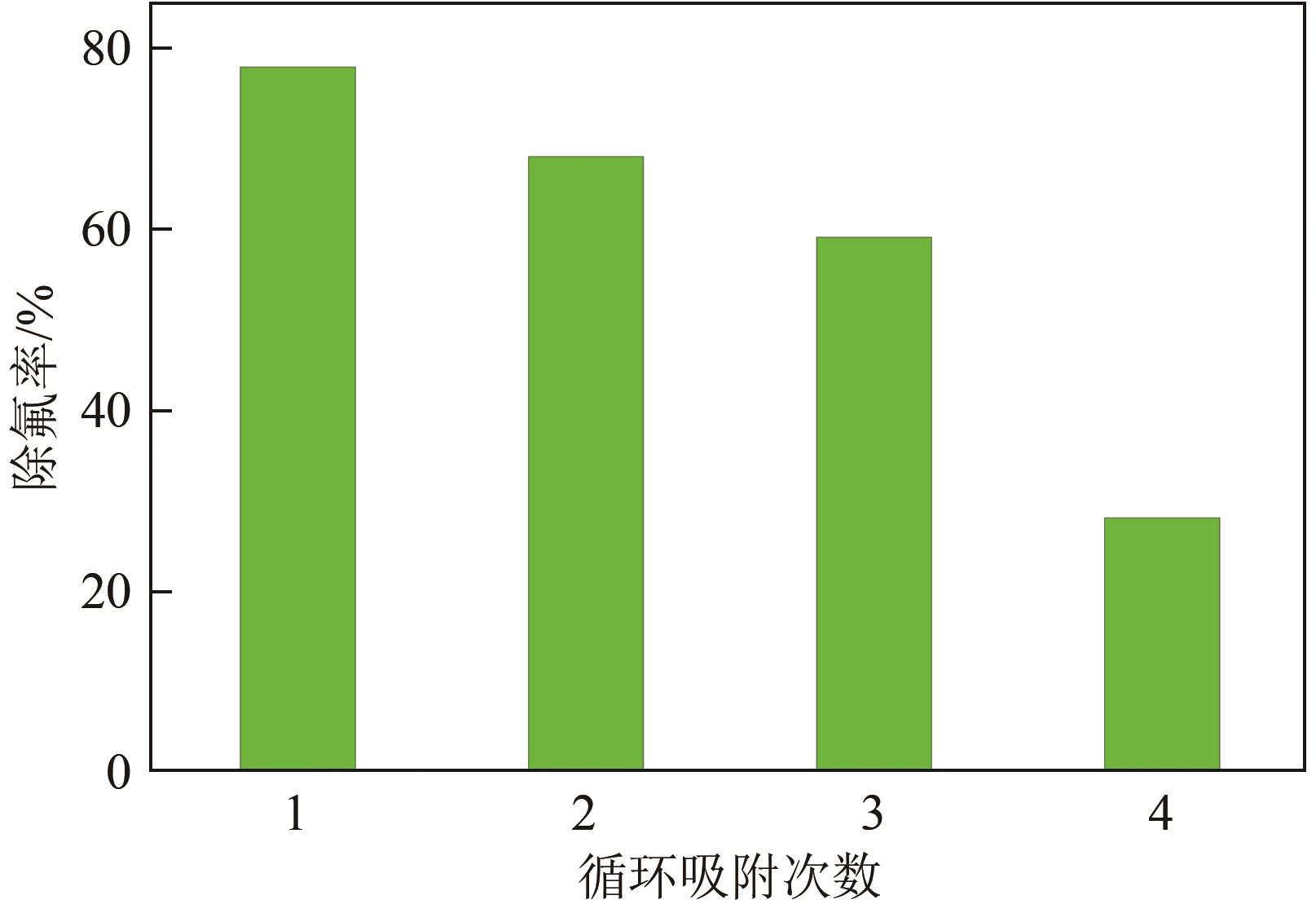
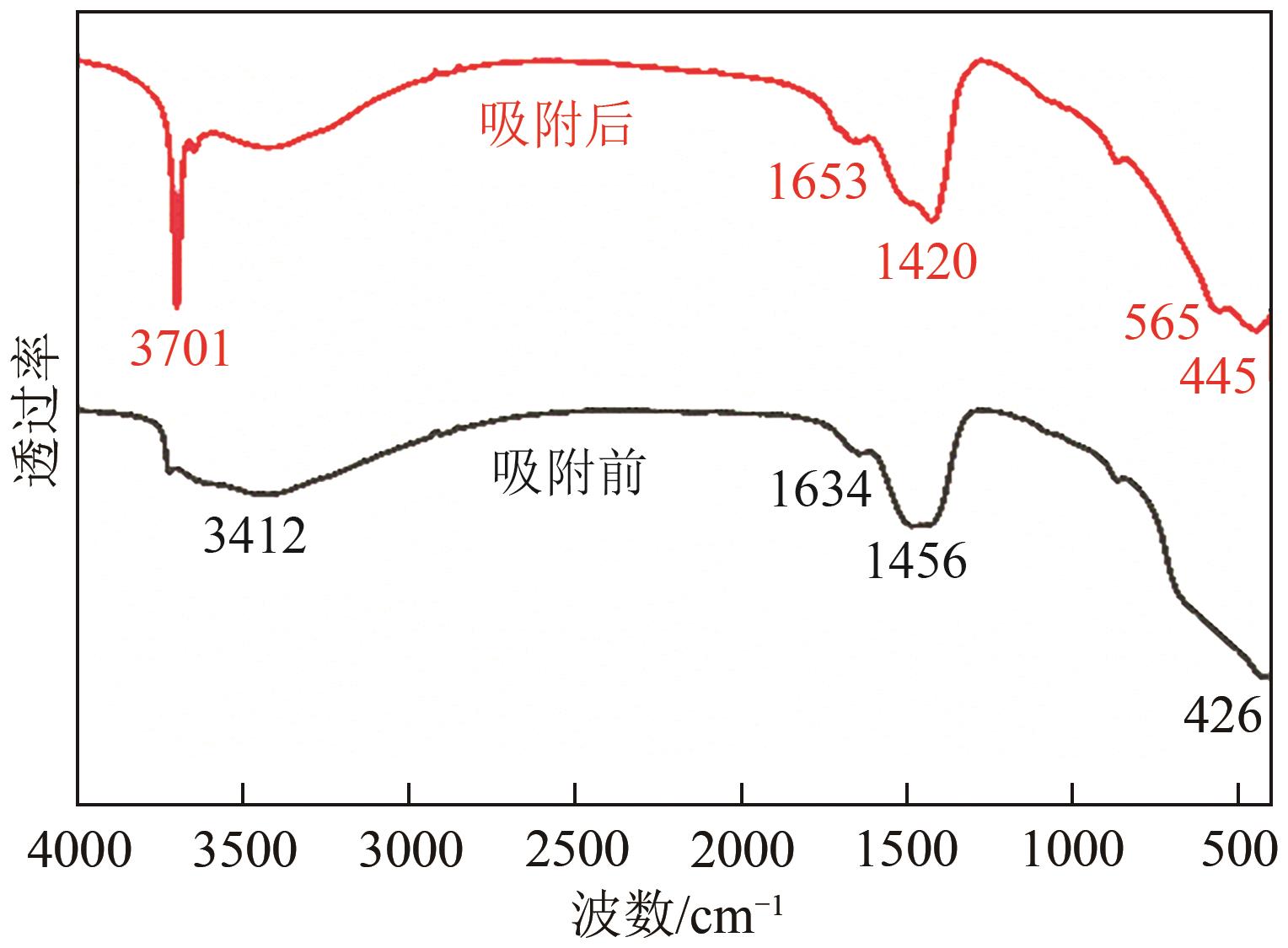
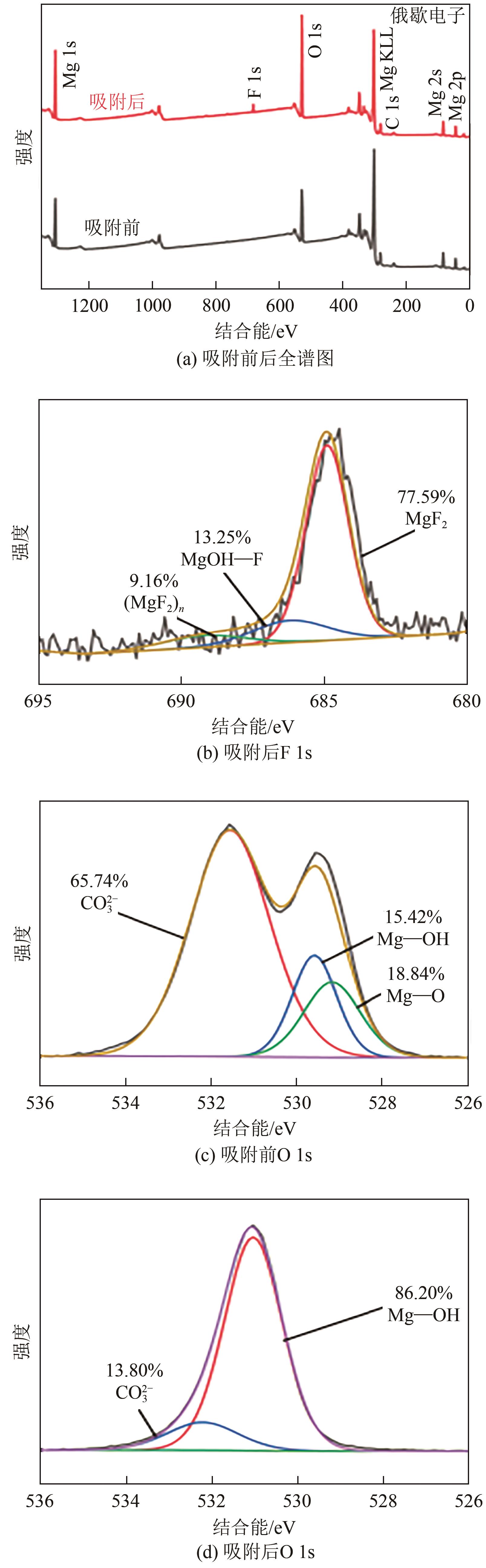
氟化物移除是全世界范围内急需解决的水处理问题。本文通过一种简单的沉淀-煅烧法合成了氧化镁吸附剂并将其应用于废水除氟研究。pH对合成的氧化镁材料比表面积影响较大,pH在10~10.5时合成的材料比表面积最大(101.1~154.8m2/g)。通过批量吸附实验及等温线研究,发现最大吸附容量为61.337mg/g;该过程符合Freundlich模型,说明吸附过程为非均相吸附;通过动力学研究,发现吸附过程符合拟二级动力学模型,说明吸附过程包含化学吸附。通过X射线衍射(XRD)、扫描电子显微镜(SEM)、傅里叶变换红外光谱(FTIR)、X射线光电子能谱(XPS)等表征结果推断出氧化镁的吸附机理为静电相互作用、离子交换机制。在pH为2~10时氧化镁能有效去除水中的氟化物;常见竞争阴离子中仅有CO32-、PO43-对氟化物的吸附有不利影响;循环吸附实验表明氧化镁吸附剂具有可再生利用的潜力。因此,通过对合成的氧化镁材料进行批次吸附实验及吸附机理的探究,为废水除氟的工业应用提供一定的理论积累。
中图分类号:
引用本文
赵鹬, 石翎, 张栋强, 李宁. 沉淀法合成氧化镁吸附剂及其对氟化物的吸附机理[J]. 化工进展, 2025, 44(2): 971-981.
ZHAO Yu, SHI Ling, ZHANG Dongqiang, LI Ning. Synthesis of magnesium oxide adsorbent through the precipitation method and its adsorption mechanism for fluoride[J]. Chemical Industry and Engineering Progress, 2025, 44(2): 971-981.
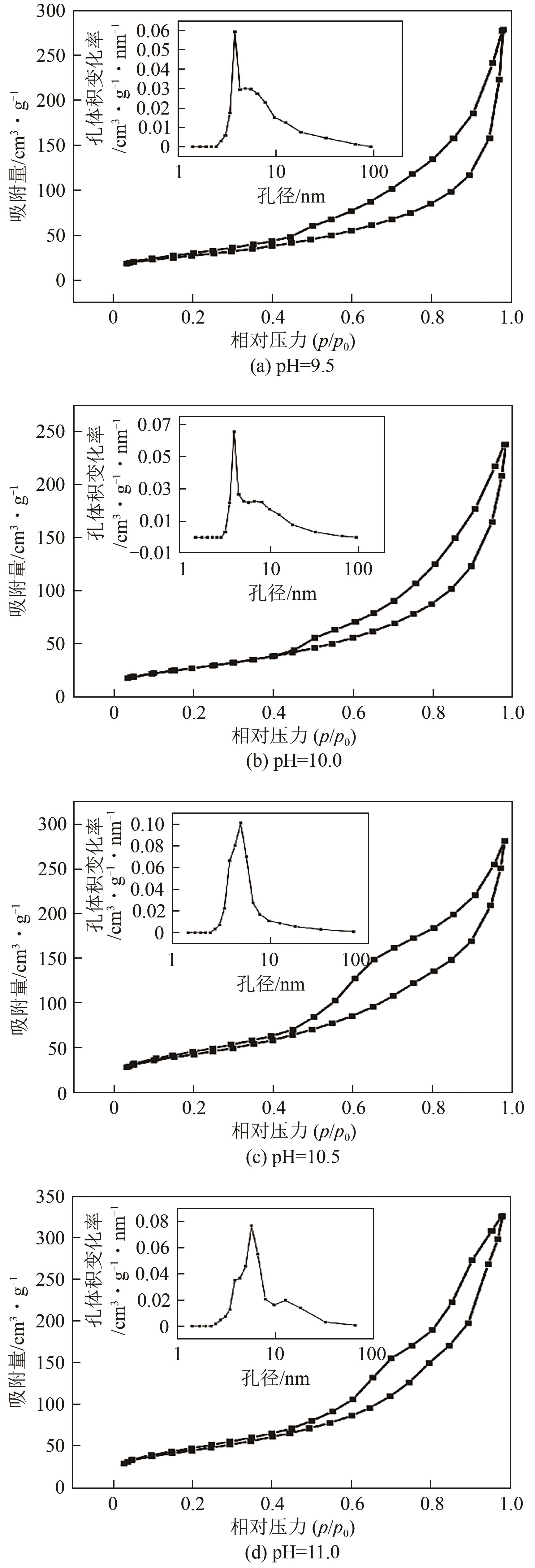
| 样品 | SBET/m2·g-1 | 总孔容/cm3·g-1 | 平均孔径/nm |
|---|---|---|---|
| MgO-9.5 | 98.5 | 0.45 | 3.80 |
| MgO-10 | 101.1 | 0.39 | 3.83 |
| MgO-10.5 | 154.8 | 0.46 | 4.95 |
| MgO-11 | 160.9 | 0.52 | 5.69 |
表1 不同pH条件下制备的MgO的结构参数
| 样品 | SBET/m2·g-1 | 总孔容/cm3·g-1 | 平均孔径/nm |
|---|---|---|---|
| MgO-9.5 | 98.5 | 0.45 | 3.80 |
| MgO-10 | 101.1 | 0.39 | 3.83 |
| MgO-10.5 | 154.8 | 0.46 | 4.95 |
| MgO-11 | 160.9 | 0.52 | 5.69 |
| 样品名称 | 吸附容量/mg·g-1 | 去除率/% |
|---|---|---|
| MgO-9.5 | 7.00 | 70.94 |
| MgO-10 | 7.60 | 77.04 |
| MgO-10.5 | 6.87 | 69.68 |
| MgO-11 | 6.31 | 63.96 |
表2 不同pH条件下制备的MgO对氟化物的吸附性能
| 样品名称 | 吸附容量/mg·g-1 | 去除率/% |
|---|---|---|
| MgO-9.5 | 7.00 | 70.94 |
| MgO-10 | 7.60 | 77.04 |
| MgO-10.5 | 6.87 | 69.68 |
| MgO-11 | 6.31 | 63.96 |
| 模型 | 参数 | 数值 |
|---|---|---|
| Langmuir吸附等温线 | qm/mg·g-1 | 61.337 |
| KL/L·mg-1 | 0.0436 | |
| R2 | 0.9294 | |
| Freundlich吸附等温线 | KF/mg·g-1 | 6.3553 |
| n | 2.0528 | |
| R2 | 0.9803 | |
| 实验值 | qe/mg·g-1 | 47.110 |
表3 MgO-10吸附氟化物的等温线模型参数
| 模型 | 参数 | 数值 |
|---|---|---|
| Langmuir吸附等温线 | qm/mg·g-1 | 61.337 |
| KL/L·mg-1 | 0.0436 | |
| R2 | 0.9294 | |
| Freundlich吸附等温线 | KF/mg·g-1 | 6.3553 |
| n | 2.0528 | |
| R2 | 0.9803 | |
| 实验值 | qe/mg·g-1 | 47.110 |
| 样品 | 浓度/g·L-1 | pH | 初始氟离子浓度 /mg·L-1 | 吸附容量 /mg·g-1 |
|---|---|---|---|---|
| 工业MgO | 1 | 7 | 10 | 7.424 |
| 制备MgO | 1 | 7 | 10 | 7.729 |
表4 工业MgO与制备MgO性能比较
| 样品 | 浓度/g·L-1 | pH | 初始氟离子浓度 /mg·L-1 | 吸附容量 /mg·g-1 |
|---|---|---|---|---|
| 工业MgO | 1 | 7 | 10 | 7.424 |
| 制备MgO | 1 | 7 | 10 | 7.729 |
| 模型 | 参数 | 数值 |
|---|---|---|
| 拟一级动力学模型 | qe/mg·g-1 | 0.409 |
| K1/min-1 | 0.0039 | |
| R2 | 0.9097 | |
| 拟二级动力学模型 | qe/mg·g-1 | 9.149 |
| K2/g·mg-1·min-1 | 0.104 | |
| R2 | 0.9999 | |
| 颗粒内扩散模型 | Ki1/min0.5 | 0.0571 |
| Ci1 | 8.5276 | |
| R | 0.8775 | |
| Ki2/min0.5 | 0.0127 | |
| Ci2 | 8.8729 | |
| R | 0.9693 | |
| 实验值 | qe/mg·g-1 | 9.215 |
表5 拟一级、拟二级和内扩散模型的吸附动力学参数
| 模型 | 参数 | 数值 |
|---|---|---|
| 拟一级动力学模型 | qe/mg·g-1 | 0.409 |
| K1/min-1 | 0.0039 | |
| R2 | 0.9097 | |
| 拟二级动力学模型 | qe/mg·g-1 | 9.149 |
| K2/g·mg-1·min-1 | 0.104 | |
| R2 | 0.9999 | |
| 颗粒内扩散模型 | Ki1/min0.5 | 0.0571 |
| Ci1 | 8.5276 | |
| R | 0.8775 | |
| Ki2/min0.5 | 0.0127 | |
| Ci2 | 8.8729 | |
| R | 0.9693 | |
| 实验值 | qe/mg·g-1 | 9.215 |
| 1 | GHOSH Aniruddha, MUKHERJEE Kakali, GHOSH Sumanta K, et al. Sources and toxicity of fluoride in the environment[J]. Research on Chemical Intermediates, 2013, 39(7): 2881-2915. |
| 2 | KANNO Cynthia M, SANDERS Rebecca L, FLYNN Steven M, et al. Novel apatite-based sorbent for defluoridation: Synthesis and sorption characteristics of nano-micro-crystalline hydroxyapatite-coated-limestone[J]. Environmental Science & Technology, 2014, 48(10): 5798-5807. |
| 3 | KIM Eun-Ah, PARK Ji Hye, HAN Sung-Hee, et al. Exploratory factor analysis of fluoride removal efficiency associated with the chemical properties of geomaterials[J]. Journal of Hazardous Materials, 2017, 334: 178-184. |
| 4 | GAN Yonghai, WANG Xiaomeng, ZHANG Li, et al. Coagulation removal of fluoride by zirconium tetrachloride: Performance evaluation and mechanism analysis[J]. Chemosphere, 2019, 218: 860-868. |
| 5 | YU Chenglong, LIU Lin, WANG Xiaodong, et al. Fluoride removal performance of highly porous activated alumina[J]. Journal of Sol-Gel Science and Technology, 2023, 106: 471-479. |
| 6 | EKKA Basanti, DHAKA Rajendra S, PATEL Raj Kishore, et al. Fluoride removal in waters using ionic liquid-functionalized alumina as a novel adsorbent[J]. Journal of Cleaner Production, 2017, 151: 303-318. |
| 7 | HE Junyong, CAI Xingguo, CHEN Kai, et al. Performance of a novelly-defined zirconium metal-organic frameworks adsorption membrane in fluoride removal[J]. Journal of Colloid and Interface Science, 2016, 484: 162-172. |
| 8 | JADHAV Sachin V, BRINGAS Eugenio, YADAV Ganapati D, et al. Arsenic and fluoride contaminated groundwaters: A review of current technologies for contaminants removal[J]. Journal of Environmental Management, 2015, 162: 306-325. |
| 9 | 田追, 张震, 卢嫚, 等. 新型除氟吸附材料的研究进展[J]. 化工进展, 2022, 41(6): 3051-3062. |
| TIAN Zhui, ZHANG Zhen, LU Man, et al. New adsorption materials for removing fluoride from wastewater: A review[J]. Chemical Industry and Engineering Progress, 2022, 41(6): 3051-3062. | |
| 10 | KUMAR Eva, BHATNAGAR Amit, KUMAR Umesh, et al. Defluoridation from aqueous solutions by nano-alumina: Characterization and sorption studies[J]. Journal of Hazardous Materials, 2011, 186(2/3): 1042-1049. |
| 11 | MALIYEKKAL Shihabudheen M, SHUKLA Sanjay, PHILIP Ligy, et al. Enhanced fluoride removal from drinking water by magnesia-amended activated alumina granules[J]. Chemical Engineering Journal, 2008, 140(1/2/3): 183-192. |
| 12 | HE Yuxuan, ZHANG Liming, AN Xiao, et al. Enhanced fluoride removal from water by rare earth (La and Ce) modified alumina: Adsorption isotherms, kinetics, thermodynamics and mechanism[J]. Science of the Total Environment, 2019, 688: 184-198. |
| 13 | CHANG Qing, ZHU Lihua, LUO Zhihong, et al. Sono-assisted preparation of magnetic magnesium-aluminum layered double hydroxides and their application for removing fluoride[J]. Ultrasonics Sonochemistry, 2011, 18(2): 553-561. |
| 14 | KAMEDA Tomohito, Jumpei OBA, YOSHIOKA Toshiaki. Recyclable Mg-Al layered double hydroxides for fluoride removal: Kinetic and equilibrium studies[J]. Journal of Hazardous Materials, 2015, 300: 475-482. |
| 15 | ARAGA Ramya, KALI Suresh, SHARMA Chandra S. Coconut‐shell‐derived carbon/carbon nanotube composite for fluoride adsorption from aqueous solution[J]. CLEAN-Soil Air Water, 2019, 47(5): 1800286. |
| 16 | MEDELLÍN-CASTILLO Nahum Andres, CRUZ-BRIANO Sergio Armando, Roberto LEYVA-RAMOS, et al. Use of bone char prepared from an invasive species, pleco fish (Pterygoplichthys spp.), to remove fluoride and Cadmium(Ⅱ) in water[J]. Journal of Environmental Management, 2020, 256: 109956. |
| 17 | AFFONSO Lutiane N, MARQUES Jorge L, LIMA Valéria V C, et al. Removal of fluoride from fertilizer industry effluent using carbon nanotubes stabilized in chitosan sponge[J]. Journal of Hazardous Materials, 2020, 388: 122042. |
| 18 | SONG Jiangyan, YU Yongyi, HAN Xiaoshuai, et al. Novel MOF(Zr)-on-MOF(Ce) adsorbent for elimination of excess fluoride from aqueous solution[J]. Journal of Hazardous Materials, 2024, 463: 132843. |
| 19 | JEYASEELAN Antonysamy, ASWIN KUMAR Ilango, VISWANATHAN Natrayasamy, et al. Development and characterization of hydroxyapatite layered lanthanum organic frameworks by template method for defluoridation of water[J]. Journal of Colloid and Interface Science, 2022, 622: 228-238. |
| 20 | 冯江涛, 王睎, 赵旭阳, 等. 改性聚吡咯材料去除水中氟离子的性能[J]. 化工进展, 2021, 40(7): 4036-4046. |
| FENG Jiangtao, WANG Xi, ZHAO Xuyang, et al. Removal of fluoride from water by modified polypyrrole[J]. Chemical Industry and Engineering Progress, 2021, 40(7): 4036-4046. | |
| 21 | ZHANG Yangzhong, HUANG Kai. Grape pomace as a biosorbent for fluoride removal from groundwater[J]. RSC Advances, 2019, 9(14): 7767-7776. |
| 22 | JIN Zhen, JIA Yong, ZHANG Kaisheng, et al. Effective removal of fluoride by porous MgO nanoplates and its adsorption mechanism[J]. Journal of Alloys and Compounds, 2016, 675: 292-300. |
| 23 | 康宁, 由昆, 徐丽, 等. 多孔球状活性氧化镁的制备及除氟效能研究[J]. 工业水处理, 2022, 42(11): 65-75. |
| KANG Ning, YOU Kun, XU Li, et al. Research on porous spherical activated MgO preparation and its defluorination efficacy[J]. Industrial Water Treatment, 2022, 42(11): 65-75. | |
| 24 | LI Lianxiang, XU Di, LI Xiaoqin, et al. Excellent fluoride removal properties of porous hollow MgO microspheres[J]. New Journal of Chemistry, 2014, 38(11): 5445-5452. |
| 25 | YU Zhichao, XU Chonghe, YUAN Kangkang, et al. Template-free synthesis of MgO mesoporous nanofibers with superior adsorption for fluoride and Congo red[J]. Ceramics International, 2018, 44(8): 9454-9462. |
| 26 | GUO Wei, LIN Hongfei, ZHU Hongxiang, et al. Preparation and application of magnesium oxide nanoparticles for superiorly fluoride removal[J]. Journal of Alloys and Compounds, 2023, 960: 170935. |
| 27 | TOLKOU Athanasia K, MANOUSI Natalia, ZACHARIADIS George A, et al. Recently developed adsorbing materials for fluoride removal from water and fluoride analytical determination techniques: A review[J]. Sustainability, 2021, 13(13): 7061-7083. |
| 28 | SING K S W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (recommendations 1984)[J]. Pure and Applied Chemistry, 1985, 57(4): 603-619. |
| 29 | TANG Dandan, ZHANG Gaoke. Efficient removal of fluoride by hierarchical Ce-Fe bimetal oxides adsorbent: Thermodynamics, kinetics and mechanism[J]. Chemical Engineering Journal, 2016, 283: 721-729. |
| 30 | PARASHAR Kamya, BALLAV Niladri, DEBNATH Sushanta, et al. Rapid and efficient removal of fluoride ions from aqueous solution using a polypyrrole coated hydrous tin oxide nanocomposite[J]. Journal of Colloid and Interface Science, 2016, 476: 103-118. |
| 31 | WANG Kaixiang, WEI Tingting, LI Yinuo, et al. Flocculation-to-adsorption transition of novel salt-responsive polyelectrolyte for recycling of highly polluted saline textile effluents[J]. Chemical Engineering Journal, 2021, 413: 127410. |
| 32 | ZHU Kecheng, DUAN Yanyan, WANG Fu, et al. Silane-modified halloysite/Fe3O4 nanocomposites: Simultaneous removal of Cr(Ⅵ) and Sb(Ⅴ) and positive effects of Cr(Ⅵ) on Sb(Ⅴ) adsorption[J]. Chemical Engineering Journal, 2017, 311: 236-246. |
| 33 | ZHANG Mengxue, XU Liheng, QI Changli, et al. Highly effective removal of Tetracycline from water by hierarchical porous carbon: Batch and column adsorption[J]. Industrial & Engineering Chemistry Research, 2019, 58(43): 20036-20046. |
| 34 | TANG Yulin, GUAN Xiaohong, WANG Jianmin, et al. Fluoride adsorption onto granular ferric hydroxide: Effects of ionic strength, pH, surface loading, and major co-existing anions[J]. Journal of Hazardous Materials, 2009, 171(1/2/3): 774-779. |
| 35 | MOHAPATRA M, ROUT K, SINGH P, et al. Fluoride adsorption studies on mixed-phase nano iron oxides prepared by surfactant mediation-precipitation technique[J]. Journal of Hazardous Materials, 2011, 186(2/3): 1751-1757. |
| 36 | LANGMUIR Irving. The constitution and fundamental properties of solids and liquids. Part Ⅰ. Solids[J]. Journal of the American Chemical Society, 1916, 38(11): 2221-2295. |
| 37 | YIN Chun, HUANG Qilan, ZHU Guiping, et al. High-performance lanthanum-based metal-organic framework with ligand tuning of the microstructures for removal of fluoride from water[J]. Journal of Colloid and Interface Science, 2022, 607: 1762-1775. |
| 38 | GOGOI Sweety, DUTTA Robin K. Mechanism of fluoride removal by phosphoric acid-enhanced limestone: Equilibrium and kinetics of fluoride sorption[J]. Desalination and Water Treatment, 2016, 57(15): 6838-6851. |
| 39 | SAHU Sumanta, MALLIK Laxmi, PAHI Souman, et al. Facile synthesis of poly o-toluidine modified lanthanum phosphate nanocomposite as a superior adsorbent for selective fluoride removal: A mechanistic and kinetic study[J]. Chemosphere, 2020, 252: 126551. |
| 40 | GAO Panpan, TIAN Xike, YANG Chao, et al. Fabrication, performance and mechanism of MgO meso-/macroporous nanostructures for simultaneous removal of As(Ⅲ) and F in a groundwater system[J]. Environmental Science: Nano, 2016, 3(6): 1416-1424. |
| 41 | ZHANG Kaisheng, WU Shibiao, WANG Xuelong, et al. Wide pH range for fluoride removal from water by MHS-MgO/MgCO3 adsorbent: Kinetic, thermodynamic and mechanism studies[J]. Journal of Colloid and Interface Science, 2015, 446: 194-202. |
| 42 | NIU Haixia, YANG Qing, TANG Kaibin, et al. Large-scale synthesis of single-crystalline MgO with bone-like nanostructures[J]. Journal of Nanoparticle Research, 2006, 8(6): 881-888. |
| 43 | JIN Zhen, JIA Yong, LUO Tao, et al. Efficient removal of fluoride by hierarchical MgO microspheres: Performance and mechanism study[J]. Applied Surface Science, 2015, 357: 1080-1088. |
| 44 | XU Rong. ZENG Huachun. Dimensional control of cobalt-hydroxide-carbonate nanorods and their thermal conversion to one-dimensional arrays of Co3O4 nanoparticles[J]. Journal of Physical Chemistry B, 2003, 107(46): 12643-12649. |
| 45 | SUGAMA T, SABATINI R, PETRAKIS L. Decomposition of chrysotile asbestos by fluorosulfonic acid[J]. Industrial & Engineering Chemistry Research, 1998, 37(1): 79-88. |
| 46 | WANG Lanting, XIE Yanhua, YANG Jinglong, et al. Insight into mechanisms of fluoride removal from contaminated groundwater using lanthanum-modified bone waste[J]. RSC Advances, 2017, 7(85): 54291-54305. |
| 47 | WUTTKE Stefan, COMAN Simona M, SCHOLZ Gudrun, et al. Novel Sol-gel synthesis of acidic MgF2- x (OH) x materials[J]. Chemistry:A European Journal, 2008, 14(36): 11488-11499. |
| 48 | MAO Chunfeng, YIN Kai, YANG Chenghan, et al. Fe-based MOFs@Pd@COFs with spatial confinement effect and electron transfer synergy of highly dispersed Pd nanoparticles for Suzuki-Miyaura coupling reaction[J]. Journal of Colloid and Interface Science, 2022, 608: 809-819. |
| [1] | 白依冉, 翟玉玲, 戴晶慧, 李舟航. 微纳尺度池沸腾表面润湿性的气泡成核及强化传热机制[J]. 化工进展, 2025, 44(2): 743-751. |
| [2] | 李琢宇, 余美琪, 陈孝彦, 胡若晖, 王庆宏, 陈春茂, 詹亚力. 炼油废催化剂吸附去除水中硝基苯的特性与机制[J]. 化工进展, 2025, 44(2): 1076-1087. |
| [3] | 张琪, 王涛, 张雪冰, 李为真, 冯波, 蒋智慧, 吕毅军, 门卓武. 合成气制高级醇Co基催化剂研究进展[J]. 化工进展, 2025, 44(2): 773-787. |
| [4] | 张爱京, 王桢钰, 肖宁宁, 宋艳娜, 李军, 冯江涛, 延卫. 新型汞离子吸附材料研究进展[J]. 化工进展, 2025, 44(2): 899-913. |
| [5] | 张强, 孙楠, 郑俊杰, 吴强, 刘传海, 李元吉. 混合热力学促进剂对水合物法分离回收瓦斯的影响[J]. 化工进展, 2025, 44(1): 192-201. |
| [6] | 杨润农, 白帆飞, 林梓荣, 孙永明, 尹祥. 分子筛吸附脱除有机硫的研究进展[J]. 化工进展, 2025, 44(1): 329-340. |
| [7] | 倪鹏, 王先泓, 黄钰涵, 马晓彤, 马子轸, 谈琰, 张华伟, 刘亭. 活性炭类和磁性金属类吸附剂喷射脱汞技术应用对比及最新进展[J]. 化工进展, 2025, 44(1): 513-524. |
| [8] | 刘新维, 高珊, 王红涛, 王建成. 气化细渣、铝灰的活化及其吸附性能[J]. 化工进展, 2025, 44(1): 558-571. |
| [9] | 赵丽阳, 李倩, 何佩熹, 潘鸿辉, 刘艳, 刘细祥. 磷钼酸-Fe3O4球磨共改性污泥基生物炭对四环素的吸附特性[J]. 化工进展, 2025, 44(1): 583-595. |
| [10] | 张炜, 黄赳, 朱晓芳, 李鹏. 凹凸棒石基钴钨水滑石吸附铅的性能及机理[J]. 化工进展, 2025, 44(1): 596-606. |
| [11] | 李琳, 黄国勇, 徐盛明, 郁丰善, 翁雅青, 曹才放, 温嘉玮, 王春霞, 王俊莲, 顾斌涛, 张袁华, 刘斌, 王才平, 潘剑明, 徐泽良, 王翀, 王珂. 铝基废催化剂载体的回收与再生制备[J]. 化工进展, 2024, 43(S1): 640-649. |
| [12] | 石磊, 王倩, 赵晓胜, 刘宏臣, 车远军, 段玉, 李庆. 油页岩灰基分子筛的制备及对亚甲基蓝的吸附[J]. 化工进展, 2024, 43(S1): 650-661. |
| [13] | 李依梦, 陈运全, 何畅, 张冰剑, 陈清林. 基于物理信息神经网络的甲烷无氧芳构化反应的正反问题[J]. 化工进展, 2024, 43(9): 4817-4823. |
| [14] | 申纯宇, 李翠利, 汤建伟, 刘咏, 刘鹏飞, 丁俊祥, 申博, 王保明. 纳米氢氧化镁制备及其阻燃应用进展[J]. 化工进展, 2024, 43(9): 4980-4995. |
| [15] | 刘丽, 冯博, 文洋, 古启雄. 硅基介孔材料的合成、功能化及对金属的吸附研究进展[J]. 化工进展, 2024, 43(9): 5063-5078. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||