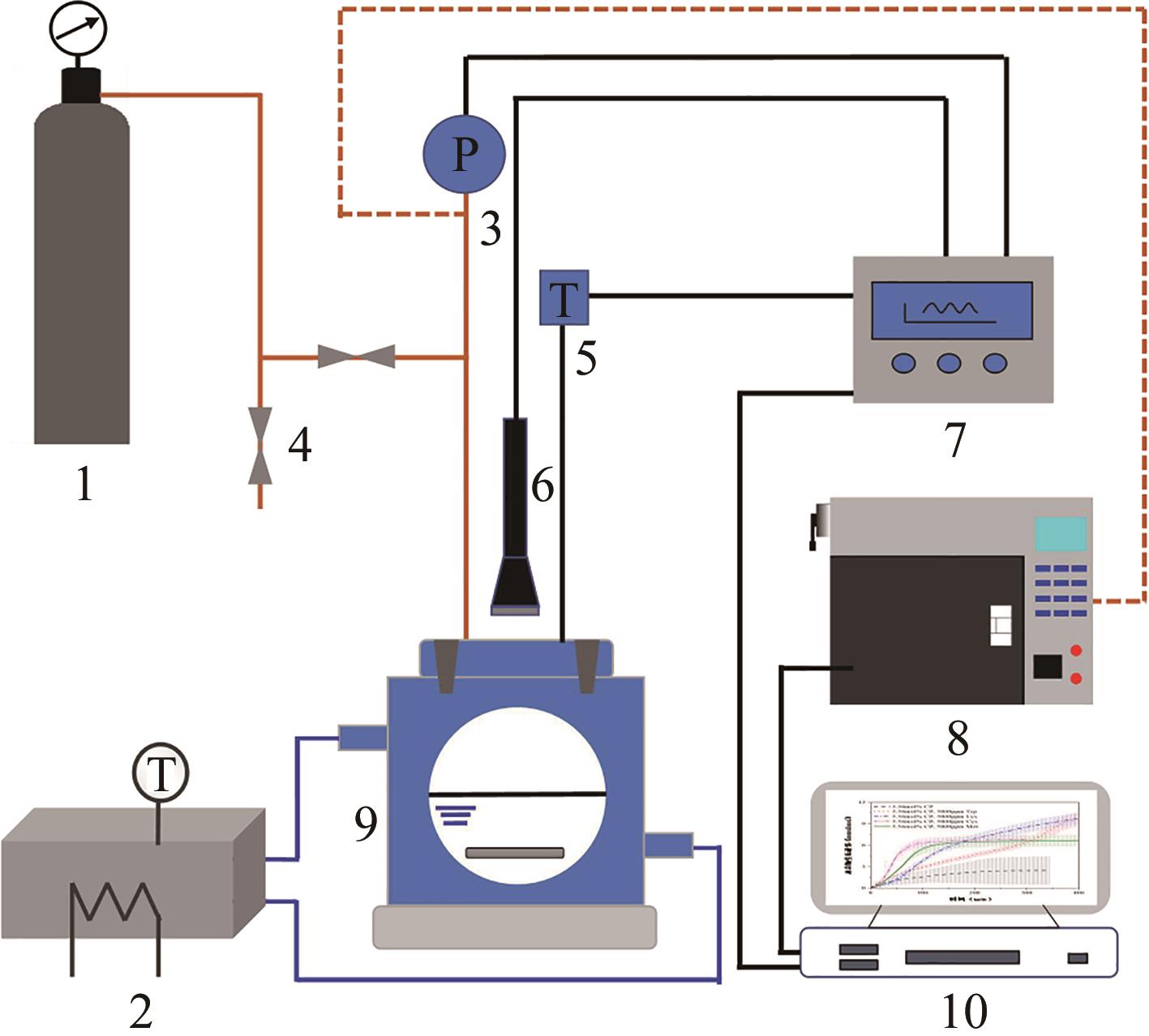
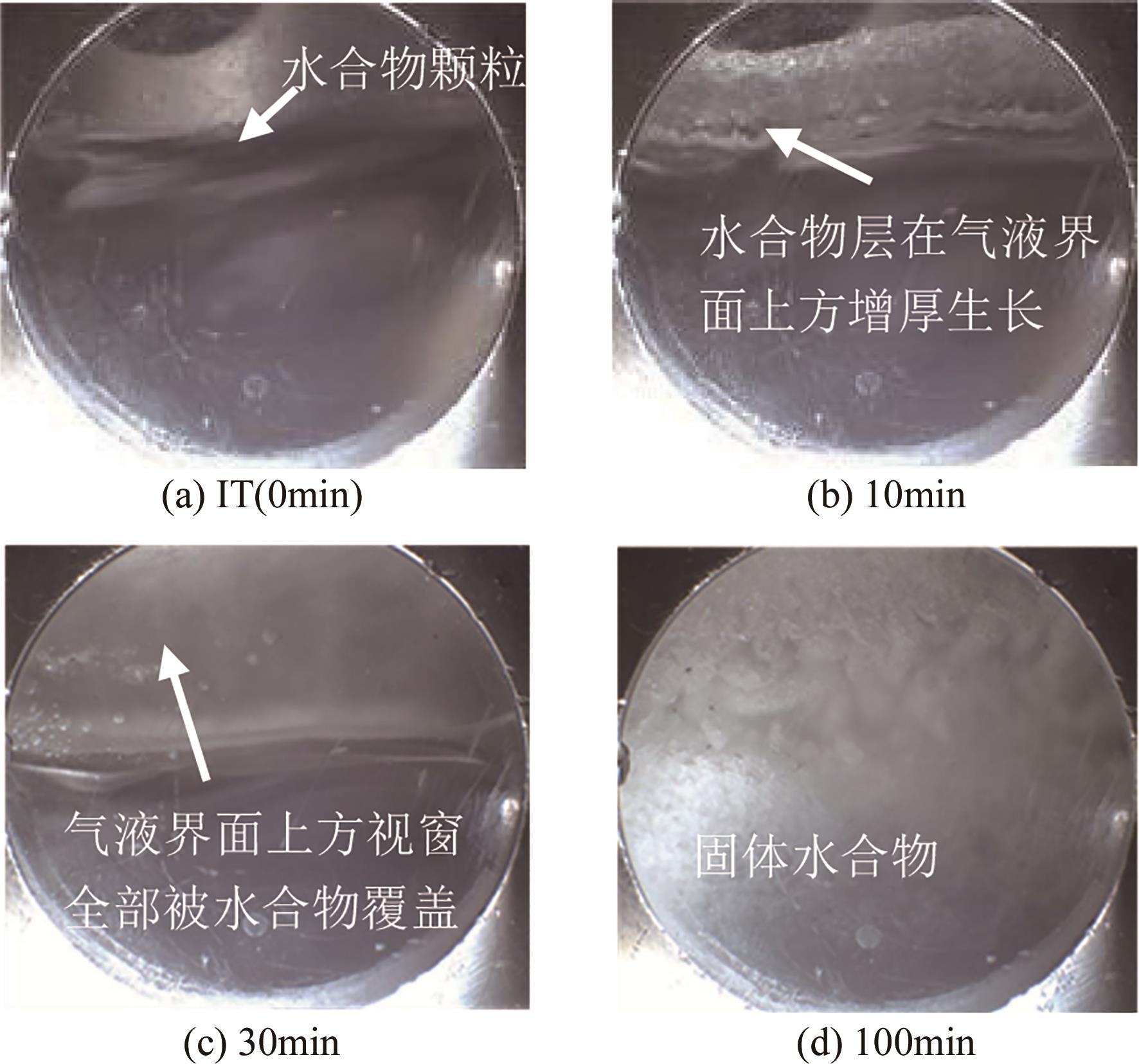
化工进展 ›› 2025, Vol. 44 ›› Issue (1): 192-201.DOI: 10.16085/j.issn.1000-6613.2024-0024
混合热力学促进剂对水合物法分离回收瓦斯的影响
张强1( ), 孙楠1, 郑俊杰2, 吴强1, 刘传海1, 李元吉1
), 孙楠1, 郑俊杰2, 吴强1, 刘传海1, 李元吉1
- 1.黑龙江科技大学安全工程学院,黑龙江 哈尔滨 150022
2.新加坡国立大学化学与生物分子工程系,新加坡 117585
-
收稿日期:2024-01-04修回日期:2024-05-16出版日期:2025-01-15发布日期:2025-02-13 -
通讯作者:张强 -
作者简介:张强(1986—),教授,博士生导师,从事瓦斯灾害防治与利用、水合物技术应用研究。E-mail:zq3946630@163.com。 -
基金资助:黑龙江省自然科学基金(YQ2022E041)
Effect of mixed thermodynamic promoters on kinetic and recovery study of hydration separation coal mine gas
ZHANG Qiang1( ), SUN Nan1, ZHENG Junjie2, WU Qiang1, LIU Chuanhai1, LI Yuanji1
), SUN Nan1, ZHENG Junjie2, WU Qiang1, LIU Chuanhai1, LI Yuanji1
- 1.College of Safety Engineering, Heilongjiang University of Science & Technology, Harbin 150022, Heilongjiang, China
2.Department of Chemical and Biomolecular Engineering, National University of Singapore, Singapore 117585, Singapore
-
Received:2024-01-04Revised:2024-05-16Online:2025-01-15Published:2025-02-13 -
Contact:ZHANG Qiang
摘要:
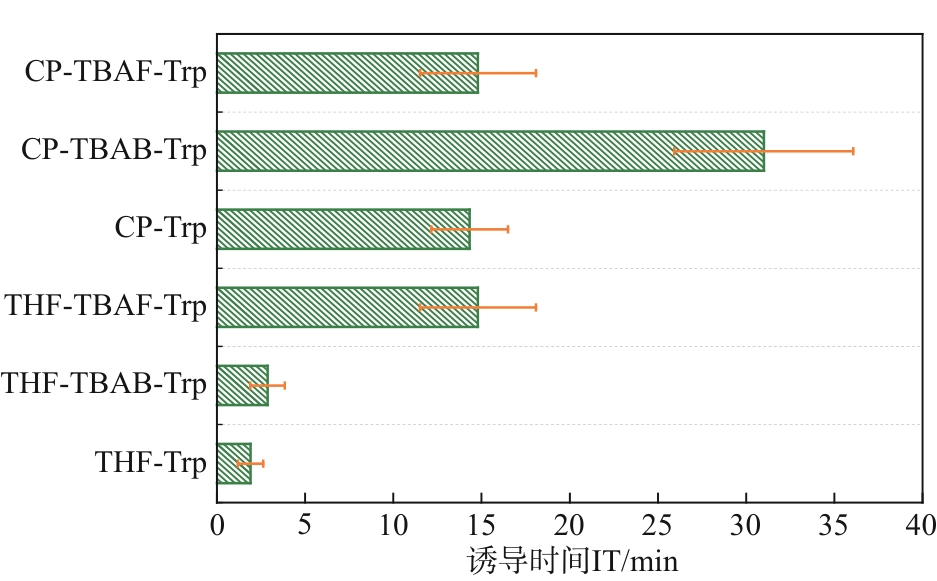
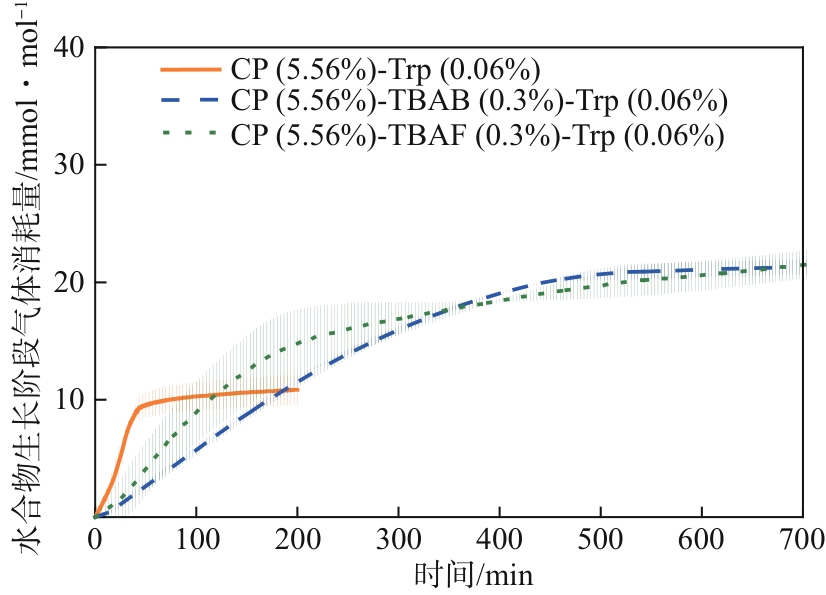
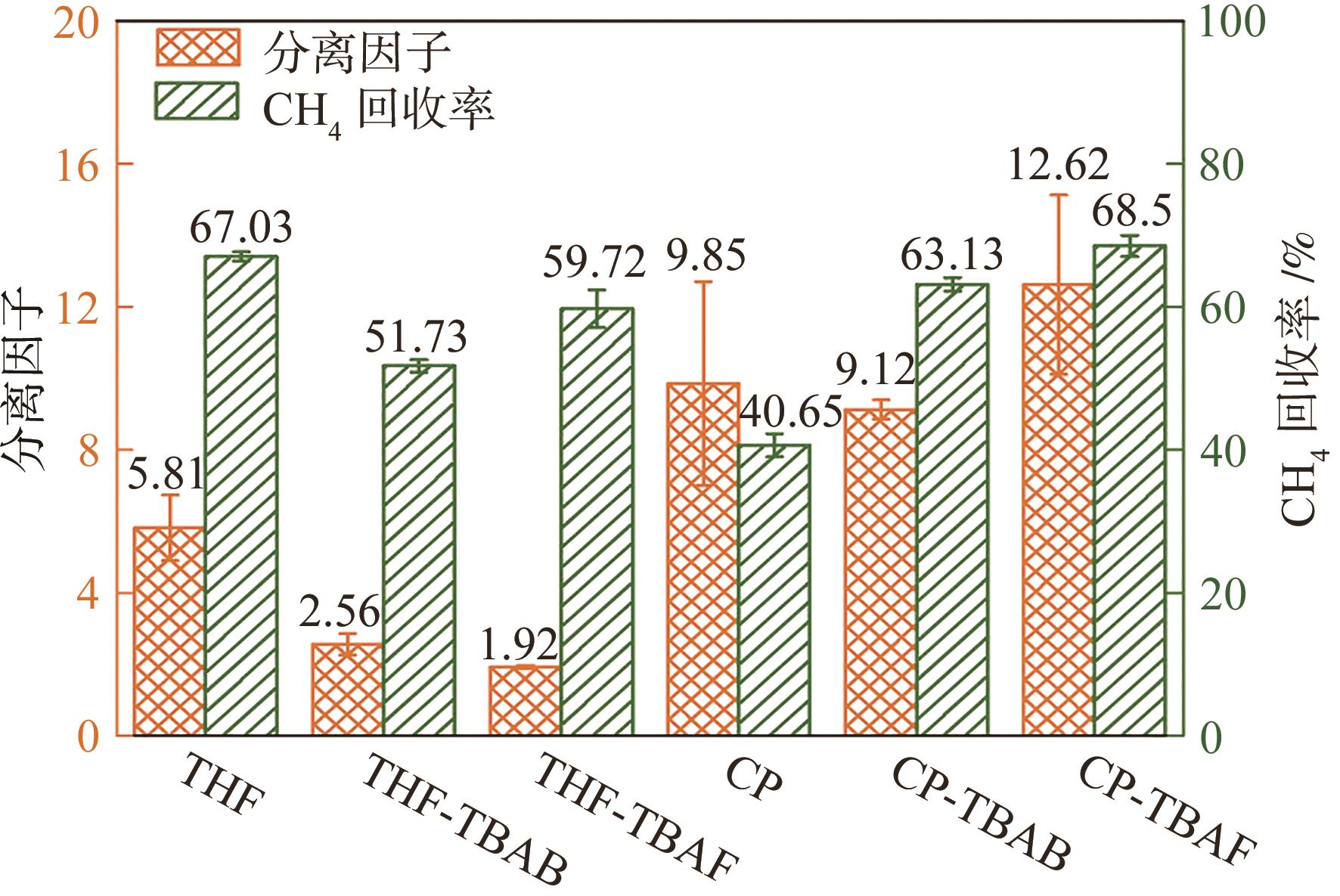
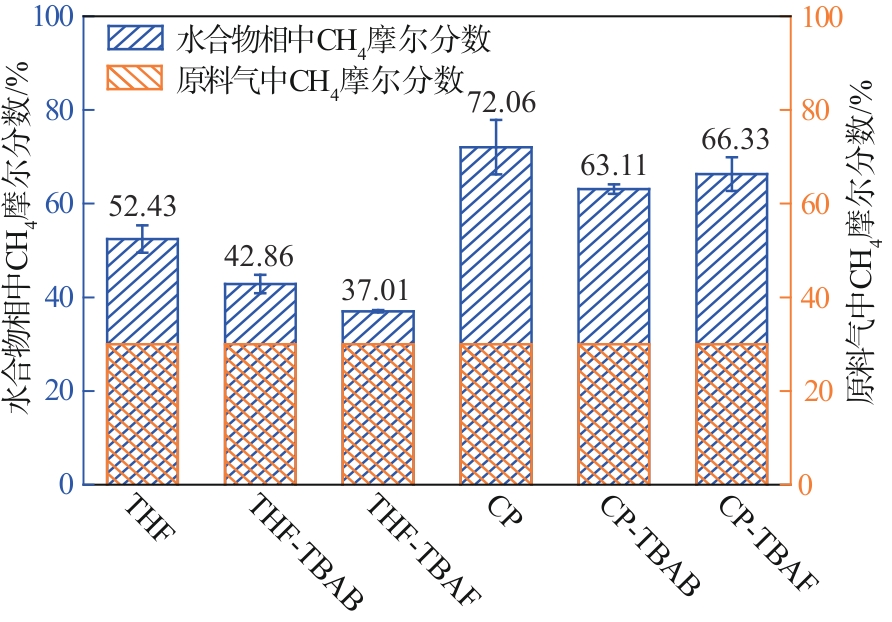
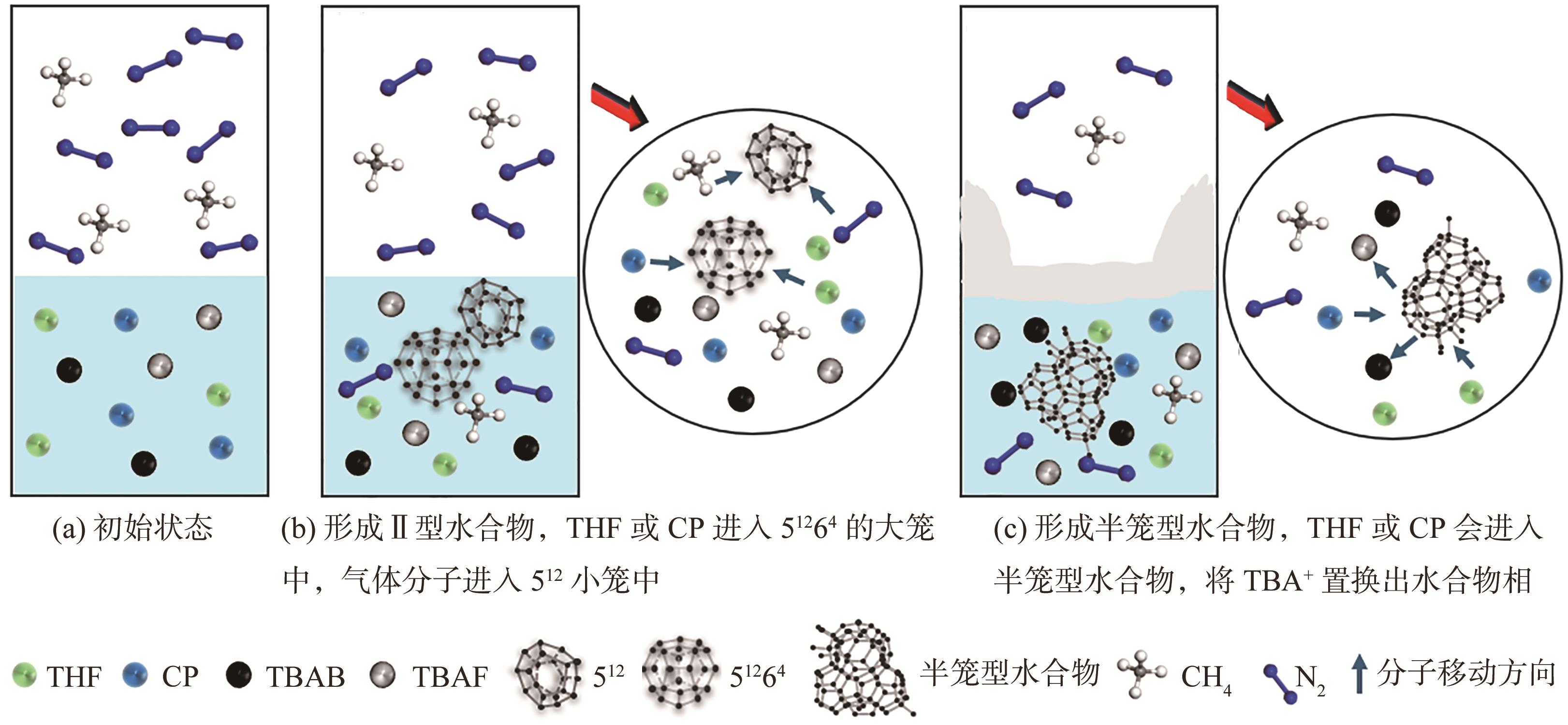
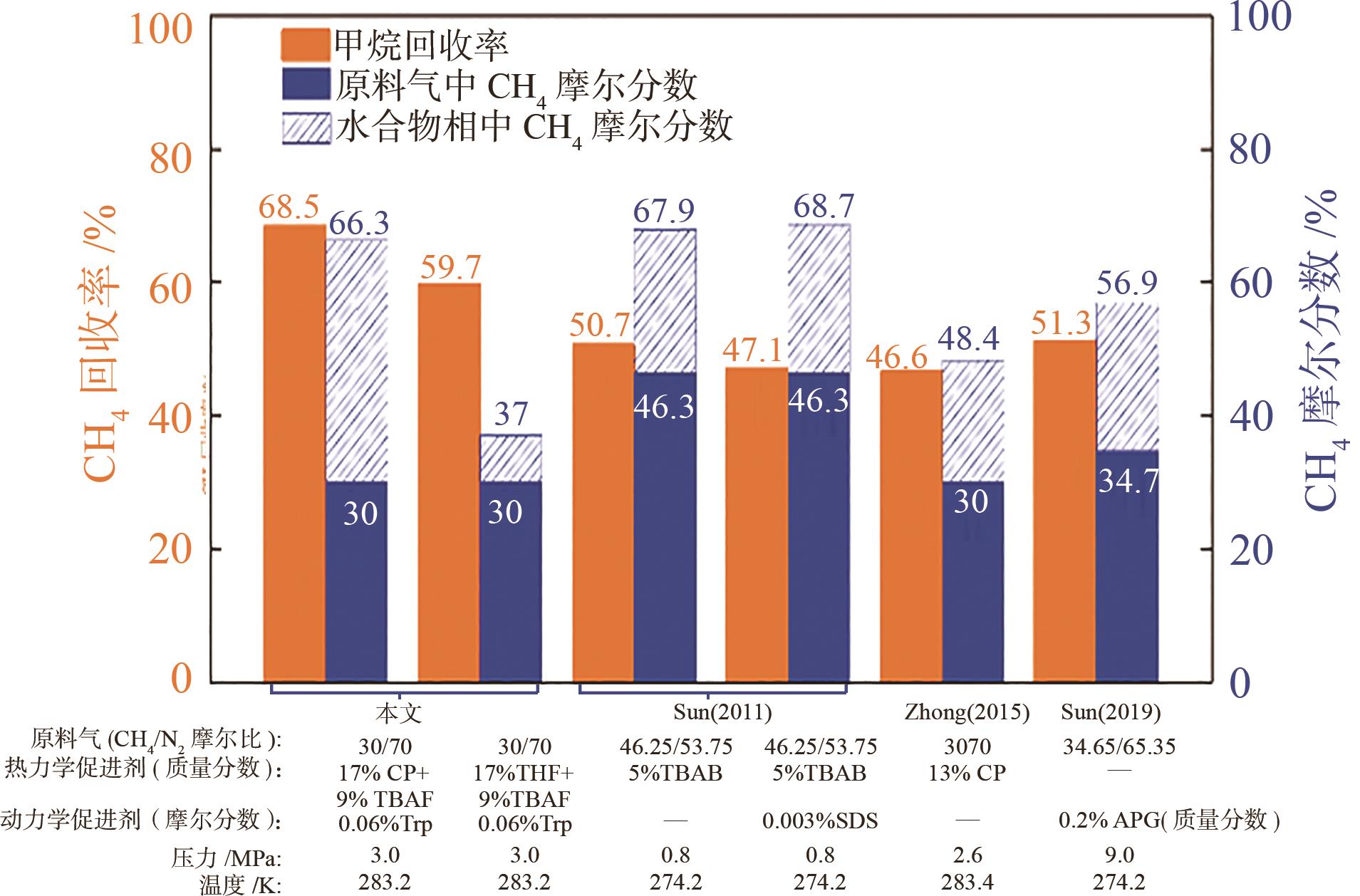
水合物法在分离瓦斯混合气体方面具有清洁、高效、安全的优势,为突破水合物储气效率的瓶颈问题,在磁力搅拌体系中考察了水合物法分离30% CH4/70% N2混合气(摩尔分数)的动力学规律与储气效率。四氢呋喃(THF)、环戊烷(CP)与四丁基溴化铵(TBAB)、四丁基氟化铵(TBAF)二元混合体系作为水合物形成热力学促进剂,0.06% L-色氨酸(Trp)作为动力学促进剂。结果表明:与THF或CP单一添加的实验体系相比,THF-TBAF或CP-TBAB、CP-TBAF体系均能延续水合物形成、提高储气量、降低形成速率,其中CP-TBAF改善效果最为明显,3h内的储气量提高了1.33倍,而水合物初期形成速率下降3倍以上。THF-TBAB-Trp、THF-TBAF-Trp体系增大了N2在水合物相的储集量,使CH4/N2的分离效果低于THF-Trp体系;CP与TBAB或TBAF在瓦斯水合分离过程中具有耦合促进作用,CP-TBAF使水合物储气量、分离因子、CH4回收率等关键指标全面提升,其中平均CH4回收率最高可达68.5%,CP-TBAF组合为突破瓦斯水合分离效率提供了参考。
中图分类号:
引用本文
张强, 孙楠, 郑俊杰, 吴强, 刘传海, 李元吉. 混合热力学促进剂对水合物法分离回收瓦斯的影响[J]. 化工进展, 2025, 44(1): 192-201.
ZHANG Qiang, SUN Nan, ZHENG Junjie, WU Qiang, LIU Chuanhai, LI Yuanji. Effect of mixed thermodynamic promoters on kinetic and recovery study of hydration separation coal mine gas[J]. Chemical Industry and Engineering Progress, 2025, 44(1): 192-201.
| 混合热力学促进剂体系 | 相平衡条件 | ∆p/MPa | |
|---|---|---|---|
| peq/MPa | T/K | ||
| (30%CH4/70%N2)THF-TBAB | 0.57 | 282.87 | 2.43 |
| (30%CH4/70%N2)THF-TBAF | 0.57 | 282.9 | 2.43 |
| (30%CH4/70%N2)CP-TBAB | 0.47 | 283.8 | 2.53 |
| (30%CH4/70%N2)CP-TBAF | 0.42 | 284.49 | 2.58 |
表1 混合热力学促进剂体系生成水合物的相平衡数据表
| 混合热力学促进剂体系 | 相平衡条件 | ∆p/MPa | |
|---|---|---|---|
| peq/MPa | T/K | ||
| (30%CH4/70%N2)THF-TBAB | 0.57 | 282.87 | 2.43 |
| (30%CH4/70%N2)THF-TBAF | 0.57 | 282.9 | 2.43 |
| (30%CH4/70%N2)CP-TBAB | 0.47 | 283.8 | 2.53 |
| (30%CH4/70%N2)CP-TBAF | 0.42 | 284.49 | 2.58 |
| 实验 编号 | 添加剂及 摩尔分数 | 诱导时间 IT/min | t90/min | IT时刻气体消耗量/mmol·mol-1 | IT+3h时刻气体消耗量/mmol·mol-1 | 初始水合物生长速率NR30 /mmol·mol-1·h-1 | 水合物相中CH4摩尔分数/% | 分离因子 | CH4回收率/% |
|---|---|---|---|---|---|---|---|---|---|
| A-1 | THF(5.56%)-Trp(0.06%) | 2.70 | 54.67 | 0.19 | 26.00 | 0.68 | 54.68 | 6.61 | 67.65 |
| A-2 | 1.30 | 55.00 | 0.16 | 25.23 | 0.65 | 53.50 | 6.01 | 66.36 | |
| A-3 | 1.70 | 40.67 | 0.08 | 30.05 | 0.79 | 49.12 | 4.80 | 67.08 | |
| 均值 | 1.9(±0.72) | 50.11(±8.18) | 0.14(±0.06) | 27.09(±2.59) | 0.71(±0.07) | 52.43(±2.93) | 5.81(±0.92) | 67.03(±0.65) | |
| A-4 | THF(5.56%)-TBAB(0.3%)-Trp(0.06%) | 2.30 | 476.00 | 0.02 | 17.50 | 0.21 | 40.72 | 2.23 | 50.96 |
| A-5 | 2.30 | 450.67 | 0.02 | 17.46 | 0.20 | 43.25 | 2.63 | 52.68 | |
| A-6 | 4.00 | 479.00 | 0.06 | 17.79 | 0.19 | 44.61 | 2.82 | 51.56 | |
| 均值 | 2.87(±0.98) | 468.56(±15.56) | 0.03(±0.02) | 17.58(±0.18) | 0.2(±0.01) | 42.86(±1.97) | 2.56(±0.3) | 51.73(±0.87) | |
| A-7 | THF(5.56%)-TBAF(0.3%)-Trp(0.06%) | 11.00 | 277.00 | 0.04 | 24.65 | 0.15 | 37.25 | 1.89 | 56.68 |
| A-8 | 16.70 | 271.67 | 0.05 | 29.36 | 0.30 | 37.02 | 1.97 | 61.55 | |
| A-9 | 16.70 | 209.00 | 0.02 | 31.17 | 0.19 | 36.75 | 1.91 | 60.93 | |
| 均值 | 14.8(±3.29) | 252.56(±37.82) | 0.04(±0.02) | 28.39(±3.37) | 0.21(±0.08) | 37.01(±0.25) | 1.92(±0.04) | 59.72(±2.65) | |
| B-1 | CP(5.56%)-Trp(0.06%) | 16.30 | 61.67 | 0.03 | 9.56 | 0.29 | 77.57 | 12.62 | 39.18 |
| B-2 | 14.70 | 65.00 | 0.01 | 12.06 | 0.21 | 65.97 | 6.91 | 40.36 | |
| B-3 | 12.00 | 68.33 | 0.06 | 10.65 | 0.17 | 72.63 | 10.02 | 42.40 | |
| 均值 | 14.33(±2.17) | 65(±3.33) | 0.03(±0.03) | 10.76(±1.25) | 0.22(±0.06) | 72.06(±5.82) | 9.85(±2.86) | 40.65(±1.63) | |
| B-4 | CP(5.56%)-TBAB(0.3%)-Trp(0.06%) | 36.70 | 384.33 | 0.01 | 10.77 | 0.05 | 64.04 | 9.32 | 62.10 |
| B-5 | 27.00 | 409.33 | 0.02 | 10.59 | 0.06 | 63.26 | 9.23 | 63.32 | |
| B-6 | 29.30 | 436.00 | 0.02 | 9.93 | 0.03 | 62.04 | 8.81 | 63.97 | |
| 均值 | 31.00(±5.07) | 409.89(±25.84) | 0.02(±0.01) | 10.43(±0.44) | 0.05(±0.02) | 63.11(±1.01) | 9.12(±0.27) | 63.13(±0.95) | |
| B-7 | CP(5.56%)-TBAF(0.3%)-Trp(0.06%) | 11.00 | 423.00 | 0.01 | 15.11 | 0.00 | 70.32 | 15.25 | 68.23 |
| B-8 | 16.70 | 641.00 | 0.02 | 16.45 | 0.14 | 65.32 | 12.36 | 70.12 | |
| B-9 | 16.70 | 467.67 | 0.01 | 10.62 | 0.06 | 63.36 | 10.24 | 67.15 | |
| 均值 | 14.8(±3.29) | 510.56(±115.15) | 0.01(±0.01) | 14.06(±3.05) | 0.07(±0.07) | 66.33(±3.59) | 12.62(±2.51) | 68.5(±1.50) | |
| C | 纯水 | — | — | — | — | — | — | — | — |
表2 在283.2K和3.0MPa(初始压力)条件下不同体系中水合物形成动力学参数、耗气量、水合分离结果参数的实验结果
| 实验 编号 | 添加剂及 摩尔分数 | 诱导时间 IT/min | t90/min | IT时刻气体消耗量/mmol·mol-1 | IT+3h时刻气体消耗量/mmol·mol-1 | 初始水合物生长速率NR30 /mmol·mol-1·h-1 | 水合物相中CH4摩尔分数/% | 分离因子 | CH4回收率/% |
|---|---|---|---|---|---|---|---|---|---|
| A-1 | THF(5.56%)-Trp(0.06%) | 2.70 | 54.67 | 0.19 | 26.00 | 0.68 | 54.68 | 6.61 | 67.65 |
| A-2 | 1.30 | 55.00 | 0.16 | 25.23 | 0.65 | 53.50 | 6.01 | 66.36 | |
| A-3 | 1.70 | 40.67 | 0.08 | 30.05 | 0.79 | 49.12 | 4.80 | 67.08 | |
| 均值 | 1.9(±0.72) | 50.11(±8.18) | 0.14(±0.06) | 27.09(±2.59) | 0.71(±0.07) | 52.43(±2.93) | 5.81(±0.92) | 67.03(±0.65) | |
| A-4 | THF(5.56%)-TBAB(0.3%)-Trp(0.06%) | 2.30 | 476.00 | 0.02 | 17.50 | 0.21 | 40.72 | 2.23 | 50.96 |
| A-5 | 2.30 | 450.67 | 0.02 | 17.46 | 0.20 | 43.25 | 2.63 | 52.68 | |
| A-6 | 4.00 | 479.00 | 0.06 | 17.79 | 0.19 | 44.61 | 2.82 | 51.56 | |
| 均值 | 2.87(±0.98) | 468.56(±15.56) | 0.03(±0.02) | 17.58(±0.18) | 0.2(±0.01) | 42.86(±1.97) | 2.56(±0.3) | 51.73(±0.87) | |
| A-7 | THF(5.56%)-TBAF(0.3%)-Trp(0.06%) | 11.00 | 277.00 | 0.04 | 24.65 | 0.15 | 37.25 | 1.89 | 56.68 |
| A-8 | 16.70 | 271.67 | 0.05 | 29.36 | 0.30 | 37.02 | 1.97 | 61.55 | |
| A-9 | 16.70 | 209.00 | 0.02 | 31.17 | 0.19 | 36.75 | 1.91 | 60.93 | |
| 均值 | 14.8(±3.29) | 252.56(±37.82) | 0.04(±0.02) | 28.39(±3.37) | 0.21(±0.08) | 37.01(±0.25) | 1.92(±0.04) | 59.72(±2.65) | |
| B-1 | CP(5.56%)-Trp(0.06%) | 16.30 | 61.67 | 0.03 | 9.56 | 0.29 | 77.57 | 12.62 | 39.18 |
| B-2 | 14.70 | 65.00 | 0.01 | 12.06 | 0.21 | 65.97 | 6.91 | 40.36 | |
| B-3 | 12.00 | 68.33 | 0.06 | 10.65 | 0.17 | 72.63 | 10.02 | 42.40 | |
| 均值 | 14.33(±2.17) | 65(±3.33) | 0.03(±0.03) | 10.76(±1.25) | 0.22(±0.06) | 72.06(±5.82) | 9.85(±2.86) | 40.65(±1.63) | |
| B-4 | CP(5.56%)-TBAB(0.3%)-Trp(0.06%) | 36.70 | 384.33 | 0.01 | 10.77 | 0.05 | 64.04 | 9.32 | 62.10 |
| B-5 | 27.00 | 409.33 | 0.02 | 10.59 | 0.06 | 63.26 | 9.23 | 63.32 | |
| B-6 | 29.30 | 436.00 | 0.02 | 9.93 | 0.03 | 62.04 | 8.81 | 63.97 | |
| 均值 | 31.00(±5.07) | 409.89(±25.84) | 0.02(±0.01) | 10.43(±0.44) | 0.05(±0.02) | 63.11(±1.01) | 9.12(±0.27) | 63.13(±0.95) | |
| B-7 | CP(5.56%)-TBAF(0.3%)-Trp(0.06%) | 11.00 | 423.00 | 0.01 | 15.11 | 0.00 | 70.32 | 15.25 | 68.23 |
| B-8 | 16.70 | 641.00 | 0.02 | 16.45 | 0.14 | 65.32 | 12.36 | 70.12 | |
| B-9 | 16.70 | 467.67 | 0.01 | 10.62 | 0.06 | 63.36 | 10.24 | 67.15 | |
| 均值 | 14.8(±3.29) | 510.56(±115.15) | 0.01(±0.01) | 14.06(±3.05) | 0.07(±0.07) | 66.33(±3.59) | 12.62(±2.51) | 68.5(±1.50) | |
| C | 纯水 | — | — | — | — | — | — | — | — |
| 1 | EDENHOFER O, MADRUGA R P, SOKONA Y, et al. Climate change 2014: mitigation of climate change[M]. Cambridge (UK): Cambridge University Press, 2015: 46-48. |
| 2 | 桑树勋, 袁亮, 刘世奇, 等. 碳中和地质技术及其煤炭低碳化应用前瞻[J]. 煤炭学报, 2022, 47(4): 1430-1451. |
| SANG Shuxun, YUAN Liang, LIU Shiqi, et al. Geological technology for carbon neutrality and its application prospect for low carbon coal exploitation and utilization[J]. Journal of China Coal Society, 2022, 47(4): 1430-1451. | |
| 3 | 游小叶. 与瓦斯共——舞产学研联动, 谱瓦斯治理利用新曲[J]. 中国高新科技, 2022(3): 5-10. |
| YOU Xiaoye. Dancing with gas—Industry-academia-research linkage, a new song of gas treatment and utilization [J] China High-Tech, 2022(3): 5-10. | |
| 4 | 黄中伟, 李国富, 杨睿月, 等. 我国煤层气开发技术现状与发展趋势[J]. 煤炭学报, 2022, 47(9): 3212-3238. |
| HUANG Zhongwei, LI Guofu, YANG Ruiyue, et al. Review and development trends of coalbed methane exploitation technology in China[J]. Journal of China Coal Society, 2022, 47(9): 3212-3238. | |
| 5 | 吴强, 张保勇. THF-SDS对矿井瓦斯水合分离影响研究[J]. 中国矿业大学学报, 2010, 39(4): 484-489. |
| WU Qiang, ZHANG Baoyong. The effect of THF-SDS on separation of methane-hydrate from mine gas[J]. Journal of China University of Mining & Technology, 2010, 39(4): 484-489. | |
| 6 | ZHANG Qiang, WU Qiang, ZHANG Hui, et al. Effect of montmorillonite on hydrate-based methane separation from mine gas[J]. Journal of Central South University, 2018, 25(1): 38-50. |
| 7 | 魏纳, 白睿玲, 周守为, 等. 碳达峰目标下中国深海天然气水合物开发战略[J]. 天然气工业, 2022, 42(2): 156-165. |
| WEI Na, BAI Ruiling, ZHOU Shouwei, et al. China’s deepwater gas hydrate development strategies under the goal of carbon peak[J]. Natural Gas Industry, 2022, 42(2): 156-165. | |
| 8 | 李占东, 干毕成, 李中, 等. 天然气水合物降压开采与出砂实验研究[J]. 中国矿业大学学报, 2020, 49(6): 1128-1136. |
| LI Zhandong, GAN Bicheng, LI Zhong, et al. An experimental study of natural gas hydrates sand production using depressurization[J]. Journal of China University of Mining & Technology, 2020, 49(6): 1128-1136. | |
| 9 | 曹代勇, 李靖, 王丹, 等. 青海木里煤田天然气水合物稳定带研究[J]. 中国矿业大学学报, 2013, 42(1): 76-82. |
| CAO Daiyong, LI Jing, WANG Dan, et al. Study of the gas hydrate stability zone in Muri Coalfield, Qinghai Province, China[J]. Journal of China University of Mining & Technology, 2013, 42(1): 76-82. | |
| 10 | LI Haoyang, LI Xiaosen, YU Yisong, et al. Morphologies, kinetics and structures of methane hydrate in the system containing tetrahydrofuran and cyclopentane[J]. Fuel, 2023, 340: 127585. |
| 11 | FU Juan, MO Jiamei, LIU Shijun, et al. Thermodynamic characteristics of methane hydrate formation in high-pressure microcalorimeter under different reaction kinetics[J]. Fuel, 2023, 332: 126072. |
| 12 | ZHANG Qiang, ZHENG Junjie, ZHANG Baoyong, et al. Coal mine gas separation of methane via clathrate hydrate process aided by tetrahydrofuran and amino acids[J]. Applied Energy, 2021, 287: 116576. |
| 13 | ZHENG Junjie, ZHANG Baoyong, WU Qiang, et al. Kinetic evaluation of cyclopentane as a promoter for CO2 capture via a clathrate process employing different contact modes[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(9): 11913-11921. |
| 14 | ZHONG Dongliang, DARABOINA Nagu, ENGLEZOS Peter. Recovery of CH4 from coal mine model gas mixture (CH4/N2) by hydrate crystallization in the presence of cyclopentane[J]. Fuel, 2013, 106: 425-430. |
| 15 | SUN Qiang, GUO Xuqiang, LIU Aixian, et al. Experimental study on the separation of CH4 and N2 via hydrate formation in TBAB solution[J]. Industrial & Engineering Chemistry Research, 2011, 50(4): 2284-2288. |
| 16 | ZHONG Dongliang, ENGLEZOS Peter. Methane separation from coal mine methane gas by tetra-n-butyl ammonium bromide semiclathrate hydrate formation[J]. Energy & Fuels, 2012, 26(4): 2098-2106. |
| 17 | ZHENG Junjie, BHATNAGAR Krittika, KHURANA Maninder, et al. Semiclathrate based CO2 capture from fuel gas mixture at ambient temperature: Effect of concentrations of tetra-n-butylammonium fluoride (TBAF) and kinetic additives[J]. Applied Energy, 2018, 217: 377-389. |
| 18 | MOHAMMADI Abolfazl. The roles TBAF and SDS on the kinetics of methane hydrate formation as a cold storage material[J]. Journal of Molecular Liquids, 2020, 309: 113175. |
| 19 | VELUSWAMY Hari Prakash, LEE Pei Yit, PREMASINGHE Kulesha, et al. Effect of biofriendly amino acids on the kinetics of methane hydrate formation and dissociation[J]. Industrial & Engineering Chemistry Research, 2017, 56(21): 6145-6154. |
| 20 | 何京玲, 诸林. SDS和THF对水合物法捕集模拟烟气中CO2的影响[J]. 石油与天然气化工, 2019, 48(2): 63-69. |
| HE Jingling, ZHU Lin. Effect of SDS and THF on CO2 captured from simulated flue gas by hydrate based gas separation[J]. Chemical Engineering of Oil & Gas, 2019, 48(2): 63-69. | |
| 21 | LI Xiaosen, XU Chungang, CHEN Zhaoyang, et al. Synergic effect of cyclopentane and tetra-n-butyl ammonium bromide on hydrate-based carbon dioxide separation from fuel gas mixture by measurements of gas uptake and X-ray diffraction patterns[J]. International Journal of Hydrogen Energy, 2012, 37(1): 720-727. |
| 22 | LI Xiaosen, XU Chungang, CHEN Zhaoyang, et al. Hydrate-based pre-combustion carbon dioxide capture process in the system with tetra-n-butyl ammonium bromide solution in the presence of cyclopentane[J]. Energy, 2011, 36(3): 1394-1403. |
| 23 | YANG Mingjun, ZHOU Hang, WANG Pengfei, et al. Effects of additives on continuous hydrate-based flue gas separation[J]. Applied Energy, 2018, 221: 374-385. |
| 24 | 王银, 赵建忠, 高强, 等. L-色氨酸+四氢呋喃体系下水合物法分离煤层气研究[J]. 化学工程, 2022, 50(5): 17-21, 24. |
| WANG Yin, ZHAO Jianzhong, GAO Qiang, et al. Separation of coalbed methane by hydrates method in L-tryptophan+tetrahydrofuran system[J]. Chemical Engineering (China), 2022, 50(5): 17-21, 24. | |
| 25 | 吕秋楠, 李小森, 李刚, 等. 水合物法分离低浓度煤层气中的甲烷[J]. 过程工程学报, 2019, 19(6): 1129-1134. |
| Qiunan LYU, LI Xiaosen, LI Gang, et al. Separation of methane from low concentration coal bed methane by hydrate-based process[J]. The Chinese Journal of Process Engineering, 2019, 19(6): 1129-1134. | |
| 26 | 王燕鸿, 姚凯, 郎雪梅, 等. 高含水油包水乳液的水合物储气性能研究[J]. 化工学报, 2021, 72(9): 4872-4880. |
| WANG Yanhong, YAO Kai, LANG Xuemei, et al. Investigation on hydrate-based methane storage properties in water-in-oil emulsion with high water content[J]. CIESC Journal, 2021, 72(9): 4872-4880. | |
| 27 | VELUSWAMY Hari Prakash, KUMAR Rajnish, LINGA Praveen. Hydrogen storage in clathrate hydrates: Current state of the art and future directions[J]. Applied Energy, 2014, 122: 112-132. |
| 28 | GAIKWAD Namrata, BHATTACHARJEE Gaurav, SANGWAI Jitendra S, et al. Kinetic and morphology study of equimolar CO2-CH4 hydrate formation in the presence of cyclooctane and L-tryptophan[J]. Energy & Fuels, 2021, 35(1): 636-648. |
| 29 | BABU Ponnivalavan, KUMAR Rajnish, LINGA Praveen. A new porous material to enhance the kinetics of clathrate process: Application to precombustion carbon dioxide capture[J]. Environmental Science & Technology, 2013, 47(22): 13191-13198. |
| 30 | LINGA Praveen, ADEYEMO Adebola, ENGLEZOS Peter. Medium-pressure clathrate hydrate/membrane hybrid process for postcombustion capture of carbon dioxide[J]. Environmental Science & Technology, 2008, 42(1): 315-320. |
| 31 | 徐纯刚, 李小森, 陈朝阳. 水合物法分离二氧化碳的研究现状[J]. 化工进展, 2011, 30(4): 701-708. |
| XU Chungang, LI Xiaosen, CHEN Zhaoyang. Research on hydrate-based carbon dioxide separation[J]. Chemical Industry and Engineering Progress, 2011, 30(4): 701-708. | |
| 32 | FAN Shuanshi, LI Shifeng, WANG Jingqu, et al. Efficient capture of CO2 from simulated flue gas by formation of TBAB or TBAF semiclathrate hydrates[J]. Energy & Fuels, 2009, 23(8): 4202-4208. |
| 33 | SÁNCHEZ-MORA María F, GALICIA-LUNA Luis A, Alfredo PIMENTEL-RODAS, et al. Experimental determination of gas hydrates dissociation conditions in CO2/N2+ethanol/1-propanol/TBAB/TBAF+water systems[J]. Journal of Chemical & Engineering Data, 2019, 64(2): 763-770. |
| 34 | SLOAN E Dendy. Introductory overview: Hydrate knowledge development[J]. American Mineralogist, 2004, 89(8/9): 1155-1161. |
| 35 | ZHANG Jibao, LI Yan, YIN Zhenyuan, et al. Coupling amino acid L-Val with THF for superior hydrogen hydrate kinetics: Implication for hydrate-based hydrogen storage[J]. Chemical Engineering Journal, 2023, 467: 143459. |
| 36 | LIU Xuejian, LI Yan, CHEN Guangjin, et al. Coupling amino acid with THF for the synergistic promotion of CO2 hydrate micro kinetics: Implication for hydrate-based CO2 sequestration[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(15): 6057-6069. |
| 37 | ZHANG Qiang, ZHENG Junjie, ZHANG Baoyong, et al. Kinetic evaluation of hydrate-based coalbed methane recovery process promoted by structure II thermodynamic promoters and amino acids[J]. Energy, 2023, 274: 127322. |
| 38 | SUN Qiang, AZAMAT Amankulov, CHEN Bo, et al. The effects of alkyl polyglucosides on the formation of CH4 hydrate and separation of CH4/N2 via hydrates formation[J]. Separation Science and Technology, 2020, 55(1): 81-87. |
| [1] | 李琢宇, 余美琪, 陈孝彦, 胡若晖, 王庆宏, 陈春茂, 詹亚力. 炼油废催化剂吸附去除水中硝基苯的特性与机制[J]. 化工进展, 2025, 44(2): 1076-1087. |
| [2] | 李依梦, 陈运全, 何畅, 张冰剑, 陈清林. 基于物理信息神经网络的甲烷无氧芳构化反应的正反问题[J]. 化工进展, 2024, 43(9): 4817-4823. |
| [3] | 何海霞, 万亚萌, 李帆帆, 牛心雨, 张静雯, 李涛, 任保增. 盐酸萘甲唑啉在甲醇-乙酸乙酯体系中的动力学及结晶工艺[J]. 化工进展, 2024, 43(8): 4230-4245. |
| [4] | 殷晨阳, 刘永峰, 陈睿哲, 张璐, 宋金瓯, 刘海峰. 基于量子化学计算的正己烷热解反应动力学模拟[J]. 化工进展, 2024, 43(8): 4273-4282. |
| [5] | 谢娟, 贺文, 赵勖丞, 李帅辉, 卢真真, 丁哲宇. 分子动力学模拟在沥青体系中的应用研究进展[J]. 化工进展, 2024, 43(8): 4432-4449. |
| [6] | 刘玉灿, 高中鲁, 徐心怡, 纪现国, 张岩, 孙洪伟, 王港. 钙改性水葫芦基生物炭吸附水中敌草隆的效能与机理[J]. 化工进展, 2024, 43(8): 4630-4641. |
| [7] | 曾武清, 王予, 卜庆国, 马硕, 白东明, 张宗建, 张鹏, 马丹丹, 王圣博, 王润其, 武丽雯, 刘晨, 马洪亭. 陈腐垃圾掺烧对垃圾炉焚烧特性的影响[J]. 化工进展, 2024, 43(8): 4642-4653. |
| [8] | 怀立业, 仲兆平, 杨宇轩. 脱硫石膏转化α-半水石膏的特征及机理:实验与模拟[J]. 化工进展, 2024, 43(8): 4694-4703. |
| [9] | 丁路, 王培尧, 孔令学, 白进, 于广锁, 李文, 王辅臣. 煤气化过程反应模型研究进展[J]. 化工进展, 2024, 43(7): 3593-3612. |
| [10] | 曹景沛, 姚乃瑜, 庞新博, 赵小燕, 赵静平, 蔡士杰, 徐敏, 冯晓博, 伊凤娇. 煤热解研究进展及其发展历程[J]. 化工进展, 2024, 43(7): 3620-3636. |
| [11] | 顾颂琦, 孙凡飞, 韦尧, 宋兴飞, 南兵, 李丽娜, 黄宇营. 时间分辨热化学原位XAFS方法[J]. 化工进展, 2024, 43(7): 3747-3755. |
| [12] | 张昊, 陆小明. 纳米钛酸钡前体热分解反应动力学及颗粒演化机理[J]. 化工进展, 2024, 43(7): 3987-3995. |
| [13] | 张东旭, 刘成, 宋乐春, 黄启玉, 王唯. 乳状液体系中气体水合物成核过程研究进展[J]. 化工进展, 2024, 43(6): 3007-3020. |
| [14] | 马栋, 解桂林, 田治华, 王勤辉, 张建国, 宋慧林, 钟晋. 流化床中煤气化细渣高温还原磷石膏过程[J]. 化工进展, 2024, 43(6): 3479-3491. |
| [15] | 江安迪, 丁雪兴, 王世鹏, 丁俊华, 力宁. 超临界CO2干气密封热动力学性能研究进展[J]. 化工进展, 2024, 43(5): 2354-2369. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||