化工进展 ›› 2024, Vol. 43 ›› Issue (6): 3007-3020.DOI: 10.16085/j.issn.1000-6613.2023-0767
• 能源加工与技术 • 上一篇
乳状液体系中气体水合物成核过程研究进展
- 1.中石化(大连)石油化工研究院有限公司,辽宁 大连 116045
2.中国石油大学(北京)油气管道输送安全国家工程实验室/城市油气输配技术北京市重点实验室,北京 102249
-
收稿日期:2023-05-09修回日期:2023-06-12出版日期:2024-06-15发布日期:2024-07-02 -
通讯作者:张东旭 -
作者简介:张东旭(1994—),男,博士,工程师,研究方向为管道流动保障及沥青基原油组分。E-mail:Dongxu_Henry_Zhang@163.com。 -
基金资助:国家自然科学基金(51374224)
Nucleation process of gas hydrates in the emulsion system: A review
ZHANG Dongxu1( ), LIU Cheng1, SONG Lechun1, HUANG Qiyu2, WANG Wei1
), LIU Cheng1, SONG Lechun1, HUANG Qiyu2, WANG Wei1
- 1.SINOPEC (Dalian) Research Institute of Petroleum and Petrochemical Company Limited, Dalian 116045, Liaoning, China
2.National Engineering Laboratory for Pipeline Safety/Beijing Key Laboratory of Urban Oil and Gas Distribution Technology, China University of Petroleum-Beijing, Beijing 102249, China
-
Received:2023-05-09Revised:2023-06-12Online:2024-06-15Published:2024-07-02 -
Contact:ZHANG Dongxu
摘要:
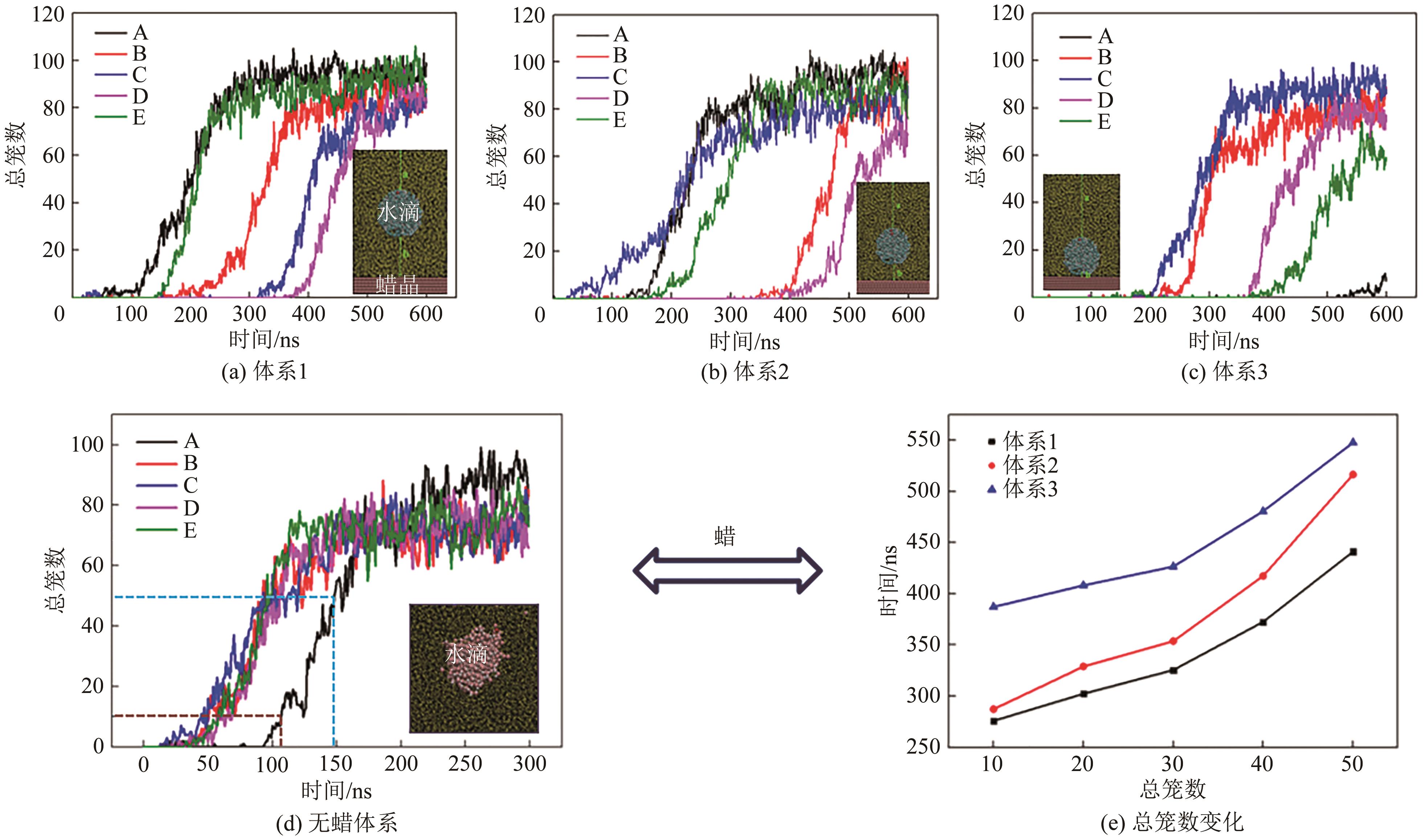
乳状液中气体水合物成核行为研究可用于评估油-气-水体系在亚稳态下稳定存在的时间,对低温环境下多相混输管道的安全运行和水合物风险控制策略的制定具有重大意义,因而成为深水管道流动安全保障的重点问题和研究热点。本文着眼于油水乳状液体系中气体水合物的成核过程,总结了实验研究中水合物成核速率的量化方法,细致分析和比较了当前研究中诱导期定义方法的异同,梳理了温度、压力、扰动强度、含水率和原油组分等环境条件和体系组成对水合物成核行为影响的研究成果及水合物成核速率预测模型。总体来看,油水乳状液中水合物的成核行为已经得到了较为深入的研究,在影响因素定性分析和模型定量描述等方面取得了一定成果,但在诱导期定义方法的统一和模型的工程化应用等方面仍需进行更为细致的研究。未来,应逐步建立统一的诱导期定义标准和更为普适性的诱导期预测模型,借助谱学仪器和分子动力学模拟等手段加深对水合物成核过程分子尺度信息的认识,更加深入理解水合物成核机理并逐渐实现量化模型的工程化应用。
中图分类号:
引用本文
张东旭, 刘成, 宋乐春, 黄启玉, 王唯. 乳状液体系中气体水合物成核过程研究进展[J]. 化工进展, 2024, 43(6): 3007-3020.
ZHANG Dongxu, LIU Cheng, SONG Lechun, HUANG Qiyu, WANG Wei. Nucleation process of gas hydrates in the emulsion system: A review[J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3007-3020.
| 计算依据 | 量化指标 | 表达式 | 方法特点 | 应用实例 |
|---|---|---|---|---|
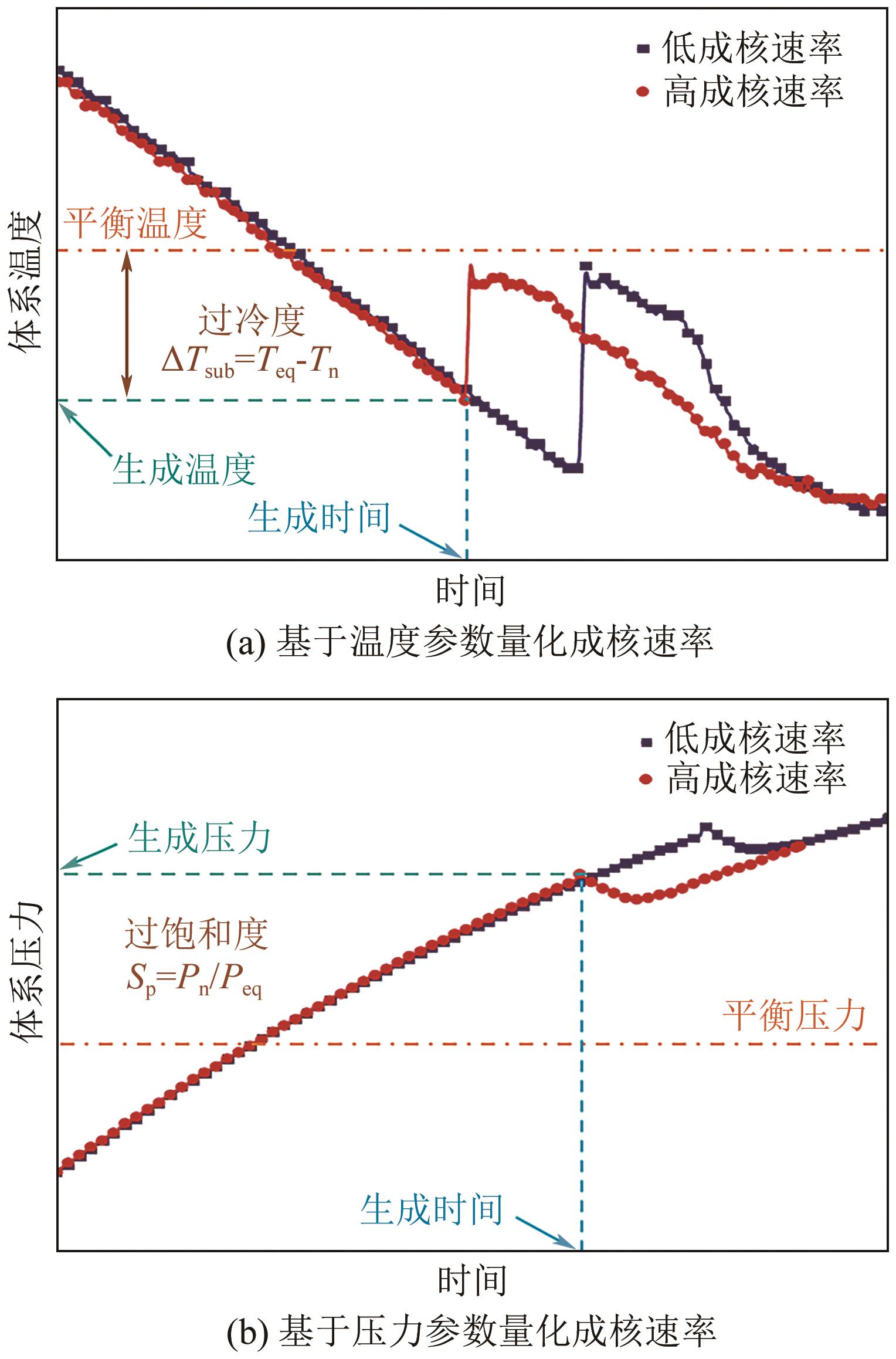
| 基于温度 | 生成温度 | Tn | ①用于降温水合物生成体系;②需借助温度传感器 | Daraboina等 |
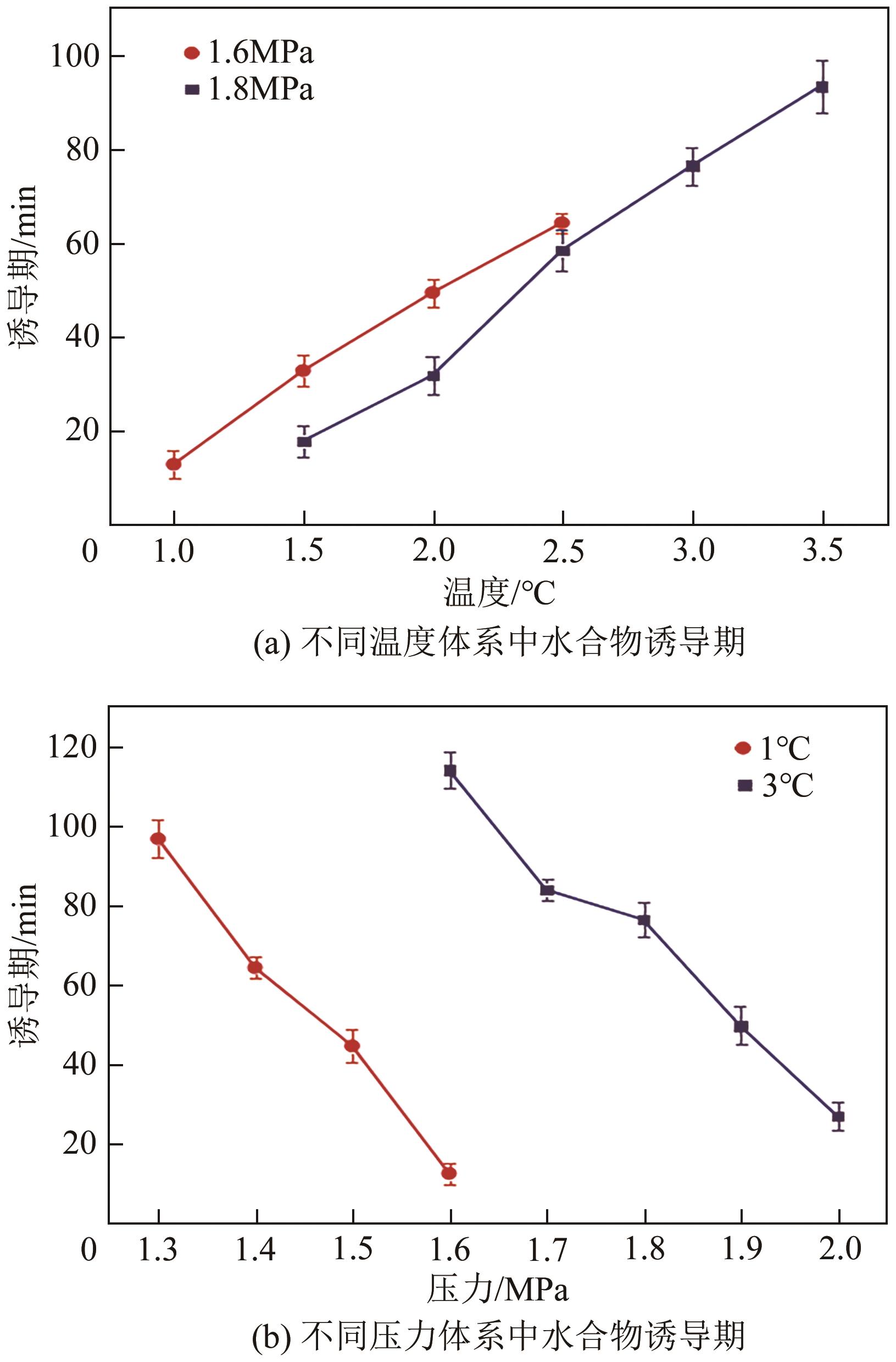
| 过冷度 | ΔT=Teq-Tn | ①多用于降温水合物生成体系;②需借助温度传感器;③需进行操作压力下平衡温度的计算 | Stoporev等 | |
| 基于压力 | 生成压力 | Pn | ①用于升压水合物生成体系;②需借助压力传感器 | Wang等 |
| 过饱和度 | Sp=Pn/Peq | ①多用于升压水合物生成体系;②需借助压力传感器;③需进行操作温度下平衡压力的计算 | 王唯 | |
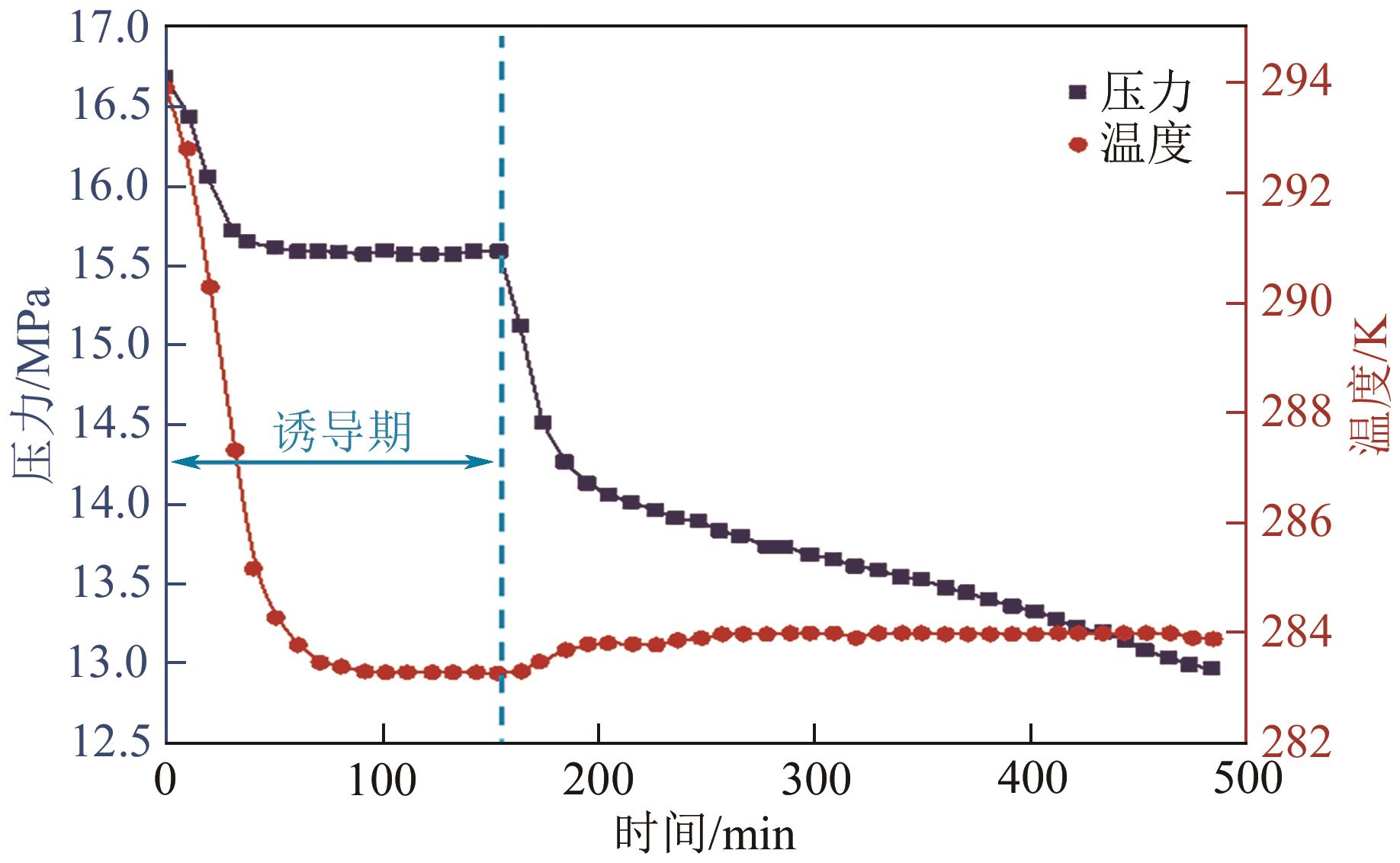
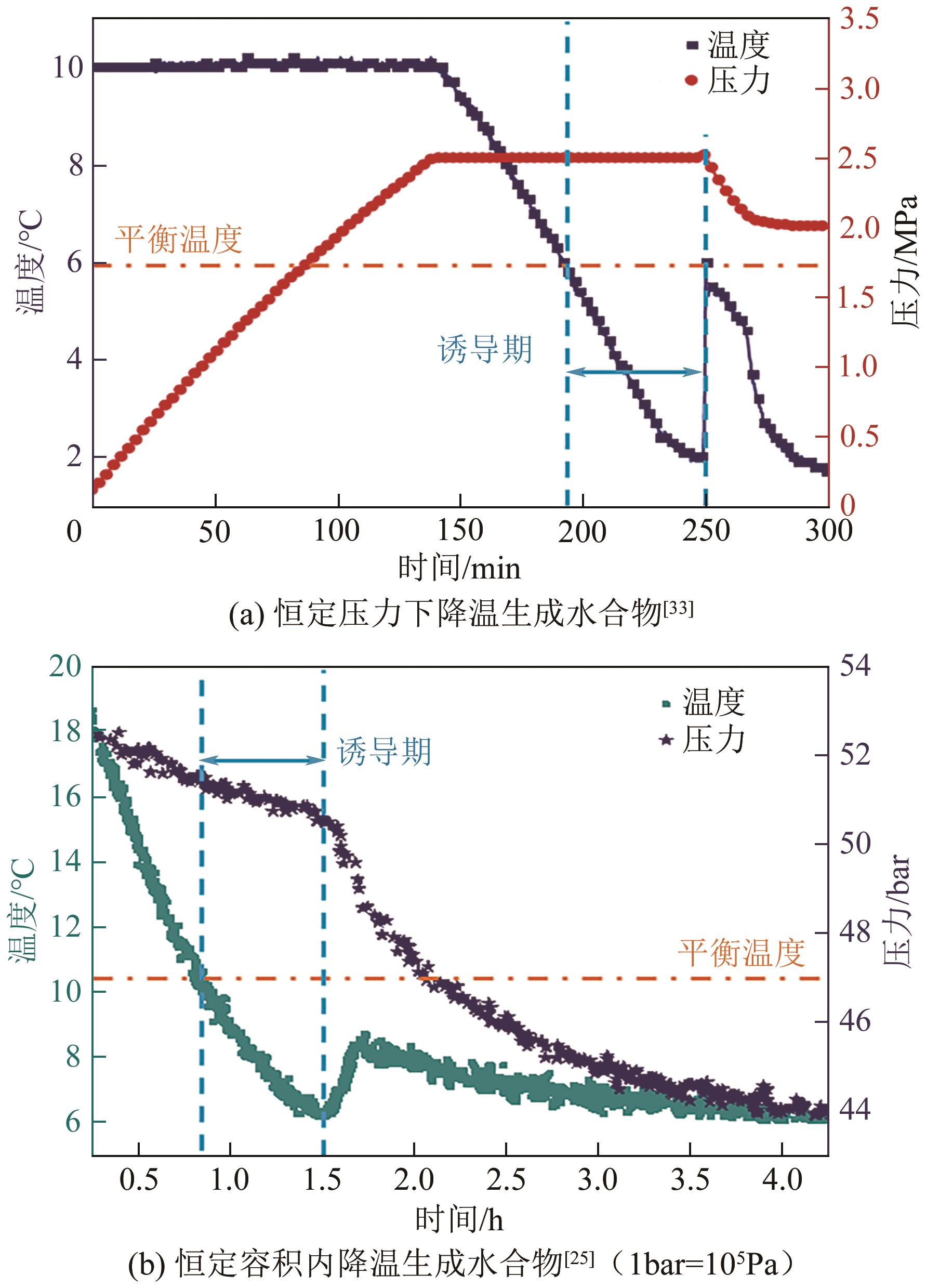
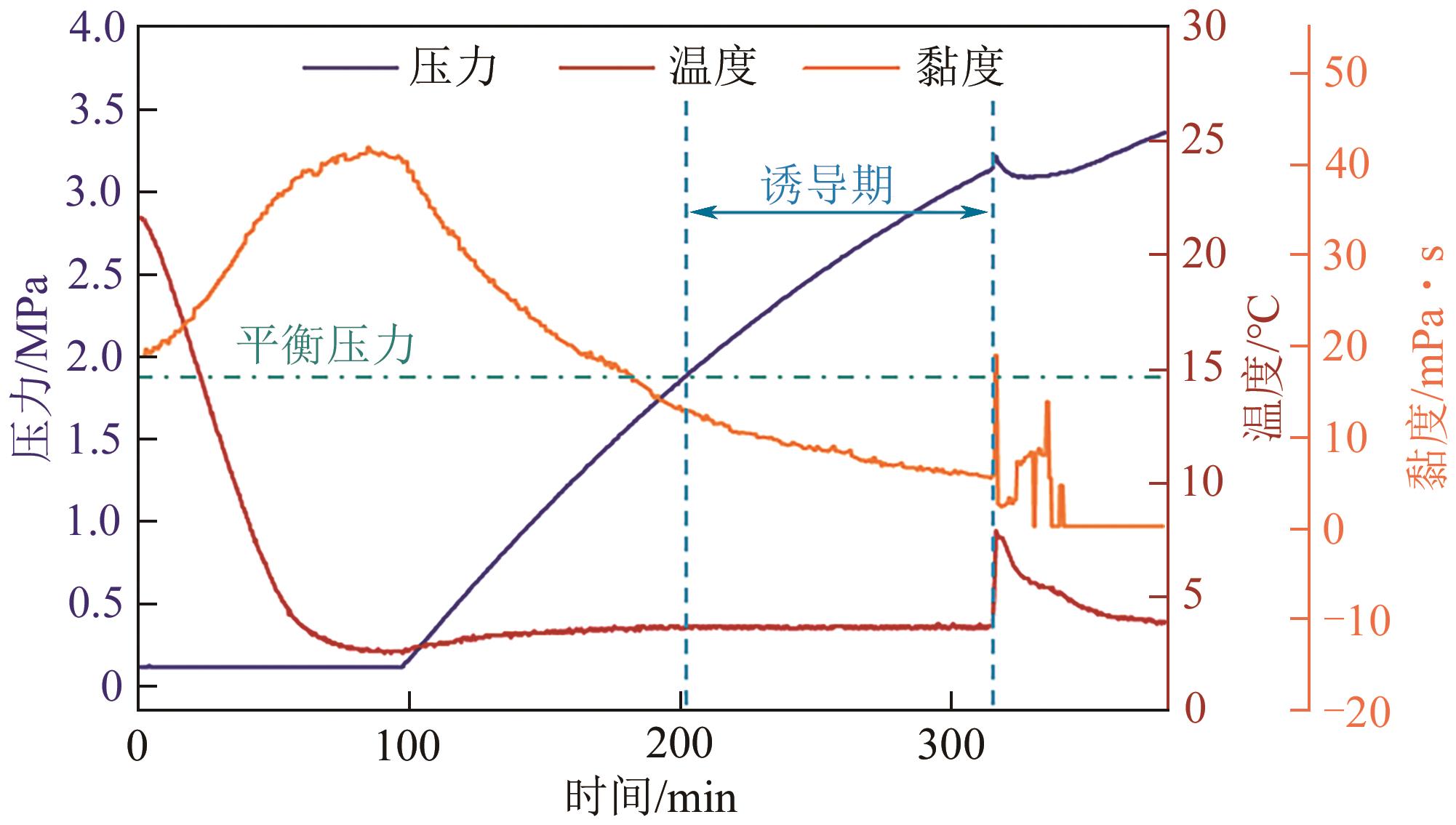
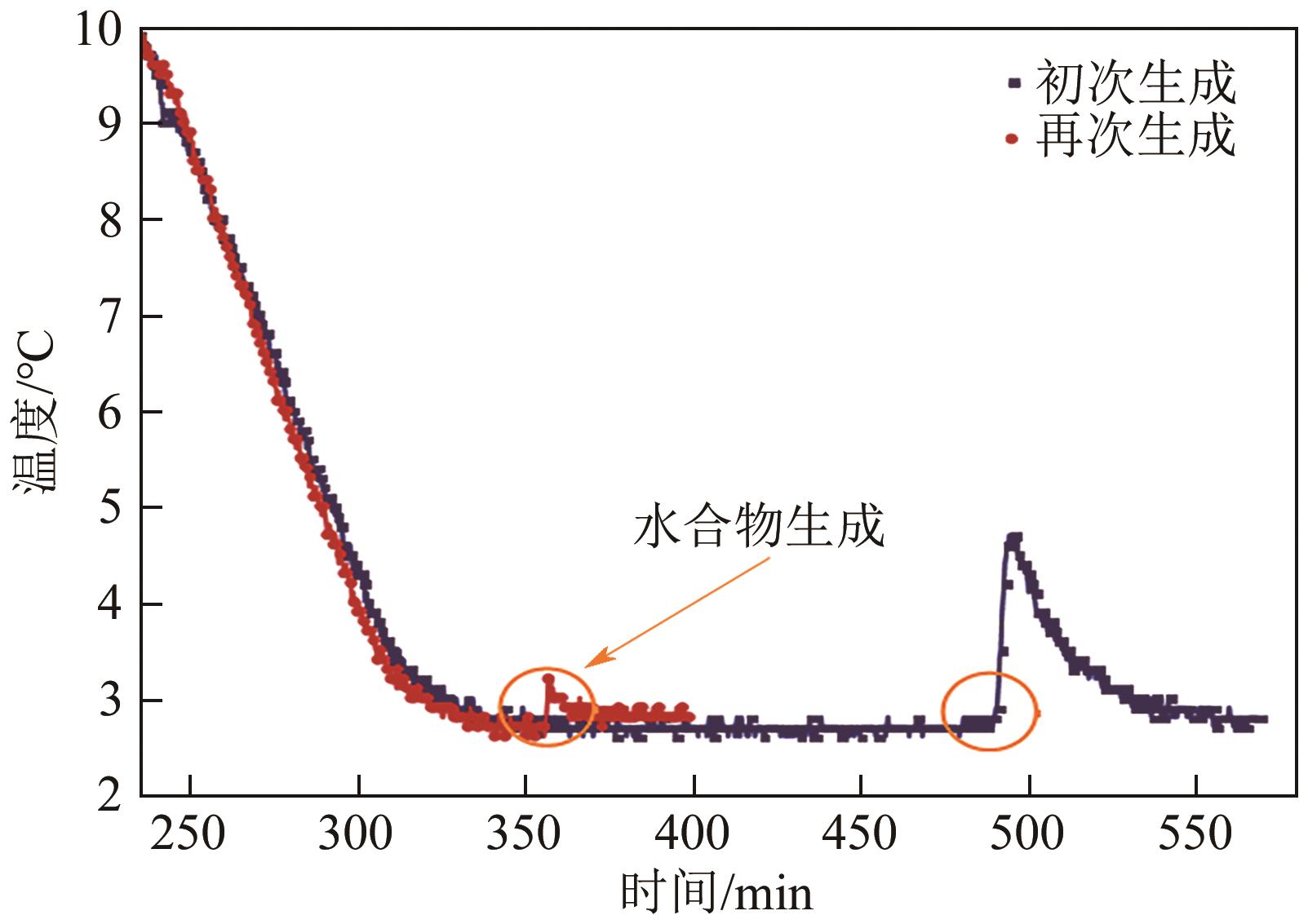
| 基于时间 | 生成时间 | tn | ①相同条件下较短的生成时间反映较快的成核速率;②降温和升压水合物生成体系均适用;③水合物生成识别可借助温度和压力等多种参数 | Zhang等 |
| 诱导期 | tind=te-ts | ①相同条件下较短的诱导期反映较快的成核速率;②适用于大部分水合物生成方法和实验装置;③应用最为广泛 | Prasad等 |
表1 乳状液体系中气体水合物成核速率量化方法
| 计算依据 | 量化指标 | 表达式 | 方法特点 | 应用实例 |
|---|---|---|---|---|
| 基于温度 | 生成温度 | Tn | ①用于降温水合物生成体系;②需借助温度传感器 | Daraboina等 |
| 过冷度 | ΔT=Teq-Tn | ①多用于降温水合物生成体系;②需借助温度传感器;③需进行操作压力下平衡温度的计算 | Stoporev等 | |
| 基于压力 | 生成压力 | Pn | ①用于升压水合物生成体系;②需借助压力传感器 | Wang等 |
| 过饱和度 | Sp=Pn/Peq | ①多用于升压水合物生成体系;②需借助压力传感器;③需进行操作温度下平衡压力的计算 | 王唯 | |
| 基于时间 | 生成时间 | tn | ①相同条件下较短的生成时间反映较快的成核速率;②降温和升压水合物生成体系均适用;③水合物生成识别可借助温度和压力等多种参数 | Zhang等 |
| 诱导期 | tind=te-ts | ①相同条件下较短的诱导期反映较快的成核速率;②适用于大部分水合物生成方法和实验装置;③应用最为广泛 | Prasad等 |
| 文献 | 研究装置 | 油相 | 气相 | 诱导期起始时刻 | 诱导期结束时刻 |
|---|---|---|---|---|---|
| Kakati等 | 高压搅拌釜 | 原油 | CH4 | 体系开始降温 | 温度突变 |
| Zi等 | 高压搅拌釜 | 正庚烷/甲苯 | CH4 | 体系开始降温 | 压力突变 |
| Ning等 | 高压搅拌釜 | 矿物油 | CH4 | 体系开始降温 | 温度突变 |
| Wang等 | 高压搅拌釜 | 柴油 | CO2 | 体系降温至平衡状态 | 温度突变 |
| Lyu等 | 高压环道 | 柴油 | 天然气 | 体系降温至平衡状态 | 温度突变 |
| Zhang等 | 高压搅拌釜 | 矿物油 | CO2 | 压力升高至平衡状态 | 压力突变 |
| Mu | 高压蓝宝石釜 | 柴油 | CH4 | 体系参数稳定 | 压力突变 |
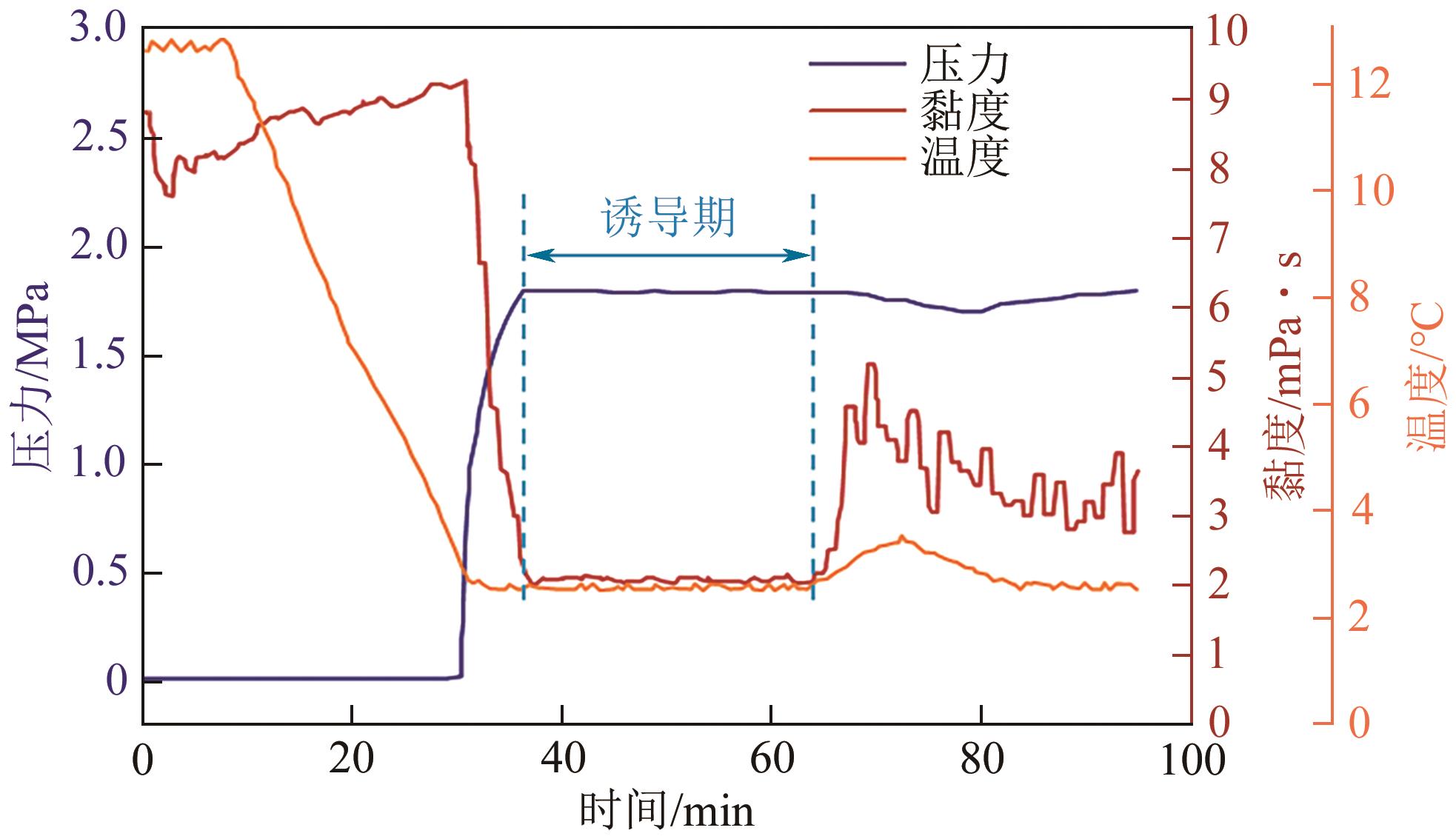
| Zheng等 | 高压搅拌釜 | 柴油 | CO2 | 体系参数稳定 | 黏度突变 |
| Ning等 | 高压搅拌釜 | 矿物油 | CH4 | 体系压力饱和 | 压力突变 |
表2 油水乳状液中水合物诱导期定义方法
| 文献 | 研究装置 | 油相 | 气相 | 诱导期起始时刻 | 诱导期结束时刻 |
|---|---|---|---|---|---|
| Kakati等 | 高压搅拌釜 | 原油 | CH4 | 体系开始降温 | 温度突变 |
| Zi等 | 高压搅拌釜 | 正庚烷/甲苯 | CH4 | 体系开始降温 | 压力突变 |
| Ning等 | 高压搅拌釜 | 矿物油 | CH4 | 体系开始降温 | 温度突变 |
| Wang等 | 高压搅拌釜 | 柴油 | CO2 | 体系降温至平衡状态 | 温度突变 |
| Lyu等 | 高压环道 | 柴油 | 天然气 | 体系降温至平衡状态 | 温度突变 |
| Zhang等 | 高压搅拌釜 | 矿物油 | CO2 | 压力升高至平衡状态 | 压力突变 |
| Mu | 高压蓝宝石釜 | 柴油 | CH4 | 体系参数稳定 | 压力突变 |
| Zheng等 | 高压搅拌釜 | 柴油 | CO2 | 体系参数稳定 | 黏度突变 |
| Ning等 | 高压搅拌釜 | 矿物油 | CH4 | 体系压力饱和 | 压力突变 |
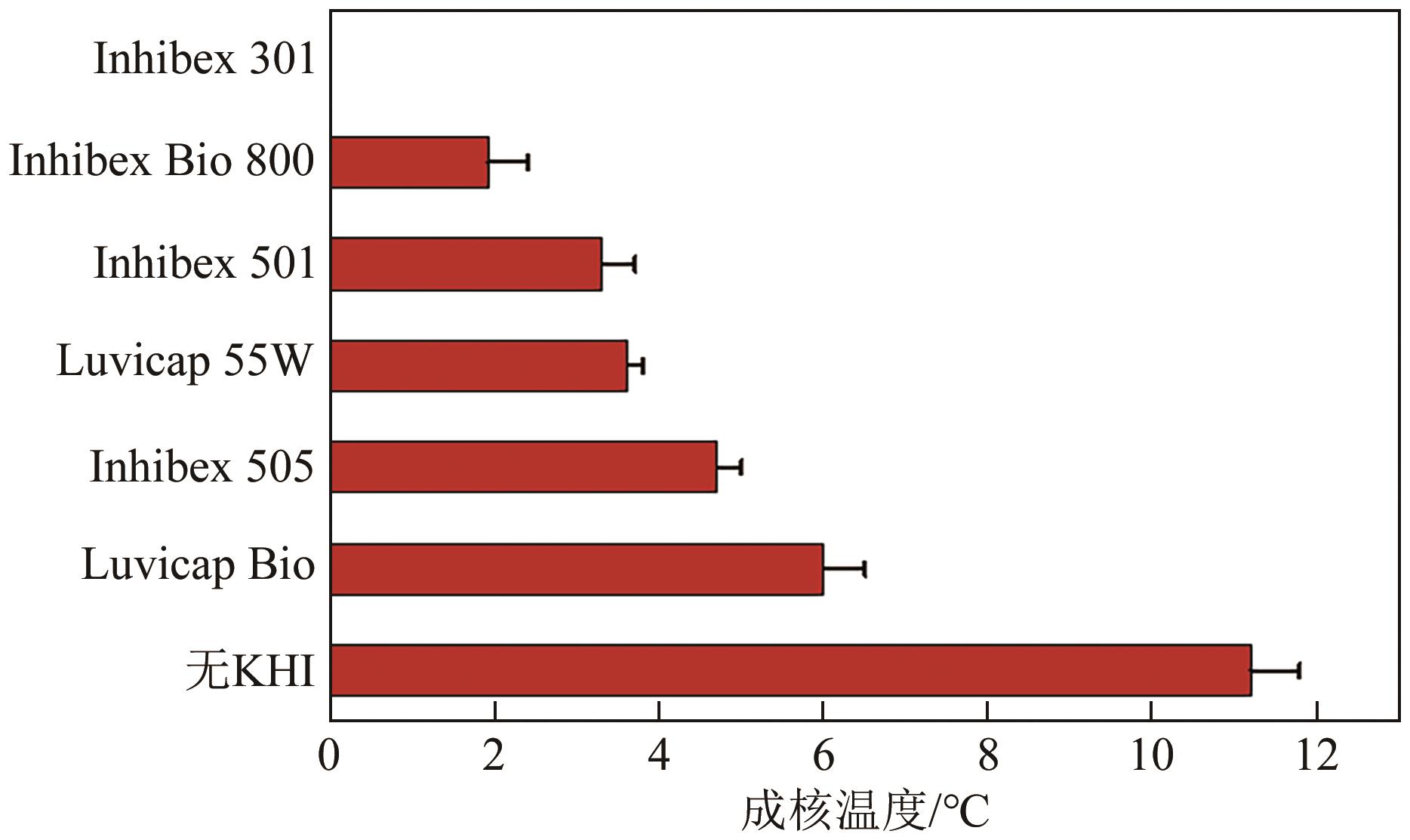
| 研究者 | 量化方法 | 研究装置 | 油相 | 气相 | 扰动强度 | 主要结论 |
|---|---|---|---|---|---|---|
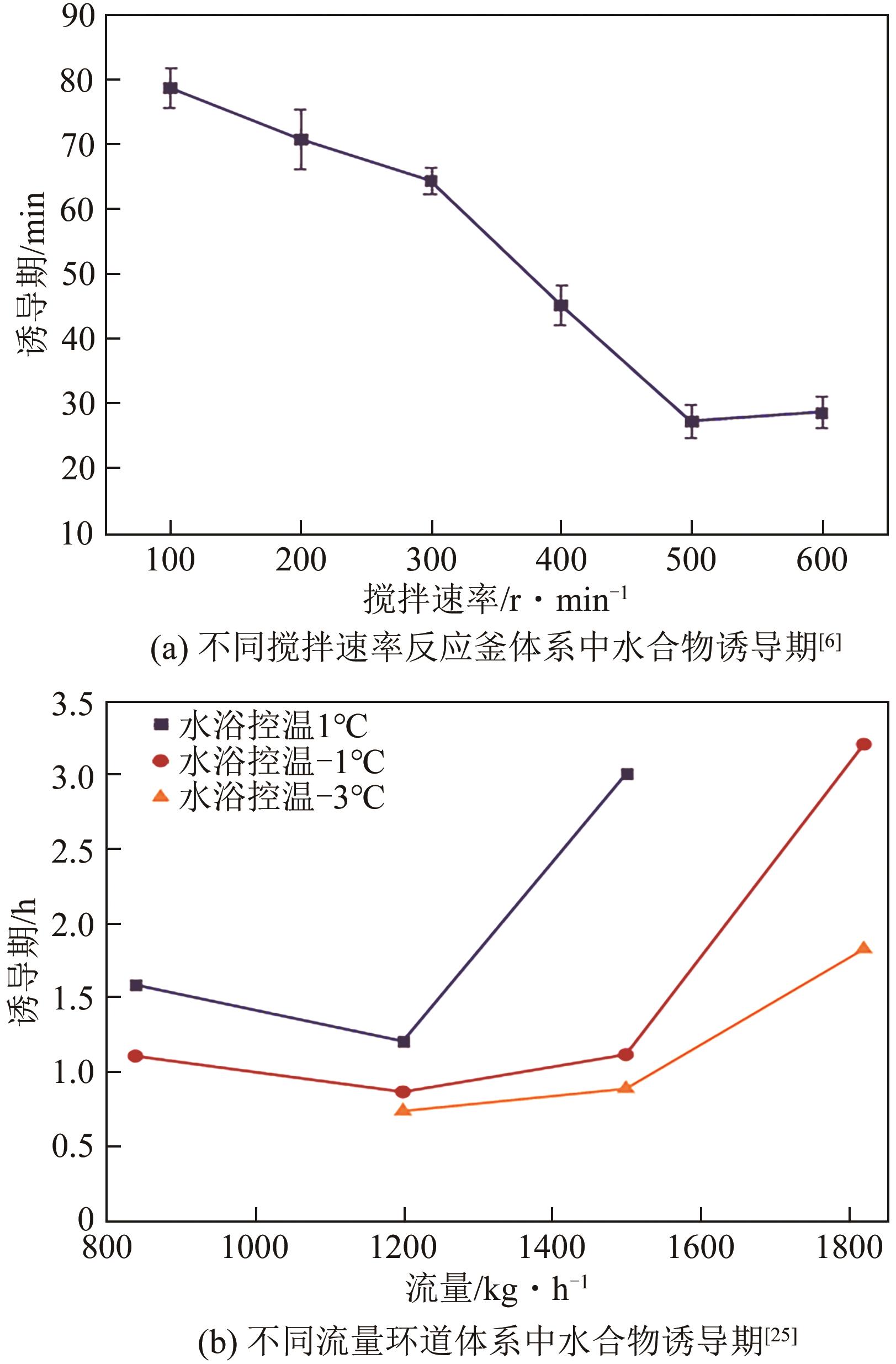
| Lyu等 | 诱导期 | 高压环道 | 柴油 | 天然气 | 840~1820kg/h | 水合物诱导期随流速升高先减小后增大 |
| Li等 | 诱导期 | 高压搅拌釜 | 癸烷 | CH4 | 300~1100r/min | 水合物诱导期随搅拌速率升高先减小后增大 |
| Zheng等 | 诱导期 | 高压搅拌釜 | 柴油 | CO2 | 100~600r/min | |
| Zhang等 | 成核时间 | 高压搅拌釜 | 含蜡柴油 | CO2 | 200~600r/min | 搅拌速率升高促进水合物成核 |
| 宋光春等 | 诱导期 | 高压搅拌釜 | 柴油 | 天然气 | 100~300r/min | |
| Stoporev等 | 过冷度 | 高压搅拌釜 | 癸烷/原油 | C1~C3混合/CH4 | 100~600r/min | 搅拌速率与成核温度无显著关系 |
| Zhang等 | 诱导期 | 高压搅拌釜 | 含沥青质矿物油 | CO2 | 100~400r/min | 搅拌速率的升高缩短水合物诱导期 |
| Dai等 | 诱导期 | 高压搅拌釜 | 正十二烷 | CO2 | 300~500r/min |
表3 油水乳状液体系扰动强度对水合物成核的影响
| 研究者 | 量化方法 | 研究装置 | 油相 | 气相 | 扰动强度 | 主要结论 |
|---|---|---|---|---|---|---|
| Lyu等 | 诱导期 | 高压环道 | 柴油 | 天然气 | 840~1820kg/h | 水合物诱导期随流速升高先减小后增大 |
| Li等 | 诱导期 | 高压搅拌釜 | 癸烷 | CH4 | 300~1100r/min | 水合物诱导期随搅拌速率升高先减小后增大 |
| Zheng等 | 诱导期 | 高压搅拌釜 | 柴油 | CO2 | 100~600r/min | |
| Zhang等 | 成核时间 | 高压搅拌釜 | 含蜡柴油 | CO2 | 200~600r/min | 搅拌速率升高促进水合物成核 |
| 宋光春等 | 诱导期 | 高压搅拌釜 | 柴油 | 天然气 | 100~300r/min | |
| Stoporev等 | 过冷度 | 高压搅拌釜 | 癸烷/原油 | C1~C3混合/CH4 | 100~600r/min | 搅拌速率与成核温度无显著关系 |
| Zhang等 | 诱导期 | 高压搅拌釜 | 含沥青质矿物油 | CO2 | 100~400r/min | 搅拌速率的升高缩短水合物诱导期 |
| Dai等 | 诱导期 | 高压搅拌釜 | 正十二烷 | CO2 | 300~500r/min |
| 1 | SLOAN E Dendy. Fundamental principles and applications of natural gas hydrates[J]. Nature, 2003, 426(6964): 353-363. |
| 2 | 周诗岽, 陈小康, 边慧, 等. CO2水合物在管道中的生成及堵塞特性[J]. 化工进展, 2018, 37(11): 4250-4256. |
| ZHOU Shidong, CHEN Xiaokang, BIAN Hui, et al. CO2 hydrate formation in pipeline and its plugging characteristics[J]. Chemical Industry and Engineering Progress, 2018, 37(11): 4250-4256. | |
| 3 | WANG Wei, HUANG Qiyu, HU Sijia, et al. Influence of wax on cyclopentane clathrate hydrate cohesive forces and interfacial properties[J]. Energy & Fuels, 2020, 34(2): 1482-1491. |
| 4 | 宫敬, 史博会, 陈玉川, 等. 含天然气水合物的海底多相管输及其堵塞风险管控[J]. 天然气工业, 2020,40(12): 133-142. |
| GONG Jing, SHI Bohui, CHEN Yuchuan, et al. Submarine multiphase pipeline transport containing natural gas hydrate and its plugging risk prevention and control[J]. Natural Gas Industry, 2020, 40(12): 133-142. | |
| 5 | JING Jiaqiang, ZHUANG Lequan, KARIMOV R, et al. Investigation of natural gas hydrate formation and slurry viscosity in non-emulsifying oil systems[J]. Chemical Engineering Research and Design, 2023, 190: 687-703. |
| 6 | ZHENG Haimin, HUANG Qiyu, WANG Wei, et al. Induction time of hydrate formation in water-in-oil emulsions[J]. Industrial & Engineering Chemistry Research, 2017, 56(29): 8330-8339. |
| 7 | XIAO Yanyun, ZHOU Shidong, LI Xiaoyan, et al. Kinetic properties of CO2 hydrate formation in the wax-containing system at different concentrations[J]. Energy & Fuels, 2023, 37(4): 2972-2982. |
| 8 | 李清平, 孙钦, 程兵, 等. 陵水17-2气田深水水下生产系统工程设计关键技术[J]. 中国海上油气, 2021, 33(3): 180-188. |
| LI Qingping, SUN Qin, CHENG Bing, et al. Key technologies for engineering design of deep water subsea production system in L S 17-2 gas field[J]. China Offshore Oil and Gas, 2021, 33(3): 180-188. | |
| 9 | ZHANG Dongxu, HUANG Qiyu, ZHENG Haimin, et al. Effect of wax crystals on nucleation during gas hydrate formation[J]. Energy & Fuels, 2019, 33(6): 5081-5090. |
| 10 | CAO Xuewen, YANG Kairan, BIAN Jiang. Investigation of CO2 hydrate slurry flow characteristics with particle dissociation for carbon storage and transportation[J]. Process Safety and Environmental Protection, 2021, 152: 427-440. |
| 11 | KIM Shol, LEE Seong Hyuk, KANG Yong Tae. Characteristics of CO2 hydrate formation/dissociation in H2O+THF aqueous solution and estimation of CO2 emission reduction by district cooling application[J]. Energy, 2017, 120: 362-373. |
| 12 | 樊栓狮, 尤莎莉, 郎雪梅, 等. 笼型水合物膜分离和捕获二氧化碳研究进展[J]. 化工进展, 2020, 39(4): 1211-1218. |
| FAN Shuanshi, YOU Shali, LANG Xuemei, et al. Separation and capture carbon dioxide by clathrate-hydrate membranes: A review[J]. Chemical Industry and Engineering Progress, 2020, 39(4): 1211-1218. | |
| 13 | 陈光进, 孙长宇, 马庆兰. 气体水合物科学与技术[M]. 2版. 北京: 化学工业出版社, 2020. |
| CHEN Guangjin, SUN Changyu, MA Qinglan. Gas hydrate science and technology[M]. 2nd ed. Beijing: Chemical Industry Press, 2020. | |
| 14 | 郑海敏. 水合物在油-气-水-蜡多相体系中诱导与生长过程研究[D]. 北京: 中国石油大学(北京), 2018. |
| ZHENG Haimin. Study on induction and growth process of hydrate in oil-gas-water-wax multiphase system[D]. Beijing: China University of Petroleum (Beijing), 2018. | |
| 15 | CHEN Yuchuan, SHI Bohui, LIU Yang, et al. Experimental and theoretical investigation of the interaction between hydrate formation and wax precipitation in water-in-oil emulsions[J]. Energy & Fuels, 2018, 32(9): 9081-9092. |
| 16 | DARABOINA Nagu, PACHITSAS Stylianos, VON SOLMS Nicolas. Natural gas hydrate formation and inhibition in gas/crude oil/aqueous systems[J]. Fuel, 2015, 148: 186-190. |
| 17 | ZI Mucong, WU Guozhong, WANG Jiang, et al. Investigation of gas hydrate formation and inhibition in oil-water system containing model asphaltene[J]. Chemical Engineering Journal, 2021, 412: 128452. |
| 18 | STOPOREV Andrey S, SEMENOV Anton P, MEDVEDEV Vladimir I, et al. Formation and agglomeration of gas hydrates in gas-organic liquid-water systems in a stirred reactor: Role of resins/asphaltenes/surfactants[J]. Journal of Petroleum Science and Engineering, 2019, 176: 952-961. |
| 19 | WANG Wei, HUANG Qiyu, ZHENG Haimin, et al. Effect of wax on hydrate formation in water-in-oil emulsions[J]. Journal of Dispersion Science and Technology, 2020, 41(12): 1821-1830. |
| 20 | ZHANG Dongxu, HUANG Qiyu, LI Rongbin, et al. Nucleation and growth of gas hydrates in emulsions of water in asphaltene-containing oils[J]. Energy & Fuels, 2021, 35(7): 5853-5866. |
| 21 | 王唯. 含蜡油包水乳状液体系水合物生成及聚集机理研究[D]. 北京: 中国石油大学(北京), 2020. |
| WANG Wei. Study on hydrate formation and aggregation mechanism of water-in-oil emulsion system containing wax oil[D]. Beijing: China University of Petroleum (Beijing), 2020. | |
| 22 | SANDOVAL Gustavo A B, THOMPSON Roney L, Cristina M S SAD, et al. Influence of adding asphaltenes and gas condensate on CO2 hydrate formation in water-CO2-oil systems[J]. Energy & Fuels, 2019, 33(8): 7138-7146. |
| 23 | SHARIFI Hassan, RIPMEESTER John, WALKER Virginia K, et al. Kinetic inhibition of natural gas hydrates in saline solutions and heptane[J]. Fuel, 2014, 117: 109-117. |
| 24 | PRASAD Siddhant Kumar, NAIR Vishnu Chandrasekharan, SANGWAI Jitendra S. Effect of asphaltenes on the kinetics of methane hydrate formation and dissociation in oil-in-water dispersion systems containing light saturated and aromatic hydrocarbons[J]. Energy & Fuels, 2021, 35(21): 17410-17423. |
| 25 | LYU X F, SHI B H, WANG Y, et al. Experimental study on hydrate induction time of gas-saturated water-in-oil emulsion using a high-pressure flow loop[J]. Oil & Gas Science and Technology: Revue D’IFP Energies Nouvelles, 2015, 70(6): 1111-1124. |
| 26 | DALMAZZONE D, HAMED N, DALMAZZONE Christine, et al. Application of high pressure DSC to the kinetics of formation of methane hydrate in water-in-oil emulsion[J]. Journal of Thermal Analysis and Calorimetry, 2006, 85(2): 361-368. |
| 27 | KASHCHIEV Dimo, FIROOZABADI Abbas. Induction time in crystallization of gas hydrates[J]. Journal of Crystal Growth, 2003, 250(3/4): 499-515. |
| 28 | WALSH Matthew R, Carolyn A KOH, SLOAN E Dendy, et al. Microsecond simulations of spontaneous methane hydrate nucleation and growth[J]. Science, 2009, 326(5956): 1095-1098. |
| 29 | ZHANG Zhengcai, WU Nengyou, LIU Changling, et al. Molecular simulation studies on natural gas hydrates nucleation and growth: A review[J]. China Geology, 2022, 5: 330-344. |
| 30 | PRASAD Siddhant Kumar, SANGWAI Jitendra S. Impact of lighter alkanes on the formation and dissociation kinetics of methane hydrate in oil-in-water dispersions relevant for flow assurance[J]. Fuel, 2023, 333: 126500. |
| 31 | KAKATI Himangshu, KAR Shranish, MANDAL Ajay, et al. Methane hydrate formation and dissociation in oil-in-water emulsion[J]. Energy & Fuels, 2014, 28(7): 4440-4446. |
| 32 | NING Yuanxing, LI Yuxing, SONG Guangchun, et al. Investigation on hydrate formation and growth characteristics in dissolved asphaltene-containing water-in-oil emulsion[J]. Langmuir, 2021, 37(37): 11072-11083. |
| 33 | ZHANG Dongxu, HUANG Qiyu, LI Rongbin, et al. Effects of waxes on hydrate behaviors in water-in-oil emulsions containing asphaltenes[J]. Chemical Engineering Science, 2021, 244: 116831. |
| 34 | Xiaofang LYU, ZHANG Jie, ZHAO Yi, et al. Experimental study of the growth kinetics of natural gas hydrates in an oil-water emulsion system[J]. ACS Omega, 2022, 7(1): 599-616. |
| 35 | LIU Yang, SHI Bohui, DING Lin, et al. Study of hydrate formation in water-in-waxy oil emulsions considering heat transfer and mass transfer[J]. Fuel, 2019, 244: 282-295. |
| 36 | ZHANG Dongxu, HUANG Qiyu, WANG Wei, et al. Effects of waxes and asphaltenes on CO2 hydrate nucleation and decomposition in oil-dominated systems[J]. Journal of Natural Gas Science and Engineering, 2021, 88: 103799. |
| 37 | MU Liang, LI Shi, MA Qinglan, et al. Experimental and modeling investigation of kinetics of methane gas hydrate formation in water-in-oil emulsion[J]. Fluid Phase Equilibria, 2014, 362: 28-34. |
| 38 | NING Fulong, GUO Dongdong, DIN Shahab Ud, et al. The kinetic effects of hydrate anti-agglomerants/surfactants[J]. Fuel, 2022, 318: 123566. |
| 39 | TONG Shikun, LI Pengfei, Fengjun LYU, et al. Promotion and inhibition effects of wax on methane hydrate formation and dissociation in water-in-oil emulsions[J]. Fuel, 2023, 337: 127211. |
| 40 | SHI Lingli, LI Junhui, HE Yong, et al. Memory effect test and analysis in methane hydrates reformation process[J]. Energy, 2023, 272: 127153. |
| 41 | LI Rong, SUN Zhigao. Improving C2H3Cl2F hydrate formation for cold storage in the presence of amino acids[J]. Journal of Energy Storage, 2023, 59: 106505. |
| 42 | SAGIDULLIN A K, STOPOREV A S, A Yu MANAKOV. Effect of temperature on the rate of methane hydrate nucleation in water-in-crude oil emulsion[J]. Energy & Fuels, 2019, 33(4): 3155-3161. |
| 43 | 张庆东, 李玉星, 王武昌, 等. 温度对甲烷水合物形成过程影响的分子动力学模拟[J]. 油气储运, 2015, 34(12): 1288-1294. |
| ZHANG Qingdong, LI Yuxing, WANG Wuchang, et al. Molecular dynamics simulation of the influence of temperature on the formation of methane hydrate[J]. Oil & Gas Storage and Transportation, 2015, 34(12): 1288-1294. | |
| 44 | LI Xingang, CHEN Chao, CHEN Yingnan, et al. Kinetics of methane clathrate hydrate formation in water-in-oil emulsion[J]. Energy & Fuels, 2015, 29(4): 2277-2288. |
| 45 | 宋光春, 施政灼, 李玉星, 等. 油水体系内水合物的生成:温度、压力和搅拌速率影响[J]. 化工进展, 2019, 38(3): 1338-1345. |
| SONG Guangchun, SHI Zhengzhuo, LI Yuxing, et al. Hydrate formation in oil-water systems: Investigations of the influences of temperature, pressure and rotation rate[J]. Chemical Industry and Engineering Progress, 2019, 38(3): 1338-1345. | |
| 46 | DAI Mengling, SUN Zhigao, SONG Jia, et al. Effect of liquid alkane on carbon dioxide hydrate formation[J]. Fuel, 2022, 323: 124335. |
| 47 | DING Kun, ZHONG Dongliang, LU Yiyu, et al. Enhanced precombustion capture of carbon dioxide by gas hydrate formation in water-in-oil emulsions[J]. Energy & Fuels, 2015, 29(5): 2971-2978. |
| 48 | 周诗岽, 于雪薇, 江坤, 等. 蜡晶析出对天然气水合物生成动力学特性的影响[J]. 天然气工业, 2018, 38(3): 103-109. |
| ZHOU Shidong, YU Xuewei, JIANG Kun, et al. Effect of wax crystal precipitation on kinetic characteristics of natural gas hydrate formation[J]. Natural Gas Industry, 2018, 38(3): 103-109. | |
| 49 | SONG Guangchun, NING Yuanxing, LI Yuxing, et al. Investigation on hydrate growth at the oil–water interface: In the presence of wax and kinetic hydrate inhibitor[J]. Langmuir, 2020, 36(48): 14881-14891. |
| 50 | 张东旭, 伍奕, 杨明, 等. 重质原油组分对水合物生成影响的研究进展[J]. 油气储运, 2022, 41(3): 264-271. |
| ZHANG Dongxu, WU Yi, YANG Ming, et al. Research progress on the effect of heavy crude oil components on hydrate formation[J]. Oil & Gas Storage and Transportation, 2022, 41(3): 264-271. | |
| 51 | LIU Jia, WANG Jing, DONG Ti, et al. Effects of wax on CH4 hydrate formation and agglomeration in oil-water emulsions[J]. Fuel, 2022, 322: 124128. |
| 52 | PENG Zeheng, WANG Wei, CHENG Lin, et al. Effect of the ethylene vinyl acetate copolymer on the induction of cyclopentane hydrate in a water-in-waxy oil emulsion system[J]. Langmuir, 2021, 37(45): 13225-13234. |
| 53 | CHENG Xianwen, HUANG Qiyu, ZHANG Dongxu, et al. Influence of asphaltene polarity on hydrate behaviors in water-in-oil emulsions[J]. Energy & Fuels, 2022, 36(1): 239-250. |
| 54 | WANG Yijie, HUANG Qiyu, ZHANG Dongxu, et al. Influence of asphaltenes on hydrate formation and decomposition in water-in-waxy oil emulsions[J]. Energy & Fuels, 2022, 36(24): 14710-14722. |
| 55 | ZHANG Jie, LI Chuanxian, YANG Fei, et al. Study on the influence mechanism of the interaction between waxes and asphaltenes on hydrate growth[J]. Fuel, 2023, 338: 127322. |
| 56 | YEGYA RAMAN Ashwin Kumar, AICHELE Clint P. Effect of particle hydrophobicity on hydrate formation in water-in-oil emulsions in the presence of wax[J]. Energy & Fuels, 2017, 31(5): 4817-4825. |
| 57 | 胡倩, 周诗岽, 郭宇, 等. 蜡晶析出对CO2水合物相平衡及诱导特性的影响[J]. 化工进展, 2021, 40(5): 2452-2460. |
| HU Qian, ZHOU Shidong, GUO Yu, et al. Influence of wax crystal precipitation on the phase equilibrium and induction characteristics of CO2 hydrate[J]. Chemical Industry and Engineering Progress, 2021, 40(5): 2452-2460. | |
| 58 | LI Zhi, LIU Bei, GONG Yinghua, et al. Molecular dynamics simulation to explore the impact of wax crystal on the formation of methane hydrate[J]. Journal of Molecular Liquids, 2022, 350: 118229. |
| 59 | LIAO Qingyun, SHI Bohui, SONG Shangfei, et al. Molecular insights into methane hydrate growth in the presence of wax molecules[J]. Fuel, 2022, 324: 124743. |
| 60 | CHEN Jun, YAN Kele, JIA Menglei, et al. Memory effect test of methane hydrate in water + diesel oil + sorbitan monolaurate dispersed systems[J]. Energy & Fuels, 2013, 27(12): 7259-7266. |
| 61 | OSHIMA Motoi, SHIMADA Wataru, HASHIMOTO Shunsuke, et al. Memory effect on semi-clathrate hydrate formation: A case study of tetragonal tetra-n-butyl ammonium bromide hydrate[J]. Chemical Engineering Science, 2010, 65(20): 5442-5446. |
| 62 | THOMPSON Helen, SOPER Alan K, BUCHANAN Piers, et al. Methane hydrate formation and decomposition: Structural studies via neutron diffraction and empirical potential structure refinement[J]. The Journal of Chemical Physics, 2006, 124(16): 164508. |
| 63 | Mark RODGER P. Methane hydrate: Melting and memory[J]. Annals of the New York Academy of Sciences, 2000, 912(1): 474-482. |
| 64 | UCHIDA Tsutomu, EBINUMA Toshiro, NARITA Hideo. Observations of CO2-hydrate decomposition and reformation processes[J]. Journal of Crystal Growth, 2000, 217(1/2): 189-200. |
| 65 | DARABOINA Nagu, PACHITSAS Stylianos, VON SOLMS Nicolas. Experimental validation of kinetic inhibitor strength on natural gas hydrate nucleation[J]. Fuel, 2015, 139: 554-560. |
| 66 | MU Liang, VON SOLMS Nicolas. Inhibition of natural gas hydrate in the system containing salts and crude oil[J]. Journal of Petroleum Science and Engineering, 2020, 188: 106940. |
| 67 | 闫柯乐, 孙长宇, 张红星, 等. 水合物动力学抑制剂在油水体系内抑制性能实验研究[J]. 科学技术与工程, 2016, 16(18): 170-175. |
| YAN KeLe, SUN Changyu, ZHANG Hongxing, et al. Experimental study on inhibition performance of hydrate kinetic inhibitor in oil-water system[J]. Science Technology and Engineering, 2016, 16(18): 170-175. | |
| 68 | FARHADIAN Abdolreza, ZHAO Yang, NAEIJI Parisa, et al. Simultaneous inhibition of natural gas hydrate formation and CO2/H2S corrosion for flow assurance inside the oil and gas pipelines[J]. Energy, 2023, 269: 126797. |
| 69 | LIAO Bo, WANG Jintang, SUN Jinsheng, et al. Microscopic insights into synergism effect of different hydrate inhibitors on methane hydrate formation: Experiments and molecular dynamics simulations[J]. Fuel, 2023, 340: 127488. |
| 70 | YI Lizhi, ZHAO Lili, TAO Shunhui. Methane hydrate formation in an oil-water system in the presence of lauroylamide propylbetaine[J]. RSC Advances, 2020, 10(21): 12255-12261. |
| 71 | LEDERHOS J P, LONG J P, SUM A, et al. Effective kinetic inhibitors for natural gas hydrates[J]. Chemical Engineering Science, 1996, 51(8): 1221-1229. |
| 72 | CARVER Tim J, DREW Michael G B, MARK RODGER P. Molecular dynamics calculations of N-methylpyrrolidone in liquid water[J]. Physical Chemistry Chemical Physics, 1999, 1(8): 1807-1816. |
| 73 | YANG Jinhai, TOHIDI Bahman. Characterization of inhibition mechanisms of kinetic hydrate inhibitors using ultrasonic test technique[J]. Chemical Engineering Science, 2011, 66(3): 278-283. |
| 74 | XU Shurui, FAN Shuanshi, FANG Songtian, et al. Pectin as an extraordinary natural kinetic hydrate inhibitor[J]. Scientific Reports, 2016, 6: 23220. |
| 75 | KASHCHIEV Dimo, FIROOZABADI Abbas. Nucleation of gas hydrates[J]. Journal of Crystal Growth, 2002, 243(3/4): 476-489. |
| 76 | JENSEN Lars, THOMSEN Kaj, VON SOLMS Nicolas. Propane hydrate nucleation: Experimental investigation and correlation[J]. Chemical Engineering Science, 2008, 63(12): 3069-3080. |
| 77 | 柳扬. 蜡与水合物共存W/O体系流动及沉积规律研究[D]. 北京: 中国石油大学(北京), 2019. |
| LIU Yang. Study on flow and deposition law of wax and hydrate coexisting W/O system[D]. Beijing: China University of Petroleum (Beijing), 2019. | |
| 78 | KANG Seong-Pil, SHIN Ju-Young, Jong-Se LIM, et al. Experimental measurement of the induction time of natural gas Hydrate and its prediction with polymeric kinetic inhibitor[J]. Chemical Engineering Science, 2014, 116: 817-823. |
| 79 | AGHAJANLOO Mahnaz, REZA EHSANI Mohammad, TAHERI Zahra, et al. Kinetics of methane+hydrogen sulfide clathrate hydrate formation in the presence/absence of poly N-vinyl pyrrolidone (PVP) and L-tyrosine: Experimental study and modeling of the induction time[J]. Chemical Engineering Science, 2022, 250: 117384. |
| [1] | 刘佳, 梁德青, 李君慧, 林德才, 吴思婷, 卢富勤. 油水体系水合物浆液流动保障研究进展[J]. 化工进展, 2023, 42(4): 1739-1759. |
| [2] | 王唯, 张东旭, 李遵照, 王晓霖, 黄启玉. 油包水乳状液体系中水合物生长行为研究进展[J]. 化工进展, 2023, 42(3): 1155-1166. |
| [3] | 李昊阳, 张炜, 李小森, 徐纯刚. 水合物储氢的研究进展[J]. 化工进展, 2022, 41(12): 6285-6294. |
| [4] | 付玮琪, 赵子贤, 于璟, 魏伟, 王志远, 黄炳香. 泡状流中水合物生成预测模型及实验[J]. 化工进展, 2022, 41(11): 5746-5754. |
| [5] | 张学民, 张梦军, 杨惠结, 李银辉, 李金平, 王英梅. 冰点以下多孔介质中气体水合物的生成动力学研究进展[J]. 化工进展, 2021, 40(S2): 101-108. |
| [6] | 谢文俊,李小森,邹颖楠,徐纯刚. 含环戊烷体系中二氧化碳水合物形成分解热特性[J]. 化工进展, 2020, 39(1): 129-136. |
| [7] | 雍宇, 史博会, 丁麟, 李文庆, 柳扬, 宋尚飞, 宫敬. 水合物生成诱导期研究进展[J]. 化工进展, 2018, 37(02): 505-516. |
| [8] | 李翠勤1,王 俊 1,魏宇佳1,孟祥勇2. 新型分子内复合抗氧剂清除自由基的性能[J]. 化工进展, 2013, 32(02): 441-445. |
| [9] | 吕秋楠,陈朝阳,李小森. 气体水合物快速生成强化技术与方法研究进展 [J]. 化工进展, 2011, 30(1): 74-. |
| [10] | 谢应明,龚金明,刘道平,李 刚,刘 妮,祁影霞. 一种新型储氢方法——水合物储氢的研究概况与发展方向 [J]. 化工进展, 2010, 29(5): 796-. |
| [11] | 李翠勤1,王 俊1,张志秋1,方 宏2. 新型分子内复合主抗氧剂的合成与性能 [J]. 化工进展, 2010, 29(11): 2154-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||