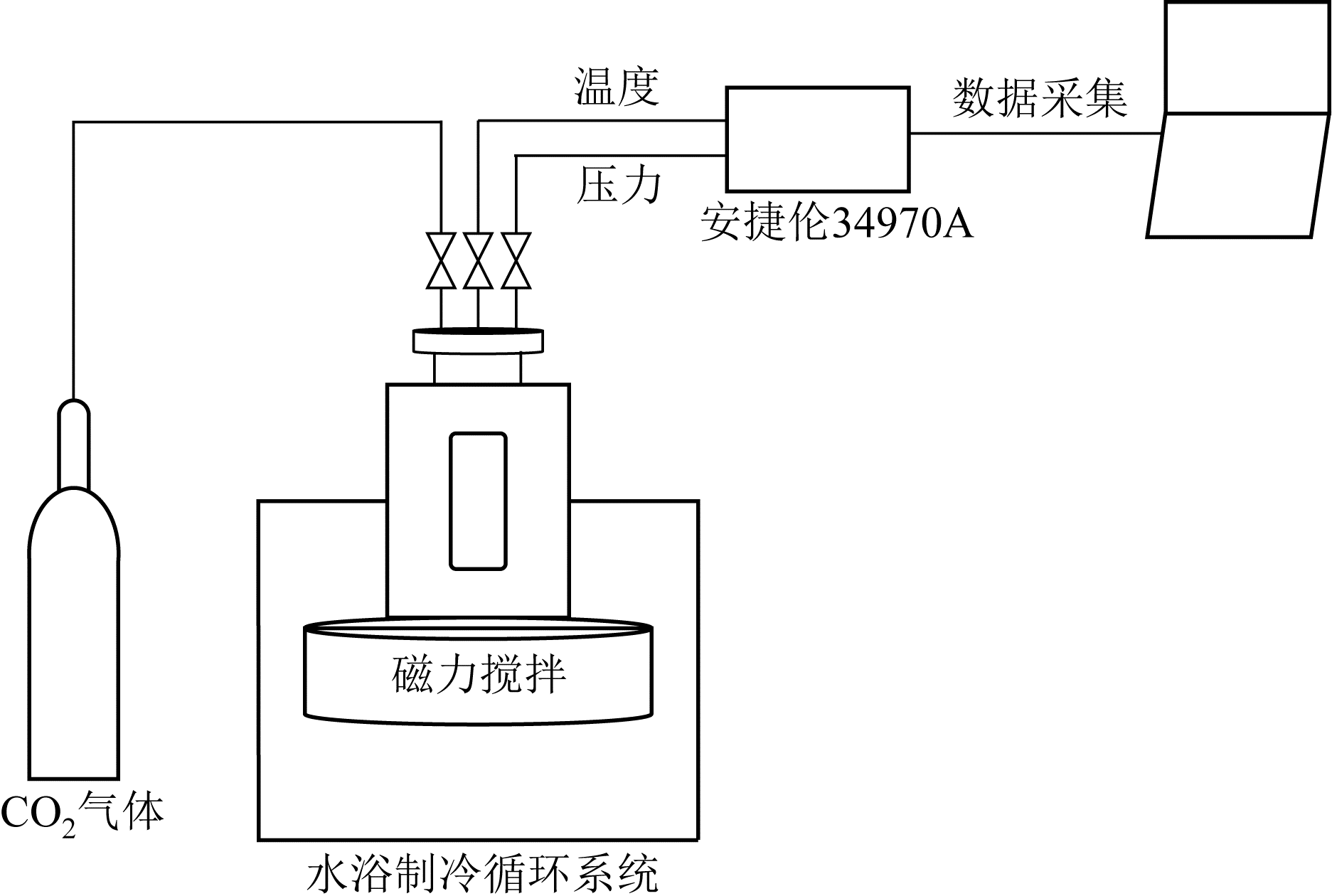
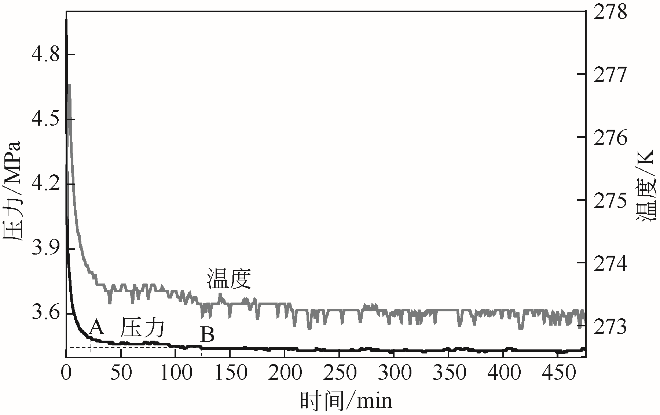
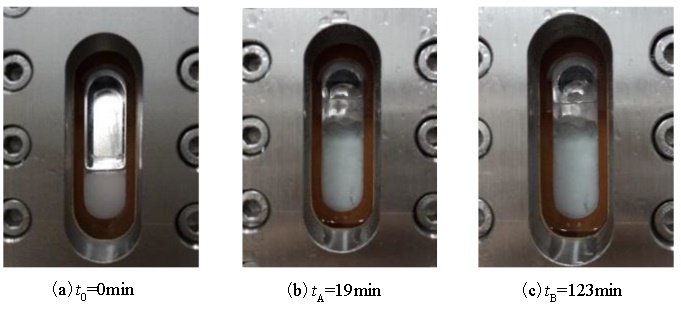
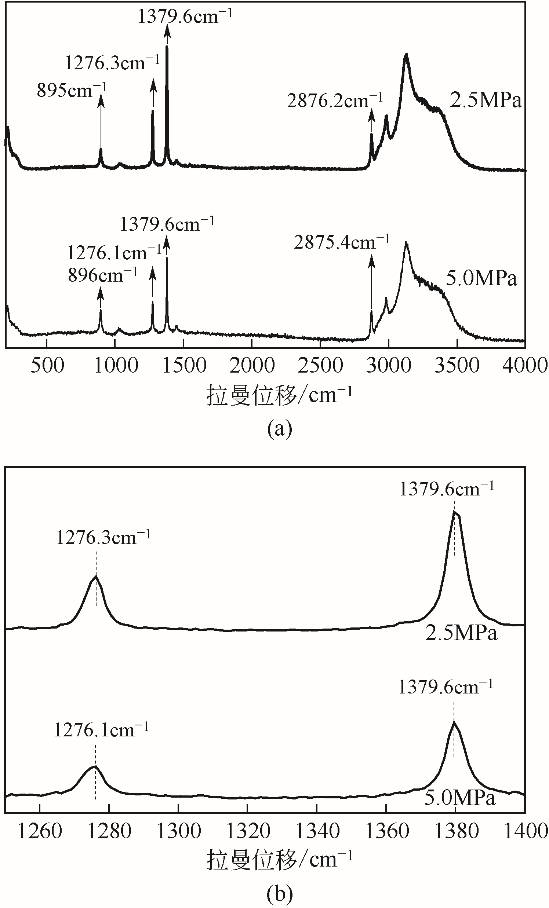
| 1 |
XU C G , CAI J , LI X S , et al . Integrated process study on hydrate-based carbon dioxide separation from integrated gasification combined cycle (IGCC) synthesis gas in scaled-up equipment[J]. Energy Fuels, 2012, 26: 6442-6448.
|
| 2 |
DIJKSTRA J W , JANSEN D . Novel concepts for CO2 capture[J]. Energy, 2004, 29(9/10): 1249-1257.
|
| 3 |
KOTOWICZ J , CHMIELNIAK T , JANUSZ-SZYMANSKA K . The influence of membrane CO2 separation on the efficiency of a coal-fired power plant[J]. Energy, 2010, 35(2): 841-850.
|
| 4 |
LI X S , XU C G , CHEN Z Y , et al . Tetra-n-butyl ammonium bromide semi-clathrate hydrate process for post-combustion capture of carbon dioxide in the presence of dodecyl trimethyl ammonium chloride[J]. Energy, 2010, 35(9): 3902-3908.
|
| 5 |
杨晓西,丁静,杨建平,等 .水合物分离二氧化碳气体的研究[J]. 东莞理工学院学报, 2006(4): 51-56.
|
|
YANG X X , DING J , YANG J P , et al . Research on separation of carbon dioxide by gas hydrate[J]. Journal of Dongguan University of Technology, 2006(4): 51-56.
|
| 6 |
SLOAN E D , KOH C A . Clathrate hydrates of natural gases[M]. 3rd ed. Boca Rator, New York: CRC Press, 2008.
|
| 7 |
SUM A K, KOH C A, SLOAN E D . Clathrate hydrates: from laboratory science to engineering practice[J]. Ind. Eng. Chem. Res., 2009, 48: 7457-7465.
|
| 8 |
徐纯刚, 李小森, 陈朝阳 . 水合物法分离二氧化碳的研究现状[J]. 化工进展, 2011, 30(4): 701-708.
|
|
XU C G , LI X S , CHEN Z Y . Research on hydrate-based carbon dioxide separation[J]. Chemical Industry and Engineering Progress, 2011, 30(4): 701-708.
|
| 9 |
XU C G , ZHANG S H , CAI J , et al . CO2 (carbon dioxide) separation from CO2-H2 (hydrogen) gas mixtures by gas hydrates in TBAB (tetra-n-butyl ammonium bromide) solution and Raman spectroscopic analysis[J]. Energy, 2013, 59: 719-725.
|
| 10 |
LIRIO C F D S , PESSOA F L P , ULLER A M C . Storage capacity of carbon dioxide hydrates in the presence of sodium dodecyl sulfate (SDS) and tetrahydrofuran (THF)[J]. Chemical Engineering Science, 2013, 96: 118-123.
|
| 11 |
LI X S , XU C G , CHEN Z Y , et al . Synergic effect of cyclopentane and tetra-n-butyl ammonium bromide on hydrate-based carbon dioxide separation from fuel gas mixture by measurements of gas uptake and X-ray diffraction patterns[J]. International Journal of Hydrogen Energy, 2012, 37(1): 720-727.
|
| 12 |
VELUSWAMY H P , CHIN W I , LINGA P . Clathrate hydrates for hydrogen storage: the impact of tetrahydrofuran, tetra-n-butylammonium bromide and cyclopentane as promoters on the macroscopic kinetics[J]. International Journal of Hydrogen Energy, 2014, 39(28): 16234-16243.
|
| 13 |
孟庆国, 刘昌岭, 贺行良, 等 . 祁连山冻土区天然气水合物激光拉曼光谱特征[J]. 地质通报, 2011, 30(12): 1863-1867.
|
|
MENG Q G , LIU C L , HE X L , et al . Laser-Raman spectroscopy characteristics of natural gas hydrates from Qilian Mountain permafrost[J]. Geological Bulletin of China, 2011, 30(12): 1863-1867.
|
| 14 |
CHEN L T , LU H L , JOHN A R . Raman spectroscopic study of CO2 in hydrate cages[J]. Chemical Engineering Science, 2015, 138: 706-711.
|
| 15 |
HANDA Y P . Calorimetric determinations of the compositions, enthalpies of dissociation, and heat capacities in the range 85 to 270 K for clathrate hydrates of xenon and krypton[J]. J. Chem. Thermodynamics, 1986, 18: 891-902.
|
| 16 |
HANDA Y P . Compositions, enthalpies of dissociation and heat capacities in the range 85 to 270 K for clathrate hydrates of methane, ethane, and propane, and enthalpy of dissociation of isobutane hydrate, as determined by a heat-flow calorimeter[J]. J. Chem. Thermodynamics, 1986, 18: 915-921.
|
| 17 |
KANG S P , LEE H, RYU B J . Enthalpies of dissociation of clathrate hydrates of carbon dioxide, nitrogen, (carbon dioxide + nitrogen), and (carbon dioxide nitrogen+tetrahydrofuran)[J]. J. Chem. Thermodynamics, 2001, 33: 513-521.
|
| 18 |
KOH C A, WESTACOTT R E , ZANG W , et al . Mechanisms of gas hydrate formation and inhibition[J]. Fluid Phase Equilibria, 2002, 151: 194-197.
|
| 19 |
DALMAZZONE D , HAMED N , DALMAZZONE C , et al . Application of high pressure DSC to the kinetics of methane hydrate in water-in-oil emulsion[J]. Journal of Thermal Analysis and Calorimetry, 2006, 85(2): 361-368.
|
| 20 |
ZHANG J S , LEE J W . Equilibrium of hydrogen + cyclopentane and carbon dioxide + cyclopentane binary hydrates[J]. J. Chem. Eng. Data, 2009, 54: 659-661.
|
| 21 |
LEE S, LEE Y, LEE J, et al . Experimental verification of methane-carbon dioxide replacement in natural gas hydrates using a differential scanning calorimeter[J]. Environ. Sci. Technol., 2013, 47(22): 13184-13190.
|
| 22 |
HO L C, BABU P , KUMAR R ,et al . HBGS(hydrate based gas separation) process for carbon dioxide capture employing an unstirred reactor with cyclopentane[J]. Energy, 2013, 63: 252-259.
|
 ),李小森1,2,3,4(
),李小森1,2,3,4( ),邹颖楠6,徐纯刚1,2,3,4(
),邹颖楠6,徐纯刚1,2,3,4( )
)
 ),Xiaosen LI1,2,3,4(
),Xiaosen LI1,2,3,4( ),Yingnan ZOU6,Chungang XU1,2,3,4(
),Yingnan ZOU6,Chungang XU1,2,3,4( )
)