化工进展 ›› 2024, Vol. 43 ›› Issue (10): 5457-5466.DOI: 10.16085/j.issn.1000-6613.2023-1692
• 能源加工与技术 • 上一篇
碳中和目标驱动下合成燃料技术的发展
- 中石化石油化工科学研究院有限公司,北京 100083
-
收稿日期:2023-09-25修回日期:2024-03-06出版日期:2024-10-15发布日期:2024-10-29 -
通讯作者:韩伟 -
作者简介:韩恒文(1973—),男,硕士,高级工程师,研究方向为石油加工和产品开发。E-mail:hanhw.ripp@sinopec.com。
Development trend of synthetic fuel technology driven by carbon neutrality
HAN Hengwen( ), HAN Wei(
), HAN Wei( ), CHENG Wei
), CHENG Wei
- SINOPEC Research Institute of Petroleum Processing Co. , Ltd. , Beijing 100083, China
-
Received:2023-09-25Revised:2024-03-06Online:2024-10-15Published:2024-10-29 -
Contact:HAN Wei
摘要:
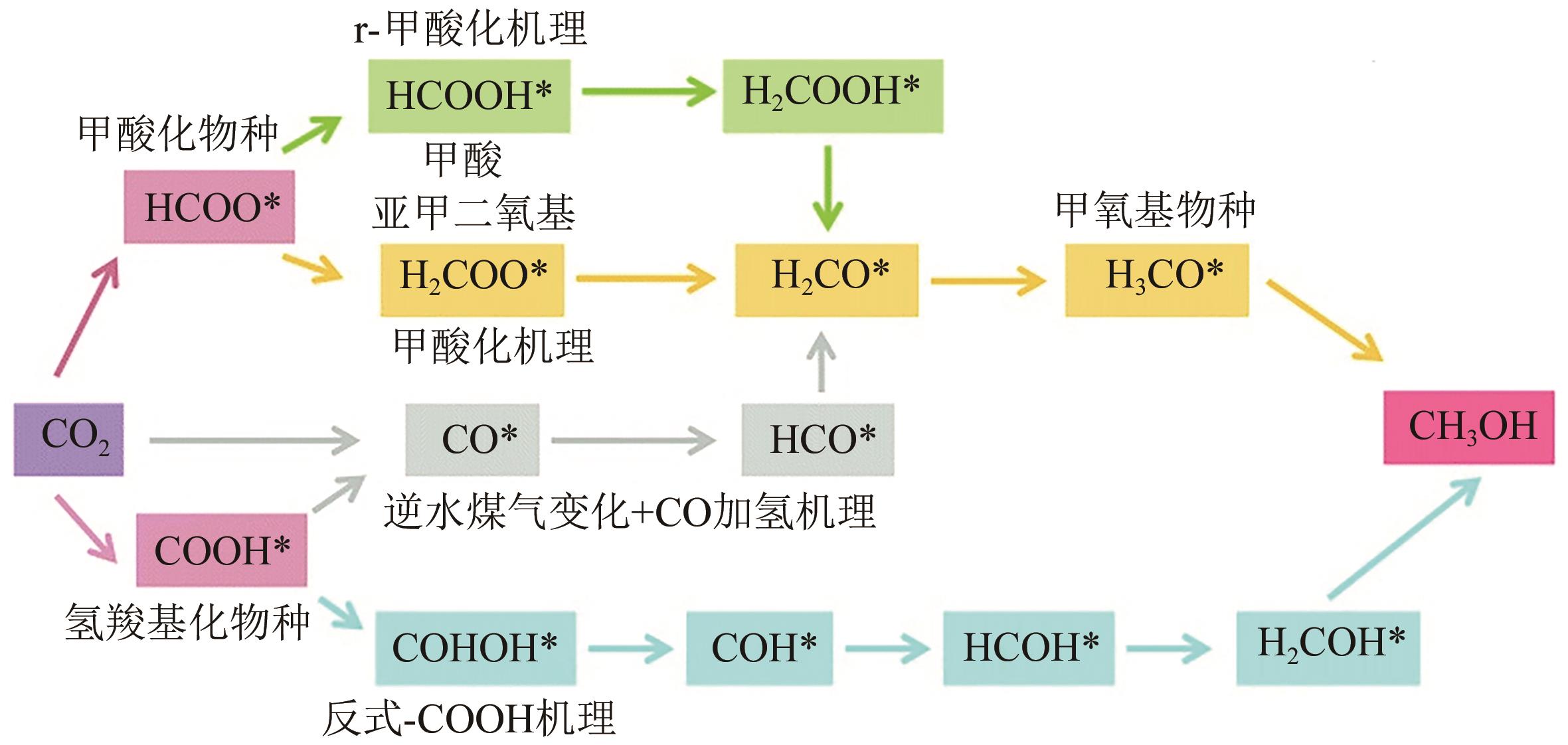
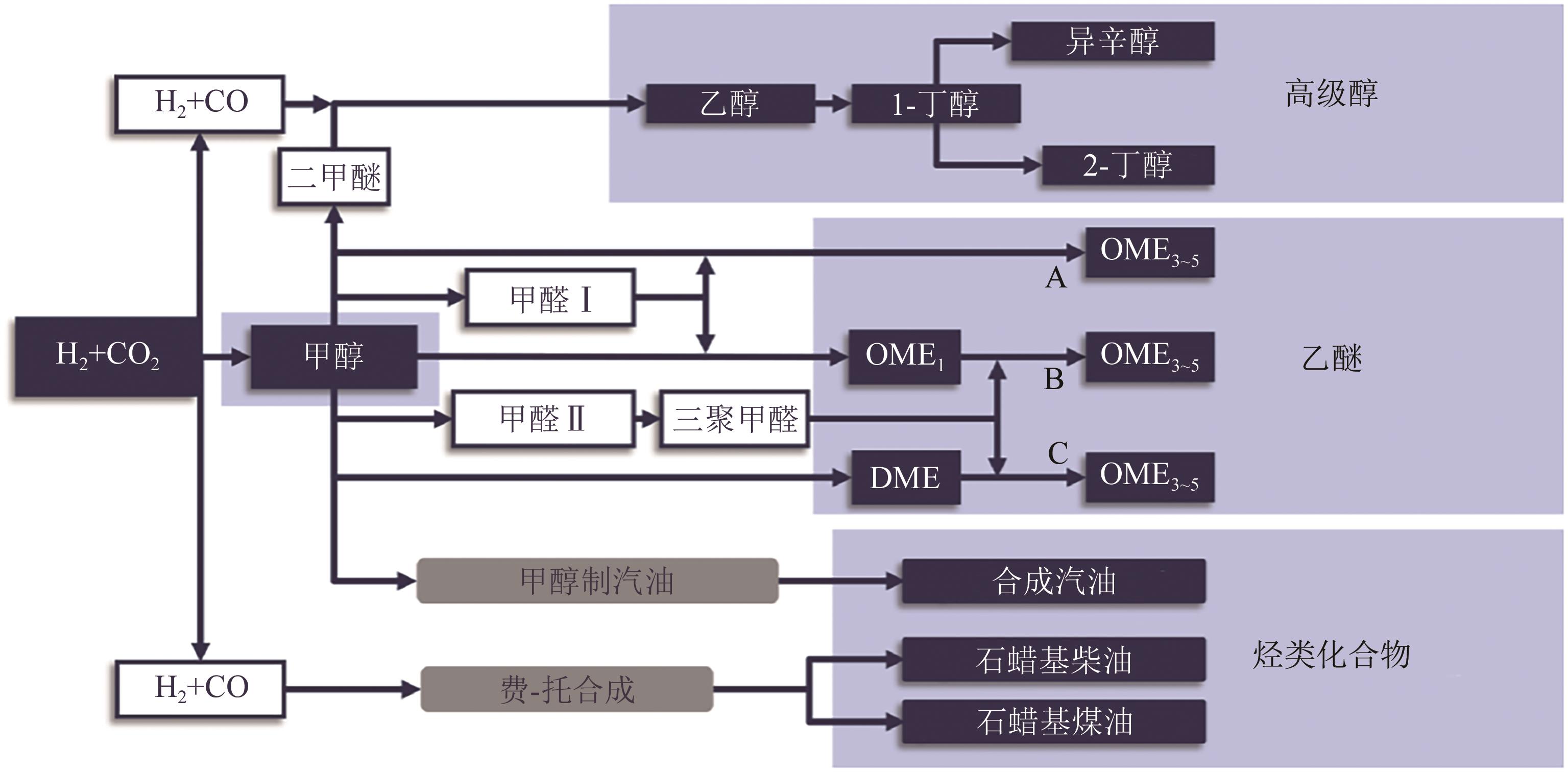
全球主要发达国家和地区均制定了实现碳中和的规划路线,我国也提出了“双碳”目标。鉴于节能改造、提升效率、用能优化等措施对炼化企业减少碳排放要求的不断提高,炼化企业实现低碳、零碳发展必须从原料构成、原始加工技术的角度出发,开发新的碳循环经济技术和零碳合成燃料技术。基于此,本文综述了国内外由捕集/储存CO2与可再生H2(绿氢)制碳氢燃料(e-Fuels)技术和由生物质制合成燃料(SNG)技术的反应机理、技术路线、催化剂、技术经济性和碳减排潜力等方面的研究进展和发展趋势,分析了未来炼化企业实现碳中和发展的技术发展路径,为炼厂转型发展提供了借鉴。
中图分类号:
引用本文
韩恒文, 韩伟, 程薇. 碳中和目标驱动下合成燃料技术的发展[J]. 化工进展, 2024, 43(10): 5457-5466.
HAN Hengwen, HAN Wei, CHENG Wei. Development trend of synthetic fuel technology driven by carbon neutrality[J]. Chemical Industry and Engineering Progress, 2024, 43(10): 5457-5466.
| 项目 | 工艺1[ | 工艺2[ | 工艺3[ | 工艺4[ | 工艺5[ |
|---|---|---|---|---|---|
| 温度/℃ | 200 | 250 | 266 | 260 | 250 |
| 压力/MPa | 3.0 | 3.0 | 3.0 | 5.0 | 5.0 |
| 催化剂 | Pd/CuO-ZnO-Al2O3-ZrO2/HZSM-5 | CuO-TiO2-ZrO2/HZSM-5 | CuO-ZnO/Al2O3 | 6CuO-3ZnO-Al2O3/HZSM-5 | CuZr-Pd/HZSM-5 |
| n(CO2)∶n(H2) | 1∶3.3 | 1∶3 | 1∶3 | 1∶3 | 1∶3 |
| 产品选择性/% | |||||
| DME | 73.56 | 47.5 | 32.4 | 75 | 51.8 |
| CO | 13.05 | 39.2 | 33.58 | 20 | 33.9 |
| CH4 | 0.1 | — | — | — | 0.2 |
| MeOH | 13.29 | 13.0 | 33.98 | 5 | 14.1 |
表1 不同催化剂作用下CO2制合成燃料的工艺条件和产品分布
| 项目 | 工艺1[ | 工艺2[ | 工艺3[ | 工艺4[ | 工艺5[ |
|---|---|---|---|---|---|
| 温度/℃ | 200 | 250 | 266 | 260 | 250 |
| 压力/MPa | 3.0 | 3.0 | 3.0 | 5.0 | 5.0 |
| 催化剂 | Pd/CuO-ZnO-Al2O3-ZrO2/HZSM-5 | CuO-TiO2-ZrO2/HZSM-5 | CuO-ZnO/Al2O3 | 6CuO-3ZnO-Al2O3/HZSM-5 | CuZr-Pd/HZSM-5 |
| n(CO2)∶n(H2) | 1∶3.3 | 1∶3 | 1∶3 | 1∶3 | 1∶3 |
| 产品选择性/% | |||||
| DME | 73.56 | 47.5 | 32.4 | 75 | 51.8 |
| CO | 13.05 | 39.2 | 33.58 | 20 | 33.9 |
| CH4 | 0.1 | — | — | — | 0.2 |
| MeOH | 13.29 | 13.0 | 33.98 | 5 | 14.1 |
| 项目 | 蒸汽气化[ | SER工艺[ | CO2气化[ |
|---|---|---|---|
| φ(干基)/% | |||
| CO | 21.2 | 8.6 | 40 |
| CO2 | 21.5 | 5.6 | 40 |
| H2 | 48 | 69.5 | 15 |
| CH4 | 8.8 | 14 | 5 |
| C x H y | 0.5 | 2.3 | 0 |
| φ(H2O)/% | 32 | 41 | 7 |
| 气化温度/℃ | 797 | 629 | >840 |
| 床层材料 | 石灰石 | 石灰石 | 橄榄石 |
| 气化效率/% | 84 | 73 | 73 |
表2 软木屑不同气化工艺参数及气化合成气的组成
| 项目 | 蒸汽气化[ | SER工艺[ | CO2气化[ |
|---|---|---|---|
| φ(干基)/% | |||
| CO | 21.2 | 8.6 | 40 |
| CO2 | 21.5 | 5.6 | 40 |
| H2 | 48 | 69.5 | 15 |
| CH4 | 8.8 | 14 | 5 |
| C x H y | 0.5 | 2.3 | 0 |
| φ(H2O)/% | 32 | 41 | 7 |
| 气化温度/℃ | 797 | 629 | >840 |
| 床层材料 | 石灰石 | 石灰石 | 橄榄石 |
| 气化效率/% | 84 | 73 | 73 |
| 工艺 | 反应器段数 | 操作温度/℃ | 原料分流数 | 一反控温方法 | 循环气温度/℃ | 催化剂参数(型号、适用温度、状态) | 应用企业 |
|---|---|---|---|---|---|---|---|
| Davy | 4 | 250~620 | 2 | 部分二反产品气循环 | 150~155 | CRG-S2(250~700℃)/氧化态或 预还原态 | 大唐克旗/阜新、伊利新天 |
| Topsoe | 5 | 250~675 | 2 | 部分一反产品气循环并添加部分蒸汽 | 180~210 | MCR-2X(250~700℃)、PK-7R (250~400℃)/氧化态 | 新疆庆华 |
| Topsoe | 4 | 250~650 | 2 | 部分二反产品气循环 | 190~210 | MCR-2X(250~700℃)、PK-7R (250~400℃)/氧化态 | 内蒙古汇能韩国浦项光阳 |
| Lurgi | 3 | 230~650 | 1或2 | 部分二反产品气循环 | 60~150 | G1-85(230~510℃)G1-86(230~650℃)/氧化态 | 工业化推广 |
| 大唐化工 | 4 | 240~650 | 4 | 部分二反产品气循环 | 170~190 | DTC-M1S(250~700℃)、DTC-M1C(250~700℃)/预还原态 | 大唐克旗 |
表3 工业化合成气甲烷化工艺参数比较[63]
| 工艺 | 反应器段数 | 操作温度/℃ | 原料分流数 | 一反控温方法 | 循环气温度/℃ | 催化剂参数(型号、适用温度、状态) | 应用企业 |
|---|---|---|---|---|---|---|---|
| Davy | 4 | 250~620 | 2 | 部分二反产品气循环 | 150~155 | CRG-S2(250~700℃)/氧化态或 预还原态 | 大唐克旗/阜新、伊利新天 |
| Topsoe | 5 | 250~675 | 2 | 部分一反产品气循环并添加部分蒸汽 | 180~210 | MCR-2X(250~700℃)、PK-7R (250~400℃)/氧化态 | 新疆庆华 |
| Topsoe | 4 | 250~650 | 2 | 部分二反产品气循环 | 190~210 | MCR-2X(250~700℃)、PK-7R (250~400℃)/氧化态 | 内蒙古汇能韩国浦项光阳 |
| Lurgi | 3 | 230~650 | 1或2 | 部分二反产品气循环 | 60~150 | G1-85(230~510℃)G1-86(230~650℃)/氧化态 | 工业化推广 |
| 大唐化工 | 4 | 240~650 | 4 | 部分二反产品气循环 | 170~190 | DTC-M1S(250~700℃)、DTC-M1C(250~700℃)/预还原态 | 大唐克旗 |
| 生物质气化方式 | 直接甲烷化 | 亚化学计量甲烷化 | 过化学计量甲烷化 |
|---|---|---|---|
| 蒸汽气化 | 气化过程中易生成焦炭,使该工艺无法工业应用 | 气化过程中的氢利用率最大化,工艺效率最高,但产物合成气需要分离未反应的CO2 | 气化过程中的碳转化实现最大化,导致氢气消耗量大,额外电耗增大,合成气需分离去除过量的氢 |
| SER气化 | 工艺可行,气化过程不生成焦炭,且不需要额外氢,但产物合成气中的焦油含量提高 | 因SER气化得到的合成气氢含量较高,工艺未获得工业应用 | 因SER气化得到的合成气氢含量过高,工艺未获得工业应用 |
| CO2气化 | 气化过程易生成焦炭,使该工艺无法工业应用 | 气化过程可实现CO2捕集和利用,但因体系氢含量过低而需要补加大量氢气,导致耗电量高 | 气化过程可实现CO2捕集和利用,但因体系氢含量过低而需要补加大量氢气,导致耗电量高 |
表4 不同合成气甲烷化策略的优势与不足
| 生物质气化方式 | 直接甲烷化 | 亚化学计量甲烷化 | 过化学计量甲烷化 |
|---|---|---|---|
| 蒸汽气化 | 气化过程中易生成焦炭,使该工艺无法工业应用 | 气化过程中的氢利用率最大化,工艺效率最高,但产物合成气需要分离未反应的CO2 | 气化过程中的碳转化实现最大化,导致氢气消耗量大,额外电耗增大,合成气需分离去除过量的氢 |
| SER气化 | 工艺可行,气化过程不生成焦炭,且不需要额外氢,但产物合成气中的焦油含量提高 | 因SER气化得到的合成气氢含量较高,工艺未获得工业应用 | 因SER气化得到的合成气氢含量过高,工艺未获得工业应用 |
| CO2气化 | 气化过程易生成焦炭,使该工艺无法工业应用 | 气化过程可实现CO2捕集和利用,但因体系氢含量过低而需要补加大量氢气,导致耗电量高 | 气化过程可实现CO2捕集和利用,但因体系氢含量过低而需要补加大量氢气,导致耗电量高 |
| 1 | BYRUM Zachary, DELLESKY Carrie. A low-carbon future in the US depends on decarbonizing petroleun refineries[R/OL]. (2021-10-21) [2023-01-08]. . |
| 2 | HEPBURN Cameron, ADLEN Ella, BEDDINGTON John, et al. The technological and economic prospects for CO2 utilization and removal[J]. Nature, 2019, 575(7781): 87-97. |
| 3 | BYRUM Zachary, Hélène PILORGÉ, WILCOX Jennifer. Technological pathways for decarbonizing petroleum refining[R/OL]. (2021-10-21) [2023-01-08]. . |
| 4 | LUNA Diego, ESTEVEZ Rafael. Optimization of biodiesel and biofuel process[J]. Energies, 2022, 15(16): 5917. |
| 5 | SCHEMME Steffen, BREUER Janos Lucian, Maximilian KÖLLER, et al. H2-based synthetic fuels: A techno-economic comparison of alcohol, ether and hydrocarbon production[J]. International Journal of Hydrogen Energy, 2020, 45(8): 5395-5414. |
| 6 | ZHONG Jiawei, YANG Xiaofeng, WU Zhilian, et al. State of the art and perspectives in heterogeneous catalysis of CO2 hydrogenation to methanol[J]. Chemical Society Reviews, 2020, 49(5): 1385-1413. |
| 7 | QI Shichao, LIU Xiaoying, ZHU Rongrong, et al. Causation of catalytic activity of Cu-ZnO for CO2 hydrogenation to methanol[J]. Chemical Engineering Journal, 2022, 430: 132784. |
| 8 | LINDSTAD Elizabeth, LAGEMANN Benjamin, RIALLAND Agathe, et al. Reduction of maritime GHG emissions and the potential role of E-fuels[J]. Transportation Research D: Transport and Environment, 2021, 101: 103075. |
| 9 | 王艳燕, 刘会贞, 韩布兴. 多相催化剂催化二氧化碳加氢合成甲醇的研究进展[J]. 高等学校化学学报, 2020, 41(11): 2393-2403. |
| WANG Yanyan, LIU Huizhen, HAN Buxing. Advances in CO2 hydrogenation to methanol by heterogeneous catalysis[J]. Chemical Journal of Chinese Universities, 2020, 41(11): 2393-2403. | |
| 10 | XIAO Shuo, ZHANG Yanfei, GAO Peng,et al. Highly efficient Cu-based catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol[J]. Catalysis Today, 2017, 281: 327-333. |
| 11 | HANSEN J B, HØJLUND NIELSEN P E. Methanol synthesis. In: Handbook of heterogeneous catalysis[G]. Weinheim: Wiley-VCH, 2008: 2920e49. . |
| 12 | SUN Kunpeng, LU Weiwei, WANG Min, et al. Low-temperature synthesis of DME from CO2/H2 over Pd-modified CuO-ZnO-Al2O3-ZrO2/HZSM-5 catalysts[J]. Catalysis Communications, 2004, 5(7): 367-370. |
| 13 | ZHANG Menghui, LIU Zhiming, LIN Guodong, et al. Pd/CNT-promoted CuZrO2/HZSM-5 hybrid catalysts for direct synthesis of DME from CO2/H2 [J]. Applied Catalysis A: General, 2013, 451: 28-35. |
| 14 | WANG Song, MAO Dongsen, GUO Xiaoming, et al. Dimethyl ether synthesis via CO2 hydrogenation over CuO-TiO2-ZrO2/HZSM-5 bifunctional catalysts[J]. Catalysis Communications, 2009, 10(10): 1367-1370. |
| 15 | ZHA Fei, DING Jian, CHANG Yue, et al. Cu-Zn-Al oxide cores packed by metal-doped amorphous silica-alumina membrane for catalyzing the hydrogenation of carbon dioxide to dimethyl ether[J]. Industrial & Engineering Chemistry Research, 2012, 51(1): 345-352. |
| 16 | NAIK Sajo P, Taegong RYU, Vy BUI, et al. Synthesis of DME from CO2/H2 gas mixture[J]. Chemical Engineering Journal, 2011, 167(1): 362-368. |
| 17 | 周紫璇, 杨海艳, 孙予罕, 等. 二氧化碳加氢制甲醇多相催化剂研究进展[J]. 高等学校化学学报, 2022, 43(7): 11-23. |
| ZHOU Zixuan, YANG Haiyan, SUN Yuhan, et al. Recent progress in heterogeneous catalysts for the hydrogenation of carbon dioxide to methanol[J]. Chemical Journal of Chinese Universities, 2022, 43(7): 11-23. | |
| 18 | WU Congyi, LIN Lili, LIU Jinjia, et al. Inverse ZrO2/Cu as a highly efficient methanol synthesis catalyst from CO2 hydrogenation[J]. Nature Communications, 2020, 11(1): 5767. |
| 19 | MUREDDU Mauro, FERRARA Francesca, PETTINAU Alberto. Highly efficient CuO/ZnO/ZrO2@SBA-15 nanocatalysts for methanol synthesis from the catalytic hydrogenation of CO2 [J]. Applied Catalysis B: Environmental, 2019, 258: 117941. |
| 20 | JIANG Xiao, NIE Xiaowa, GUO Xinwen, et al. Recent advances in carbon dioxide hydrogenation to methanol via heterogeneous catalysis[J]. Chemical Reviews, 2020, 120(15): 7984-8034. |
| 21 | ZHANG Xueqiang, KIRILIN Alexey V, ROZEVELD Steve, et al. Support effect and surface reconstruction in In2O3/m-ZrO2 catalyzed CO2 hydrogenation[J]. ACS Catalysis, 2022, 12(7): 3868-3880. |
| 22 | ZABILSKIY Maxim, SUSHKEVICH Vitaly L, NEWTON Mark A, et al. Mechanistic study of carbon dioxide hydrogenation over Pd/ZnO-based catalysts: The role of palladium-zinc alloy in selective methanol synthesis[J]. Angewandte Chemie International Edition, 2021, 60(31): 17053-17059. |
| 23 | SONG Jimin, LIU Sihang, YANG Chengsheng, et al. The role of Al doping in Pd/ZnO catalyst for CO2 hydrogenation to methanol[J]. Applied Catalysis B: Environmental, 2020, 263: 118367. |
| 24 | DYBBERT Valentin, FEHR Samuel Matthias, KLEIN Florian, et al. Oxidative fluorination of Cu/ZnO methanol catalysts[J]. Angewandte Chemie International Edition, 2019, 58(37): 12935-12939. |
| 25 | ZHOU Zixuan, GAO Peng. Direct carbon dioxide hydrogenation to produce bulk chemicals and liquid fuels via heterogeneous catalysis[J]. Chinese Journal of Catalysis, 2022, 43(8): 2045-2056. |
| 26 | SNIDER Jonathan L, STREIBEL Verena, HUBERT McKenzie A, et al. Revealing the synergy between oxide and alloy phases on the performance of bimetallic In-Pd catalysts for CO2 hydrogenation to methanol[J]. ACS Catalysis, 2019, 9(4): 3399-3412. |
| 27 | LI Molly Meng-Jung, ZOU Hanbo, ZHENG Jianwei, et al. Methanol synthesis at a wide range of H2/CO2 ratios over a Rh-In bimetallic catalyst[J]. Angewandte Chemie International Edition, 2020, 59(37): 16039-16046. |
| 28 | YU Jiahui, LIU Shuai, MU Xueliang, et al. Cu-ZrO2 catalysts with highly dispersed Cu nanoclusters derived from ZrO2@ HKUST-1 composites for the enhanced CO2 hydrogenation to methanol[J]. 2021, 419: 129656. |
| 29 | FREI Matthias S, MONDELLI Cecilia, Rodrigo GARCÍA-MUELAS, et al. Atomic-scale engineering of indium oxide promotion by palladium for methanol production via CO2 hydrogenation[J]. Nature Communications, 2019, 10: 3377. |
| 30 | RASTEIRO Letícia F, DE SOUSA Rafael A, VIEIRA Luiz H, et al. Insights into the alloy-support synergistic effects for the CO2 hydrogenation towards methanol on oxide-supported Ni5Ga3 catalysts: An experimental and DFT study[J]. Applied Catalysis B: Environmental, 2022, 302: 120842. |
| 31 | SHEN Chenyang, SUN Kaihang, ZHANG Zhitao,et al. Highly active Ir/In2O3 catalysts for selective hydrogenation of CO2 to methanol: Experimental and theoretical studies [J]. ACS Catal,2021,11(7):4036-4046 |
| 32 | PINHEIRO ARAÚJO Thaylan, Jordi MORALES-VIDAL, ZOU Tangsheng, et al. Flame spray pyrolysis as a synthesis platform to assess metal promotion in In2O3-catalyzed CO2 hydrogenation[J]. Advanced Energy Materials, 2022, 12(14): 2103707. |
| 33 | ZHU Jie, WANG Peng, ZHANG Xiaoben, et al. Dynamic structural evolution of iron catalysts involving competitive oxidation and carburization during CO2 hydrogenation[J]. Science Advances, 2022, 8(5): eabm3629. |
| 34 | Chemische OTTO A., verfahrenstechnische und Cokonomische Bewertung von Kohlendioxid als Rohstoff in der chemischen Industrie. Schriftenreihe des Forschungszentrums Jü lich: Reihe Energie & Umwelt[D]. Jü lich: Forschungszentrum Jü lich. 2015, III: 272. |
| 35 | SCHEMME Steffen, BREUER Janos Lucian, SAMSUN Remzi Can, et al. Promising catalytic synthesis pathways towards higher alcohols as suitable transport fuels based on H2 and CO2 [J]. Journal of CO2 Utilization, 2018, 27: 223-237. |
| 36 | LIN Qinjie, Kun Lin TAY, YU Wenbin, et al. Polyoxymethylene dimethyl ether 3 (PODE3) as an alternative fuel to reduce aerosol pollution[J]. Journal of Cleaner Production, 2021, 285: 124857. |
| 37 | Mar PÉREZ-FORTES, SCHÖNEBERGER Jan C, BOULAMANTI Aikaterini, et al. Methanol synthesis using captured CO2 as raw material: Techno-economic and environmental assessment[J]. Applied Energy, 2016, 161: 718-732. |
| 38 | Porsche and Siemens Energy in collaboration to build industrial plant for production of eFuel in Chile[N]. Auto Business News (ABN), 2021-09-13. |
| 39 | BOOT Michael D, TIAN Miao, HENSEN Emiel J M, et al. Impact of fuel molecular structure on auto-ignition behavior—Design rules for future high performance gasolines[J]. Progress in Energy and Combustion Science, 2017, 60: 1-25. |
| 40 | DE LUNA Phil, hristopher HAHN C, HIGGINS Drew, et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes?[J]. Science, 2019, 364(6438): eaav3506. |
| 41 | RAMIREZ Adrian, Mani SARATHY S, GASCON Jorge. CO2 derived E-fuels: Research trends, misconceptions, and future directions[J]. Trends in Chemistry, 2020, 2(9): 785-795. |
| 42 | SCHILDHAUER Tilman J, BIOLLAZ Serge M. Synthetic natural gas from coal, dry biomass, and power-to-gas applications[M]. New Jersey: Wiley, 2016. |
| 43 | HOFBAUER Hermann. Biomass gasification for electricity and fuels, large scale[M]//MEYERS R A. Encyclopedia of Sustainability Science and Technology. New York: Springer, 2012: 1426-1445. |
| 44 | KALTSCHMITT M, SPLIETHOFF H .Energie aus biomasse:Grundlagen,techniken und verfahren[M]. 2nd ed. Berlin, Germany: Springer, 2009. |
| 45 | FUCHS J. Verfahrenscharakteristika von sorption enhanced reforming in einem einem fortschrittlichen gaserzeugungssystem: Verfahrenscharakteristika von sorption enhanced reforming in einem einem fortschrittlichen gaserzeugungssystem[R]. https://repositum.tuwien.at/handle/20. 500. 12708/16812? mode=full (accessed on 27 May 2021). |
| 46 | Stefan MÜLLER, STIDL Martin, Tobias PRÖLL, et al. Hydrogen from biomass: Large-scale hydrogen production based on a dual fluidized bed steam gasification system[J]. Biomass Conversion and Biorefinery, 2011, 1(1): 55-61. |
| 47 | ZHANG Yaning, LI Bingxi, LI Hongtao, et al. Thermodynamic evaluation of biomass gasification with air in autothermal gasifiers[J]. Thermochimica Acta, 2011, 519(1/2): 65-71. |
| 48 | STEC Marcin, CZAPLICKI Andrzej, TOMASZEWICZ Grzegorz, et al. Effect of CO2 addition on lignite gasification in a CFB reactor: A pilot-scale study[J]. Korean Journal of Chemical Engineering, 2018, 35(1): 129-136. |
| 49 | JEREMIÁŠ M, POHOŘELÝ M, SVOBODA K, et al. Gasification of biomass with CO2 and H2O mixtures in a catalytic fluidised bed[J]. Fuel, 2017, 210: 605-610. |
| 50 | VALIN Sylvie, BEDEL Laurent, GUILLAUDEAU Jacques, et al. CO2 as a substitute of steam or inert transport gas in a fluidised bed for biomass gasification[J]. Fuel, 2016, 177: 288-295. |
| 51 | ZENG Liang, WEI Di, TOAN Sam, et al. Sorption-enhanced chemical looping oxidative steam reforming of methanol for on-board hydrogen supply[J]. Green Energy & Environment, 2022, 7(1): 145-155. |
| 52 | MAUERHOFER A M, MÜLLER S, BARTIK A, et al. Conversion of CO2 during the DFB biomass gasification process[J]. Biomass Conversion and Biorefinery, 2021, 11(1): 15-27. |
| 53 | FUCHS Josef, SCHMID Johannes C, Stefan MÜLLER, et al. Dual fluidized bed gasification of biomass with selective carbon dioxide removal and limestone as bed material: A review[J]. Renewable and Sustainable Energy Reviews, 2019, 107: 212-231. |
| 54 | WANG Sheng, BI Xiaotao, WANG Shudong. Thermodynamic analysis of biomass gasification for biomethane production[J]. Energy, 2015, 90: 1207-1218. |
| 55 | PIO D T, TARELHO L A C. Industrial gasification systems (>3 MWth) for bioenergy in Europe: Current status and future perspectives[J]. Renewable and Sustainable Energy Reviews, 2021, 145: 111108. |
| 56 | THUNMAN Henrik, SEEMANN Martin, BERDUGO VILCHES Teresa, et al. Advanced biofuel production via gasification-lessons learned from 200 man-years of research activity with Chalmers’ research gasifier and the GoBiGas demonstration plant[J]. Energy Science & Engineering, 2018, 6(1): 6-34. |
| 57 | Stefan RÖNSCH, SCHNEIDER Jens, MATTHISCHKE Steffi, et al. Review on methanation-From fundamentals to current projects[J]. Fuel, 2016, 166: 276-296. |
| 58 | LEHNER Markus, BIEGGER Philipp, MEDVED Ana Roza. Power-to-gas: Die rolle der chemischen speicherung in einem energiesystem mit hohen anteilen an erneuerbarer energie[J]. e & i Elektrotechnik und Informationstechnik, 2017, 134(3): 246-251. |
| 59 | MEDVED A. The Influence of Nitrogen on Catalytic Methanation[D]. Leoben, Austria: Montanuniversität Leoben, 2020. |
| 60 | BAI Xiaobo, WANG Sheng, SUN Tianjun, et al. Influence of operating conditions on carbon deposition over a Ni catalyst for the production of synthetic natural gas (SNG) from coal[J]. Catalysis Letters, 2014, 144(12): 2157-2166. |
| 61 | BARTIK Alexander, BENEDIKT Florian, LUNZER Andreas, et al. Thermodynamic investigation of SNG production based on dual fluidized bed gasification of biogenic residues[J]. Biomass Conversion and Biorefinery, 2021, 11(1): 95-110. |
| 62 | KOPYSCINSKI Jan, SCHILDHAUER Tilman J, BIOLLAZ Serge M A. Production of synthetic natural gas (SNG) from coal and dry biomass—A technology review from 1950 to 2009[J]. Fuel, 2010, 89(8): 1763-1783. |
| 63 | 李安学, 李春启, 左玉帮, 等. 合成气甲烷化工艺技术研究进展[J]. 化工进展, 2015, 34(11): 3898-3905. |
| LI Anxue, LI Chunqi, ZUO Yubang, et al. Research development syngas methanation technology[J]. Chemical Industry and Engineering Progress, 2015, 34(11): 3898-3905. | |
| 64 | TREMEL Alexander, GADERER Matthias, SPLIETHOFF Hartmut. Small-scale production of synthetic natural gas by allothermal biomass gasification[J]. International Journal of Energy Research, 2013, 37(11): 1318-1330. |
| 65 | SALBRECHTER Katrin, SCHUBERT Teresa. Combination of b-fuels and e-fuels—A technological feasibility study[J]. Energies, 2021, 14(17): 5250. |
| 66 | LARSSON Anton, KUBA Matthias, BERDUGO VILCHES Teresa, et al. Steam gasification of biomass―Typical gas quality and operational strategies derived from industrial-scale plants[J]. Fuel Processing Technology, 2021, 212: 106609. |
| [1] | 宋兴飞, 贾鑫, 安萍, 韩振南, 许光文. 热化学反应工程科学与技术发展与展望[J]. 化工进展, 2024, 43(7): 3513-3533. |
| [2] | 韩伟, 韩恒文, 程薇, 汤玮健. 碳中和目标驱动下生物质燃料技术研究进展[J]. 化工进展, 2024, 43(5): 2463-2474. |
| [3] | 解仲凯, 施伟东. 电荷极化光催化剂光转化二氧化碳制多碳化学品的研究进展[J]. 化工进展, 2024, 43(5): 2714-2722. |
| [4] | 杨梦茹, 彭琴, 常玉龙, 邱淑兴, 张溅波, 江霞. 生物炭替代煤粉/焦炭高炉炼铁碳减排技术研究进展[J]. 化工进展, 2024, 43(1): 490-500. |
| [5] | 舒斌, 陈建宏, 熊健, 吴其荣, 喻江涛, 杨平. 碳中和目标下推动绿色甲醇发展的必要性分析[J]. 化工进展, 2023, 42(9): 4471-4478. |
| [6] | 刘含笑, 吴黎明, 林青阳, 周烨, 罗象, 桂志军, 刘小伟, 单思珂, 朱前林, 陆诗建. 碳足迹评估技术及其在重点工业行业的应用[J]. 化工进展, 2023, 42(5): 2201-2218. |
| [7] | 陈崇明, 曾四鸣, 罗小娜, 宋国升, 韩忠阁, 郁金星, 孙楠楠. 基于超交联聚合物前体的碳载钾基CO2吸附剂制备和性能[J]. 化工进展, 2023, 42(3): 1540-1550. |
| [8] | 张育新, 王灿, 舒文祥. 二氧化碳的还原及其利用研究进展[J]. 化工进展, 2023, 42(2): 944-956. |
| [9] | 于锋. 关于“氮一化学”的思考与展望[J]. 化工进展, 2023, 42(12): 6136-6140. |
| [10] | 姚伦, 周雍进. 一碳化合物生物利用和转化研究进展[J]. 化工进展, 2023, 42(1): 16-29. |
| [11] | 胡兵, 徐立军, 何山, 苏昕, 汪继伟. 碳达峰与碳中和目标下PEM电解水制氢研究进展[J]. 化工进展, 2022, 41(9): 4595-4604. |
| [12] | 周颖, 周红军, 徐春明. 氢能的思考及发展路径判断和实践[J]. 化工进展, 2022, 41(8): 4587-4592. |
| [13] | 杨学萍. 碳中和背景下现代煤化工技术路径探索[J]. 化工进展, 2022, 41(7): 3402-3412. |
| [14] | 唐娇娇, 谢军祥, 陈重军, 余成, 陈德超. 城镇污水处理厂碳中和技术及案例[J]. 化工进展, 2022, 41(5): 2662-2671. |
| [15] | 黄晟, 王静宇, 李振宇. 碳中和目标下石油与化学工业绿色低碳发展路径分析[J]. 化工进展, 2022, 41(4): 1689-1703. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||