化工进展 ›› 2024, Vol. 43 ›› Issue (5): 2629-2644.DOI: 10.16085/j.issn.1000-6613.2023-2065
• 催化与材料技术 • 上一篇
吸附强化水气变换制氢复合催化剂研究进展
- 1.太原理工大学省部共建煤基能源清洁高效利用国家重点实验室,山西 太原 030024
2.山西浙大新材料与化工研究院,山西 太原 030000
-
收稿日期:2023-11-28修回日期:2024-03-16出版日期:2024-05-15发布日期:2024-06-15 -
通讯作者:荆洁颖 -
作者简介:张金鹏(1997—),女,博士研究生,研究方向为吸附强化制氢。E-mail:zhangjinpeng2502@126.com。 -
基金资助:国家重点研发计划(2022YFE0208400);山西浙大新材料与化工研究院研发项目(2021SX-FR002);中央高校基本科研业务费专项(2022ZFJH004)
Composite catalyst of sorption enhanced water gas shift for hydrogen production: A review
ZHANG Jinpeng1( ), QU Ting1, JING Jieying1,2(
), QU Ting1, JING Jieying1,2( ), LI Wenying1
), LI Wenying1
- 1.State Key Laboratory of Clean and Efficient Coal Utilization, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
2.Shanxi-Zheda Institute of Advanced Materials and Chemical Engineering, Taiyuan 030000, Shanxi, China
-
Received:2023-11-28Revised:2024-03-16Online:2024-05-15Published:2024-06-15 -
Contact:JING Jieying
摘要:
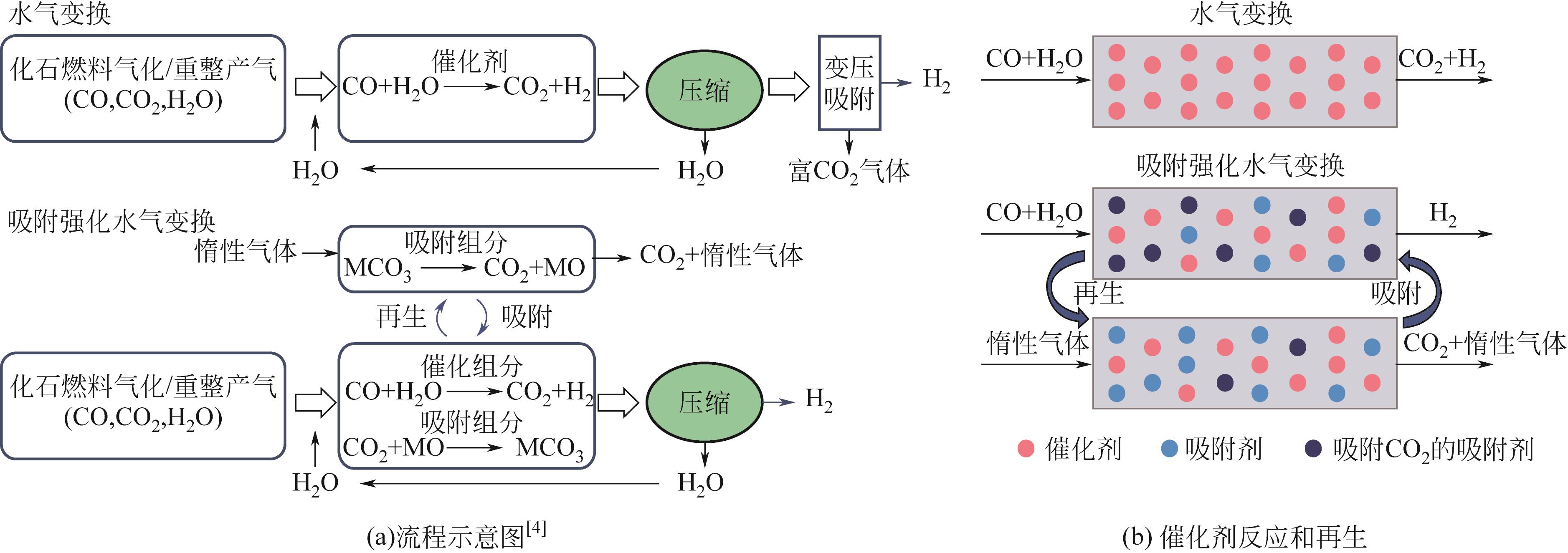
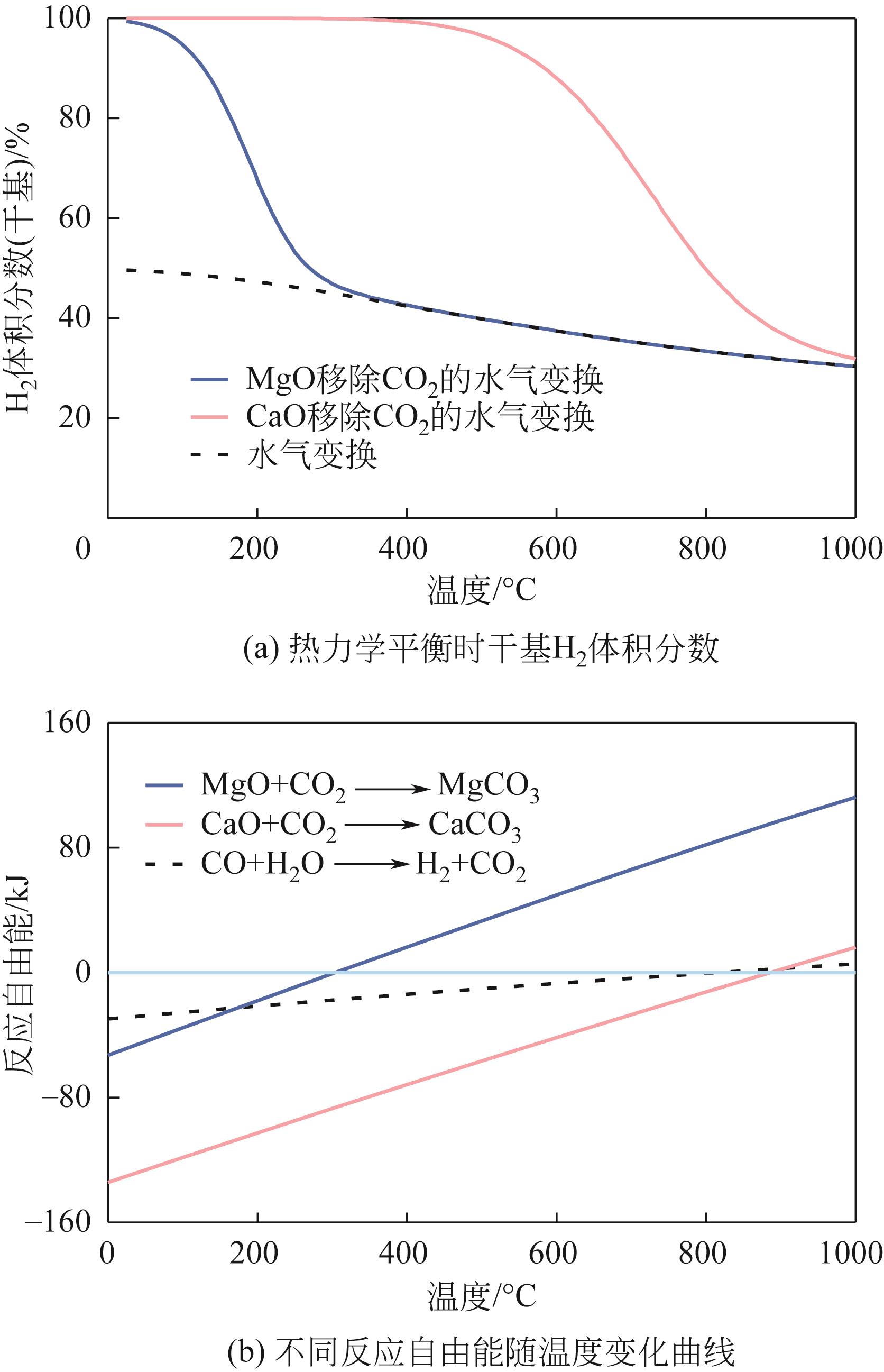
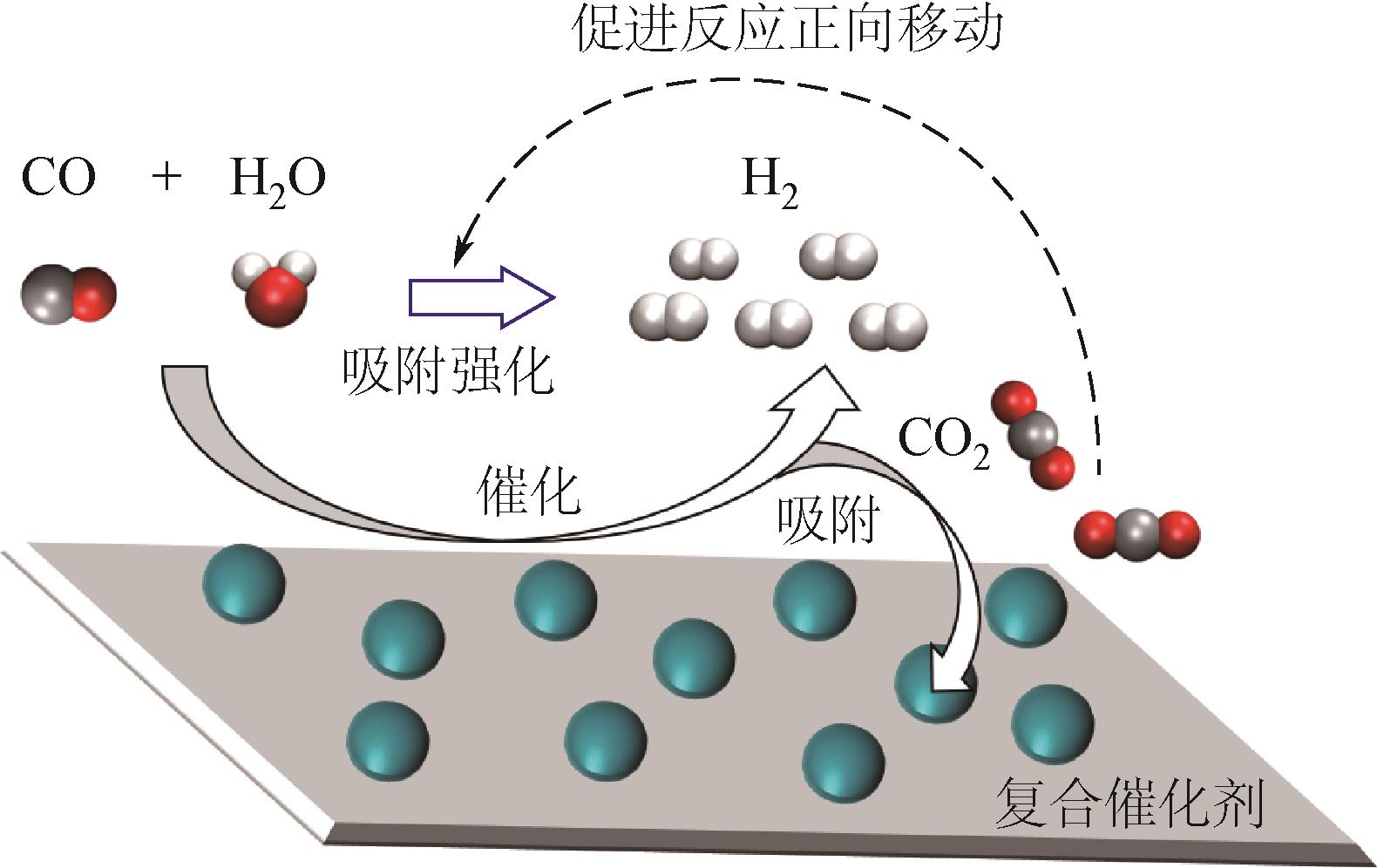
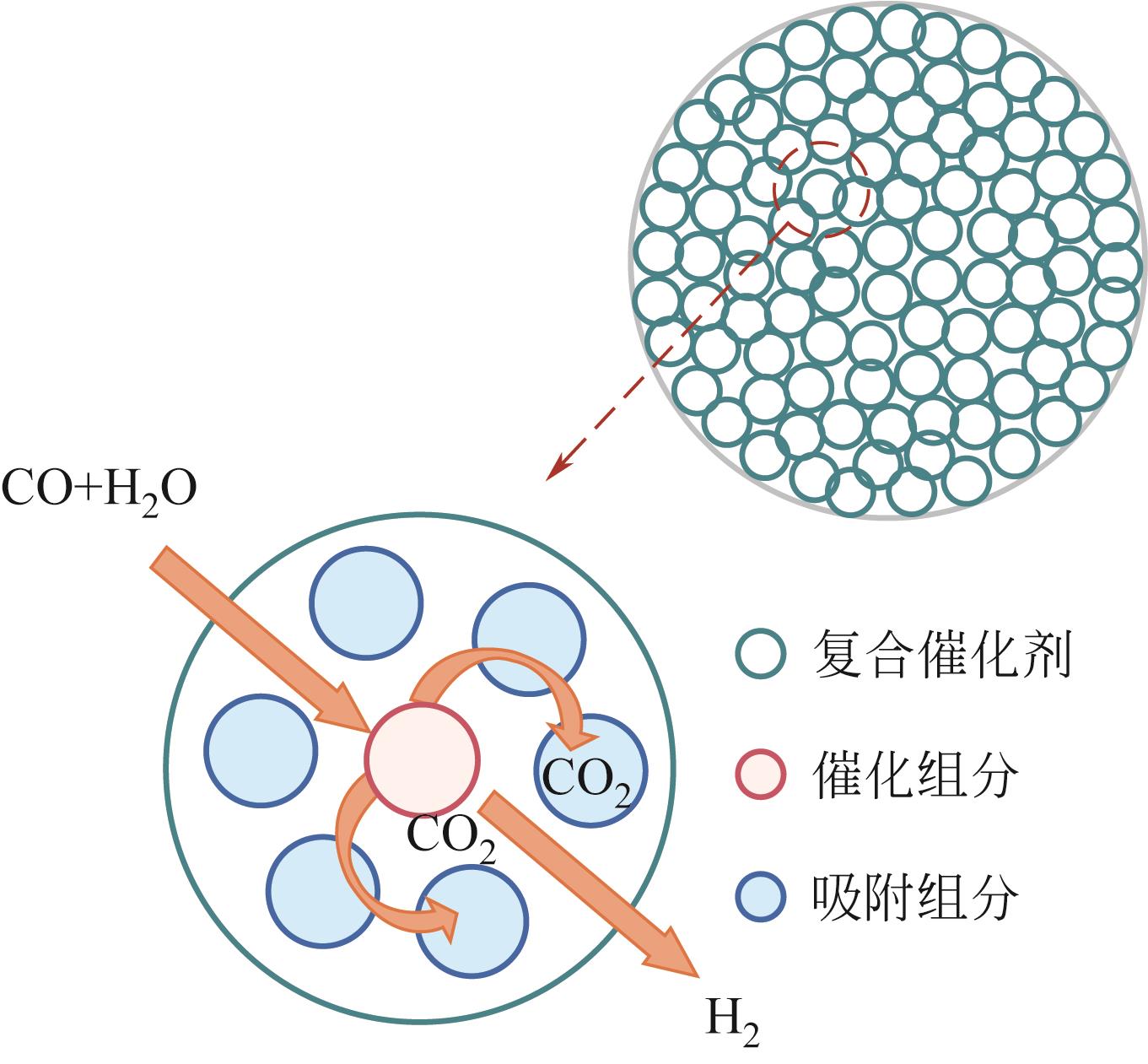
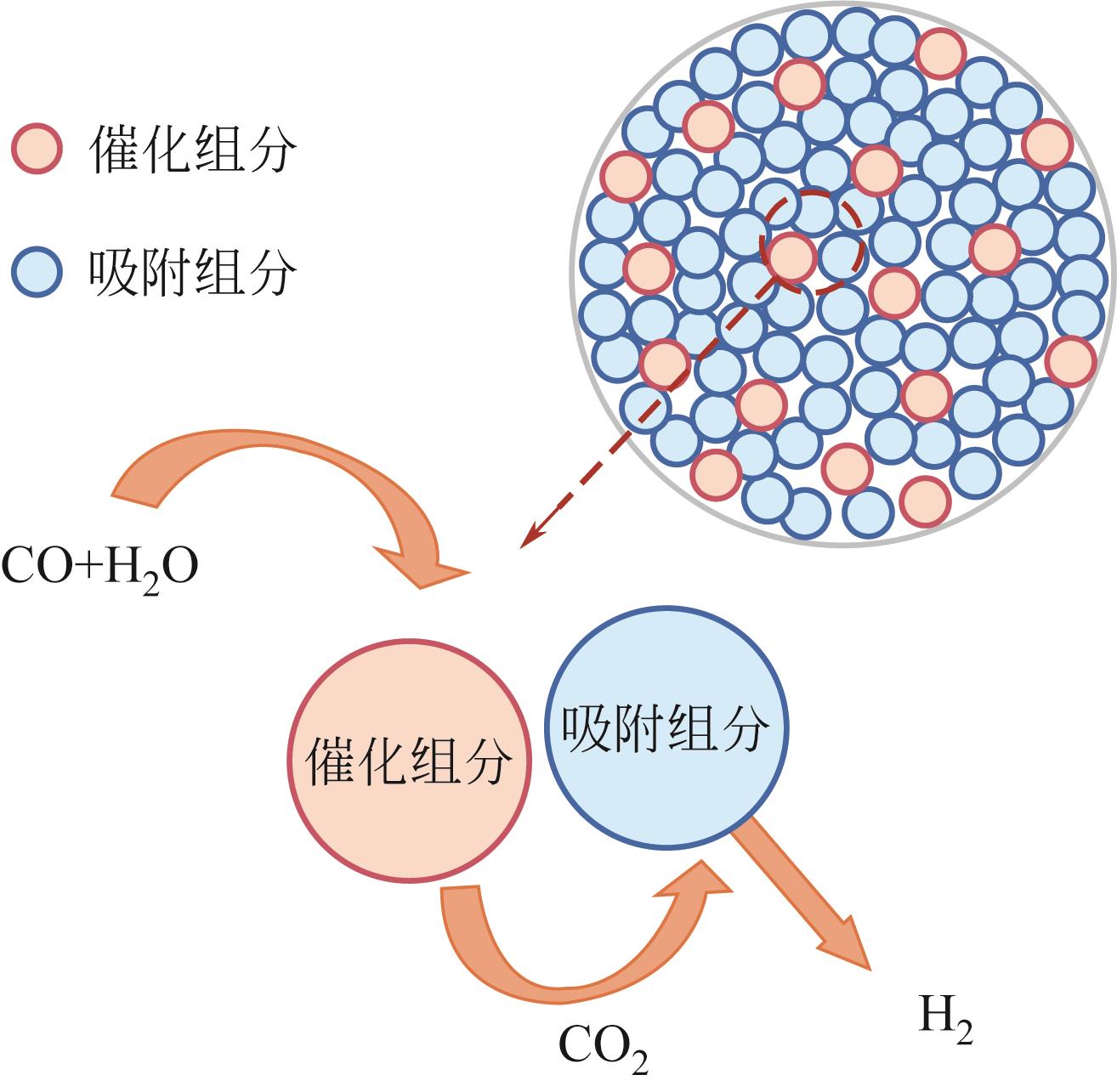
吸附强化水气变换反应(SEWGS)是实现高纯氢制备及二氧化碳(CO2)减排的关键反应之一。SEWGS借助复合催化剂将水气变换反应(制氢)和原位移除CO2反应(脱碳)耦合,打破热力学限制使反应平衡向制氢侧移动。SEWGS具有一步制取高纯氢气的特点,但复合催化剂在连续操作过程中由于烧结和CO2扩散受阻存在循环稳定性下降的问题,进而影响制氢效率。本文阐述了高温Ni/CaO基复合催化剂吸附强化制氢的研究现状,简述了Fe/CaO基复合催化剂SEWGS制氢面临的主要问题,回顾了中温Cu/MgO基和Cu/类水滑石基复合催化剂的SEWGS制氢现状及现阶段的核心问题。从复合催化剂的催化组分和吸附组分角度,分析了SEWGS制氢过程中复合催化剂循环稳定性降低的原因,简述了现阶段最有效的改性手段。进一步从增强CO2扩散和改善烧结角度入手,围绕复合催化剂设计、操作条件和床层装填方式等方面探讨提高复合催化剂循环稳定性的策略。指出设计开发组成简单、易制备、兼具高活性和高稳定性的复合催化剂实现制氢和脱碳耦合是今后SEWGS制氢的研究方向。
中图分类号:
引用本文
张金鹏, 屈婷, 荆洁颖, 李文英. 吸附强化水气变换制氢复合催化剂研究进展[J]. 化工进展, 2024, 43(5): 2629-2644.
ZHANG Jinpeng, QU Ting, JING Jieying, LI Wenying. Composite catalyst of sorption enhanced water gas shift for hydrogen production: A review[J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2629-2644.
| 吸附剂分类 | 吸附剂 | 理论吸附容量/ | (吸附/再生温度)/℃ | 使用中存在的问题 | 优点 |
|---|---|---|---|---|---|
| 高温吸附(>400℃) | 陶瓷基(以Li4SiO4为例[ | 0.367 | 450~600/720~750 | 动力学限制 | 循环稳定性好 |
| CaO基[ | 0.786 | 600~750/800~950 | 再生温度高 | 便宜易得 | |
| 中温吸附(200~400℃) | MgO基[ | 1.100 | 250~350/380~450 | 吸附速率慢 | 再生温度低 |
| 类水滑石[ | 0.022 | 300~380/400~500 | 吸附容量低 | 循环稳定性能好、层间调控 | |
| 低温吸附(≤200℃) | 碱金属碳酸盐(以Na2CO3为例[ | 0.415(NaHCO3) 0.250(Na2CO3·3NaHCO3) | 50~100/120~200 | 碳酸化速率慢,且耐久性差,工作温度易受限 | 低成本 |
| 碳基、沸石类、金属有机骨架类、聚合物类吸附剂[ | 物理/化学吸附 | 120~200 (多用于变压吸附) | 压力影响显著 | 变压解吸 |
表1 固体吸附剂分类
| 吸附剂分类 | 吸附剂 | 理论吸附容量/ | (吸附/再生温度)/℃ | 使用中存在的问题 | 优点 |
|---|---|---|---|---|---|
| 高温吸附(>400℃) | 陶瓷基(以Li4SiO4为例[ | 0.367 | 450~600/720~750 | 动力学限制 | 循环稳定性好 |
| CaO基[ | 0.786 | 600~750/800~950 | 再生温度高 | 便宜易得 | |
| 中温吸附(200~400℃) | MgO基[ | 1.100 | 250~350/380~450 | 吸附速率慢 | 再生温度低 |
| 类水滑石[ | 0.022 | 300~380/400~500 | 吸附容量低 | 循环稳定性能好、层间调控 | |
| 低温吸附(≤200℃) | 碱金属碳酸盐(以Na2CO3为例[ | 0.415(NaHCO3) 0.250(Na2CO3·3NaHCO3) | 50~100/120~200 | 碳酸化速率慢,且耐久性差,工作温度易受限 | 低成本 |
| 碳基、沸石类、金属有机骨架类、聚合物类吸附剂[ | 物理/化学吸附 | 120~200 (多用于变压吸附) | 压力影响显著 | 变压解吸 |
| 复合催化剂 | (反应温度/再生温度)/℃ | 1st/CO2吸附容量-最大循环/CO2吸附容量 | 1st/H2含量 |
|---|---|---|---|
| CaO@Ni-Al2O3[ | (500~700)/900 | 1/0.7% CO2含量 | 1/98.2% |
| NiAl-(2nm)-CaO[ | (400~600)/800 | 1/0.54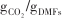 -30/0.43 -30/0.43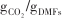 | 1/98% |
| Fe-Mn/CaO-Ca12Al14O33[ | 600/(850~920) | 1/0.53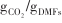 -20/0.43 -20/0.43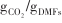 | 1/95.4% |
| K2CO3促进Cu/MgO-Al2O3[ | 300/(350~420) | 1/0.34mmol/gDFMs-10/0.25mmol/gDFMs | 1/99.9% |
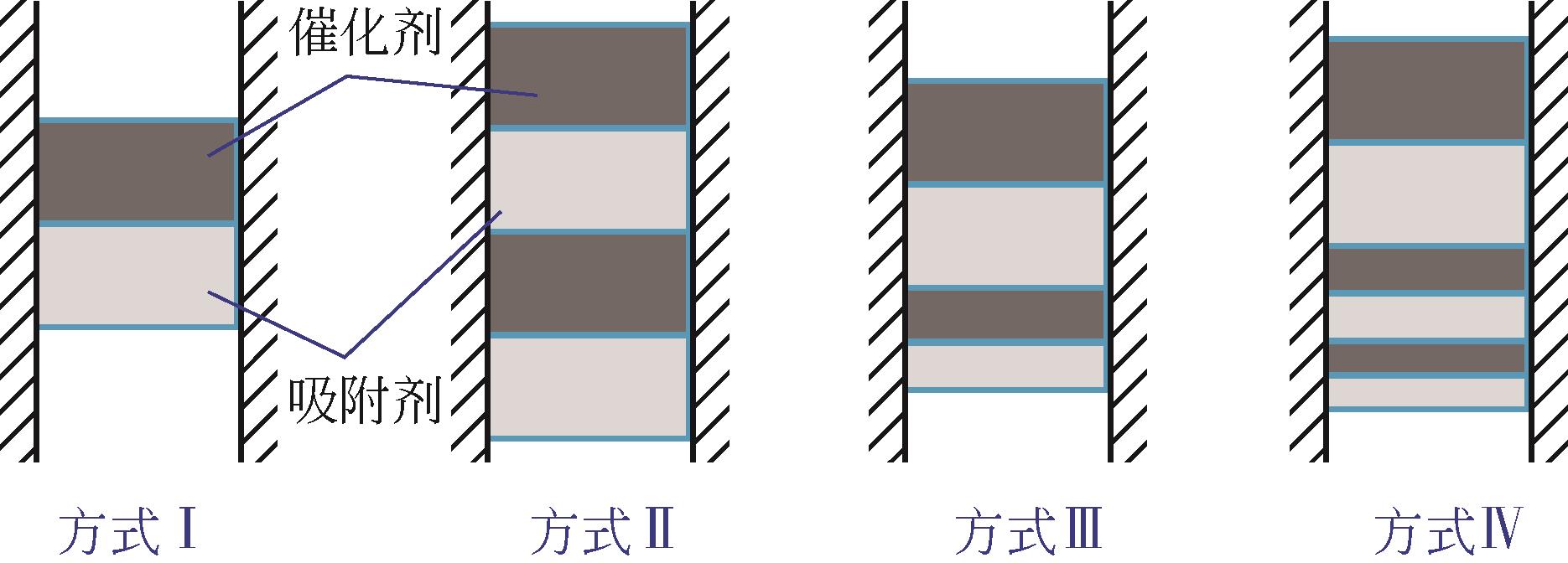
| 不同装填方式Cu/Ce0.6Zr0.4O2和AMS促进Mg95Ca5[ | 300/420 | 1/0.62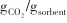 -10/0.42 -10/0.42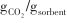 | 1/99.39% |
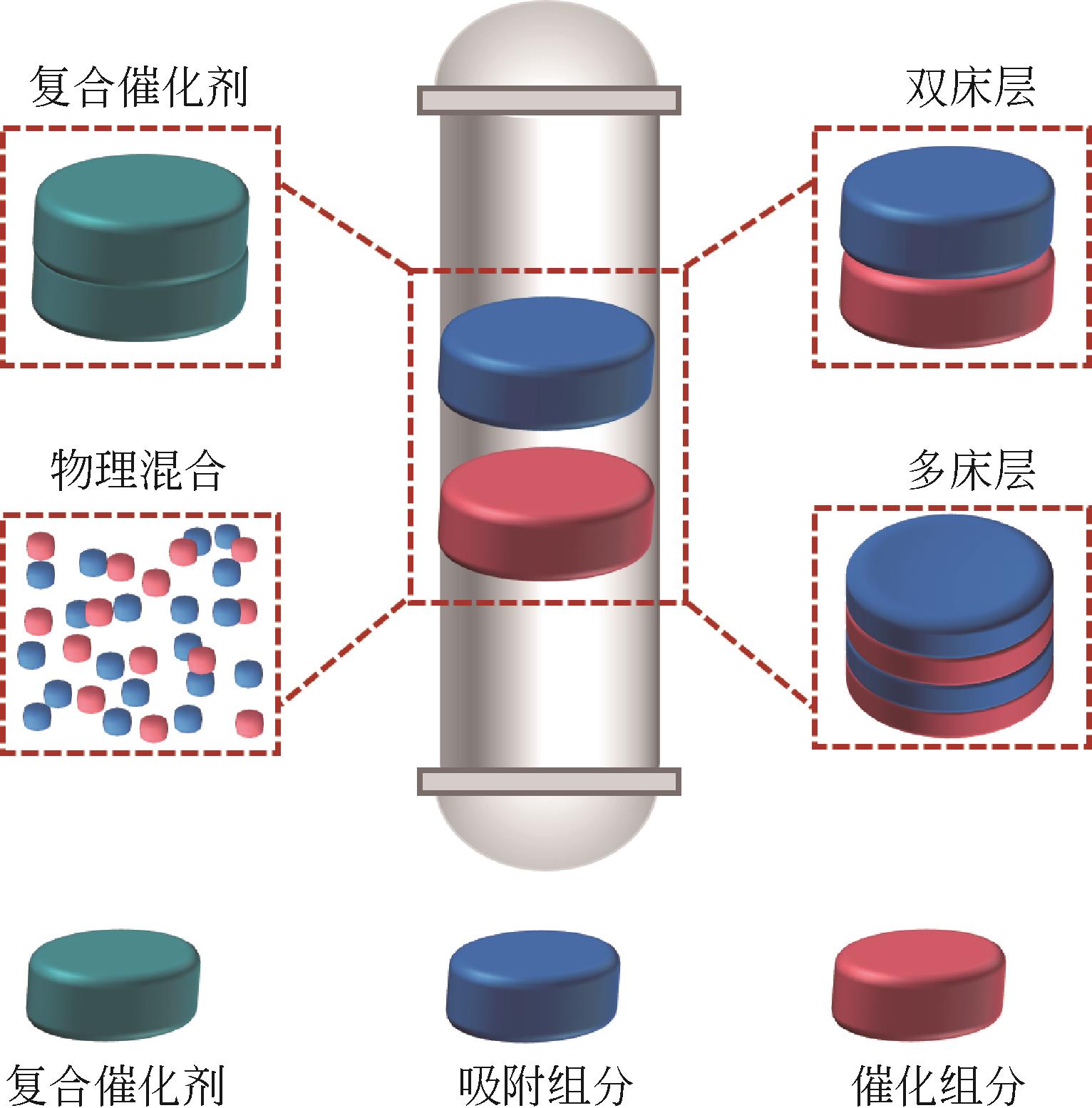
| Cu-MgHAlH和NaNO3掺杂类水滑石物理混合[ | 250 | 1/4.40mmol/gDFMs-7/2.44mmol/gDFMs | 1/99% |
| 四段床层(Cu/Ce0.6Zr0.4O2|AMS-Mg95Ca5及KLDO10)[ | 300/420 | 1/0.28mmol/gsorbent-10/0.26mmol/gsorbent | 1/99.9% |
表2 不同复合催化剂吸附强化水气变换制氢性能对比
| 复合催化剂 | (反应温度/再生温度)/℃ | 1st/CO2吸附容量-最大循环/CO2吸附容量 | 1st/H2含量 |
|---|---|---|---|
| CaO@Ni-Al2O3[ | (500~700)/900 | 1/0.7% CO2含量 | 1/98.2% |
| NiAl-(2nm)-CaO[ | (400~600)/800 | 1/0.54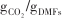 -30/0.43 -30/0.43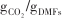 | 1/98% |
| Fe-Mn/CaO-Ca12Al14O33[ | 600/(850~920) | 1/0.53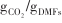 -20/0.43 -20/0.43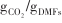 | 1/95.4% |
| K2CO3促进Cu/MgO-Al2O3[ | 300/(350~420) | 1/0.34mmol/gDFMs-10/0.25mmol/gDFMs | 1/99.9% |
| 不同装填方式Cu/Ce0.6Zr0.4O2和AMS促进Mg95Ca5[ | 300/420 | 1/0.62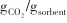 -10/0.42 -10/0.42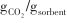 | 1/99.39% |
| Cu-MgHAlH和NaNO3掺杂类水滑石物理混合[ | 250 | 1/4.40mmol/gDFMs-7/2.44mmol/gDFMs | 1/99% |
| 四段床层(Cu/Ce0.6Zr0.4O2|AMS-Mg95Ca5及KLDO10)[ | 300/420 | 1/0.28mmol/gsorbent-10/0.26mmol/gsorbent | 1/99.9% |
| 1 | DUTTA Suman. A review on production, storage of hydrogen and its utilization as an energy resource[J]. Journal of Industrial and Engineering Chemistry, 2014, 20(4): 1148-1156. |
| 2 | BAYKARA Sema Z. Hydrogen: A brief overview on its sources, production and environmental impact[J]. International Journal of Hydrogen Energy, 2018, 43(23): 10605-10614. |
| 3 | JI Mengdi, WANG Jianlong. Review and comparison of various hydrogen production methods based on costs and life cycle impact assessment indicators[J]. International Journal of Hydrogen Energy, 2021, 46(78): 38612-38635. |
| 4 | 刘璐, 许凯, 荆洁颖, 等. CO2吸附强化CH4/H2O重整制氢催化剂研究进展[J]. 洁净煤技术, 2021, 27(1): 73-82. |
| LIU Lu, XU Kai, JING Jieying, et al. Research progress on catalysts for hydrogen production by CO2 sorption enhancement of CH4/H2O reforming[J]. Clean Coal Technology, 2021, 27(1): 73-82. | |
| 5 | HARRISON Douglas P. Sorption-enhanced hydrogen production: A review[J]. Industrial & Engineering Chemistry Research, 2008, 47(17): 6486-6501. |
| 6 | LOPEZ ORTIZ Alejandro, HARRISON Douglas P. Hydrogen production using sorption-enhanced reaction[J]. Industrial & Engineering Chemistry Research, 2001, 40(23): 5102-5109. |
| 7 | BARAJ Erlisa, Karel CIAHOTNÝ, Tomáš HLINČÍK. The water gas shift reaction: Catalysts and reaction mechanism[J]. Fuel, 2021, 288: 119817. |
| 8 | CHEN Wei-Hsin, CHEN Chia-Yang. Water gas shift reaction for hydrogen production and carbon dioxide capture: A review[J]. Applied Energy, 2020, 258: 114078. |
| 9 | ZHOU Limin, LIU Yanyan, LIU Shuling, et al. For more and purer hydrogen—The progress and challenges in water gas shift reaction[J]. Journal of Energy Chemistry, 2023, 83: 363-396. |
| 10 | 荆洁颖, 屈婷, 陶威, 等. CO2原位捕集强化水气变换制氢研究进展[J]. 煤炭学报, 2023, 48(2): 986-995. |
| JING Jieying, QU Ting, TAO Wei, et al. An overview on CO2 sorption enhanced water gas shift for hydrogen production[J]. Journal of China Coal Society, 2023, 48(2): 986-995. | |
| 11 | GAO Wanlin, ZHOU Tuantuan, GAO Yanshan, et al. Molten salts-modified MgO-based adsorbents for intermediate-temperature CO2 capture: A review[J]. Journal of Energy Chemistry, 2017, 26(5): 830-838. |
| 12 | DOU Binlin, WANG Chao, SONG Yongchen, et al. Solid sorbents for in-situ CO2 removal during sorption-enhanced steam reforming process: A review[J]. Renewable and Sustainable Energy Reviews, 2016, 53: 536-546. |
| 13 | MENDES D, MENDES A, MADEIRA L M, et al. The water-gas shift reaction: From conventional catalytic systems to Pd-based membrane reactors—A review[J]. Asia-Pacific Journal of Chemical Engineering, 2010, 5(1): 111-137. |
| 14 | ZHANG Chunxiao, LI Yingjie, HE Zirui, et al. Microtubular Fe/Mn-promoted CaO-Ca12Al14O33 bi-functional material for H2 production from sorption enhanced water gas shift[J]. Applied Catalysis B: Environmental, 2022, 314: 121474. |
| 15 | POZZO Alessandro DAL, ARMUTLULU Andaç, REKHTINA Margarita, et al. CO2 uptake and cyclic stability of MgO-based CO2 sorbents promoted with alkali metal nitrates and their eutectic mixtures[J]. ACS Applied Energy Materials, 2019, 2(2): 1295-1307. |
| 16 | HU Yingchao, LU Hongyuan, LIU Wenqiang, et al. Incorporation of CaO into inert supports for enhanced CO2 capture: A review[J]. Chemical Engineering Journal, 2020, 396: 125253. |
| 17 | 徐运飞, 李英杰, 王涛, 等. MgO吸附剂捕集CO2的研究进展[J]. 洁净煤技术, 2021, 27(1): 125-134. |
| XU Yunfei, LI Yingjie, WANG Tao, et al. Research progress on MgO sorbents for CO2 capture[J]. Clean Coal Technology, 2021, 27(1): 125-134. | |
| 18 | GAO Wanlin, XIAO Jiewen, WANG Qiang, et al. Unravelling the mechanism of intermediate-temperature CO2 interaction with molten-NaNO3-salt-promoted MgO[J]. Advanced Materials, 2022, 34(4): 2106677. |
| 19 | LU Hong, LU Yongqi, Massoud ROSTAM-ABADI. CO2 sorbents for a sorption-enhanced water-gas-shift process in IGCC plants: A thermodynamic analysis and process simulation study[J]. International Journal of Hydrogen Energy, 2013, 38(16): 6663-6672. |
| 20 | SEGGIANI M, PUCCINI M, VITOLO S. High-temperature and low concentration CO2 sorption on Li4SiO4 based sorbents: Study of the used silica and doping method effects[J]. International Journal of Greenhouse Gas Control, 2011, 5(4): 741-748. |
| 21 | 江涛, 魏小娟, 王胜平, 等. 固体吸附剂捕集CO2的研究进展[J]. 洁净煤技术, 2022, 28(1): 42-57. |
| JIANG Tao, WEI Xiaojuan, WANG Shengping, et al. Research progress on solid sorbents for CO2 capture[J]. Clean Coal Technology, 2022, 28(1): 42-57. | |
| 22 | YANG Zhongzhu, WEI Jingjing, ZENG Guangming, et al. A review on strategies to LDH-based materials to improve adsorption capacity and photoreduction efficiency for CO2 [J]. Coordination Chemistry Reviews, 2019, 386: 154-182. |
| 23 | LIU Wenqiang, AN Hui, QIN Changlei, et al. Performance enhancement of calcium oxide sorbents for cyclic CO2 capture—A review[J]. Energy & Fuels, 2012, 26(5): 2751-2767. |
| 24 | 耿一琪, 郭彦霞, 樊飙, 等. CaO基吸附剂捕集CO2及其抗烧结改性研究进展[J]. 燃料化学学报, 2021, 49(7): 998-1013. |
| GENG Yiqi, GUO Yanxia, FAN Biao, et al. Research progress of calcium-based adsorbents for CO2 capture and anti-sintering modification[J]. Journal of Fuel Chemistry and Technology, 2021, 49(7): 998-1013. | |
| 25 | 王胜平, 沈辉, 范莎莎, 等. 固体二氧化碳吸附剂研究进展[J]. 化学工业与工程, 2014, 31(1): 72-78. |
| WANG Shengping, SHEN Hui, FAN Shasha, et al. Research progress of solid adsorbents for CO2 capture[J]. Chemical Industry and Engineering, 2014, 31(1): 72-78. | |
| 26 | RODRIGUEZ J A, MA S, LIU P, et al. Activity of CeO x and TiO x nanoparticles grown on Au(111) in the water-gas shift reaction[J]. Science, 2007, 318(5857): 1757-1760. |
| 27 | LIN Lili, ZHOU Wu, GAO Rui, et al. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts[J]. Nature, 2017, 544(7648): 80-83. |
| 28 | YAO Siyu, ZHANG Xiao, ZHOU Wu, et al. Atomic-layered Au clusters on α-MoC as catalysts for the low-temperature water-gas shift reaction[J]. Science, 2017, 357(6349): 389-393. |
| 29 | LIANG Y, HARRISON D P, GUPTA R P, et al. Carbon dioxide capture using dry sodium-based sorbents[J]. Energy & Fuels, 2004, 18(2): 569-575. |
| 30 | JING Jieying, LIU Lu, XU Kai, et al. Improved hydrogen production performance of Ni-Al2O3/CaO-CaZrO3 composite catalyst for CO2 sorption enhanced CH4/H2O reforming[J]. International Journal of Hydrogen Energy, 2023, 48(7): 2558-2570. |
| 31 | KIM Sung Min, ARMUTLULU Andac, KIERZKOWSKA Agnieszka M, et al. Development of an effective bi-functional Ni-CaO catalyst-sorbent for the sorption-enhanced water gas shift reaction through structural optimization and the controlled deposition of a stabilizer by atomic layer deposition[J]. Sustainable Energy & Fuels, 2020, 4(2): 713-729. |
| 32 | DANG Chengxiong, LIU Liqiang, YANG Guangxing, et al. Mg-promoted Ni-CaO microsphere as bi-functional catalyst for hydrogen production from sorption-enhanced steam reforming of glycerol[J]. Chemical Engineering Journal, 2020, 383: 123204. |
| 33 | 方书起, 王毓谦, 李攀, 等. 生物油催化重整制氢研究进展[J]. 化工进展, 2022, 41(3): 1330-1339. |
| FANG Shuqi, WANG Yuqian, LI Pan, et al. Research progress of hydrogen production by catalytic reforming of bio-oil[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1330-1339. | |
| 34 | SAEIDI Samrand, András SÁPI, KHOJA Asif Hussain, et al. Evolution paths from gray to turquoise hydrogen via catalytic steam methane reforming: Current challenges and future developments[J]. Renewable and Sustainable Energy Reviews, 2023, 183: 113392. |
| 35 | Seong BIN JO, Jin Hyeok WOO, LEE Jong Heon, et al. CO2 green technologies in CO2 capture and direct utilization processes: Methanation, reverse water-gas shift, and dry reforming of methane[J]. Sustainable Energy & Fuels, 2020, 4(11): 5543-5549. |
| 36 | TSIOTSIAS Anastasios I, CHARISIOU Nikolaos D, YENTEKAKIS Ioannis V, et al. Bimetallic Ni-based catalysts for CO2 methanation: A review[J]. Nanomaterials, 2020, 11(1): 28. |
| 37 | KONG Meng, ALBRECHT Karl O, SHANKS Brent H, et al. Development of a combined catalyst and sorbent for the water gas shift reaction[J]. Industrial & Engineering Chemistry Research, 2014, 53(23): 9570-9577. |
| 38 | WATANABE Keita, MIYAO Toshihiro, HIGASHIYAMA Kazutoshi, et al. High temperature water-gas shift reaction over hollow Ni-Fe-Al oxide nano-composite catalysts prepared by the solution-spray plasma technique[J]. Catalysis Communications, 2009, 10(14): 1952-1955. |
| 39 | ASHOK Jangam, Ming hui WAI, KAWI Sibudjing. Nickel-based catalysts for high-temperature water gas shift reaction-methane suppression[J]. ChemCatChem, 2018, 10(18): 3927-3942. |
| 40 | SHOKROLLAHI YANCHESHMEH Marziehossadat, RADFARNIA Hamid R, ILIUTA Maria C. Sustainable production of high-purity hydrogen by sorption enhanced steam reforming of glycerol over CeO2-promoted Ca9Al6O18-CaO/NiO bifunctional material[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(11): 9774-9786. |
| 41 | SCHWACH Pierre, PAN Xiulian, BAO Xinhe. Direct conversion of methane to value-added chemicals over heterogeneous catalysts: Challenges and prospects[J]. Chemical Reviews, 2017, 117(13): 8497-8520. |
| 42 | DING Y, ALPAY E. Adsorption-enhanced steam–methane reforming[J]. Chemical Engineering Science, 2000, 55(18): 3929-3940. |
| 43 | YI Kwang Bok, HARRISON Douglas P. Low-pressure sorption-enhanced hydrogen production[J]. Industrial & Engineering Chemistry Research, 2005, 44(6): 1665-1669. |
| 44 | AHMED Shabbir, LEE Sheldon H D, FERRANDON Magali S. Catalytic steam reforming of biogas—Effects of feed composition and operating conditions[J]. International Journal of Hydrogen Energy, 2015, 40(2): 1005-1015. |
| 45 | SABOKMALEK Saleh, ALAVI Seyed Mehdi, REZAEI Mehran, et al. Fabrication and catalytic evaluation of Ni/CaO-Al2O3 in glycerol steam reforming: Effect of Ni loading[J]. Journal of the Energy Institute, 2023, 109: 101270. |
| 46 | LI Zhenshan, CAI Ningsheng, HUANG Yuyu, et al. Synthesis, experimental studies, and analysis of a new calcium-based carbon dioxide absorbent[J]. Energy & Fuels, 2005, 19(4): 1447-1452. |
| 47 | MARTAVALTZI Christina S, PEFKOS Tilemachos D, LEMONIDOU Angeliki A. Operational window of sorption enhanced steam reforming of methane over CaO-Ca12Al14O33 [J]. Industrial & Engineering Chemistry Research, 2011, 50(2): 539-545. |
| 48 | XIE Miaomiao, ZHOU Zhiming, QI Yang, et al. Sorption-enhanced steam methane reforming by in situ CO2 capture on a CaO-Ca9Al6O18 sorbent[J]. Chemical Engineering Journal, 2012, 207/208: 142-150. |
| 49 | KIM Jong-Nam, Chang Hyun KO, YI Kwang Bok. Sorption enhanced hydrogen production using one-body CaO-Ca12Al14O33-Ni composite as catalytic absorbent[J]. International Journal of Hydrogen Energy, 2013, 38(14): 6072-6078. |
| 50 | CHEN X, YANG L, ZHOU Z M, et al. Core-shell structured CaO-Ca9Al6O18@Ca5Al6O14/Ni bifunctional material for sorption-enhanced steam methane reforming[J]. Chemical Engineering Science, 2017, 163: 114-122. |
| 51 | XU Jiayan, XUE Xiaochong, WU Sufang. Stability of the Ni-TiO2@nano CaO/Al2O3 complex catalyst used in ReSER process for hydrogen production[J]. International Journal of Hydrogen Energy, 2016, 41(16): 6781-6786. |
| 52 | 许凯. 吸附强化制氢CaO-Ca3Al2O6@Ni-SiO2复合催化剂制备及结构调控[D]. 太原: 太原理工大学, 2022. |
| XU Kai. Preparation and structure regulation of CaO-Ca3Al2O6@Ni-SiO2 composite catalyst for adsorption-enhanced hydrogen production[D].Taiyuan: Taiyuan University of Technology, 2022. | |
| 53 | YALCIN Ozgen, SOURAV Sagar, WACHS Israel E. Design of Cr-free promoted copper-iron oxide-based high-temperature water-gas shift catalysts[J]. ACS Catalysis, 2023, 13(19): 12681-12691. |
| 54 | ZAMBONI I, COURSON C, KIENNEMANN A. Fe-Ca interactions in Fe-based/CaO catalyst/sorbent for CO2 sorption and hydrogen production from toluene steam reforming[J]. Applied Catalysis B: Environmental, 2017, 203: 154-165. |
| 55 | MÜLLER Christoph R, PACCIANI Roberta, BOHN Christopher D, et al. Investigation of the enhanced water gas shift reaction using natural and synthetic sorbents for the capture of CO2 [J]. Industrial & Engineering Chemistry Research, 2009, 48(23): 10284-10291. |
| 56 | DAMMA Devaiah, JAMPAIAH Deshetti, WELTON Aaron, et al. Effect of Nb modification on the structural and catalytic property of Fe/Nb/M (M = Mn, Co, Ni, and Cu) catalyst for high temperature water-gas shift reaction[J]. Catalysis Today, 2020, 355: 921-931. |
| 57 | PARK Yong Min, CHO Jae Min, HAN Gui Young, et al. Roles of highly ordered mesoporous structures of Fe-Ni bimetal oxides for an enhanced high-temperature water-gas shift reaction activity[J]. Catalysis Science & Technology, 2021, 11(9): 3251-3260. |
| 58 | HAN Long Han, MA Kaili, WU Yuelun, et al. Promoted calcium looping H2 production via catalytic reforming of polycyclic aromatic hydrocarbon using a synthesized CO2 absorbent prepared by impregnation[J]. International Journal of Energy Research, 2021, 45(7): 10409-10424. |
| 59 | DI FELICE L, COURSON C, NIZNANSKY D, et al. Biomass gasification with catalytic tar reforming: A model study into activity enhancement of calcium- and magnesium-oxide-based catalytic materials by incorporation of iron[J]. Energy & Fuels, 2010, 24(7): 4034-4045. |
| 60 | AZHARUDDIN M, TSUDA H, WU S, et al. Catalytic decomposition of biomass tars with iron oxide catalysts[J]. Fuel, 2008, 87(4/5): 451-459. |
| 61 | TWIGG Martyn V, SPENCER Michael S. Deactivation of supported copper metal catalysts for hydrogenation reactions[J]. Applied Catalysis A: General, 2001, 212(1/2): 161-174. |
| 62 | SUN Zheyi, SHAO Bin, ZHANG Yun, et al. Integrated CO2 capture and methanation from the intermediate-temperature flue gas on dual functional hybrids of AMS/CaMgO||NiCo[J]. Separation and Purification Technology, 2023, 307: 122680. |
| 63 | HU Yuanwu, CUI Hongjie, CHENG Zhenmin, et al. Sorption-enhanced water gas shift reaction by in situ CO2 capture on an alkali metal salt-promoted MgO-CaCO3 sorbent[J]. Chemical Engineering Journal, 2019, 377: 119823. |
| 64 | HU Yuanwu, CHENG Zhenmin, ZHOU Zhiming. High-purity H2 production by sorption-enhanced water gas shift on a K2CO3-promoted Cu/MgO-Al2O3 difunctional material[J]. Sustainable Energy & Fuels, 2021, 5(13): 3340-3350. |
| 65 | HUANG Pu, GUO Yafei, WANG Guodong, et al. Insights into nickel-based dual function materials for CO2 sorption and methanation: Effect of reduction temperature[J]. Energy & Fuels, 2021, 35(24): 20185-20196. |
| 66 | GAO Wanlin, VASILIADES Michalis A, DAMASKINOS Constantinos M, et al. Molten salt-promoted MgO adsorbents for CO2 capture: Transient kinetic studies[J]. Environmental Science & Technology, 2021, 55(8): 4513-4521. |
| 67 | GAO Wanlin, ZHOU Tuantuan, GAO Yanshan, et al. Study on MNO3/NO2 (M = Li, Na, and K)/MgO composites for intermediate-temperature CO2 capture[J]. Energy & Fuels, 2019, 33(3): 1704-1712. |
| 68 | HUANG Pu, CHU Jie, FU Jiali, et al. Influence of reduction conditions on the structure-activity relationships of NaNO3-promoted Ni/MgO dual function materials for integrated CO2 capture and methanation[J]. Chemical Engineering Journal, 2023, 467: 143431. |
| 69 | LEE Chan Hyun, KIM Suji, YOON Hyung Jin, et al. Water gas shift and sorption-enhanced water gas shift reactions using hydrothermally synthesized novel Cu-Mg-Al hydrotalcite-based catalysts for hydrogen production[J]. Renewable and Sustainable Energy Reviews, 2021, 145: 111064. |
| 70 | 刘璐. Ni-Al2O3/CaO-CaZrO3复合催化剂制备及吸附强化制氢性能调变[D]. 太原: 太原理工大学, 2022. |
| LIU Lu. Synthesis of Ni-Al2O3/CaO-CaZrO3 composite catalyst and modulation of its sorption enhanced hydrogen production performance[D]. Taiyuan: Taiyuan University of Technology, 2022. | |
| 71 | XU Huawu, HU Yuanwu, CHENG Zhenmin, et al. Production of high-purity H2 through sorption-enhanced water gas shift over a combination of two intermediate-temperature CO2 sorbents[J]. International Journal of Hydrogen Energy, 2023, 48(64): 25185-25196. |
| 72 | LYSIKOV A I, OKUNEV A G, NETSKINA O V. Study of a nickel catalyst under conditions of the SER process: Influence of RedOx cycling[J]. International Journal of Hydrogen Energy, 2013, 38(25): 10354-10363. |
| 73 | ROGERS Kyle A, FU Jile, XU Yiyi, et al. Guaiacol deoxygenation using ceria-zirconia based catalysts with hydrogen produced internally via water-gas-shift reaction[J]. Catalysis Today, 2023, 407: 68-79. |
| 74 | GINÉS M J L, AMADEO N, LABORDE M, et al. Activity and structure-sensitivity of the water-gas shift reaction over CuZnAl mixed oxide catalysts[J]. Applied Catalysis A: General, 1995, 131(2): 283-296. |
| 75 | HOSSAIN Mohammad M, AHMED Shakeel. Cu-based mixed metal oxide catalysts for WGSR: Reduction kinetics and catalytic activity[J]. The Canadian Journal of Chemical Engineering, 2013, 91(8): 1450-1458. |
| 76 | LUNKENBEIN Thomas, SCHUMANN Julia, BEHRENS Malte, et al. Formation of a ZnO overlayer in industrial Cu/ZnO/Al2O3 catalysts induced by strong metal-support interactions[J]. Angewandte Chemie International Edition, 2015, 54(15): 4544-4548. |
| 77 | ZHOU Yan, CHEN Aling, NING Jing, et al. Electronic and geometric structure of the copper-ceria interface on Cu/CeO2 catalysts[J]. Chinese Journal of Catalysis, 2020, 41(6): 928-937. |
| 78 | LIN Jiann-Horng, BISWAS Prakash, GULIANTS Vadim V, et al. Hydrogen production by water-gas shift reaction over bimetallic Cu-Ni catalysts supported on La-doped mesoporous ceria[J]. Applied Catalysis A: General, 2010, 387(1/2): 87-94. |
| 79 | MABOUDI N, MESHKANI Fereshteh, REZAEI M. Influence of group IIA metals on the performance of the Ni Cu/CeO2Al2O3 catalysts in high-temperature water gas shift reaction[J]. International Journal of Hydrogen Energy, 2019, 44(5): 2694-2703. |
| 80 | ZANG Pengchao, TANG Jiyun, ZHANG Xiaoyang, et al. Strategies to improve CaO absorption cycle stability and progress of catalysts in Ca-based DFMs for integrated CO2 capture-conversion: A critical review[J]. Journal of Environmental Chemical Engineering, 2023, 11(5): 111047. |
| 81 | DEREVSCHIKOV V S, LYSIKOV A I, OKUNEV A G. High temperature CaO/Y2O3 carbon dioxide absorbent with enhanced stability for sorption-enhanced reforming applications[J]. Industrial & Engineering Chemistry Research, 2011, 50(22): 12741-12749. |
| 82 | LIU Hao, WU Sufang. Preparation of high sorption durability nano-CaO-ZnO CO2 adsorbent[J]. Energy & Fuels, 2019, 33(8): 7626-7633. |
| 83 | NAEEM Muhammad Awais, ARMUTLULU Andac, IMTIAZ Qasim, et al. Optimization of the structural characteristics of CaO and its effective stabilization yield high-capacity CO2 sorbents[J]. Nature Communications, 2018, 9(1): 2408. |
| 84 | HU Yingchao, LIU Wenqiang, CHEN Hongqiang, et al. Screening of inert solid supports for CaO-based sorbents for high temperature CO2 capture[J]. Fuel, 2016, 181: 199-206. |
| 85 | JING Jieying, LI Tingyu, ZHANG Xuewei, et al. Enhanced CO2 sorption performance of CaO/Ca3Al2O6 sorbents and its sintering-resistance mechanism[J]. Applied Energy, 2017, 199: 225-233. |
| 86 | 李清. 高效抗烧结钙基二氧化碳吸附剂的制备及性能研究[D]. 太原: 太原理工大学, 2019. |
| LI Qing. Preparation and performance of high-efficiency anti-sintering calcium-based CO2 sorbents[D].Taiyuan: Taiyuan University of Technology, 2019. | |
| 87 | CUI Hongjie, CHENG Zhenmin, ZHOU Zhiming. Unravelling the role of alkaline earth metal carbonates in intermediate temperature CO2 capture using alkali metal salt-promoted MgO-based sorbents[J]. Journal of Materials Chemistry A, 2020, 8(35): 18280-18291. |
| 88 | ZHANG Keling, LI Xiaohong Shari, LI Weizhen, et al. Phase transfer-catalyzed fast CO2 absorption by MgO-based absorbents with high cycling capacity[J]. Advanced Materials Interfaces, 2014, 1(3): 1400030. |
| 89 | HARADA Takuya, SIMEON Fritz, HAMAD Esam Z, et al. Alkali metal nitrate-promoted high-capacity MgO adsorbents for regenerable CO2 capture at moderate temperatures[J]. Chemistry of Materials, 2015, 27(6): 1943-1949. |
| 90 | KWAK Jin-Su, KIM Kang-Yeong, YOON Ji Woong, et al. Interfacial interactions govern the mechanisms of CO2 absorption and desorption on A2CO3-promoted MgO (A=Na, K, Rb, and Cs) absorbents[J]. The Journal of Physical Chemistry C, 2018, 122(35): 20289-20300. |
| 91 | Anh-Tuan VU, Keon HO, JIN Seongmin, et al. Double sodium salt-promoted mesoporous MgO sorbent with high CO2 sorption capacity at intermediate temperatures under dry and wet conditions[J]. Chemical Engineering Journal, 2016, 291: 161-173. |
| 92 | 邓少碧, 边洲峰. 核壳结构在甲烷干重整中的应用[J]. 化工进展, 2023, 42(1): 247-254. |
| DENG Shaobi, BIAN Zhoufeng. Application of core-shell structure catalyst in dry reforming of methane[J]. Chemical Industry and Engineering Progress, 2023, 42(1): 247-254. | |
| 93 | 曹敏, 毛玉娇, 王倩倩, 等. 金属催化剂烧结机制及抗烧结策略[J]. 化工进展, 2023, 42(2): 744-755. |
| CAO Min, MAO Yujiao, WANG Qianqian, et al. Sintering mechanism and sintering-resistant strategies for metal-based catalyst[J]. Chemical Industry and Engineering Progress, 2023, 42(2): 744-755. | |
| 94 | SHISHIDO Tetsuya, YAMAMOTO Manabu, ATAKE Ikuo, et al. Cu/Zn-based catalysts improved by adding magnesium for water-gas shift reaction[J]. Journal of Molecular Catalysis A: Chemical, 2006, 253(1/2): 270-278. |
| 95 | LI Didi, XU Fang, TANG Xuan, et al. Induced activation of the commercial Cu/ZnO/Al2O3 catalyst for the steam reforming of methanol[J]. Nature Catalysis, 2022, 5: 99-108. |
| 96 | YU J, QIN X, YANG Y, et al. Highly stable Pt/CeO2 catalyst with embedding structure toward water-gas shift reaction[J]. Journal of the American Chemical Society, 2024, 146(1): 1071-1080. |
| 97 | SHAO Bin, ZHANG Yun, SUN Zheyi, et al. CO2 capture and in situ conversion: Recent progresses and perspectives[J]. Green Chemical Engineering, 2022, 3(3): 189-198. |
| 98 | OLIVEIRA Eduardo L G, GRANDE Carlos A, RODRIGUES Alírio E. Effect of catalyst activity in SMR-SERP for hydrogen production: Commercial vs. large-pore catalyst[J]. Chemical Engineering Science, 2011, 66(3): 342-354. |
| [1] | 周安宁, 江雨寒, 刘墨宣, 赵伟, 李振. 电解煤浆制氢过程中煤阶及矿物的影响与煤结构演化研究进展[J]. 化工进展, 2024, 43(5): 2294-2310. |
| [2] | 李思, 陶艺月, 肖振翀, 张亮, 李俊, 朱恂, 廖强. 热再生电池堆-二氧化碳电化学还原池系统耦合特性[J]. 化工进展, 2024, 43(5): 2568-2575. |
| [3] | 李凯, 魏鹤琳, 左夏华, 杨卫民, 阎华, 安瑛. 水基炭黑-胶原蛋白纳米流体制备及稳定性实验[J]. 化工进展, 2024, 43(4): 1944-1952. |
| [4] | 齐亚兵, 吴子波, 杨清翠. Pickering乳液制备及稳定性研究进展[J]. 化工进展, 2024, 43(4): 2017-2030. |
| [5] | 刘涵, 曲明璐, 叶振东, 杨帆, 黄蓓佳, 张亚宁, 刘洪芝. 钙镁二元盐复合材料的储热性能[J]. 化工进展, 2024, 43(4): 1764-1773. |
| [6] | 吴晨赫, 刘彧旻, 杨昕旻, 崔记伟, 姜韶堃, 叶金花, 刘乐全. 粉体光催化全水分解技术研究进展[J]. 化工进展, 2024, 43(4): 1810-1822. |
| [7] | 卢志强, 石雨, 陈鹏宇, 张亮, 李俊, 付乾, 朱恂, 廖强. 具有高浓度氨腔室的立式热再生氨电池性能特性[J]. 化工进展, 2024, 43(3): 1224-1231. |
| [8] | 刘泽鹏, 曾纪珺, 唐晓博, 赵波, 韩升, 廖袁淏, 张伟. 四种烷基咪唑磷酸酯离子液体的热力学性质[J]. 化工进展, 2024, 43(3): 1484-1491. |
| [9] | 孙宏军, 李腾, 李金霞, 丁红兵. 基于Kelvin-Helmholtz不稳定性和界面剪切作用的扰动波高预测模型[J]. 化工进展, 2024, 43(2): 609-618. |
| [10] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [11] | 张亚娟, 徐惠, 胡贝, 史星伟. 化学镀法制备NiCoP/rGO/NF高效电解水析氢催化剂[J]. 化工进展, 2023, 42(8): 4275-4282. |
| [12] | 王鑫, 王兵兵, 杨威, 徐志明. 金属表面PDA/PTFE超疏水涂层抑垢与耐腐蚀性能[J]. 化工进展, 2023, 42(8): 4315-4321. |
| [13] | 陆洋, 周劲松, 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛. CeO2/TiO2吸附剂煤气脱汞产物的浸出规律[J]. 化工进展, 2023, 42(7): 3875-3883. |
| [14] | 吴展华, 盛敏. 绝热加速量热仪在反应安全风险评估应用中的常见问题[J]. 化工进展, 2023, 42(7): 3374-3382. |
| [15] | 王蕴青, 杨国锐, 延卫. 过渡金属磷化物的改性方法及其在电化学析氢中的应用[J]. 化工进展, 2023, 42(7): 3532-3549. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||