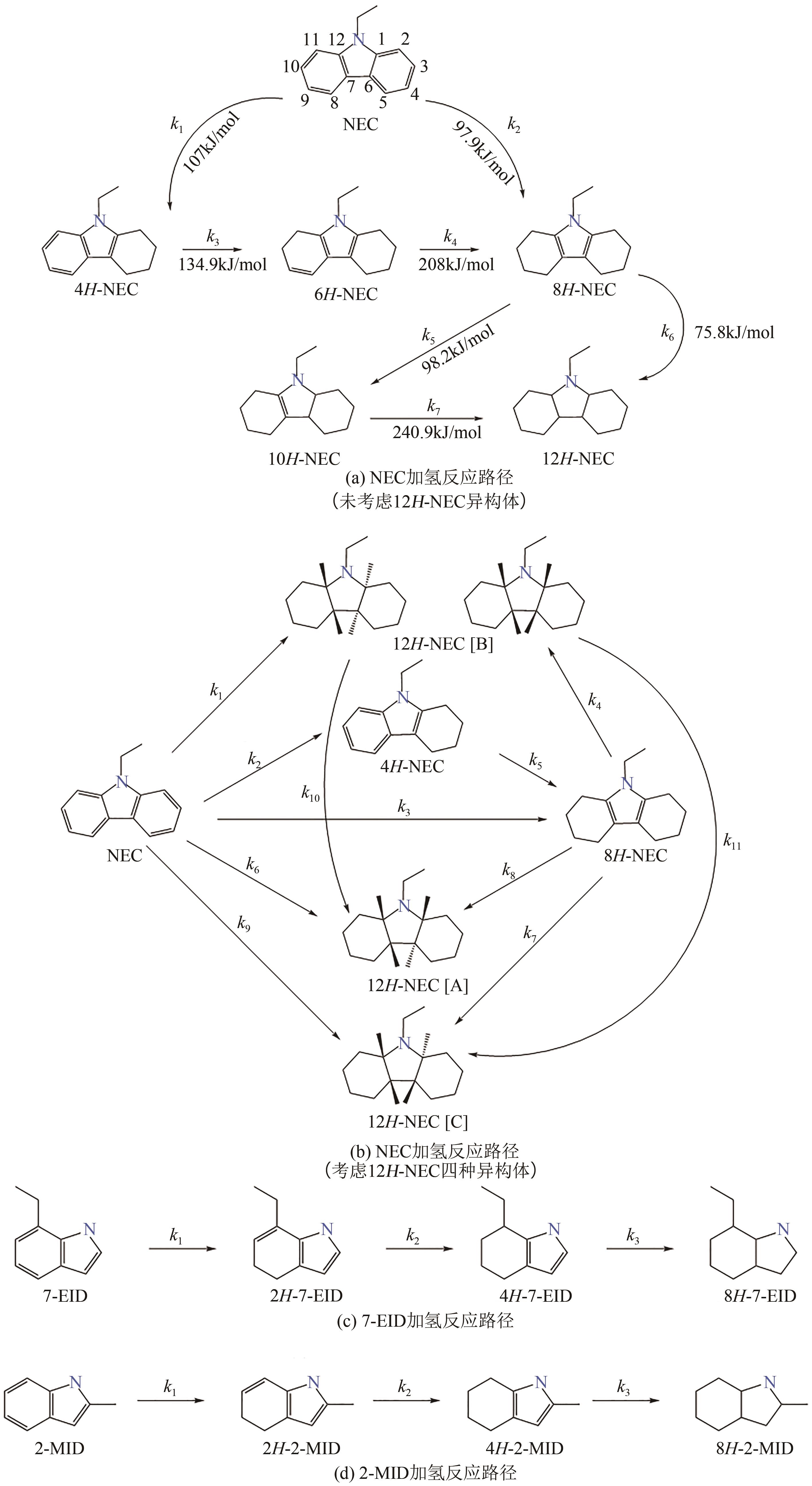
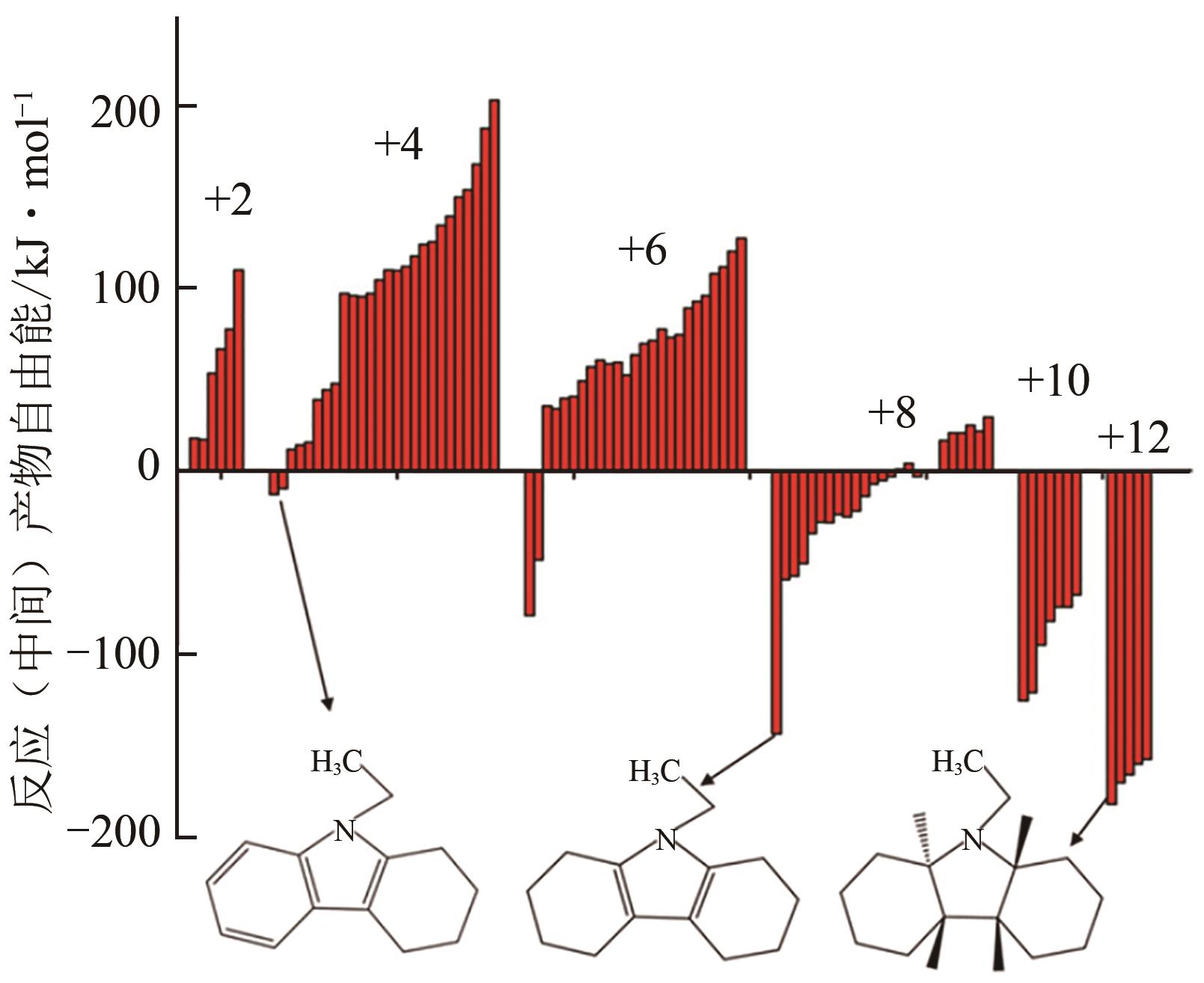
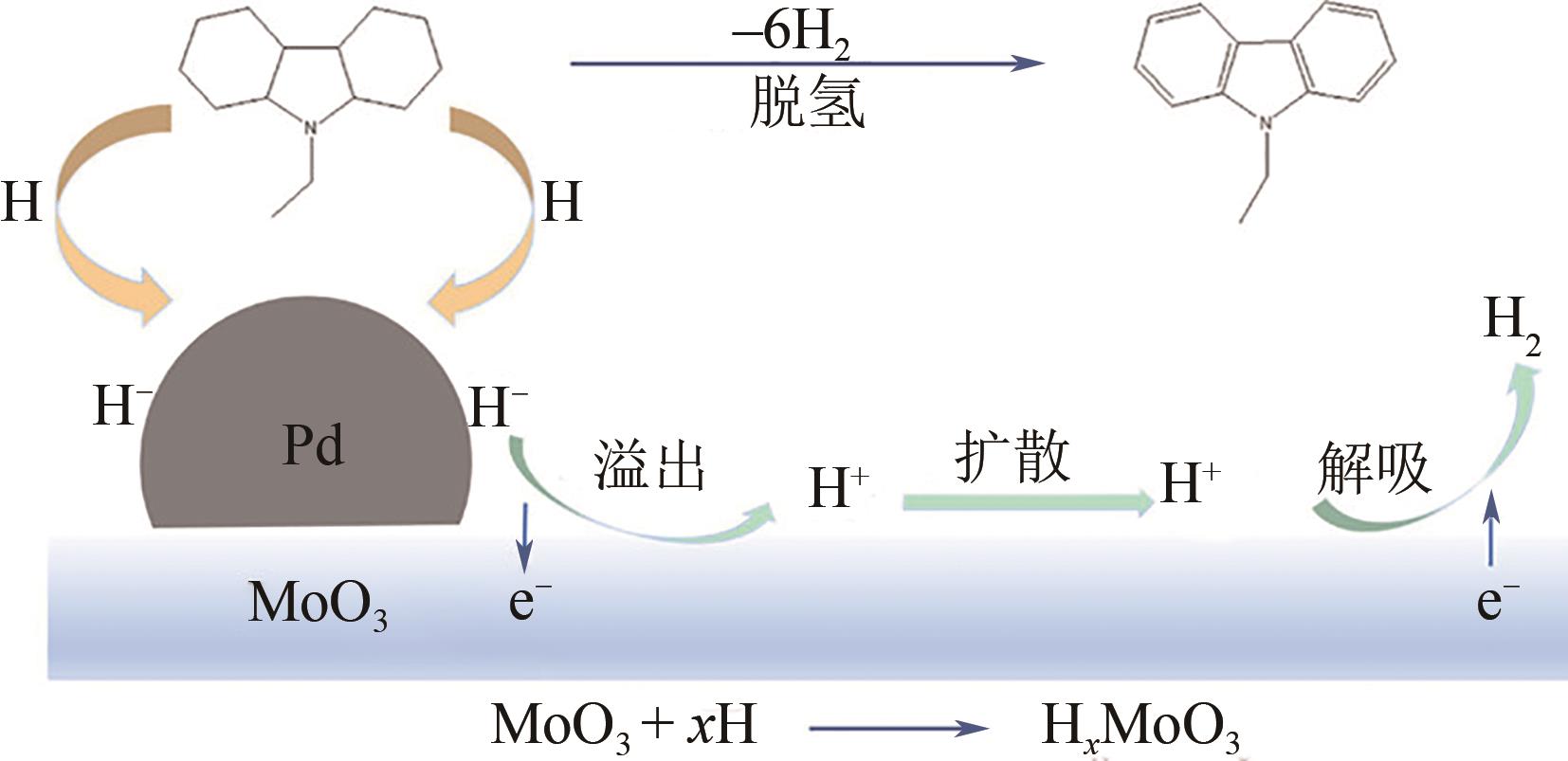
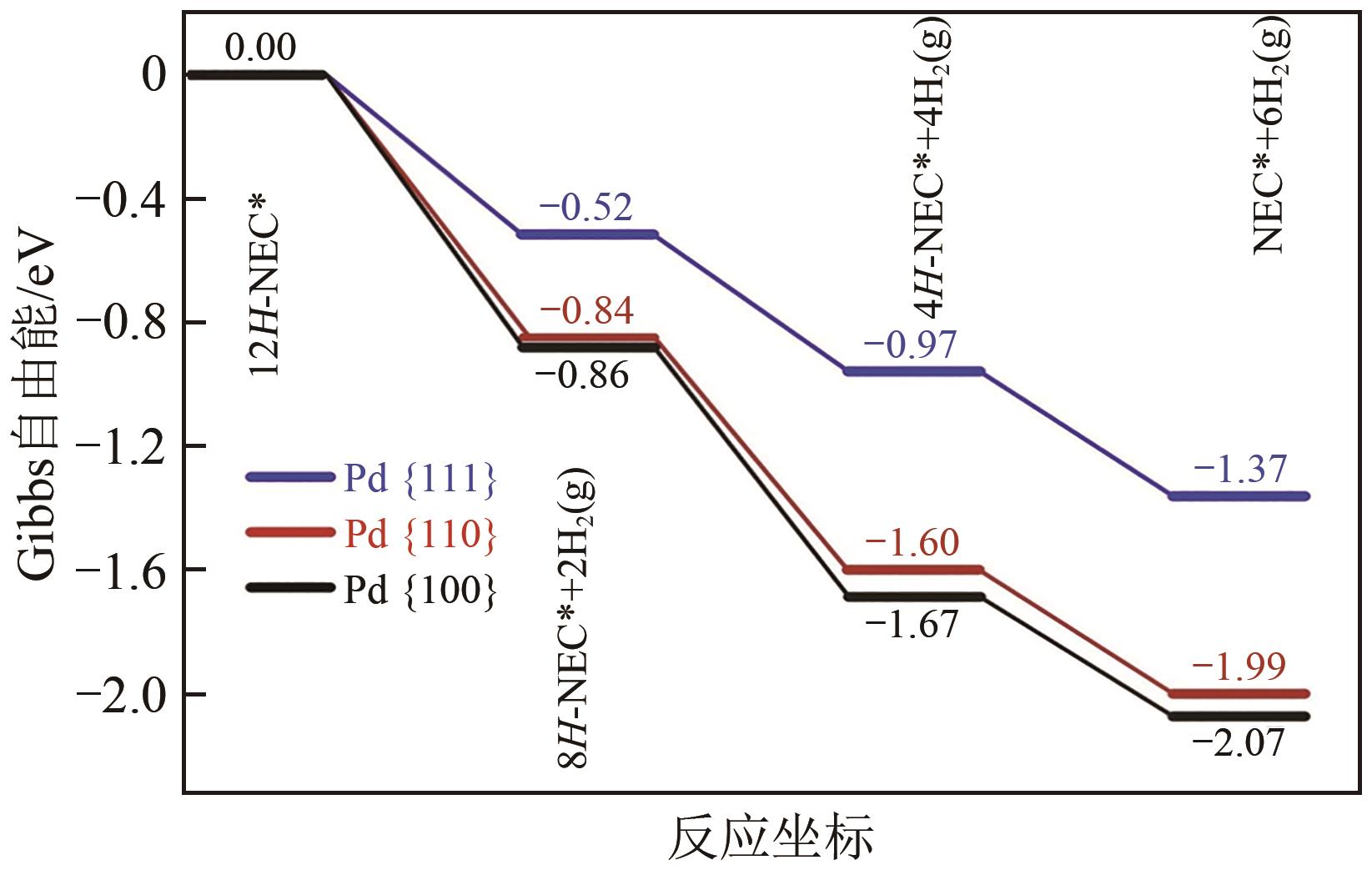
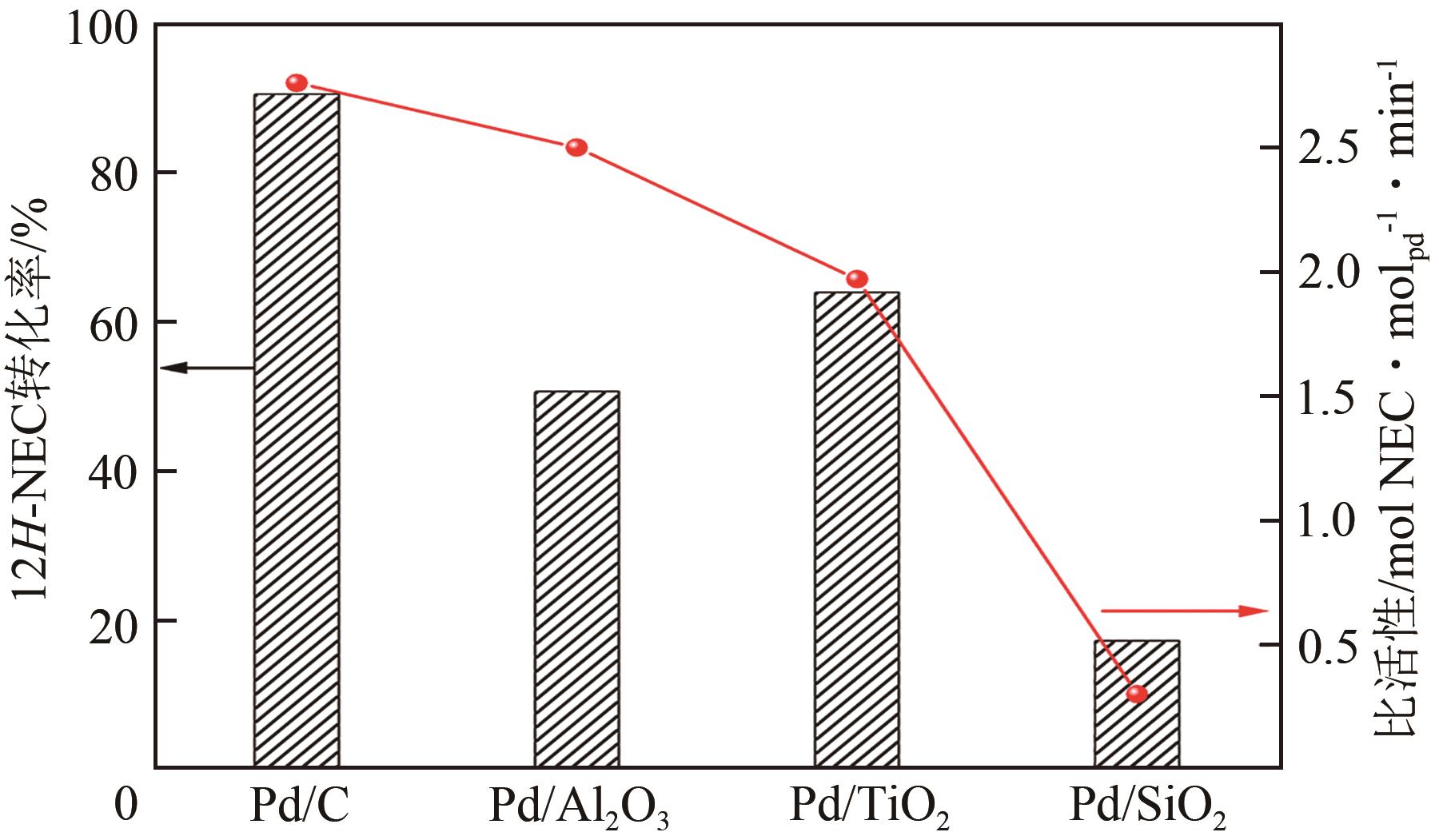
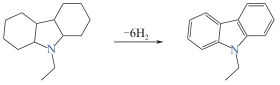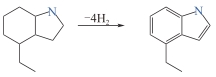化工进展 ›› 2023, Vol. 42 ›› Issue (12): 6325-6344.DOI: 10.16085/j.issn.1000-6613.2023-0089
• 工业催化 • 上一篇
含氮有机液体储放氢催化体系研究进展
李佳豪1( ), 杨锦2, 潘伦1(
), 杨锦2, 潘伦1( ), 钟勇斌2, 王志敏2, 王锦生2(
), 钟勇斌2, 王志敏2, 王锦生2( ), 张香文1, 邹吉军1
), 张香文1, 邹吉军1
- 1.天津大学化工学院,绿色合成与转化教育部重点实验室,天津 300072
2.东方电气集团东方锅炉 股份有限公司,四川 成都 610000
-
收稿日期:2023-01-19修回日期:2023-04-11出版日期:2023-12-25发布日期:2024-01-08 -
通讯作者:潘伦,王锦生 -
作者简介:李佳豪(1999—),男,硕士研究生,研究方向为有机液体化合物储氢技术。E-mail:jiahao_li1999@163.com。 -
基金资助:国家自然科学基金(22222808)
Research progress in catalytic system for hydrogen storage and release from nitrogen-containing liquid organic carriers
LI Jiahao1( ), YANG Jin2, PAN Lun1(
), YANG Jin2, PAN Lun1( ), ZHONG Yongbin2, WANG Zhimin2, WANG Jinsheng2(
), ZHONG Yongbin2, WANG Zhimin2, WANG Jinsheng2( ), ZHANG Xiangwen1, ZOU Jijun1
), ZHANG Xiangwen1, ZOU Jijun1
- 1.Key Laboratory for Green Chemical Technology of the Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China
2.DongFang Boiler Group Co. , Ltd. , Chengdu 610000, Sichuan, China
-
Received:2023-01-19Revised:2023-04-11Online:2023-12-25Published:2024-01-08 -
Contact:PAN Lun, WANG Jinsheng
摘要:
氢能源作为重要的二次能源,能量密度大、环境友好且用途广泛,是人类战略能源发展的重要方向。然而,氢气储运仍面临较大的成本和安全难题,有机液体储氢化合物(LOHCs)储放氢技术以其储氢密度较高、储存条件温和、运输方便等优势成为氢气储运可供选择的技术之一。相比稠环芳烃类化合物,含氮有机储氢化合物具有更温和的催化加氢和脱氢条件,可有效提高储放氢鲁棒性和反应能效。基于此,本文系统综述了含氮有机储氢化合物加氢及脱氢反应研究进展,阐述了两类反应的路径和催化作用机制,从催化剂活性中心和载体、双金属协同效应、反应条件、催化剂稳定性等方面系统分析了加氢/脱氢催化剂,并详细总结了基于连串反应、反应网络等模型的反应动力学。介绍了含氮有机储氢化合物储氢技术目前面临的挑战并提出未来的研究思路及展望。但是该技术仍存在较多问题,应在有机储氢化合物配方体系、储放氢连续反应系统、催化剂设计与制备、催化剂构效关系、精准反应动力学和全面理化性质数据库等方面进行深入研究。
中图分类号:
引用本文
李佳豪, 杨锦, 潘伦, 钟勇斌, 王志敏, 王锦生, 张香文, 邹吉军. 含氮有机液体储放氢催化体系研究进展[J]. 化工进展, 2023, 42(12): 6325-6344.
LI Jiahao, YANG Jin, PAN Lun, ZHONG Yongbin, WANG Zhimin, WANG Jinsheng, ZHANG Xiangwen, ZOU Jijun. Research progress in catalytic system for hydrogen storage and release from nitrogen-containing liquid organic carriers[J]. Chemical Industry and Engineering Progress, 2023, 42(12): 6325-6344.
| LOHCs的转化 | 储氢量(质量分数) /% | 脱氢反应焓 /kJ | 熔点(0.1MPa)/℃ | 沸点(0.1MPa)/℃ | ||
|---|---|---|---|---|---|---|
| 富氢分子 | 贫氢分子 | 富氢分子 | 贫氢分子 | |||
12H-NEC
| 5.8 | 50.6 | -85 | 68 | 280 | 190 |
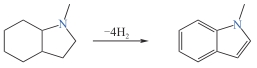
8H-1-甲基吲哚
| 5.76 | 51.9 | <-25 | <-20 | >230 | 238 |
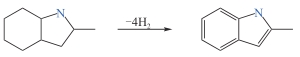
8H-2-甲基吲哚
| 5.76 | — | 25 | 57 | 205 | 272 |
8H-7-乙基吲哚
| 5.23 | — | < -20 | -14 | > 230 | 230 |
8H-2,3-二甲基吲哚
| 5.23 | — | <-10 | 105 | ≥285 | 285 |
10H-喹啉
| 7.2 | 61.9 | -45 | -17 | 200 | 237 |
表1 典型的含氮LOHCs的转化及其理化性质[46,62-65]
| LOHCs的转化 | 储氢量(质量分数) /% | 脱氢反应焓 /kJ | 熔点(0.1MPa)/℃ | 沸点(0.1MPa)/℃ | ||
|---|---|---|---|---|---|---|
| 富氢分子 | 贫氢分子 | 富氢分子 | 贫氢分子 | |||
12H-NEC
| 5.8 | 50.6 | -85 | 68 | 280 | 190 |
8H-1-甲基吲哚
| 5.76 | 51.9 | <-25 | <-20 | >230 | 238 |
8H-2-甲基吲哚
| 5.76 | — | 25 | 57 | 205 | 272 |
8H-7-乙基吲哚
| 5.23 | — | < -20 | -14 | > 230 | 230 |
8H-2,3-二甲基吲哚
| 5.23 | — | <-10 | 105 | ≥285 | 285 |
10H-喹啉
| 7.2 | 61.9 | -45 | -17 | 200 | 237 |
| 序号 | 底物 | 催化剂 | 循环 次数 | 储氢 性能①/% | 参考文献 |
|---|---|---|---|---|---|
| 1 | 12H-NEC | 1%Pd-IP/S15 | 7 | 94.6 | [ |
| 2 | 1%Pd1Ni1/K6 | 6 | 87 | [ | |
| 3 | 2.5%Pd/LDHs-us | 6 | 93 | [ | |
| 4 | 1%PdRh0.6/γ-Al2O3 | 5 | 97.7 | [ | |
| 5 | 5%Pd2Co1/NGC | 6 | 95.6 | [ | |
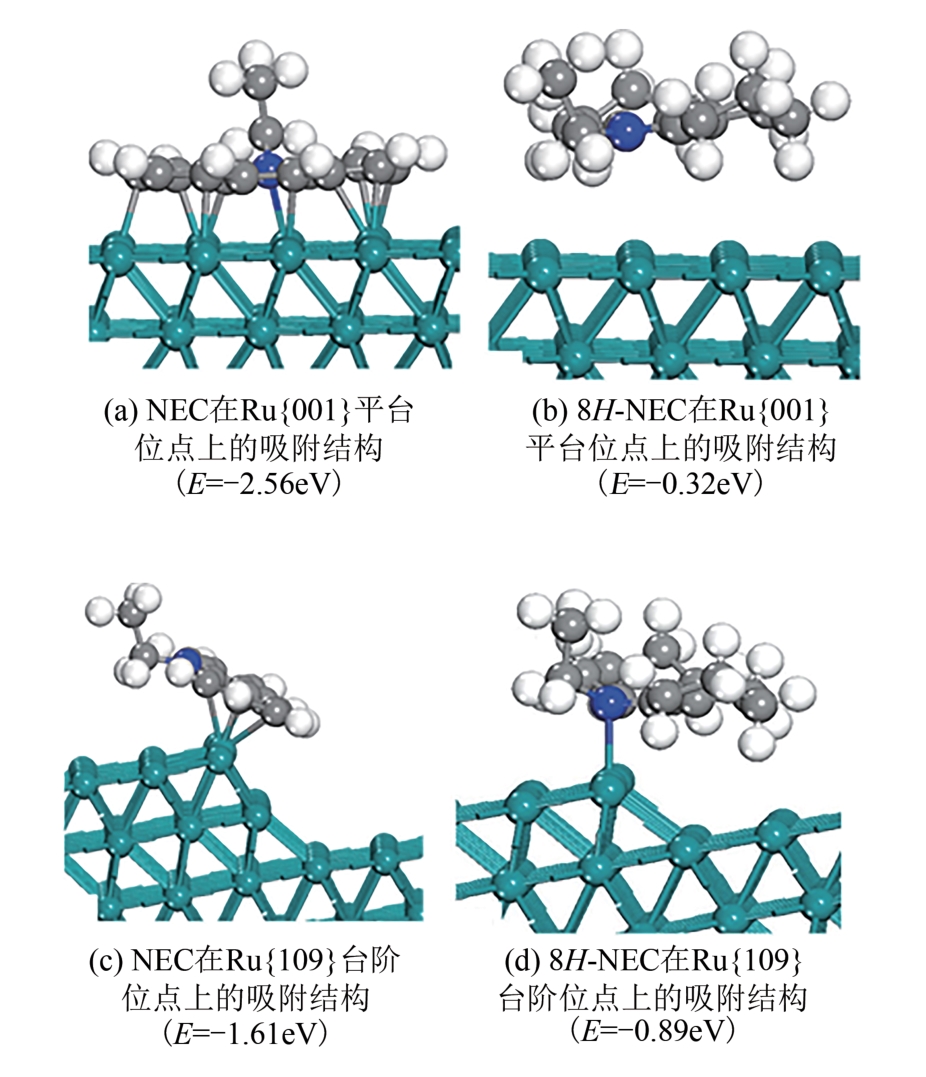
| 6 | 2.5%Pd/LDHs-c | 6 | 86 | [ | |
| 7 | Pd3Cu1/SiO2 | 5 | 95.85 | [ | |
| 8 | Pd3Ni1/SiO2 | 5 | 92.4 | [ | |
| 9 | 1%Pd-IP/S15 | 7 | 97 | [ | |
| 10 | 5%Pd/Al2O3-YH3 | 3 | 97 | [ | |
| 11 | 5%Pd/C | 4 | 92.2 | [ | |
| 12 | Au1Pd1.3/rGO | 5 | 98 | [ | |
| 13 | 3.0%Pd/CNTs | 5 | 90 | [ | |
| 14 | 5%Pd/Al2O3 | 10 | 98 | [ |
表2 含氮有机储氢化合物脱氢催化剂重复性
| 序号 | 底物 | 催化剂 | 循环 次数 | 储氢 性能①/% | 参考文献 |
|---|---|---|---|---|---|
| 1 | 12H-NEC | 1%Pd-IP/S15 | 7 | 94.6 | [ |
| 2 | 1%Pd1Ni1/K6 | 6 | 87 | [ | |
| 3 | 2.5%Pd/LDHs-us | 6 | 93 | [ | |
| 4 | 1%PdRh0.6/γ-Al2O3 | 5 | 97.7 | [ | |
| 5 | 5%Pd2Co1/NGC | 6 | 95.6 | [ | |
| 6 | 2.5%Pd/LDHs-c | 6 | 86 | [ | |
| 7 | Pd3Cu1/SiO2 | 5 | 95.85 | [ | |
| 8 | Pd3Ni1/SiO2 | 5 | 92.4 | [ | |
| 9 | 1%Pd-IP/S15 | 7 | 97 | [ | |
| 10 | 5%Pd/Al2O3-YH3 | 3 | 97 | [ | |
| 11 | 5%Pd/C | 4 | 92.2 | [ | |
| 12 | Au1Pd1.3/rGO | 5 | 98 | [ | |
| 13 | 3.0%Pd/CNTs | 5 | 90 | [ | |
| 14 | 5%Pd/Al2O3 | 10 | 98 | [ |
| 序号 | 底物 | 反应级数 | 催化剂 | 温度范围/℃ | 压力/MPa | 表观活化能/kJ·mol-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 1 | NPC | 1C | 70%Ni/AlSiO-1/1 | 130~160 | 7 | 10.88 | [ |
| 2 | NPC | 1C | 0.5%Ru/WO3 | 100~130 | 6 | 70.19 | [ |
| 3 | NPC | 1C | 5%Ru2.5Ni2.5/Al2O3 | 150~180 | 4 | 12.95 | [ |
| 4 | NPC | 1C | 5%Ru/Al2O3① | 120~150 | 7 | 18.4 | [ |
| 5 | NEC | 1C | 0.5%Ru/Beta | 90~120 | 6 | 45.7 | [ |
| 6 | NEC | 1C | 0.5%Ru/Al2O3 | 90~120 | 6 | 88.3 | [ |
| 7 | NEC | 1C | 5%Ru/Al2O3 | 140~170 | 7 | 67.6 | [ |
| 8 | NEC | 1P | Ni0.5Ru4.5/pg-BC | 110~140 | 6 | 57.3 | [ |
| 9 | NEC | 1P | Ru4Pd1/LDH | 110~140 | 6 | 56.1 | [ |
| 10 | NEC | 1P | 5.89%Ru/pg-BC | 110~140 | 6 | 55.9 | [ |
| 11 | NEC | 1P | 5%Ru/NiFe-LDH | 110~130 | 6 | 25.15 | [ |
| 12 | NEC | 1P | 5%Ru/LDH | 110~140 | 6 | 35.78 | [ |
| 13 | NEC | 1C | Raney-Ni | 120~180 | 5 | 115 | [ |
| 14 | NEC | 1C | 5%Ru/Al2O3① | 150~180 | 8 | 71.2 | [ |
| 15 | NEC | 1P | 5%Ru/γ-Al2O3① | 120~160 | 6 | 30.94 | [ |
| 16 | NEC | 1P | 5%Ru/γ-Al2O3① | 95~160 | 6 | 27.01 | [ |
| 17 | NEC | 1C | 5%Ru, Rh, Pd/AC① | 130 | 7 | — | [ |
| 18 | NEC | 1P | Raney-Ni | 120~200 | 5 | 65.17 | [ |
| 19 | NEC | 1C | 5%Ru/Al2O3① | 120~170 | 7 | 58 | [ |
| 20 | NEC | 1C | 5%Ru/Al2O3① | 130~150 | 7 | 99.5 | [ |
| 21 | 咔唑 | 1C | Raney-Ni | 170~230 | 5 | 90 | [ |
| 22 | 1,2-DMID | 1C | 5%Ru/Al2O3① | 130~170 | 7 | 85.1 | [ |
| 23 | 7-EID | 1C | 5%Ru/Al2O3① | 130~160 | 7 | 51.5 | [ |
| 24 | 1-EID | 1C | 5%Ru/Al2O3① | 160~190 | 9 | 62.4 | [ |
| 25 | 2-MID | 1C | 5%Ru/Al2O3① | 120~170 | 7 | 21 | [ |
| 26 | NEC | 1C | 钌黑① 铂黑① 钯黑① 65%Ni/SiO2-Al2O3① | 130 | 7 | — | [ |
| 27 | NEC | 1C | 5%Ru/Al2O3① 钌黑① 5%Ru/SiO2-Al2O3 | 130 | 7 | — | [ |
| 28 | NEC | 1C | 5%Ru/Al2O3① | 150~170 | 7 | NEC NEC 4H-NEC 6H-NEC 8H-NEC 8H-NEC 10H-NEC | [ |
表3 含氮LOHCs贫氢分子加氢反应动力学参数
| 序号 | 底物 | 反应级数 | 催化剂 | 温度范围/℃ | 压力/MPa | 表观活化能/kJ·mol-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 1 | NPC | 1C | 70%Ni/AlSiO-1/1 | 130~160 | 7 | 10.88 | [ |
| 2 | NPC | 1C | 0.5%Ru/WO3 | 100~130 | 6 | 70.19 | [ |
| 3 | NPC | 1C | 5%Ru2.5Ni2.5/Al2O3 | 150~180 | 4 | 12.95 | [ |
| 4 | NPC | 1C | 5%Ru/Al2O3① | 120~150 | 7 | 18.4 | [ |
| 5 | NEC | 1C | 0.5%Ru/Beta | 90~120 | 6 | 45.7 | [ |
| 6 | NEC | 1C | 0.5%Ru/Al2O3 | 90~120 | 6 | 88.3 | [ |
| 7 | NEC | 1C | 5%Ru/Al2O3 | 140~170 | 7 | 67.6 | [ |
| 8 | NEC | 1P | Ni0.5Ru4.5/pg-BC | 110~140 | 6 | 57.3 | [ |
| 9 | NEC | 1P | Ru4Pd1/LDH | 110~140 | 6 | 56.1 | [ |
| 10 | NEC | 1P | 5.89%Ru/pg-BC | 110~140 | 6 | 55.9 | [ |
| 11 | NEC | 1P | 5%Ru/NiFe-LDH | 110~130 | 6 | 25.15 | [ |
| 12 | NEC | 1P | 5%Ru/LDH | 110~140 | 6 | 35.78 | [ |
| 13 | NEC | 1C | Raney-Ni | 120~180 | 5 | 115 | [ |
| 14 | NEC | 1C | 5%Ru/Al2O3① | 150~180 | 8 | 71.2 | [ |
| 15 | NEC | 1P | 5%Ru/γ-Al2O3① | 120~160 | 6 | 30.94 | [ |
| 16 | NEC | 1P | 5%Ru/γ-Al2O3① | 95~160 | 6 | 27.01 | [ |
| 17 | NEC | 1C | 5%Ru, Rh, Pd/AC① | 130 | 7 | — | [ |
| 18 | NEC | 1P | Raney-Ni | 120~200 | 5 | 65.17 | [ |
| 19 | NEC | 1C | 5%Ru/Al2O3① | 120~170 | 7 | 58 | [ |
| 20 | NEC | 1C | 5%Ru/Al2O3① | 130~150 | 7 | 99.5 | [ |
| 21 | 咔唑 | 1C | Raney-Ni | 170~230 | 5 | 90 | [ |
| 22 | 1,2-DMID | 1C | 5%Ru/Al2O3① | 130~170 | 7 | 85.1 | [ |
| 23 | 7-EID | 1C | 5%Ru/Al2O3① | 130~160 | 7 | 51.5 | [ |
| 24 | 1-EID | 1C | 5%Ru/Al2O3① | 160~190 | 9 | 62.4 | [ |
| 25 | 2-MID | 1C | 5%Ru/Al2O3① | 120~170 | 7 | 21 | [ |
| 26 | NEC | 1C | 钌黑① 铂黑① 钯黑① 65%Ni/SiO2-Al2O3① | 130 | 7 | — | [ |
| 27 | NEC | 1C | 5%Ru/Al2O3① 钌黑① 5%Ru/SiO2-Al2O3 | 130 | 7 | — | [ |
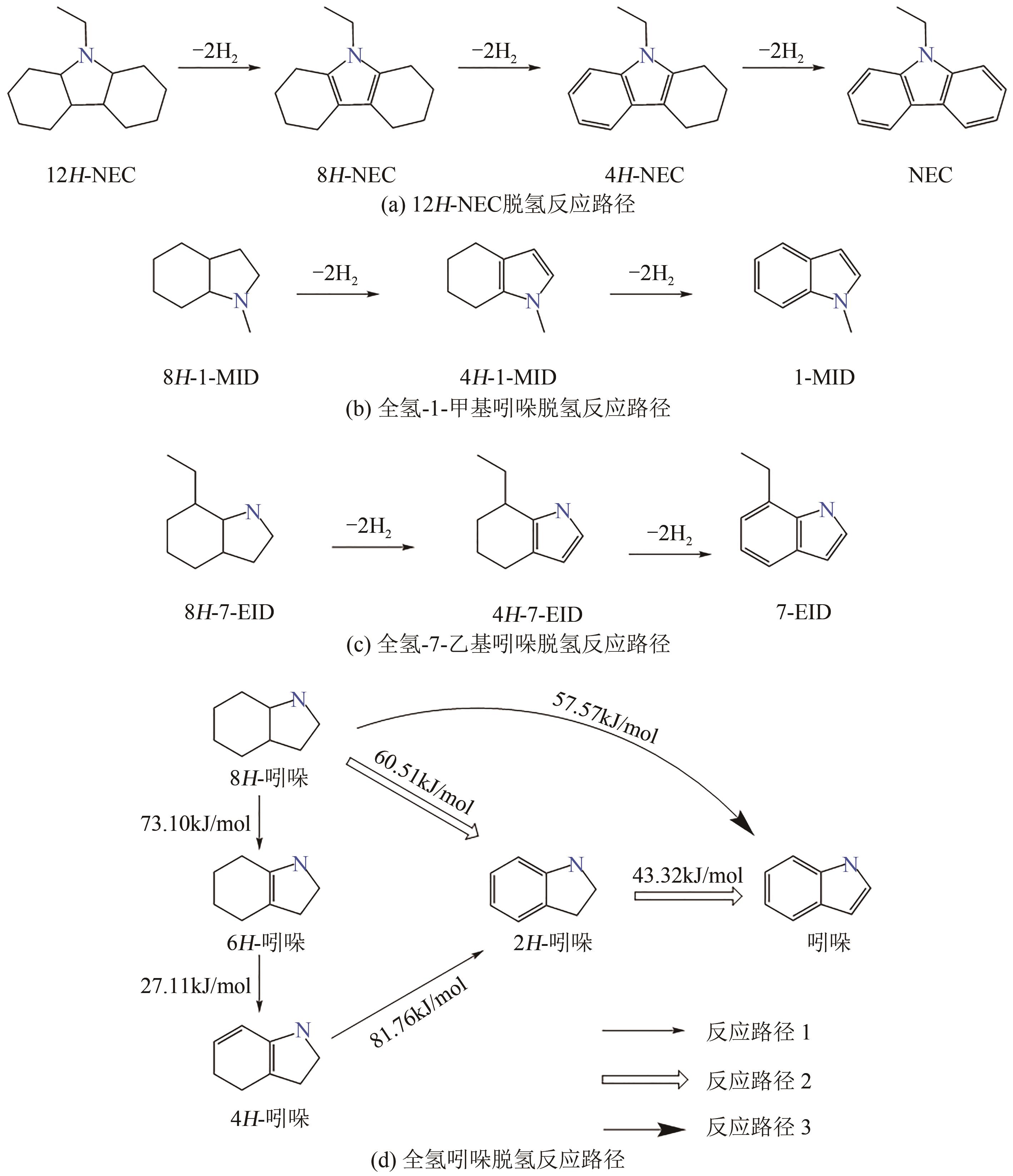
| 28 | NEC | 1C | 5%Ru/Al2O3① | 150~170 | 7 | NEC NEC 4H-NEC 6H-NEC 8H-NEC 8H-NEC 10H-NEC | [ |
| 序号 | 底物 | 反应级数 | 催化剂 | 表观活化能/kJ·mol-1 | 参考文献 |
|---|---|---|---|---|---|
| 1 | 12H-NPC | 1C | 3%Pd/MIL-101 | 12H-NPC 8H-NPC 4H-NPC | [ |
| 2 | 1C | 5%Pd/Al2O3① | 12H-NPC 8H-NPC 4H-NPC | [ | |
| 3 | 12H-NEC | 1C | 1%Pt/TiO2 | 12H-NEC 8H-NEC 4H-NEC | [ |
| 4 | 1C | 5%Pd/Al2O3 | 12H-NEC 8H-NEC 4H-NEC | [ | |
| 5 | 1C | 5%Pd/SiO2 | 126.7 | [ | |
12H-NEC 12H-NEC | |||||
| 6 | 1C | 2.5%Pd/LDHs-us | 90.97 | [ | |
| 7 | 1C | PdCo/NGC | 67.2 | [ | |
| 8 | 1C | 5%Pd/MoO3 | 55.19 | [ | |
| 9 | 1C | 1%Pd4Ni1/KIT-6 | 84.8 | [ | |
| 10 | 1C | Pd-M (M=Cu, Ni)/SiO2 | — | [ | |
| 11 | 1C | Pd-M (M=Co, Ni, Cu)/Al2O3 | — | [ | |
| 12 | 12H-NPC | 1C | 5%Pd-Ni/Al2O3 | 72.03 | [ |
| 13 | 12H-NEC | 1C | 5%Pd/C, Al2O3, TiO2, SiO2① | — | [ |
| 14 | 1C | 5%M (M=Pt, Pd, Ru, Rh, Au)/TiO2 and Pd/Al2O3① | — | [ | |
| 15 | 1C | 5%Pd3Au1/SiO2 | — | [ | |
| 16 | 1C | 2.32%Pd/rGO | — | [ | |
| 17 | 1C | 3%Pd/CNTs | 43.8 | [ | |
| 18 | 1C | Pt/γ-Al2O3 | 203 (本征活化能) | [ | |
| 19 | 1C | 钳形Ir | 115 | [ | |
| 20 | 1C | 5%Pd (Pt, Ru, Rh)/Al2O3① | — | [ | |
| 22 | 1C | 5%Pd/SiO2 | — | [ | |
| 22 | 12H-咔唑 | 1C | 5%Pd/C① | 208.9 | [ |
| 23 | 4H-咔唑 | 0C | 5%Pd/SiO2 | 67.7 | [ |
| 24 | 8H-2,3-DMID | 1C | 5%Pd/Al2O3① | 39.6 | [ |
| 25 | 8H-1,2-DMID | 1C | 5%Pd/Al2O3① | 111.9 | [ |
| 26 | 8H-1-MID | 1C | 5%Pd/Al2O3① | 122.99 | [ |
| 27 | 1C | 钳形Ir | 142 | [ | |
| 28 | 1-丁基吡咯烷 | 1C | 钳形Ir | 166 | [ |
| 29 | 8H-7-EID | 1C | 5%Pd/Al2O3 | 101.9 | [ |
| 30 | 8H-2-MID | 1C | 5%Pd/Al2O3① | 27.1 | [ |
表4 含氮LOHCs富氢分子脱氢反应动力学参数
| 序号 | 底物 | 反应级数 | 催化剂 | 表观活化能/kJ·mol-1 | 参考文献 |
|---|---|---|---|---|---|
| 1 | 12H-NPC | 1C | 3%Pd/MIL-101 | 12H-NPC 8H-NPC 4H-NPC | [ |
| 2 | 1C | 5%Pd/Al2O3① | 12H-NPC 8H-NPC 4H-NPC | [ | |
| 3 | 12H-NEC | 1C | 1%Pt/TiO2 | 12H-NEC 8H-NEC 4H-NEC | [ |
| 4 | 1C | 5%Pd/Al2O3 | 12H-NEC 8H-NEC 4H-NEC | [ | |
| 5 | 1C | 5%Pd/SiO2 | 126.7 | [ | |
12H-NEC 12H-NEC | |||||
| 6 | 1C | 2.5%Pd/LDHs-us | 90.97 | [ | |
| 7 | 1C | PdCo/NGC | 67.2 | [ | |
| 8 | 1C | 5%Pd/MoO3 | 55.19 | [ | |
| 9 | 1C | 1%Pd4Ni1/KIT-6 | 84.8 | [ | |
| 10 | 1C | Pd-M (M=Cu, Ni)/SiO2 | — | [ | |
| 11 | 1C | Pd-M (M=Co, Ni, Cu)/Al2O3 | — | [ | |
| 12 | 12H-NPC | 1C | 5%Pd-Ni/Al2O3 | 72.03 | [ |
| 13 | 12H-NEC | 1C | 5%Pd/C, Al2O3, TiO2, SiO2① | — | [ |
| 14 | 1C | 5%M (M=Pt, Pd, Ru, Rh, Au)/TiO2 and Pd/Al2O3① | — | [ | |
| 15 | 1C | 5%Pd3Au1/SiO2 | — | [ | |
| 16 | 1C | 2.32%Pd/rGO | — | [ | |
| 17 | 1C | 3%Pd/CNTs | 43.8 | [ | |
| 18 | 1C | Pt/γ-Al2O3 | 203 (本征活化能) | [ | |
| 19 | 1C | 钳形Ir | 115 | [ | |
| 20 | 1C | 5%Pd (Pt, Ru, Rh)/Al2O3① | — | [ | |
| 22 | 1C | 5%Pd/SiO2 | — | [ | |
| 22 | 12H-咔唑 | 1C | 5%Pd/C① | 208.9 | [ |
| 23 | 4H-咔唑 | 0C | 5%Pd/SiO2 | 67.7 | [ |
| 24 | 8H-2,3-DMID | 1C | 5%Pd/Al2O3① | 39.6 | [ |
| 25 | 8H-1,2-DMID | 1C | 5%Pd/Al2O3① | 111.9 | [ |
| 26 | 8H-1-MID | 1C | 5%Pd/Al2O3① | 122.99 | [ |
| 27 | 1C | 钳形Ir | 142 | [ | |
| 28 | 1-丁基吡咯烷 | 1C | 钳形Ir | 166 | [ |
| 29 | 8H-7-EID | 1C | 5%Pd/Al2O3 | 101.9 | [ |
| 30 | 8H-2-MID | 1C | 5%Pd/Al2O3① | 27.1 | [ |
| 50 | JORSCHICK H, PREUSTER P, DÜRR S, et al. Hydrogen storage using a hot pressure swing reactor[J]. Energy & Environmental Science, 2017, 10(7): 1652-1659. |
| 51 | DO G, PREUSTER P, ASLAM R, et al. Hydrogenation of the liquid organic hydrogen carrier compound dibenzyltoluene-reaction pathway determination by 1H NMR spectroscopy[J]. Reaction Chemistry & Engineering, 2016, 1(3): 313-320. |
| 52 | STARK Katharina, KEIL Philipp, SCHUG Sebastian, et al. Melting points of potential liquid organic hydrogen carrier systems consisting of N-alkylcarbazoles[J]. Journal of Chemical & Engineering Data, 2016, 61(4): 1441-1448. |
| 53 | YANG Ming, DONG Yuan, FEI Shunxin, et al. A comparative study of catalytic dehydrogenation of perhydro-N-ethylcarbazole over noble metal catalysts[J]. International Journal of Hydrogen Energy, 2014, 39(33): 18976-18983. |
| 54 | ZHANG Yujing, WANG Jixue, ZHOU Feng, et al. An effective strategy for hydrogen supply: Catalytic acceptorless dehydrogenation of N-heterocycles[J]. Catalysis Science & Technology, 2021, 11(12): 3990-4007. |
| 55 | CRABTREE Robert H. Nitrogen-containing liquid organic hydrogen carriers: Progress and prospects[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(6): 4491-4498. |
| 56 | EBLAGON Katarzyna Morawa, RENTSCH Daniel, FRIEDRICHS Oliver, et al. Hydrogenation of 9-ethylcarbazole as a prototype of a liquid hydrogen carrier[J]. International Journal of Hydrogen Energy, 2010, 35(20): 11609-11621. |
| 57 | SOTOODEH Farnaz, ZHAO Liang, SMITH Kevin J. Kinetics of H2 recovery from dodecahydro-N-ethylcarbazole over a supported Pd catalyst[J]. Applied Catalysis A: General, 2009, 362(1/2): 155-162. |
| 58 | SOTOODEH Farnaz, SMITH Kevin J. Kinetics of hydrogen uptake and release from heteroaromatic compounds for hydrogen storage[J]. Industrial & Engineering Chemistry Research, 2010, 49(3): 1018-1026. |
| 59 | SUN Feifei, AN Yue, LEI Lecheng, et al. Identification of the starting reaction position in the hydrogenation of (N-ethyl)carbazole over Raney-Ni[J]. Journal of Energy Chemistry, 2015, 24(2): 219-224. |
| 60 | EBLAGON K M, TAM K, YU K M K, et al. Comparative study of catalytic hydrogenation of 9-ethylcarbazole for hydrogen storage over noble metal surfaces[J]. The Journal of Physical Chemistry C, 2012, 116(13): 7421-7429. |
| 61 | LI Linlin, YANG Ming, DONG Yuan, et al. Hydrogen storage and release from a new promising Liquid organic hydrogen storage carrier (LOHC): 2-Methylindole[J]. International Journal of Hydrogen Energy, 2016, 41(36): 16129-16134. |
| 62 | RAO Purna Chandra, YOON Minyoung. Potential liquid-organic hydrogen carrier (LOHC) systems: A review on recent progress[J]. Energies, 2020, 13(22): 6040. |
| 63 | DONG Yuan, ZHAO Haoming, ZHAO Yinheng, et al. Study of catalytic hydrogenation and dehydrogenation of 2,3-dimethylindole for hydrogen storage application[J]. RSC Advances, 2021, 11(26): 15729-15737. |
| 64 | DONG Yuan, YANG Ming, LI Linlin, et al. Study on reversible hydrogen uptake and release of 1,2-dimethylindole as a new liquid organic hydrogen carrier[J]. International Journal of Hydrogen Energy, 2019, 44(10): 4919-4929. |
| 65 | AAKKO-SAKSA Päivi T, COOK Chris, KIVIAHO Jari, et al. Liquid organic hydrogen carriers for transportation and storing of renewable energy—Review and discussion[J]. Journal of Power Sources, 2018, 396: 803-823. |
| 66 | YU Hongen, YANG Xue, WU Yong, et al. Bimetallic Ru-Ni/TiO2 catalysts for hydrogenation of N-ethylcarbazole: Role of TiO2 crystal structure[J]. Journal of Energy Chemistry, 2020, 40: 188-195. |
| 67 | YANG Ming, CHENG Guoe, XIE Dandan, et al. Study of hydrogenation and dehydrogenation of 1-methylindole for reversible onboard hydrogen storage application[J]. International Journal of Hydrogen Energy, 2018, 43(18): 8868-8876. |
| 68 | OUMA Cecil N M, MODISHA Phillimon M, BESSARABOV Dmitri. Catalytic dehydrogenation of the liquid organic hydrogen carrier octahydroindole on Pt (111) surface: Ab initio insights from density functional theory calculations[J]. Applied Surface Science, 2019, 471: 1034-1040. |
| 69 | SOBOTA Marek, NIKIFORIDIS Ioannis, AMENDE Max, et al. Dehydrogenation of dodecahydro-N-ethylcarbazole on Pd/Al2O3 model catalysts[J]. Chemistry-A European Journal, 2011, 17(41): 11542-11552. |
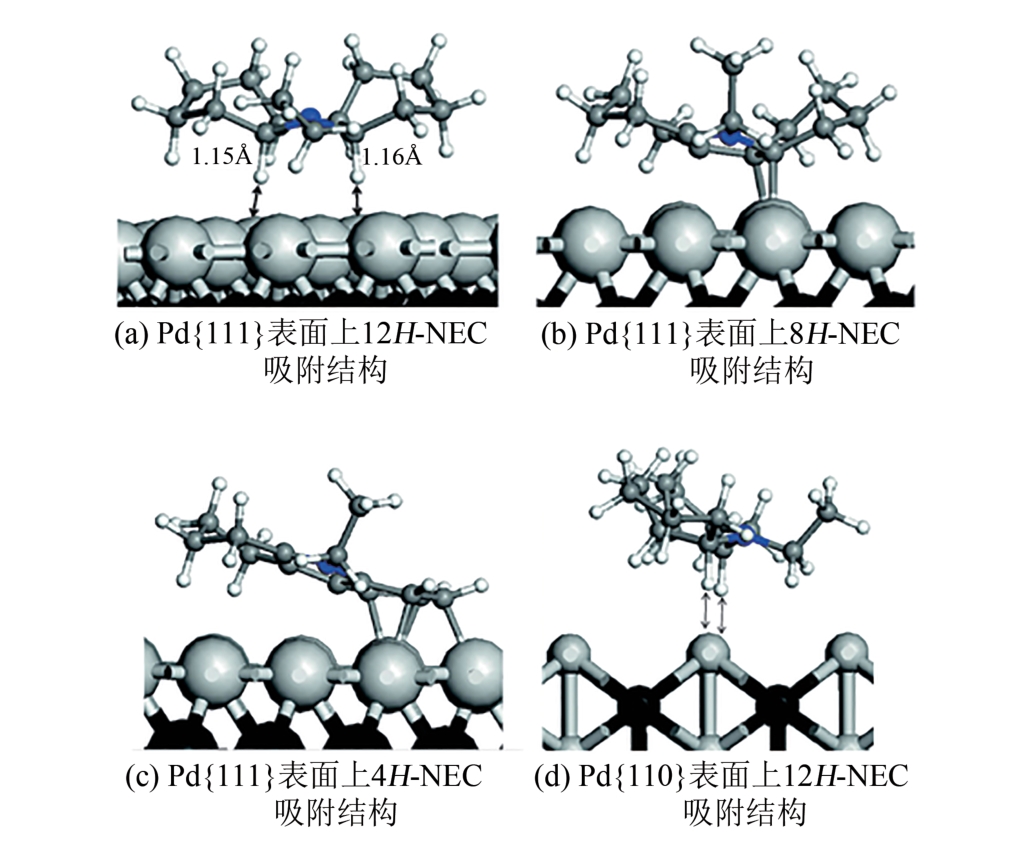
| 70 | AMENDE Max, SCHERNICH Stefan, SOBOTA Marek, et al. Dehydrogenation mechanism of liquid organic hydrogen carriers: Dodecahydro-N-ethylcarbazole on Pd(111)[J]. Chemistry-A European Journal, 2013, 19(33): 10854-10865. |
| 71 | AMENDE Max, GLEICHWEIT Christoph, WERNER Kristin, et al. Model catalytic studies of liquid organic hydrogen carriers: Dehydrogenation and decomposition mechanisms of dodecahydro-N-ethylcarbazole on Pt(111)[J]. ACS Catalysis, 2014, 4(2): 657-665. |
| 72 | SCHWARZ Matthias, BACHMANN Philipp, SILVA Thais Nascimento, et al. Model catalytic studies of novel liquid organic hydrogen carriers: Indole, indoline and octahydroindole on Pt(111)[J]. Chemistry-A European Journal, 2017, 23(59): 14806-14818. |
| 73 | SOTOODEH Farnaz, SMITH Kevin J. Structure sensitivity of dodecahydro-N-ethylcarbazole dehydrogenation over Pd catalysts[J]. Journal of Catalysis, 2011, 279(1): 36-47. |
| 74 | FENG Zhaolu, CHEN Xiaomin, BAI Xuefeng. Hydrogen production from the catalytic dehydrogenation of dodecahydro-N-ethylcarbazole: Effect of Pd precursor on the catalytic performance of Pd/C catalysts[J]. Environmental Science and Pollution Research, 2021, 28(43): 61623-61635. |
| 75 | JIANG Zhao, GONG Xiang, GUO Shuyi, et al. Engineering PdCu and PdNi bimetallic catalysts with adjustable alloying degree for the dehydrogenation reaction of dodecahydro-N-ethylcarbazole[J]. International Journal of Hydrogen Energy, 2021, 46(2): 2376-2389. |
| 76 | JIANG Zhao, GONG Xiang, WANG Bin, et al. A experimental study on the dehydrogenation performance of dodecahydro-N-ethylcarbazole on M/TiO2 catalysts[J]. International Journal of Hydrogen Energy, 2019, 44(5): 2951-2959. |
| 77 | GONG Xiang, JIANG Zhao, FANG Tao. Enhancing selectivity and reducing cost for dehydrogenation of dodecahydro-N-ethylcarbazole by supporting platinum on titanium dioxide[J]. International Journal of Hydrogen Energy, 2020, 45(11): 6838-6847. |
| 78 | WANG Bin, CHEN Youtao, CHANG Tieyan, et al. Facet-dependent catalytic activities of Pd/rGO: Exploring dehydrogenation mechanism of dodecahydro-N-ethylcarbazole[J]. Applied Catalysis B: Environmental, 2020, 266: 118658. |
| 79 | WANG Bin, CHANG Tieyan, GONG Xiang, et al. One-pot synthesis of Au/Pd core/shell nanoparticles supported on reduced graphene oxide with enhanced dehydrogenation performance for dodecahydro-N-ethylcarbazole[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(1): 1760-1768. |
| 80 | SHI Jiaming, BAI Xuefeng. In situ preparation of ultrafine Ru nanocatalyst supported on nitrogen-doped layered double hydroxide by nitrogen glow discharge plasma for catalytic hydrogenation of N-ethylcarbazole[J]. Applied Organometallic Chemistry, 2020, 34(9): e5777. |
| 81 | ZHU Mengyang, XU Lixin, DU Lin, et al. Palladium supported on carbon nanotubes as a high-performance catalyst for the dehydrogenation of dodecahydro-N-ethylcarbazole[J]. Catalysts, 2018, 8(12): 638. |
| 82 | QIN Yibo, BAI Xuefeng. Hydrogenation of N-ethylcarbazole over Ni-Ru alloy nanoparticles loaded on graphitized carbon prepared by carbothermal reduction[J]. Fuel, 2022, 307: 121921. |
| 83 | LIU Xiaoran, SHI Jiaming, BAI Xuefeng, et al. Ultrasonic-assisted synthesis of highly stable RuPd bimetallic catalysts supported on MgAl-layered double hydroxide for N-ethylcarbazole hydrogenation[J]. Environmental Science and Pollution Research, 2022, 29(32): 48558-48572. |
| 84 | MODISHA Phillimon, BESSARABOV Dmitri. Stress tolerance assessment of dibenzyltoluene-based liquid organic hydrogen carriers[J]. Sustainable Energy & Fuels, 2020, 4(9): 4662-4670. |
| 85 | 薛景文, 于鹏飞, 张彦康, 等. 液态有机氢载体储氢系统脱氢反应器研究进展[J]. 热力发电, 2022, 51(11): 1-10. |
| XUE Jingwen, YU Pengfei, ZHANG Yankang, et al. Review on advances of dehydrogenation reactor for hydrogen storage system using liquid organic hydrogen carrier[J]. Thermal Power Generation, 2022, 51(11): 1-10. | |
| 86 | YANG Xue, WU Yiman, YU Hongen, et al. A YH3 promoted palladium catalyst for reversible hydrogen storage of N-ethylcarbazole[J]. International Journal of Hydrogen Energy, 2020, 45(58): 33657-33662. |
| 87 | WU Yong, YU Hongen, GUO Yanru, et al. A rare earth hydride supported ruthenium catalyst for the hydrogenation of N-heterocycles: Boosting the activity via a new hydrogen transfer path and controlling the stereoselectivity[J]. Chemical Science, 2019, 10(45): 10459-10465. |
| 88 | SHI Libin, QI Suitao, QU Jifeng, et al. Integration of hydrogenation and dehydrogenation based on dibenzyltoluene as liquid organic hydrogen energy carrier[J]. International Journal of Hydrogen Energy, 2019, 44(11): 5345-5354. |
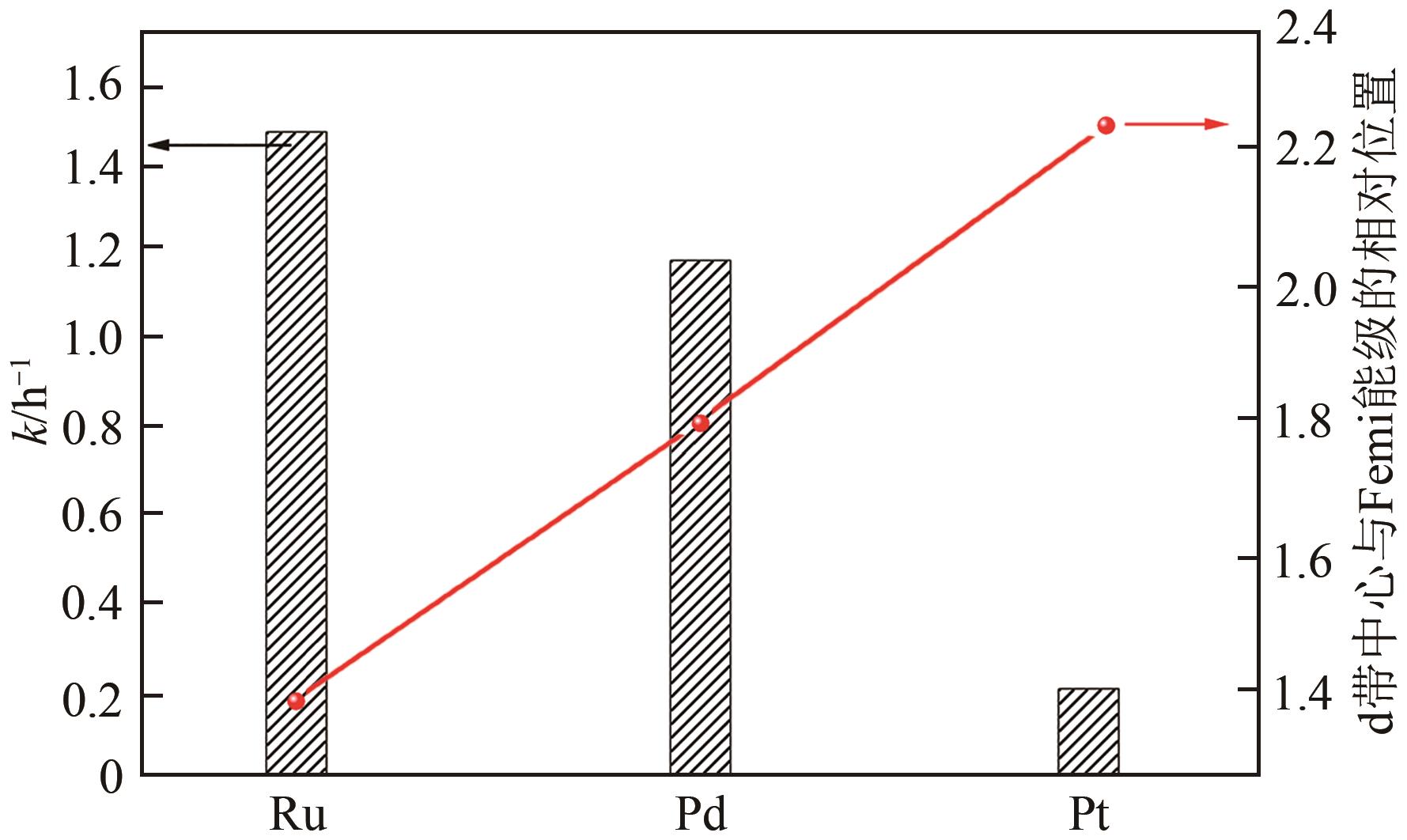
| 89 | HAMMER B. Special sites at noble and late transition metal catalysts[J]. Topics in Catalysis, 2006, 37(1): 3-16. |
| 90 | LIU Xiaoran, SHI Jiaming, BAI Xuefeng, et al. Ultrasound-excited hydrogen radical from NiFe layered double hydroxide for preparation of ultrafine supported Ru nanocatalysts in hydrogen storage of N-ethylcarbazole[J]. Ultrasonics Sonochemistry, 2021, 81: 105840. |
| 91 | YE Xufeng, AN Yue, XU Guohua. Kinetics of 9-ethylcarbazole hydrogenation over Raney-Ni catalyst for hydrogen storage[J]. Journal of Alloys and Compounds, 2011, 509(1): 152-156. |
| 92 | WANG Yingwen, ZHANG Yajing, WANG Kangjun, et al. Preparation of Ni/SiO2 by ammonia evaporation method for synthesis of 2-MTHF from 2-MF hydrogenation[J]. Journal of Fuel Chemistry and Technology, 2021, 49(1): 97-103. |
| 93 | GE Lixia, QIU Minghuang, ZHU Yanfeng, et al. Synergistic catalysis of Ru single-atoms and zeolite boosts high-efficiency hydrogen storage[J]. Applied Catalysis B: Environmental, 2022, 319: 121958. |
| 94 | WANG Weicong, HE Tianou, YANG Xiaolong, et al. General synthesis of amorphous PdM (M = Cu, Fe, Co, Ni) alloy nanowires for boosting HCOOH dehydrogenation[J]. Nano Letters, 2021, 21(8): 3458-3464. |
| 95 | WU Yong, YU Hongen, GUO Yanru, et al. Promoting hydrogen absorption of liquid organic hydrogen carriers by solid metal hydrides[J]. Journal of Materials Chemistry A, 2019, 7(28): 16677-16684. |
| 96 | 宋林, 安越, 容丽春, 等. Ni/γ-Al2O3催化乙基咔唑加氢性能研究[J]. 化学工程, 2015, 43(10): 50-53, 59. |
| SONG Lin, AN Yue, RONG Lichun, et al. Catalytic hydrogenation of N-ethylcarbazole over Ni/γ-Al2O3 catalyst[J]. Chemical Engineering, 2015, 43(10): 50-53, 59. | |
| 97 | QIN Yibo, SHI Jiaming, BAI Xuefeng. Preparing ultra-stable Ru nanocatalysts supported on partially graphitized biochar via carbothermal reduction for hydrogen storage of N-ethylcarbazole[J]. International Journal of Hydrogen Energy, 2021, 46(50): 25543-25554. |
| 98 | CHEN Xuedi, LI Gen, GAO Min, et al. Wet-impregnated bimetallic Pd-Ni catalysts with enhanced activity for dehydrogenation of perhydro-N-propylcarbazole[J]. International Journal of Hydrogen Energy, 2020, 45(56): 32168-32178. |
| 99 | GONG Xiang, GUO Shuyi, JIANG Zhao, et al. Tuning the alloy degree for Pd-M/Al2O3 (M=Co/Ni/Cu) bimetallic catalysts to enhance the activity and selectivity of dodecahydro-N-ethylcarbazole dehydrogenation[J]. International Journal of Hydrogen Energy, 2021, 46(68): 33835-33848. |
| 100 | TRITSARIS Georgios A, NØRSKOV Jens K, ROSSMEISL Jan. Trends in oxygen reduction and methanol activation on transition metal chalcogenides[J]. Electrochimica Acta, 2011, 56(27): 9783-9788. |
| 101 | WANG Bin, CHANG Tieyan, JIANG Zhao, et al. Catalytic dehydrogenation study of dodecahydro-N-ethylcarbazole by noble metal supported on reduced graphene oxide[J]. International Journal of Hydrogen Energy, 2018, 43(15): 7317-7325. |
| 102 | LI Jinjian, TONG Fengya, LI Yi, et al. Dehydrogenation of dodecahydro-N-ethylcarbazole over spinel supporting catalyst in a continuous flow fixed bed reactor[J]. Fuel, 2022, 321: 124034. |
| 103 | Yongxiao TUO, MENG Ying, CHEN Chen, et al. Partial positively charged Pt in Pt/MgAl2O4 for enhanced dehydrogenation activity[J]. Applied Catalysis B: Environmental, 2021, 288: 119996. |
| 104 | CAO Xinrui, JI Yongfei, LUO Yi. Dehydrogenation of propane to propylene by a Pd/Cu single-atom catalyst: Insight from first-principles calculations[J]. The Journal of Physical Chemistry C, 2015, 119(2): 1016-1023. |
| 105 | FEI Shunxin, WANG Yueying, WU Heng, et al. Synergistic strategy for the fast dehydrogenation of liquid organic hydrogen carriers over a Pd/MoO3 catalyst[J]. ACS Applied Energy Materials, 2022, 5(9): 10562-10571. |
| 106 | ZHANG Hui, JIN Mingshang, XIONG Yujie, et al. Shape-controlled synthesis of Pd nanocrystals and their catalytic applications[J]. Accounts of Chemical Research, 2013, 46(8): 1783-1794. |
| 107 | JIN Mingshang, ZHANG Hui, XIE Zhaoxiong, et al. Palladium nanocrystals enclosed by {100} and {111} facets in controlled proportions and their catalytic activities for formic acid oxidation[J]. Energy & Environmental Science, 2012, 5(4): 6352-6357. |
| 108 | MA Xianyin, CHEN Yafeng, WANG Han, et al. Electrocatalytic oxidation of ethanol and ethylene glycol on cubic, octahedral and rhombic dodecahedral palladium nanocrystals[J]. Chemical Communications, 2018, 54(20): 2562-2565. |
| 109 | JIANG Zhao, GUO Shuyi, FANG Tao. Enhancing the catalytic activity and selectivity of PdAu/SiO2 bimetallic catalysts for dodecahydro-N-ethylcarbazole dehydrogenation by controlling the particle size and dispersion[J]. ACS Applied Energy Materials, 2019, 2(10): 7233-7243. |
| 110 | AMENDE Max, GLEICHWEIT Christoph, SCHERNICH Stefan, et al. Size and structure effects controlling the stability of the liquid organic hydrogen carrier dodecahydro-N-ethylcarbazole during dehydrogenation over Pt model catalysts[J]. The Journal of Physical Chemistry Letters, 2014, 5(8): 1498-1504. |
| 111 | NAGATAKE Satoshi, HIGO Takuma, Shuhei OGO, et al. Dehydrogenation of methylcyclohexane over Pt/TiO2 catalyst[J]. Catalysis Letters, 2016, 146(1): 54-60. |
| 112 | IIDA Hajime, IGARASHI Akira. Characterization of a Pt/TiO2 (rutile) catalyst for water gas shift reaction at low-temperature[J]. Applied Catalysis A: General, 2006, 298: 152-160. |
| 113 | ZHAO Yonghui, LI Shenggang, SUN Yuhan. Theoretical study on the dissociative adsorption of CH4 on Pd-doped Ni surfaces[J]. Chinese Journal of Catalysis, 2013, 34(5): 911-922. |
| 114 | TAMARANY Rizcky, SHIN Dongyun, KANG Sukho, et al. Formic acid dehydrogenation over PdNi alloys supported on N-doped carbon: Synergistic effect of Pd-Ni alloying on hydrogen release[J]. Physical Chemistry Chemical Physics, 2021, 23(19): 11515-11527. |
| 115 | FENG Zhaolu, BAI Xuefeng. Enhanced activity of bimetallic Pd-Ni nanoparticles on KIT-6 for production of hydrogen from dodecahydro-N-ethylcarbazole[J]. Fuel, 2022, 329: 125473. |
| 116 | MAKARYAN I A, SEDOV I V. Hydrogenation/dehydrogenation catalysts for hydrogen storage systems based on liquid organic carriers (A review)[J]. Petroleum Chemistry, 2021, 61(9): 977-988. |
| 117 | AMENDE Max, KAFTAN Andre, BACHMANN Philipp, et al. Regeneration of LOHC dehydrogenation catalysts: In-situ IR spectroscopy on single crystals, model catalysts, and real catalysts from UHV to near ambient pressure[J]. Applied Surface Science, 2016, 360: 671-683. |
| 118 | YANG Ming, HAN Chaoqun, NI Gang, et al. Temperature controlled three-stage catalytic dehydrogenation and cycle performance of perhydro-9-ethylcarbazole[J]. International Journal of Hydrogen Energy, 2012, 37(17): 12839-12845. |
| 119 | WAN Chao, AN Yue, CHEN Fengqiu, et al. Kinetics of N-ethylcarbazole hydrogenation over a supported Ru catalyst for hydrogen storage[J]. International Journal of Hydrogen Energy, 2013, 38(17): 7065-7069. |
| 1 | 国家发改委. 国家发改委发布《氢能产业发展中长期规划(2021—2035年)》[J]. 稀土信息, 2022(4): 26-32. |
| National Development and Reform Commission. The national development and reform commission issued the medium and long-term plan for the development of hydrogen energy industry (2021—2035)[J]. Rare Earth Information, 2022(4): 26-32. | |
| 2 | SHI Libin, ZHOU Yiming, TAN Xiao, et al. Dielectric barrier discharge plasma grafting carboxylate groups on Pt/Al2O3 catalysts for highly efficient hydrogen release from perhydro-dibenzyltoluene[J]. Catalysis Science & Technology, 2022, 12(5): 1441-1449. |
| 3 | ZAKGEYM Dina, ENGL Timo, MAHAYNI Yazan, et al. Development of an efficient Pt/SiO2 catalyst for the transfer hydrogenation from perhydro-dibenzyltoluene to acetone[J]. Applied Catalysis A: General, 2022, 639: 118644. |
| 4 | LEE Sanghun, LEE Jaemyung, KIM Taehong, et al. Pt/CeO2 catalyst synthesized by combustion method for dehydrogenation of perhydro-dibenzyltoluene as liquid organic hydrogen carrier: Effect of pore size and metal dispersion[J]. International Journal of Hydrogen Energy, 2021, 46(7): 5520-5529. |
| 5 | WU Yanpeng, LIU Xiaoran, BAI Xuefeng, et al. Ultrasonic-assisted preparation of ultrafine Pd nanocatalysts loaded on Cl--intercalated MgAl layered double hydroxides for the catalytic dehydrogenation of dodecahydro-N-ethylcarbazole[J]. Ultrasonics Sonochemistry, 2022, 88: 106097. |
| 6 | 程一步. 2022年国内氢能产业发展动态及新政策对产业影响浅析[J]. 石油石化绿色低碳, 2022, 7(5): 1-6. |
| CHENG Yibu. China’s hydrogen energy industry development and new policy implications in 2022[J]. Green Petroleum & Petrochemicals, 2022, 7(5): 1-6. | |
| 7 | 程明睿, 高宏. 绿氢已成为未来维护能源安全的重要方向[J]. 科技中国, 2022(10): 60-65. |
| CHENG Mingrui, GAO Hong. Green hydrogen has become an important direction to maintain energy security in the future[J]. Scitech in China, 2022(10): 60-65. | |
| 8 | 杜忠明, 郑津洋, 戴剑锋, 等. 我国绿氢供应体系建设思考与建议[J]. 中国工程科学, 2022, 24(6): 64-71. |
| DU Zhongming, ZHENG Jinyang, DAI Jianfeng, et al. Construction of green-hydrogen supply system in China: Reflections and suggestions[J]. Strategic Study of CAE, 2022, 24(6): 64-71. | |
| 120 | WAN Chao, AN Yue, XU Guohua, et al. Study of catalytic hydrogenation of N-ethylcarbazole over ruthenium catalyst[J]. International Journal of Hydrogen Energy, 2012, 37(17): 13092-13096. |
| 121 | YANG Ming, DONG Yuan, CHENG Hansong. Hydrogenation kinetics of N-ethylcarbaozle as a heteroaromatic liquid organic hydrogen carrier[J]. Advanced Materials Research, 2014, 953/954: 981-984. |
| 122 | DONG Yuan, YANG Ming, ZHU Ting, et al. Hydrogenation kinetics of N-ethylindole on a supported Ru catalyst[J]. Energy Technology, 2018, 6(3): 558-562. |
| 123 | LI Peijie, DONG Yuan, DING Yuhang, et al. Effect of hydrogen spillover on the surface of tungsten oxide on hydrogenation of cyclohexene and N-propylcarbazole[J]. International Journal of Hydrogen Energy, 2021, 46(5): 3945-3953. |
| 124 | LI Chenguang, YANG Ming, LIU Zhenjie, et al. Ru-Ni/Al2O3 bimetallic catalysts with high catalytic activity for N-propylcarbazole hydrogenation[J]. Catalysis Science & Technology, 2020, 10(7): 2268-2276. |
| 125 | YANG Ming, DONG Yuan, FEI Shunxin, et al. Hydrogenation of N-propylcarbazole over supported ruthenium as a new prototype of liquid organic hydrogen carriers (LOHC)[J]. RSC Advances, 2013, 3(47): 24877-24881. |
| 126 | EBLAGON Katarzyna Morawa, Kin TAM, TSANG Shik Chi Edman. Comparison of catalytic performance of supported ruthenium and rhodium for hydrogenation of 9-ethylcarbazole for hydrogen storage applications[J]. Energy & Environmental Science, 2012, 5(9): 8621-8630. |
| 127 | DONG Yuan, YANG Ming, ZHU Ting, et al. Fast dehydrogenation kinetics of perhydro-N-propylcarbazole over a supported Pd catalyst[J]. ACS Applied Energy Materials, 2018, 1(8): 4285-4292. |
| 128 | DONG Yuan, YANG Ming, MEI Pan, et al. Dehydrogenation kinetics study of perhydro-N-ethylcarbazole over a supported Pd catalyst for hydrogen storage application[J]. International Journal of Hydrogen Energy, 2016, 41(20): 8498-8505. |
| 129 | PETERS Willi, SEIDEL Alexander, HERZOG Stefan, et al. Macrokinetic effects in perhydro-N-ethylcarbazole dehydrogenation and H2 productivity optimization by using egg-shell catalysts[J]. Energy & Environmental Science, 2015, 8(10): 3013-3021. |
| 130 | BRAYTON Daniel F, JENSEN Craig M. Dehydrogenation of pyrrolidine based liquid organic hydrogen carriers by an iridium pincer catalyst, an isothermal kinetic study[J]. International Journal of Hydrogen Energy, 2015, 40(46): 16266-16270. |
| 131 | SOTOODEH Farnaz, HUBER Benjamin J M, SMITH Kevin J. Dehydrogenation kinetics and catalysis of organic heteroaromatics for hydrogen storage[J]. International Journal of Hydrogen Energy, 2012, 37(3): 2715-2722. |
| 9 | FELDERHOFF Michael, WEIDENTHALER Claudia, VON HELMOLT Rittmar, et al. Hydrogen storage: The remaining scientific and technological challenges[J]. Physical Chemistry Chemical Physics, 2007, 9(21): 2643-2653. |
| 10 | 李璐伶, 樊栓狮, 陈秋雄, 等. 储氢技术研究现状及展望[J]. 储能科学与技术, 2018, 7(4): 586-594. |
| LI Luling, FAN Shuanshi, CHEN Qiuxiong, et al. Hydrogen storage technology: Current status and prospects[J]. Energy Storage Science and Technology, 2018, 7(4): 586-594. | |
| 11 | JORSCHICK H, PREUSTER P, BÖSMANN A, et al. Hydrogenation of aromatic and heteroaromatic compounds-a key process for future logistics of green hydrogen using liquid organic hydrogen carrier systems[J]. Sustainable Energy & Fuels, 2021, 5(5): 1311-1346. |
| 12 | ZHOU Liu, SUN Lin, XU Lixin, et al. Recent developments of effective catalysts for hydrogen storage technology using N-ethylcarbazole[J]. Catalysts, 2020, 10(6): 648. |
| 13 | JIANG Zhao, PAN Qi, XU Jie, et al. Current situation and prospect of hydrogen storage technology with new organic liquid[J]. International Journal of Hydrogen Energy, 2014, 39(30): 17442-17451. |
| 14 | SINGH Rasmeet, SINGH Mandeep, GAUTAM Sanjeev. Hydrogen economy, energy, and liquid organic carriers for its mobility[J]. Materials Today: Proceedings, 2021, 46: 5420-5427. |
| 15 | KIM Tae Wan, JEONG Hwiram, BAIK Joon Hyun, et al. State-of-the-art catalysts for hydrogen storage in liquid organic hydrogen carriers[J]. Chemistry Letters, 2022, 51(3): 239-255. |
| 16 | 刘安鼐, 任靖, 赵保槐, 等. 液相有机氢载体的催化研究与应用[J]. 北京化工大学学报(自然科学版), 2021, 48(4): 1-18. |
| LIU Annai, REN Jing, ZHAO Baohuai, et al. Catalysis and applications of liquid organic hydrogen carriers[J]. Journal of Beijing University of Chemical Technology (Natural Science Edition), 2021, 48(4): 1-18. | |
| 17 | 王亚雄, 钟顺彬, 孙逢春. 车载高质量密度固态储氢材料研究进展[J]. 稀有金属, 2022, 46(6): 796-812. |
| WANG Yaxiong, ZHONG Shunbin, SUN Fengchun. Research progress in vehicular high mass density solid hydrogen storage materials[J]. Chinese Journal of Rare Metals, 2022, 46(6): 796-812. | |
| 18 | MORAWA Eblagon Katarzyna, Kin TAM, YU K M Kerry,et al. Study of catalytic sites on ruthenium for hydrogenation of N-ethylcarbazole: Implications of hydrogen storage via reversible catalytic hydrogenation[J]. The Journal of Physical Chemistry C, 2010, 114(21): 9720-9730. |
| 19 | TEICHMANN Daniel, ARLT Wolfgang, WASSERSCHEID Peter, et al. A future energy supply based on liquid organic hydrogen carriers (LOHC)[J]. Energy & Environmental Science, 2011, 4(8): 2767-2773. |
| 20 | Katarína SISÁKOVÁ, Natália PODROJKOVÁ, Renáta ORIŇAKOVÁ, et al. Novel catalysts for dibenzyltoluene as a potential liquid organic hydrogen carrier use—A mini-review[J]. Energy & Fuels, 2021, 35(9): 7608-7623. |
| 21 | LIU Hu, XUE Jingwen, YU Pengfei, et al. Hydrogenation of N-ethylcarbazole with hydrogen-methane mixtures for hydrogen storage[J]. Fuel, 2023, 331: 125920. |
| 22 | FENG Zhaolu, CHEN Xiaomin, BAI Xuefeng. Catalytic dehydrogenation of liquid organic hydrogen carrier dodecahydro-N-ethylcarbazole over palladium catalysts supported on different supports[J]. Environmental Science and Pollution Research, 2020, 27(29): 36172-36185. |
| 23 | MODISHA Phillimon M, OUMA Cecil N M, GARIDZIRAI Rudaviro, et al. The prospect of hydrogen storage using liquid organic hydrogen carriers[J]. Energy & Fuels, 2019, 33(4): 2778-2796. |
| 24 | TANG Chenxi, FENG Zhaolu, BAI Xuefeng. Magnetic N-doped partially graphitized carbon-loaded Pd-Co alloy nanoparticles for efficient hydrogen production[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2022, 648: 129348. |
| 25 | DING Yuhang, DONG Yuan, ZHANG Heshun, et al. A highly adaptable Ni catalyst for liquid organic hydrogen carriers hydrogenation[J]. International Journal of Hydrogen Energy, 2021, 46(53): 27026-27036. |
| 26 | TAKISE Kent, SATO Ayaka, MURAKAMI Kota, et al. Irreversible catalytic methylcyclohexane dehydrogenation by surface protonics at low temperature[J]. RSC Advances, 2019, 9(11): 5918-5924. |
| 27 | TAKISE Kent, SATO Ayaka, Shuhei OGO, et al. Low-temperature selective catalytic dehydrogenation of methylcyclohexane by surface protonics[J]. RSC Advances, 2019, 9(48): 27743-27748. |
| 28 | KOSAKA Misato, HIGO Takuma, Shuhei OGO, et al. Low-temperature selective dehydrogenation of methylcyclohexane by surface protonics over Pt/anatase-TiO2 catalyst[J]. International Journal of Hydrogen Energy, 2020, 45(1): 738-743. |
| 29 | WANG Weiyan, MIAO Lei, WU Kui, et al. Hydrogen evolution in the dehydrogenation of methylcyclohexane over Pt/CeMgAlO catalysts derived from their layered double hydroxides[J]. International Journal of Hydrogen Energy, 2019, 44(5): 2918-2925. |
| 30 | PARK Sanghyoun, NASEEM Mujahid, LEE Sangyong. Experimental assessment of perhydro-dibenzyltoluene dehydrogenation reaction kinetics in a continuous flow system for stable hydrogen supply[J]. Materials, 2021, 14(24): 7613. |
| 132 | SOTOODEH Farnaz, HUBER Benjamin J M, SMITH Kevin J. The effect of the N atom on the dehydrogenation of heterocycles used for hydrogen storage[J]. Applied Catalysis A: General, 2012, 419/420: 67-72. |
| 31 | CHEN Ximeng, GIERLICH Christian H, Simon SCHÖTZ, et al. Hydrogen production based on liquid organic hydrogen carriers through sulfur doped platinum catalysts supported on TiO2 [J]. ACS Sustainable Chemistry & Engineering, 2021, 9(19): 6561-6573. |
| 32 | DÜRR S, ZILM S, GEIßELBRECHT M, et al. Experimental determination of the hydrogenation/dehydrogenation—Equilibrium of the LOHC system H0/H18-dibenzyltoluene[J]. International Journal of Hydrogen Energy, 2021, 46(64): 32583-32594. |
| 33 | Ahsan ALI, Udaya KUMAR G, LEE Hee Joon. Investigation of hydrogenation of dibenzyltoluene as liquid organic hydrogen carrier[J]. Materials Today: Proceedings, 2021, 45: 1123-1127. |
| 34 | KIM Chan Hun, LEE Min-Woo, JANG Ji Soo, et al. Enhanced activity of a WO x -incorporated Pt/Al2O3 catalyst for the dehydrogenation of homocyclic LOHCs: Effects of impregnation sequence on Pt-WO x interactions[J]. Fuel, 2022, 313: 122654. |
| 35 | Nicole BRÜCKNER, OBESSER Katharina, Andreas BÖSMANN, et al. Evaluation of industrially applied heat-transfer fluids as liquid organic hydrogen carrier systems[J]. ChemSusChem, 2014, 7(1): 229-235. |
| 36 | LEINWEBER Anatol, Karsten MÜLLER. Hydrogenation of the liquid organic hydrogen carrier compound monobenzyl toluene: Reaction pathway and kinetic effects[J]. Energy Technology, 2018, 6(3): 513-520. |
| 37 | JORSCHICK H, GEIßELBRECHT M, EßL M, et al. Benzyltoluene/dibenzyltoluene-based mixtures as suitable liquid organic hydrogen carrier systems for low temperature applications[J]. International Journal of Hydrogen Energy, 2020, 45(29): 14897-14906. |
| 38 | KIM Tae Wan, KIM Minseok, KIM Seok Ki, et al. Remarkably fast low-temperature hydrogen storage into aromatic benzyltoluenes over MgO-supported Ru nanoparticles with homolytic and heterolytic H2 adsorption[J]. Applied Catalysis B: Environmental, 2021, 286: 119889. |
| 39 | Timo RÜDE, Stefan DÜRR, PREUSTER Patrick, et al. Benzyltoluene/perhydro benzyltoluene-pushing the performance limits of pure hydrocarbon liquid organic hydrogen carrier (LOHC) systems[J]. Sustainable Energy & Fuels, 2022, 6(6): 1541-1553. |
| 40 | FENG Zhaolu, WANG Yindong, BAI Xuefeng. Preparation of highly dispersed Pd/SBA-15 catalysts for dodecahydro-N-ethylcarbazole dehydrogenation reaction by ion exchange-glow discharge[J]. Environmental Science and Pollution Research, 2022, 29(26): 39266-39280. |
| 41 | YU Hongen, YANG Xue, JIANG Xiaojing, et al. LaNi5.5 particles for reversible hydrogen storage in N-ethylcarbazole[J]. Nano Energy, 2021, 80: 105476. |
| 42 | XUE Wenjie, LIU Hongxia, MAO Baohua, et al. Reversible hydrogenation and dehydrogenation of N-ethylcarbazole over bimetallic Pd-Rh catalyst for hydrogen storage[J]. Chemical Engineering Journal, 2021, 421: 127781. |
| 43 | LIU Honglei, ZHOU Cunhui, LI Wenqian, et al. Ultralow Rh bimetallic catalysts with high catalytic activity for the hydrogenation of N-ethylcarbazole[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(15): 5260-5267. |
| 44 | DING Chenghan, ZHU Ting, WANG Fanyi, et al. High active Pd@mil-101 catalyst for dehydrogenation of liquid organic hydrogen carrier[J]. International Journal of Hydrogen Energy, 2020, 45(32): 16144-16152. |
| 45 | STEINHAUER J, BACHMANN P, FREIBERGER E M, et al. Model catalytic studies of liquid organic hydrogen carriers: Indole/indoline/octahydroindole on Ni(111)[J]. The Journal of Physical Chemistry C, 2020, 124(41): 22559-22567. |
| 46 | CHEN Zhiwen, YANG Ming, ZHU Ting, et al. 7-Ethylindole: A new efficient liquid organic hydrogen carrier with fast kinetics[J]. International Journal of Hydrogen Energy, 2018, 43(28): 12688-12696. |
| 47 | PREUSTER Patrick, PAPP Christian, WASSERSCHEID Peter. Liquid organic hydrogen carriers (LOHCs): Toward a hydrogen-free hydrogen economy[J]. Accounts of Chemical Research, 2017, 50(1): 74-85. |
| 48 | Karsten MÜLLER, STARK Katharina, EMEL’YANENKO Vladimir N, et al. Liquid organic hydrogen carriers: Thermophysical and thermochemical studies of benzyl- and dibenzyl-toluene derivatives[J]. Industrial & Engineering Chemistry Research, 2015, 54(32): 7967-7976. |
| 49 | Karsten MÜLLER, Johannes VÖLKL, ARLT Wolfgang. Thermodynamic evaluation of potential organic hydrogen carriers[J]. Energy Technology, 2013, 1(1): 20-24. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [6] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [7] | 杨建平. 降低HPPO装置反应系统原料消耗的PSE[J]. 化工进展, 2023, 42(S1): 21-32. |
| [8] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [9] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [10] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [11] | 葛全倩, 徐迈, 梁铣, 王凤武. MOFs材料在光电催化领域应用的研究进展[J]. 化工进展, 2023, 42(9): 4692-4705. |
| [12] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [13] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [14] | 史柯柯, 刘木子, 赵强, 李晋平, 刘光. 镁基储氢材料的性能及研究进展[J]. 化工进展, 2023, 42(9): 4731-4745. |
| [15] | 刘木子, 史柯柯, 赵强, 李晋平, 刘光. 固体储氢材料的研究进展[J]. 化工进展, 2023, 42(9): 4746-4769. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||