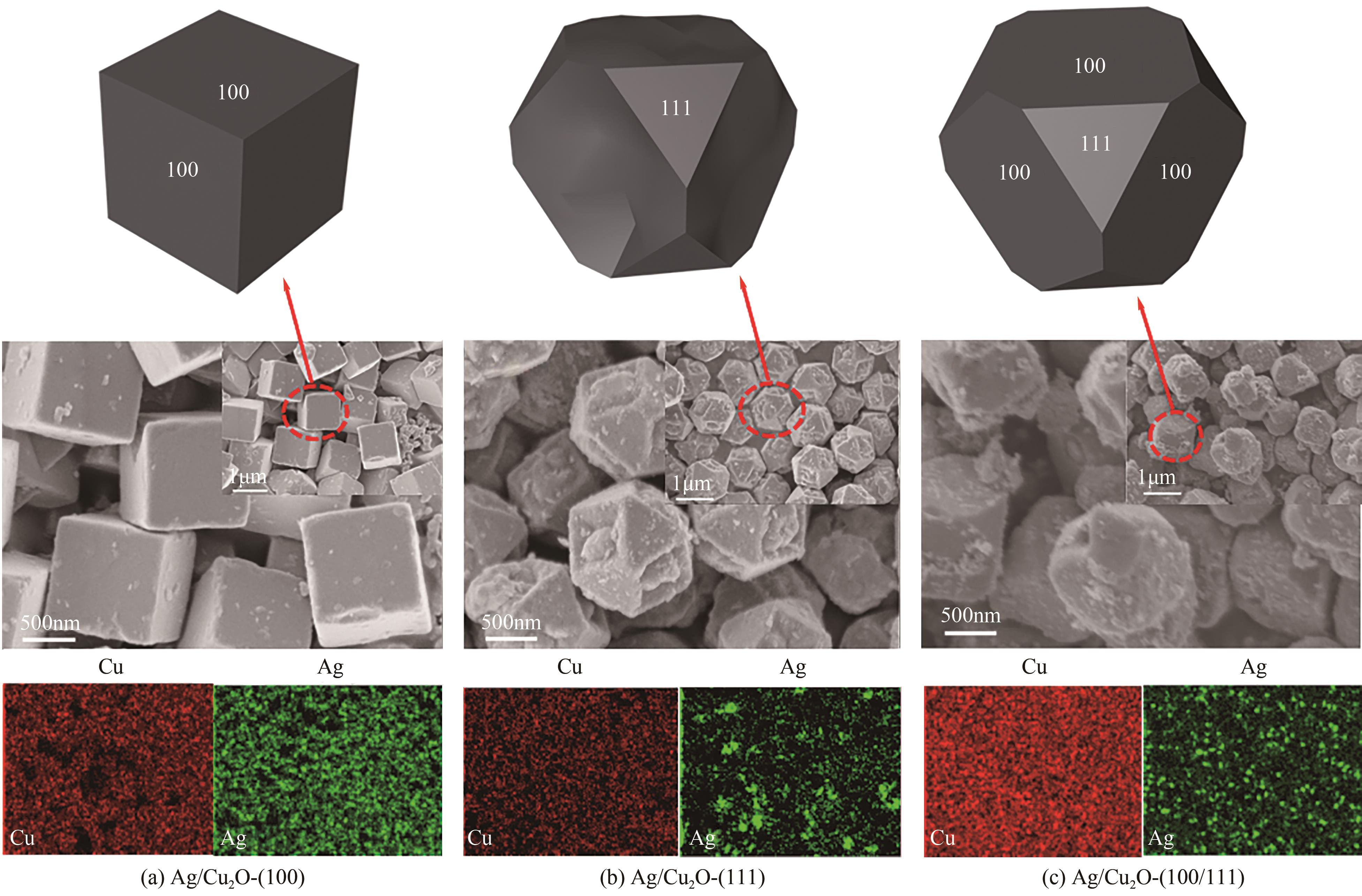
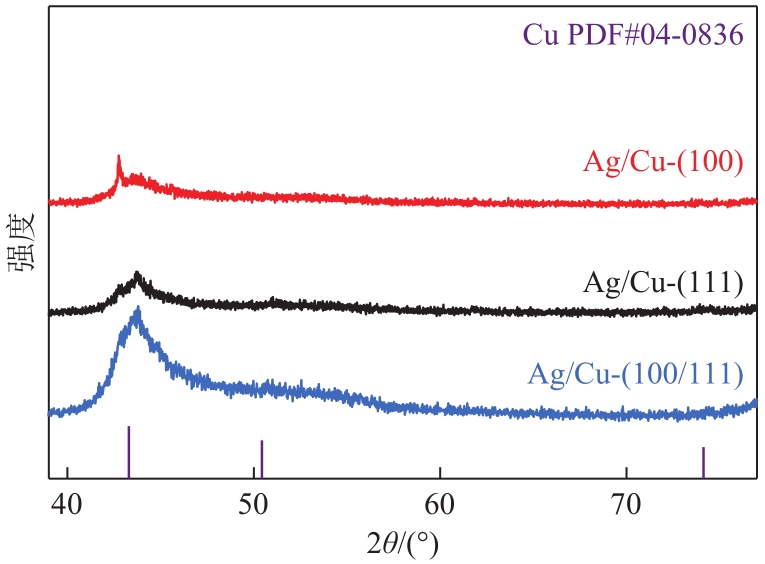
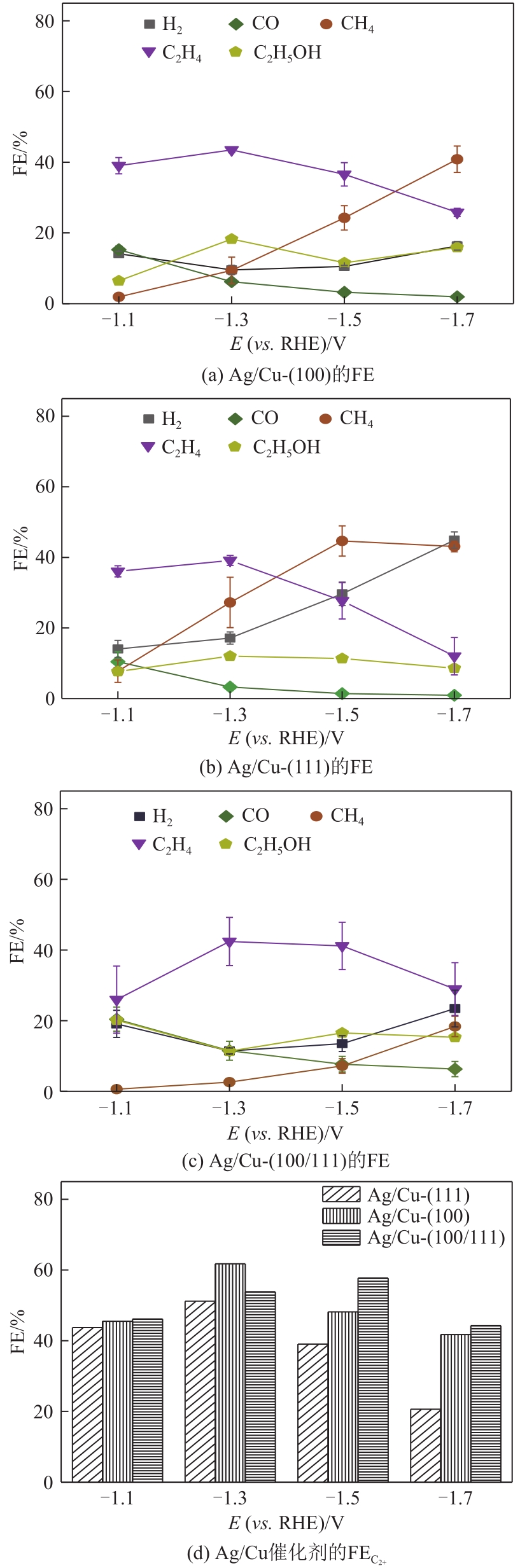
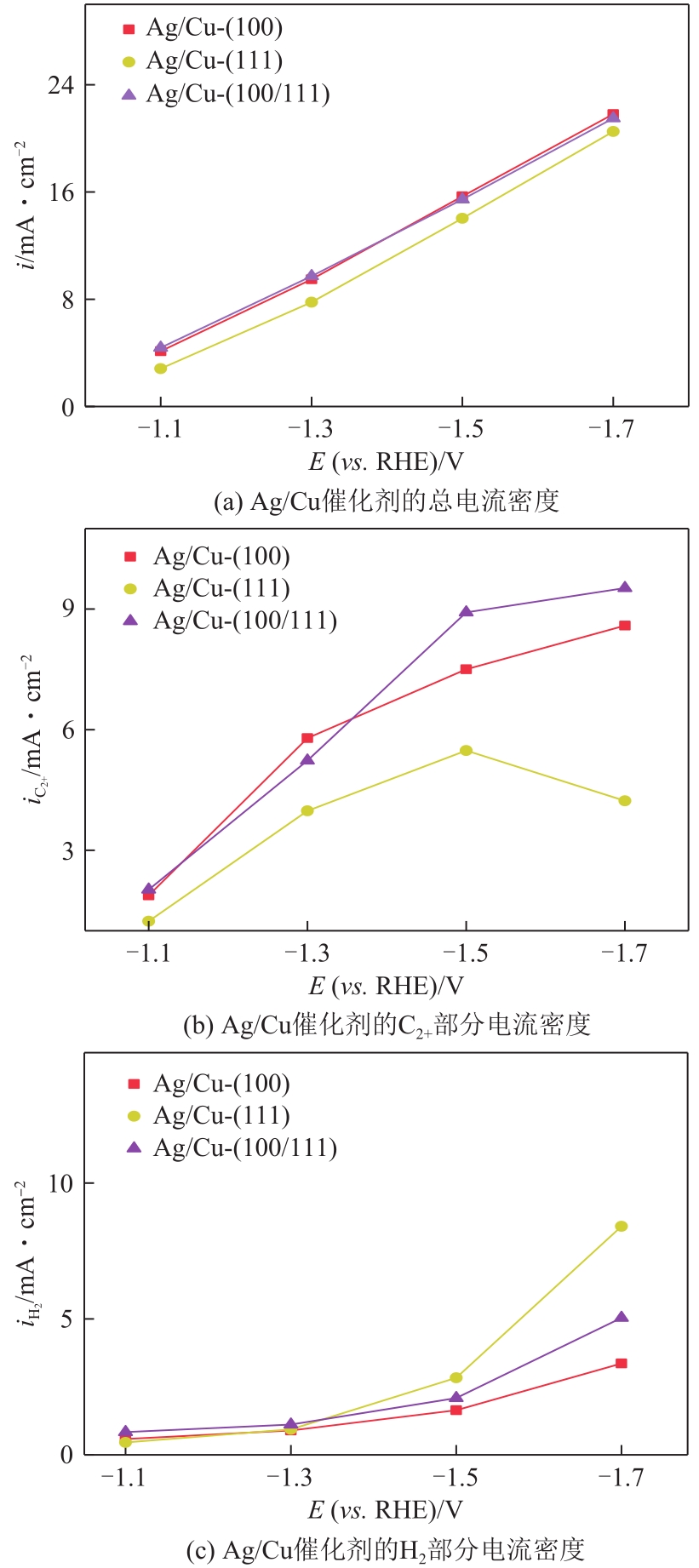
| 1 |
BAKER H S, MILLAR R J, KAROLY D J, et al. Higher CO2 concentrations increase extreme event risk in a 1.5℃ world[J]. Nature Climate Change, 2018, 8(7): 604-608.
|
| 2 |
ROGELJ J, HUPPMANN D, KREY V, et al. A new scenario logic for the Paris Agreement long-term temperature goal[J]. Nature, 2019, 573(7774): 357-363.
|
| 3 |
SHAKUN J D, CLARK P U, HE F, et al. Global warming preceded by increasing carbon dioxide concentrations during the last deglaciation[J]. Nature, 2012, 484(7392): 49-54.
|
| 4 |
HOU L, HAN J Y, WANG C, et al. Ag nanoparticle embedded Cu nanoporous hybrid arrays for the selective electrocatalytic reduction of CO2 towards ethylene[J]. Inorganic Chemistry Frontiers, 2020, 7(10): 2097-2106.
|
| 5 |
BIRDJA Y Y, PEREZ G E, FIGUEIREDO M C, et al. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels[J]. Nature Energy, 2019, 4(9): 732-745.
|
| 6 |
JONES J P, PRAKASH G K S, OLAH G A. Electrochemical CO2 Reduction: Recent advances and current trends[J]. Israel Journal of Chemistry, 2014, 54(10): 1451-1466.
|
| 7 |
KIBRIA M G, EDWARDS J P, GABARDO C M, et al. Electrochemical CO2 reduction into chemical feedstocks: From mechanistic electrocatalysis models to system design[J]. Advanced Materials, 2019, 31(31): 1807166.
|
| 8 |
GAO D F, ARAN A R, JEON H S, et al. Rational catalyst and electrolyte design for CO2 electroreduction towards multicarbon products[J]. Nature Catalysis, 2019, 2(3): 198-210.
|
| 9 |
NITOPI S, BERTHEUSSEN E, SCOTT S B, et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte[J]. Chemical Reviews, 2019, 119(12): 7610-7672.
|
| 10 |
QIAO J L, LIU Y Y, HONG F, et al. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels[J]. Chemical Society Reviews, 2014, 43(2): 631-675.
|
| 11 |
RACITI D, WANG C. Recent advances in CO2 reduction electrocatalysis on copper[J]. ACS Energy Letters, 2018, 3(7): 1545-1556.
|
| 12 |
WANG P T, YANG H, XU Y, et al. Synergized Cu/Pb core/shell electrocatalyst for high-efficiency CO2 reduction to C2+ liquids[J]. ACS Nano, 2020, 15(1): 1039-1047.
|
| 13 |
SU X S, SUN Y M, JIN L, et al. Hierarchically porous Cu/Zn bimetallic catalysts for highly selective CO2 electroreduction to liquid C2 products[J]. Applied Catalysis B: Environmental, 2020, 269: 118800.
|
| 14 |
ZENG J Q, FIORENTIN M R, FONTANA M, et al. Novel insights into Sb-Cu catalysts for electrochemical reduction of CO2 [J]. Applied Catalysis B: Environmental, 2022, 306: 121089.
|
| 15 |
HU H J, TANG Y, HU Q, et al. In-situ grown nanoporous Zn-Cu catalysts on brass foils for enhanced electrochemical reduction of carbon dioxide[J]. Applied Surface Science, 2018, 445: 281-286.
|
| 16 |
GAO D F, ZHANG Y, ZHOU Z W, et al. Enhancing CO2 electroreduction with the metal-oxide interface[J]. Journal of the American Chemical Society, 2017, 139(16): 5652-5655.
|
| 17 |
CHU S L, YAN X P, CHOI C, et al. Stabilization of Cu+ by tuning a CuO-CeO2 interface for selective electrochemical CO2 reduction to ethylene[J]. Green Chemistry, 2020, 22(19): 6540-6546.
|
| 18 |
LI Q, ZHU W L, FU J J, et al. Controlled assembly of Cu nanoparticles on pyridinic-N rich graphene for electrochemical reduction of CO2 to ethylene[J]. Nano Energy, 2016, 24: 1-9.
|
| 19 |
YIN Z Y, YU C, ZHAO Z L, et al. Cu3N nanocubes for selective electrochemical reduction of CO2 to ethylene[J]. Nano Letters, 2019, 19(12): 8658-8663.
|
| 20 |
YANG D X, ZHU Q G, SUN X F, et al. Nanoporous Cu/Ni oxide composites: Efficient catalysts for electrochemical reduction of CO2 in aqueous electrolytes[J]. Green Chemistry, 2018, 20(16): 3705-3710.
|
| 21 |
GUO S X, LI F W, CHEN L, et al. Polyoxometalate-promoted electrocatalytic CO2 reduction at nanostructured silver in dimethylformamide[J]. ACS Applied Materials & Interfaces, 2018, 10(15): 12690-12697.
|
| 22 |
LI C W, CISTON J, KANAN M W. Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper[J]. Nature, 2014, 508(7497): 504-507.
|
| 23 |
WANG J Q, LI Z, DONG C K, et al. Silver/copper interface for relay electroreduction of carbon dioxide to ethylene[J]. ACS Applied Materials & Interfaces, 2019, 11(3): 2763-2767.
|
| 24 |
ZHONG Y Z, KONG X D, SONG Z M, et al. Adjusting local CO confinement in porous-shell Ag@Cu catalysts for enhancing C—C coupling toward CO2 eletroreduction[J]. Nano Letters, 2022, 22(6): 2554-2560.
|
| 25 |
KIBRIA M G, DINH C T, SEIFITOKALDANI A, et al. A surface reconstruction route to high productivity and selectivity in CO2 electroreduction toward C2+ hydrocarbons[J]. Advanced Materials, 2018, 30(49): 1804867.
|
| 26 |
WU Z Z, ZHANG X L, NIU Z Z, et al. Identification of Cu(100)/Cu(111) interfaces as superior active sites for CO dimerization during CO2 electroreduction[J]. Journal of the American Chemical Society, 2021, 144(1): 259-269.
|
| 27 |
MARKOSE K, SHAJI M, BHATIA S, et al. Novel boron-doped p-type Cu2O thin films as a hole-selective contact in C-Si solar cells[J]. ACS Applied Materials & Interfaces, 2020, 12(11): 12972-12981.
|
| 28 |
SUN Y G, MAYERS B, HERRICKS T, et al. Polyol synthesis of uniform silver nanowires: A plausible growth mechanism and the supporting evidence[J]. Nano Letters, 2003, 3(7): 955-960.
|
| 29 |
LI J Q, MEI Z X, LIU L S, et al. Probing defects in nitrogen-doped Cu2O[J]. Scientific Reports, 2014, 4(1): 7240.
|
| 30 |
ZHEN W L, JIAO W J, WU Y Q, et al. The role of a metallic copper interlayer during visible photocatalytic hydrogen generation over a Cu/Cu2O/Cu/TiO2 catalyst[J]. Catalysis Science & Technology, 2017, 7(21): 5028-5037.
|
| 31 |
SCHON G, TUMMAVUORI J, LINDSTROM B, et al. ESCA studies of Ag, Ag2O and AgO[J]. Acta Chem. Scand, 1973, 27(7): 2623.
|
| 32 |
ZHANG Y Z, WANG X Y, DONG P M, et al. TiO2 surfaces self-doped with Ag nanoparticles exhibit efficient CO2 photoreduction under visible light[J]. RSC Advances, 2018, 8(29): 15991-15998.
|
| 33 |
LIU B Q, YAO X, ZHANG Z J, et al. Synthesis of Cu2O nanostructures with tunable crystal facets for electrochemical CO2 reduction to alcohols[J]. ACS Applied Materials & Interfaces, 2021, 13(33): 39165-39177.
|
 ), 房强1,2, 钟达忠1,2, 赵强1,2(
), 房强1,2, 钟达忠1,2, 赵强1,2( ), 李晋平1,2(
), 李晋平1,2( )
)
 ), FANG Qiang1,2, ZHONG Dazhong1,2, ZHAO Qiang1,2(
), FANG Qiang1,2, ZHONG Dazhong1,2, ZHAO Qiang1,2( ), LI Jinping1,2(
), LI Jinping1,2( )
)