化工进展 ›› 2023, Vol. 42 ›› Issue (5): 2702-2716.DOI: 10.16085/j.issn.1000-6613.2022-1239
退役锂电池正极材料资源化回收技术研究进展
王昊1,2,3( ), 霍进达2,3, 曲国瑞1,2,3, 杨家琪1,2, 周世伟1,2,3, 李博1,2,3, 魏永刚1,2,3(
), 霍进达2,3, 曲国瑞1,2,3, 杨家琪1,2, 周世伟1,2,3, 李博1,2,3, 魏永刚1,2,3( )
)
- 1.昆明理工大学省部共建复杂有色金属资源清洁利用国家重点实验室,云南 昆明 650093
2.昆明理工大学冶金 节能减排教育部工程研究中心,云南 昆明 650093
3.昆明理工大学冶金与能源工程学院,云南 昆明 650093
-
收稿日期:2022-07-04修回日期:2022-08-25出版日期:2023-05-10发布日期:2023-06-02 -
通讯作者:魏永刚 -
作者简介:王昊(1997—),男,硕士研究生,主要研究方向为废旧锂离子电池资源化回收。E-mail:haowang970126@163.com。 -
基金资助:国家自然科学基金(52064033);云南省重点基础研究发展计划(202101AS070025)
Research progress of positive electrode material recycling technology for retired lithium batteries
WANG Hao1,2,3( ), HUO Jinda2,3, QU Guorui1,2,3, YANG Jiaqi1,2, ZHOU Shiwei1,2,3, LI Bo1,2,3, WEI Yonggang1,2,3(
), HUO Jinda2,3, QU Guorui1,2,3, YANG Jiaqi1,2, ZHOU Shiwei1,2,3, LI Bo1,2,3, WEI Yonggang1,2,3( )
)
- 1.State Key Laboratory of Complex Nonferrous Metal Resources Clean Utilization, Kunming University of Science and Technology, Kunming 650093, Yunnan, China
2.Engineering Research Center of Metallurgical Energy Conservation and Emission Reduction of Ministry of Education, Kunming University of Science and Technology, Kunming 650093, Yunnan, China
3.Faculty of Metallurgy and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, Yunnan, China
-
Received:2022-07-04Revised:2022-08-25Online:2023-05-10Published:2023-06-02 -
Contact:WEI Yonggang
摘要:
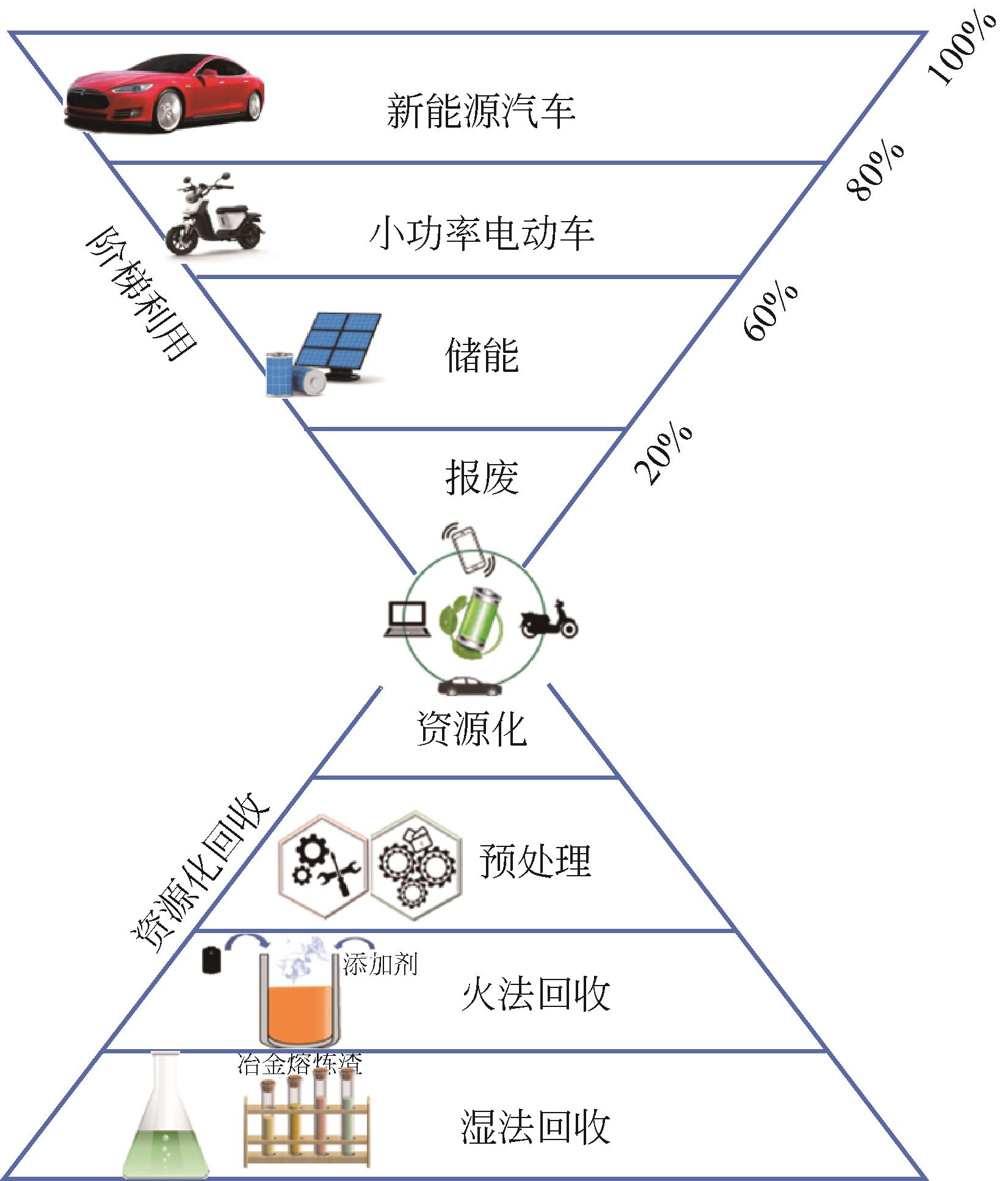
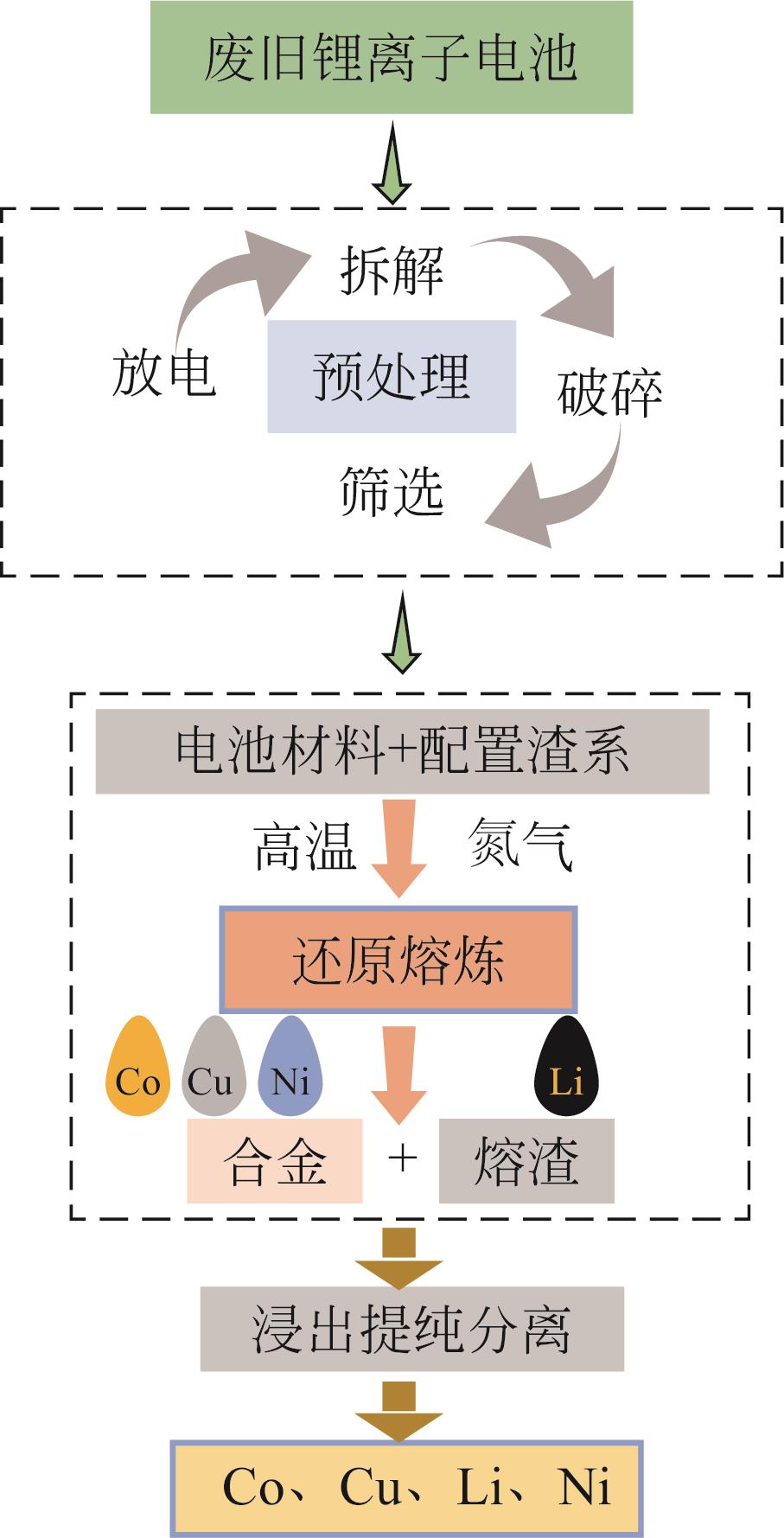
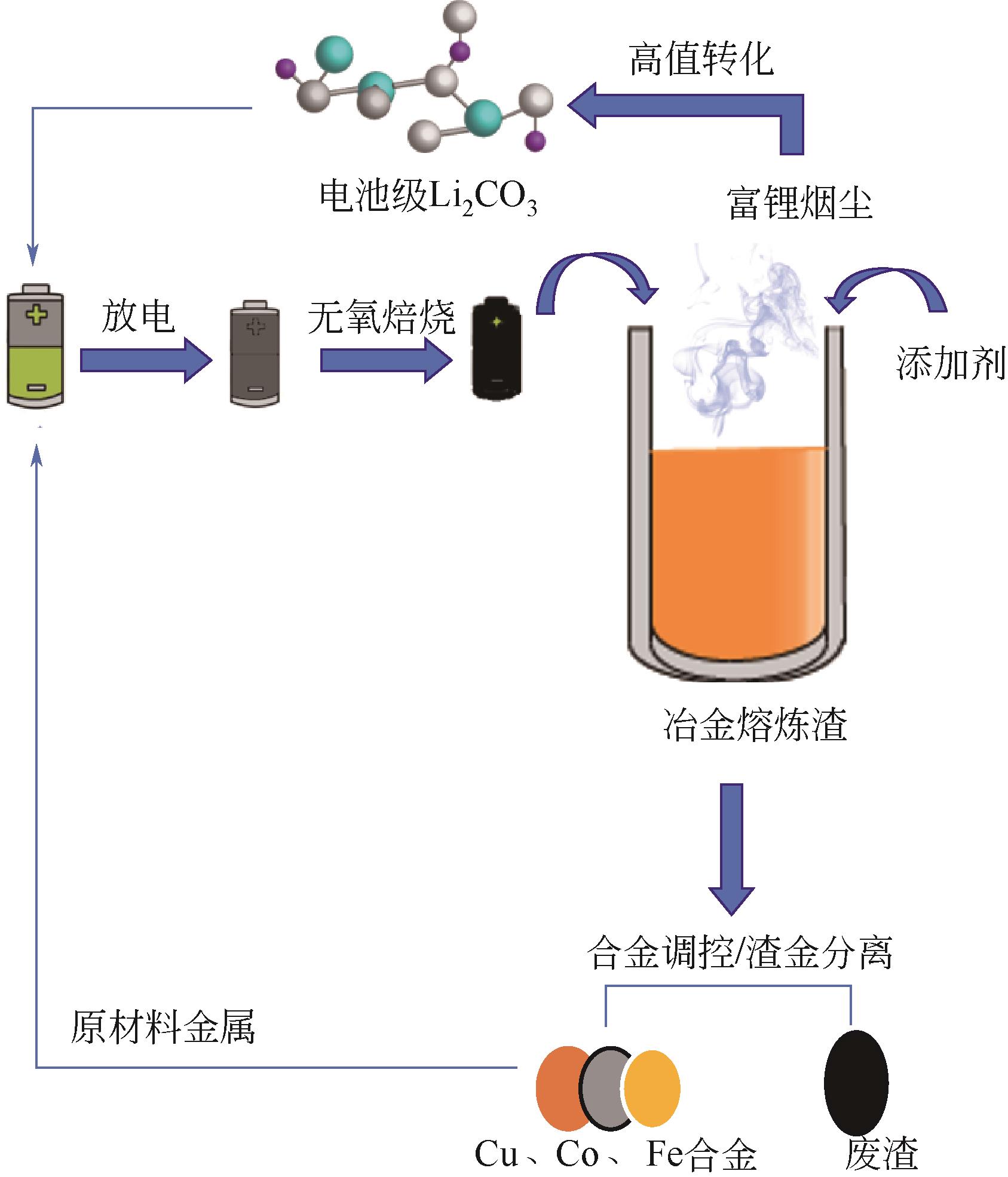
新能源汽车产业快速发展带动锂离子电池消费不断增加,直接导致用于生产电池材料的钴、锂、镍等能源金属严重短缺。未来退役锂离子电池产量将呈指数增加,其资源化回收受到广泛关注。资源化回收不仅可以缓解电池材料紧缺现状,还解决了废旧电池堆积而引起的危害。本文针对退役锂离子电池放电预处理和湿法、火法两种资源化回收工艺最新研究现状进行了综述,并就未来发展趋势进行了讨论。在现有火法回收工艺基础上提出一种利用高温熔融冶炼渣处理废旧锂离子电池回收有价金属的新方法,通过添加适宜的氯化剂将渣中锂转化为高温易挥发的LiCl,实现从烟尘中富集并高效回收锂的新思路,解决了传统火法工艺需从渣中对锂进行二次提取的技术缺陷。
中图分类号:
引用本文
王昊, 霍进达, 曲国瑞, 杨家琪, 周世伟, 李博, 魏永刚. 退役锂电池正极材料资源化回收技术研究进展[J]. 化工进展, 2023, 42(5): 2702-2716.
WANG Hao, HUO Jinda, QU Guorui, YANG Jiaqi, ZHOU Shiwei, LI Bo, WEI Yonggang. Research progress of positive electrode material recycling technology for retired lithium batteries[J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2702-2716.
| 成分 | 所用材料 | 成本占比/% | 化学特性 | 潜在危害性 |
|---|---|---|---|---|
| 正极材料 | 钴酸锂/锰酸锂/镍酸锂/磷酸铁锂 | 30~35 | 与水、酸、还原剂或强氧化剂发生反应 | 重金属污染 |
| 负极材料 | 碳材料/石墨 | 10~15 | 遇明火或高温易爆炸 | 粉尘污染 |
| 电解质 | LiPF6/LiBF4/LiAsF6 | 10~15 | 强腐蚀性,遇水生成HF,氧化产生P2O5等有毒物质 | 氟污染,有害气体污染 |
| 电解质溶剂 | 碳酸乙烯酯/碳酸二甲酯 | 10~15 | 水解产生醛和酸,燃烧产生CO、CO2等 | 有机物污染 |
| 隔膜材料 | 聚丙烯/聚乙烯 | 20~30 | 燃烧可产生CO、醛等 | 有机物污染 |
| 黏合剂 | 聚偏氟乙烯/偏氟乙烯 | 10~15 | 受热分解产生HF | 氟污染 |
表1 锂离子电池主要组成成分的化学特性及对环境的潜在危害性[11]
| 成分 | 所用材料 | 成本占比/% | 化学特性 | 潜在危害性 |
|---|---|---|---|---|
| 正极材料 | 钴酸锂/锰酸锂/镍酸锂/磷酸铁锂 | 30~35 | 与水、酸、还原剂或强氧化剂发生反应 | 重金属污染 |
| 负极材料 | 碳材料/石墨 | 10~15 | 遇明火或高温易爆炸 | 粉尘污染 |
| 电解质 | LiPF6/LiBF4/LiAsF6 | 10~15 | 强腐蚀性,遇水生成HF,氧化产生P2O5等有毒物质 | 氟污染,有害气体污染 |
| 电解质溶剂 | 碳酸乙烯酯/碳酸二甲酯 | 10~15 | 水解产生醛和酸,燃烧产生CO、CO2等 | 有机物污染 |
| 隔膜材料 | 聚丙烯/聚乙烯 | 20~30 | 燃烧可产生CO、醛等 | 有机物污染 |
| 黏合剂 | 聚偏氟乙烯/偏氟乙烯 | 10~15 | 受热分解产生HF | 氟污染 |
| 材料 | 钴酸锂电池(LCO) /% | 锰酸锂电池(LMO) /% | 镍、锰、钴三元电池(NMC) /% | 磷酸铁锂电池(LFP) /% | 镍钴铝三元电池(NCA) /% |
|---|---|---|---|---|---|
| 活性正极材料 | 35.3 | 40.8 | 34.7 | 32.7 | 30.6 |
| 石墨 | 20.9 | 16.8 | 21.7 | 19 | 24.2 |
| 黏结剂 | 3.0 | 3.0 | 3.0 | 2.7 | 2.9 |
| 铜 | 16.1 | 15.0 | 15.7 | 13.9 | 16.7 |
| 铝 | 8.1 | 7.8 | 8.2 | 7.5 | 8.6 |
| 电解液 | 14.2 | 14.4 | 14.6 | 22.2 | 14.9 |
| 其他(塑料隔膜) | 2.4 | 2.1 | 2.1 | 1.9 | 2.3 |
表2 不同种类锂电池各种材料占比[15]
| 材料 | 钴酸锂电池(LCO) /% | 锰酸锂电池(LMO) /% | 镍、锰、钴三元电池(NMC) /% | 磷酸铁锂电池(LFP) /% | 镍钴铝三元电池(NCA) /% |
|---|---|---|---|---|---|
| 活性正极材料 | 35.3 | 40.8 | 34.7 | 32.7 | 30.6 |
| 石墨 | 20.9 | 16.8 | 21.7 | 19 | 24.2 |
| 黏结剂 | 3.0 | 3.0 | 3.0 | 2.7 | 2.9 |
| 铜 | 16.1 | 15.0 | 15.7 | 13.9 | 16.7 |
| 铝 | 8.1 | 7.8 | 8.2 | 7.5 | 8.6 |
| 电解液 | 14.2 | 14.4 | 14.6 | 22.2 | 14.9 |
| 其他(塑料隔膜) | 2.4 | 2.1 | 2.1 | 1.9 | 2.3 |
| 方法 | 机理 | 优点 | 缺点 | 浸出溶液 | 参考文献 |
|---|---|---|---|---|---|
| 酸浸出 | |||||
| 无机酸浸出 | 利用溶液的酸特性,氢离子溶解并与电池材料发生反应,使电池材料中的有价金属成分转移到浸出液中 | 浸出速度快、浸出效率高、成本低、适用面广 | 会产生有毒废气以及酸性废水、对设备要求较高 | 盐酸、硫酸、磷酸、硝酸等 | [ |
| 有机酸浸出 | 更加绿色环保、减少废气和废水的排放 | 浸出不完全、浸出速度慢、有机酸成本较高 | 柠檬酸、抗坏血酸、草酸、苹果酸等 | [ | |
| 碱浸出 | 利用溶液中强碱性环境下氨根离子与金属离子之间的相互络合作用 | 浸出后不会产生强酸废水、低毒性和高选择性 | 成本较高、浸出速率较慢 | 硫酸铵、碳酸铵、氯化铵、氨水溶液 | [ |
| 生物浸出 | 通过微生物代谢产生酸,将不溶性金属氧化物转化为可溶金属离子 | 绿色环保无污染 | 浸出效率低、反应过程缓慢、周期长、耗时久 | 硫氧化铁杆菌、黑曲霉等 | [ |
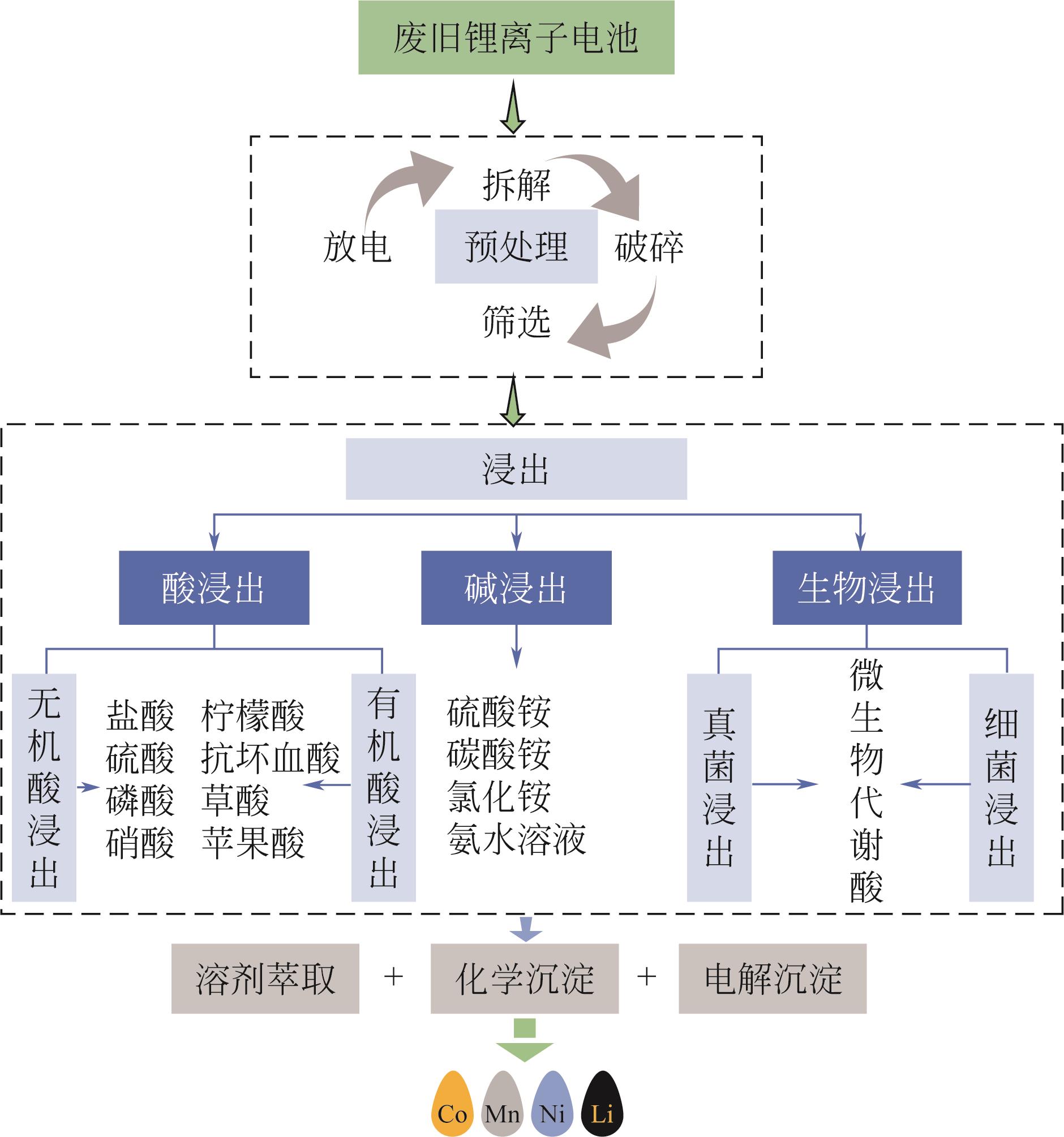
表3 不同湿法浸出方法的介绍
| 方法 | 机理 | 优点 | 缺点 | 浸出溶液 | 参考文献 |
|---|---|---|---|---|---|
| 酸浸出 | |||||
| 无机酸浸出 | 利用溶液的酸特性,氢离子溶解并与电池材料发生反应,使电池材料中的有价金属成分转移到浸出液中 | 浸出速度快、浸出效率高、成本低、适用面广 | 会产生有毒废气以及酸性废水、对设备要求较高 | 盐酸、硫酸、磷酸、硝酸等 | [ |
| 有机酸浸出 | 更加绿色环保、减少废气和废水的排放 | 浸出不完全、浸出速度慢、有机酸成本较高 | 柠檬酸、抗坏血酸、草酸、苹果酸等 | [ | |
| 碱浸出 | 利用溶液中强碱性环境下氨根离子与金属离子之间的相互络合作用 | 浸出后不会产生强酸废水、低毒性和高选择性 | 成本较高、浸出速率较慢 | 硫酸铵、碳酸铵、氯化铵、氨水溶液 | [ |
| 生物浸出 | 通过微生物代谢产生酸,将不溶性金属氧化物转化为可溶金属离子 | 绿色环保无污染 | 浸出效率低、反应过程缓慢、周期长、耗时久 | 硫氧化铁杆菌、黑曲霉等 | [ |
| 种类 | 浸出剂 | 添加剂 | 回收率 | 参考文献 |
|---|---|---|---|---|
| 无机酸浸出 | 4.0mol/L HCl | 4.0% H2O2 | 100% Co、99.99% Ni | [ |
| 2mol/L H2SO4 | 8.0% H2O2 | 98% Co | [ | |
| 0.7mol/L H3PO4 | 4.0% H2O2 | 99.7 Co、99.9% Li | [ | |
| 2.5mol/L H2SO4 | 无 | 97% Li | [ | |
| 有机酸浸出 | 1.5mol/L C3H6O3 | 5.0% H2O2 | 98.90% Co、97.70% Li | [ |
| 1.25mol/L C6H8O6 | 无 | 98.50% Co、94.80% Li | [ | |
| 1.5mol/L C4H6O4 | 4.0% H2O2 | 100% Co、96.% Li | [ | |
| 1.5mol/L C4H6O4 | 2.0% H2O2 | 93.% Co、99% Li | [ | |
| 1.5mol/L C4H7NO4 | 4.0% H2O2 | 60.00% Co、Li | [ | |
| 2.0mol/L C6H8O7 | 1.0% H2O2 | 81.50% Co、92.35% Li | [ | |
| 1.25mol/L C4H6O6 | 0.9% H2O2 | 99.50% Co、90.20% Li | [ | |
| 碱浸出 | 1.5mol/L (NH4)2SO4 | 0.5mol/L Na2SO3 | 94.8% Ni、88.4% Co、96.7% Li | [ |
| 3.1mol/L NH4OH+1.5mol/L NH4HCO3 | 5.0% H2O2 | 99.28% Li、99.63% Ni、99.76% Co | [ | |
| 1mol/L NH3+0.5mol/L (NH4)2CO3 | 0.5mol/L (NH4)2SO3 | 100% Ni | [ | |
| 生物浸出 | 硫氧化细菌+铁氧化细菌 | 无 | 80% Co、73% Li | [ |
| 嗜酸硫氧化菌+铁氧化菌 | 无 | 60% Li、48% Ni、74% Cu、53% Co | [ | |
| 氧化亚铁硫杆菌+氧化硫杆菌 | 无 | 99% Li、89% Ni、50% Co | [ | |
| 黑曲霉 | 无 | 100% Li、82% Co | [ |
表4 湿法浸出电池正极活性材料成果展示
| 种类 | 浸出剂 | 添加剂 | 回收率 | 参考文献 |
|---|---|---|---|---|
| 无机酸浸出 | 4.0mol/L HCl | 4.0% H2O2 | 100% Co、99.99% Ni | [ |
| 2mol/L H2SO4 | 8.0% H2O2 | 98% Co | [ | |
| 0.7mol/L H3PO4 | 4.0% H2O2 | 99.7 Co、99.9% Li | [ | |
| 2.5mol/L H2SO4 | 无 | 97% Li | [ | |
| 有机酸浸出 | 1.5mol/L C3H6O3 | 5.0% H2O2 | 98.90% Co、97.70% Li | [ |
| 1.25mol/L C6H8O6 | 无 | 98.50% Co、94.80% Li | [ | |
| 1.5mol/L C4H6O4 | 4.0% H2O2 | 100% Co、96.% Li | [ | |
| 1.5mol/L C4H6O4 | 2.0% H2O2 | 93.% Co、99% Li | [ | |
| 1.5mol/L C4H7NO4 | 4.0% H2O2 | 60.00% Co、Li | [ | |
| 2.0mol/L C6H8O7 | 1.0% H2O2 | 81.50% Co、92.35% Li | [ | |
| 1.25mol/L C4H6O6 | 0.9% H2O2 | 99.50% Co、90.20% Li | [ | |
| 碱浸出 | 1.5mol/L (NH4)2SO4 | 0.5mol/L Na2SO3 | 94.8% Ni、88.4% Co、96.7% Li | [ |
| 3.1mol/L NH4OH+1.5mol/L NH4HCO3 | 5.0% H2O2 | 99.28% Li、99.63% Ni、99.76% Co | [ | |
| 1mol/L NH3+0.5mol/L (NH4)2CO3 | 0.5mol/L (NH4)2SO3 | 100% Ni | [ | |
| 生物浸出 | 硫氧化细菌+铁氧化细菌 | 无 | 80% Co、73% Li | [ |
| 嗜酸硫氧化菌+铁氧化菌 | 无 | 60% Li、48% Ni、74% Cu、53% Co | [ | |
| 氧化亚铁硫杆菌+氧化硫杆菌 | 无 | 99% Li、89% Ni、50% Co | [ | |
| 黑曲霉 | 无 | 100% Li、82% Co | [ |
| 1 | 刘彦龙. 中国锂离子电池产业发展现状及市场发展趋势[J]. 电源技术, 2019, 43(2): 181-187. |
| LIU Yanlong. Development status and market trend of China’s lithium-ion battery industry[J]. Chinese Journal of Power Sources, 2019, 43(2): 181-187. | |
| 2 | NATARAJAN Subramanian, ARAVINDAN Vanchiappan. Burgeoning prospects of spent lithium-ion batteries in multifarious applications[J]. Advanced Energy Materials, 2018, 8(33): 1802303. |
| 3 | SUN Jie, LI Jigang, ZHOU Tian, et al. Toxicity, a serious concern of thermal runaway from commercial Li-ion battery[J]. Nano Energy, 2016, 27: 313-319. |
| 4 | ZAGHIB K, DONTIGNY M, GUERFI A, et al. Safe and fast-charging Li-ion battery with long shelf life for power applications[J]. Journal of Power Sources, 2011, 196(8): 3949-3954. |
| 5 | LIU Xianming, LIU Guoping. Study of a lithium battery automatic shell control system algorithm[J]. Applied Mechanics and Materials, 2015, 738/739: 1039-1047. |
| 6 | JIANG Yong, LU Cai, LIU Xing, et al. Lithium acetate modified PU/graphene composites as separator for advanced Li-ion batteries[J]. Micro & Nano Letters, 2020, 15(4): 213-217. |
| 7 | 唐子威, 侯旭, 裴波, 等. 锂离子电池电解液研究进展[J]. 船电技术, 2017, 37(6): 14-15, 19. |
| TANG Ziwei, HOU Xu, PEI Bo, et al. Research progress of the electrolyte for lithium-ion battery[J]. Marine Electric & Electronic Engineering, 2017, 37(6): 14-15, 19. | |
| 8 | 梁波, 陈栩, 张帅. 水溶性羧甲基壳聚糖粘结剂在锂离子电池石墨负极中的应用[J]. 中国有色金属学报, 2018, 28(7): 1379-1386. |
| LIANG Bo, CHEN Xu, ZHANG Shuai. Application of water-soluble carboxymethyl chitosan as binder in graphite anode of lithium-ion battery[J]. The Chinese Journal of Nonferrous Metals, 2018, 28(7): 1379-1386. | |
| 9 | RABAH M A, FARGHALY F E, ABD-EL MOTALEB M A. Recovery of nickel, cobalt and some salts from spent Ni-MH batteries[J]. Waste Management, 2008, 28(7): 1159-1167. |
| 10 | 孙杰, 李吉刚, 党胜男, 等. 锂离子电池及其材料热失控毒物研究[J]. 储能科学与技术, 2015, 4(6): 609-615. |
| SUN Jie, LI Jigang, DANG Shengnan, et al. Research of toxic productions from thermal runaway processes of Li-ion battery and materials[J]. Energy Storage Science and Technology, 2015, 4(6): 609-615. | |
| 11 | 李洪枚, 姜亢. 废旧锂离子电池对环境污染的分析与对策[J]. 上海环境科学, 2004(5): 201-203, 230. |
| LI Hongmei, JIANG Kang. An analysis of waste lithium-ion battery contamination to environment and its countermeasures[J]. Shanghai Environmental Sciences, 2004(5): 201-203, 230. | |
| 12 | 朱景和, 陈新林, 马忠臣. 赞比亚某铜钴硫化矿浮选工艺研究[J]. 有色矿冶, 2014, 30(2): 13-17. |
| ZHU Jinghe, CHEN Xinlin, MA Zhongchen. A flotation process for a copper-cobalt sulfide ore from Zambia[J]. Non-Ferrous Mining and Metallurgy, 2014, 30(2): 13-17. | |
| 13 | GRATZ Eric, Qina SA, APELIAN Diran, et al. A closed loop process for recycling spent lithium ion batteries[J]. Journal of Power Sources, 2014, 262: 255-262. |
| 14 | QU Guorui, WEI Yonggang, LIU Cuiping, et al. Efficient separation and recovery of lithium through volatilization in the recycling process of spent lithium-ion batteries[J]. Waste Management, 2022, 150: 66-74. |
| 15 | Hayder ALI, KHAN Hassan A, PECHT Michael G. Circular economy of Li batteries: Technologies and trends[J]. Journal of Energy Storage, 2021, 40: 102690. |
| 16 | WANG Qingsong, JIANG Lihua, YU Yan, et al. Progress of enhancing the safety of lithium ion battery from the electrolyte aspect[J]. Nano Energy, 2019, 55: 93-114. |
| 17 | CARDARELLI F, DUBE J. Method for recycling spent lithium metal polymer rechargeable batteries and related materials: US07192564B2[P]. 2007-03-20. |
| 18 | HUA Yang, ZHOU Sida, HUANG Yi, et al. Sustainable value chain of retired lithium-ion batteries for electric vehicles[J]. Journal of Power Sources, 2020, 478: 228753. |
| 19 | OJANEN Severi, Mari LUNDSTRÖM, Annukka SANTASALO-AARNIO, et al. Challenging the concept of electrochemical discharge using salt solutions for lithium-ion batteries recycling[J]. Waste Management, 2018, 76: 242-249. |
| 20 | James SHAW-STEWART, Anna ALVAREZ-REGUERA, GRESZTA Agata, et al. Aqueous solution discharge of cylindrical lithium-ion cells[J]. Sustainable Materials and Technologies, 2019, 22: e00110. |
| 21 | ZHANG Tao, HE Yaqun, GE Linhan, et al. Characteristics of wet and dry crushing methods in the recycling process of spent lithium-ion batteries[J]. Journal of Power Sources, 2013, 240: 766-771. |
| 22 | KIM Seoa, BANG Jaeyeon, YOO Junsang, et al. A comprehensive review on the pretreatment process in lithium-ion battery recycling[J]. Journal of Cleaner Production, 2021, 294: 126329. |
| 23 | LI Jia, WANG Guangxu, XU Zhenming. Generation and detection of metal ions and volatile organic compounds (VOCs) emissions from the pretreatment processes for recycling spent lithium-ion batteries[J]. Waste Management, 2016, 52: 221-227. |
| 24 | 齐婷. 废旧锂离子电池的放电特性与电极材料的无氧焙烧还原回收[D]. 上海: 上海交通大学, 2017. |
| QI Ting. Discharge properties of waste lithium ion batteries and oxygen-free roasting to recycle electrode materials[D]. Shanghai: Shanghai Jiao Tong University, 2017. | |
| 25 | 宋秀玲, 戴书琪, 徐永胜, 等. 废旧锂离子电池放电的实验研究[J]. 应用化工, 2015, 44(4): 594-597. |
| SONG Xiuling, DAI Shuqi, XU Yongsheng, et al. Experimental study on the discharge of the waste lithium ion battery[J]. Applied Chemical Industry, 2015, 44(4): 594-597. | |
| 26 | XIAO Jiefeng, GUO Jie, ZHAN Lu, et al. A cleaner approach to the discharge process of spent lithium ion batteries in different solutions[J]. Journal of Cleaner Production, 2020, 255: 120064. |
| 27 | YAO Linpeng, ZENG Qi, QI Ting, et al. An environmentally friendly discharge technology to pretreat spent lithium-ion batteries[J]. Journal of Cleaner Production, 2020, 245: 118820. |
| 28 | 苏勇, 卢世杰, 周宏喜, 等. 废旧动力锂离子电池安全放电研究[J]. 中国资源综合利用, 2020, 38(10): 40-42, 52. |
| SU Yong, LU Shijie, ZHOU Hongxi, et al. Research on the safe discharge of waste power lithium-ion battery[J]. China Resources Comprehensive Utilization, 2020, 38(10): 40-42, 52. | |
| 29 | 谢英豪, 欧彦楠, 余海军, 等. 废旧车用动力电池安全放电研究[J]. 工业安全与环保, 2017, 43(9): 44-47. |
| XIE Yinghao, Yannan OU, YU Haijun, et al. Research on safe discharge for used EV traction battery[J]. Industrial Safety and Environmental Protection, 2017, 43(9): 44-47. | |
| 30 | ROUHI Hassan, KAROLA Eero, Rodrigo SERNA-GUERRERO, et al. Voltage behavior in lithium-ion batteries after electrochemical discharge and its implications on the safety of recycling processes[J]. Journal of Energy Storage, 2021, 35: 102323. |
| 31 | WANG Hao, QU Guorui, YANG Jiaqi, et al. An effective and cleaner discharge method of spent lithium batteries[J]. Journal of Energy Storage, 2022, 54: 105383. |
| 32 | XUAN Wen, OTSUKI Akira, CHAGNES Alexandre. Investigation of the leaching mechanism of NMC 811 (LiNi0.8Mn0.1Co0.1O2) by hydrochloric acid for recycling lithium ion battery cathodes[J]. RSC Advances, 2019, 9(66): 38612-38618. |
| 33 | LI Dongmin, ZHANG Bao, YE Long, et al. Regeneration of high-performance Li1.2Mn0.54Ni0.13Co0.13O2 cathode material from mixed spent lithium-ion batteries through selective ammonia leaching[J]. Journal of Cleaner Production, 2022, 349: 131373. |
| 34 | MOAZZAM Parisa, BOROUMAND Yasaman, RABIEI Parisa, et al. Lithium bioleaching: An emerging approach for the recovery of Li from spent lithium ion batteries[J]. Chemosphere, 2021, 277: 130196. |
| 35 | SETHURAJAN Manivannan, GAYDARDZHIEV Stoyan. Bioprocessing of spent lithium ion batteries for critical metals recovery—A review[J]. Resources, Conservation and Recycling, 2021, 165: 105225. |
| 36 | GUO Yang, LI Feng, ZHU Haochen, et al. Leaching lithium from the anode electrode materials of spent lithium-ion batteries by hydrochloric acid (HCl)[J]. Waste Management, 2016, 51: 227-233. |
| 37 | XU Meiling, KANG Shumei, JIANG Feng, et al. A process of leaching recovery for cobalt and lithium from spent lithium-ion batteries by citric acid and salicylic acid[J]. RSC Advances, 2021, 11(44): 27689-27700. |
| 38 | KU Heesuk, JUNG Yeojin, Minsang JO, et al. Recycling of spent lithium-ion battery cathode materials by ammoniacal leaching[J]. Journal of Hazardous Materials, 2016, 313: 138-146. |
| 39 | ROY Joseph Jegan, CAO Bin, MADHAVI Srinivasan. A review on the recycling of spent lithium-ion batteries (LIBs) by the bioleaching approach[J]. Chemosphere, 2021, 282: 130944. |
| 40 | SWAIN Basudev, JEONG Jinki, LEE Jae chun, et al. Hydrometallurgical process for recovery of cobalt from waste cathodic active material generated during manufacturing of lithium ion batteries[J]. Journal of Power Sources, 2007, 167(2): 536-544. |
| 41 | CHEN Weisheng, Hsing Jung HO. Recovery of valuable metals from lithium-ion batteries NMC cathode waste materials by hydrometallurgical methods[J]. Metals, 2018, 8(5): 321. |
| 42 | CHEN Xiangping, CHEN Yongbin, ZHOU Tao, et al. Hydrometallurgical recovery of metal values from sulfuric acid leaching liquor of spent lithium-ion batteries[J]. Waste Management, 2015, 38: 349-356. |
| 43 | PRASETYO Erik, MURYANTA Widya Aryani, ANGGRAINI Astria Gesta, et al. Tannic acid as a novel and green leaching reagent for cobalt and lithium recycling from spent lithium-ion batteries[J]. Journal of Material Cycles and Waste Management, 2022, 24(3): 927-938. |
| 44 | QI Yaping, MENG Fansong, YI Xiaoxia, et al. A novel and efficient ammonia leaching method for recycling waste lithium ion batteries[J]. Journal of Cleaner Production, 2020, 251: 119665. |
| 45 | WANG Shubin, WANG Chao, LAI Fengjiao, et al. Reduction-ammoniacal leaching to recycle lithium, cobalt, and nickel from spent lithium-ion batteries with a hydrothermal method: Effect of reductants and ammonium salts[J]. Waste Management, 2020, 102: 122-130. |
| 46 | ZHENG Xiaohong, GAO Wenfang, ZHANG Xihua, et al. Spent lithium-ion battery recycling—Reductive ammonia leaching of metals from cathode scrap by sodium sulphite[J]. Waste Management, 2017, 60: 680-688. |
| 47 | WINSLOW Kevin M, LAUX Steven J, TOWNSEND Timothy G. A review on the growing concern and potential management strategies of waste lithium-ion batteries[J]. Resources, Conservation and Recycling, 2018, 129: 263-277. |
| 48 | BISWAL Basanta Kumar, JADHAV Umesh U, MADHAIYAN Munusamy, et al. Biological leaching and chemical precipitation methods for recovery of Co and Li from spent lithium-ion batteries[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(9): 12343-12352. |
| 49 | HEYDARIAN Ahmad, MOUSAVI Seyyed Mohammad, VAKILCHAP Farzane, et al. Application of a mixed culture of adapted acidophilic bacteria in two-step bioleaching of spent lithium-ion laptop batteries[J]. Journal of Power Sources, 2018, 378: 19-30. |
| 50 | GUZOLU Jafar Sharrivar, GHARABAGHI Mahdi, MOBIN Mohammad, et al. Extraction of Li and Co from Li-ion batteries by chemical methods[J]. Journal of the Institution of Engineers: Series D, 2017, 98(1): 43-48. |
| 51 | PENG Chao, HAMUYUNI Joseph, WILSON Benjamin P, et al. Selective reductive leaching of cobalt and lithium from industrially crushed waste Li-ion batteries in sulfuric acid system[J]. Waste Management, 2018, 76: 582-590. |
| 52 | TYLER Or, W D Gourley STORM, KARTHIKEYAN Kaliyappan, et al. Recycling of mixed cathode lithium-ion batteries for electric vehicles: Current status and future outlook[J]. Carbon Energy, 2020, 2: 6-43. |
| 53 | FAN Bailin, CHEN Xiangping, ZHOU Tao, et al. A sustainable process for the recovery of valuable metals from spent lithium-ion batteries[J]. Waste Management & Research: the Journal for a Sustainable Circular Economy, 2016, 34(5): 474-481. |
| 54 | ZHENG Y, LONG H, ZHOU L, et al. Leaching procedure and kinetic studies of cobalt in cathode materials from spent lithium ion batteries using organic citric acid as leachant[J]. International Journal of Environmental Research, 2016, 10: 159-168. |
| 55 | NAYAKA G P, PAI K V, SANTHOSH G, et al. Dissolution of cathode active material of spent Li-ion batteries using tartaric acid and ascorbic acid mixture to recover Co[J]. Hydrometallurgy, 2016, 161: 54-57. |
| 56 | SUN Conghao, XU Liping, CHEN Xiangping, et al. Sustainable recovery of valuable metals from spent lithium-ion batteries using DL-malic acid: Leaching and kinetics aspect[J]. Waste Management & Research, 2018, 36(2): 113-120. |
| 57 | ZENG Xianlai, LI Jinhui, SHEN Bingyu. Novel approach to recover cobalt and lithium from spent lithium-ion battery using oxalic acid[J]. Journal of Hazardous Materials, 2015, 295: 112-118. |
| 58 | LI Li, LU Jun, REN Yang, et al. Ascorbic-acid-assisted recovery of cobalt and lithium from spent Li-ion batteries[J]. Journal of Power Sources, 2012, 218: 21-27. |
| 59 | REFLY S, FLOWERI O, MAYANGSARI T R, et al. Regeneration of LiNi1/3Co1/3Mn1/3O2 cathode active materials from end-of-life lithium-ion batteries through ascorbic acid leaching and oxalic acid coprecipitation processes[J]. ACS Sustainable Chemistry & Engineering, 2020(8): 16104-16114. |
| 60 | BARIK S P, PRABAHARAN G, KUMAR L. Leaching and separation of Co and Mn from electrode materials of spent lithium-ion batteries using hydrochloric acid: Laboratory and pilot scale study[J]. Journal of Cleaner Production, 2017, 147: 37-43. |
| 61 | YI Aifei, ZHU Zhaowu, LIU Yahui, et al. Using highly concentrated chloride solutions to leach valuable metals from cathode-active materials in spent lithium-ion batteries[J]. Rare Metals, 2021, 40(7): 1971-1978. |
| 62 | GUIMARÃES L F, BOTELHO A B, ESPINOSA D C R. Sulfuric acid leaching of metals from waste Li-ion batteries without using reducing agent[J]. Minerals Engineering, 2022, 183: 107597. |
| 63 | CHEN Xiangping, MA Hongrui, LUO Chuanbao, et al. Recovery of valuable metals from waste cathode materials of spent lithium-ion batteries using mild phosphoric acid[J]. Journal of Hazardous Materials, 2017, 326: 77-86. |
| 64 | FU Yuanpeng, HE Yaqun, CHEN Hangchao, et al. Effective leaching and extraction of valuable metals from electrode material of spent lithium-ion batteries using mixed organic acids leachant[J]. Journal of Industrial and Engineering Chemistry, 2019, 79: 154-162. |
| 65 | YANG Jingbo, FAN Ersha, LIN Jiao, et al. Recovery and reuse of anode graphite from spent lithium-ion batteries via citric acid leaching[J]. ACS Applied Energy Materials, 2021, 4(6): 6261-6268. |
| 66 | ISLAM Aminul, AHMED Tofayal, AWUAL Md Rabiul, et al. Advances in sustainable approaches to recover metals from e-waste—A review[J]. Journal of Cleaner Production, 2020, 244: 118815. |
| 67 | Nazanin BAHALOO-HOREH, MOUSAVI Seyyed Mohammad, BANIASADI Mahsa. Use of adapted metal tolerant Aspergillus niger to enhance bioleaching efficiency of valuable metals from spent lithium-ion mobile phone batteries[J]. Journal of Cleaner Production, 2018, 197: 1546-1557. |
| 68 | WU Weijin, LIU Xiaocui, ZHANG Xu, et al. Mechanism underlying the bioleaching process of LiCoO2 by sulfur-oxidizing and iron-oxidizing bacteria[J]. Journal of Bioscience and Bioengineering, 2019, 128(3): 344-354. |
| 69 | Nazanin BAHALOO-HOREH, MOUSAVI Seyyed Mohammad. Enhanced recovery of valuable metals from spent lithium-ion batteries through optimization of organic acids produced by Aspergillus niger [J]. Waste Management, 2017, 60: 666-679. |
| 70 | JOULIÉ M, LAUCOURNET R, BILLY E. Hydrometallurgical process for the recovery of high value metals from spent lithium nickel cobalt aluminum oxide based lithium-ion batteries[J]. Journal of Power Sources, 2014, 247: 551-555. |
| 71 | BERTUOL Daniel A, MACHADO Caroline M, SILVA Mariana L, et al. Recovery of cobalt from spent lithium-ion batteries using supercritical carbon dioxide extraction[J]. Waste Management, 2016, 51: 245-251. |
| 72 | ZHENG Rujuan, ZHAO Li, WANG Wenhui, et al. Optimized Li and Fe recovery from spent lithium-ion batteries via a solution-precipitation method[J]. RSC Advances, 2016, 6(49): 43613-43625. |
| 73 | LI Li, FAN Ersha, GUAN Yibiao, et al. Sustainable recovery of cathode materials from spent lithium-ion batteries using lactic acid leaching system[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(6): 5224-5233. |
| 74 | LI Li, QU Wenjie, ZHANG Xiaoxiao, et al. Succinic acid-based leaching system: a sustainable process for recovery of valuable metals from spent Li-ion batteries[J]. Journal of Power Sources, 2015, 282: 544-551. |
| 75 | LI Li, DUNN Jennifer B, ZHANG Xiao xiao, et al. Recovery of metals from spent lithium-ion batteries with organic acids as leaching reagents and environmental assessment[J]. Journal of Power Sources, 2013, 233: 180-189. |
| 76 | GOLMOHAMMADZADEH Rabeeh, RASHCHI Fereshteh, VAHIDI Ehsan. Recovery of lithium and cobalt from spent lithium-ion batteries using organic acids: process optimization and kinetic aspects[J]. Waste Management, 2017, 64: 244-254. |
| 77 | ZHENG Xiaohong, GAO Wenfang, ZHANG Xinhua, et al. Spent lithium-ion battery recycling—Reductive ammonia leaching of metals from cathode scrap by sodium sulphite[J]. Waste Management, 2017, 60: 680-688. |
| 78 | XIN Baoping, ZHANG Di, ZHANG Xian, et al. Bioleaching mechanism of Co and Li from spent lithium-ion battery by the mixed culture of acidophilic sulfur-oxidizing and iron-oxidizing bacteria[J]. Bioresource Technology, 2009, 100(24): 6163-6169. |
| 79 | BOXALL Naomi J, CHENG Ka Yu, BRUCKARD Warren, et al. Application of indirect non-contact bioleaching for extracting metals from waste lithium-ion batteries[J]. Journal of Hazardous Materials, 2018, 360: 504-511. |
| 80 | TRAN Thanh Tuan, MOON Hyun Seung, LEE Man Seung. Recovery of valuable metals from the hydrochloric leaching solution of reduction smelted metallic alloys from spent lithium-ion batteries[J]. Journal of Chemical Technology & Biotechnology, 2022, 97(5): 1247-1258. |
| 81 | QU Xin, XIE Hongwei, CHEN Xiang, et al. Recovery of LiCoO2 from spent lithium-ion batteries through a low-temperature ammonium chloride roasting approach: Thermodynamics and reaction mechanisms[J]. ACS Sustainable Chemistry & Engineering, 2020(8): 6524-6532. |
| 82 | HUANG Yong, SHAO Penghui, YANG Liming, et al. Thermochemically driven crystal phase transfer via chlorination roasting toward the selective extraction of lithium from spent LiNi1/3Co1/3Mn1/3O2 [J]. Resources, Conservation and Recycling, 2021, 174: 105757. |
| 83 | YUAN Wenhui, QIU Dingfan, WAN Chengyan. Co-Cu-Fe alloy recycled from spent lithium ion batteries by reducing smelting process[J]. 2010, 26(4): 455-458. |
| 84 | REN Guoxing, XIAO Songwen, XIE Meiqiu, et al. Recovery of valuable metals from spent lithium ion batteries by smelting reduction process based on FeO-SiO2-Al2O3 slag system[J]. Transactions of Nonferrous Metals Society of China, 2017, 27(2): 450-456. |
| 85 | XIAO Songwen, REN Guoxing, XIE Meiqiu, et al. Recovery of valuable metals from spent lithium-ion batteries by smelting reduction process based on MnO-SiO2-Al2O3 slag system[J]. Journal of Sustainable Metallurgy, 2017, 3: 703-710. |
| 86 | DANG Hui, LI Na, CHANG Zhidong, et al. Lithium leaching via calcium chloride roasting from simulated pyrometallurgical slag of spent lithium ion battery[J]. Separation and Purification Technology, 2020, 233: 116025. |
| 87 | WANG Dahui, ZHANG Xiaodong, CHEN Huaijing, et al. Separation of Li and Co from the active mass of spent Li-ion batteries by selective sulfating roasting with sodium bisulfate and water leaching[J]. Minerals Engineering, 2018, 126: 28-35. |
| 88 | LIN Jiao, LIU Chunwei, CAO Hongbin, et al. Environmentally benign process for selective recovery of valuable metals from spent lithium-ion batteries by using conventional sulfation roasting[J]. Green Chemistry, 2019, 21(21): 5904-5913. |
| 89 | TANG Yiqi, QU Xin, ZHANG Beilei, et al. Recycling of spent lithium nickel cobalt manganese oxides via a low-temperature ammonium sulfation roasting approach[J]. Journal of Cleaner Production, 2021, 279: 123633. |
| 90 | XIAO Jiefeng, NIU Bo, SONG Qingming, et al. Novel targetedly extracting lithium: An environmental-friendly controlled chlorinating technology and mechanism of spent lithium ion batteries recovery[J]. Journal of Hazardous Materials, 2021, 404: 123947. |
| 91 | LI Jia, WANG Guangxu, XU Zhenming. Environmentally-friendly oxygen-free roasting/wet magnetic separation technology for in situ recycling cobalt, lithium carbonate and graphite from spent LiCoO2/graphite lithium batteries[J]. Journal of Hazardous Materials, 2016, 302: 97-104. |
| 92 | ZHOU Fengyin, QU Xin, WU Yongxin, et al. Vacuum pyrolysis of pine sawdust to recover spent lithium ion batteries: The synergistic effect of carbothermic reduction and pyrolysis gas reduction[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(3): 1287-1297. |
| 93 | PINEGAR Haruka, MARTHI Rajashekhar, YANG Peilin, et al. Reductive thermal treatment of LiCoO2 from end-of-life lithium-ion batteries with hydrogen[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(22): 7447-7453. |
| [1] | 马伊, 曹世伟, 王家骏, 林立群, 邢延, 曹腾良, 卢峰, 赵振伦, 张志军. 低共熔溶剂回收废旧锂离子电池正极材料的研究进展[J]. 化工进展, 2023, 42(S1): 219-232. |
| [2] | 于捷, 张文龙. 锂离子电池隔膜的发展现状与进展[J]. 化工进展, 2023, 42(4): 1760-1768. |
| [3] | 马文杰, 姚卫棠. 共价有机框架(COFs)在锂离子电池中的应用[J]. 化工进展, 2023, 42(10): 5339-5352. |
| [4] | 王玥, 郑晓洪, 陶天一, 刘秀庆, 李丽, 孙峙. 废锂离子电池正极材料中锂元素选择性回收的研究进展[J]. 化工进展, 2022, 41(8): 4530-4543. |
| [5] | 程明强, 汝娟坚, 华一新, 王丁, 耿笑, 张文文, 黄皓铭, 王道祥. 低共熔溶剂在废旧锂离子电池正极材料回收中的研究进展[J]. 化工进展, 2022, 41(6): 3293-3305. |
| [6] | 马续, 邹明贵, 崔巍巍, 付安然, 廖小龙, 巩桂芬. 一种水性负极黏结剂的合成及性能[J]. 化工进展, 2022, 41(6): 3138-3145. |
| [7] | 段曼华, 程丹, 肖伟, 杨占旭. 聚丙烯腈/聚酯无纺布微孔复合锂电隔膜的制备及性能[J]. 化工进展, 2022, 41(5): 2615-2622. |
| [8] | 龚鑫, 刘小冬, 温福山, 师楠, 刘东. 中间相炭微球乳化-聚合法制备及电化学性能[J]. 化工进展, 2022, 41(5): 2379-2388. |
| [9] | 温嘉玮, 杨晨林, 成建凤, 黄国勇, 郭学益. 尖晶石型三元高电压正极材料的合成及其性能[J]. 化工进展, 2022, 41(11): 5968-5976. |
| [10] | 胡华坤, 薛文东, 蒋朋, 李勇. 锂离子电池安全添加剂的研究进展[J]. 化工进展, 2022, 41(10): 5441-5455. |
| [11] | 邱治文, 吴爱民, 王杰, 黄昊. Si基锂离子电池负极材料研究进展[J]. 化工进展, 2021, 40(S1): 253-269. |
| [12] | 宋洁尘, 夏青, 徐宇兴, 谭强强. 全固态锂离子电池的研究进展与挑战[J]. 化工进展, 2021, 40(9): 5045-5060. |
| [13] | 邹文洪, 樊佑, 张焱焱, 白正帅, 汤育欣. 安全固态锂电池室温聚合物基电解质的研究进展[J]. 化工进展, 2021, 40(9): 5029-5044. |
| [14] | 俞明浩, 顾梦旋, 吴正颖, 孙林兵. 锰氧化物的合成及在锂离子电池中的应用进展[J]. 化工进展, 2021, 40(9): 5012-5028. |
| [15] | 王策, 王国庆, 王二锐, 吴天昊, 尉海军. 锂离子电池正极材料合成及改性[J]. 化工进展, 2021, 40(9): 4998-5011. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||