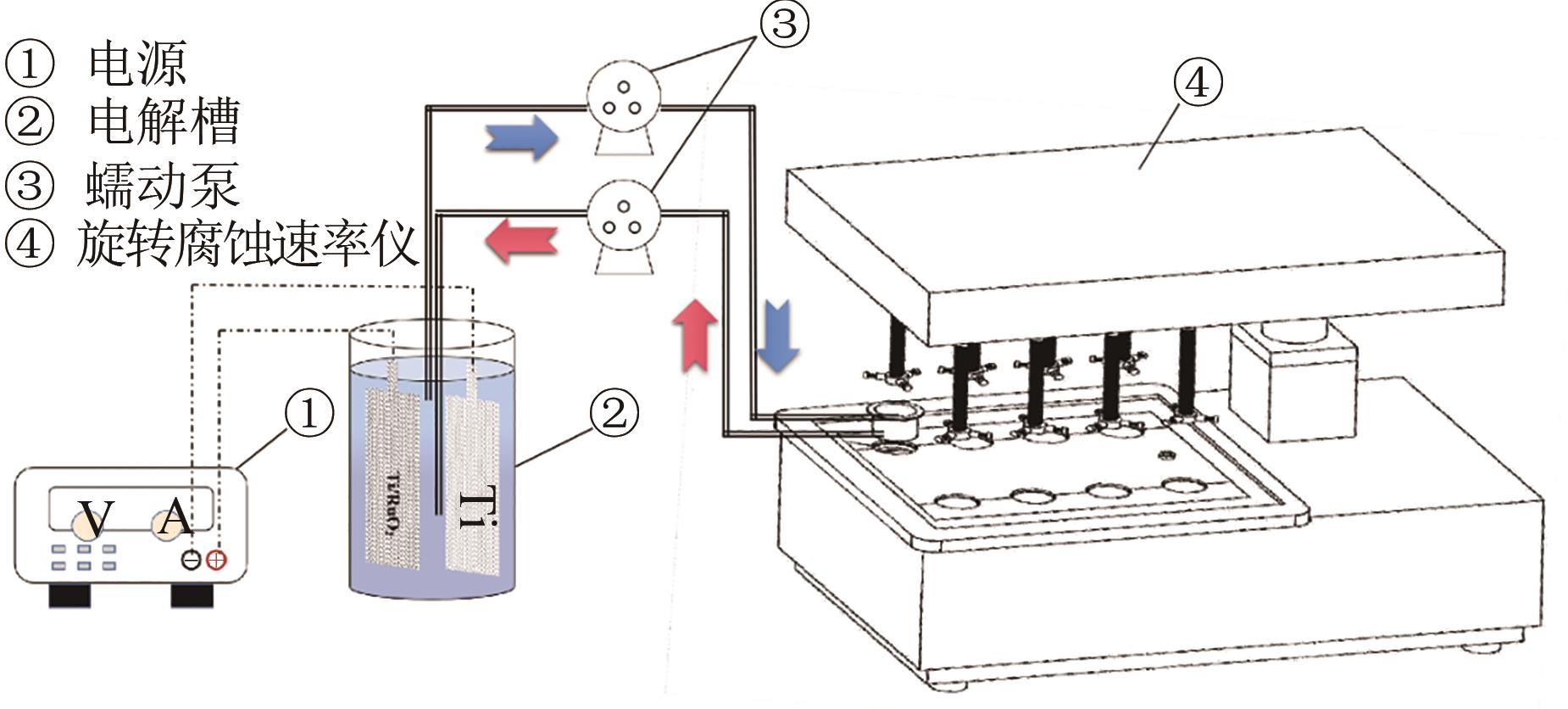
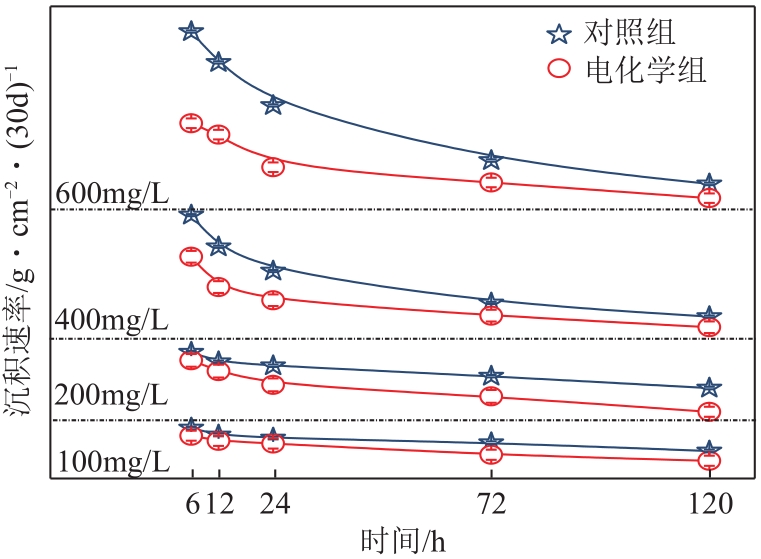
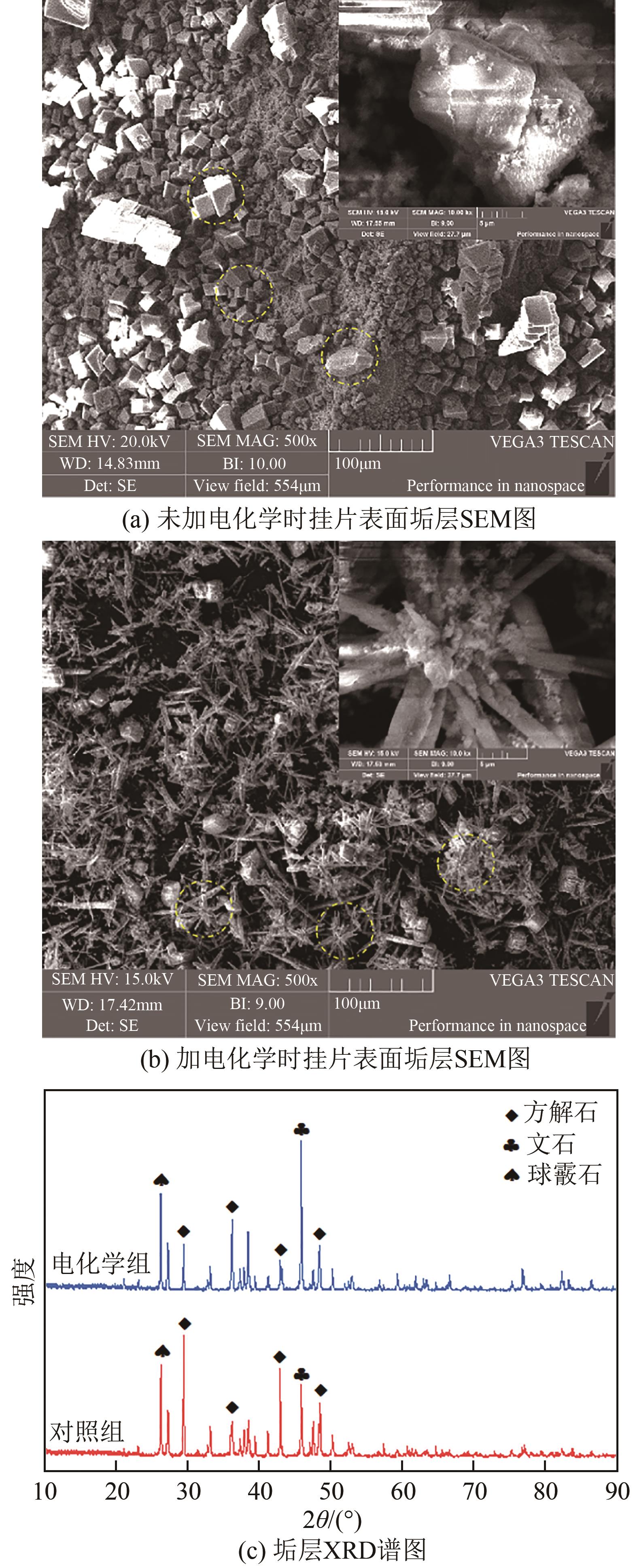
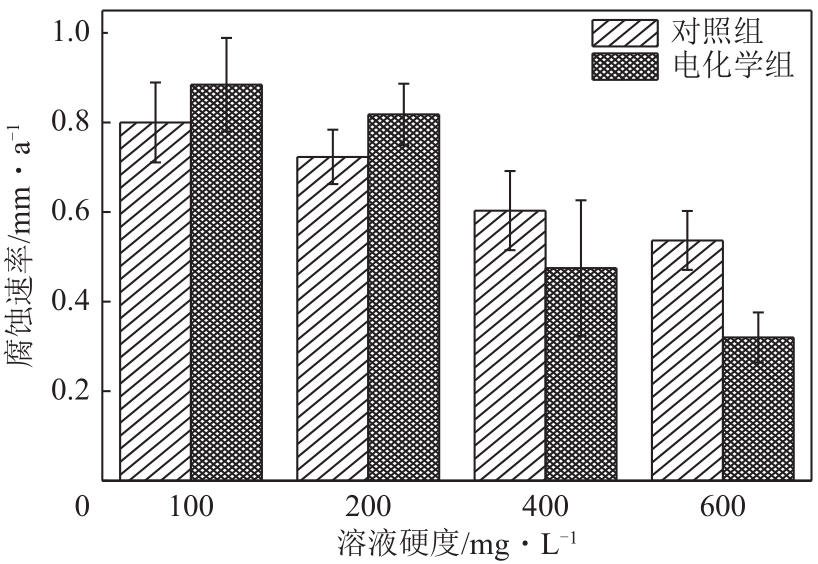
| 1 |
徐志明, 张一龙, 王景涛, 等. 两种阴离子对析晶污垢沉积的影响[J]. 化工学报, 2015, 66(6): 2268-2273.
|
|
XU Zhiming, ZHANG Yilong, WANG Jingtao, et al. Effect of two kinds of anions on crystallization fouling deposition[J]. CIESC Journal, 2015, 66(6): 2268-2273.
|
| 2 |
ZHU Haifeng, et al. Effect of electrochemical pretreatment on the control of scaling and fouling caused by circulating cooling water on heat exchanger and side-stream reverse osmosis membrane[J]. Journal of Water Process Engineering, 2021, 43: 102261.
|
| 3 |
彭力, 许萍, 张雅君, 等. 工业循环冷却水系统中运行条件对污垢的影响[J]. 水处理技术, 2016, 42(4): 46-50.
|
|
PENG Li, XU Ping, ZHANG Yajun, et al. Effects of running conditions on fouling in industrial circulating cooling water system[J]. Technology of Water Treatment, 2016, 42(4): 46-50.
|
| 4 |
HU Jiayuan, CAO Shunan, XIE Jianli. EIS study on the corrosion behavior of rusted carbon steel in 3% NaCl solution[J]. Anti-Corrosion Methods and Materials, 2013, 60(2): 100-105.
|
| 5 |
时利香, 王信粉, 李薇, 等. 循环冷却水系统新型缓蚀剂应用进展[J]. 应用化工, 2019, 48(10): 2427-2430.
|
|
SHI Lixiang, WANG Xinfen, LI Wei, et al. Progress in application of new corrosion inhibitors for circulating cooling water systems[J]. Applied Chemical Industry, 2019, 48(10): 2427-2430.
|
| 6 |
吕胜杰, 程学群, 段振国, 等. 循环水中氯离子含量对碳钢腐蚀行为影响规律的研究[J]. 石油炼制与化工, 2011, 42(3): 84-87.
|
|
Shengjie LYU, CHENG Xuequn, DUAN Zhenguo, et al. Effect of chloride ion content in circulating water on the corrosion behavior of carbon steel[J]. Petroleum Processing and Petrochemicals, 2011, 42(3): 84-87.
|
| 7 |
WANG Yetao, CHEN Hualin, ZHANG Zhijian, et al. Synthesis and characterization of PBTCA-modified hyperbranched polyether corrosion and scale inhibitors[J]. Journal of Applied Polymer Science, 2019, 136(38): 48041.
|
| 8 |
LI Shunling, QU Qing, LI Lei, et al. Bacillus cereus s-EPS as a dual bio-functional corrosion and scale inhibitor in artificial seawater[J]. Water Research, 2019, 166: 115094.
|
| 9 |
LI Heng. Control of mineral scale deposition in cooling systems using secondary-treated municipal wastewater[J]. Water Research, 2011, 45(2): 748-760.
|
| 10 |
钱凯凯, 胡将军. 电解参数对循环冷却水处理及倒极除垢效果的影响[J]. 工业水处理, 2020, 40(1): 83-86.
|
|
QIAN Kaikai, HU Jiangjun. Influence of electrolysis parameters on the treatment of circulating cooling water and the descaling effect of reversing electrodes[J]. Industrial Water Treatment, 2020, 40(1): 83-86.
|
| 11 |
ZHI Suli. Hardness removal by a novel electrochemical method[J]. Desalination, 2016, 381: 8-14.
|
| 12 |
HASSON David. Calcium carbonate hardness removal by a novel electrochemical seeds system[J]. Desalination, 2010, 263(1/2/3): 285-289.
|
| 13 |
JIN Huachang, et al. Polarity reversal electrochemical process for water softening[J]. Separation and Purification Technology, 2019, 210: 943-949.
|
| 14 |
LI Xianhui. Intermediate concentrate demineralization techniques for enhanced brackish water reverse osmosis water recovery—A review[J]. Desalination, 2019, 466: 24-35.
|
| 15 |
LIU Bo, GUO Wanqian, REN Nanqi. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A review[J]. Advanced Materials Research, 2013, 788: 405-408.
|
| 16 |
路思佳, 郑兴, 李晓良. 化学还原氧化石墨烯修饰PbO2电极及催化降解酸性红G[J]. 中国环境科学, 2021, 41(8): 3635-3641.
|
|
LU Sijia, ZHENG Xing, LI Xiaoliang. Chemically reduced graphene oxide modified PbO2 electrodes and the degradation of acidic red G[J]. China Environmental Science, 2021, 41(8): 3635-3641.
|
| 17 |
YU Yang, JIN Huachang, MENG Pengjun, et al. Electrochemical water softening using air-scoured washing for scale detachment[J]. Separation and Purification Technology, 2018, 191: 216-224.
|
| 18 |
徐浩, 郭艺菲, 郭思远, 等. 循环冷却水系统使用电化学除垢设备的选择方法[C]//中国环境科学学会2019年科学技术年会论文集, 西安, 2019.
|
|
Xu Hao, Guo Yifei, Guo Siyuan, et al. Selection of electrochemical scale removal equipment for circulating cooling water system[C]//2019 Annual Conference of Science and Technology of Chinese Society for Environmental Sciences, Xi'an, 2019.
|
| 19 |
徐浩, 延卫. 阴极电流密度对电化学除垢技术生成水垢的影响[J]. 西安交通大学学报, 2013, 47(7): 47-51.
|
|
XU Hao, YAN Wei. Effect of cathode current density on scale generated by electrochemical scale removal technique[J]. Journal of Xi'an Jiaotong University, 2013, 47(7): 47-51.
|
| 20 |
XU Hao, XU Zhicheng, GUO Yifei, Y et al. Research and application progress of electrochemical water quality stabilization technology for recirculating cooling water in China: A short review[J]. Journal of Water Process Engineering, 2020, 37: 101433.
|
| 21 |
徐浩, 延卫, 汤成莉. 水垢的电化学去除工艺与机理研究[J]. 西安交通大学学报, 2009, 43(5): 104-108.
|
|
XU Hao, YAN Wei, TANG Chengli. Technology and mechanism of water scale removal by electrochemical method[J]. Journal of Xi'an Jiaotong University, 2009, 43(5): 104-108.
|
| 22 |
徐浩, 袁孟孟, 罗清林, 等. 电化学水垢去除技术中试实验研究[J]. 工业水处理, 2019, 39(7): 37-41.
|
|
XU Hao, YUAN Mengmeng, LUO Qinglin, et al. Experimental study on pilot test of electrochemical scale removal technology[J]. Industrial Water Treatment, 2019, 39(7): 37-41.
|
| 23 |
李森, 王海峰. 电化学法处理冷却循环水技术的应用[J]. 化工进展, 2013, 32(10): 2514-2517.
|
|
LI Sen, WANG Haifeng. Application of electrochemical technology in the treatment of circulating cooling water[J]. Chemical Industry and Engineering Progress, 2013, 32(10): 2514-2517.
|
| 24 |
ZHANG Chunhui. Investigation on an electrochemical pilot equipment for water softening with an automatic descaling system: Parameter optimization and energy consumption analysis[J]. Journal of Cleaner Production, 2020, 276: 123178.
|
| 25 |
CLAUWAERT Peter. Electrochemical tap water softening: A zero chemical input approach[J]. Water Research, 2020, 169: 115263.
|
| 26 |
段慧萍. 电化学新工艺在循环水处理装置中的应用[J]. 煤化工, 2018, 46(3): 30-33.
|
|
DUAN Huiping. Application of new electrochemical process in circulating water treatment unit[J]. Coal Chemical Industry, 2018, 46(3): 30-33.
|
| 27 |
王仕文, 贺胜如, 张连波, 等. 循环水电化学处理技术在大榭石化的工业化应用[J]. 工业水处理, 2018, 38(6): 96-99.
|
|
WANG Shiwen, HE Shengru, ZHANG Lianbo, et al. Industrial application of electrochemical treatment technology to the circulating water system in Daxie Petrochemical Co., Ltd.[J]. Industrial Water Treatment, 2018, 38(6): 96-99.
|
| 28 |
刘上月, 磨冠龙. 浅谈电化学设备在钢铁企业循环冷却水处理中的运用[J]. 科学技术创新, 2021(18): 185-186.
|
|
LIU Shangyue, MO Guanlong. Talking about the application of electrochemical equipment in the treatment of circulating cooling water in iron and steel enterprises[J]. Scientific and Technological Innovation, 2021(18): 185-186.
|
| 29 |
胡艳华, 郦和生. Ca2+浓度和碱度对循环水结垢和腐蚀的影响[J]. 石化技术, 2012, 19(2): 9-11.
|
|
HU Yanhua, LI Hesheng. The scaling and corrosion in circulated water influenced from alkalinity and concentration of Ca2+ [J]. Petrochemical Industry Technology, 2012, 19(2): 9-11.
|
| 30 |
ZHANG Chunli, HE Yi, XU Zhonghao, et al. Fabrication of Fe3O4 @SiO2 nanocomposites to enhance anticorrosion performance of epoxy coatings[J]. Polymers for Advanced Technologies, 2016, 27(6): 740-747.
|
| 31 |
刘卫国, 刘建东, 赵陈龙. 水的磁处理防腐蚀机理研究[J]. 腐蚀与防护, 2006, 27(4): 196-198.
|
|
LIU Weiguo, LIU Jiandong, ZHAO Chenlong. Corrosion-prevention mechanism in magnetic treatment of water[J]. Corrosion & Protection, 2006, 27(4): 196-198.
|
| 32 |
欧阳志, 弓志定, 高晶, 等. 无磷配方在煤化工循环冷却水系统中的应用研究[J]. 工业水处理, 2019, 39(12): 110-112.
|
|
OUYANG Zhi, GONG Zhiding, GAO Jing, et al. Application of non-phosphorus formula in coal chemical circulating cooling water system[J]. Industrial Water Treatment, 2019, 39(12): 110-112.
|
| 33 |
曹生现, 赵振超, 李思博, 等. 循环冷却水处理在线监控评价设备的开发[J]. 化工学报, 2015, 66(7): 2649-2655.
|
|
CAO Shengxian, ZHAO Zhenchao, LI Sibo, et al. Development of online monitoring and evaluation device for treatment of circulating cooling water[J]. CIESC Journal, 2015, 66(7): 2649-2655.
|
 ), 李晓良1,2(
), 李晓良1,2( ), 赵会艳2, 田志娟2, 郑兴1(
), 赵会艳2, 田志娟2, 郑兴1( )
)
 ), LI Xiaoliang1,2(
), LI Xiaoliang1,2( ), ZHAO Huiyan2, TIAN Zhijuan2, ZHENG Xing1(
), ZHAO Huiyan2, TIAN Zhijuan2, ZHENG Xing1( )
)