化工进展 ›› 2023, Vol. 42 ›› Issue (2): 1028-1038.DOI: 10.16085/j.issn.1000-6613.2022-0689
离子型有机多孔聚合物的制备及其烟气脱硫耦合脱碳性质
- 西安交通大学化学工程与技术学院,陕西 西安 710049
-
收稿日期:2022-04-18修回日期:2022-06-09出版日期:2023-02-25发布日期:2023-03-13 -
通讯作者:马和平 -
作者简介:陈姝晖(1981—),女,博士研究生,研究方向为多孔材料的制备及工业应用。E-mail:applechen2018@stu.xjtu.edu.cn。 -
基金资助:国家自然科学基金(21773223);陕西省自然科学基础研究计划(2020JM-005)
Preparation of ionic organic porous polymer and its coupled desulfurization and decarbonization properties in flue gas
CHEN Shuhui( ), WU Yue, ZHANG Wenxiang, WANG Shanshan, MA Heping(
), WU Yue, ZHANG Wenxiang, WANG Shanshan, MA Heping( )
)
- School of Chemical Engineering and Technology, Xi’an Jiaotong University, Xi’an 710049, Shaanxi, China
-
Received:2022-04-18Revised:2022-06-09Online:2023-02-25Published:2023-03-13 -
Contact:MA Heping
摘要:
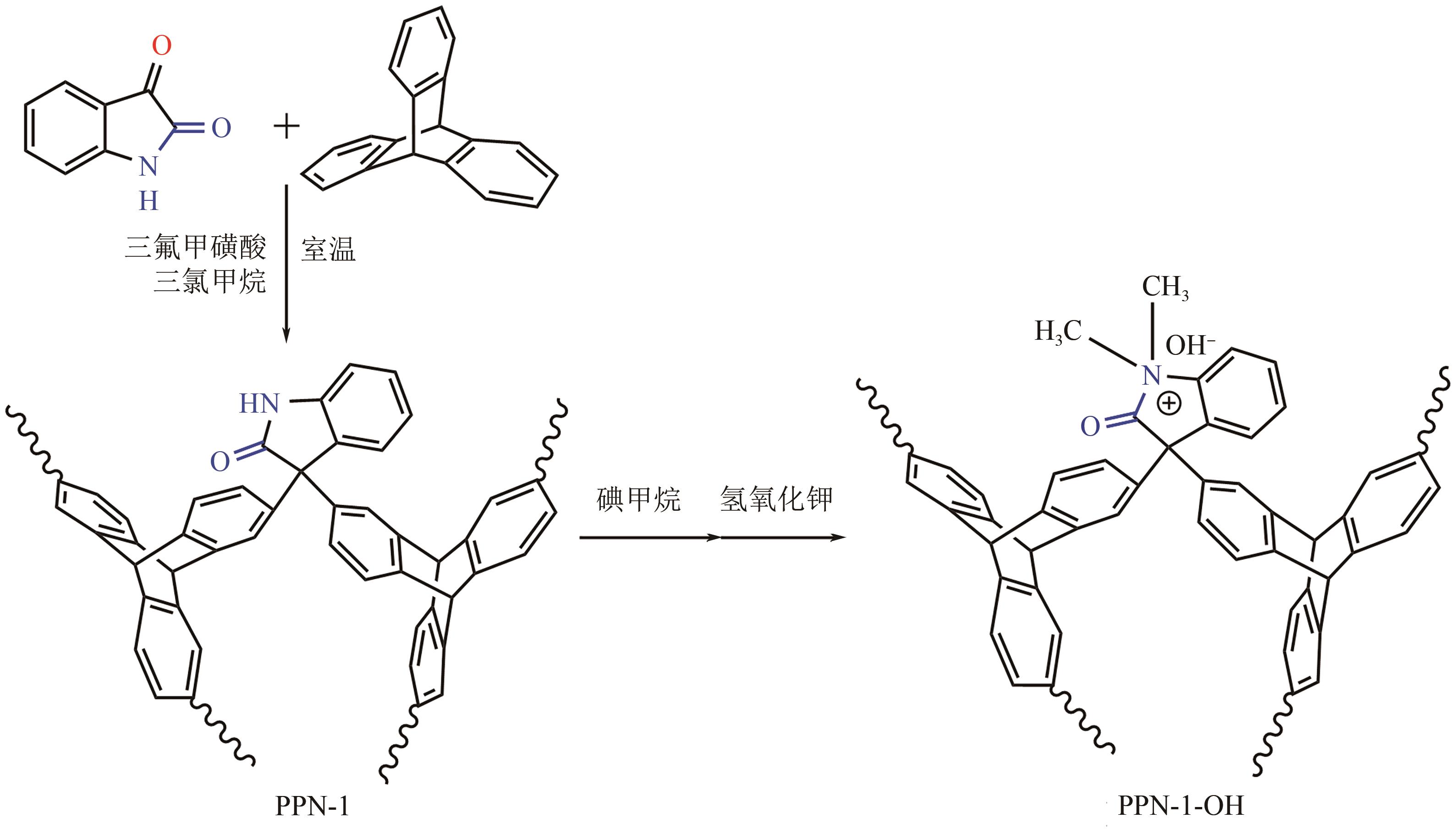
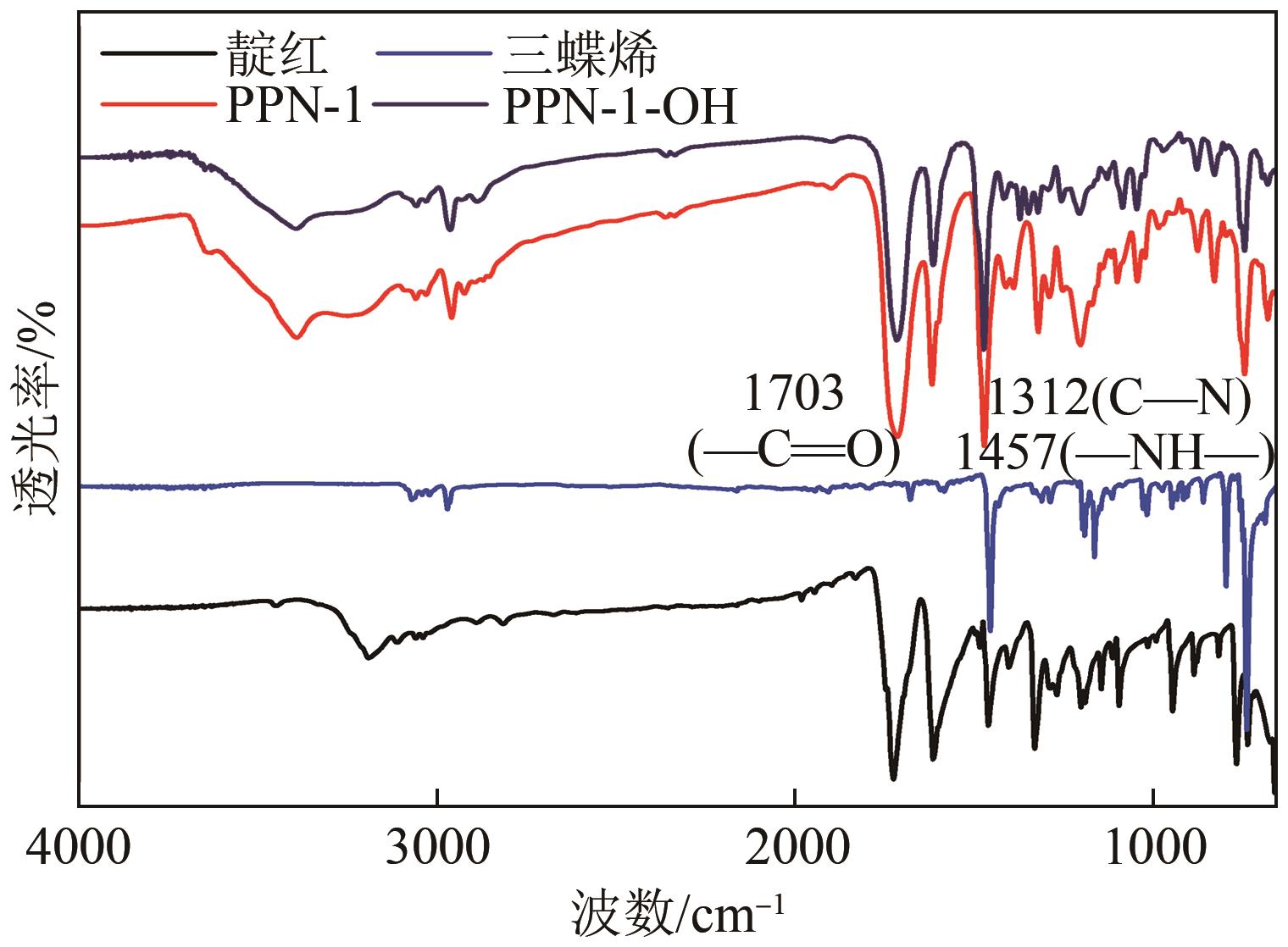
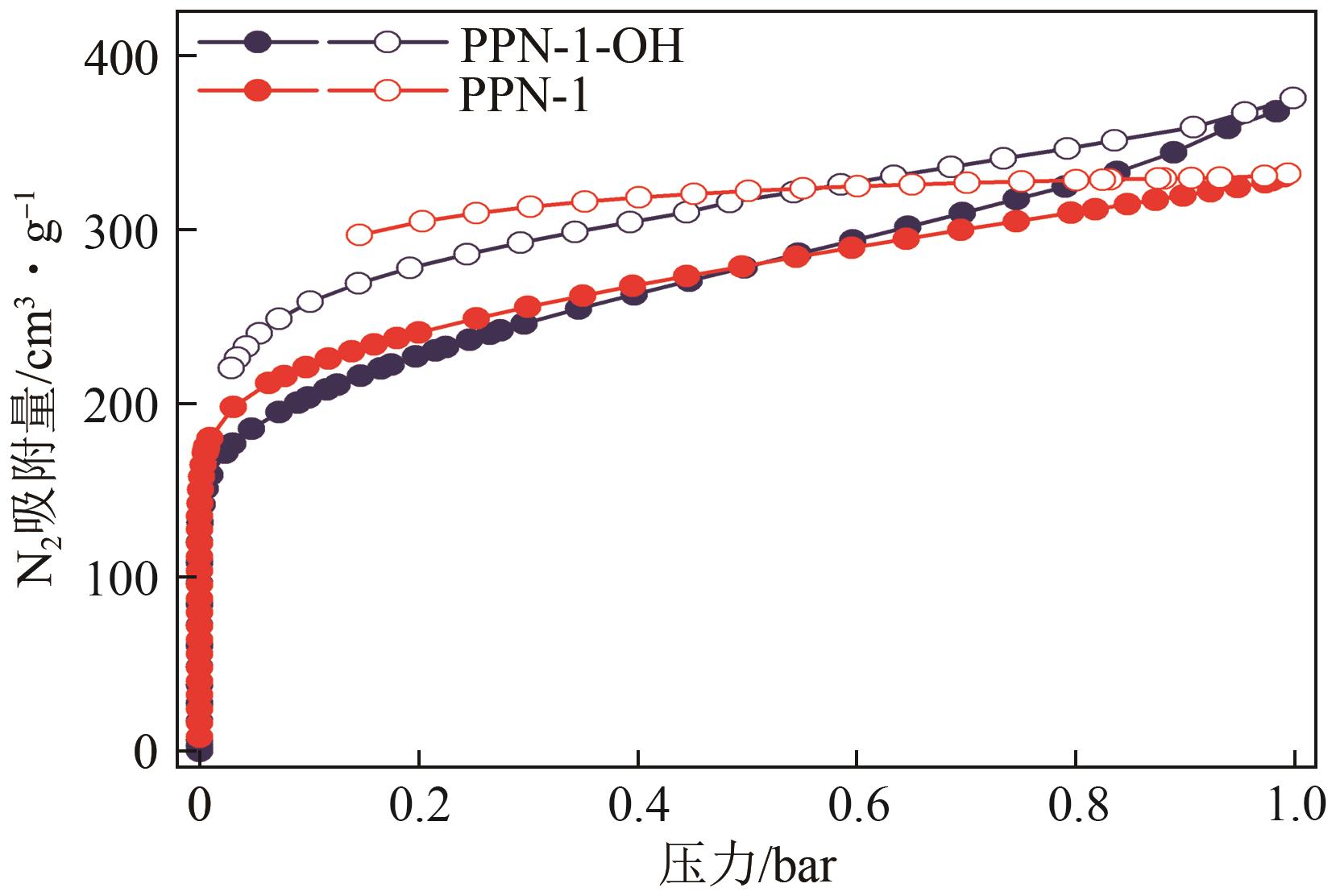
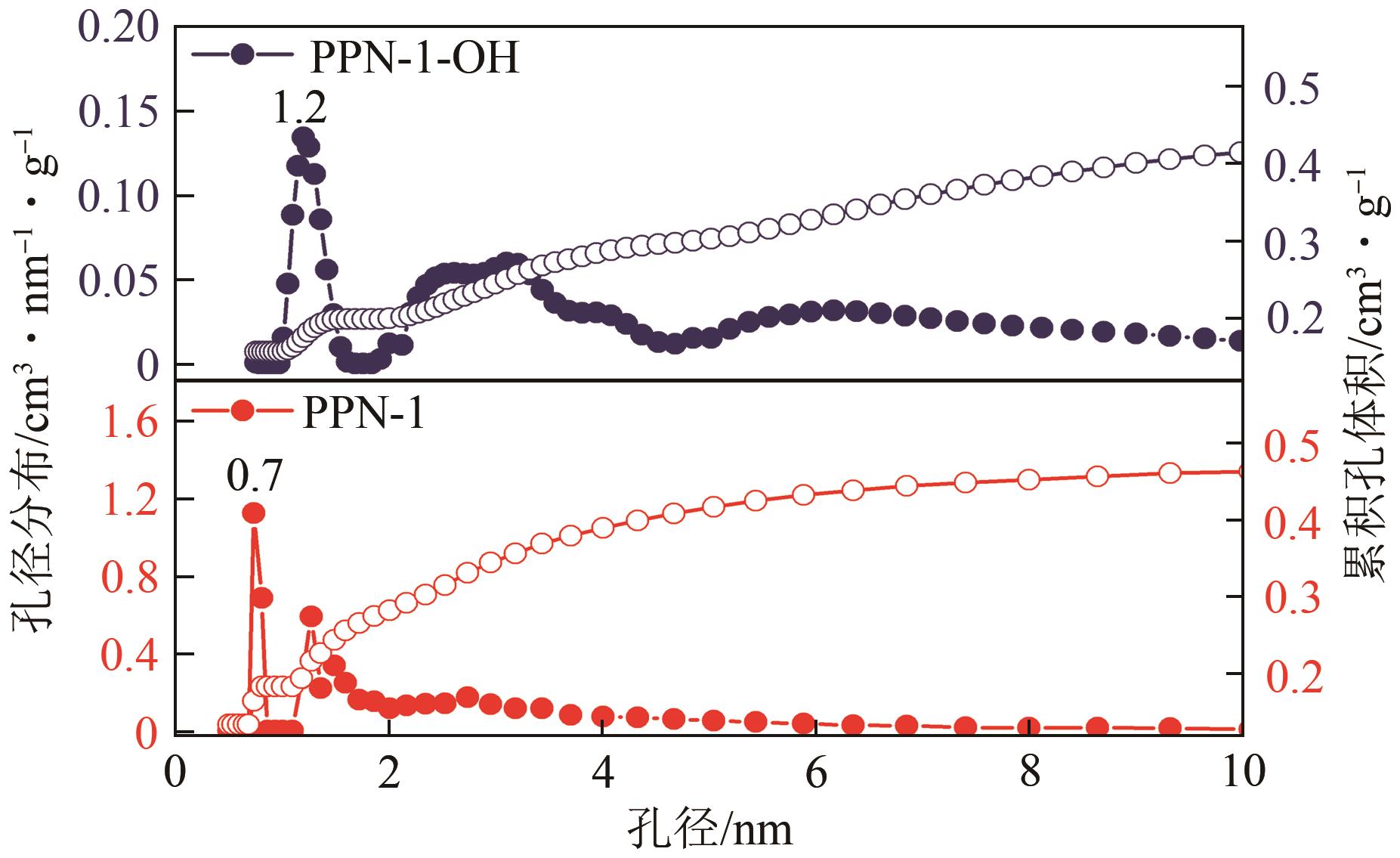
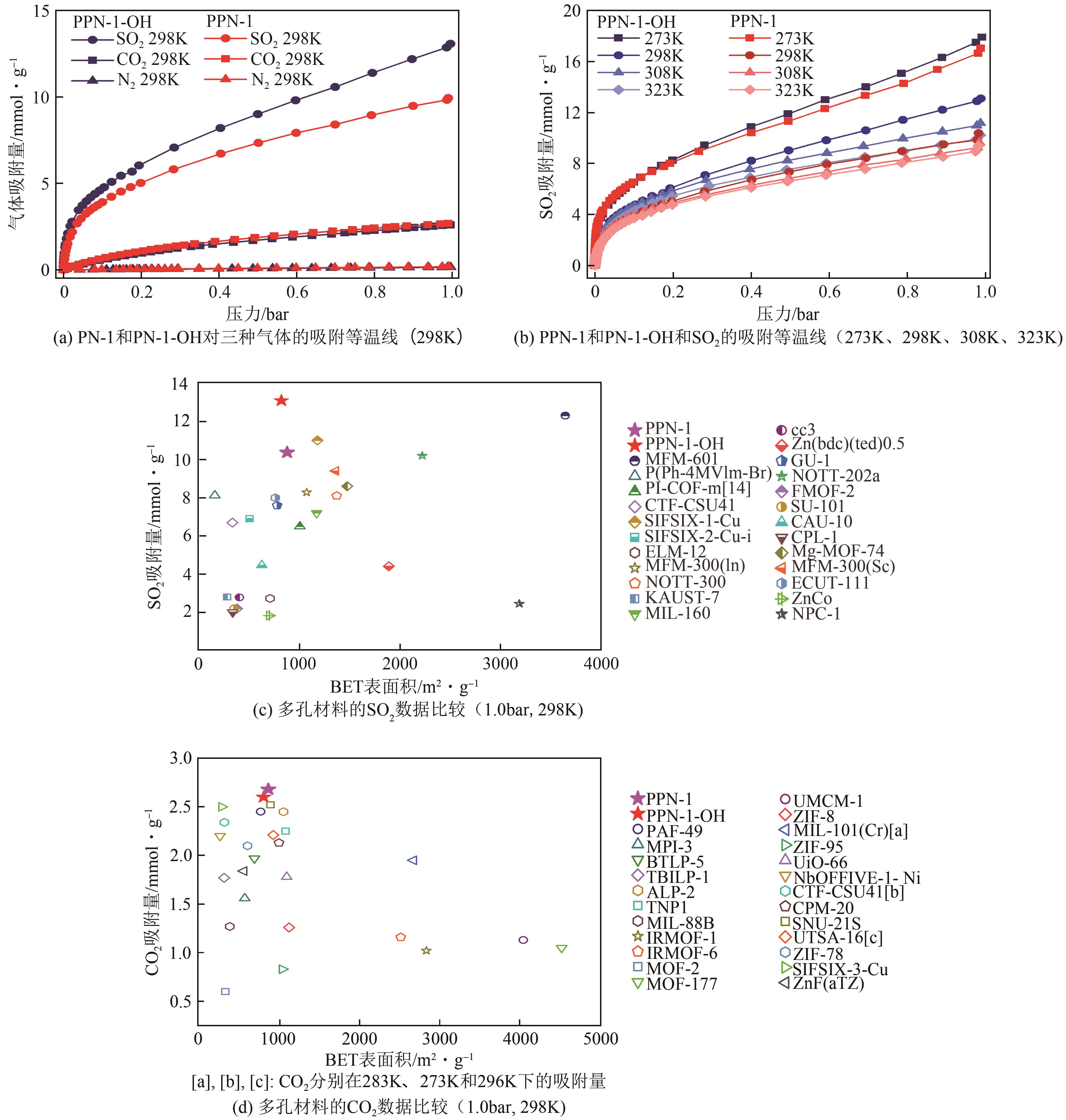
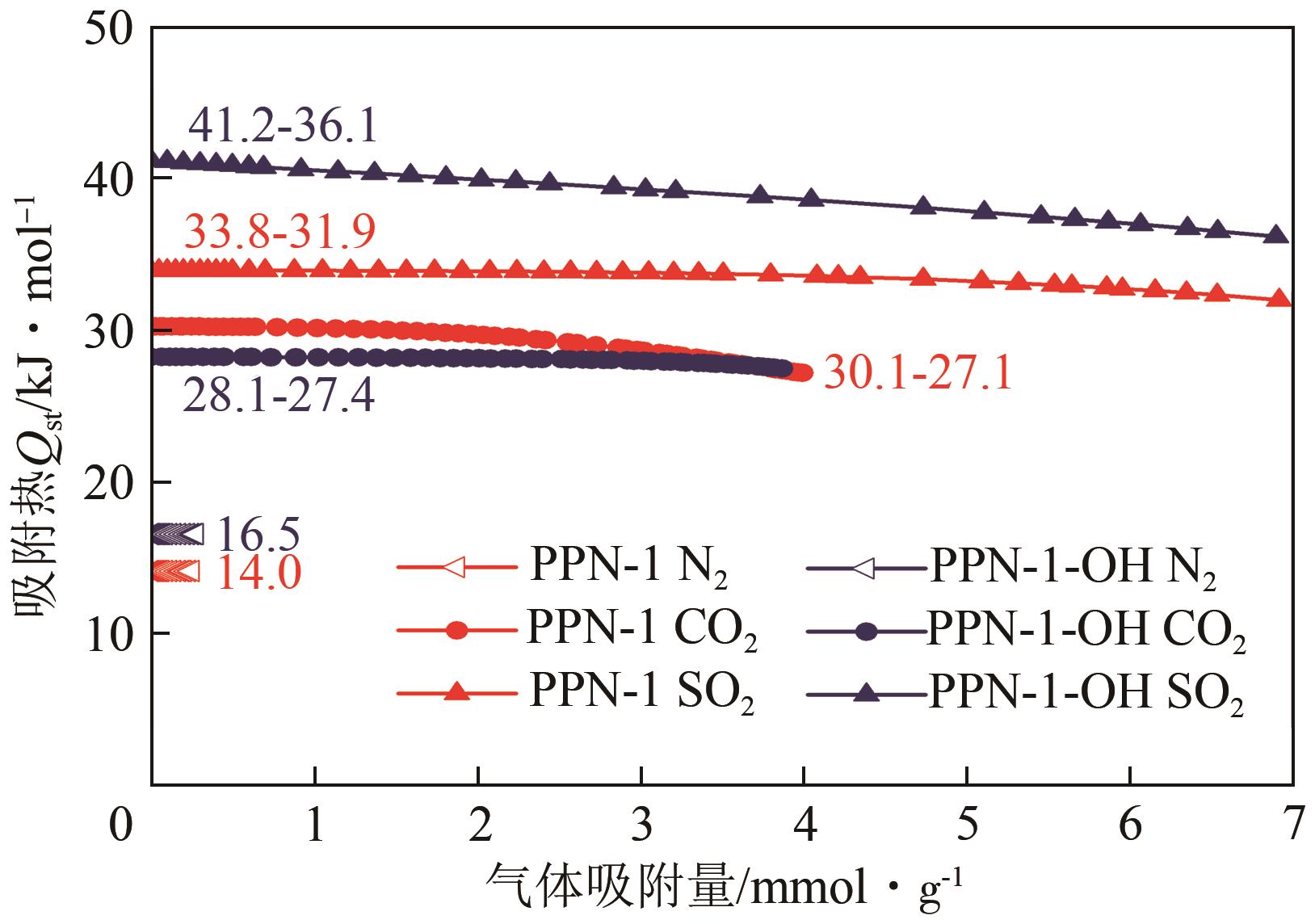
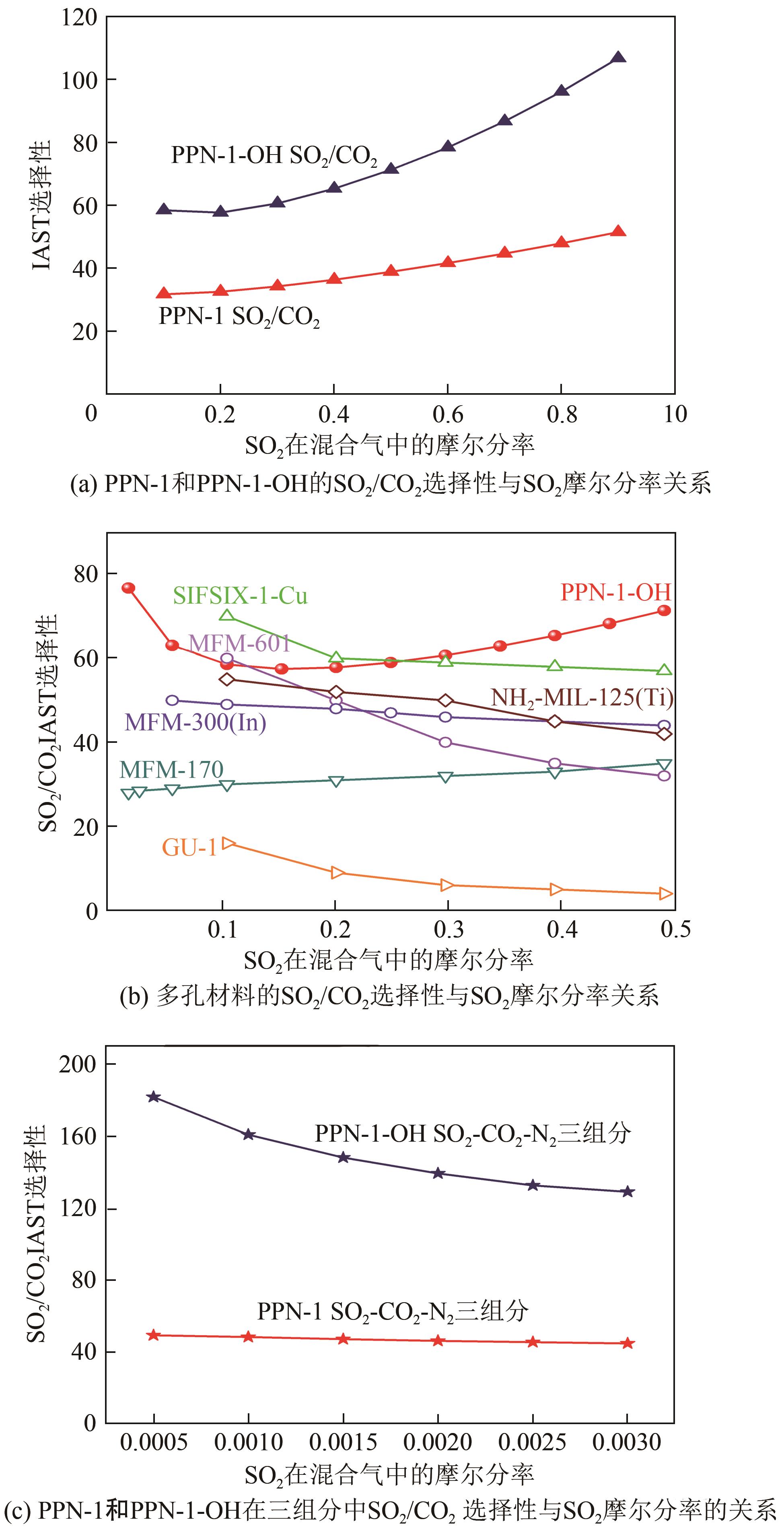
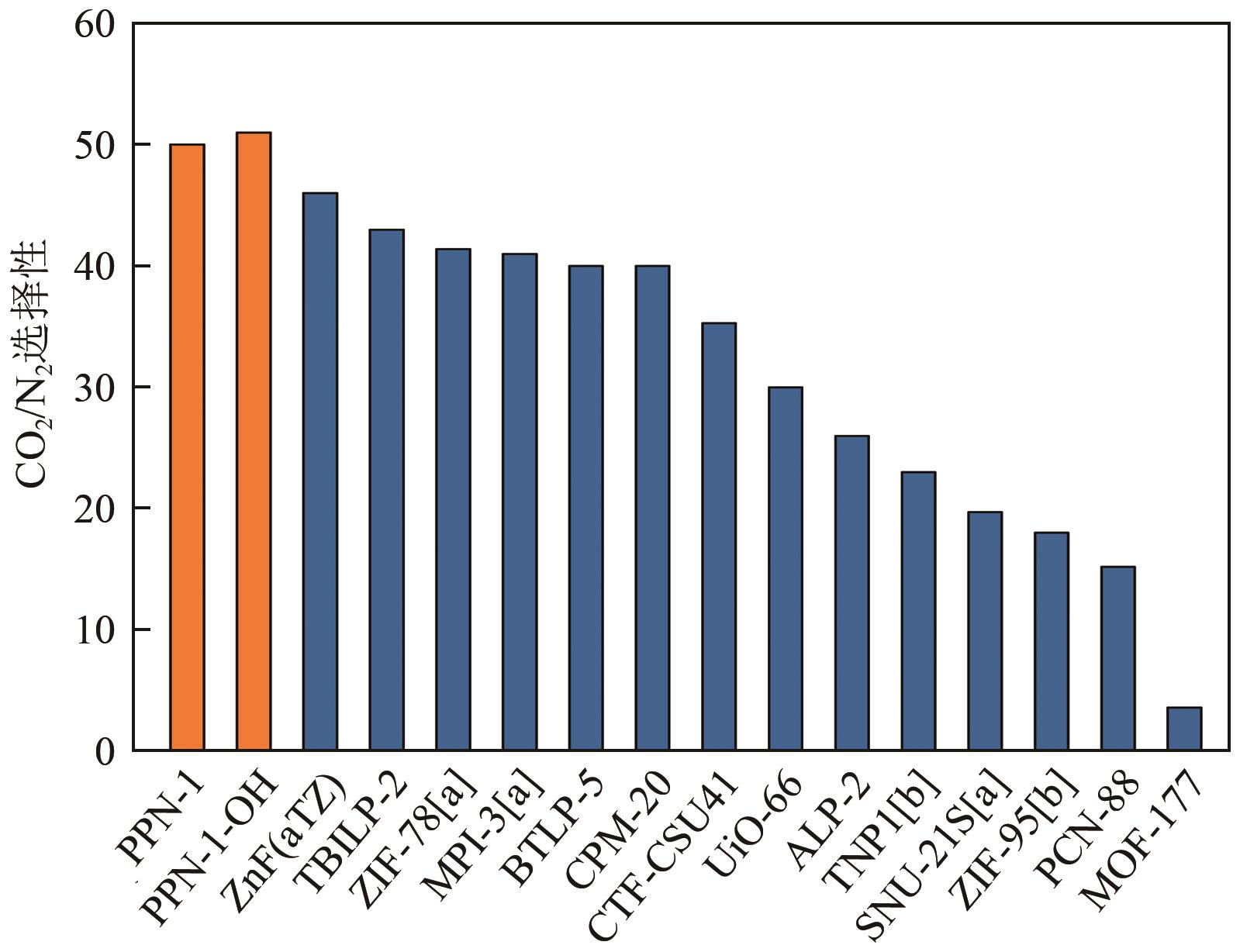
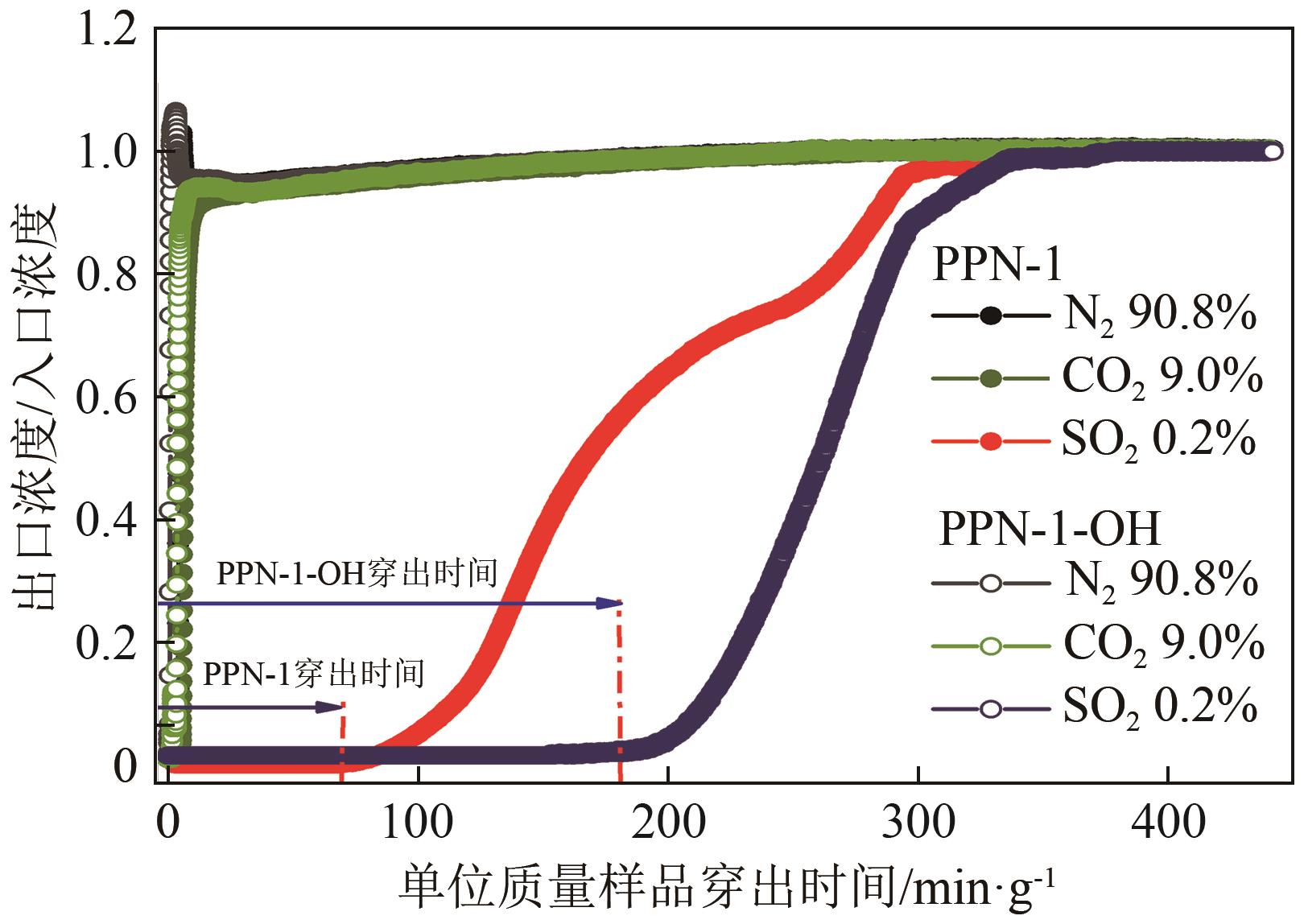
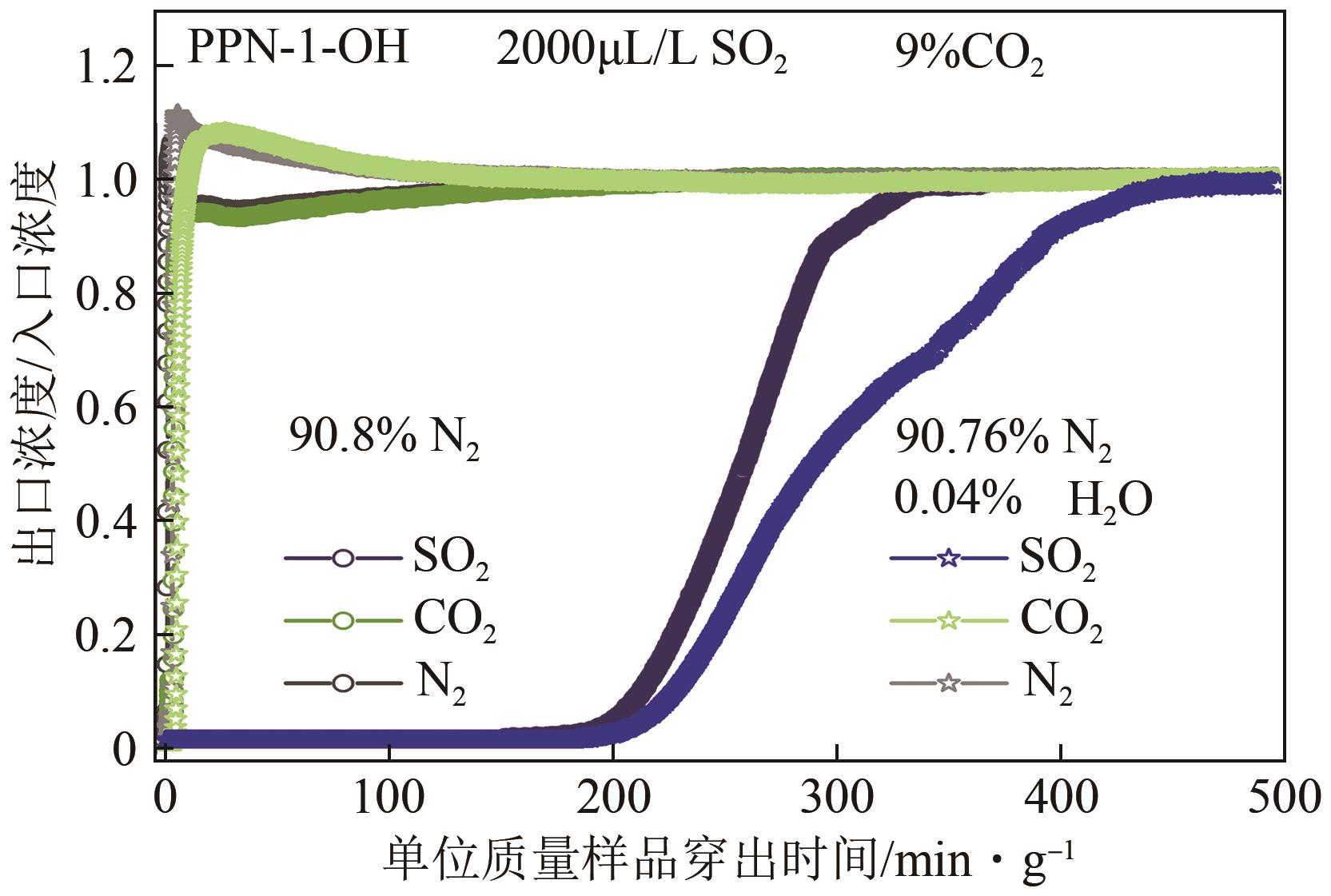
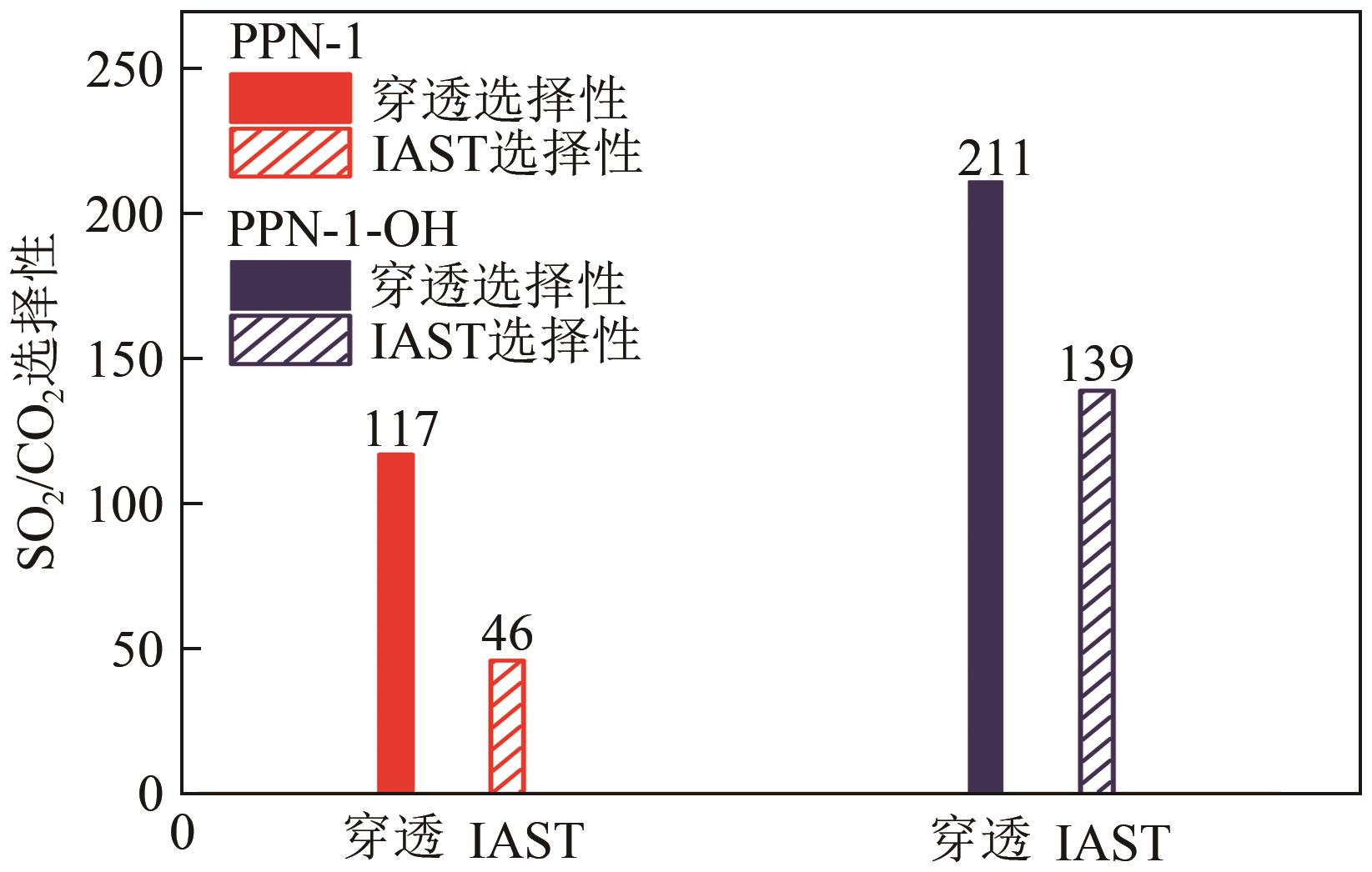
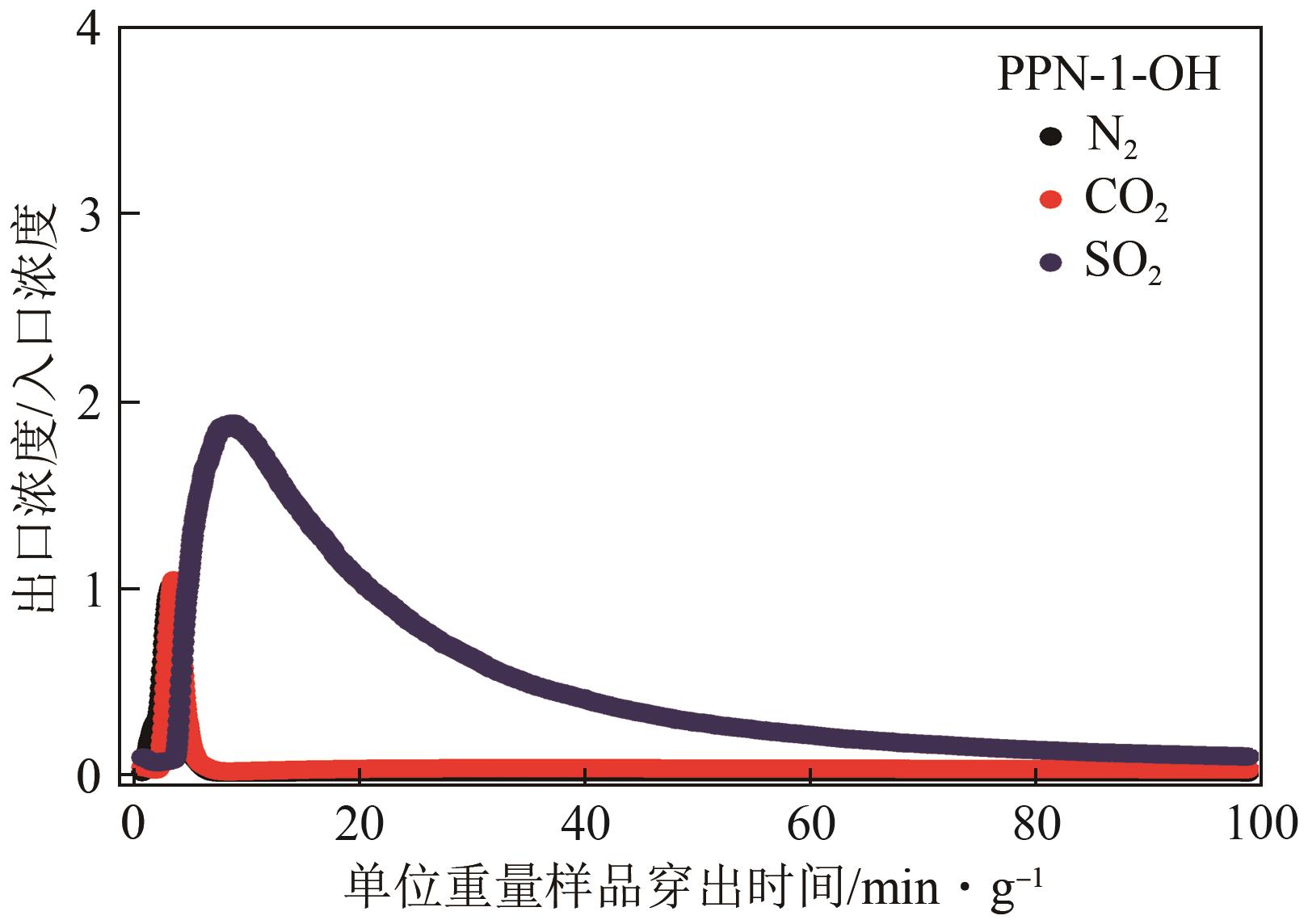
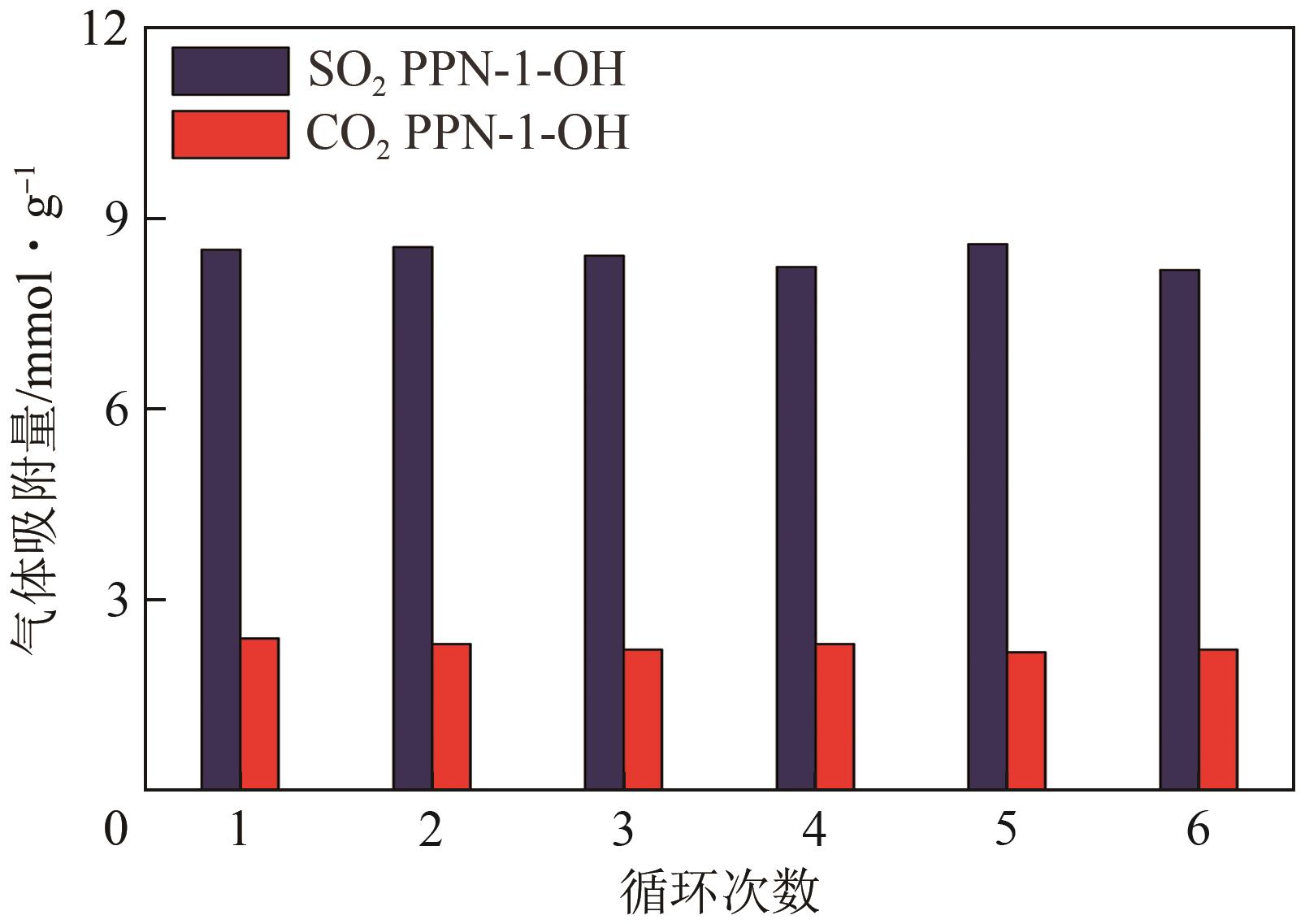
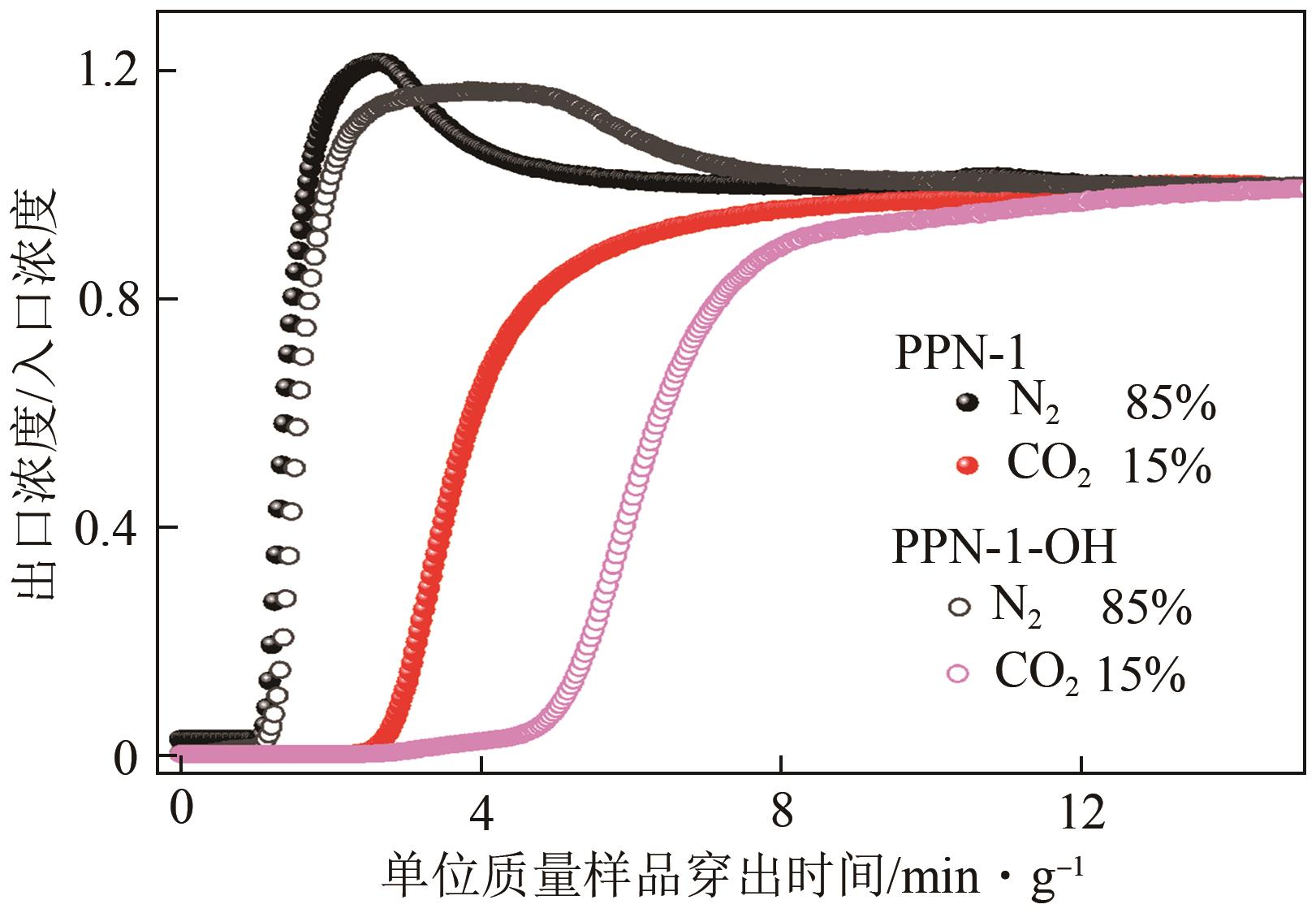
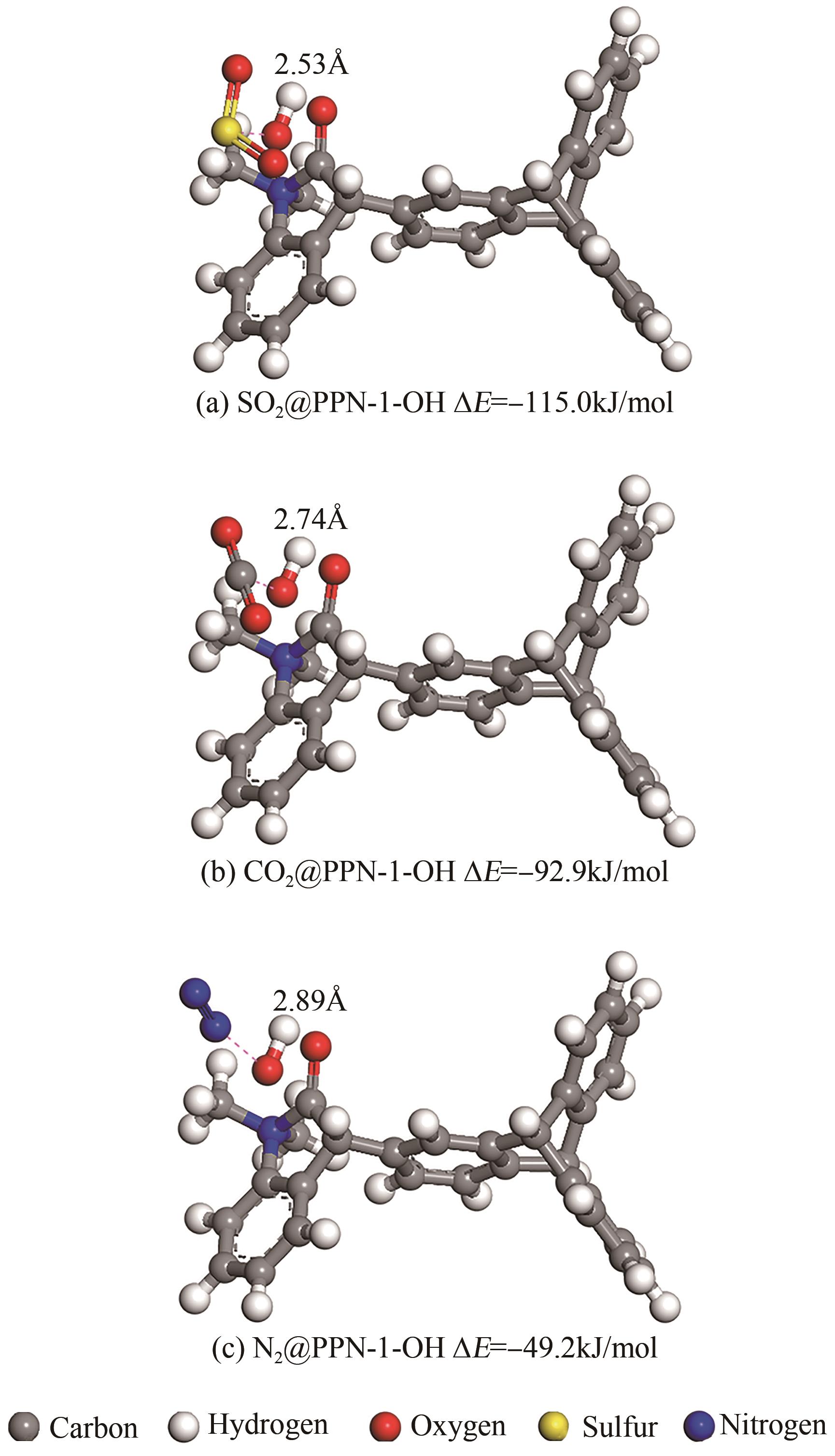
高效经济地消除燃煤烟气中的SO2和CO2对生态环境具有重要意义,但开发具有高捕集能力、高选择性和良好稳定性的吸附剂仍然是一个挑战。本文通过靛红与芳香族单体(三蝶烯)在超酸性条件下反应得到了性质稳定的有机多孔聚合物吸附剂PPN-1,并对PPN-1季胺化以及离子交换获得离子型多孔聚合物PPN-1-OH。由于两种有机多孔吸附剂拥有合适的BET表面积、丰富的微孔孔道以及大量的富电子基团,PPN-1和PPN-1-OH对SO2和CO2具有很强的亲和力。尤其是离子型有机多孔聚合物PPN-1-OH显示出超高的静态吸附能力,在298K、0.1MPa下其SO2的吸附量高达13.09mmol/g,超过了此前报道的多孔材料。基于瞬时吸附速率,理想吸附溶液理论模拟和固定床穿透实验的研究证明PPN-1-OH具有卓越的SO2动态吸附容量和选择性。在三组分混合气体(SO2/CO2/N2=0.2/9/90.8,体积比)动态固定床穿透实验中,PPN-1-OH的SO2吸附量高达1.81mmol/g,SO2/CO2选择性突破211。理论分析表明PPN-1-OH微孔孔道内大量的羟基基团能显著增强骨架与SO2之间的结合力,有效地提高吸附容量和选择性。
中图分类号:
引用本文
陈姝晖, 伍岳, 张文祥, 王闪闪, 马和平. 离子型有机多孔聚合物的制备及其烟气脱硫耦合脱碳性质[J]. 化工进展, 2023, 42(2): 1028-1038.
CHEN Shuhui, WU Yue, ZHANG Wenxiang, WANG Shanshan, MA Heping. Preparation of ionic organic porous polymer and its coupled desulfurization and decarbonization properties in flue gas[J]. Chemical Industry and Engineering Progress, 2023, 42(2): 1028-1038.
| 材料 | SO2吸附量/mmol·g-1 | CO2吸附量/mmol·g-1 | N2吸附量/mmol·g-1 | |||||
|---|---|---|---|---|---|---|---|---|
| 273K | 298K | 308K | 323K | 273K | 298K | 273K | 298K | |
| PPN-1 | 17.01 | 10.38 | 9.64 | 9.37 | 3.99 | 2.68 | 0.27 | 0.18 |
| PPN-1-OH | 17.89 | 13.09 | 11.16 | 10.22 | 3.87 | 2.60 | 0.27 | 0.15 |
表1 PPN-1和PPN-1-OH在1.0bar时的SO2、CO2和N2吸附量
| 材料 | SO2吸附量/mmol·g-1 | CO2吸附量/mmol·g-1 | N2吸附量/mmol·g-1 | |||||
|---|---|---|---|---|---|---|---|---|
| 273K | 298K | 308K | 323K | 273K | 298K | 273K | 298K | |
| PPN-1 | 17.01 | 10.38 | 9.64 | 9.37 | 3.99 | 2.68 | 0.27 | 0.18 |
| PPN-1-OH | 17.89 | 13.09 | 11.16 | 10.22 | 3.87 | 2.60 | 0.27 | 0.15 |
| 1 | JIANG Xiao, NIE Xiaowa, GUO Xinwen, et al. Recent advances in carbon dioxide hydrogenation to methanol via heterogeneous catalysis[J]. Chemical Reviews, 2020, 120(15): 7984-8034. |
| 2 | LUCAS Francisco W S, Gary GRIM R, TACEY Sean A, et al. Electrochemical routes for the valorization of biomass-derived feedstocks: From chemistry to application[J]. ACS Energy Letters, 2021, 6(4): 1205-1270. |
| 3 | NOVOTNIK Breda, NANDY Arpita, VENKATESAN Senthil Velan, et al. Can fossil fuel energy be recovered and used without any CO2 emissions to the atmosphere?[J]. Reviews in Environmental Science and Bio/Technology, 2020, 19(1): 217-240. |
| 4 | LIU Songtao, HAO Hongke, JIA Wenbo, et al. Effects of ultralow-emission retrofitting on mercury emission from a coal-fired power plant[J]. Energy & Fuels, 2020, 34(6): 7502-7508. |
| 5 | ZHANG Yan, ZHANG Peixin, YU Weikang, et al. Highly selective and reversible sulfur dioxide adsorption on a microporous metal-organic framework via polar sites[J]. ACS Applied Materials & Interfaces, 2019, 11(11): 10680-10688. |
| 6 | HASHEMI Ali, PAJOUM SHARIATI Farshid, SOHANI Elnaz, et al. CO2 biofixation by Synechococcus elongatus from the power plant flue gas under various light-dark cycles[J]. Clean Technologies and Environmental Policy, 2020, 22(8): 1735-1743. |
| 7 | LI Xiaosen, ZHAN Hao, XU Chungang, et al. Effects of tetrabutyl-(ammonium/phosphonium) salts on clathrate hydrate capture of CO2 from simulated flue gas[J]. Energy & Fuels, 2012, 26(4): 2518-2527. |
| 8 | SHEKHAH Osama, BELMABKHOUT Youssef, CHEN Zhijie, et al. Made-to-order metal-organic frameworks for trace carbon dioxide removal and air capture[J]. Nature Communications, 2014, 5: 4228. |
| 9 | WANG Yuxuan, ZHANG Qian, HE Kebin, et al. Sulfate-nitrate-ammonium aerosols over China: Response to 2000—2015 emission changes of sulfur dioxide, nitrogen oxides, and ammonia[J]. Atmospheric Chemistry and Physics, 2013, 13: 2635-2652. |
| 10 | HAN Xue, YANG Sihai, Martin SCHRÖDER. Porous metal-organic frameworks as emerging sorbents for clean air[J]. Nature Reviews Chemistry, 2019, 3(2): 108-118. |
| 11 | Jonghun LIM, CHOI Yeongryeol, KIM Geonyeol, et al. Modeling of the wet flue gas desulfurization system to utilize low-grade limestone[J]. Korean Journal of Chemical Engineering, 2020, 37(12): 2085-2093. |
| 12 | YANG Lifeng, QIAN Siheng, WANG Xiaobing, et al. Energy-efficient separation alternatives: Metal-organic frameworks and membranes for hydrocarbon separation[J]. Chemical Society Reviews, 2020, 49(15): 5359-5406. |
| 13 | WANG Jun, ZHANG Yan, ZHANG Peixin, et al. Optimizing pore space for flexible-robust metal-organic framework to boost trace acetylene removal[J]. Journal of the American Chemical Society, 2020, 142(21): 9744-9751. |
| 14 | XIAO Jing, SONG Chunshan, MA Xiaoliang, et al. Effects of aromatics, diesel additives, nitrogen compounds, and moisture on adsorptive desulfurization of diesel fuel over activated carbon[J]. Industrial & Engineering Chemistry Research, 2012, 51(8): 3436-3443. |
| 15 | YI Honghong, WANG Zhixiang, LIU Haiyan, et al. Adsorption of SO2, NO, and CO2 on activated carbons: equilibrium and thermodynamics[J]. Journal of Chemical & Engineering Data, 2014, 59(5): 1556-1563. |
| 16 | CHEN Xi, SHEN Benxian, SUN Hui, et al. Ion-exchange modified zeolites X for selective adsorption desulfurization from Claus tail gas: Experimental and computational investigations[J]. Microporous and Mesoporous Materials, 2018, 261: 227-236. |
| 17 | LIAO Junjie, ZHANG Yunfei, FAN Lijun, et al. Insight into the acid sites over modified NaY zeolite and their adsorption mechanisms for thiophene and benzene[J]. Industrial & Engineering Chemistry Research, 2019, 58(11): 4572-4580. |
| 18 | HUO Quan, LI Jianshu, QI Xiaoran, et al. Cu, Zn-embedded MOF-derived bimetallic porous carbon for adsorption desulfurization[J]. Chemical Engineering Journal, 2019, 378: 122106. |
| 19 | CHEN Fuqiang, LAI Dan, GUO Lidong, et al. Deep desulfurization with record SO2 adsorption on the metal-organic frameworks[J]. Journal of the American Chemical Society, 2021, 143(24): 9040-9047. |
| 20 | GRAPE Erik Svensson, Gabriel FLORES J, HIDALGO Tania, et al. A robust and biocompatible bismuth ellagate MOF synthesized under green ambient conditions[J]. Journal of the American Chemical Society, 2020, 142(39): 16795-16804. |
| 21 | SUO Xian, YU Ying, QIAN Siheng, et al. Tailoring the pore size and chemistry of ionic ultramicroporous polymers for trace sulfur dioxide capture with high capacity and selectivity[J]. Angewandte Chemie International Edition, 2021, 60(13): 6986-6991. |
| 22 | BRANDT Philipp, NUHNEN Alexander, LANGE Marcus, et al. Metal-organic frameworks with potential application for SO2 separation and flue gas desulfurization[J]. ACS Applied Materials & Interfaces, 2019, 11(19): 17350-17358. |
| 23 | MA Heping, LIU Bailing, LI Bin, et al. Cationic covalent organic frameworks: A simple platform of anionic exchange for porosity tuning and proton conduction[J]. Journal of the American Chemical Society, 2016, 138(18): 5897-5903. |
| 24 | RAMEZANIPOUR PENCHAH Hamid, GHAEMI Ahad, GANADZADEH GILANI Hossein. Benzene-based hyper-cross-linked polymer with enhanced adsorption capacity for CO2 capture[J]. Energy & Fuels, 2019, 33(12): 12578-12586. |
| 25 | LI Lina, CAI Kun, WANG Pengyuan, et al. Construction of sole benzene ring porous aromatic frameworks and their high adsorption properties[J]. ACS Applied Materials & Interfaces, 2015, 7(1): 201-208. |
| 26 | CHEN Tongfan, ZHANG Wenxiang, LI Bin, et al. Adsorptive separation of aromatic compounds from alkanes by π-π interactions in a carbazole-based conjugated microporous polymer[J]. ACS Applied Materials & Interfaces, 2020, 12(50): 56385-56392. |
| 27 | FU Yu, WANG Zhiqiang, LI Sizhe, et al. Functionalized covalent triazine frameworks for effective CO2 and SO2 removal[J]. ACS Applied Materials & Interfaces, 2018, 10(42): 36002-36009. |
| 28 | RABBANI Mohammad Gulam, ISLAMOGLU Timur, EL-KADERI Hani M. Benzothiazole-and benzoxazole-linked porous polymers for carbon dioxide storage and separation[J]. Journal of Materials Chemistry A, 2017, 5(1): 258-265. |
| 29 | WU Linbo, AN Dong, DONG Jie, et al. Preparation and SO2 absorption/desorption properties of crosslinked poly(1,1,3,3-tetramethylguanidine acrylate) porous particles[J]. Macromolecular Rapid Communications, 2006, 27(22): 1949-1954. |
| 30 | DENG Hua, YI Honghong, TANG Xiaolong, et al. Adsorption equilibrium for sulfur dioxide, nitric oxide, carbon dioxide, nitrogen on 13X and 5A zeolites[J]. Chemical Engineering Journal, 2012, 188: 77-85. |
| 31 | CUI Xili, YANG Qiwei, YANG Lifeng, et al. Ultrahigh and selective SO2 uptake in inorganic anion-pillared hybrid porous materials[J]. Advanced Materials, 2017, 29(28): 1606929. |
| 32 | CARTER Joseph H, HAN Xue, MOREAU Florian Y, et al. Exceptional adsorption and binding of sulfur dioxide in a robust zirconium-based metal-organic framework[J]. Journal of the American Chemical Society, 2018, 140(46): 15564-15567. |
| 33 | YANG Sihai, LIU Leifeng, SUN Junliang, et al. Irreversible network transformation in a dynamic porous host catalyzed by sulfur dioxide[J]. Journal of the American Chemical Society, 2013, 135(13): 4954-4957. |
| 34 | LI Guiyang, WANG Zhonggang. Microporous polyimides with uniform pores for adsorption and separation of CO2 gas and organic vapors[J]. Macromolecules, 2013, 46(8): 3058-3066. |
| 35 | SEKIZKARDES Ali Kemal, ALTARAWNEH Suha, KAHVECI Zafer, et al. Highly selective CO2 capture by triazine-based benzimidazole-linked polymers[J]. Macromolecules, 2014, 47(23): 8328-8334. |
| 36 | ARAB Pezhman, RABBANI Mohammad Gulam, SEKIZKARDES Ali Kemal, et al. Copper(I)-catalyzed synthesis of nanoporous azo-linked polymers: Impact of textural properties on gas storage and selective carbon dioxide capture[J]. Chemistry of Materials, 2014, 26(3): 1385-1392. |
| 37 | Aysu YURDUŞEN, Yuda YÜRÜM. A controlled synthesis strategy to enhance the CO2 adsorption capacity of MIL-88B type MOF crystallites by the crucial role of narrow micropores[J]. Industrial & Engineering Chemistry Research, 2019, 58(31): 14058-14072. |
| 38 | CMARIK Gregory E, KIM Min, COHEN Seth M, et al. Tuning the adsorption properties of UiO-66 via ligand functionalization[J]. Langmuir: the ACS Journal of Surfaces and Colloids, 2012, 28(44): 15606-15613. |
| 39 | SMITH Gemma L, EYLEY Jennifer E, HAN Xue, et al. Reversible coordinative binding and separation of sulfur dioxide in a robust metal-organic framework with open copper sites[J]. Nature Materials, 2019, 18(12): 1358-1365. |
| 40 | SAVAGE Mathew, CHENG Yongqiang, EASUN Timothy L, et al. Selective adsorption of sulfur dioxide in a robust metal-organic framework material[J]. Advanced Materials, 2016, 28(39): 8705-8711. |
| [1] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [2] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [3] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [4] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [5] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [6] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [7] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [8] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [9] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [10] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| [11] | 黄玉飞, 李子怡, 黄杨强, 金波, 罗潇, 梁志武. 光催化CO2和CH4重整催化剂研究进展[J]. 化工进展, 2023, 42(8): 4247-4263. |
| [12] | 娄宝辉, 吴贤豪, 张驰, 陈臻, 冯向东. 纳米流体用于二氧化碳吸收分离研究进展[J]. 化工进展, 2023, 42(7): 3802-3815. |
| [13] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| [14] | 张雪伟, 黄亚继, 许月阳, 程好强, 朱志成, 李金壘, 丁雪宇, 王圣, 张荣初. 碱性吸附剂对燃煤烟气中SO3的吸附特性[J]. 化工进展, 2023, 42(7): 3855-3864. |
| [15] | 吕超, 张习文, 金理健, 杨林军. 新型两相吸收剂-离子液体系统高效捕获CO2[J]. 化工进展, 2023, 42(6): 3226-3232. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||