化工进展 ›› 2022, Vol. 41 ›› Issue (11): 6053-6060.DOI: 10.16085/j.issn.1000-6613.2022-0153
爱德万甜中间体3-羟基-4-甲氧基肉桂醛的合成
- 厦门大学化学化工学院,福建 厦门 361005
-
收稿日期:2022-01-24修回日期:2022-03-25出版日期:2022-11-25发布日期:2022-11-28 -
通讯作者:黎四芳 -
作者简介:方聪(1993—),男,硕士研究生,研究方向为精细化工。E-mail:congfang@stu.xmu.edu.cn。
Synthesis of 3-hydroxy-4-methoxy cinnamaldehyde as intermediate of advantame
FANG Cong( ), LIU Yixue, LI Sifang(
), LIU Yixue, LI Sifang( )
)
- College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, Fujian, China
-
Received:2022-01-24Revised:2022-03-25Online:2022-11-25Published:2022-11-28 -
Contact:LI Sifang
摘要:
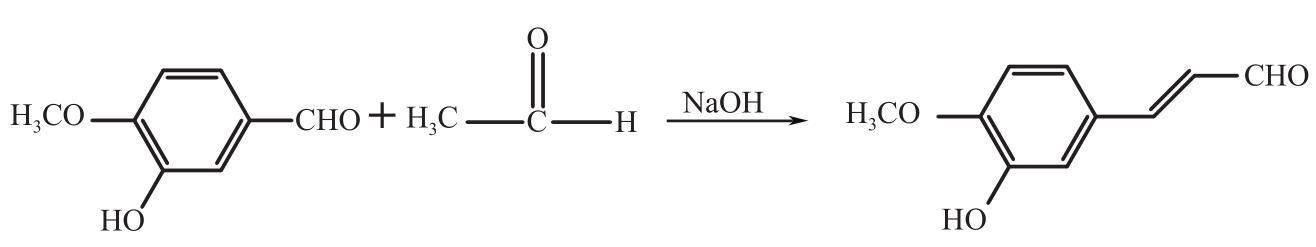
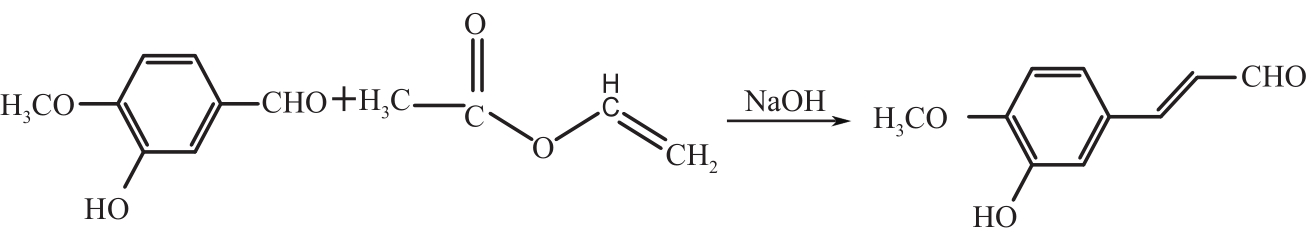
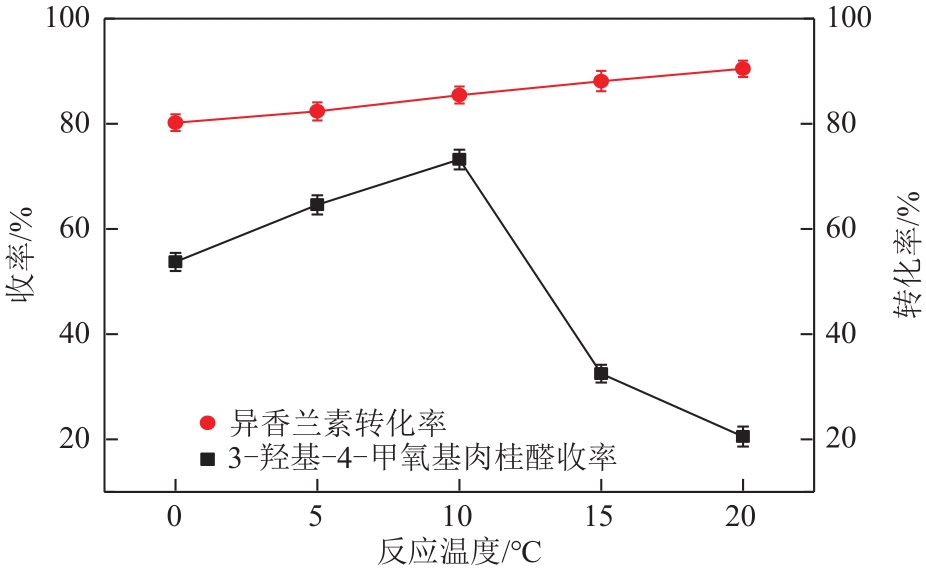
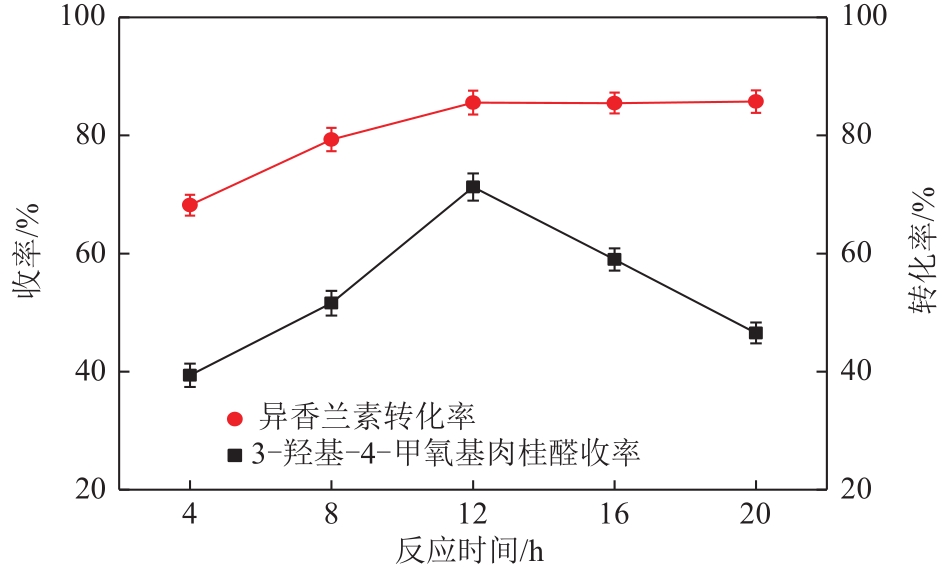
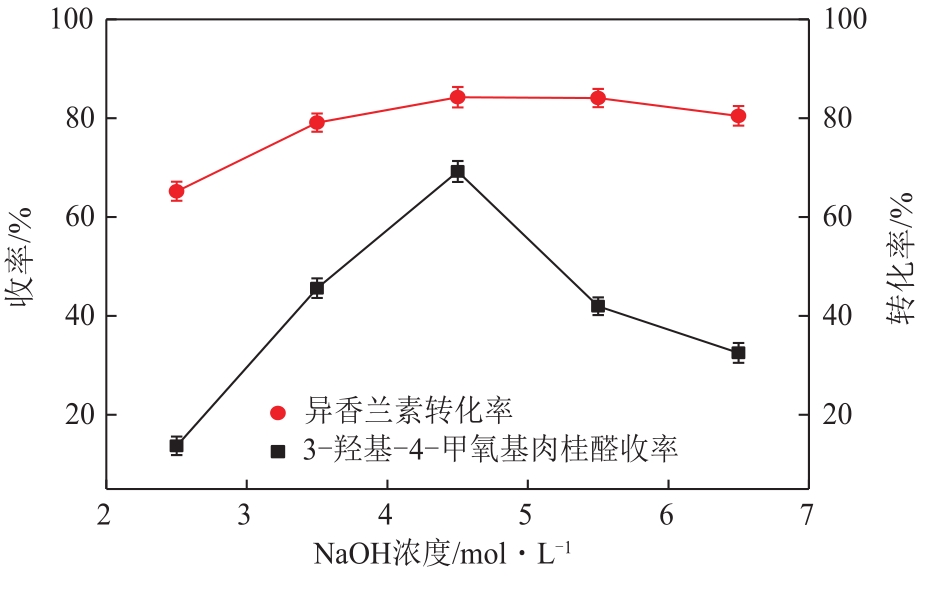
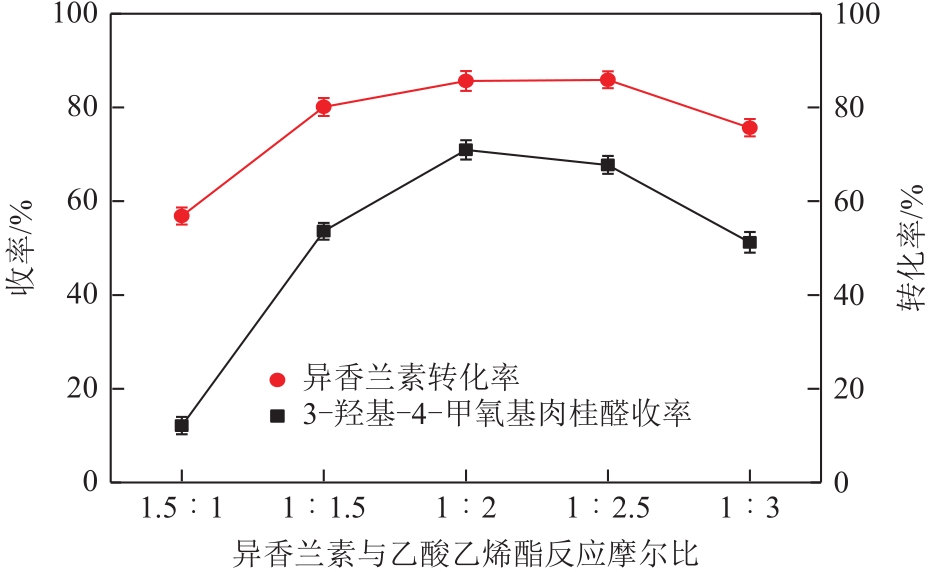
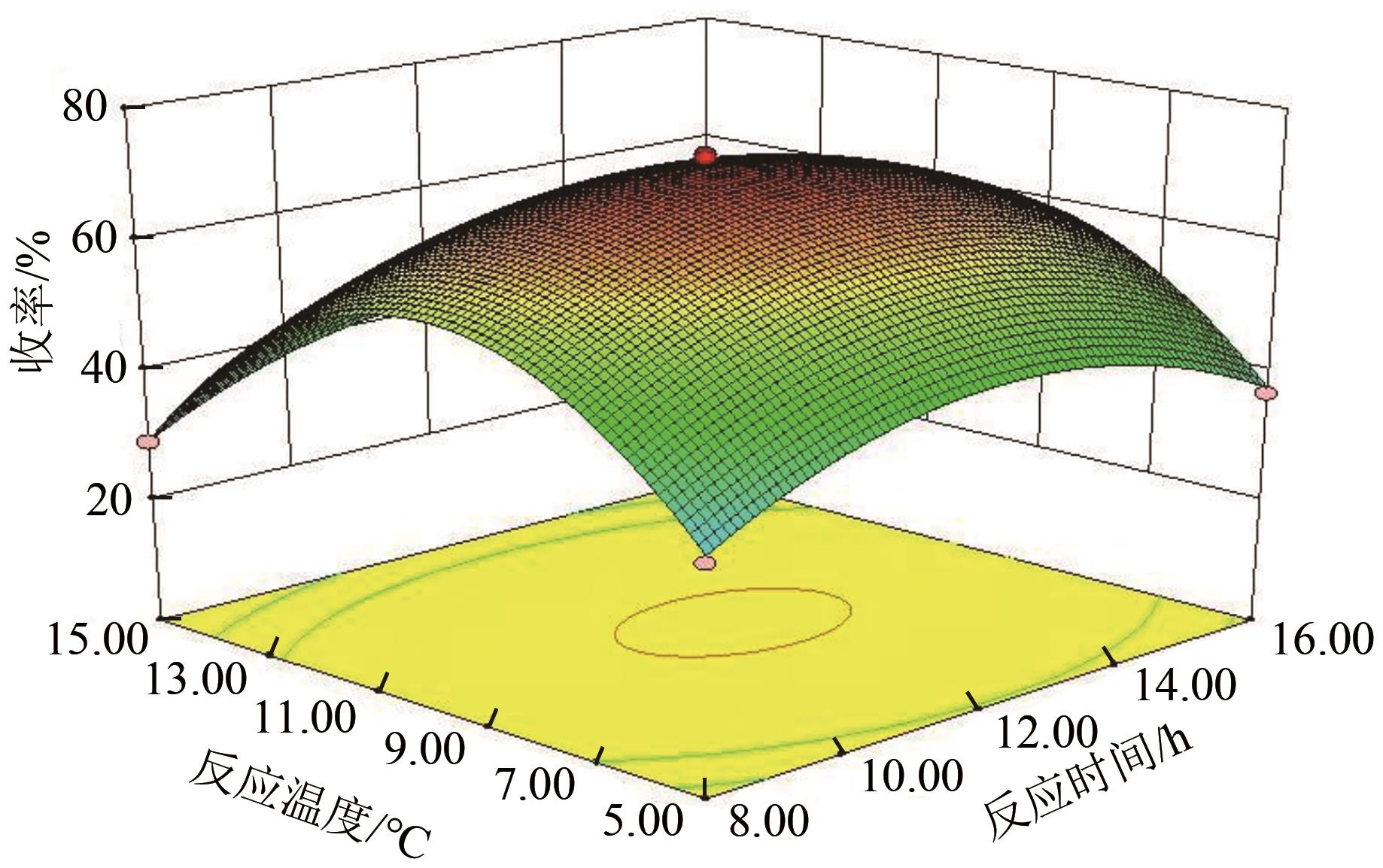
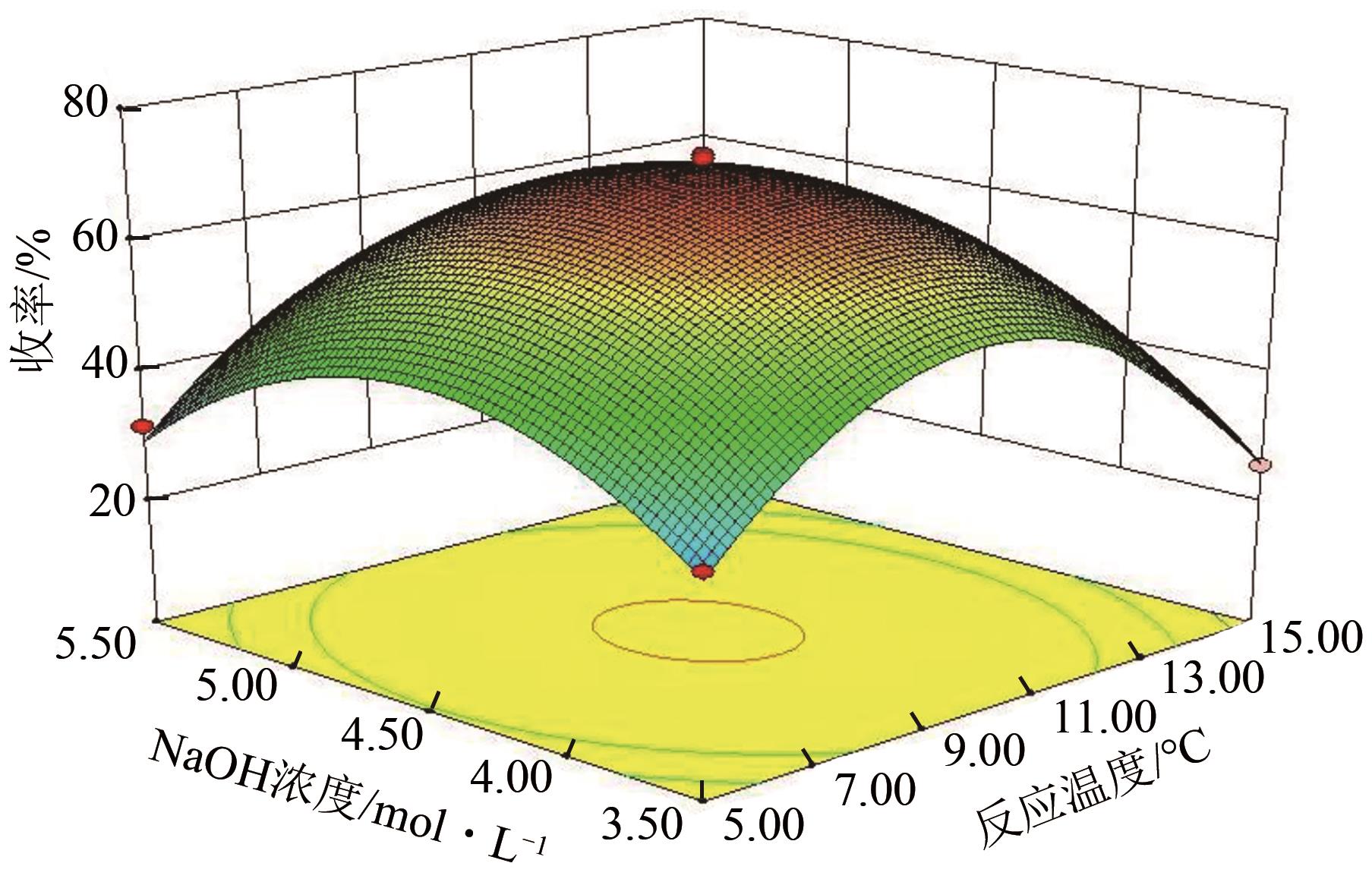
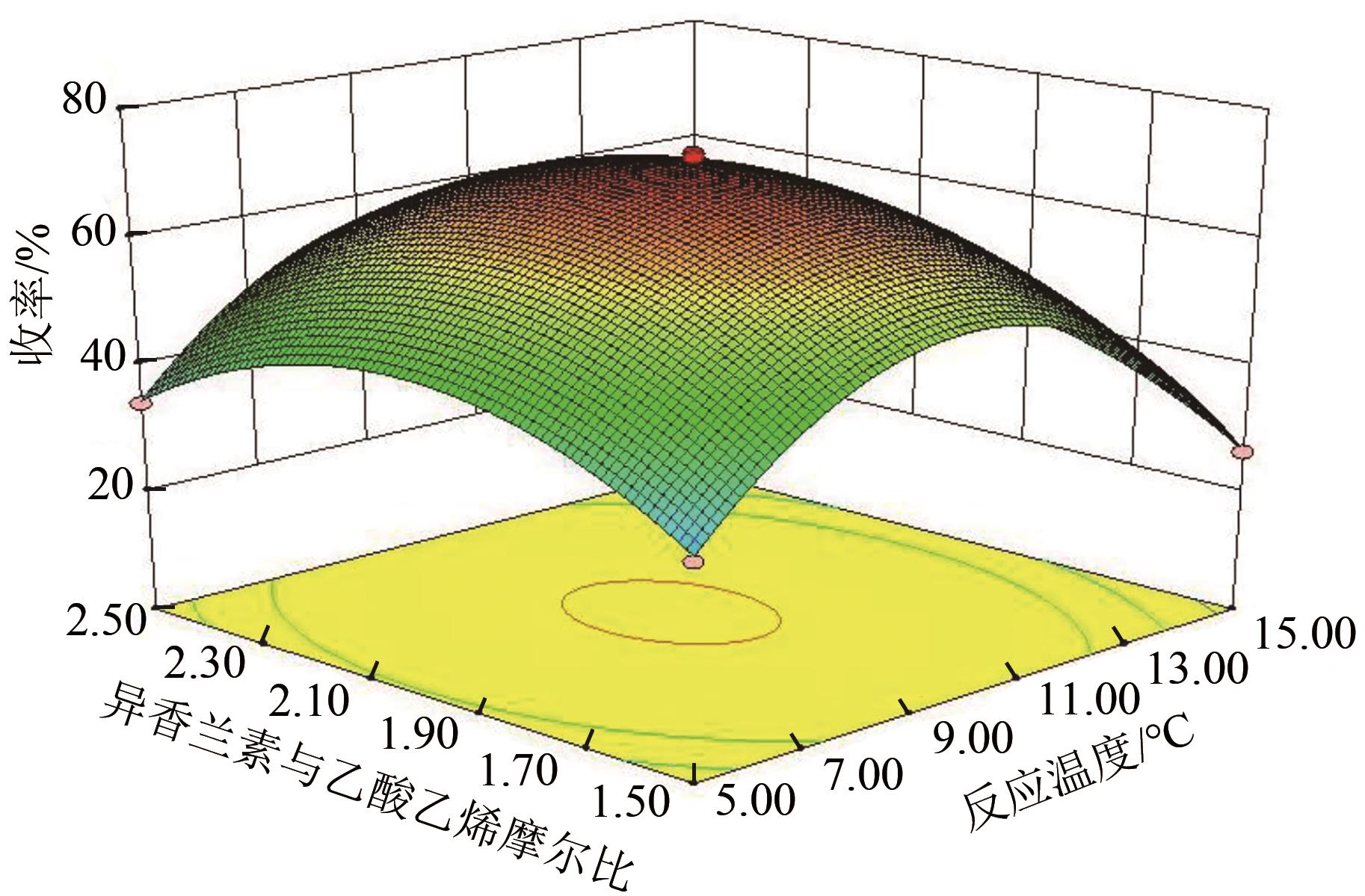
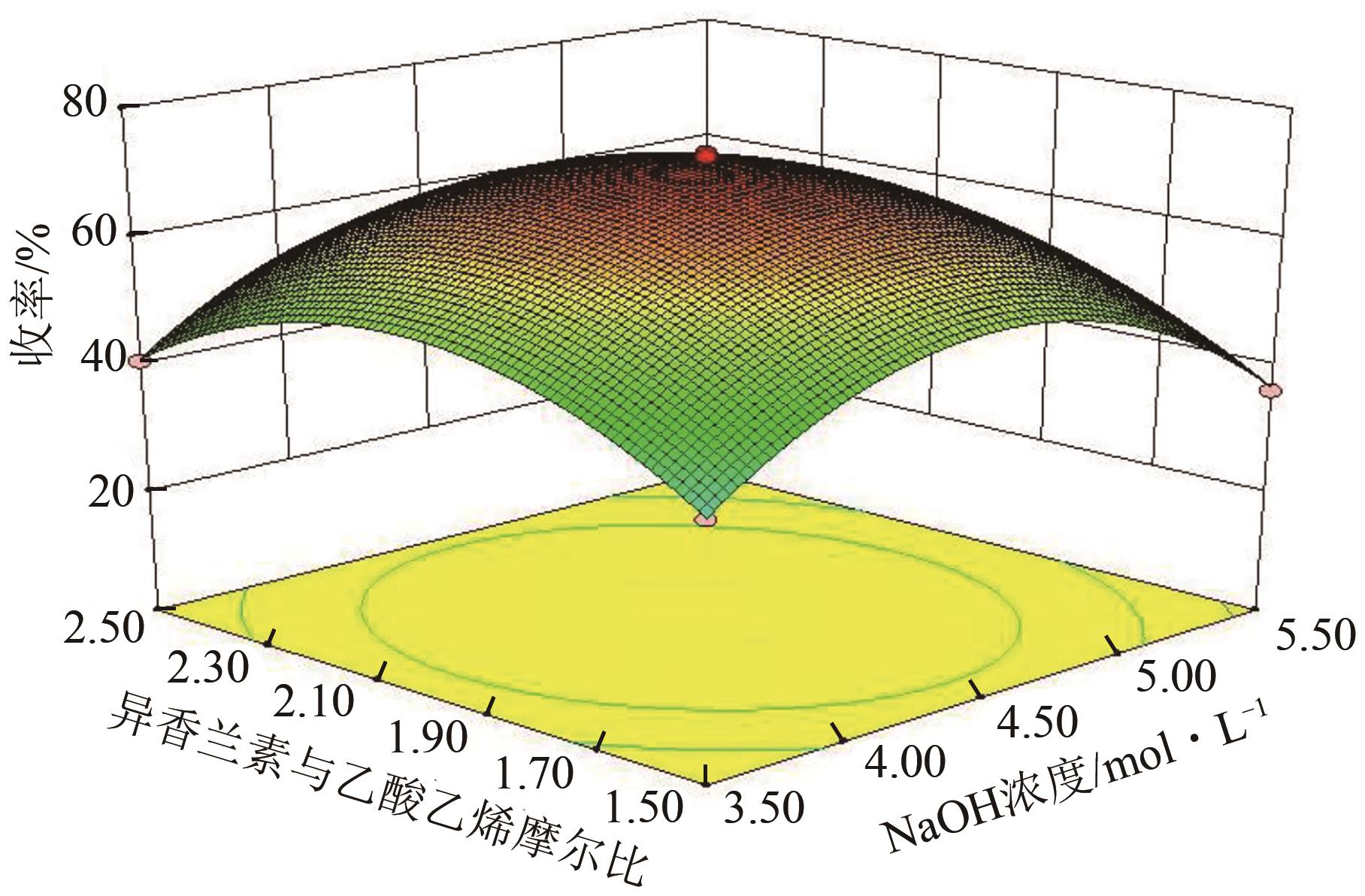
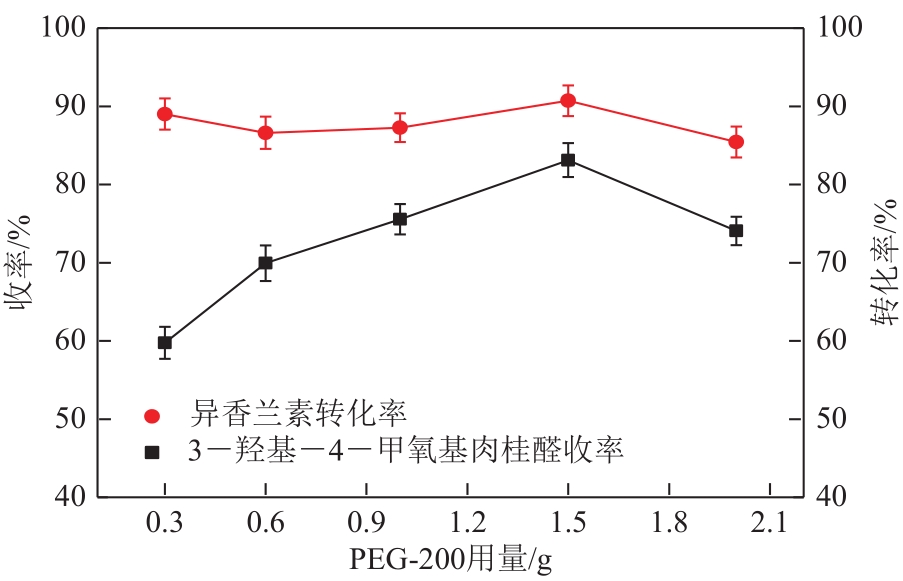
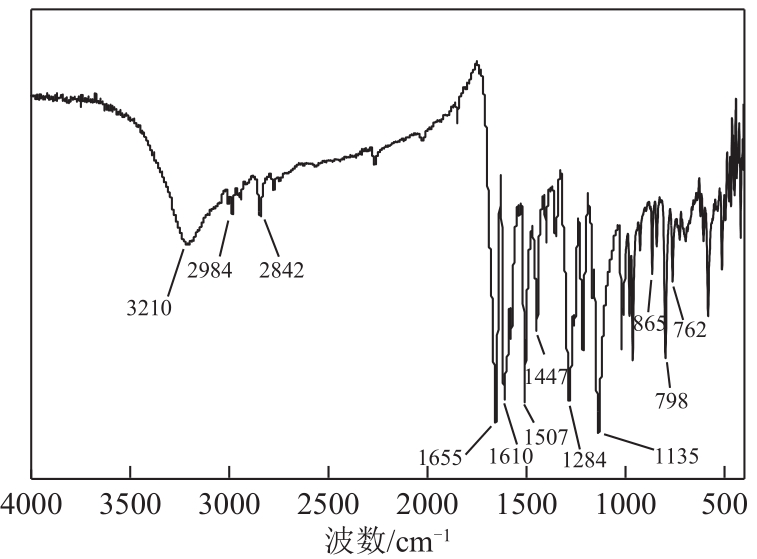
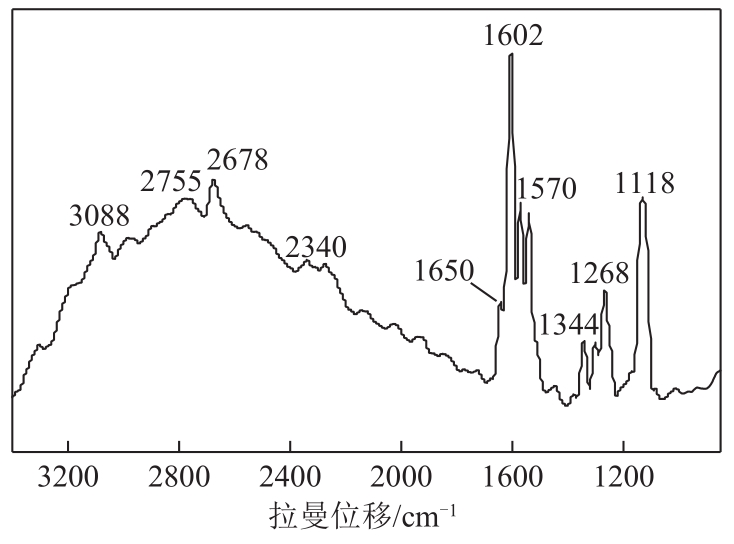
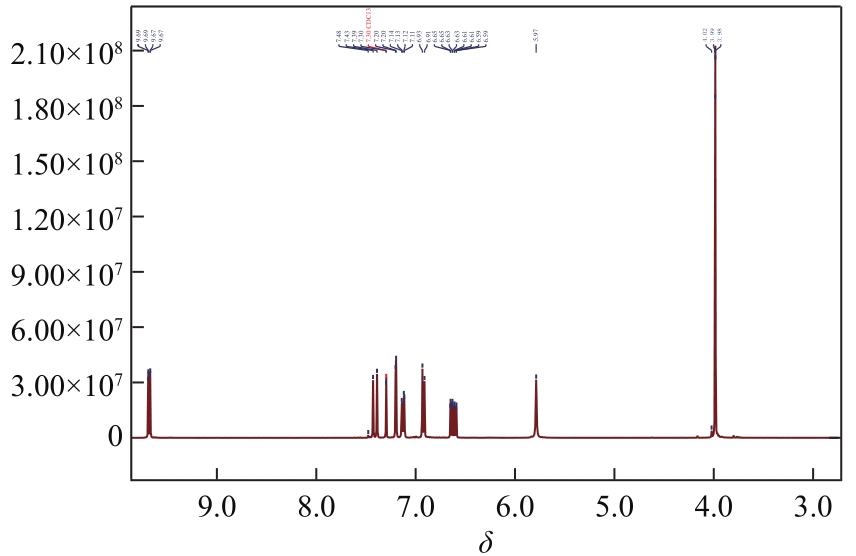
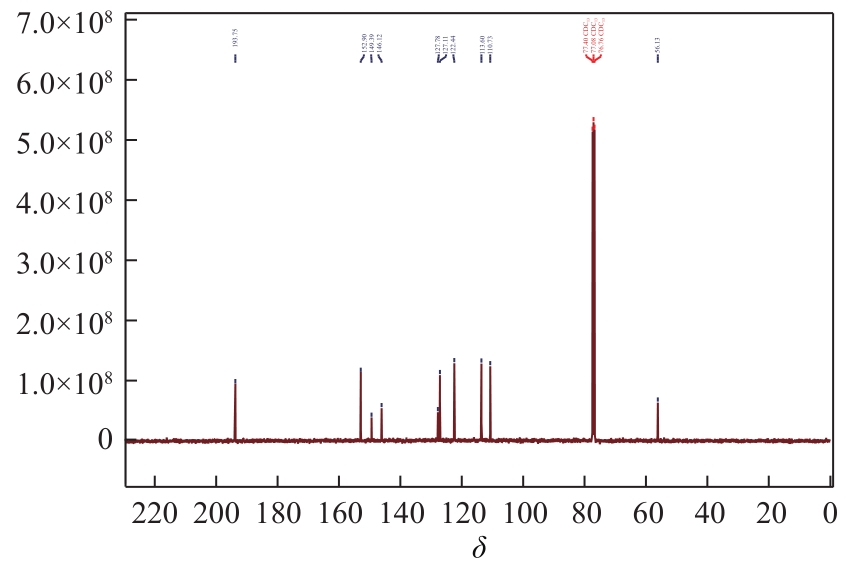
3-羟基-4-甲氧基肉桂醛是制备新型的二肽超高甜度甜味剂爱德万甜的重要中间体。目前制备该中间体的主要方法是通过异香兰素与乙醛在强碱存在下进行羟醛缩合反应,但由于乙醛容易发生自缩合反应从而存在反应条件极为苛刻和3-羟基-4-甲氧基肉桂醛收率低等问题,本文以乙酸乙烯作为反应物替代乙醛与异香兰素发生反应合成3-羟基-4-甲氧基肉桂醛,采用单因素实验考察了反应温度、反应时间、NaOH浓度和异香兰素与乙酸乙烯配比对该反应的影响,并在此基础上进行了响应面优化。在优化的反应条件(NaOH浓度4.5mol/L,反应温度10℃,反应时间12h,异香兰素与乙酸乙烯摩尔比1∶2)下,3-羟基-4-甲氧基肉桂醛收率为71.44%±2.21%。为了进一步提高产品收率,探索了添加相转移催化剂对该反应的强化作用,发现PEG-200对强化该反应有显著作用,3-羟基-4-甲氧基肉桂醛收率可达83.12%±2.18%。
中图分类号:
引用本文
方聪, 刘怡雪, 黎四芳. 爱德万甜中间体3-羟基-4-甲氧基肉桂醛的合成[J]. 化工进展, 2022, 41(11): 6053-6060.
FANG Cong, LIU Yixue, LI Sifang. Synthesis of 3-hydroxy-4-methoxy cinnamaldehyde as intermediate of advantame[J]. Chemical Industry and Engineering Progress, 2022, 41(11): 6053-6060.
| 因素 | 水平 | ||
|---|---|---|---|
| -1 | 0 | 1 | |
| A 反应时间/h | 8 | 12 | 16 |
| B 反应温度/℃ | 5 | 10 | 15 |
| C NaOH浓度/mol·L-1 | 0.14 | 0.18 | 0.22 |
| D 异香兰素与乙酸乙烯摩尔比 | 1∶1.5 | 1∶2 | 1∶2.5 |
表1 响应面实验因素水平设计
| 因素 | 水平 | ||
|---|---|---|---|
| -1 | 0 | 1 | |
| A 反应时间/h | 8 | 12 | 16 |
| B 反应温度/℃ | 5 | 10 | 15 |
| C NaOH浓度/mol·L-1 | 0.14 | 0.18 | 0.22 |
| D 异香兰素与乙酸乙烯摩尔比 | 1∶1.5 | 1∶2 | 1∶2.5 |
| 序列 | 因素 | 收率/% | ||||
|---|---|---|---|---|---|---|
| A:反应时间 | B:反应温度 | C:NaOH 浓度 | D:异香兰素与乙酸乙烯摩尔比 | 实际值 | 预测值 | |
| 1 | -1 | 0 | 1 | 0 | 37.94 | 38.92 |
| 2 | 0 | -1 | 1 | 0 | 31.55 | 29.23 |
| 3 | 1 | -1 | 0 | 0 | 36.61 | 37.56 |
| 4 | -1 | -1 | 0 | 0 | 34.17 | 35.31 |
| 5 | 1 | 1 | 0 | 0 | 30.57 | 30.59 |
| 6 | -1 | 1 | 0 | 0 | 28.88 | 29.09 |
| 7 | 0 | 1 | -1 | 0 | 25.36 | 25.66 |
| 8 | -1 | 0 | -1 | 0 | 41.13 | 40.71 |
| 9 | 0 | 0 | 0 | 0 | 69.23 | 71.44 |
| 10 | 0 | 0 | 0 | 0 | 71.25 | 71.44 |
| 11 | 0 | 0 | 0 | 0 | 70.98 | 71.44 |
| 12 | 1 | 0 | -1 | 0 | 43.95 | 43.83 |
| 13 | 0 | 1 | 1 | 0 | 23.25 | 22.44 |
| 14 | 0 | 0 | 0 | 0 | 72.54 | 71.44 |
| 15 | 0 | 0 | 0 | 0 | 73.21 | 71.44 |
| 16 | 0 | -1 | -1 | 0 | 33.29 | 32.07 |
| 17 | 1 | 0 | 1 | 0 | 39.59 | 40.21 |
| 18 | -1 | 0 | 0 | 1 | 45.82 | 44.61 |
| 19 | 0 | 1 | 0 | 1 | 28.79 | 28.75 |
| 20 | 0 | 0 | -1 | 1 | 40.77 | 41.27 |
| 21 | 0 | -1 | 0 | 1 | 33.98 | 34.52 |
| 22 | 1 | 0 | 0 | 1 | 46.78 | 46.12 |
| 23 | 0 | 0 | 1 | 1 | 37.02 | 37.89 |
| 24 | 0 | 0 | -1 | -1 | 39.11 | 39.40 |
| 25 | 0 | -1 | 0 | -1 | 32.93 | 33.83 |
| 26 | 0 | 0 | 1 | -1 | 36.07 | 36.73 |
| 27 | 1 | 0 | 0 | -1 | 45.78 | 44.97 |
| 28 | 0 | 1 | 0 | -1 | 26.07 | 26.40 |
| 29 | -1 | 0 | 0 | -1 | 44.10 | 42.73 |
表2 响应面实验结果表
| 序列 | 因素 | 收率/% | ||||
|---|---|---|---|---|---|---|
| A:反应时间 | B:反应温度 | C:NaOH 浓度 | D:异香兰素与乙酸乙烯摩尔比 | 实际值 | 预测值 | |
| 1 | -1 | 0 | 1 | 0 | 37.94 | 38.92 |
| 2 | 0 | -1 | 1 | 0 | 31.55 | 29.23 |
| 3 | 1 | -1 | 0 | 0 | 36.61 | 37.56 |
| 4 | -1 | -1 | 0 | 0 | 34.17 | 35.31 |
| 5 | 1 | 1 | 0 | 0 | 30.57 | 30.59 |
| 6 | -1 | 1 | 0 | 0 | 28.88 | 29.09 |
| 7 | 0 | 1 | -1 | 0 | 25.36 | 25.66 |
| 8 | -1 | 0 | -1 | 0 | 41.13 | 40.71 |
| 9 | 0 | 0 | 0 | 0 | 69.23 | 71.44 |
| 10 | 0 | 0 | 0 | 0 | 71.25 | 71.44 |
| 11 | 0 | 0 | 0 | 0 | 70.98 | 71.44 |
| 12 | 1 | 0 | -1 | 0 | 43.95 | 43.83 |
| 13 | 0 | 1 | 1 | 0 | 23.25 | 22.44 |
| 14 | 0 | 0 | 0 | 0 | 72.54 | 71.44 |
| 15 | 0 | 0 | 0 | 0 | 73.21 | 71.44 |
| 16 | 0 | -1 | -1 | 0 | 33.29 | 32.07 |
| 17 | 1 | 0 | 1 | 0 | 39.59 | 40.21 |
| 18 | -1 | 0 | 0 | 1 | 45.82 | 44.61 |
| 19 | 0 | 1 | 0 | 1 | 28.79 | 28.75 |
| 20 | 0 | 0 | -1 | 1 | 40.77 | 41.27 |
| 21 | 0 | -1 | 0 | 1 | 33.98 | 34.52 |
| 22 | 1 | 0 | 0 | 1 | 46.78 | 46.12 |
| 23 | 0 | 0 | 1 | 1 | 37.02 | 37.89 |
| 24 | 0 | 0 | -1 | -1 | 39.11 | 39.40 |
| 25 | 0 | -1 | 0 | -1 | 32.93 | 33.83 |
| 26 | 0 | 0 | 1 | -1 | 36.07 | 36.73 |
| 27 | 1 | 0 | 0 | -1 | 45.78 | 44.97 |
| 28 | 0 | 1 | 0 | -1 | 26.07 | 26.40 |
| 29 | -1 | 0 | 0 | -1 | 44.10 | 42.73 |
| 方差来源 | 平方和 | 自由度 | 均方 | F | P | 显著性 |
|---|---|---|---|---|---|---|
| 回归方程 | 6249.78 | 14 | 446.41 | 223.75 | < 0.0001 | + + |
| A | 10.53 | 1 | 10.53 | 5.28 | 0.0376 | |
| B | 130.75 | 1 | 130.75 | 65.53 | < 0.0001 | + + |
| C | 27.57 | 1 | 27.57 | 13.82 | 0.0023 | + |
| D | 6.90 | 1 | 6.90 | 3.46 | 0.0841 | |
| AB | 0.14 | 1 | 0.14 | 0.070 | 0.7945 | |
| AC | 0.34 | 1 | 0.34 | 0.17 | 0.6850 | |
| AD | 0.13 | 1 | 0.13 | 0.065 | 0.8025 | |
| BC | 0.034 | 1 | 0.034 | 0.017 | 0.8977 | |
| BD | 0.70 | 1 | 0.70 | 0.35 | 0.5638 | |
| CD | 0.13 | 1 | 0.13 | 0.063 | 0.8052 | |
| A2 | 979.04 | 1 | 979.04 | 490.72 | < 0.0001 | + + |
| B2 | 4391.39 | 1 | 4391.39 | 2201.09 | < 0.0001 | + + |
| C2 | 2118.43 | 1 | 2118.43 | 1061.82 | < 0.0001 | + + |
| D2 | 1372.84 | 1 | 1372.84 | 688.11 | < 0.0001 | + + |
| 残差 | 27.93 | 14 | 2.00 | — | — | |
| 失拟项 | 18.46 | 10 | 1.85 | 0.78 | 0.6603 | |
| 纯误差 | 9.47 | 4 | 2.37 | — | — | |
| 总和 | 6277.71 | 28 | — | — | — |
表3 方差分析
| 方差来源 | 平方和 | 自由度 | 均方 | F | P | 显著性 |
|---|---|---|---|---|---|---|
| 回归方程 | 6249.78 | 14 | 446.41 | 223.75 | < 0.0001 | + + |
| A | 10.53 | 1 | 10.53 | 5.28 | 0.0376 | |
| B | 130.75 | 1 | 130.75 | 65.53 | < 0.0001 | + + |
| C | 27.57 | 1 | 27.57 | 13.82 | 0.0023 | + |
| D | 6.90 | 1 | 6.90 | 3.46 | 0.0841 | |
| AB | 0.14 | 1 | 0.14 | 0.070 | 0.7945 | |
| AC | 0.34 | 1 | 0.34 | 0.17 | 0.6850 | |
| AD | 0.13 | 1 | 0.13 | 0.065 | 0.8025 | |
| BC | 0.034 | 1 | 0.034 | 0.017 | 0.8977 | |
| BD | 0.70 | 1 | 0.70 | 0.35 | 0.5638 | |
| CD | 0.13 | 1 | 0.13 | 0.063 | 0.8052 | |
| A2 | 979.04 | 1 | 979.04 | 490.72 | < 0.0001 | + + |
| B2 | 4391.39 | 1 | 4391.39 | 2201.09 | < 0.0001 | + + |
| C2 | 2118.43 | 1 | 2118.43 | 1061.82 | < 0.0001 | + + |
| D2 | 1372.84 | 1 | 1372.84 | 688.11 | < 0.0001 | + + |
| 残差 | 27.93 | 14 | 2.00 | — | — | |
| 失拟项 | 18.46 | 10 | 1.85 | 0.78 | 0.6603 | |
| 纯误差 | 9.47 | 4 | 2.37 | — | — | |
| 总和 | 6277.71 | 28 | — | — | — |
| 1 | AMINO Yusuke, MORI Kenich, TOMIYAMA Yasuyuki, et al. Development of new, low calorie sweetener: new aspartame derivative[M]//WEERASINGHE Deepthi K, DUBOIS Grant E. Sweetness and sweeteners: biology, chemistry, and psychophysics. Washington D C: ACS, 2008: 463-480. |
| 2 | BISHAY I E, BURSEY R G. Advantame[M]//O’BRIEN-Nabors Lyn. Alternative sweeteners. 4th edition. Boca Raton: CRC Press, 2012: 31-45. |
| 3 | OTABE A, FUJIEDA T, MASUYAMA T, et al. Advantame-an overview of the toxicity data[J]. Food and Chemical Toxicology: an International Journal Published for the British Industrial Biological Research Association, 2011, 49(S1): S2-S7. |
| 4 | RENWICK A G. Postscript on advantame: a novel high-potency low-calorie sweetener[J]. Food and Chemical Toxicology, 2011, 49: S1. |
| 5 | Food Safety Commission of Japan. Advantame: summary[R]. Tokyo: FSCJ, 2013. |
| 6 | EFSA Panel on Food Additives and Nutrient Sources added to Food ANS). Scientific opinion on the safety of advantame for the proposed uses as a food additive[J]. EFSA Journal, 2013, 11(7): 3301-3368. |
| 7 | ZHANG Jiyue, ZHANG Jianbo, YU Hangyu, et al. Theoretical risk assessment of dietary exposure to advantame among the Chinese population[J]. Biomedical and Environmental Sciences: BES, 2019, 32(12): 930-933. |
| 8 | DUBOIS Grant E, PRAKASH Indra. Non-caloric sweeteners, sweetness modulators, and sweetener enhancers[J]. Annual Review of Food Science and Technology, 2012, 3: 353-380. |
| 9 | Kay O’DONNELL. Aspartame, neotame and advantame[M]// Sweeteners and sugar alternatives in food technology. Oxford: John Wiley & Sons Inc., 2012: 117-136. |
| 10 | 黎四芳. 高甜度甜味剂的生产与应用[M]. 厦门: 厦门大学出版社, 2021. |
| LI Sifang. Production and application of high potency sweeteners[M]. Xiamen: Xiamen University Press, 2021. | |
| 11 | 方聪, 刘怡雪, 黎四芳. 新型超高甜度二肽甜味剂爱德万甜的研究进展[J]. 中国食品添加剂, 2021, 32(2): 128-136. |
| FANG Cong, LIU Yixue, LI Sifang. Research progress in new ultra-high potency dipeptide sweetener advantame[J]. China Food Additives, 2021, 32(2): 128-136. | |
| 12 | NAGASHIMA Kazutaka, AOKI Yuuichi, TAKEMOTO Tadashi, et al. Process for production of aspartyl dipeptide ester derivative, novel production intermediate therefor, and process for production thereof: US6794531[P]. 2004-09-21. |
| 13 | MORI Kenichi, FUJITA Shinji, FUNAKOSHI Nao, et al. Process for producing cinnamaldehyde derivatives, use thereof and the like: US7141263[P]. 2006-11-28. |
| 14 | 陈博儒, 陈良, 朱思明. 甜味剂中间体3-羟基-4-甲氧基苯丙烯醛的合成工艺优化及其结构表征[J]. 食品工业科技, 2018, 39(22): 144-149. |
| CHEN Boru, CHEN Liang, ZHU Siming. Optimization of synthesis process and structural characterization of 3-hydroxy-4-methoxy benzal acrolein—A aweetener intermediate[J]. Science and Technology of Food Industry, 2018, 39(22): 144-149. | |
| 15 | CHEN Boru, LIU Qiang, WANG Huan, et al. Purification, characterization, and identification of 3-hydroxy-4-methoxy benzal acrolein—An intermediate of synthesizing advantame[J]. Food Science & Nutrition, 2020, 8(2): 744-753. |
| 16 | 吴美红, 郑建仙. 超高倍甜味剂N-[3-(3-羟基-4-甲氧基苯基)丙基]-阿斯巴甜的合成研究[J]. 食品与发酵工业, 2009, 35(6): 1-5. |
| WU Meihong, ZHENG Jianxian. Synthesis of high potency sweetener N-[3-(3-hydroxy-4-methoxyphenyl) propyl]-aspartyl-L-henylalaninel-methylester[J]. Food and Fermentation Industries, 2009, 35(6): 1-5. | |
| 17 | 刘怡雪. Advantame的合成[D]. 厦门: 厦门大学, 2018. |
| LIU Yixue. The synthesis of advantame[D]. Xiamen: Xiamen University, 2018. | |
| 18 | DIVI M K P, RAO M A N, NOWSHUDDIN S. Process for the preparation of advantame:US9512063[P]. 2016-12-06. |
| 19 | Gardner SWAIN C, POWELL Arnet L, SHEPPARD William A, et al. Mechanism of the cannizzaro reaction[J]. Journal of the American Chemical Society, 1979, 101(13): 3576-3583. |
| 20 | J J Vanden EYNDE, MUTONKOLE K, VAN HAVERBEKE Y. Surfactant-assisted organic reactions in water. Effect of ultrasound on condensation reactions between active methylene compounds and arylaldehydes[J]. Ultrasonics Sonochemistry, 2001, 8(1): 35-39. |
| 21 | 丁秋平. 胶束中氨基酸直接催化Aldol反应研究[D]. 南昌: 江西师范大学, 2003. |
| DING Qiuping. Study of the aldol reactions directly catalyzed by amino acids in aqueous micelles[D]. Nanchang: Jiangxi Normal University, 2003. | |
| 22 | VASHISHTHA Manu, MISHRA Manish, SHAH Dinesh O. A novel approach for selective cross aldol condensation using reusable NaOH-cationic micellar systems[J]. Applied Catalysis A: General, 2013, 466: 38-44. |
| 23 | 张媛媛, 郎万中, 褚联峰, 等. PVP相转移催化合成辛烯醛[J]. 广州化工, 2009, 37(9): 155-157, 160. |
| ZHANG Yuanyuan, LANG Wanzhong, CHU Lianfeng, et al. Synthesis of 2-ethylhexenal with phase transfer catalyst PVP[J]. Guangzhou Chemical Industry, 2009, 37(9): 155-157, 160. | |
| 24 | 陈逸. 表面活性剂及其在相转移催化方面的应用研究[J]. 当代化工研究, 2019(1): 105-106. |
| CHEN Yi. Surfactant and its application in phase transfer catalysis[J]. Modern Chemical Research, 2019(1): 105-106. |
| [1] | 李梦圆, 郭凡, 李群生. 聚乙烯醇生产中回收工段第三、第四精馏塔的模拟与优化[J]. 化工进展, 2023, 42(S1): 113-123. |
| [2] | 张瑞杰, 刘志林, 王俊文, 张玮, 韩德求, 李婷, 邹雄. 水冷式复叠制冷系统的在线动态模拟与优化[J]. 化工进展, 2023, 42(S1): 124-132. |
| [3] | 王福安. 300kt/a环氧丙烷工艺反应器降耗减排分析[J]. 化工进展, 2023, 42(S1): 213-218. |
| [4] | 陈匡胤, 李蕊兰, 童杨, 沈建华. 质子交换膜燃料电池气体扩散层结构与设计研究进展[J]. 化工进展, 2023, 42(S1): 246-259. |
| [5] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [6] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [7] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [8] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [9] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [10] | 赵巍, 赵德银, 李世瀚, 刘洪达, 孙进, 郭艳秋. 三嗪型天然气管道缓蚀型减阻剂合成与应用[J]. 化工进展, 2023, 42(S1): 391-399. |
| [11] | 王正坤, 黎四芳. 双子表面活性剂癸炔二醇的绿色合成[J]. 化工进展, 2023, 42(S1): 400-410. |
| [12] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [13] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [14] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [15] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||