化工进展 ›› 2022, Vol. 41 ›› Issue (8): 4147-4158.DOI: 10.16085/j.issn.1000-6613.2021-2140
过渡金属单原子催化剂活化H2O2/PMS/PDS降解有机污染物的研究进展
- 南华大学土木工程学院,湖南 衡阳 421001
-
收稿日期:2021-10-18修回日期:2022-01-14出版日期:2022-08-25发布日期:2022-08-22 -
通讯作者:周书葵 -
作者简介:段毅(1987—),男,博士,工程师,研究方向为水质净化与水污染控制等。E-mail:duanyi1987@163.com 。 -
基金资助:国家自然科学基金(51174117);湖南省教育厅科研平台项目(15K106);湖南省创新平台开放基金(17K078);南华大学科研启动基金(200XQD039)
Progress in the degradation of organic pollutants by H2O2/PMS/PDS activated by transition metal single-atom catalysts
DUAN Yi( ), ZOU Ye, ZHOU Shukui(
), ZOU Ye, ZHOU Shukui( ), YANG Liu
), YANG Liu
- School of Civil Engineering, University of South China, Hengyang 421001, Hunan, China
-
Received:2021-10-18Revised:2022-01-14Online:2022-08-25Published:2022-08-22 -
Contact:ZHOU Shukui
摘要:
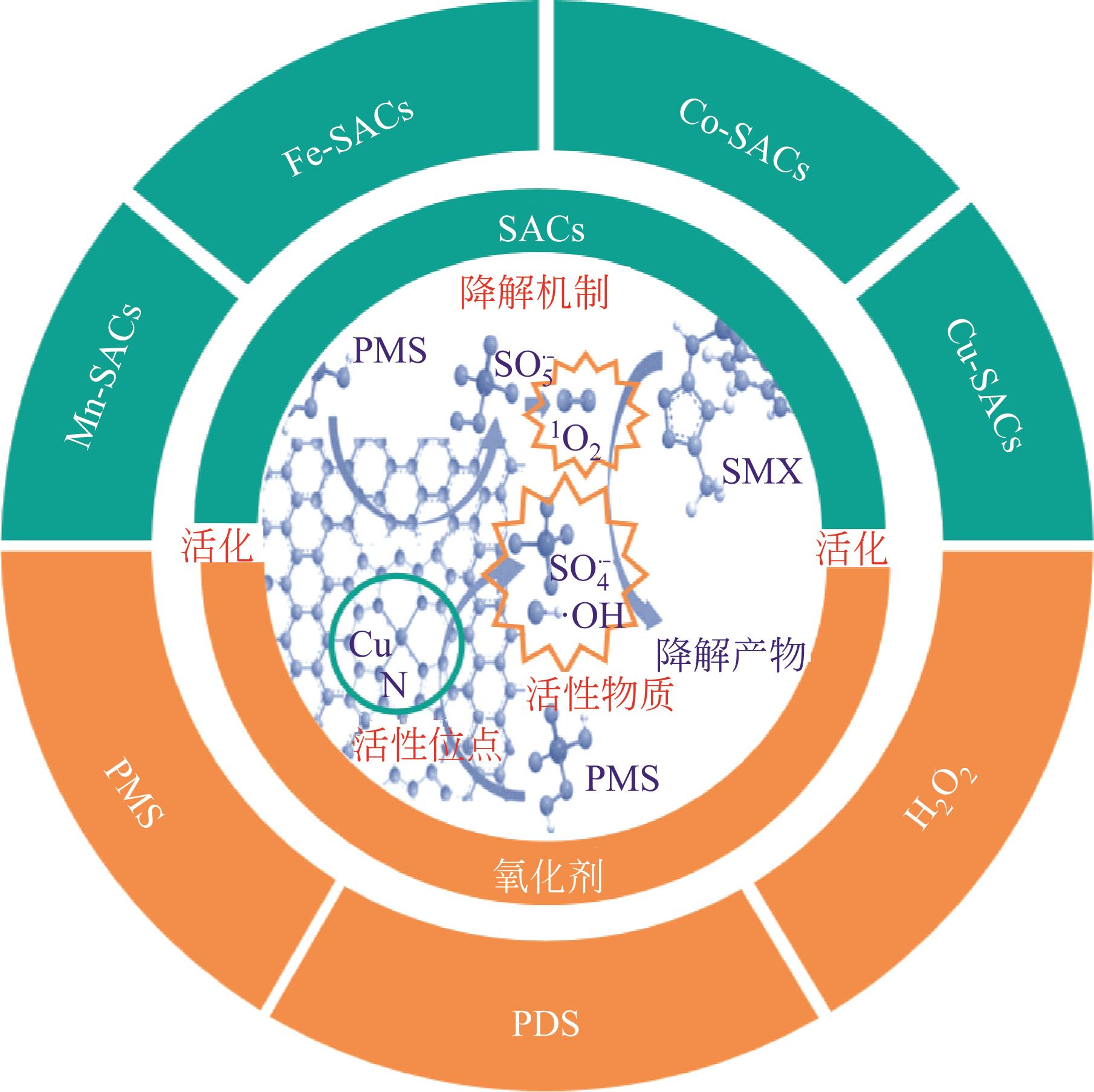
单原子催化剂(SACs)是一种将金属以原子态负载于载体上的新型材料,具有原子利用率高、催化活性强和易回收等优点,使其在催化降解有机污染物方面备受关注。本文介绍了SACs的催化影响因素,总结了SACs催化降解有机污染物在环境领域中的应用。此外,着重综述了不同过渡金属(Fe、Co、Mn、Cu等)单原子催化剂在基于双氧水或过硫酸盐的高级氧化技术中的催化机理,单原子金属(M)一般与N键合形成活性位点M—N x,活化氧化剂生成自由基或单线态氧,高效降解有机污染物。最后,提出未来SACs在催化降解有机污染物的研究方向是合成金属负载量高、稳定性高、pH适用范围更广的SACs,以及根据SACs的结构-性能关系和催化机理,对目标污染物设计特定催化剂。
中图分类号:
引用本文
段毅, 邹烨, 周书葵, 杨柳. 过渡金属单原子催化剂活化H2O2/PMS/PDS降解有机污染物的研究进展[J]. 化工进展, 2022, 41(8): 4147-4158.
DUAN Yi, ZOU Ye, ZHOU Shukui, YANG Liu. Progress in the degradation of organic pollutants by H2O2/PMS/PDS activated by transition metal single-atom catalysts[J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4147-4158.
| 金属 | 催化剂/g·L-1 | 合成方法 | 有机物及浓度 /mg·L-1 | 氧化剂及浓度 /mmol·L-1 | 循环次数 (效率) | 降解效率 /%(min) | 主要活性基团 | 参考 文献 |
|---|---|---|---|---|---|---|---|---|
| Fe | Fe x Mo1-x S2(0.1) | 水热解法 | PPA(20) | PDS(1) | 5(62.1%) | 90(30) | SO | [ |
| Fe/MnO2(0.2) | 热处理法 | MB(20) | H2O2(4.4) | — | 82(80) | ·OH | [ | |
| Fe-N-C(0.02) | 球磨法 | 2,4-DCP(3.3) | PDS(0.2) | — | 90(60) | Fe(Ⅴ) | [ | |
| Fe3O4/MIL-101(1) | 超声法 | OPD(50) | H2O2(0.66) | 5(95%) | 97.79(25) | ·OH | [ | |
| FePC/石墨烯(0.2) | 煅烧酸洗法 | 苯酚(50) | H2O2(4.4) | 5(55%) | 77.1(180) | ·OH | [ | |
| Fe-g-C3N4(0.2) | 高温热解法 | MB(20) | H2O2(77) | — | 99.16(80) | ·OH、1O2 | [ | |
| FeSA-N/C(0.15) | 热处理法 | BPA(20) | PMS(11.77) | 5(81%) | 99.3(20) | 1O2 | [ | |
| SA Fe-g-C3N4(0.1) | 高温煅烧法 | TC(50) | PMS(0.5) | 4(91%) | 93.29(40) | ·OH、SO | [ | |
| Co | SA Co-N/C(0.05) | 高温煅烧法 | NPX(10) | PMS(0.5) | 4(96%) | 100(50) | ·OH、SO | [ |
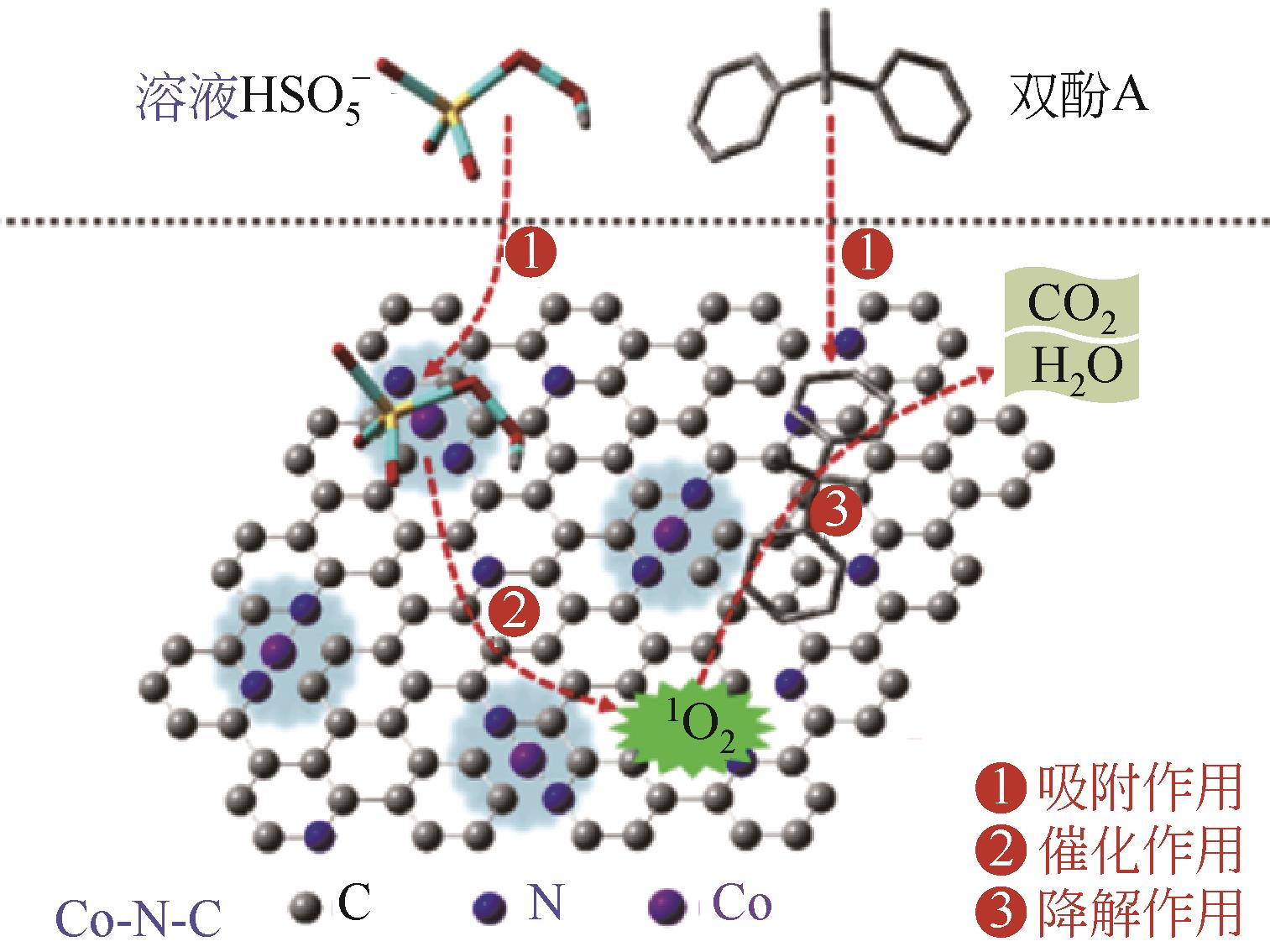
| Co-N-C(0.5) | 煅烧酸洗法 | BPA(80) | PMS(0.98) | 3(96.3%) | 100(60) | 1O2 | [ | |
| Co-C-N(0.5) | 模板蚀刻法 | AO7(50) | PMS(0.1) | 6(99.3%) | 100(10) | SO | [ | |
| SA Co-N-C(0.1) | 热解法 | CQP(10) | PMS(1) | 3(80%) | 98(20) | SO | [ | |
| BCN/CoN(0.03) | 高温煅烧法 | TC(50) | PMS(8.82) | 5(100%) | 100(60) | 1O2 | [ | |
| FeCo-NC-2(0.1) | 热处理法 | BPA(20) | PMS(0.65) | 8(85%) | 98(60) | SO | [ | |
| Mn | Mn-ISAs@CN(0.2) | 热解法 | BPA(20) | PMS(0.65) | 5(80%) | 90(60) | ·OH | [ |
| Mn-CN(0.1) | 热解法 | OA(10) | H2O2(—) | 5(82%) | 100(40) | ·OH | [ | |
| SA-Mn/NG(0.1) | 高温煅烧法 | SMX(10) | PMS(1) | 4(84%) | 97(40) | ·OH、SO | [ | |
| SA-Mn/g-C3N4(0.1) | 高温煅烧法 | TBBPA(50) | PMS(5) | 5(93%) | 100(30) | 1O2、SO | [ | |
| Cu | Cu-C3N4(1) | 热解法 | RhB(10) | H2O2(29.4) | — | 99.97(60) | ·OH | [ |
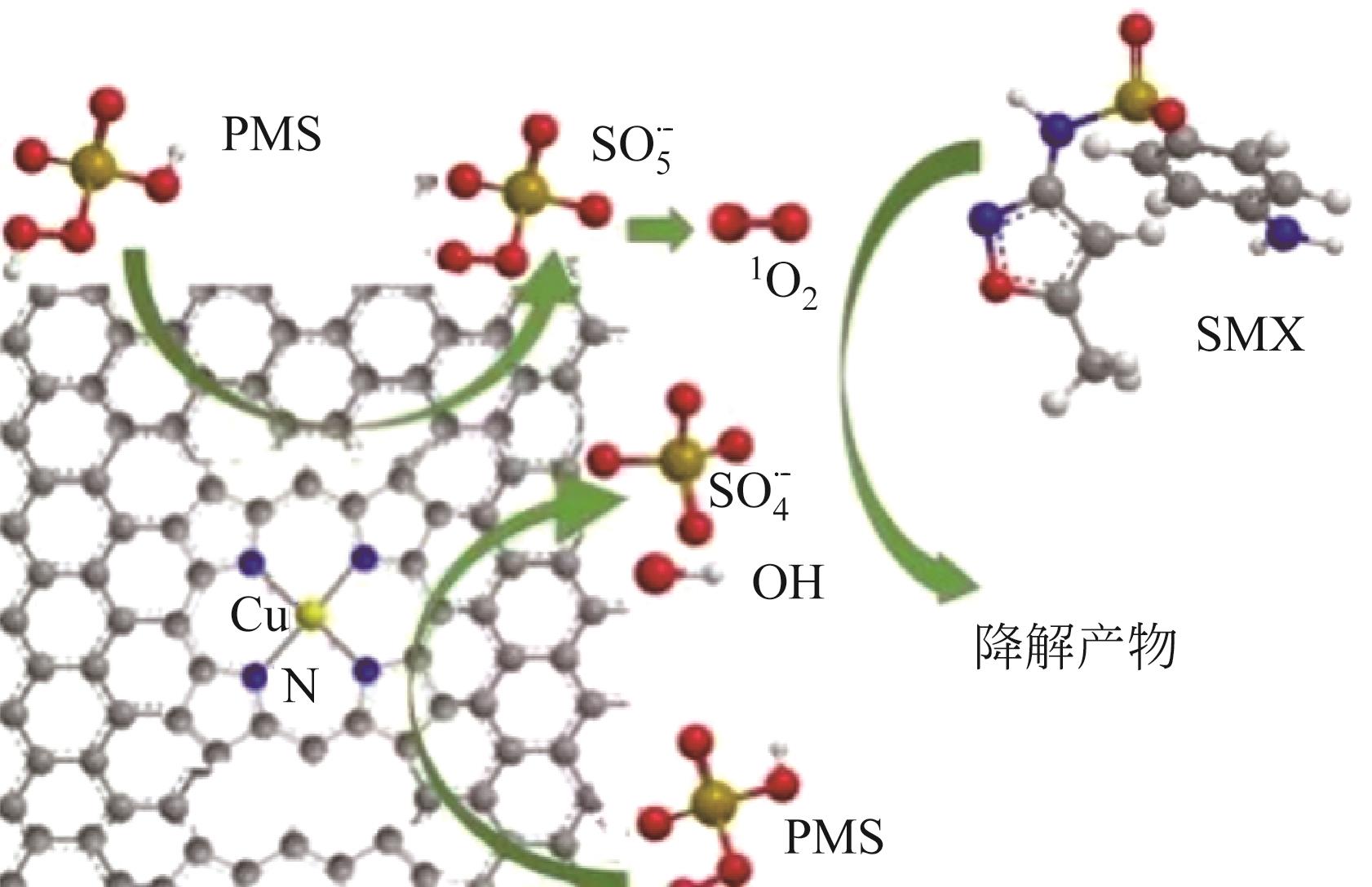
| SA-Cu/rGO(0.1) | 球磨法 | SMX(10) | PMS(1.3) | 5(91.6%) | 99.6(60) | ·OH、SO | [ | |
| SA-Cu@NBC(0.1) | 高温煅烧法 | BPA(20) | PMS(11.77) | 4(97%) | 100(60) | SO | [ | |
| 双金属 | Co/Fe-N-C(0.5) | 热解法 | 苯酚(100) | PDS(10) | 5(70.4%) | 79.2(120) | SO | [ |
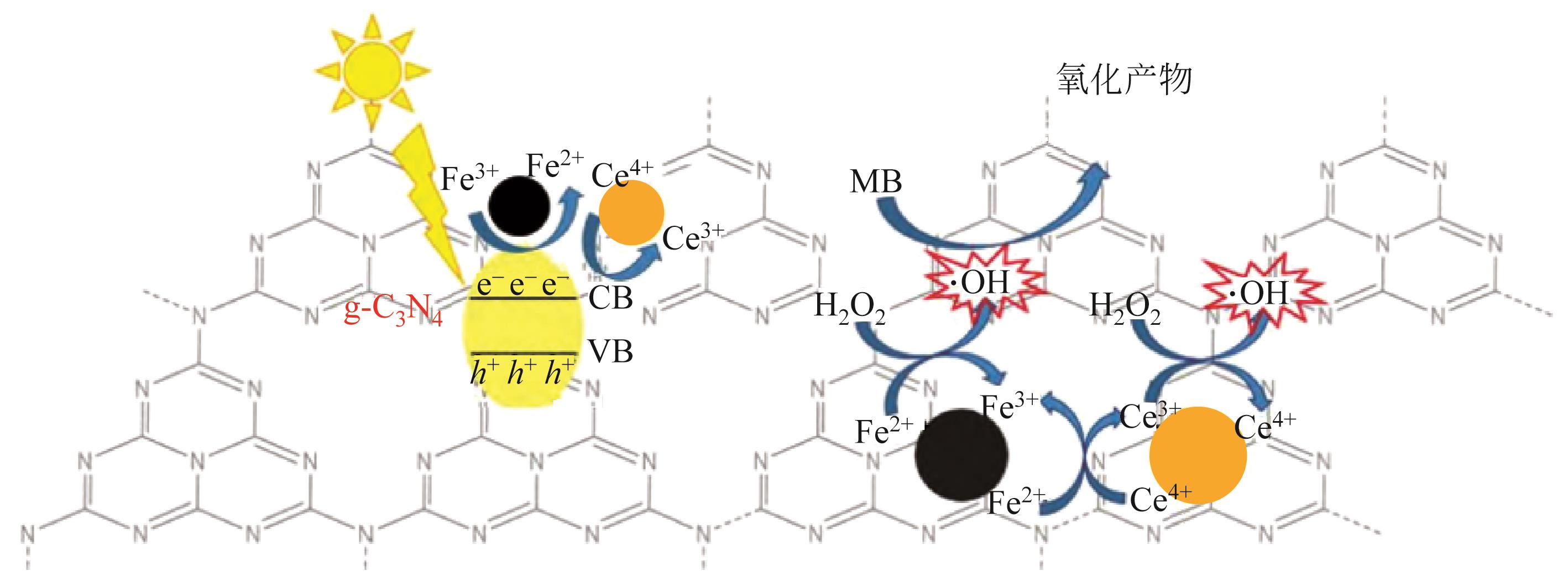
| Fe-Ce/g-C3N4(0.5) | 高温煅烧法 | MB(200) | H2O2(4.4) | 3(90%) | 100(40) | ·OH、·OOH | [ | |
| FeBi-NC(0.03) | 热解法 | RhB(30) | PMS(4.41) | 5(99%) | 100(5) | ·OH、SO | [ | |
| Fe/Cu-N-C(0.1) | 热解法 | CAP(20) | PDS(5) | 5(90.8%) | 90.8(60) | ·OH、SO | [ | |
| Pt | Pt/Al2O3(0.2) | 热处理法 | 1,4-D(20) | H2O2(3.5) | 4(76%) | 95(60) | SO | [ |
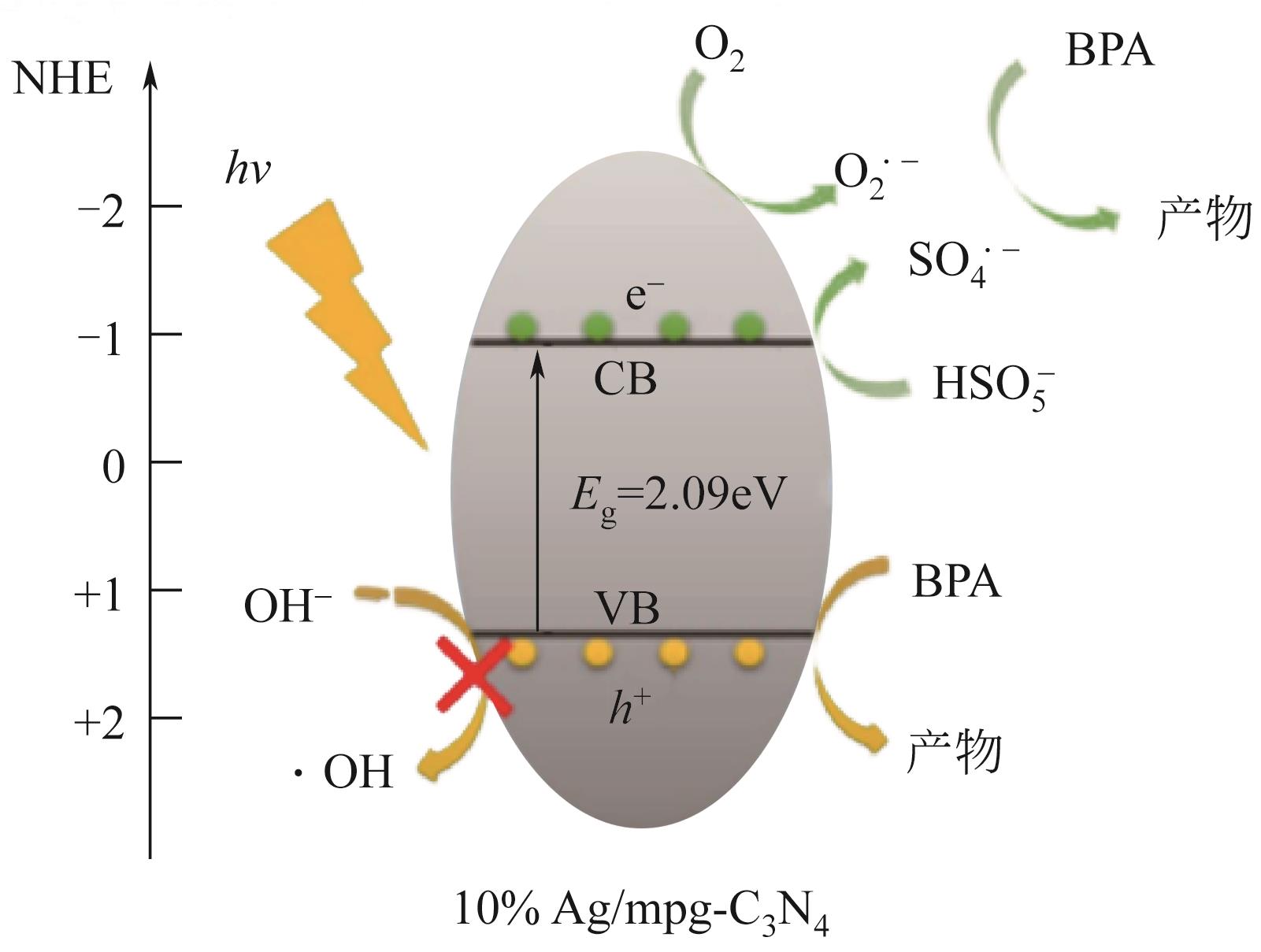
| Ag | Ag/mpg-C3N4(0.1) | 高温煅烧法 | BPA(20) | PMS(1) | 4(76%) | 98(60) | SO | [ |
表1 单原子催化剂用于高级氧化中降解有机污染物的应用
| 金属 | 催化剂/g·L-1 | 合成方法 | 有机物及浓度 /mg·L-1 | 氧化剂及浓度 /mmol·L-1 | 循环次数 (效率) | 降解效率 /%(min) | 主要活性基团 | 参考 文献 |
|---|---|---|---|---|---|---|---|---|
| Fe | Fe x Mo1-x S2(0.1) | 水热解法 | PPA(20) | PDS(1) | 5(62.1%) | 90(30) | SO | [ |
| Fe/MnO2(0.2) | 热处理法 | MB(20) | H2O2(4.4) | — | 82(80) | ·OH | [ | |
| Fe-N-C(0.02) | 球磨法 | 2,4-DCP(3.3) | PDS(0.2) | — | 90(60) | Fe(Ⅴ) | [ | |
| Fe3O4/MIL-101(1) | 超声法 | OPD(50) | H2O2(0.66) | 5(95%) | 97.79(25) | ·OH | [ | |
| FePC/石墨烯(0.2) | 煅烧酸洗法 | 苯酚(50) | H2O2(4.4) | 5(55%) | 77.1(180) | ·OH | [ | |
| Fe-g-C3N4(0.2) | 高温热解法 | MB(20) | H2O2(77) | — | 99.16(80) | ·OH、1O2 | [ | |
| FeSA-N/C(0.15) | 热处理法 | BPA(20) | PMS(11.77) | 5(81%) | 99.3(20) | 1O2 | [ | |
| SA Fe-g-C3N4(0.1) | 高温煅烧法 | TC(50) | PMS(0.5) | 4(91%) | 93.29(40) | ·OH、SO | [ | |
| Co | SA Co-N/C(0.05) | 高温煅烧法 | NPX(10) | PMS(0.5) | 4(96%) | 100(50) | ·OH、SO | [ |
| Co-N-C(0.5) | 煅烧酸洗法 | BPA(80) | PMS(0.98) | 3(96.3%) | 100(60) | 1O2 | [ | |
| Co-C-N(0.5) | 模板蚀刻法 | AO7(50) | PMS(0.1) | 6(99.3%) | 100(10) | SO | [ | |
| SA Co-N-C(0.1) | 热解法 | CQP(10) | PMS(1) | 3(80%) | 98(20) | SO | [ | |
| BCN/CoN(0.03) | 高温煅烧法 | TC(50) | PMS(8.82) | 5(100%) | 100(60) | 1O2 | [ | |
| FeCo-NC-2(0.1) | 热处理法 | BPA(20) | PMS(0.65) | 8(85%) | 98(60) | SO | [ | |
| Mn | Mn-ISAs@CN(0.2) | 热解法 | BPA(20) | PMS(0.65) | 5(80%) | 90(60) | ·OH | [ |
| Mn-CN(0.1) | 热解法 | OA(10) | H2O2(—) | 5(82%) | 100(40) | ·OH | [ | |
| SA-Mn/NG(0.1) | 高温煅烧法 | SMX(10) | PMS(1) | 4(84%) | 97(40) | ·OH、SO | [ | |
| SA-Mn/g-C3N4(0.1) | 高温煅烧法 | TBBPA(50) | PMS(5) | 5(93%) | 100(30) | 1O2、SO | [ | |
| Cu | Cu-C3N4(1) | 热解法 | RhB(10) | H2O2(29.4) | — | 99.97(60) | ·OH | [ |
| SA-Cu/rGO(0.1) | 球磨法 | SMX(10) | PMS(1.3) | 5(91.6%) | 99.6(60) | ·OH、SO | [ | |
| SA-Cu@NBC(0.1) | 高温煅烧法 | BPA(20) | PMS(11.77) | 4(97%) | 100(60) | SO | [ | |
| 双金属 | Co/Fe-N-C(0.5) | 热解法 | 苯酚(100) | PDS(10) | 5(70.4%) | 79.2(120) | SO | [ |
| Fe-Ce/g-C3N4(0.5) | 高温煅烧法 | MB(200) | H2O2(4.4) | 3(90%) | 100(40) | ·OH、·OOH | [ | |
| FeBi-NC(0.03) | 热解法 | RhB(30) | PMS(4.41) | 5(99%) | 100(5) | ·OH、SO | [ | |
| Fe/Cu-N-C(0.1) | 热解法 | CAP(20) | PDS(5) | 5(90.8%) | 90.8(60) | ·OH、SO | [ | |
| Pt | Pt/Al2O3(0.2) | 热处理法 | 1,4-D(20) | H2O2(3.5) | 4(76%) | 95(60) | SO | [ |
| Ag | Ag/mpg-C3N4(0.1) | 高温煅烧法 | BPA(20) | PMS(1) | 4(76%) | 98(60) | SO | [ |
| 1 | CHENG Min, ZENG Guangming, HUANG Danlian, et al. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: a review[J]. Chemical Engineering Journal, 2016, 284: 582-598. |
| 2 | PETRIE B, BARDEN R, KASPZYK-HORDERN B. A review on emerging contaminants in wastewaters and the environment: current knowledge, understudied areas and recommendations for future monitoring[J]. Water Research, 2015, 72: 3-27. |
| 3 | TRAN N H, REINHARD M, GIN K Y H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions: a review[J]. Water Research, 2018, 133: 182-207. |
| 4 | FLYTZANI-STEPHANOPOULOS M. Gold atoms stabilized on various supports catalyze the water-gas shift reaction[J]. Accounts of Chemical Research, 2014, 47(3): 783-792. |
| 5 | WANG Xun, PENG Qing, LI Yadong. Interface-mediated growth of monodispersed nanostructures[J]. Accounts of Chemical Research, 2007, 40(8): 635-643. |
| 6 | CHEN Guangxu, XU Chaofa, HUANG Xiaoqing, et al. Interfacial electronic effects control the reaction selectivity of platinum catalysts[J]. Nature Materials, 2016, 15(5): 564-569. |
| 7 | DENG Dehui, NOVOSELOV K S, FU Qiang, et al. Catalysis with two-dimensional materials and their heterostructures[J]. Nature Nanotechnology, 2016, 11(3): 218-230. |
| 8 | ZHAO Guofeng, YANG Fan, CHEN Zongjia, et al. Metal/oxide interfacial effects on the selective oxidation of primary alcohols[J]. Nature Communications, 2017, 8: 14039. |
| 9 | JIN Huanyu, GUO Chunxian, LIU Xin, et al. Emerging two-dimensional nanomaterials for electrocatalysis[J]. Chemical Reviews, 2018, 118(13): 6337-6408. |
| 10 | JIAO Long, JIANG Hailong. Metal-organic-framework-based single-atom catalysts for energy applications[J]. Chem, 2019, 5(4): 786-804. |
| 11 | YANG Xiaofeng, WANG Aiqin, QIAO Botao, et al. Single-atom catalysts: a new frontier in heterogeneous catalysisy[J]. Accounts of Chemical Research, 2013, 46(8): 1740-1748. |
| 12 | WEON S, HUANG Dahong, RIGBY K, et al. Environmental materials beyond and below the nanoscale: single-atom catalysts[J]. ACS ES&T Engineering, 2021, 1(2): 157-172. |
| 13 | HUANG Bingkun, WU Zelin, ZHOU Hongyu, et al. Recent advances in single-atom catalysts for advanced oxidation processes in water purification[J]. Journal of Hazardous Materials, 2021, 412: 125253. |
| 14 | SHANG Yanan, XU Xing, GAO Baoyu, et al. Single-atom catalysis in advanced oxidation processes for environmental remediation[J]. Chemical Society Reviews, 2021, 50(8): 5281-5322. |
| 15 | NEYENS E, BAEYENS J. A review of classic Fenton’s peroxidation as an advanced oxidation technique[J]. Journal of Hazardous Materials, 2003, 98(1/2/3): 33-50. |
| 16 | 韩旭, 漆舒羽, 张锋伟, 等. 原子级单分散Fe催化剂的高效合成及在可见光下染料降解性能的研究[J].山西大学学报(自然科学版), 2020, 43(3): 552-558. |
| HAN Xu, QI Shuyu, ZHANG Fengwei, et al. Study on high-efficiency synthesis of monodisperse Fe catalyst and properties of visible light degradation[J]. Journal of Shanxi University(Natural Science Edition), 2020, 43(3): 552-558. | |
| 17 | YIN Yu, SHI Lei, LI Wenlang, et al. Boosting Fenton-like reactions via single atom Fe catalysis[J]. Environmental Science & Technology, 2019, 53(19): 11391-11400. |
| 18 | GAO Yaowen, ZHU Yue, Lai LYU, et al. Electronic structure modulation of graphitic carbon nitride by oxygen doping for enhanced catalytic degradation of organic pollutants through peroxymonosulfate activation[J]. Environmental Science & Technology, 2018, 52(24): 14371-14380. |
| 19 | YAO Yunjin, YIN Hongyu, GAO Mengxue, et al. Electronic structure modulation of covalent organic frameworks by single-atom Fe doping for enhanced oxidation of aqueous contaminants[J]. Chemical Engineering Science, 2019, 209: 115211. |
| 20 |
JIANG Ning, XU Haodan, WANG Lihong, et al. Nonradical oxidation of pollutants with single-atom-Fe( )-activated persulfate: Fe( )-activated persulfate: Fe( ) being the possible intermediate oxidant[J]. Environmental Science & Technology, 2020, 54(21): 14057-14065. ) being the possible intermediate oxidant[J]. Environmental Science & Technology, 2020, 54(21): 14057-14065.
|
| 21 | HUANG Lizhi, WEI Xiuli, GAO Enlai, et al. Single Fe atoms confined in two-dimensional MoS2 for sulfite activation: a biomimetic approach towards efficient radical generation[J]. Applied Catalysis B: Environmental, 2020, 268: 118459. |
| 22 | ANIPSITAKIS G P, DIONYSIOU D D. Radical generation by the interaction of transition metals with common oxidants[J]. Environmental Science & Technology, 2004, 38(13): 3705-3712. |
| 23 | LIU Wengang, ZHANG Leilei, YAN Wensheng, et al. Single-atom dispersed Co-N-C catalyst: structure identification and performance for hydrogenative coupling of nitroarenes[J]. Chemical Science, 2016, 7(9): 5758-5764. |
| 24 | CHEN Mantang, WANG Nan, ZHU Lihua. Single-atom dispersed Co-N-C: a novel adsorption-catalysis bifunctional material for rapid removing bisphenol A[J]. Catalysis Today, 2020, 348: 187-193. |
| 25 | 徐劼, 王柯晴, 田丹, 等. 单原子Co-C-N催化过一硫酸盐降解金橙Ⅱ[J]. 中国环境科学, 2021, 41(1): 151-160. |
| XU Jie, WANG Keqing, TIAN Dan, et al. Degradation of AO7 with peroxymonosulfate catalyzed by Co-C-N single atom[J]. China Environmental Science, 2021, 41(1): 151-160. | |
| 26 | CHU Chiheng, YANG Ji, ZHOU Xuechen, et al. Cobalt single atoms on tetrapyridomacrocyclic support for efficient peroxymonosulfate activation[J]. Environmental Science & Technology, 2021, 55(2): 1242-1250. |
| 27 | YANG Jingren, ZENG Deqian, ZHANG Qinggang, et al. Single Mn atom anchored on N-doped porous carbon as highly efficient Fenton-like catalyst for the degradation of organic contaminants[J]. Applied Catalysis B: Environmental, 2020, 279: 119363. |
| 28 | ZHONG Yuanhong, LIANG Xiaoliang, HE Zisen, et al. The constraints of transition metal substitutions (Ti, Cr, Mn, Co and Ni) in magnetite on its catalytic activity in heterogeneous Fenton and UV/Fenton reaction: from the perspective of hydroxyl radical generation[J]. Applied Catalysis B: Environmental, 2014, 150/151: 612-618. |
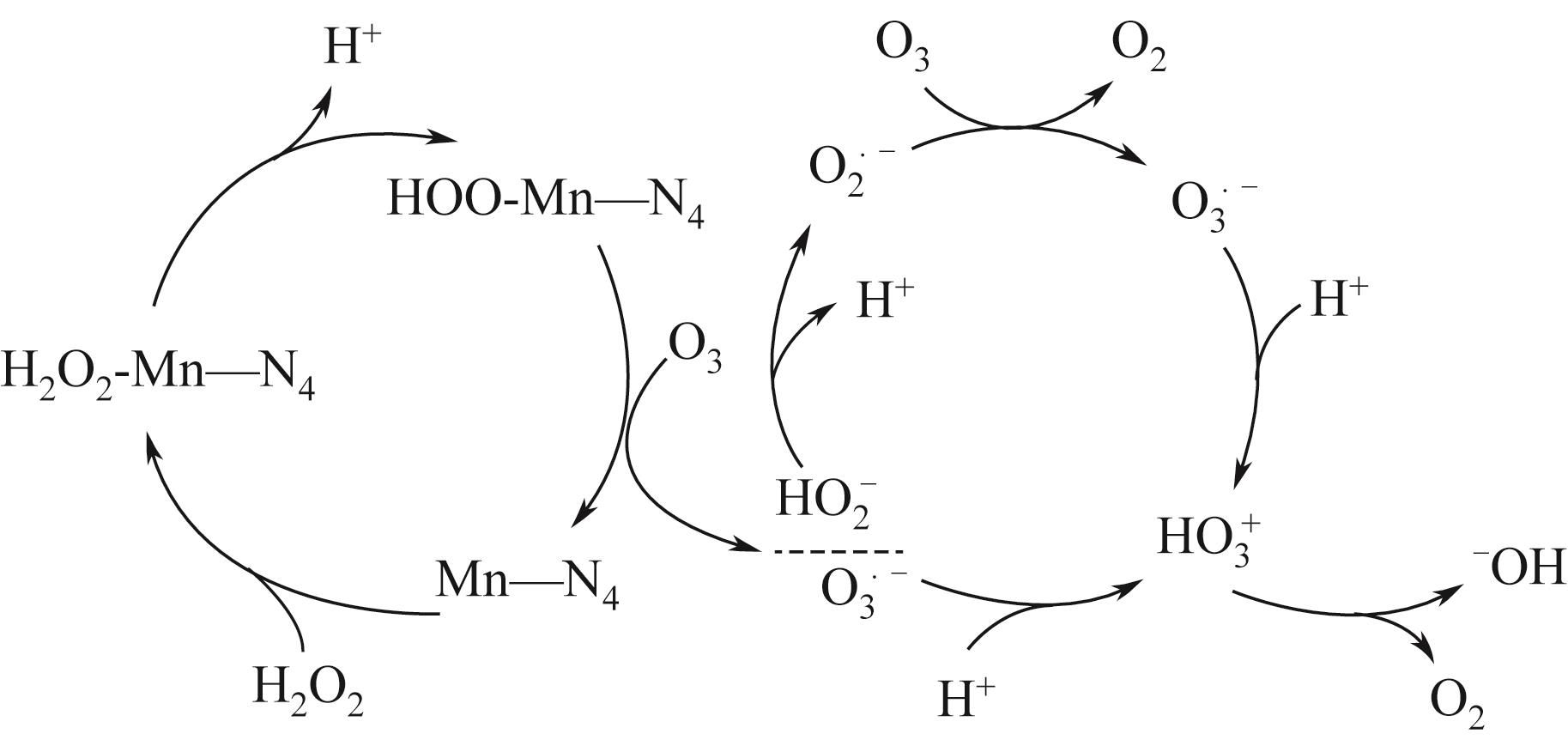
| 29 | GUO Zhuang, XIE Yongbing, XIAO Jiadong, et al. Single-atom Mn-N4 site-catalyzed peroxone reaction for the efficient production of hydroxyl radicals in an acidic solution[J]. Journal of the American Chemical Society, 2019, 141(30): 12005-12010. |
| 30 | 柯倩. 过渡金属单原子负载石墨相氮化碳的制备及其降解污染物的应用研究[D]. 金华: 浙江师范大学, 2020. |
| KE Qian. Preparation of graphitic carbon nitride supported by transition mental single atom and application of pollutants degradation[D]. Jinhua: Zhejiang Normal University, 2020. | |
| 31 | XU Jinwei, ZHENG Xueli, FENG Zhiping, et al. Organic wastewater treatment by a single-atom catalyst and electrolytically produced H2O2 [J]. Nature Sustainability, 2021, 4(3): 233-241. |
| 32 | CHEN Feng, WU Xilin, YANG Liu, et al. Efficient degradation and mineralization of antibiotics via heterogeneous activation of peroxymonosulfate by using graphene supported single-atom Cu catalyst[J]. Chemical Engineering Journal, 2020, 394: 124904. |
| 33 | 邓方鑫. 钴铁双金属单原子催化剂活化过硫酸盐处理含酚废水的研究[D]. 湘潭: 湘潭大学, 2020. |
| DENG Fangxin. Research on treatment of phenolic wastewater by catalyzed peroxydisulfate activation with isolated diatomic Co-Fe metal-nitrogen sites[D]. Xiangtan: Xiangtan University, 2020. | |
| 34 | CHEN Qiumeng, LIU Yuan, LU Yuwan, et al. Atomically dispersed Fe/Bi dual active sites single-atom nanozymes for cascade catalysis and peroxymonosulfate activation to degrade dyes[J]. Journal of Hazardous Materials, 2022, 422: 126929. |
| 35 | WU Huihui, YAN Jingjing, XU Xin, et al. Synergistic effects for boosted persulfate activation in a designed Fe-Cu dual-atom site catalyst[J]. Chemical Engineering Journal, 2022, 428: 132611. |
| 36 | 梁言, 王婷雯, 赵永琴, 等. Fe-Ce/g-C3N4芬顿催化剂的制备及其降解有机污染物性能研究[J]. 现代化工, 2021, 41(3): 190-195. |
| LIANG Yan, WANG Tingwen, ZHAO Yongqin, et al. Preparation of Fenton catalyst Fe-Ce/g-C3N4 and its performance for degradation of organic pollutants[J]. Modern Chemical Industry, 2021, 41(3): 190-195. | |
| 37 | 陈枫. 碳材料负载过渡金属单原子催化剂应用于水中微污染物的催化降解研究[D]. 金华: 浙江师范大学, 2020. |
| CHEN Feng. Synthesis of tranition metal single atom-doped carbon materials catalysts and applied to the degradation of micro-pollutants in water[D]. Jinhua: Zhejiang Normal University, 2020. | |
| 38 | HUANG Dahong, DE VERA G A, CHU Chiheng, et al. Single-atom Pt catalyst for effective C-F bond activation via hydrodefluorination[J]. ACS Catalysis, 2018, 8(10): 9353-9358. |
| 39 | FENG Yong, LEE Poheng, WU Deli, et al. Surface-bound sulfate radical-dominated degradation of 1, 4-dioxane by alumina-supported palladium (Pd/Al2O3) catalyzed peroxymonosulfate[J]. Water Research, 2017, 120: 12-21. |
| 40 | WANG Yanbin, ZHAO Xu, CAO Di, et al. Peroxymonosulfate enhanced visible light photocatalytic degradation bisphenol A by single-atom dispersed Ag mesoporous g-C3N4 hybrid[J]. Applied Catalysis B: Environmental, 2017, 211: 79-88. |
| 41 | XUE Yudong, PHAM N N T, NAM G, et al. Persulfate activation by ZIF-67-derived cobalt/nitrogen-doped carbon composites: kinetics and mechanisms dependent on persulfate precursor[J]. Chemical Engineering Journal, 2021, 408: 127305. |
| 42 | QI Yuanfeng, LI Jing, ZHANG Yanqing, et al. Novel lignin-based single atom catalysts as peroxymonosulfate activator for pollutants degradation: role of single cobalt and electron transfer pathway[J]. Applied Catalysis B: Environmental, 2021, 286: 119910. |
| 43 | ZHANG Danyu, YIN Kai, TANG Yanhong, et al. Hollow sea-urchin-shaped carbon-anchored single-atom iron as dual-functional electro-Fenton catalysts for degrading refractory thiamphenicol with fast reaction kinetics in a wide pH range[J]. Chemical Engineering Journal, 2022, 427: 130996. |
| 44 | YANG Ting, FAN Shisuo, LI Yang, et al. Fe-N/C single-atom catalysts with high density of Fe-N x sites toward peroxymonosulfate activation for high-efficient oxidation of bisphenol A: electron-transfer mechanism[J]. Chemical Engineering Journal, 2021, 419: 129590 |
| 45 | ZHANG Longshuai, JIANG Xunheng, ZHONG Ziai, et al. Carbon nitride supported high-loading Fe single-atom catalyst for activation of peroxymonosulfate to generate 1O2 with 100% selectivity[J]. Angewandte Chemie International Edition, 2021, 60(40): 21751-21755. |
| 46 | PAN Jingwen, GAO Baoyu, DUAN Pijun,et al. Improving peroxymonosulfate activation by copper ion-saturated adsorbent-based single atom catalysts for the degradation of organic contaminants: electron-transfer mechanism and the key role of Cu single atoms[J]. Journal of Materials Chemistry A, 2021, 9(19): 11604-11613. |
| 47 | ZHAO Xue, LI Xue, ZHU Zhu, et al. Single-atom Co embedded in BCN matrix to achieve 100% conversion of peroxymonosulfate into singlet oxygen[J]. Applied Catalysis B: Environmental, 2022, 300: 120759. |
| 48 | PENG Xiaoming, WU Jianqun, ZHAO Zilong, et al. Activation of peroxymonosulfate by single atom Co-N-C catalysts for high-efficient removal of chloroquine phosphate via non-radical pathways: electron-transfer mechanism[J]. Chemical Engineering Journal, 2022, 429: 132245. |
| 49 | ZHAO Shiyong, CHEN Guangxu, ZHOU Guangmin, et al. A universal seeding strategy to synthesize single atom catalysts on 2D materials for electrocatalytic applications[J]. Advanced Functional Materials, 2020, 30(6): 1906157. |
| 50 | CHEN Zhe, ZHAO Jingxiang, CABRERA C R, et al. Computational screening of efficient single-atom catalysts based on graphitic carbon nitride (g-C3N4) for nitrogen electroreduction[J]. Small Methods, 2019, 3(6): 1800368. |
| 51 | PENG Xiaoming, WU Jianqun, ZHAO Zilong, et al. Activation of peroxymonosulfate by single-atom Fe-g-C3N4 catalysts for high efficiency degradation of tetracycline via nonradical pathways: role of high-valent iron-oxo species and Fe-N x sites[J]. Chemical Engineering Journal, 2022, 427: 130803. |
| 52 | ZHAO Chaocheng, DONG Pei, LIU Zongmei, et al. Facile synthesis of Fe3O4/MIL-101 nanocomposite as an efficient heterogeneous catalyst for degradation of pollutants in Fenton-like system[J]. RSC Advances, 2017, 7(39): 24453-24461. |
| 53 | HUANG Ruting, LIU Yanyu, CHEN Zhiwen, et al. Fe-species-loaded mesoporous MnO2 superstructural requirements for enhanced catalysis[J]. ACS Applied Materials & Interfaces, 2015, 7(7): 3949-3959. |
| 54 | WANG Qinglong, LI Haiyan, YANG Jinghe, et al. Iron phthalocyanine-graphene donor-acceptor hybrids for visible-light-assisted degradation of phenol in the presence of H2O2 [J]. Applied Catalysis B: Environmental, 2016, 192: 182-192. |
| 55 | AN Sufeng, ZHANG Guanghui, WANG Tingwen, et al. High-density ultra-small clusters and single-atom Fe sites embedded in graphitic carbon nitride (g-C3N4) for highly efficient catalytic advanced oxidation processes[J]. ACS Nano, 2018, 12(9): 9441-9450. |
| 56 | LI Xuning, HUANG Xiang, XI Shibo, et al. Single cobalt atoms anchored on porous N-doped graphene with dual reaction sites for efficient Fenton-like catalysis[J]. Journal of the American Chemical Society, 2018, 140(39): 12469-12475. |
| [1] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [2] | 王谨航, 何勇, 史伶俐, 龙臻, 梁德青. 气体水合物阻聚剂研究进展[J]. 化工进展, 2023, 42(9): 4587-4602. |
| [3] | 葛全倩, 徐迈, 梁铣, 王凤武. MOFs材料在光电催化领域应用的研究进展[J]. 化工进展, 2023, 42(9): 4692-4705. |
| [4] | 宋伟涛, 宋慧平, 范朕连, 樊飙, 薛芳斌. 粉煤灰在防腐涂料中的研究进展[J]. 化工进展, 2023, 42(9): 4894-4904. |
| [5] | 杨静, 李博, 李文军, 刘晓娜, 汤刘元, 刘月, 钱天伟. 焦化污染场地中萘降解菌的分离及降解特性[J]. 化工进展, 2023, 42(8): 4351-4361. |
| [6] | 吴亚, 赵丹, 方荣苗, 李婧瑶, 常娜娜, 杜春保, 王文珍, 史俊. 用于复杂原油乳液的高效破乳剂开发及应用研究进展[J]. 化工进展, 2023, 42(8): 4398-4413. |
| [7] | 储甜甜, 刘润竹, 杜高华, 马嘉浩, 张孝阿, 王成忠, 张军营. 有机胍催化脱氢型RTV硅橡胶的制备和可降解性能[J]. 化工进展, 2023, 42(7): 3664-3673. |
| [8] | 欧阳素芳, 周道伟, 黄伟, 贾凤. 新型耐迁移橡胶防老剂的研究进展[J]. 化工进展, 2023, 42(7): 3708-3719. |
| [9] | 徐伟, 李凯军, 宋林烨, 张兴惠, 姚舜华. 光催化及其协同电化学降解VOCs的研究进展[J]. 化工进展, 2023, 42(7): 3520-3531. |
| [10] | 龚鹏程, 严群, 陈锦富, 温俊宇, 苏晓洁. 铁酸钴复合碳纳米管活化过硫酸盐降解铬黑T的性能及机理[J]. 化工进展, 2023, 42(7): 3572-3581. |
| [11] | 吴锋振, 刘志炜, 谢文杰, 游雅婷, 赖柔琼, 陈燕丹, 林冠烽, 卢贝丽. 生物质基铁/氮共掺杂多孔炭的制备及其活化过一硫酸盐催化降解罗丹明B[J]. 化工进展, 2023, 42(6): 3292-3301. |
| [12] | 杨红梅, 高涛, 鱼涛, 屈撑囤, 高家朋. 高铁酸盐处理难降解有机物磺化酚醛树脂[J]. 化工进展, 2023, 42(6): 3302-3308. |
| [13] | 吕学东, 罗发亮, 林海涛, 宋丹青, 刘义, 牛瑞雪, 郑柳春. 聚丁二酸丁二醇酯的合成工艺及气体阻隔性最新进展[J]. 化工进展, 2023, 42(5): 2546-2554. |
| [14] | 高江雨, 张耀君, 贺攀阳, 刘礼才, 张枫烨. 磷酸基地质聚合物的制备及其性能研究进展[J]. 化工进展, 2023, 42(3): 1411-1425. |
| [15] | 薛博, 杨婷婷, 王雪峰. 聚苯胺/碳纳米管气敏材料的研究进展[J]. 化工进展, 2023, 42(3): 1448-1456. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||