化工进展 ›› 2022, Vol. 41 ›› Issue (7): 3925-3937.DOI: 10.16085/j.issn.1000-6613.2021-1900
黄原酸改性交联面包酵母的制备及对Pb(Ⅱ)的吸附特性
- 1.楚雄师范学院资源环境与化学学院,云南 楚雄 675000
2.云南农业大学资源与环境学院,云南 昆明 650201
-
收稿日期:2021-09-06修回日期:2021-10-24出版日期:2022-07-25发布日期:2022-07-23 -
通讯作者:李天国 -
作者简介:段正洋(1990—),男,博士,讲师,主要从事含重金属及染料污染水体控制与治理方面的研究。E-mail:zyduan@cxtc.edu.cn 。 -
基金资助:国家自然科学基金(41701362);云南省地方高校联合专项——青年项目(202101BA070001-012);云南省教育厅基金(2019J0397);楚雄师范学院博士科研启动基金(JBS2002)
Preparation of xanthate-functionalized cross-linked baker's yeast and its adsorption characteristics for Pb(Ⅱ)
DUAN Zhengyang1( ), HU Ningmeng1, LI Tianguo2(
), HU Ningmeng1, LI Tianguo2( )
)
- 1.College of Resources, Environment and Chemistry, Chuxiong Normal University, Chuxiong 675000, Yunnan, China
2.College of Resources and Environment, Yunnan Agricultural University, Kunming 650201, Yunnan, China
-
Received:2021-09-06Revised:2021-10-24Online:2022-07-25Published:2022-07-23 -
Contact:LI Tianguo
摘要:
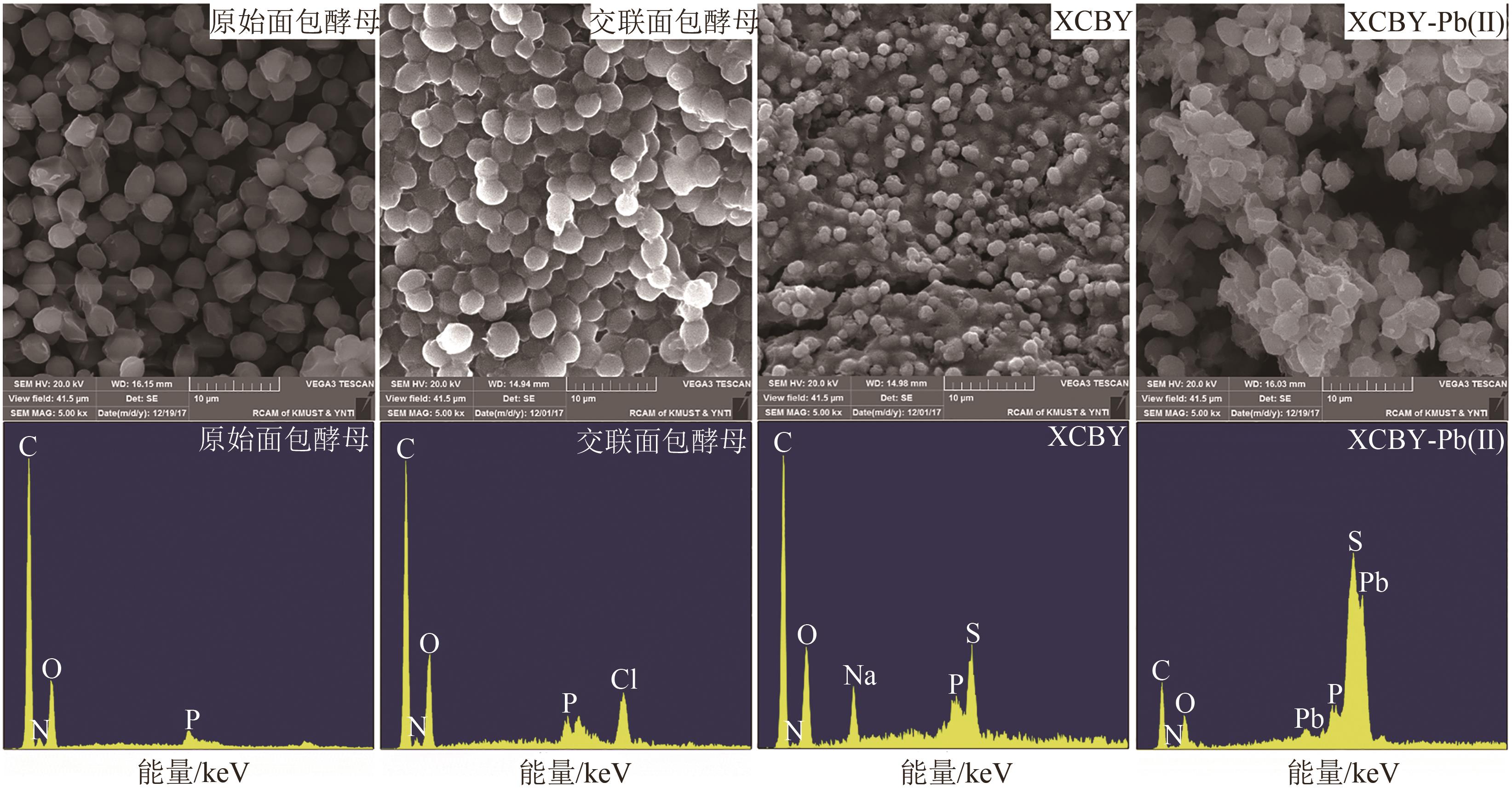
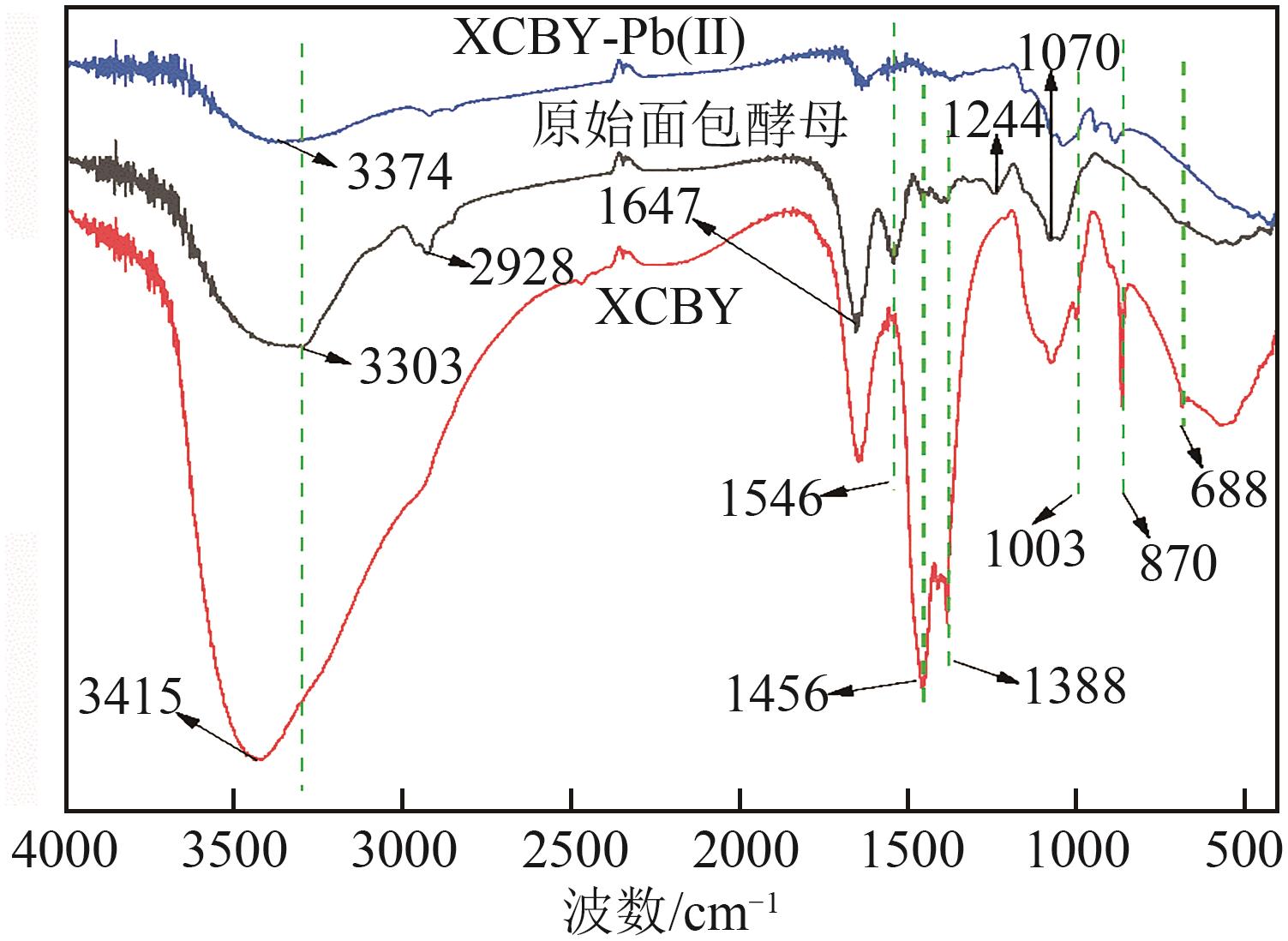
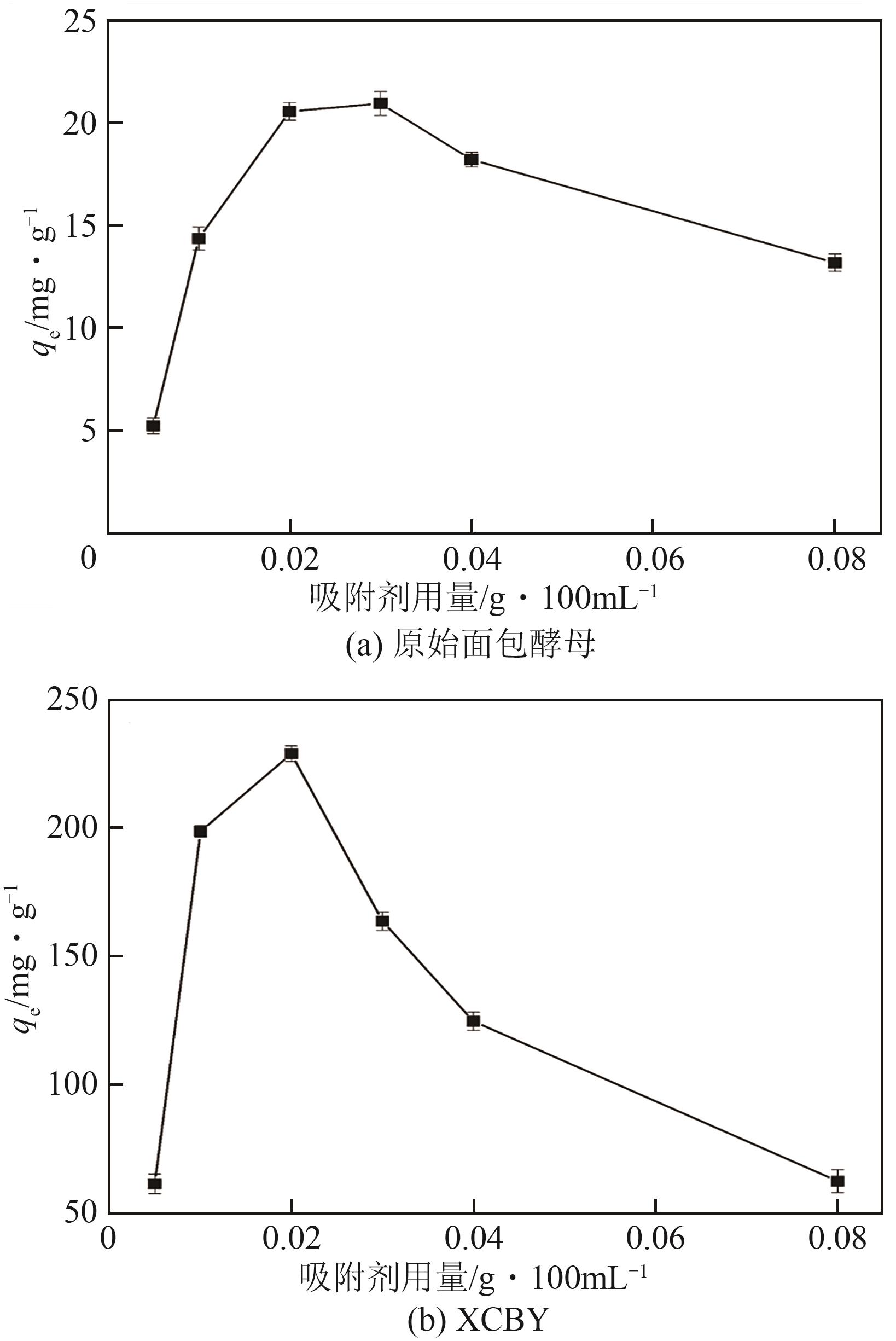
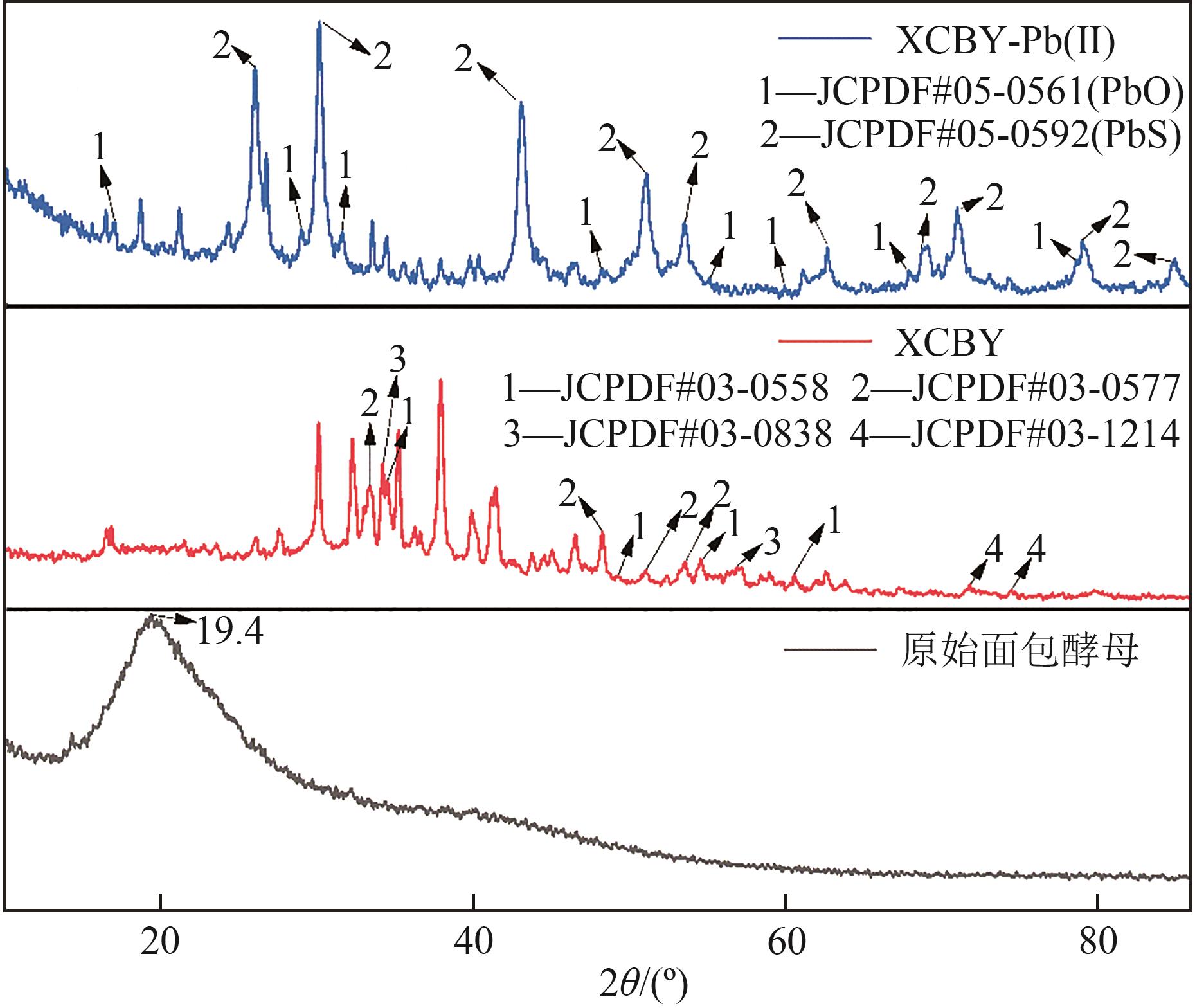
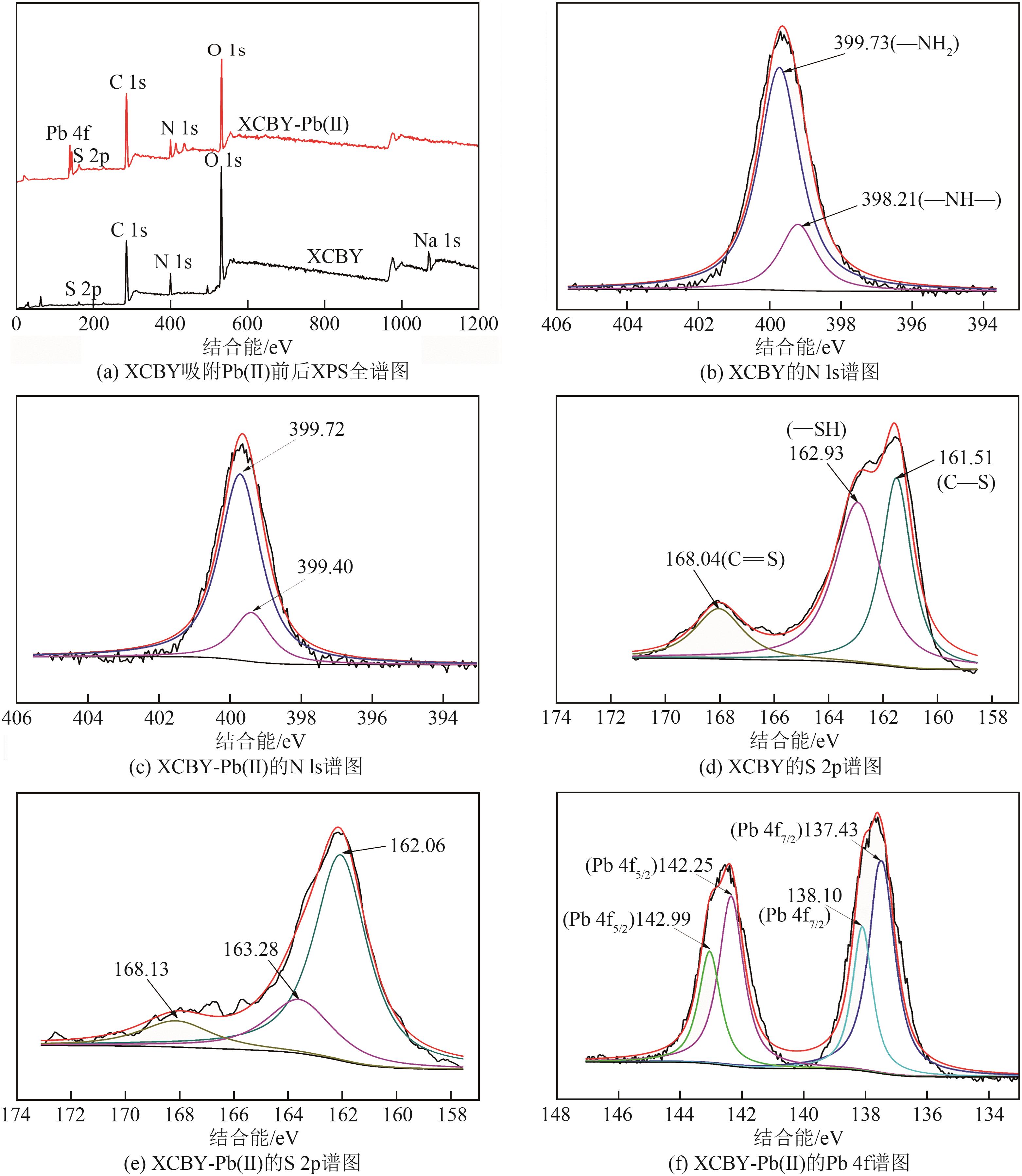
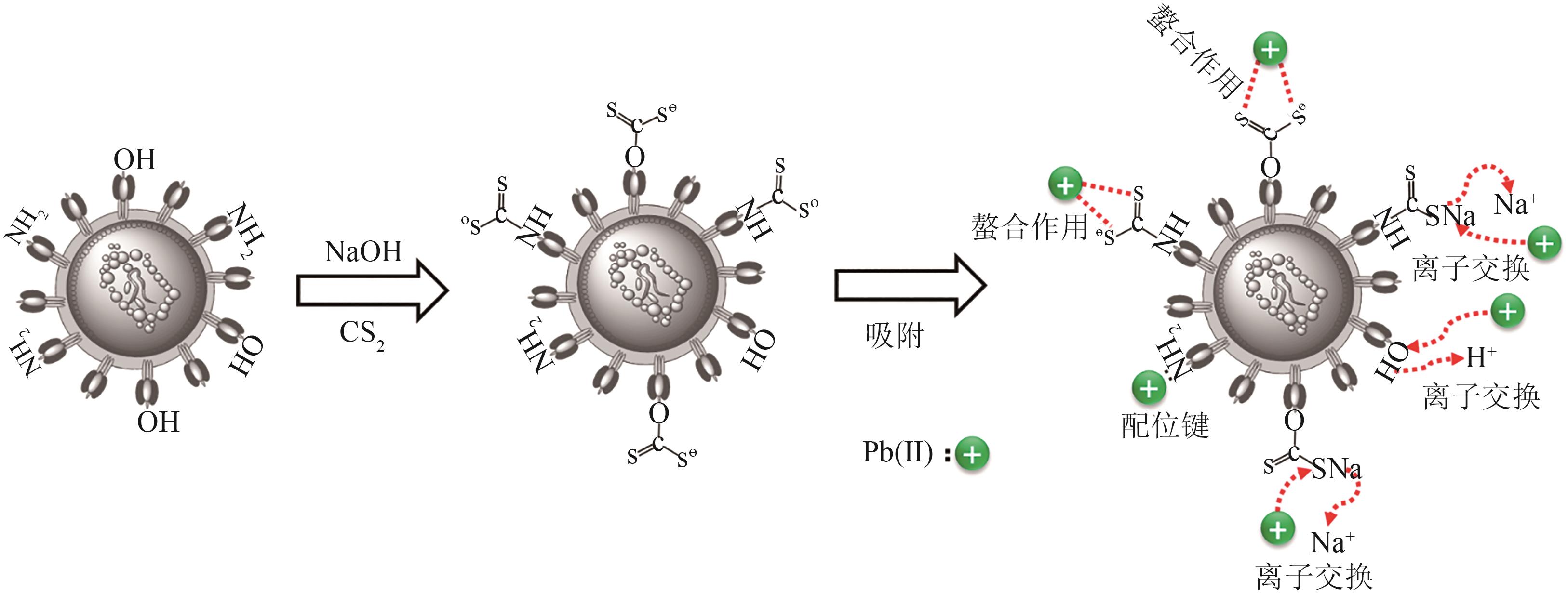
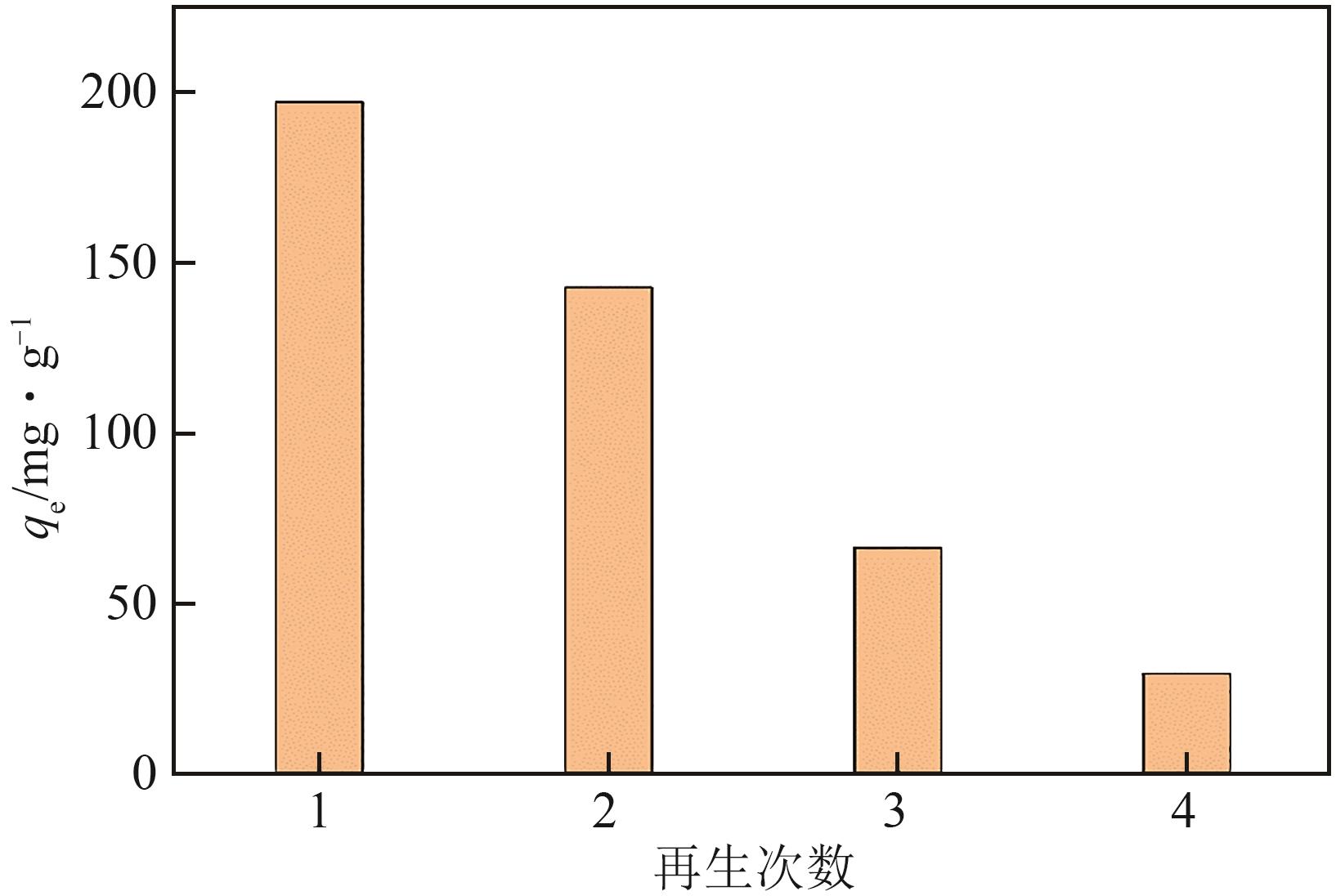
以面包酵母为基材,通过两步法制备得到黄原酸改性交联面包酵母(XCBY),并应用于水溶液中Pb(Ⅱ)的去除研究,原始面包酵母、XCBY和XCBY-Pb(Ⅱ)的理化特性分别采用SRA、SEM、EDS、FTIR、XRD和XPS等手段进行表征,研究了溶液pH、吸附剂用量、Pb(Ⅱ)初始浓度等因素对XCBY 吸附性能的影响,并探讨了吸附动力学、等温线、热力学和XCBY的再生性能。结果表明,原始面包酵母经交联和黄原酸改性后,其细胞形态没有明显变化,且对Pb(Ⅱ)的吸附性能得到明显提高。XCBY对Pb(Ⅱ)的吸附过程更符合伪二阶动力学和Langmuir吸附等温线模型,热力学分析表明吸附过程为自发的吸热反应。在30℃、吸附时间为40min的条件下,Langmuir吸附等温线拟合结果显示XCBY对Pb(Ⅱ)的最大吸附量达到319.91mg/g。此外,Pb(Ⅱ)在XCBY上的吸附机制主要依赖于XCBY表面—NH2的配位作用、—OH的离子交换以及—C(
中图分类号:
引用本文
段正洋, 胡柠檬, 李天国. 黄原酸改性交联面包酵母的制备及对Pb(Ⅱ)的吸附特性[J]. 化工进展, 2022, 41(7): 3925-3937.
DUAN Zhengyang, HU Ningmeng, LI Tianguo. Preparation of xanthate-functionalized cross-linked baker's yeast and its adsorption characteristics for Pb(Ⅱ)[J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3925-3937.
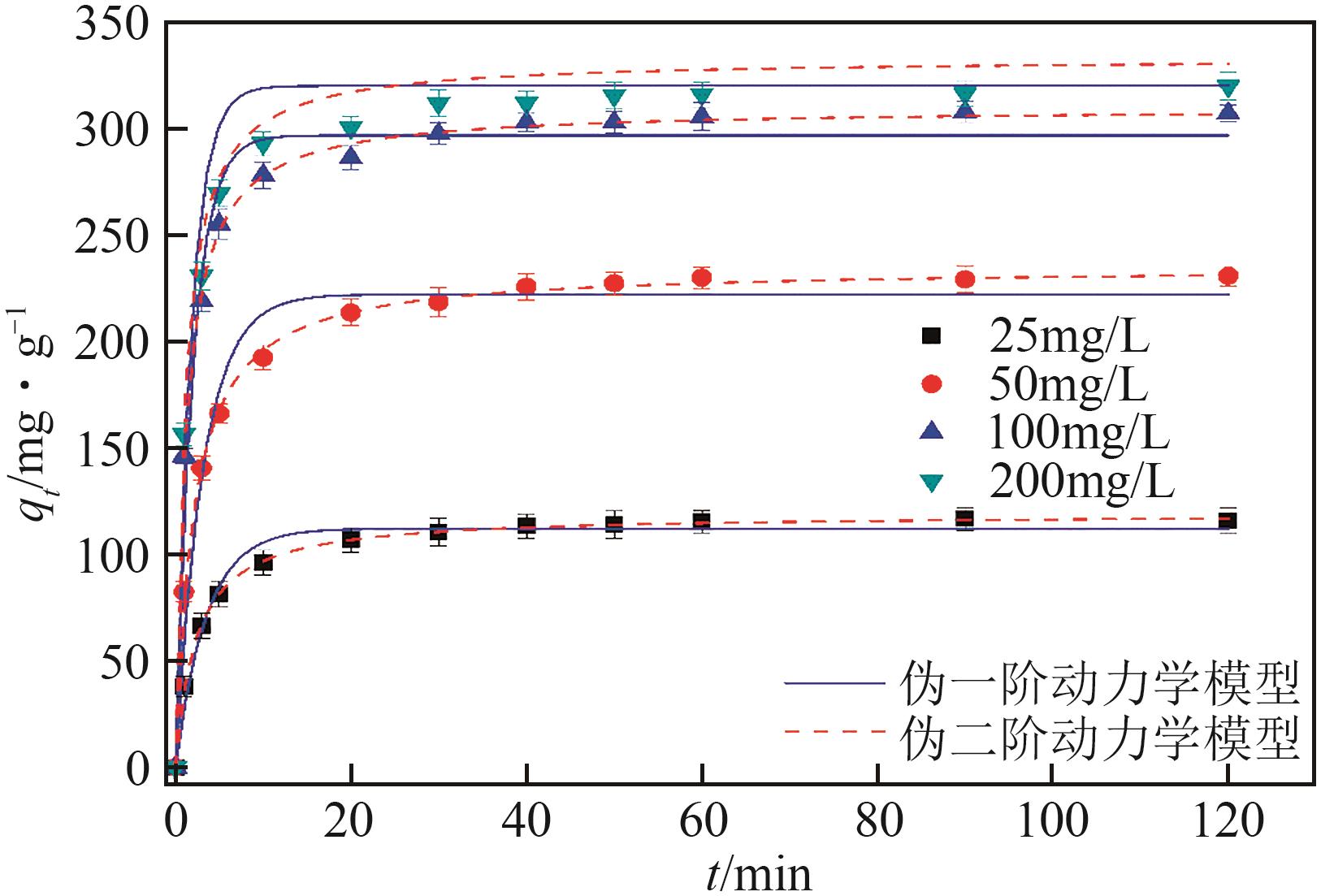
| C0/mg·L -1 | qe, exp/mg·g -1 | 伪一阶动力学模型 | 伪二阶动力学模型 | ||||
|---|---|---|---|---|---|---|---|
| qe/mg·g -1 | k1/min -1 | R2 | qe/mg·g -1 | k2/g·mg -1·min -1 | R2 | ||
| 25 | 117.78 | 112.25 | 0.285 | 0.9788 | 119.21 | 0.00367 | 0.9995 |
| 50 | 231.77 | 222.28 | 0.320 | 0.9749 | 235.04 | 0.00214 | 0.9991 |
| 100 | 307.58 | 296.96 | 0.509 | 0.9757 | 309.93 | 0.00280 | 0.9988 |
| 200 | 317.10 | 308.74 | 0.537 | 0.9785 | 321.62 | 0.00289 | 0.9989 |
表1 XCBY吸附Pb(Ⅱ)的动力学参数
| C0/mg·L -1 | qe, exp/mg·g -1 | 伪一阶动力学模型 | 伪二阶动力学模型 | ||||
|---|---|---|---|---|---|---|---|
| qe/mg·g -1 | k1/min -1 | R2 | qe/mg·g -1 | k2/g·mg -1·min -1 | R2 | ||
| 25 | 117.78 | 112.25 | 0.285 | 0.9788 | 119.21 | 0.00367 | 0.9995 |
| 50 | 231.77 | 222.28 | 0.320 | 0.9749 | 235.04 | 0.00214 | 0.9991 |
| 100 | 307.58 | 296.96 | 0.509 | 0.9757 | 309.93 | 0.00280 | 0.9988 |
| 200 | 317.10 | 308.74 | 0.537 | 0.9785 | 321.62 | 0.00289 | 0.9989 |
| t /℃ | qe, exp/mg·g -1 | Langmuir模型 | Freundlich模型 | Langmuir-Freundlich模型 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| qmax/mg·g -1 | KL /L·mg -1 | R2 | KF /mg·g -1 | n | R2 | qmax /mg·g -1 | KLF/L·mg -1 | nLF | R2 | ||
| 25 | 306.51 | 308.95 | 0.433 | 0.9972 | 122.38 | 4.746 | 0.9217 | 317.79 | 0.392 | 0.868 | 0.9988 |
| 30 | 317.87 | 319.91 | 0.496 | 0.9927 | 131.23 | 4.906 | 0.9069 | 323.59 | 0.478 | 0.932 | 0.9922 |
| 35 | 321.90 | 322.39 | 0.871 | 0.9954 | 149.63 | 5.531 | 0.9103 | 331.11 | 0.795 | 0.829 | 0.9982 |
表2 Langmuir、Freundlich和L-F模型对吸附数据的拟合结果
| t /℃ | qe, exp/mg·g -1 | Langmuir模型 | Freundlich模型 | Langmuir-Freundlich模型 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| qmax/mg·g -1 | KL /L·mg -1 | R2 | KF /mg·g -1 | n | R2 | qmax /mg·g -1 | KLF/L·mg -1 | nLF | R2 | ||
| 25 | 306.51 | 308.95 | 0.433 | 0.9972 | 122.38 | 4.746 | 0.9217 | 317.79 | 0.392 | 0.868 | 0.9988 |
| 30 | 317.87 | 319.91 | 0.496 | 0.9927 | 131.23 | 4.906 | 0.9069 | 323.59 | 0.478 | 0.932 | 0.9922 |
| 35 | 321.90 | 322.39 | 0.871 | 0.9954 | 149.63 | 5.531 | 0.9103 | 331.11 | 0.795 | 0.829 | 0.9982 |
| 吸附剂 | 吸附量/mg·g -1 | 参考文献 |
|---|---|---|
| 黄原酸改性交联面包酵母 | 319.91 | 本研究 |
| 黄原酸改性壳聚糖海绵 | 216.45 | [ |
| 黄原酸改性纤维素 | 531.29 | [ |
| 黄原酸改性玉米秸秆 | 20.58 | [ |
| 黄原酸改性多孔木质素 | 64.90 | [ |
| 黄原酸功能化蛋壳膜 | 33.11 | [ |
| 黄原酸改性交联淀粉 | 47.11 | [ |
| 黄原酸改性交联磁性壳聚糖/聚乙烯醇颗粒 | 59.86 | [ |
| 黄原酸改性多壁纳米碳管 | 83.01 | [ |
| 超声协助黄原酸改性纤维素 | 134.41 | [ |
| 黄原酸改性板栗壳 | 124.84 | [ |
表3 XCBY与其他类似吸附剂对Pb(Ⅱ)的吸附性能比较
| 吸附剂 | 吸附量/mg·g -1 | 参考文献 |
|---|---|---|
| 黄原酸改性交联面包酵母 | 319.91 | 本研究 |
| 黄原酸改性壳聚糖海绵 | 216.45 | [ |
| 黄原酸改性纤维素 | 531.29 | [ |
| 黄原酸改性玉米秸秆 | 20.58 | [ |
| 黄原酸改性多孔木质素 | 64.90 | [ |
| 黄原酸功能化蛋壳膜 | 33.11 | [ |
| 黄原酸改性交联淀粉 | 47.11 | [ |
| 黄原酸改性交联磁性壳聚糖/聚乙烯醇颗粒 | 59.86 | [ |
| 黄原酸改性多壁纳米碳管 | 83.01 | [ |
| 超声协助黄原酸改性纤维素 | 134.41 | [ |
| 黄原酸改性板栗壳 | 124.84 | [ |
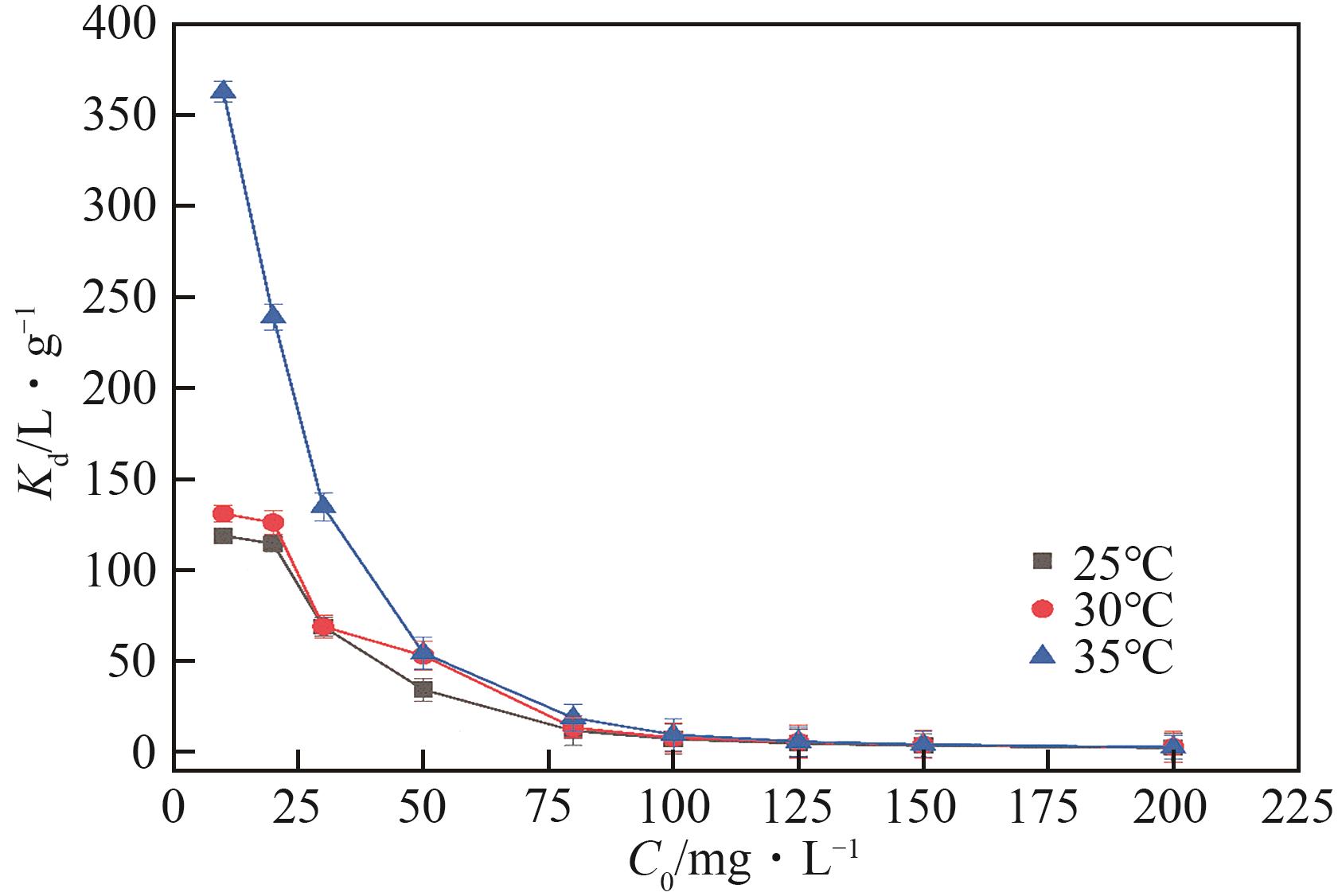
| 温度/℃ | Kd/cm3·g -1 | ΔG?/kJ·mol -1 | ΔH? /kJ·mol -1 | ΔS?/J·mol -1·K -1 |
|---|---|---|---|---|
| 25 | 4.639 | -3.803 | 17.29 | 70.04 |
| 30 | 5.565 | -4.325 | ||
| 35 | 5.709 | -4.463 |
表4 Pb(Ⅱ)在XCBY上吸附的热力学参数
| 温度/℃ | Kd/cm3·g -1 | ΔG?/kJ·mol -1 | ΔH? /kJ·mol -1 | ΔS?/J·mol -1·K -1 |
|---|---|---|---|---|
| 25 | 4.639 | -3.803 | 17.29 | 70.04 |
| 30 | 5.565 | -4.325 | ||
| 35 | 5.709 | -4.463 |
| 1 | WANG N N, XU X J, LI H Y, et al. Enhanced selective adsorption of Pb(Ⅱ) from aqueous solutions by one-pot synthesis of xanthate-modified chitosan sponge: behaviors and mechanisms[J]. Industrial & Engineering Chemistry Research, 2016, 55(47): 12222-12231. |
| 2 | WANG N N, XU X J, LI H Y, et al. Preparation and application of a xanthate-modified thiourea chitosan sponge for the removal of Pb(Ⅱ) from aqueous solutions[J]. Industrial & Engineering Chemistry Research, 2016, 55(17): 4960-4968. |
| 3 | JIA Q J, ZHANG W T, LI D P, et al. Hydrazinolyzed cellulose-g-polymethyl acrylate as adsorbent for efficient removal of Cd(Ⅱ) and Pb(Ⅱ) ions from aqueous solution[J]. Water Science and Technology, 2017, 75(5): 1051-1058. |
| 4 | 徐大勇, 张苗, 杨伟伟, 等. 氧化铝改性污泥生物炭粒制备及其对Pb(Ⅱ)的吸附特性[J]. 化工进展, 2020, 39(3): 1153-1166. |
| XU Dayong, ZHANG Miao, YANG Weiwei, et al. Preparation of alumina modified sludge biocharcoal particles and their adsorption characteristics for Pb(Ⅱ)[J]. Chemical Industry and Engineering Progress, 2020, 39(3): 1153-1166. | |
| 5 | PENG X L, XU F, ZHANG W Z, et al. Magnetic Fe3O4@silica-xanthan gum composites for aqueous removal and recovery of Pb2+ [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2014, 443: 27-36. |
| 6 | 刘江龙, 郭焱, 席艺慧. FeCl3和十六烷基三甲基溴化铵改性赤泥对水中铜离子的吸附性能和机理[J]. 化工进展, 2020, 39(2): 776-789. |
| LIU Jianglong, GUO Yan, XI Yihui. Adsorption and mechanism of copper ions in water by red mud modified with FeCl3 and hexadecyl trimethyl ammonium bromide (CTAB)[J]. Chemical Industry and Engineering Progress, 2020, 39(2): 776-789. | |
| 7 | ZONG P F, CAO D L, WANG S F, et al. Synthesis of Fe3O4/CD magnetic nanocomposite via low temperature plasma technique with high enrichment of Ni(Ⅱ) from aqueous solution[J]. Journal of the Taiwan Institute of Chemical Engineers, 2017, 70: 134-140. |
| 8 | ZHOU G Y, LUO J M, LIU C B, et al. A highly efficient polyampholyte hydrogel sorbent based fixed-bed process for heavy metal removal in actual industrial effluent[J]. Water Research, 2016, 89: 151-160. |
| 9 | YUAN M L, XIE T F, YAN G J, et al. Effective removal of Pb2+ from aqueous solutions by magnetically modified zeolite[J]. Powder Technology, 2018, 332: 234-241. |
| 10 | KUL A R, KOYUNCU H. Adsorption of Pb(Ⅱ) ions from aqueous solution by native and activated bentonite: Kinetic, equilibrium and thermodynamic study[J]. Journal of Hazardous Materials, 2010, 179(1/2/3): 332-339. |
| 11 | OKOLI C P, DIAGBOYA P N, ANIGBOGU I O, et al. Competitive biosorption of Pb(Ⅱ) and Cd(Ⅱ) ions from aqueous solutions using chemically modified moss biomass (Barbula lambarenensis)[J]. Environmental Earth Sciences, 2016, 76(1): 33-42. |
| 12 | TONG M, YU J X, SUN X M, et al. Polymer modified biomass of baker’s yeast for treating simulated wastewater containing nickel and lead[J]. Polymers for Advanced Technologies, 2007, 18(10): 829-834. |
| 13 | XIA Y X, MENG L Y, JIANG Y J, et al. Facile preparation of MnO2 functionalized baker’s yeast composites and their adsorption mechanism for Cadmium[J]. Chemical Engineering Journal, 2015, 259: 927-935. |
| 14 | ZHANG W, MENG L Y, MU G Q, et al. A facile strategy for fabrication of nano-ZnO/yeast composites and their adsorption mechanism towards lead (Ⅱ) ions[J]. Applied Surface Science, 2016, 378: 196-206. |
| 15 | GÖKSUNGUR Y, ÜREN S, GÜVENÇ U. Biosorption of cadmium and lead ions by ethanol treated waste baker's yeast biomass[J]. Bioresource Technology, 2005, 96(1): 103-109. |
| 16 | LI T T, LIU Y G, PENG Q Q, et al. Removal of lead(Ⅱ) from aqueous solution with ethylenediamine-modified yeast biomass coated with magnetic chitosan microparticles: kinetic and equilibrium modeling[J]. Chemical Engineering Journal, 2013, 214: 189-197. |
| 17 | XU M, ZHANG Y S, ZHANG Z M, et al. Study on the adsorption of Ca2+, Cd2+ and Pb2+ by magnetic Fe3O4 yeast treated with EDTA dianhydride[J]. Chemical Engineering Journal, 2011, 168(2): 737-745. |
| 18 | BEDIAKO J K, WEI W, KIM S, et al. Removal of heavy metals from aqueous phases using chemically modified waste Lyocell fiber[J]. Journal of Hazardous Materials, 2015, 299: 550-561. |
| 19 | VIEIRA M G A, NETO A F A, GIMENES M L, et al. Removal of nickel on Bofe bentonite calcined clay in porous bed[J]. Journal of Hazardous Materials, 2010, 176(1/2/3): 109-118. |
| 20 | ÁLVAREZ-AYUSO E, GARCÍA-SÁNCHEZ A. Removal of heavy metals from waste waters by natural and Na-exchanged bentonites[J]. Clays and Clay Minerals, 2003, 51(5): 475-480. |
| 21 | OUARDI Y EL, LENOBLE V, BRANGER C, et al. Enhancing clay adsorption properties: a comparison between chemical and combined chemical/thermal treatments[J]. Groundwater for Sustainable Development, 2021, 12: 100544. |
| 22 | ZHU Y H, HU J, WANG J L. Competitive adsorption of Pb(Ⅱ), Cu(Ⅱ) and Zn(Ⅱ) onto xanthate-modified magnetic chitosan[J]. Journal of Hazardous Materials, 2012, 221/222: 155-161. |
| 23 | DENG S B, TING Y P. Characterization of PEI-modified biomass and biosorption of Cu(Ⅱ), Pb(Ⅱ) and Ni(Ⅱ)[J]. Water Research, 2005, 39(10): 2167-2177. |
| 24 | XIA L, HU Y X, ZHANG B H. Kinetics and equilibrium adsorption of copper(Ⅱ) and nickel(Ⅱ) ions from aqueous solution using sawdust xanthate modified with ethanediamine[J]. Transactions of Nonferrous Metals Society of China, 2014, 24(3): 868-875. |
| 25 | ZHENG L C, MENG P P. Preparation, characterization of corn stalk xanthates and its feasibility for Cd (Ⅱ) removal from aqueous solution[J]. Journal of the Taiwan Institute of Chemical Engineers, 2016, 58: 391-400. |
| 26 | LIANG S, GUO X Y, FENG N C, et al. Application of orange peel xanthate for the adsorption of Pb2+ from aqueous solutions[J]. Journal of Hazardous Materials, 2009, 170(1): 425-429. |
| 27 | KANNAMBA B, REDDY K L, APPARAO B V. Removal of Cu(Ⅱ) from aqueous solutions using chemically modified chitosan[J]. Journal of Hazardous Materials, 2010, 175(1/2/3): 939-948. |
| 28 | LAGERGREN S. Zur theorie der sogenannten adsorption gelöster stoffe, Kungliga Svenska Vetenskapsakademiens[J]. Handlingar, 1898, 24(4): 1-39. |
| 29 | HO Y S, MCKAY G. Pseudo-second order model for sorption processes[J]. Process Biochemistry, 1999, 34(5): 451-465. |
| 30 | CHEN Y W, WANG J L. The characteristics and mechanism of Co(Ⅱ) removal from aqueous solution by a novel xanthate-modified magnetic chitosan[J]. Nuclear Engineering and Design, 2012, 242: 452-457. |
| 31 | KAMARI A, NGAH W S W. Isotherm, kinetic and thermodynamic studies of lead and copper uptake by H2SO4 modified chitosan[J]. Colloids and Surfaces B: Biointerfaces, 2009, 73(2): 257-266. |
| 32 | LI Z L, KONG Y, GE Y Y. Synthesis of porous lignin xanthate resin for Pb2+ removal from aqueous solution[J]. Chemical Engineering Journal, 2015, 270: 229-234. |
| 33 | LANGMUIR I. The adsorption of gases on plane surfaces of glass, mica and platinum[J]. Journal of the American Chemical Society, 1918, 40(9): 1361-1403. |
| 34 | FREUNDLICH H. Of the adsorption of gases. Section Ⅱ. Kinetics and energetics of gas adsorption. Introductory paper to section Ⅱ[J]. Transactions of the Faraday Society, 1932, 28: 195. |
| 35 | SIPS R. Combined form of Langmuir and freundlich equations[J]. Journal of Chemical Physics, 1948, 16: 490-495. |
| 36 | GUERRA D J L, MELLO I, RESENDE R, et al. Application as absorbents of natural and functionalized Brazilian bentonite in Pb2+ adsorption: equilibrium, kinetic, pH, and thermodynamic effects[J]. Water Resources and Industry, 2013, 4: 32-50. |
| 37 | 邹雪, 龚正君. 黄原酸功能化蛋壳膜及其对Pb(Ⅱ)的吸附研究[J]. 西南大学学报(自然科学版), 2020, 42(11): 141-146. |
| ZOU Xue, GONG Zhengjun. Study on adsorption removal of Pb(Ⅱ) by xanthated eggshell membrane[J]. Journal of Southwest University (Natural Science Edition), 2020, 42(11): 141-146. | |
| 38 | FENG K, WEN G H. Absorbed Pb2+ and Cd2+ ions in water by cross-linked starch xanthate[J]. International Journal of Polymer Science, 2017, 2017: 1-9. |
| 39 | LV L, CHEN N, FENG C P, et al. Heavy metal ions removal from aqueous solution by xanthate-modified cross-linked magnetic chitosan/poly(vinyl alcohol) particles[J]. RSC Advances, 2017, 7(45): 27992-28000. |
| 40 | GAO T T, YU J G, ZHOU Y, et al. Performance of xanthate-modified multi-walled carbon nanotubes on adsorption of lead ions[J]. Water, Air, & Soil Pollution, 2017, 228(5): 172-183. |
| 41 | WANG C Q, WANG H, GU G H. Ultrasound-assisted xanthation of cellulose from lignocellulosic biomass optimized by response surface methodology for Pb(Ⅱ) sorption[J]. Carbohydrate Polymers, 2018, 182: 21-28. |
| 42 | CHEN M, WANG X F, ZHANG H. Comparative research on selective adsorption of Pb(Ⅱ) by biosorbents prepared by two kinds of modifying waste biomass: highly-efficient performance, application and mechanism[J]. Journal of Environmental Management, 2021, 288: 112388. |
| 43 | EVERETT D H. The thermodynamics of adsorption. Part Ⅱ.—Thermodynamics of monolayers on solids[J]. Transactions of the Faraday Society, 1950, 46: 942-957. |
| 44 | DENG J Q, LIU Y G, LIU S B, et al. Competitive adsorption of Pb(Ⅱ), Cd(Ⅱ) and Cu(Ⅱ) onto chitosan-pyromellitic dianhydride modified biochar[J]. Journal of Colloid and Interface Science, 2017, 506: 355-364. |
| 45 | GÖK Ö, ÖZCAN A, ERDEM B, et al. Prediction of the kinetics, equilibrium and thermodynamic parameters of adsorption of copper(Ⅱ) ions onto 8-hydroxy quinoline immobilized bentonite[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2008, 317(1/2/3): 174-185. |
| 46 | QIN F, BAI B, JING D W, et al. CdS nanoparticles anchored on the surface of yeast via a hydrothermal processes for environmental applications[J]. RSC Advances, 2014, 4(66): 34864-34872. |
| 47 | CHANG Y K, LEU M H, CHANG J E, et al. Combined two-stage xanthate processes for the treatment of copper-containing wastewater[J]. Engineering in Life Sciences, 2007, 7(1): 75-80. |
| 48 | HE J, LU Y C, LUO G S. Ca(Ⅱ) imprinted chitosan microspheres: an effective and green adsorbent for the removal of Cu(II), Cd(Ⅱ) and Pb(Ⅱ) from aqueous solutions[J]. Chemical Engineering Journal, 2014, 244: 202-208. |
| 49 | ZHAO F P, REPO E, SONG Y, et al. Polyethylenimine-cross-linked cellulose nanocrystals for highly efficient recovery of rare earth elements from water and a mechanism study[J]. Green Chemistry, 2017, 19(20): 4816-4828. |
| 50 | ODIO O F, LARTUNDO-ROJAS L, PALACIOS E G, et al. Synthesis of a novel poly-thiolated magnetic nano-platform for heavy metal adsorption. Role of thiol and carboxyl functions[J]. Applied Surface Science, 2016, 386: 160-177. |
| 51 | GEDAM N, NETI N R, KORMUNDA M, et al. Novel lead dioxide-graphite-polymer composite anode for electrochemical chlorine generation[J]. Electrochimica Acta, 2015, 169: 109-116. |
| 52 | ZINGG D S, HERCULES D M. ChemInform abstract: electron spectroscopy for chemical analysis studies of lead sulfide oxidation[J]. Chemischer Informationsdienst, 1978, 9(50): 1992-1995. |
| 53 | LIANG X F, XU Y M, SUN G H, et al. Preparation, characterization of thiol-functionalized silica and application for sorption of Pb2+ and Cd2+ [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2009, 349(1/2/3): 61-68. |
| [1] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [2] | 赵景超, 谭明. 表面活性剂对电渗析减量化工业含盐废水的影响[J]. 化工进展, 2023, 42(S1): 529-535. |
| [3] | 王晨, 白浩良, 康雪. 大功率UV-LED散热与纳米TiO2光催化酸性红26耦合系统性能[J]. 化工进展, 2023, 42(9): 4905-4916. |
| [4] | 王琦, 寇丽红, 王冠宇, 王吉坤, 刘敏, 李兰廷, 王昊. 焦化废水生物出水中可溶解性有机物的分子识别[J]. 化工进展, 2023, 42(9): 4984-4993. |
| [5] | 史天茜, 石永辉, 武新颖, 张益豪, 秦哲, 赵春霞, 路达. Fe2+对厌氧氨氧化EGSB反应器运行性能的影响[J]. 化工进展, 2023, 42(9): 5003-5010. |
| [6] | 郑梦启, 王成业, 汪炎, 王伟, 袁守军, 胡真虎, 何春华, 王杰, 梅红. 菌藻共生技术在工业废水零排放中的应用与展望[J]. 化工进展, 2023, 42(8): 4424-4431. |
| [7] | 于静文, 宋璐娜, 刘砚超, 吕瑞东, 武蒙蒙, 冯宇, 李忠, 米杰. 一种吲哚基超交联聚合物In-HCP对水中碘的吸附作用[J]. 化工进展, 2023, 42(7): 3674-3683. |
| [8] | 陈娜, 张肖静, 张楠, 马冰冰, 张涵, 杨浩洁, 张宏忠. 淬灭酶对亚硝化-混合自养脱氮系统的影响[J]. 化工进展, 2023, 42(7): 3816-3823. |
| [9] | 陈香李, 李倩倩, 张甜, 李彪, 李康康. 自愈合油水分离膜的研究进展[J]. 化工进展, 2023, 42(7): 3600-3610. |
| [10] | 李白雪, 信欣, 朱羽蒙, 刘琴, 刘鑫. SASD-A体系构建及进水不同S/N对脱氮工艺的影响机制[J]. 化工进展, 2023, 42(6): 3261-3271. |
| [11] | 杨红梅, 高涛, 鱼涛, 屈撑囤, 高家朋. 高铁酸盐处理难降解有机物磺化酚醛树脂[J]. 化工进展, 2023, 42(6): 3302-3308. |
| [12] | 李华华, 李逸航, 金北辰, 李隆昕, 成少安. 厌氧氨氧化-生物电化学耦合废水处理系统的研究进展[J]. 化工进展, 2023, 42(5): 2678-2690. |
| [13] | 朱昊, 刘汉飞, 高源, 白蓉蓉, 倪嵩波, 黄益平, 李庆同, 李小东, 韩卫清. 催化臭氧化体系射流曝气系统参数优化及苯酚阶段氧化分析[J]. 化工进展, 2023, 42(5): 2717-2723. |
| [14] | 张涵, 张肖静, 马冰冰, 佴灿, 刘烁烁, 马永鹏, 宋亚丽. 以城市废弃污泥为种泥启动厌氧氨氧化工艺的可行性[J]. 化工进展, 2023, 42(2): 1080-1088. |
| [15] | 黄起中, 刘冰, 马红鹏, 吕文杰. 基于新型微通道分离技术的甲醇制烯烃废水处理[J]. 化工进展, 2023, 42(2): 669-676. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||