化工进展 ›› 2022, Vol. 41 ›› Issue (7): 3915-3924.DOI: 10.16085/j.issn.1000-6613.2021-1827
2-吡啶甲醛功能化SBA-15介孔材料的制备及其对Cr(Ⅲ)离子的吸附
- 辽宁工业大学化学与环境工程学院,交叉科学研究院,辽宁 锦州 121001
-
收稿日期:2021-08-25修回日期:2021-11-09出版日期:2022-07-25发布日期:2022-07-23 -
通讯作者:郭宇 -
作者简介:姜晓庆(1995—),女,硕士研究生,研究方向为化工新材料。E-mail:2502726134@qq.com 。 -
基金资助:国家自然科学基金(21601075);辽宁省“兴辽英才计划”项目(XLYC2007171);辽宁省“百千万人才工程”项目(辽人社函〔2020〕78号)
Synthesis of 2-pyridinecarboxaldehyde functionalized SBA-15 mesoporous material for the adsorption of Cr(Ⅲ) ions from aqueous solution
JIANG Xiaoqing( ), GUO Yu(
), GUO Yu( ), WU Hongmei
), WU Hongmei
- Institute of Interdisciplinary Research, School of Chemical and Environmental Engineering, Liaoning University of Technology, Jinzhou 121001, Liaoning, China
-
Received:2021-08-25Revised:2021-11-09Online:2022-07-25Published:2022-07-23 -
Contact:GUO Yu
摘要:
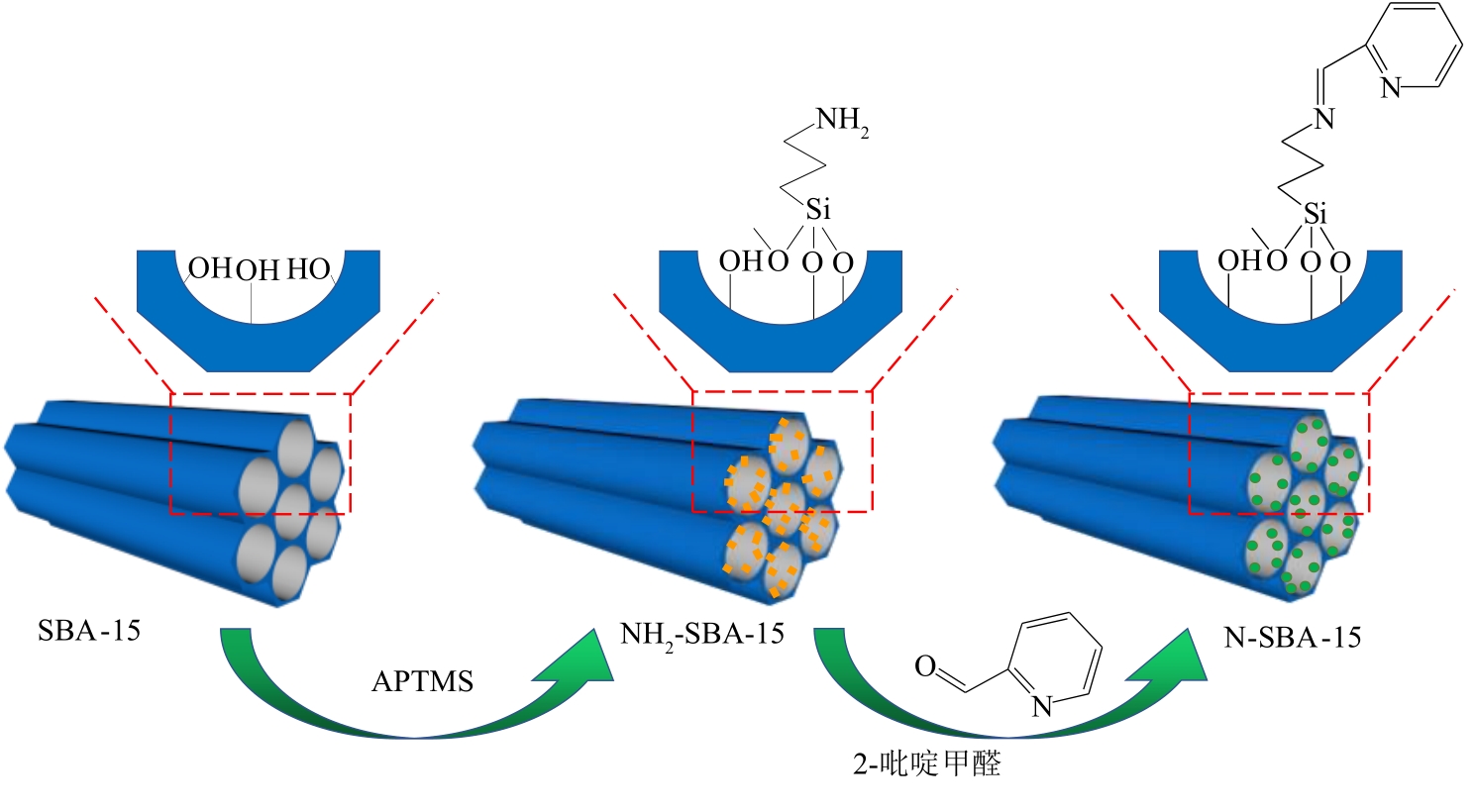
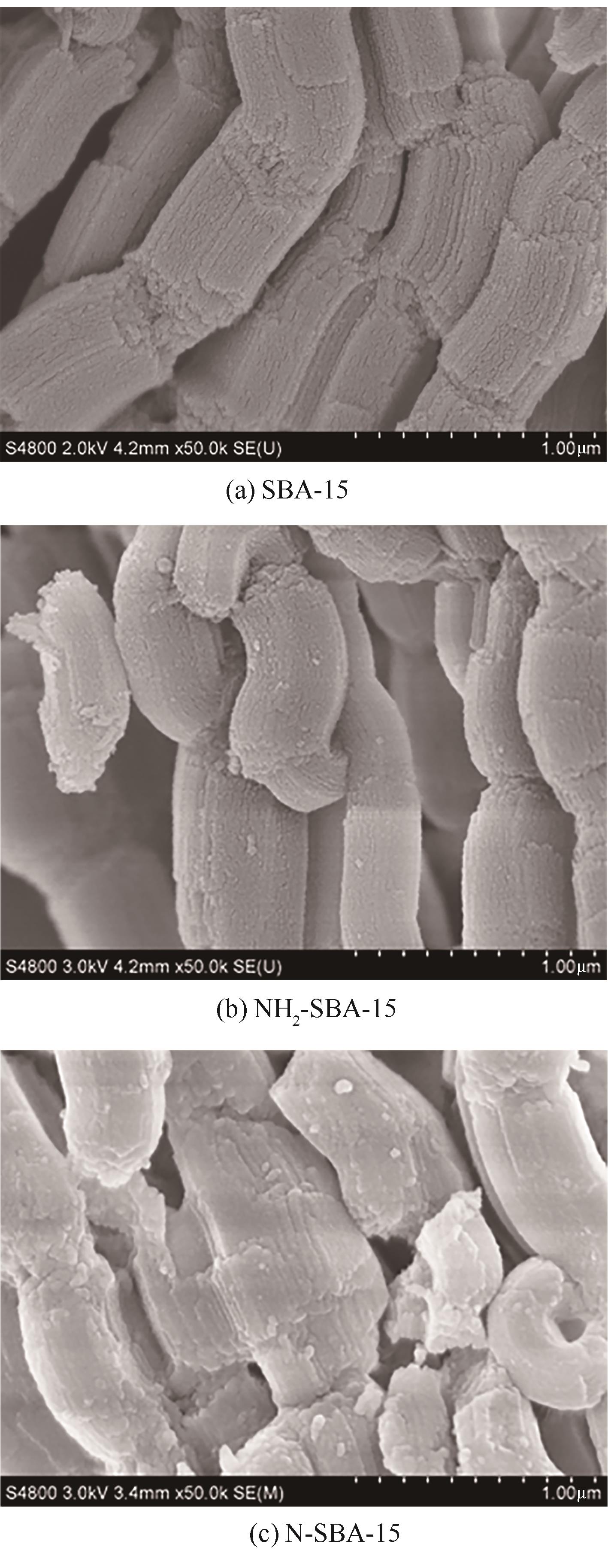
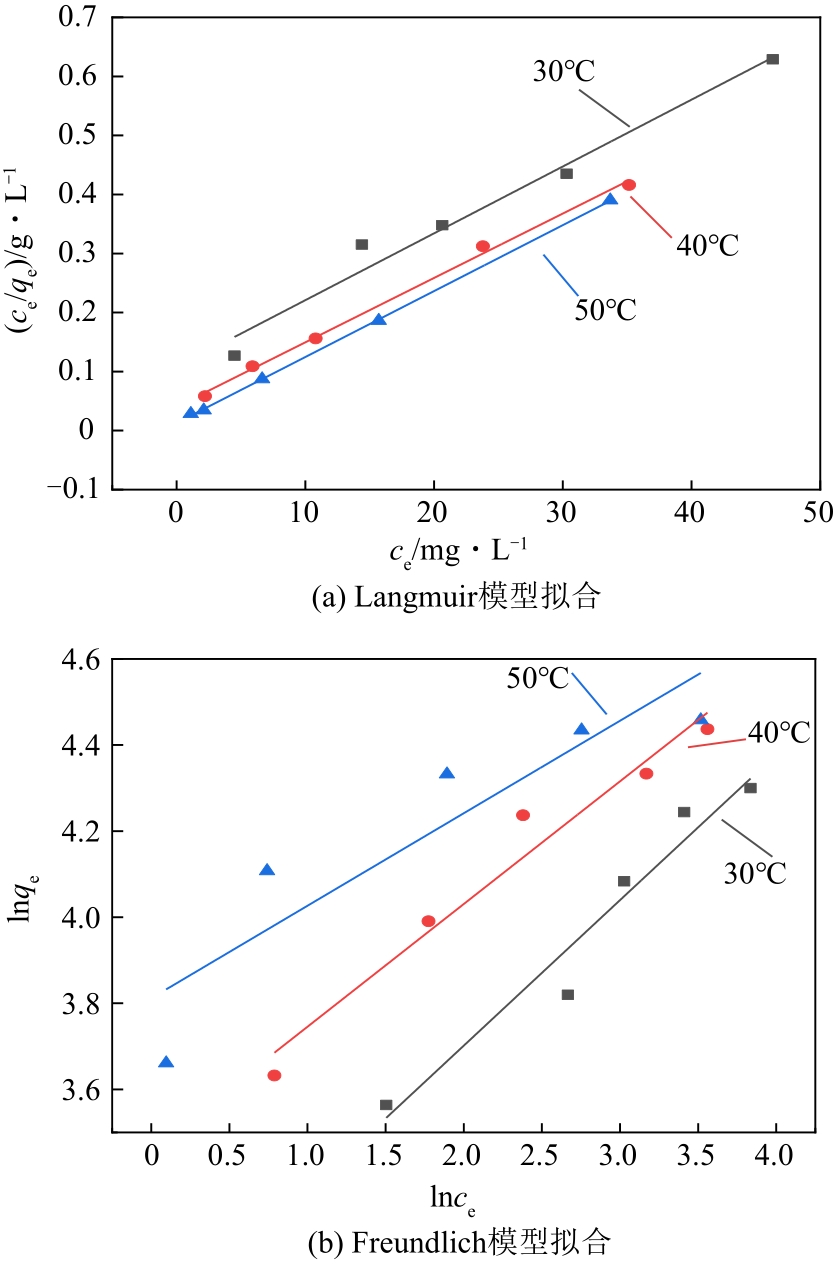
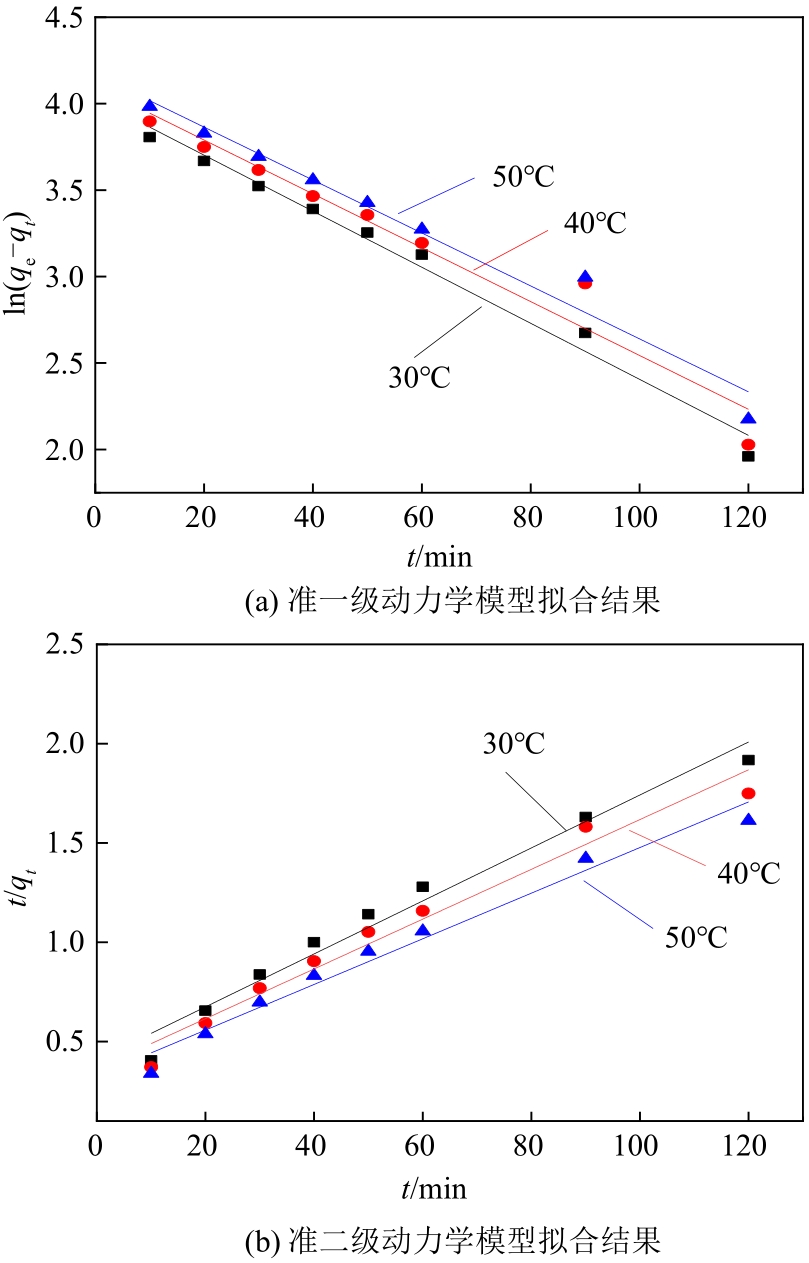
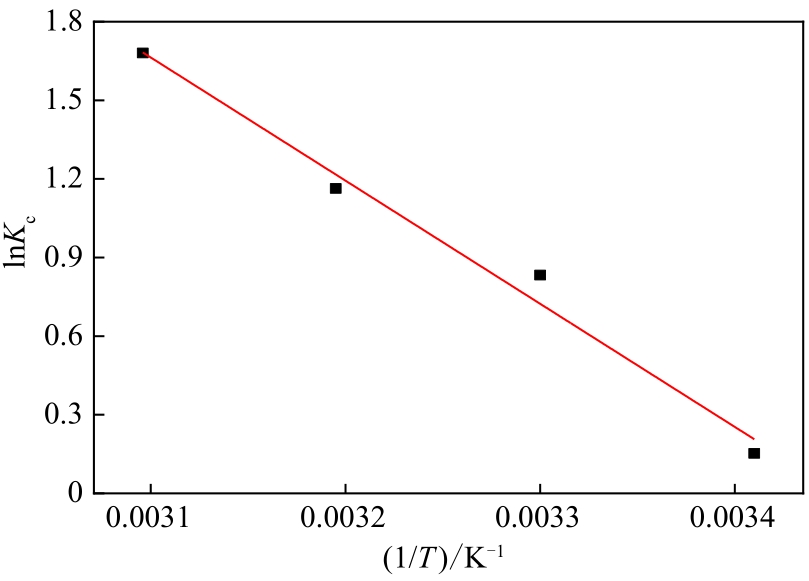
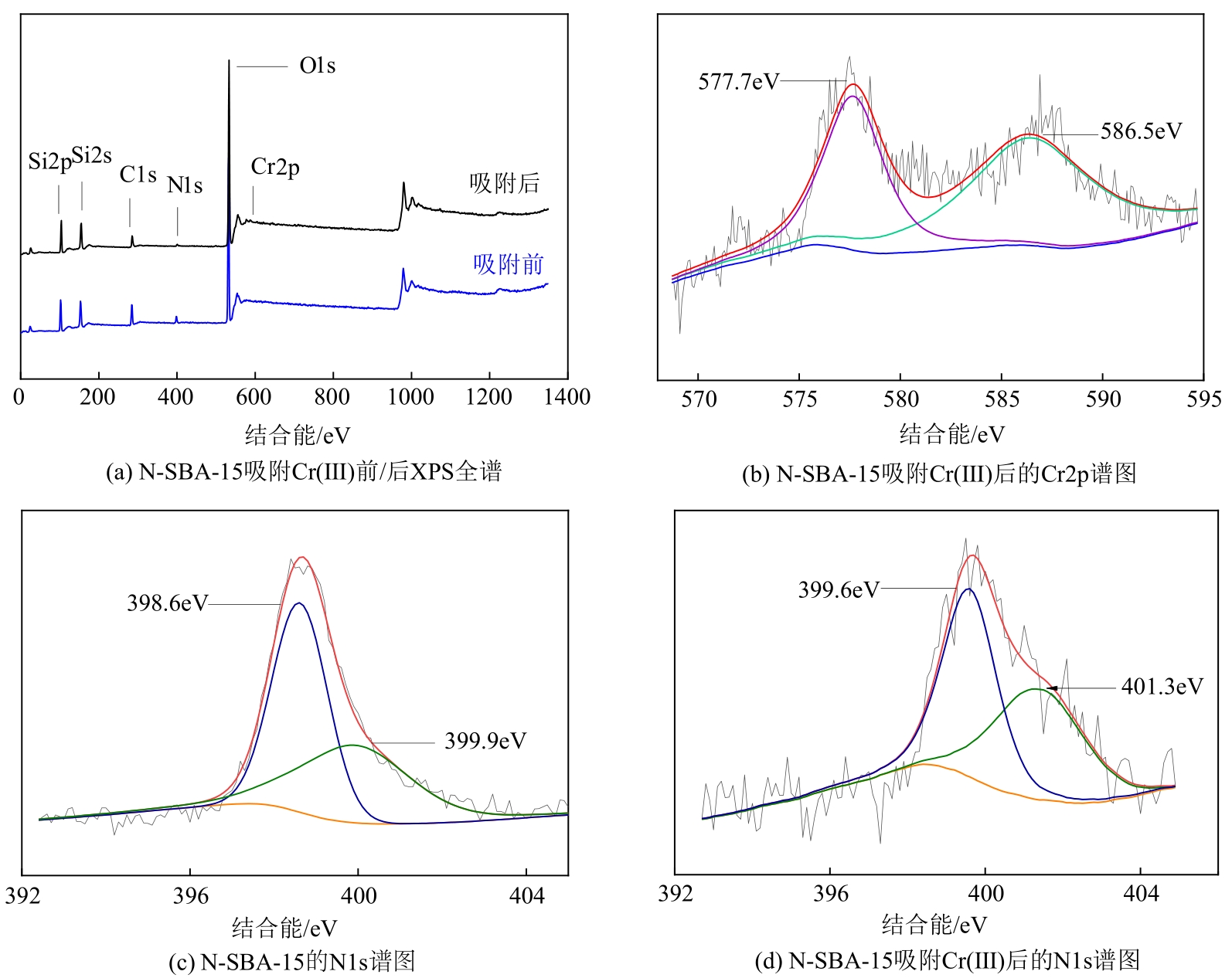
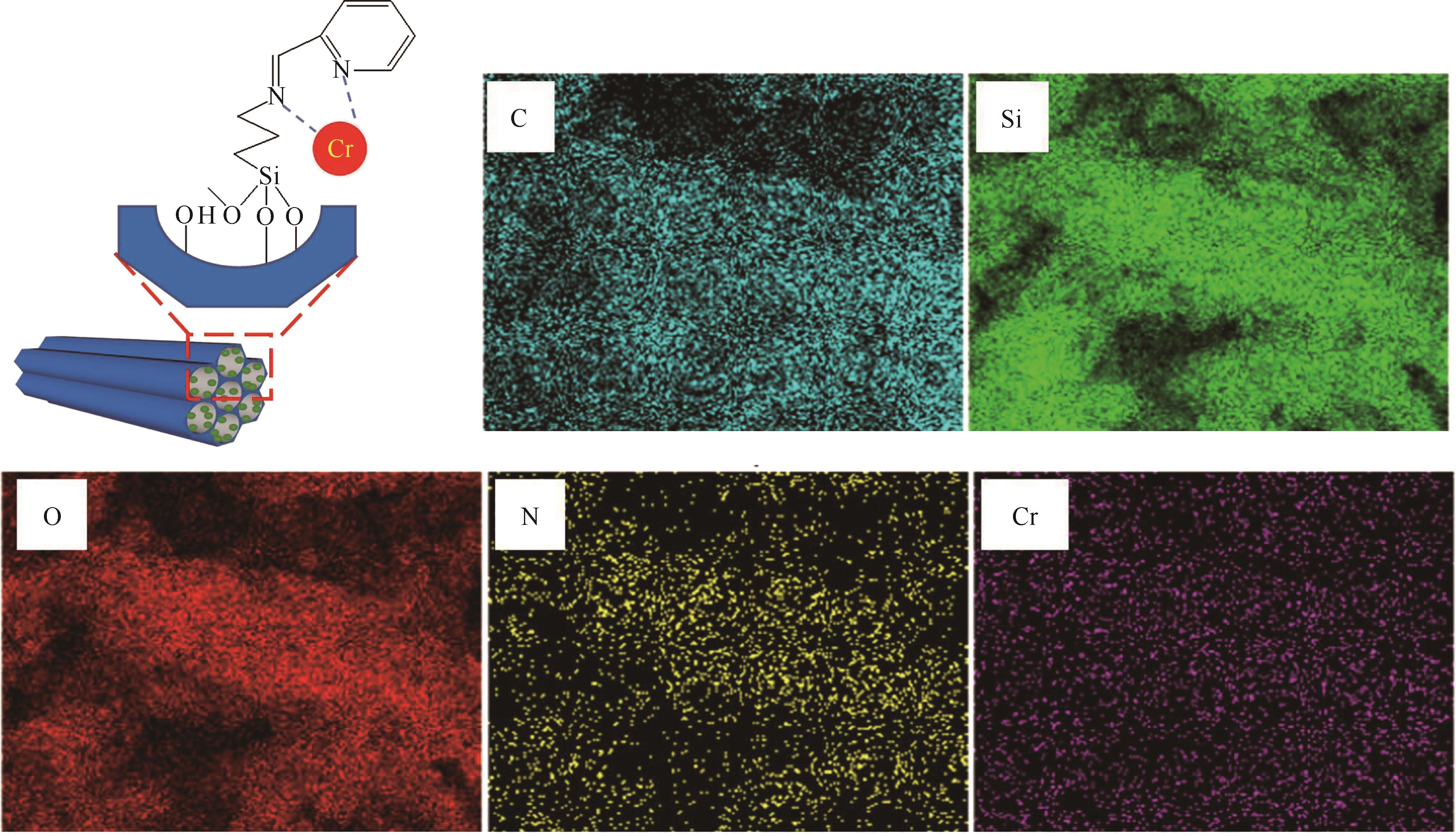
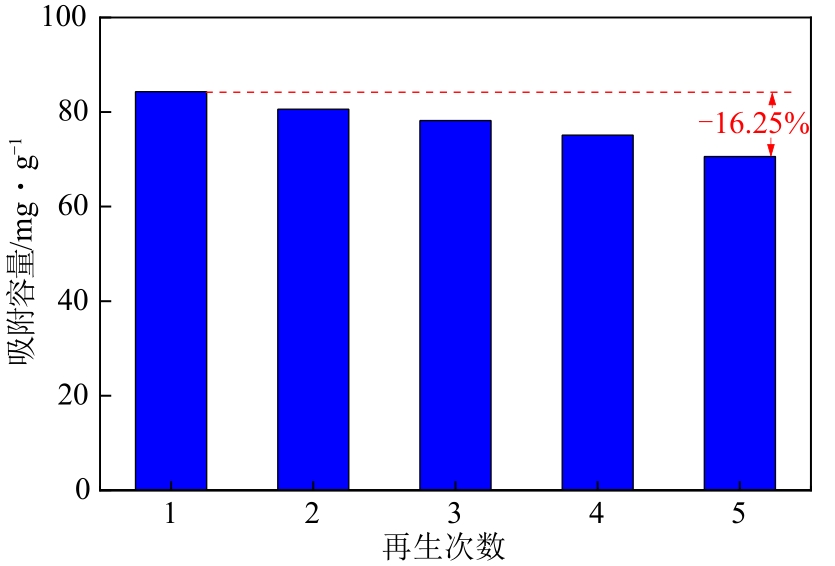
通过席夫碱反应将2-吡啶甲醛成功嫁接到氨基化的SBA-15介孔分子筛上,本文获得了一种新型功能化SBA-15吸附剂(N-SBA-15)。采用傅里叶变换红外光谱、X射线衍射、元素分析、扫描电子显微镜、透射电子显微镜、X射线光电子能谱、热重分析和氮气吸附-脱附等手段对N-SBA-15的表面官能团、形貌、孔道结构和表面化学性质进行了详细的表征分析。利用N-SBA-15对水溶液中的Cr(Ⅲ)进行了吸附实验,其最大吸附容量为84.3mg/g。动力学分析和等温吸附研究结果表明,N-SBA-15对Cr(Ⅲ)的吸附过程符合准二级动力学模型和Langmuir模型。吸附热力学分析表明,该吸附过程是自发的吸热过程(?G<0、?S>0、?H>0)。吸附机理分析表明该吸附过程主要是由N-SBA-15表面有机官能团与Cr(Ⅲ)的配位作用实现的。而且,N-SBA-15吸附剂经过5次吸附-脱附测试,仍然对Cr(Ⅲ)具有较高的吸附容量。
中图分类号:
引用本文
姜晓庆, 郭宇, 吴红梅. 2-吡啶甲醛功能化SBA-15介孔材料的制备及其对Cr(Ⅲ)离子的吸附[J]. 化工进展, 2022, 41(7): 3915-3924.
JIANG Xiaoqing, GUO Yu, WU Hongmei. Synthesis of 2-pyridinecarboxaldehyde functionalized SBA-15 mesoporous material for the adsorption of Cr(Ⅲ) ions from aqueous solution[J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3915-3924.
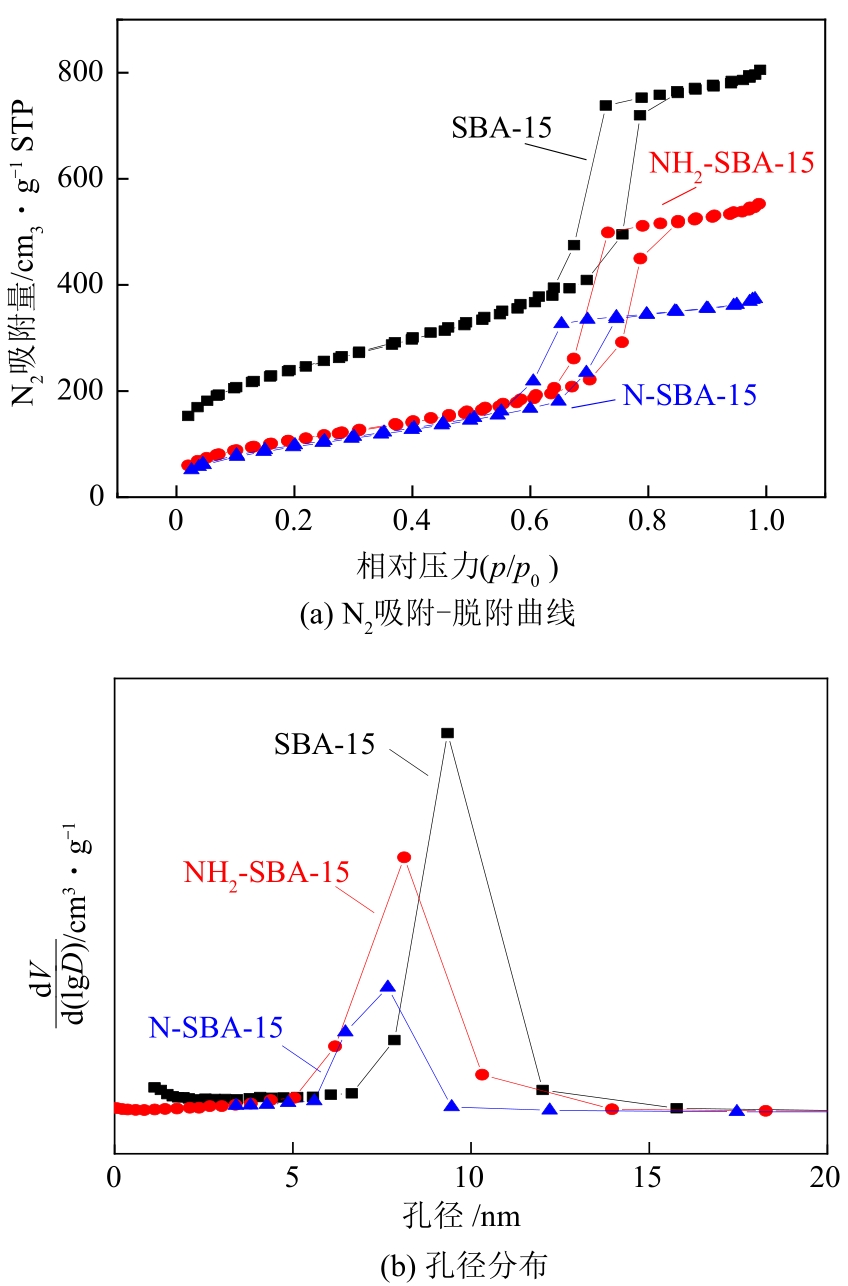
| 样品 | 比表面积/cm3·g-1 | 孔径/nm | 孔容/cm3·g-1 |
|---|---|---|---|
| SBA-15 | 757.40 | 9.60 | 1.04 |
| NH2-SBA-15 | 462.92 | 8.12 | 0.91 |
| N-SBA-15 | 335.04 | 7.67 | 0.54 |
表1 SBA-15、NH2-SBA-15和N-SBA-15分子筛的孔结构分析
| 样品 | 比表面积/cm3·g-1 | 孔径/nm | 孔容/cm3·g-1 |
|---|---|---|---|
| SBA-15 | 757.40 | 9.60 | 1.04 |
| NH2-SBA-15 | 462.92 | 8.12 | 0.91 |
| N-SBA-15 | 335.04 | 7.67 | 0.54 |
| 温度/℃ | Langmuir模型 | Freundlich模型 | ||||
|---|---|---|---|---|---|---|
| qm/g·mg-1 | KL/L·mg-1 | R2 | KF/mg·g-1 | n | R2 | |
| 30 | 88.34 | 0.105 | 0.968 | 20.60 | 2.96 | 0.935 |
| 40 | 91.66 | 0.269 | 0.996 | 31.84 | 3.51 | 0.950 |
| 50 | 91.74 | 0.859 | 0.999 | 45.24 | 4.65 | 0.775 |
表2 不同温度下N-SBA-15吸附Cr(Ⅲ)的吸附等温线拟合常数
| 温度/℃ | Langmuir模型 | Freundlich模型 | ||||
|---|---|---|---|---|---|---|
| qm/g·mg-1 | KL/L·mg-1 | R2 | KF/mg·g-1 | n | R2 | |
| 30 | 88.34 | 0.105 | 0.968 | 20.60 | 2.96 | 0.935 |
| 40 | 91.66 | 0.269 | 0.996 | 31.84 | 3.51 | 0.950 |
| 50 | 91.74 | 0.859 | 0.999 | 45.24 | 4.65 | 0.775 |
| 温度 /℃ | qe(实验) /mg·g-1 | 准一级动力学模型 | 准二级动力学模型 | ||||
|---|---|---|---|---|---|---|---|
| K1/g·mg-1·min-1 | qe(计算)/mg·g-1 | R2 | K2/g·mg-1·min-1 | qe(计算)/mg·g-1 | R2 | ||
| 30 | 84.3 | 0.0162 | 56.07 | 0.983 | 4.36×10-4 | 74.96 | 0.972 |
| 40 | 0.0156 | 60.42 | 0.944 | 4.32×10-4 | 79.74 | 0.966 | |
| 50 | 0.0145 | 64.89 | 0.969 | 4.02×10-4 | 87.03 | 0.973 | |
表3 N-SBA-15吸附Cr(Ⅲ)的动力学方程拟合参数
| 温度 /℃ | qe(实验) /mg·g-1 | 准一级动力学模型 | 准二级动力学模型 | ||||
|---|---|---|---|---|---|---|---|
| K1/g·mg-1·min-1 | qe(计算)/mg·g-1 | R2 | K2/g·mg-1·min-1 | qe(计算)/mg·g-1 | R2 | ||
| 30 | 84.3 | 0.0162 | 56.07 | 0.983 | 4.36×10-4 | 74.96 | 0.972 |
| 40 | 0.0156 | 60.42 | 0.944 | 4.32×10-4 | 79.74 | 0.966 | |
| 50 | 0.0145 | 64.89 | 0.969 | 4.02×10-4 | 87.03 | 0.973 | |
| 温度/K | Kc | ΔG/kJ·mol-1 | ΔS/kJ·mol-1·K-1 | ΔH/kJ·mol-1 |
|---|---|---|---|---|
| 293 | 1.165 | -0.485 | 0.135 | 39.07 |
| 303 | 2.300 | -1.835 | ||
| 313 | 3.202 | -3.185 | ||
| 323 | 5.369 | -4.535 |
表4 N-SBA-15吸附Cr(Ⅲ)的热力学参数
| 温度/K | Kc | ΔG/kJ·mol-1 | ΔS/kJ·mol-1·K-1 | ΔH/kJ·mol-1 |
|---|---|---|---|---|
| 293 | 1.165 | -0.485 | 0.135 | 39.07 |
| 303 | 2.300 | -1.835 | ||
| 313 | 3.202 | -3.185 | ||
| 323 | 5.369 | -4.535 |
| 吸附剂 | 最大吸附容量/mg·g-1 | 参考文献 |
|---|---|---|
| coir pith | 11.56 | [ |
| NZVI-SDBC | 28.89 | [ |
| XPB | 56.50 | [ |
| Fe3O4@SiO2 | 39.27 | [ |
| NH2-SBA-15 | 24.88 | [ |
| N-SBA-15 | 84.3 | 本文 |
表5 不同吸附剂对Cr(Ⅲ)的吸附性能对比
| 吸附剂 | 最大吸附容量/mg·g-1 | 参考文献 |
|---|---|---|
| coir pith | 11.56 | [ |
| NZVI-SDBC | 28.89 | [ |
| XPB | 56.50 | [ |
| Fe3O4@SiO2 | 39.27 | [ |
| NH2-SBA-15 | 24.88 | [ |
| N-SBA-15 | 84.3 | 本文 |
| 1 | SELVARAJ R, SANTHANAM M, SELVAMANI V, et al. A membrane electroflotation process for recovery of recyclable chromium(Ⅲ) from tannery spent liquor effluent[J]. Journal of Hazardous Materials, 2018, 346: 133-139. |
| 2 | CHOW Y N, LEE L K, ZAKARIA N A, et al. Phytotoxic effects of trivalent chromium-enriched water irrigation in Vigna unguiculata seedling[J]. Journal of Cleaner Production, 2018, 202: 101-108. |
| 3 | LIU W, YU Y X. Removal of recalcitrant trivalent chromium complexes from industrial wastewater under strict discharge standards[J]. Environmental Technology & Innovation, 2021, 23: 101644. |
| 4 | LIU G J, CUI C C, JIANG L, et al. Visible light-induced hydrogels towards reversible adsorption and desorption based on trivalent chromium in aqueous solution[J]. Reactive and Functional Polymers, 2021, 163: 104886. |
| 5 | LYU T, MA R G, KE K, et al. Synthesis of gallic acid functionalized magnetic hydrogel beads for enhanced synergistic reduction and adsorption of aqueous chromium[J]. Chemical Engineering Journal, 2021, 408: 127327. |
| 6 | KYZIOŁ-KOMOSIŃSKA J, AUGUSTYNOWICZ J, LASEK W, et al. Callitriche cophocarpa biomass as a potential low-cost biosorbent for trivalent chromium[J]. Journal of Environmental Management, 2018, 214: 295-304. |
| 7 | LATIF A, SHENG D, SUN K, et al. Remediation of heavy metals polluted environment using Fe-based nanoparticles: mechanisms, influencing factors, and environmental implications[J]. Environmental Pollution, 2020, 264:114728. |
| 8 | RAMALINGAM B, VENKATACHALAM S S, KIRAN M S, et al. Rationally designed shewanella oneidensis biofilm toilored graphene-magnetite hybrid nanobiocomposite as reusable living functional nanomaterial for effective removal of trivalent chromium[J]. Environmental Pollution, 2021, 278: 116847. |
| 9 | LI M J, MA C X, YIN X X, et al. Investigating trivalent chromium biosorption-driven extracellular polymeric substances changes of Synechocystis sp. PCC 7806 by parallel factor analysis (PARAFAC) analysis[J]. Bioresource Technology Reports, 2019, 7: 100249. |
| 10 | EL-SHAHAWI M S, HASSAN S S M, OTHMAN A M, et al. Retention profile and subsequent chemical speciation of chromium (Ⅲ) and (Ⅵ) in industrial wastewater samples employing some onium cations loaded polyurethane foams[J]. Microchemical Journal, 2008, 89(1): 13-19. |
| 11 | JIANG D M, YANG Y H, HUANG C T, et al. Removal of the heavy metal ion nickel (Ⅱ) via an adsorption method using flower globular magnesium hydroxide[J]. Journal of Hazardous Materials, 2019, 373: 131-140. |
| 12 | 张振国, 张铭栋, 顾平, 等. 沸石材料吸附水中放射性锶和铯的研究进展[J]. 化工进展, 2019, 38(4): 1984-1995. |
| ZHANG Zhenguo, ZHANG Mingdong, GU Ping, et al. Progress in adsorption of radioactive strontium and cesium from aqueous solution on zeolite materials[J]. Chemical Industry and Engineering Progress, 2019, 38(4): 1984-1995. | |
| 13 | DU X, ZHANG Q, QIAO W L, et al. Controlled self-assembly of oligomers-grafted fibrous polyaniline/single zirconium phosphate nanosheet hybrids with potential-responsive ion exchange properties[J]. Chemical Engineering Journal, 2016, 302: 516-525. |
| 14 | MARCINIAK M, GOSCIANSKA J, FRANKOWSKI M, et al. Optimal synthesis of oxidized mesoporous carbons for the adsorption of heavy metal ions[J]. Journal of Molecular Liquids, 2019, 276: 630-637. |
| 15 | WU H M, XIAO Y, GUO Y, et al. Functionalization of SBA-15 mesoporous materials with 2-acetylthiophene for adsorption of Cr(Ⅲ) ions[J]. Microporous and Mesoporous Materials, 2020, 292: 109754. |
| 16 | RADI S, TIGHADOUINI S, MASSAOUDI M EL, et al. Thermodynamics and kinetics of heavy metals adsorption on silica particles chemically modified by conjugated β-ketoenol furan[J]. Journal of Chemical & Engineering Data, 2015, 60(10): 2915-2925. |
| 17 | ORTIZ-BUSTOS J, MARTÍN A, MORALES V, et al. Surface-functionalization of mesoporous SBA-15 silica materials for controlled release of methylprednisolone sodium hemisuccinate: influence of functionality type and strategies of incorporation[J]. Microporous and Mesoporous Materials, 2017, 240: 236-245. |
| 18 | О DUDARKO, KOBYLINSKA N, MISHRA B, et al. Facile strategies for synthesis of functionalized mesoporous silicas for the removal of rare-earth elements and heavy metals from aqueous systems[J]. Microporous and Mesoporous Materials, 2021, 315: 110919. |
| 19 | LI S L, LI S Q, WEN N, et al. Highly effective removal of lead and cadmium ions from wastewater by bifunctional magnetic mesoporous silica[J]. Separation and Purification Technology, 2021, 265: 118341. |
| 20 | DINDAR M H, YAFTIAN M R, ROSTAMNIA S. Potential of functionalized SBA-15 mesoporous materials for decontamination of water solutions from Cr(Ⅵ), As(Ⅴ) and Hg(Ⅱ) ions[J]. Journal of Environmental Chemical Engineering, 2015, 3(2): 986-995. |
| 21 | 肖昱, 郭宇, 吴红梅, 等. 氨基功能化介孔硅吸附剂的制备及其对铬(Ⅲ)的吸附行为[J]. 化工进展, 2020, 39(1): 257-266. |
| XIAO Yu, GUO Yu, WU Hongmei, et al. Adsorption of chromium(Ⅲ) ions with amino functionalized mesoporous silica adsorbent[J]. Chemical Industry and Engineering Progress, 2020, 39(1): 257-266. | |
| 22 | LIU S, CUI H Z, LI Y L, et al. Bis-pyrazolyl functionalized mesoporous SBA-15 for the extraction of Cr(Ⅲ) and detection of Cr(Ⅵ) in artificial jewelry samples[J]. Microchemical Journal, 2017, 131: 130-136. |
| 23 | CORREIA L M M, SOLIMAN M M A, GRANADEIRO C M, et al. Vanadium C-scorpionate supported on mesoporous aptes-functionalized SBA-15 as catalyst for the peroxidative oxidation of benzyl alcohol[J]. Microporous and Mesoporous Materials, 2021, 320: 111111. |
| 24 | RIBEIRO S O, GRANADEIRO C M, ALMEIDA P L, et al. Effective zinc-substituted keggin composite to catalyze the removal of sulfur from real diesels under a solvent-free system[J]. Industrial & Engineering Chemistry Research, 2019, 58(40): 18540-18549. |
| 25 | ZHOU Y, BAO R L, YUE B, et al. Synthesis, characterization and catalytic application of SBA-15 immobilized rare earth metal sandwiched polyoxometalates[J]. Journal of Molecular Catalysis A: Chemical, 2007, 270(1/2): 50-55. |
| 26 | HAO S Y, ZHONG Y J, PEPE F, et al. Adsorption of Pb2+ and Cu2+ on anionic surfactant-templated amino-functionalized mesoporous silicas[J]. Chemical Engineering Journal, 2012, 189/190: 160-167. |
| 27 | GOLLAKOTA A R K, MUNAGAPATI V S, SHADANGI K P, et al. Encapsulating toxic Rhodamine 6G dye, and Cr(Ⅵ) metal ions from liquid phase using AlPO4-5 molecular sieves. Preparation, characterization, and adsorption parameters[J]. Journal of Molecular Liquids, 2021, 336: 116549. |
| 28 | HERNÁNDEZ-MORALES V, NAVA R, ACOSTA-SILVA Y J, et al. Adsorption of lead (Ⅱ) on SBA-15 mesoporous molecular sieve functionalized with-NH2 groups[J]. Microporous and Mesoporous Materials, 2012, 160: 133-142. |
| 29 | LIU C, JIN R N, OUYANG X K, et al. Adsorption behavior of carboxylated cellulose nanocrystal-polyethyleneimine composite for removal of Cr(Ⅵ) ions[J]. Applied Surface Science, 2017, 408: 77-87. |
| 30 | SALMANI M H, EHRAMPOUSH M H, ESLAMI H, et al. Synthesis, characterization and application of mesoporous silica in removal of cobalt ions from contaminated water[J]. Groundwater for Sustainable Development, 2020, 11: 100425. |
| 31 | MAHMOUDI F, AMINI M M, SILLANPÄÄ M. Hydrothermal synthesis of novel MIL-100(Fe)@SBA-15 composite material with high adsorption efficiency towards dye pollutants for wastewater remediation[J]. Journal of the Taiwan Institute of Chemical Engineers, 2020, 116: 303-313. |
| 32 | SHARMA R, KUMAR D. Adsorption of Cr(Ⅲ) and Cu(Ⅱ) on hydrothermally synthesized graphene oxide-calcium-zinc nanocomposite[J]. Journal of Chemical & Engineering Data, 2018, 63(12): 4560-4572. |
| 33 | 张永德, 黄松涛, 罗学刚, 等. 膨化稻壳对铀及伴生重金属离子的吸附机理[J]. 化工进展, 2016, 35(9): 2707-2714. |
| ZHANG Yongde, HUANG Songtao, LUO Xuegang, et al. Adsorption characteristics and mechanism of U(Ⅵ) and associated heavy metals on expanded rice husk[J]. Chemical Industry and Engineering Progress, 2016, 35(9): 2707-2714. | |
| 34 | 王重庆, 王晖, 江小燕, 等. 生物炭吸附重金属离子的研究进展[J]. 化工进展, 2019, 38(1): 692-706. |
| WANG Chongqing, WANG Hui, JIANG Xiaoyan, et al. Research advances on adsorption of heavy metals by biochar[J]. Chemical Industry and Engineering Progress, 2019, 38(1): 692-706. | |
| 35 | DENG S B, TING Y P. Polyethylenimine-modified fungal biomass as a high-capacity biosorbent for Cr(Ⅵ) anions: sorption capacity and uptake mechanisms[J]. Environmental Science & Technology, 2005, 39(21): 8490-8496. |
| 36 | LEI Z M, AN Q D, FAN Y, et al. Monolithic magnetic carbonaceous beads for efficient Cr(Ⅵ) removal from water[J]. New Journal of Chemistry, 2016, 40(2): 1195-1204. |
| 37 | PARAB H, JOSHI S, SHENOY N, et al. Determination of kinetic and equilibrium parameters of the batch adsorption of Co(Ⅱ), Cr(Ⅲ) and Ni(Ⅱ) onto coir pith[J]. Process Biochemistry, 2006, 41(3): 609-615. |
| 38 | QIU Y, ZHANG Q, GAO B, et al. Removal mechanisms of Cr(Ⅵ) and Cr(Ⅲ) by biochar supported nanosized zero-valent iron: synergy of adsorption, reduction and transformation[J]. Environmental Pollution, 2020, 265: 115018. |
| 39 | ARIM A L, QUINA M J, GANDO-FERREIRA L M. Uptake of trivalent chromium from aqueous solutions by xanthate pine bark: characterization, batch and column studies[J]. Process Safety and Environmental Protection, 2019, 121:374-386. |
| 40 | WANG J H, MAO M, ATIF S, et al. Adsorption behavior and mechanism of aqueous Cr(Ⅲ) and Cr(Ⅲ)-EDTA chelates on DTPA-chitosan modified Fe3O4@SiO2 [J]. Reactive and Functional Polymers, 2020, 156: 104720. |
| [1] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [2] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [3] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [4] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [5] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [6] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [7] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [8] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [9] | 姜晶, 陈霄宇, 张瑞妍, 盛光遥. 载锰生物炭制备及其在环境修复中应用研究进展[J]. 化工进展, 2023, 42(8): 4385-4397. |
| [10] | 张振, 李丹, 陈辰, 吴菁岚, 应汉杰, 乔浩. 吸附树脂对唾液酸的分离纯化[J]. 化工进展, 2023, 42(8): 4153-4158. |
| [11] | 陆洋, 周劲松, 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛. CeO2/TiO2吸附剂煤气脱汞产物的浸出规律[J]. 化工进展, 2023, 42(7): 3875-3883. |
| [12] | 张雪伟, 黄亚继, 许月阳, 程好强, 朱志成, 李金壘, 丁雪宇, 王圣, 张荣初. 碱性吸附剂对燃煤烟气中SO3的吸附特性[J]. 化工进展, 2023, 42(7): 3855-3864. |
| [13] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| [14] | 李艳玲, 卓振, 池亮, 陈曦, 孙堂磊, 刘鹏, 雷廷宙. 氮掺杂生物炭的制备与应用研究进展[J]. 化工进展, 2023, 42(7): 3720-3735. |
| [15] | 于静文, 宋璐娜, 刘砚超, 吕瑞东, 武蒙蒙, 冯宇, 李忠, 米杰. 一种吲哚基超交联聚合物In-HCP对水中碘的吸附作用[J]. 化工进展, 2023, 42(7): 3674-3683. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||