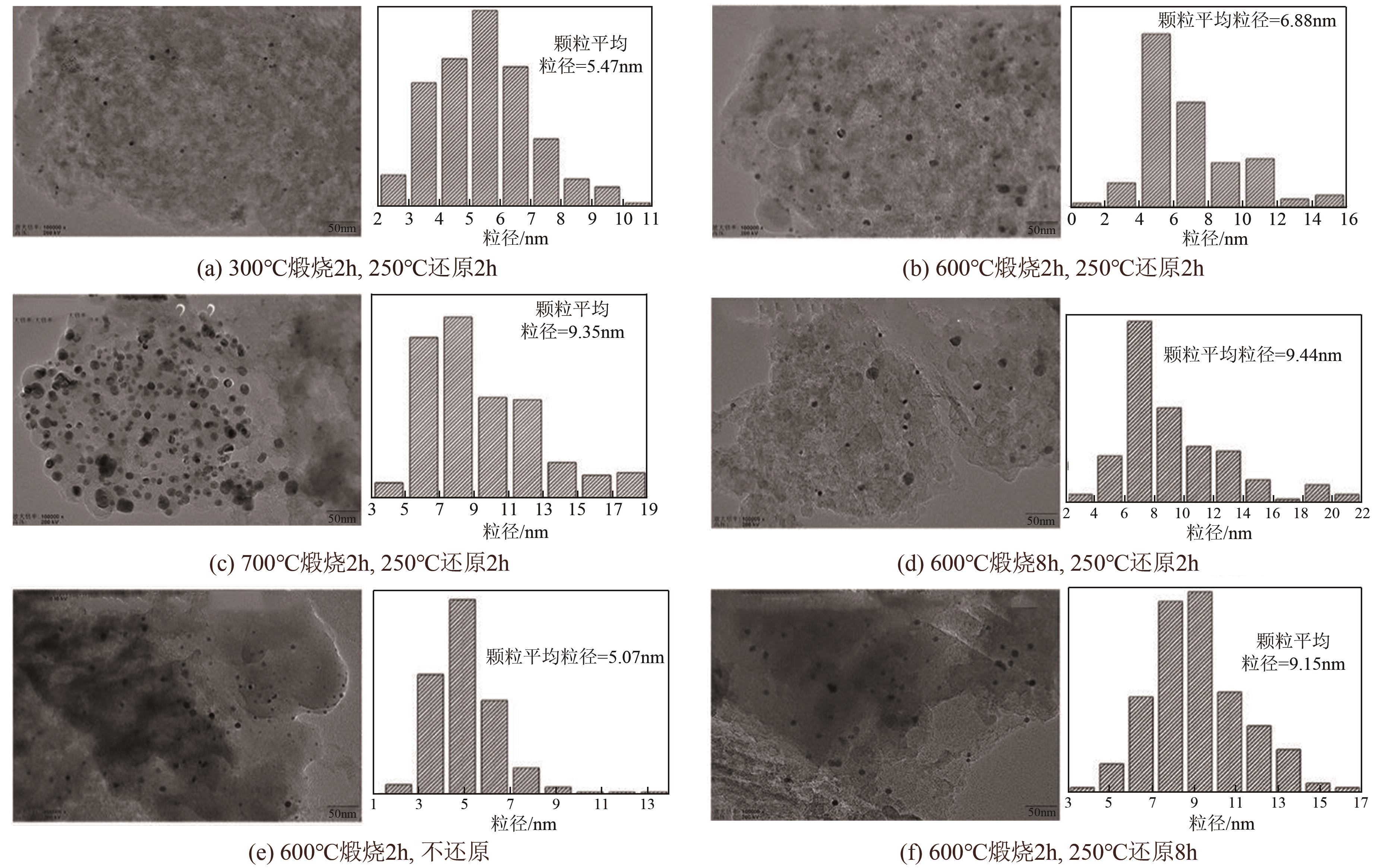
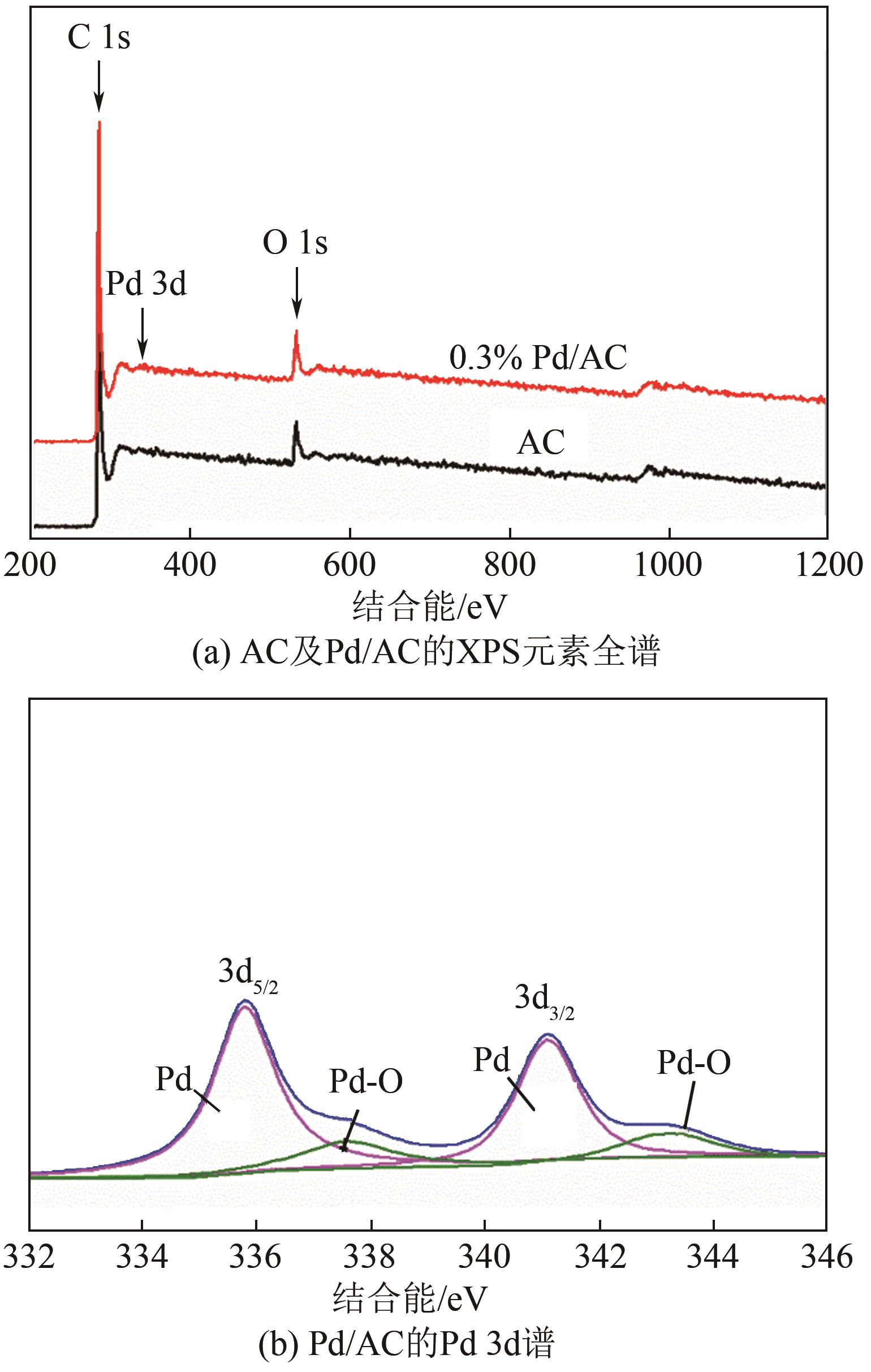
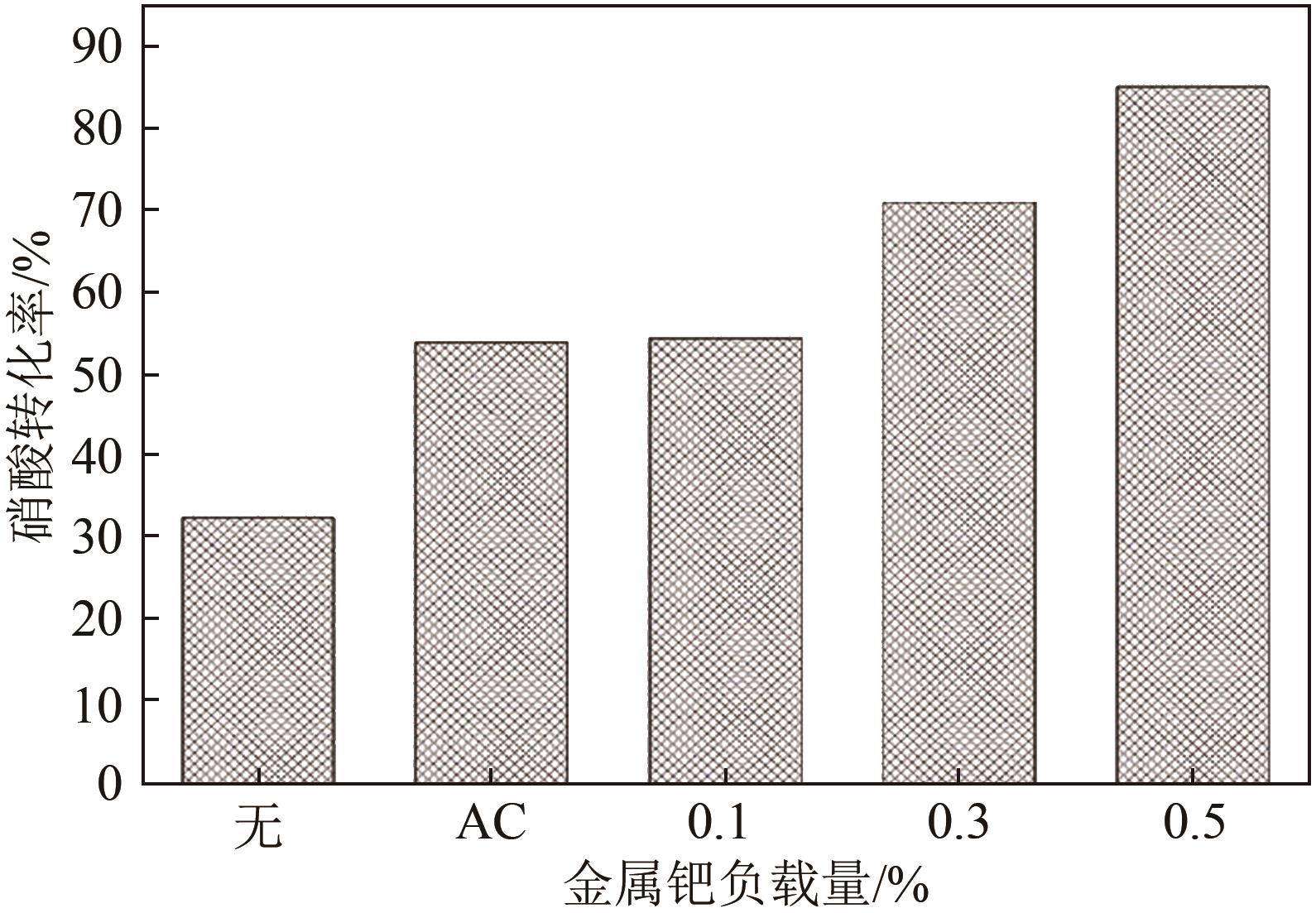
| 1 |
宗弘元, 孙凤侠, 刘俊涛, 等. 一氧化氮与硝酸和烷基醇反应制亚硝酸烷基酯的方法: CN201510657092.5[P]. 2018-07-13.
|
|
ZONG Hongyuan, SUN Fengxia, LIU Juntao, et al. Method for producing alkyl nitrite ester by reaction of nitric oxide with nitric acid and alkyl alcohol: CN201510657092.5[P]. 2018-07-13.
|
| 2 |
孙凤侠, 刘俊涛, 龚海燕. 亚硝酸甲酯的生产方法: CN106565498B[P]. 2018-07-17.
|
|
SUN Fengxia, LIU Juntao, GONG Haiyan. Production method of methyl nitrite: CN106565498B[P]. 2018-07-17.
|
| 3 |
周张锋, 李兆基, 潘鹏斌, 等. 煤制乙二醇技术进展[J]. 化工进展, 2010, 29(11): 2003-2009.
|
|
ZHOU Zhangfeng, LI Zhaoji, PAN Pengbin, et al. Progress in technologies of coal-based ethylene glycol synthesis[J]. Chemical Industry and Engineering Progress, 2010, 29(11): 2003-2009.
|
| 4 |
马继平, 徐杰, 贾秀全, 等. 一种还原稀硝酸到亚硝酸的方法: CN201410062618.0 [P]. 2015-08-26.
|
|
MA Jiping, XU Jie, JIA Xiuquan, et al. A method for reducing dilute nitric acid to nitrite: CN201410062618.0 [P]. 2015-08-26.
|
| 5 |
郑卫, 孔会娜. 煤制乙二醇废水处理技术及发展趋势[J]. 河南化工, 2019, 36(2): 9-11, 28.
|
|
ZHENG Wei, KONG Huina. Technology and development trend of coal-to-ethylene glycol wastewater treatment[J]. Henan Chemical Industry, 2019, 36(2): 9-11, 28.
|
| 6 |
杉瀬良二, 田中秀二, 井伊宏文, 等. 生产亚硝酸烷基酯的方法: CN1267401C[P]. 2006-08-02.
|
|
SEO Sugase, HIDEJI Tanaka, IKWANG Man, et al. Method for producing arrcostab nitrite: CN1267401C[P]. 2006-08-02.
|
| 7 |
李文龙, 王玮涵, 李振花, 等. 一氧化氮与硝酸和甲醇反应制亚硝酸甲酯[J]. 化学反应工程与工艺, 2015, 31(2): 188-192.
|
|
LI Wenlong, WANG Weihan, LI Zhenhua, et al. Synthesis of methyl nitrite from nitric oxide, nitric acid and methanol[J]. Chemical Reaction Engineering and Technology, 2015, 31(2): 188-192.
|
| 8 |
陈鹏. 自吸式搅拌鼓泡反应器中硝酸还原反应的研究[J]. 化学工程与装备, 2016(9): 59-61.
|
|
CHEN Peng. Study of nitrate reduction reaction in a self-priming bubbling reactor[J]. Chemical Engineering & Equipment, 2016(9): 59-61.
|
| 9 |
张向凯. 硝酸还原技术在煤制乙二醇装置中的应用及评价[J]. 化肥设计, 2019, 57(4): 41-43.
|
|
ZHANG Xiangkai. The application of nitric acid reduction technology in coal-to-glycol unit and evaluation[J]. Chemical Fertilizer Design, 2019, 57(4): 41-43.
|
| 10 |
吴晓金, 孔国杰, 梁鹏, 等. 一种处理稀硝酸并生成亚硝酸烷基酯的催化剂的制备方法: CN104338550A[P]. 2015-02-11.
|
|
WU Xiaojin, KONG Guojie, LIANG Peng, et al. Preparation method of catalyst used in dilute nitric acid treating and alkyl nitrite generating: CN104338550A[P]. 2015-02-11.
|
| 11 |
孙凤侠, 刘俊涛, 刘国强. NO与硝酸和甲醇反应制亚硝酸甲酯的方法: CN106565494B[P]. 2018-08-17.
|
|
SUN Fengxia, LIU Juntao, LIU Guoqiang. Methyl nitrite preparation method by reaction of NO and nitric acid and methanol: CN106565494B[P]. 2018-08-17.
|
| 12 |
高明亮. 乙二醇生产中硝酸催化还原的实验研究及应用[J]. 河南化工, 2016, 33(8): 33-37.
|
|
GAO Mingliang. Experimental research and application of nitric acid reduction in ethylene glycol production[J]. Henan Chemical Industry, 2016, 33(8): 33-37.
|
| 13 |
李文龙, 李扬, 王科, 等. 乙醇中硝酸还原工艺条件及Pd-Cu催化剂研究[J]. 化学反应工程与工艺, 2017, 33(5): 402-408.
|
|
LI Wenlong, LI Yang, WANG Ke, et al. Study on process conditions and Pd-Cu catalysts for nitrate acid reduction in ethanol[J]. Chemical Reaction Engineering and Technology, 2017, 33(5): 402-408.
|
| 14 |
李文龙, 宋元江, 李扬, 等. 用于催化稀硝酸还原反应的贵金属催化剂研究[J]. 天然气化工(Cl化学与化工), 2019, 44(5): 32-36.
|
|
LI Wenlong, SONG Yuanjiang, LI Yang, et al. Noble metal catalysts for dilute nitric acid reduction[J]. Natural Gas Chemical Industry, 2019, 44(5): 32-36.
|
| 15 |
TRAWCZYŃSKI J, GHEEK P, OKAL J, et al. Reduction of nitrate on active carbon supported Pd-Cu catalysts[J]. Applied Catalysis A: General, 2011, 409/410: 39-47.
|
| 16 |
谭贺云,孙巧萍,肖忠连, 等. 煅烧温度对Pr6O11@C光催化性能的影响[J/OL]. 中国稀土学报. .
|
|
TAN Heyun, SUN Qiaoping; XIAO Zhonglian, et al. Effect of calcination on the photocatalytic efficiency of Pr6O11@C[J/OL]. Journal of the Chinese Society of Rare Earths. .
|
| 17 |
卢东亮, 代广周, 姚英邦, 等. 煅烧温度对Li0.33La0.56TiO3固态离子电容器性能的影响[J]. 无机材料学报, 2018, 33(10): 1077-1082.
|
|
LU Dongliang, DAI Guangzhou, YAO Yingbang, et al. Influence of calcining temperature on the property of Li0.33La0.56TiO3 solid-state ionic capacitor[J]. Journal of Inorganic Materials, 2018, 33(10): 1077-1082.
|
| 18 |
DAI Zhongmin, MENG Jun, MUHAMMAD Niaz, et al. The potential feasibility for soil improvement, based on the properties of biochars pyrolyzed from different feedstocks[J]. Journal of Soils and Sediments, 2013, 13(6): 989-1000.
|
| 19 |
薛嵩, 钱林波, 晏井春, 等. 生物炭携载纳米零价铁对溶液中Cr(Ⅵ)的去除[J]. 环境工程学报, 2016, 10(6): 2895-2901.
|
|
XUE Song, QIAN Linbo, YAN Jingchun, et al. Removal of Cr(Ⅵ) from aqueous using biochar carried nanoscal zero-velent iron[J]. Chinese Journal of Environmental Engineering, 2016, 10(6): 2895-2901.
|
| 20 |
LI Zelong, LI Jinlei, LIU Jianhua, et al. Palladium nanoparticles supported on nitrogen-functionalized active carbon: a stable and highly efficient catalyst for the selective hydrogenation of nitroarenes[J]. ChemCatChem, 2014, 6(5): 1333-1339.
|
| 21 |
张伟, 杨柳, 蒋海燕, 等. 污泥活性炭的表征及其对Cr(Ⅵ)的吸附特性[J]. 环境工程学报, 2014, 8(4): 1439-1446.
|
|
ZHANG Wei, YANG Liu, JIANG Haiyan, et al. Characterization of sludge-based activated carbon and its adsorption properties on Cr(Ⅵ)[J]. Chinese Journal of Environmental Engineering, 2014, 8(4): 1439-1446.
|
| 22 |
林珈羽, 张越, 刘沅, 等. 不同原料和炭化温度下制备的生物炭结构及性质[J]. 环境工程学报, 2016, 10(6): 3200-3206.
|
|
LIN Jiayu, ZHANG Yue, LIU Yuan, et al. Structure and properties of biochar under different materials and carbonization temperatures[J]. Chinese Journal of Environmental Engineering, 2016, 10(6): 3200-3206.
|
| 23 |
JIN Haiyan, XIONG Tianyi, LI Yi, et al. Improved electrocatalytic activity for ethanol oxidation by Pd@N-doped carbon from biomass[J]. Chemical Communications, 2014, 50(84): 12637-12640.
|
| 24 |
LIANG Ruowen, LUO Shuiguang, JING Fenfen, et al. A simple strategy for fabrication of Pd@MIL-100(Fe) nanocomposite as a visible-light-driven photocatalyst for the treatment of pharmaceuticals and personal care products (PPCPs)[J]. Applied Catalysis B: Environmental, 2015, 176/177: 240-248.
|
| 25 |
ZHANG Jiawei, SUN Kangkang, LI Dandan, et al. Pd-Ni bimetallic nanoparticles supported on active carbon as an efficient catalyst for hydrodeoxygenation of aldehydes[J]. Applied Catalysis A: General, 2019, 569: 190-195.
|
| 26 |
THUY VU Hang Thi, Viet le NAM VO, CHUNG Young Min. Geometric, electronic, and synergistic effect in the sulfonated carbon-supported Pd catalysts for the direct synthesis of hydrogen peroxide[J]. Applied Catalysis A: General, 2020, 607: 117867.
|
| 27 |
谢炳炎, 蔡海林, 徐竹生, 等. 钯/炭催化剂在不同气氛中热处理的考察[J]. 燃料化学学报, 1992, 20(2): 213-218.
|
|
XIE Bingyan, CAI Hailin, XU Zhusheng, et al. Performance of Pd/C catalyst after heat treatment in various gases[J]. Journal of Fuel Chemistry and Technology, 1992, 20(2): 213-218.
|
| 28 |
陈琛, 金汉强. 钯碳催化剂的制备条件对苯酚气相加氢制环己酮的影响[J]. 能源化工, 2015, 36(6): 1-4.
|
|
CHEN Chen, JIN Hanqiang. Effect of preparing conditions of Pd/C catalysts on vapour phase hydrogenation of phenol to cyclohexanone[J]. Energy Chemical Industry, 2015, 36(6): 1-4.
|
 ), 徐垒3, 陈晨3, 徐忠宁4, 付明来1,3(
), 徐垒3, 陈晨3, 徐忠宁4, 付明来1,3( )
)
 ), XU Lei3, CHEN Chen3, XU Zhongning4, FU Minglai1,3(
), XU Lei3, CHEN Chen3, XU Zhongning4, FU Minglai1,3( )
)