化工进展 ›› 2022, Vol. 41 ›› Issue (3): 1528-1538.DOI: 10.16085/j.issn.1000-6613.2021-1712
鼓泡床中电石渣加速碳酸化分析与响应面优化
郑鹏( ), 李蔚玲(
), 李蔚玲( ), 郭亚飞, 孙健, 王瑞林, 赵传文
), 郭亚飞, 孙健, 王瑞林, 赵传文
- 南京师范大学能源与机械工程学院,江苏 南京 210046
-
收稿日期:2021-08-11修回日期:2021-09-29出版日期:2022-03-23发布日期:2022-03-28 -
通讯作者:李蔚玲 -
作者简介:郑鹏(1997—),男,硕士研究生,研究方向为鼓泡床多相流反应器。E-mail:411533280@qq.com 。 -
基金资助:国家自然科学基金(51706108);江苏省高校自然科学研究面上项目(17KJB470008);南京师范大学科研启动项目(184080H202B73)
Analysis of carbide slag accelerated carbonation in bubble column and response surface optimization
ZHENG Peng( ), LI Weiling(
), LI Weiling( ), GUO Yafei, SUN Jian, WANG Ruilin, ZHAO Chuanwen
), GUO Yafei, SUN Jian, WANG Ruilin, ZHAO Chuanwen
- School of Energy and Mechanical Engineering, Nanjing Normal University, Nanjing 210046, Jiangsu, China
-
Received:2021-08-11Revised:2021-09-29Online:2022-03-23Published:2022-03-28 -
Contact:LI Weiling
摘要:
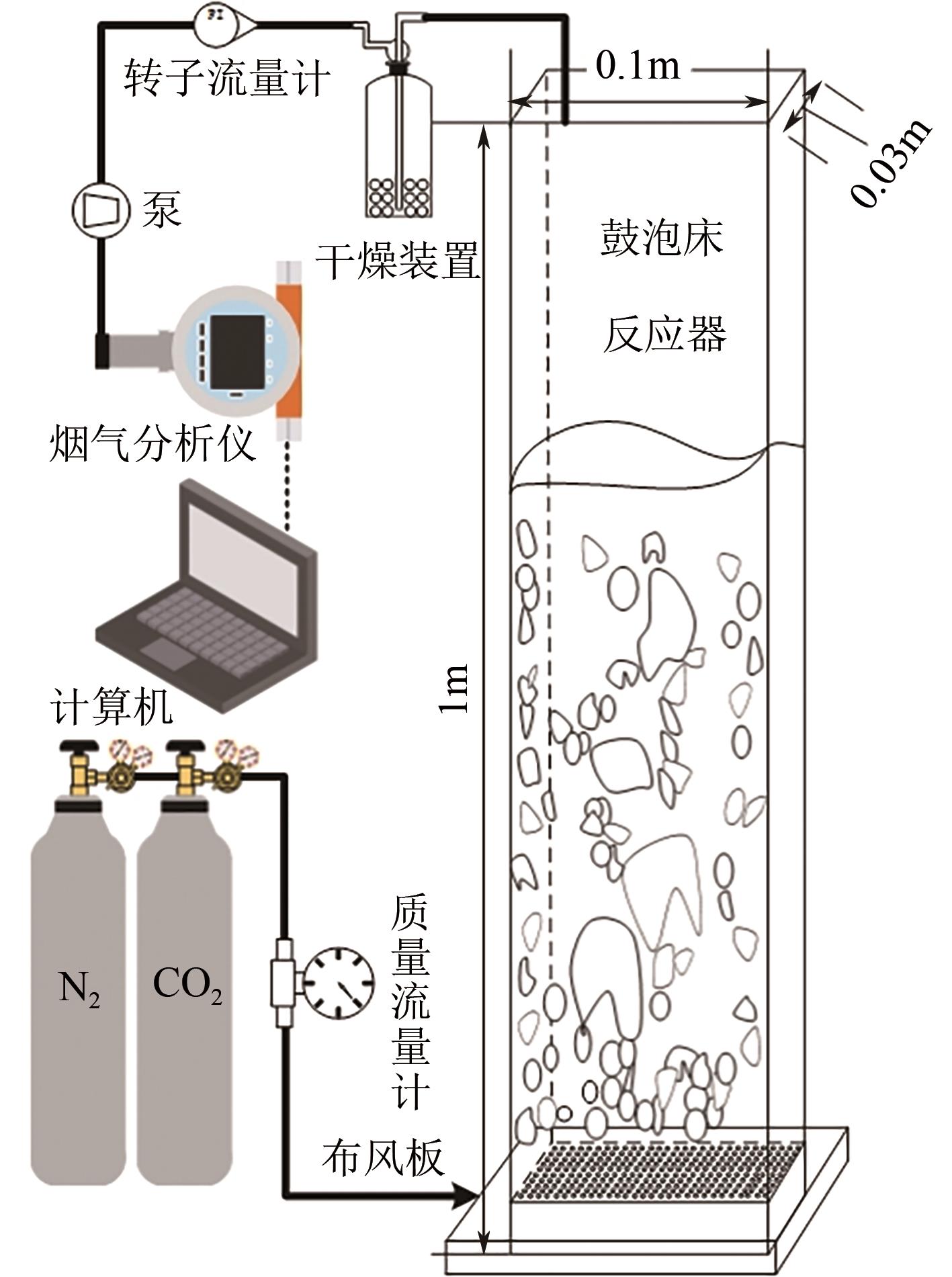
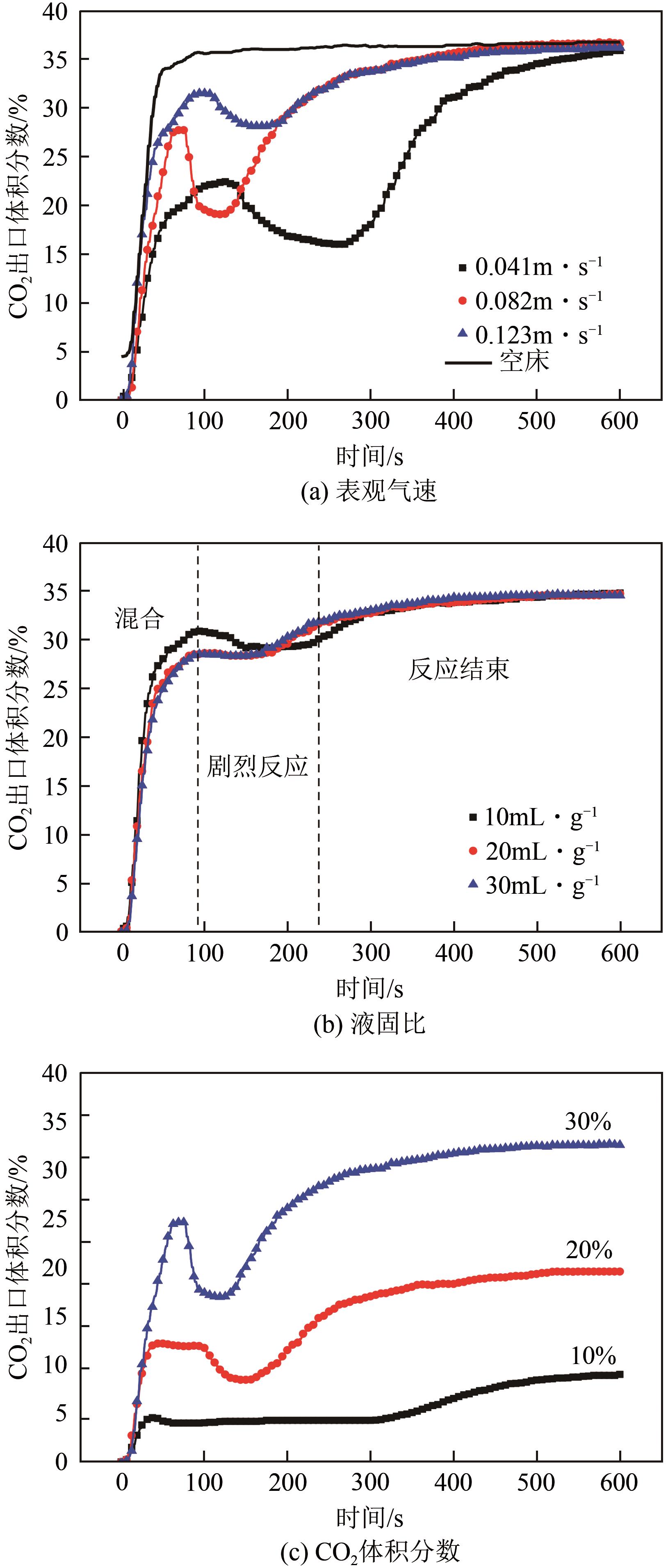
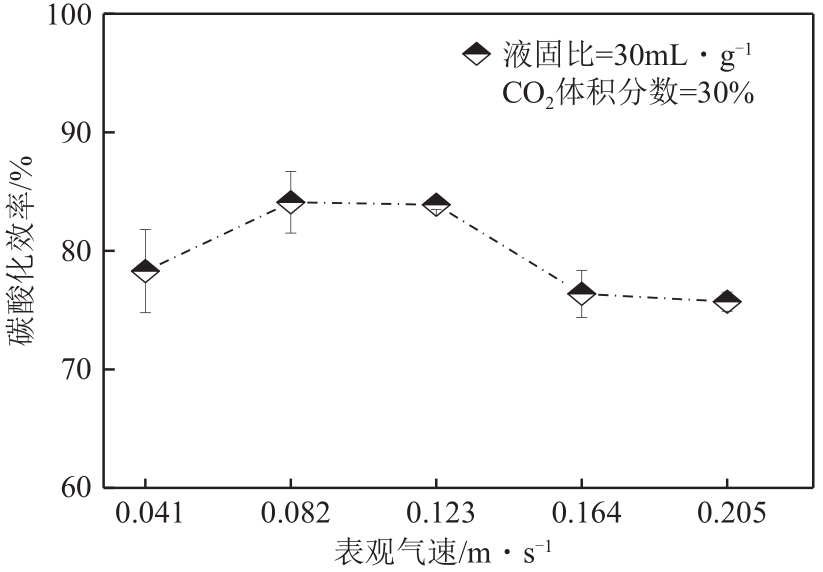
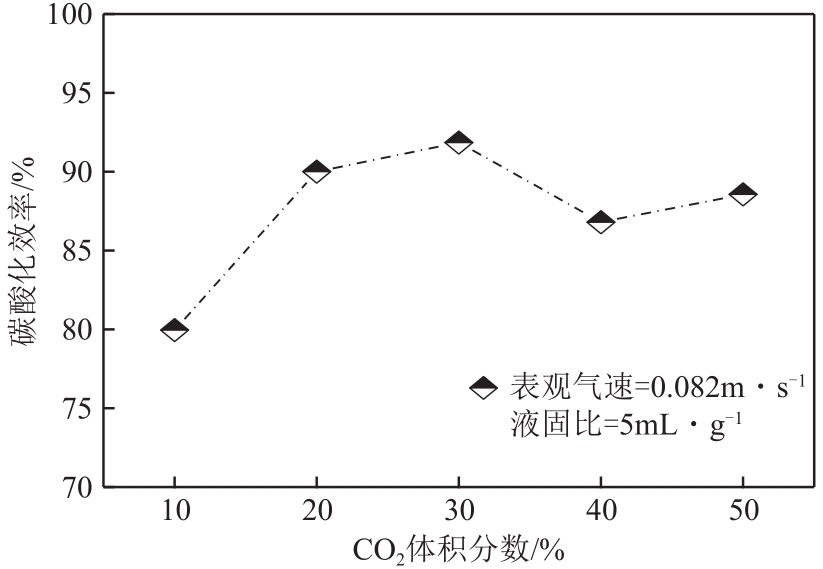
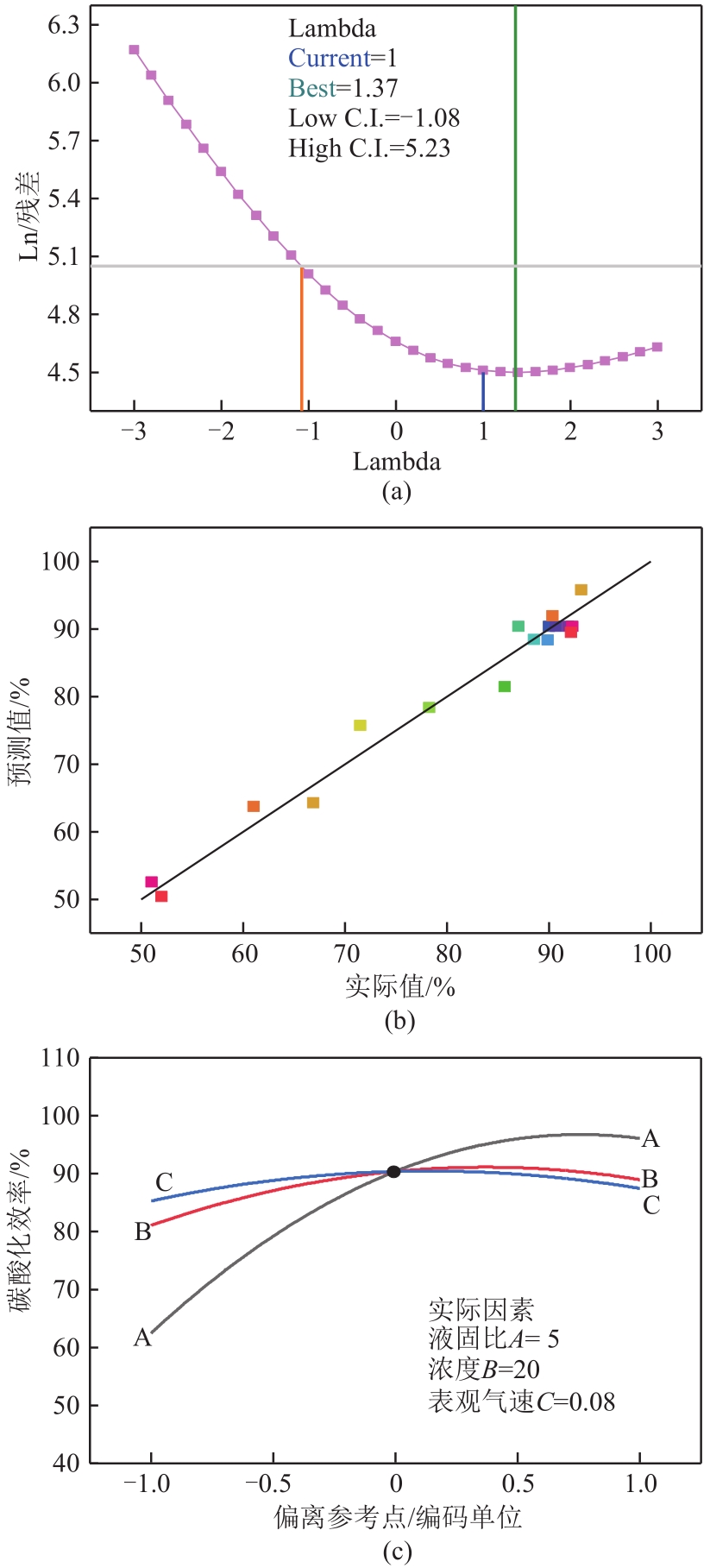
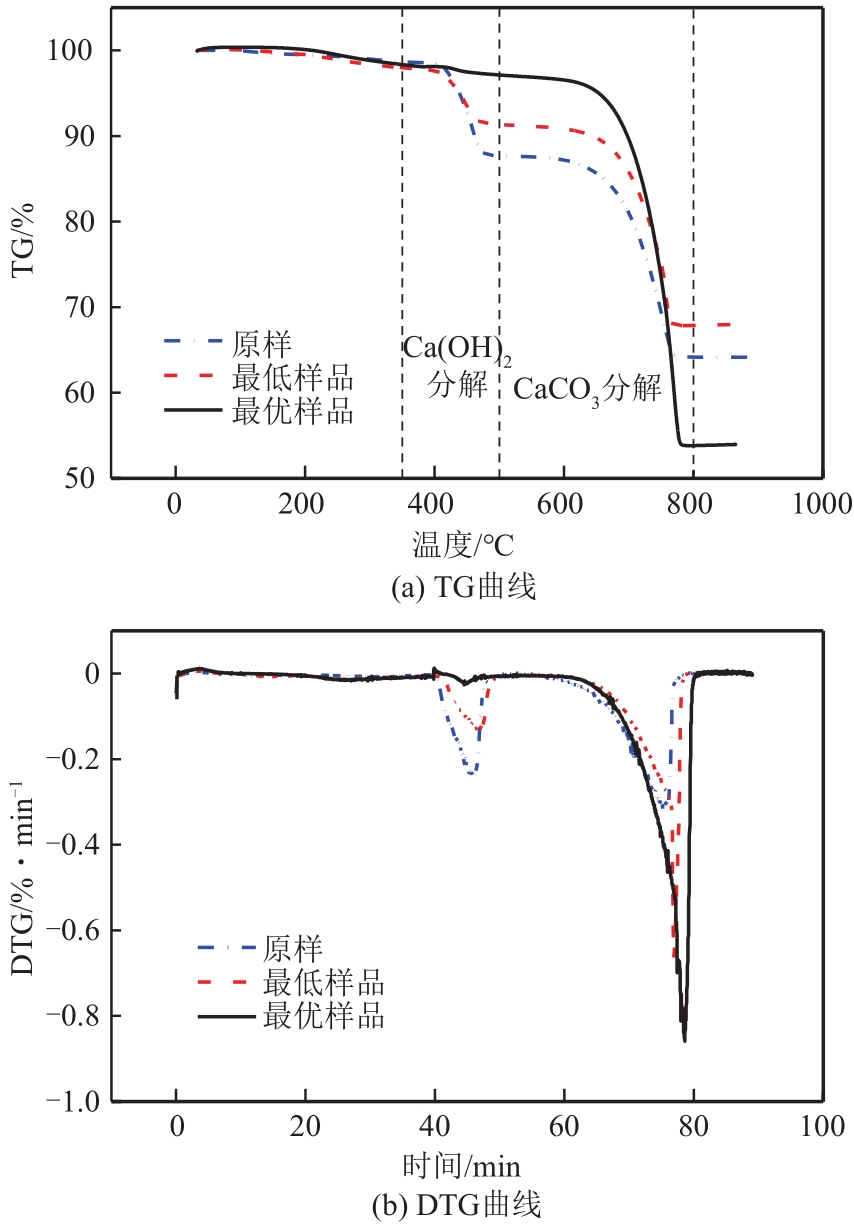
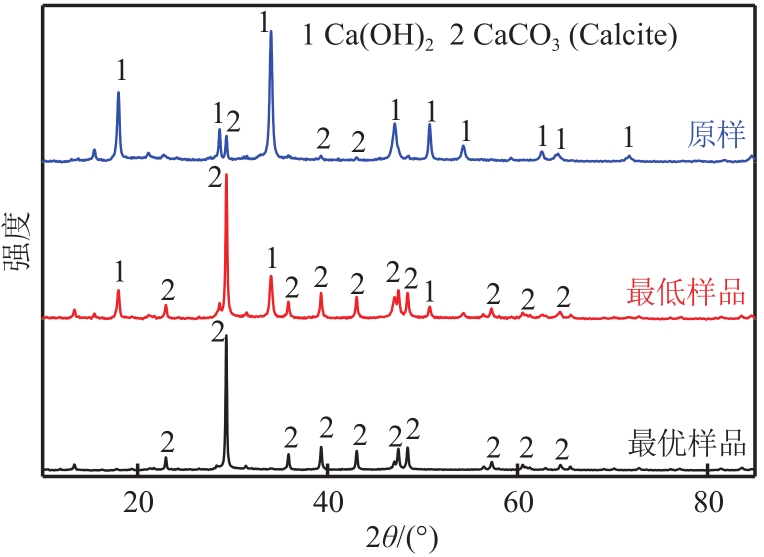
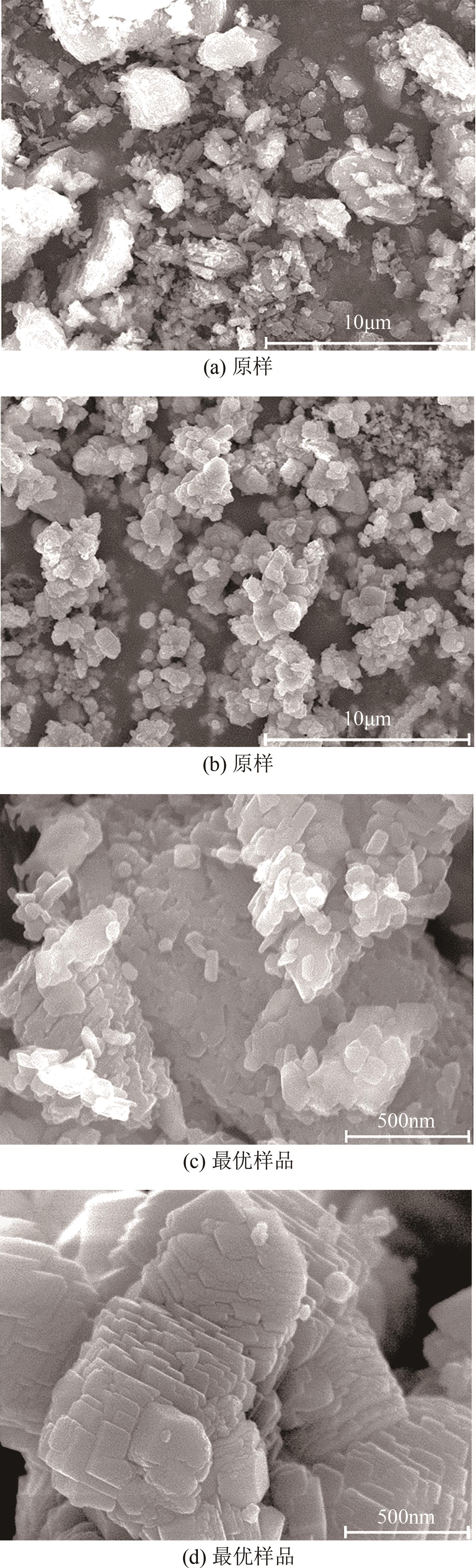
搭建了鼓泡床碳酸化反应器,研究常温常压下电石渣直接液相碳酸化矿化封存CO2的能力,揭示了重要操作参数表观气速、液固比和CO2浓度对电石渣矿化封存CO2能力和碳酸化效率的影响规律。同时构建响应面模型,分析各参数对电石渣碳酸化效率的影响强度,优化获得最大碳酸化效率及相应操作工况。结果表明,增加气速有利于钙离子溶解和CO2吸收,但反应器中过高气速易导致气相通道效应,不利于气液充分接触。当液固比降低,溶液中钙离子浓度提高,更有利于碳酸化反应,但液固比过低会影响固液间传质。适当增加CO2浓度有利于提高碳酸化效率,但CO2浓度增至到一定值后,对碳酸化效率影响降低。响应面建模分析发现,各因素对碳酸化效率影响顺序为:液固比>CO2浓度>表观气速。优化结果发现碳酸化效率最高为93.58%,工况为表观气速0.07m/s,液固比为8.26mL/g和CO2体积分数为20.91%。研究可知,鼓泡床中常温常压下电石渣直接液相加速碳酸化反应,具有较大的CO2固定量和高的碳酸化效率,实验结果为电石渣加速矿化封存CO2技术的发展提供了基础数据。
中图分类号:
引用本文
郑鹏, 李蔚玲, 郭亚飞, 孙健, 王瑞林, 赵传文. 鼓泡床中电石渣加速碳酸化分析与响应面优化[J]. 化工进展, 2022, 41(3): 1528-1538.
ZHENG Peng, LI Weiling, GUO Yafei, SUN Jian, WANG Ruilin, ZHAO Chuanwen. Analysis of carbide slag accelerated carbonation in bubble column and response surface optimization[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1528-1538.
| 水平 | 液固比A/mL?g-1 | 浓度B/% | 表观气速C/m?s-1 |
|---|---|---|---|
| -1 | 1 | 10 | 0.041 |
| 0 | 5 | 20 | 0.082 |
| 1 | 9 | 30 | 0.123 |
表1 Box-Behnken Design因素及水平
| 水平 | 液固比A/mL?g-1 | 浓度B/% | 表观气速C/m?s-1 |
|---|---|---|---|
| -1 | 1 | 10 | 0.041 |
| 0 | 5 | 20 | 0.082 |
| 1 | 9 | 30 | 0.123 |
| 实验 | 液固比A/mL?g-1 | 浓度B/% | 表观气速C/m?s-1 | 碳酸化效率Y/% |
|---|---|---|---|---|
| 2 | 9 | 10 | 0.082 | 92.17 |
| 1 | 1 | 10 | 0.082 | 52.01 |
| 6 | 9 | 20 | 0.041 | 93.17 |
| 12 | 5 | 30 | 0.123 | 88.53 |
| 14 | 5 | 20 | 0.082 | 87.00 |
| 17 | 5 | 20 | 0.082 | 90.01 |
| 5 | 1 | 20 | 0.041 | 51.04 |
| 3 | 1 | 30 | 0.082 | 61.04 |
| 11 | 5 | 10 | 0.123 | 71.49 |
| 4 | 9 | 30 | 0.082 | 90.36 |
| 7 | 1 | 20 | 0.123 | 66.89 |
| 8 | 9 | 20 | 0.123 | 89.89 |
| 9 | 5 | 10 | 0.041 | 78.26 |
| 16 | 5 | 20 | 0.082 | 92.3 |
| 10 | 5 | 30 | 0.041 | 85.67 |
| 13 | 5 | 20 | 0.082 | 91.88 |
| 15 | 5 | 20 | 0.082 | 90.71 |
表2 Box-Behnken Design实验设计及结果
| 实验 | 液固比A/mL?g-1 | 浓度B/% | 表观气速C/m?s-1 | 碳酸化效率Y/% |
|---|---|---|---|---|
| 2 | 9 | 10 | 0.082 | 92.17 |
| 1 | 1 | 10 | 0.082 | 52.01 |
| 6 | 9 | 20 | 0.041 | 93.17 |
| 12 | 5 | 30 | 0.123 | 88.53 |
| 14 | 5 | 20 | 0.082 | 87.00 |
| 17 | 5 | 20 | 0.082 | 90.01 |
| 5 | 1 | 20 | 0.041 | 51.04 |
| 3 | 1 | 30 | 0.082 | 61.04 |
| 11 | 5 | 10 | 0.123 | 71.49 |
| 4 | 9 | 30 | 0.082 | 90.36 |
| 7 | 1 | 20 | 0.123 | 66.89 |
| 8 | 9 | 20 | 0.123 | 89.89 |
| 9 | 5 | 10 | 0.041 | 78.26 |
| 16 | 5 | 20 | 0.082 | 92.3 |
| 10 | 5 | 30 | 0.041 | 85.67 |
| 13 | 5 | 20 | 0.082 | 91.88 |
| 15 | 5 | 20 | 0.082 | 90.71 |
| 方差来源 | 平方和 | 自由度 | 均方值 | F值 | P值 | 显著性 |
|---|---|---|---|---|---|---|
| Model | 3316.757 | 9 | 368.5286 | 28.33906 | 0.0001 | 显著 |
| 液固比A | 2264.982 | 1 | 2264.982 | 174.1723 | <0.0001 | |
| 浓度B | 125.3736 | 1 | 125.3736 | 9.640964 | 0.0172 | |
| 表观气速C | 9.37445 | 1 | 9.37445 | 0.720875 | 0.4239 | |
| AB | 29.3764 | 1 | 29.3764 | 2.258983 | 0.1765 | |
| AC | 91.48923 | 1 | 91.48923 | 7.035327 | 0.0328 | |
| BC | 23.18423 | 1 | 23.18423 | 1.782818 | 0.2236 | |
| A2 | 519.948 | 1 | 519.948 | 39.9829 | 0.0004 | |
| B2 | 121.5316 | 1 | 121.5316 | 9.345522 | 0.0184 | |
| C2 | 68.04379 | 1 | 68.04379 | 5.232423 | 0.0560 | |
| 残差 | 91.02983 | 7 | 13.00426 | |||
| 失拟误差 | 73.42323 | 3 | 24.47441 | 5.56028 | 0.0654 | 不显著 |
| 纯误差 | 17.6066 | 4 | 4.40165 | |||
| 总和 | 3407.787 | 16 |
表3 响应面方差分析
| 方差来源 | 平方和 | 自由度 | 均方值 | F值 | P值 | 显著性 |
|---|---|---|---|---|---|---|
| Model | 3316.757 | 9 | 368.5286 | 28.33906 | 0.0001 | 显著 |
| 液固比A | 2264.982 | 1 | 2264.982 | 174.1723 | <0.0001 | |
| 浓度B | 125.3736 | 1 | 125.3736 | 9.640964 | 0.0172 | |
| 表观气速C | 9.37445 | 1 | 9.37445 | 0.720875 | 0.4239 | |
| AB | 29.3764 | 1 | 29.3764 | 2.258983 | 0.1765 | |
| AC | 91.48923 | 1 | 91.48923 | 7.035327 | 0.0328 | |
| BC | 23.18423 | 1 | 23.18423 | 1.782818 | 0.2236 | |
| A2 | 519.948 | 1 | 519.948 | 39.9829 | 0.0004 | |
| B2 | 121.5316 | 1 | 121.5316 | 9.345522 | 0.0184 | |
| C2 | 68.04379 | 1 | 68.04379 | 5.232423 | 0.0560 | |
| 残差 | 91.02983 | 7 | 13.00426 | |||
| 失拟误差 | 73.42323 | 3 | 24.47441 | 5.56028 | 0.0654 | 不显著 |
| 纯误差 | 17.6066 | 4 | 4.40165 | |||
| 总和 | 3407.787 | 16 |
| 1 | PAN Shuyuan, CHIANG A, CHANG E E, et al. An innovative approach to integrated carbon mineralization and waste utilization: a review[J]. Aerosol and Air Quality Research, 2015, 15(3): 1072-1091. |
| 2 | 孙健. 高钙废弃物衍生吸收剂脱碳性能及其成型改性机制的研究[D]. 武汉: 华中科技大学, 2017. |
| SUN Jian. Investigation on CO2 capture properties and pelletization modification mechanism of CaO-based sorbent derived from calcium-rich wastes[D]. Wuhan: Huazhong University of Science and Technology, 2017. | |
| 3 | BOOT-HANDFORD M E, ABANADES J C, ANTHONY E J, et al. Carbon capture and storage update[J]. Energy & Environmental Science, 2014, 7(1): 130-189. |
| 4 | CORTÉS C G, TZIMAS E, PETEVES S. Technologies for coal based hydrogen and electricity co-production power plants with CO2 capture[R]. JRC Scientific and Technical Reports, EUR, 2009: 23661. |
| 5 | HO H J, IIZUKA A, SHIBATA E. Carbon capture and utilization technology without carbon dioxide purification and pressurization: a review on its necessity and available technologies[J]. Industrial & Engineering Chemistry Research, 2019, 58(21): 8941-8954. |
| 6 | 王中辉, 苏胜, 尹子骏, 等. CO2矿化及吸收-矿化一体化(IAM)方法研究进展[J]. 化工进展, 2021, 40(4): 2318-2327. |
| WANG Zhonghui, SU Sheng, YIN Zijun, et al. Research progress of CO2 mineralization and integrated absorption-mineralization (IAM) method[J]. Chemical Industry and Engineering Progress, 2021, 40(4): 2318-2327. | |
| 7 | SEIFRITZ W. CO2 disposal by means of silicates[J]. Nature, 1990, 345(6275): 486. |
| 8 | 王超, 杨保俊, 周金刚, 等. 由电石渣制备高分散纳米碳酸钙[J]. 化工进展, 2017, 36(S1): 346-352. |
| WANG Chao, YANG Baojun, ZHOU Jingang, et al. Preparation of highly dispersed nano calcium carbonate from calcium carbide residue[J]. Chemical Industry and Engineering Progress, 2017, 36(S1): 346-352. | |
| 9 | 孙荣岳, 叶江明, 毕小龙, 等. 丙酸改性提高电石渣捕集CO2性能的动力学分析[J]. 化工进展, 2017, 36(6): 2325-2330. |
| SUN Rongyue, YE Jiangming, BI Xiaolong, et al. Kinetic analysis on CO2 capture performance of carbide slag modified by propionic acid[J]. Chemical Industry and Engineering Progress, 2017, 36(6): 2325-2330. | |
| 10 | 马晓彤, 李英杰, 王文静, 等. 间歇氯化对电石渣循环捕集CO2性能的影响[J]. 化工学报, 2016, 67(12): 5268-5275. |
| MA Xiaotong, LI Yingjie, WANG Wenjing, et al. Effect of indirect chlorination on cyclic CO2 capture performance of carbide slag[J]. CIESC Journal, 2016, 67(12): 5268-5275. | |
| 11 | ZHANG Junqiang, WANG Zhishuai, LI Tong, et al. Preparation of CaO-containing carbon pellet from recycling of carbide slag: effects of temperature and H3PO4 [J]. Waste Management, 2019, 84: 64-73. |
| 12 | NIU Shengli, LIU Mengqi, LU Chunmei, et al. Thermogravimetric analysis of carbide slag[J]. Journal of Thermal Analysis and Calorimetry, 2014, 115(1): 73-79. |
| 13 | LI Yingjie, WANG Wenjing, CHENG Xingxing, et al. Simultaneous CO2/HCl removal using carbide slag in repetitive adsorption/desorption cycles[J]. Fuel, 2015, 142: 21-27. |
| 14 | WU Shuimu, LI Yingjie, ZHAO Jianli, et al. Simultaneous CO2/SO2 adsorption performance of carbide slag in adsorption/desorption cycles[J]. The Canadian Journal of Chemical Engineering, 2016, 94(1): 33-40. |
| 15 | YANG Jie, LIU Shengyu, MA Liping, et al. Mechanism analysis of carbide slag capture of CO2 via a gas-liquid-solid three-phase fluidization system[J]. Journal of Cleaner Production, 2021, 279: 123712. |
| 16 | YOU Quan, WU Shiyong, WU Youqing, et al. Product distributions and characterizations for integrated mild-liquefaction and carbonization of low rank coals[J]. Fuel Processing Technology, 2017, 156: 54-61. |
| 17 | AIL S S, DASAPPA S. Biomass to liquid transportation fuel via Fischer Tropsch synthesis-technology review and current scenario[J]. Renewable and Sustainable Energy Reviews, 2016, 58: 267-286. |
| 18 | WU Weize, HAN Buxing, GAO Haixiang, et al. Desulfurization of flue gas: SO2 absorption by an ionic liquid[J]. Angewandte Chemie International Edition, 2004, 43(18): 2415-2417. |
| 19 | MOTA A, VICENTE A A, TEIXEIRA J. Effect of spent grains on flow regime transition in bubble column[J]. Chemical Engineering Science, 2011, 66(14): 3350-3357. |
| 20 | SONG H S, RAMKRISHNA D, TRINH S, et al. Multiplicity and sensitivity analysis of Fischer-Tropsch bubble column slurry reactors: plug-flow gas and well-mixed slurry model[J]. Chemical Engineering Science, 2003, 58(12): 2759-2766. |
| 21 | BARGHI S, PRAKASH A, MARGARITIS A, et al. Flow regime identification in a slurry bubble column from gas holdup and pressure fluctuations analysis[J]. The Canadian Journal of Chemical Engineering, 2004, 82(5): 865-870. |
| 22 | JHAWAR A K, PRAKASH A. Heat transfer in a slurry bubble column reactor: a critical overview[J]. Industrial & Engineering Chemistry Research, 2012, 51(4): 1464-1473. |
| 23 | CHANG E E, PAN Shuyuan, CHEN Y H, et al. CO2 sequestration by carbonation of steelmaking slags in an autoclave reactor[J]. Journal of Hazardous Materials, 2011, 195: 107-114. |
| 24 | COSTA G, BACIOCCHI R, POLETTINI A, et al. Current status and perspectives of accelerated carbonation processes on municipal waste combustion residues[J]. Environmental Monitoring and Assessment, 2007, 135(1/2/3): 55-75. |
| 25 | LI Xiaomin, BERTOS M F, HILLS C D, et al. Accelerated carbonation of municipal solid waste incineration fly ashes[J]. Waste Management, 2007, 27(9): 1200-1206. |
| 26 | HUIJGEN W J J, WITKAMP G J, COMANS R N J. Mechanisms of aqueous wollastonite carbonation as a possible CO2 sequestration process[J]. Chemical Engineering Science, 2006, 61(13): 4242-4251. |
| 27 | TAN Wenyi, ZHANG Zixin, LI Hongyi, et al. Carbonation of gypsum from wet flue gas desulfurization process: experiments and modeling[J]. Environmental Science and Pollution Research International, 2017, 24(9): 8602-8608. |
| 28 | DING Wenjin, YANG Huaming, OUYANG Jing. Mineral carbonation of a desulfurization residue for CO2 sequestration[J]. RSC Advances, 2015, 5(82): 67184-67194. |
| 29 | LI Ruibing, ZHANG Ting’an, LIU Yan, et al. Characteristics of red mud slurry flow in carbonation reactor[J]. Powder Technology, 2017, 311: 66-76. |
| 30 | KANTARCI N, BORAK F, ULGEN K O. Bubble column reactors[J]. Process Biochemistry, 2005, 40(7): 2263-2283. |
| 31 | WANG Tiefeng, WANG Jinfu, JIN Yong. Slurry reactors for gas-to-liquid processes: a review[J]. Industrial & Engineering Chemistry Research, 2007, 46(18): 5824-5847. |
| 32 | LIU Weizao, TENG Liumei, ROHANI S, et al. CO2 mineral carbonation using industrial solid wastes: a review of recent developments[J]. Chemical Engineering Journal, 2021, 416: 129093. |
| 33 | HUIJGEN W J J, COMANS R N J. Carbon dioxide sequestration by mineral carbonation: literature review[D]. Petten: Energy Research Centre of the Netherlands, 2003. |
| 34 | TAMILSELVI DANANJAYAN R R, KANDASAMY P, ANDIMUTHU R. Direct mineral carbonation of coal fly ash for CO2 sequestration[J]. Journal of Cleaner Production, 2016, 112: 4173-4182. |
| 35 | TEBBICHE I, PASQUIER L C, MERCIER G, et al. Thermally activated serpentine leaching under flue gas conditions in a bubble column reactor operated at ambient pressure and temperature[J]. Hydrometallurgy, 2020, 195: 105391. |
| 36 | CHANG E E, CHIU A C, PAN Shuyuan, et al. Carbonation of basic oxygen furnace slag with metalworking wastewater in a slurry reactor[J]. International Journal of Greenhouse Gas Control, 2013, 12: 382-389. |
| 37 | FERNÁNDEZ BERTOS M, LI X, SIMONS S J R, et al. Investigation of accelerated carbonation for the stabilisation of MSW incinerator ashes and the sequestration of CO2 [J]. Green Chem, 2004, 6(8): 428-436. |
| 38 | YADAV V S, PRASAD M, KHAN J, et al. Sequestration of carbon dioxide (CO2) using red mud[J]. Journal of Hazardous Materials, 2010, 176(1/2/3): 1044-1050. |
| 39 | HO H J, IIZUKA A, SHIBATA E, et al. CO2 utilization via direct aqueous carbonation of synthesized concrete fines under atmospheric pressure[J]. ACS Omega, 2020, 5(26): 15877-15890. |
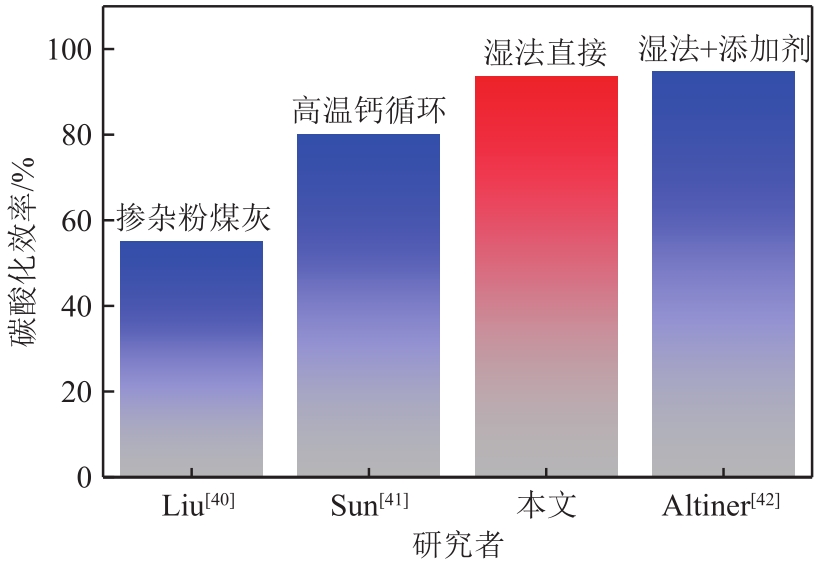
| 40 | LIU Rong, WANG Xiaolong, GAO Shiwang. CO2 capture and mineralization using carbide slag doped fly ash[J]. Greenhouse Gases: Science and Technology, 2020, 10(1): 103-115. |
| 41 | SUN Jian, LIU Wenqiang, HU Yingchao, et al. Enhanced performance of extruded-spheronized carbide slag pellets for high temperature CO2 capture[J]. Chemical Engineering Journal, 2016, 285: 293-303. |
| 42 | ALTINER M. Use of Taguchi approach for synthesis of calcite particles from calcium carbide slag for CO2 fixation by accelerated mineral carbonation[J]. Arabian Journal of Chemistry, 2019, 12(4): 531-540. |
| 43 | KITAMURA M, KONNO H, YASUI A, et al. Controlling factors and mechanism of reactive crystallization of calcium carbonate polymorphs from calcium hydroxide suspensions[J]. Journal of Crystal Growth, 2002, 236(1/2/3): 323-332. |
| [1] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [2] | 张杰, 王放放, 夏忠林, 赵光金, 马双忱. “双碳”目标下SF6排放现状、减排手段分析及未来展望[J]. 化工进展, 2023, 42(S1): 447-460. |
| [3] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [4] | 李佳, 樊星, 陈莉, 李坚. 硝酸生产尾气中NO x 和N2O联合脱除技术研究进展[J]. 化工进展, 2023, 42(7): 3770-3779. |
| [5] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| [6] | 顾诗亚, 董亚超, 刘琳琳, 张磊, 庄钰, 都健. 考虑中间节点的碳捕集管路系统设计与优化[J]. 化工进展, 2023, 42(6): 2799-2808. |
| [7] | 符乐, 杨阳, 徐文青, 耿錾卜, 朱廷钰, 郝润龙. 新型相变有机胺吸收捕集CO2技术研究进展[J]. 化工进展, 2023, 42(4): 2068-2080. |
| [8] | 尚玉, 肖满, 崔秋芳, 涂特, 晏水平. CO2捕集工艺中热再生气余热的PVDF/BN-OH平板复合膜回收特性[J]. 化工进展, 2023, 42(3): 1618-1628. |
| [9] | 刘丹, 范云洁, 王慧敏, 严政, 李鹏飞, 李家成, 曹雪波. 基于废弃PET的高值化功能性多孔碳材料及其应用进展[J]. 化工进展, 2023, 42(2): 969-984. |
| [10] | 沈天绪, 沈来宏. 基于3kW塔式串行流化床差异燃料的化学链燃烧解析[J]. 化工进展, 2023, 42(1): 138-147. |
| [11] | 王璐, 张磊, 都健. 机器学习高效筛选用于CO2/N2选择性吸附分离的沸石材料[J]. 化工进展, 2023, 42(1): 148-158. |
| [12] | 王一茹, 宋小三, 水博阳, 王三反. 胺功能化介孔二氧化硅捕集CO2的研究进展[J]. 化工进展, 2022, 41(S1): 536-544. |
| [13] | 慕彦君, 宋倩倩, 王红秋, 付凯妹, 雪晶, 王春娇. 美国炼化行业低碳发展策略与启示[J]. 化工进展, 2022, 41(6): 2797-2805. |
| [14] | 周红军, 周颖, 徐春明. 中国碳达峰碳中和目标下炼化一体化新路径与实践[J]. 化工进展, 2022, 41(4): 2226-2230. |
| [15] | 张卫风, 周武, 王秋华. 相变吸收捕集烟气中CO2技术的发展现状[J]. 化工进展, 2022, 41(4): 2090-2101. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||