化工进展 ›› 2025, Vol. 44 ›› Issue (6): 3486-3496.DOI: 10.16085/j.issn.1000-6613.2025-0008
• 工业催化 • 上一篇
甲基环己烷脱氢催化体系的研究进展
- 中石化石油化工科学研究院有限公司,北京 100083
-
收稿日期:2025-01-02修回日期:2025-02-21出版日期:2025-06-25发布日期:2025-07-08 -
通讯作者:刘诗哲 -
作者简介:刘诗哲(1989—),男,博士,高级工程师,研究方向为炼油与石化技术。E-mail:liushizhe.ripp@sinopec.com。
Advances in catalytic system for methylcyclohexane dehydrogenation
- SINOPEC Research Institute of Petroleum Processing Co. , Ltd. , Beijing 100083, China
-
Received:2025-01-02Revised:2025-02-21Online:2025-06-25Published:2025-07-08 -
Contact:LIU Shizhe
摘要:
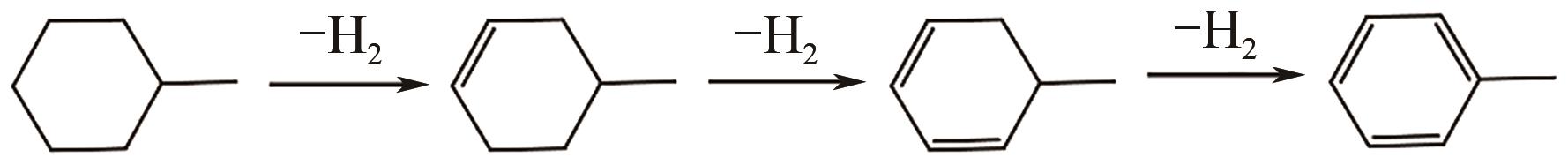
有机液体储氢技术是氢能储存和运输的一种很有潜力的解决方案。甲基环己烷具有储氢密度高、储存和运输状态下化学性质稳定等特点,可作为优良的储氢载体。通过甲基环己烷-甲苯-氢(MTH)体系的可逆加氢-脱氢反应,可以有效实现氢的储存和释放。目前,甲苯加氢技术已较为成熟,但甲基环己烷脱氢技术中催化剂的活性和稳定性仍不能满足工业应用的需要。本文分析了国内外甲基环己烷脱氢催化体系的研究现状,介绍了贵金属催化剂和非贵金属催化剂在活性组分与载体的选择、制备方法和催化脱氢性能等方面的研究进展,并对脱氢催化剂未来的发展方向进行了展望。开发具有良好的催化活性、产物选择性和稳定性的脱氢催化剂是MTH体系应用于储氢技术的关键。
中图分类号:
引用本文
刘诗哲. 甲基环己烷脱氢催化体系的研究进展[J]. 化工进展, 2025, 44(6): 3486-3496.
LIU Shizhe. Advances in catalytic system for methylcyclohexane dehydrogenation[J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3486-3496.
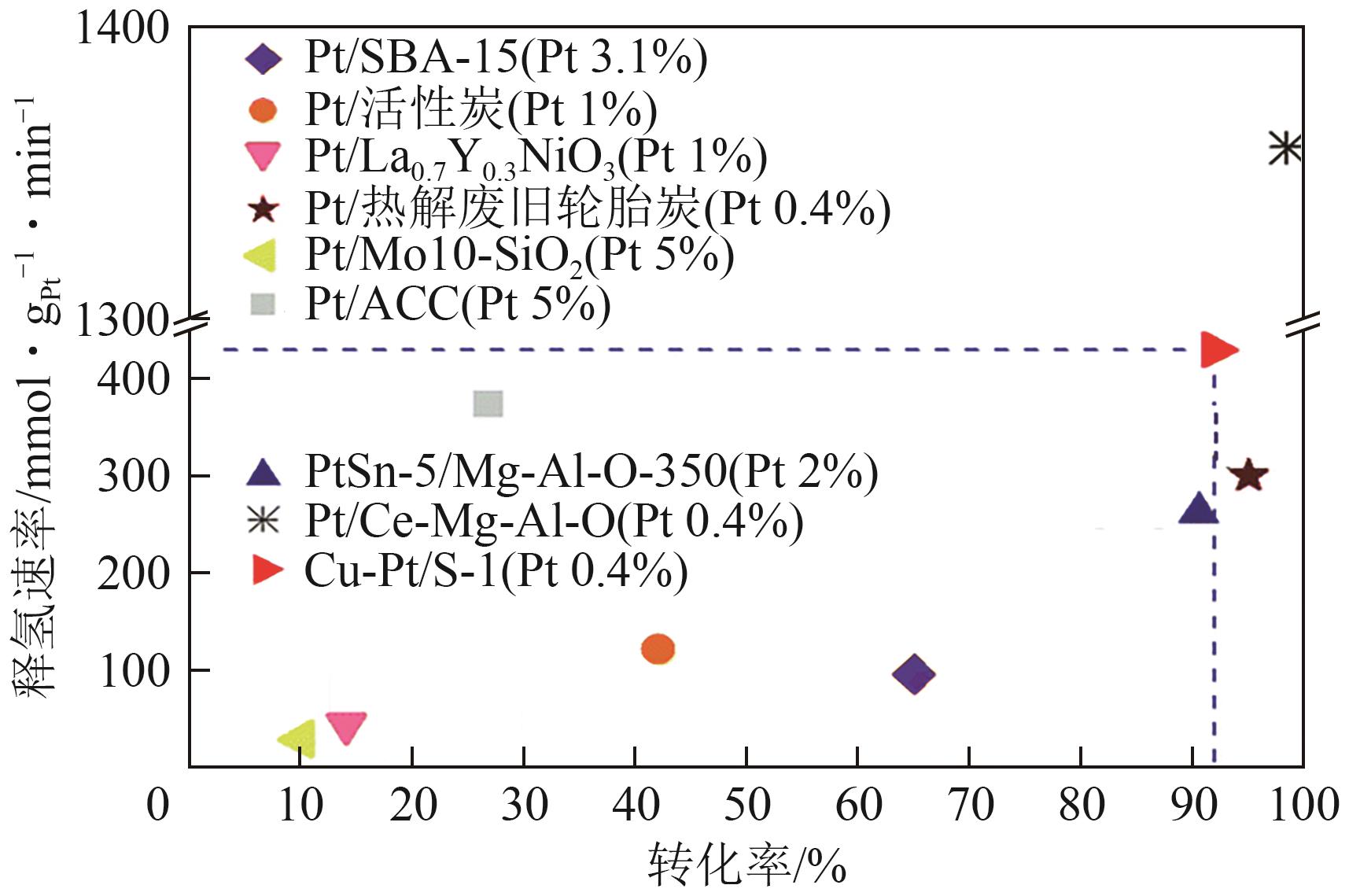
| 催化剂种类 | 反应器 | 反应条件 | 释氢速率① | 转化率/% | 选择性/% |
|---|---|---|---|---|---|
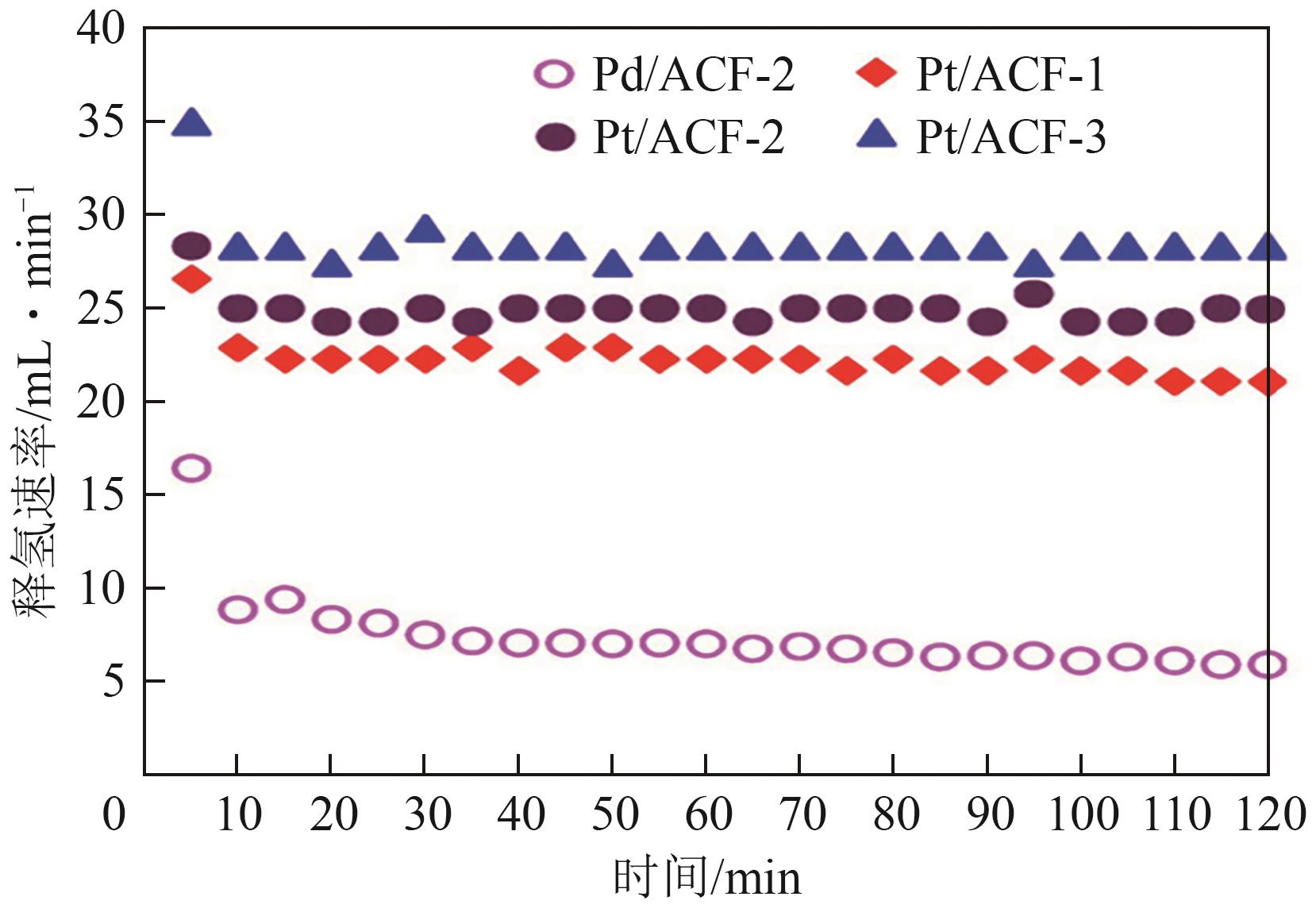
| 0.7%Pt/ACF[ | 固定床 | T=300℃, 0.004mmolmet, MCH 4mL/min+N2 5mL/min | 1659.14mmol/(gPt·min)-1 (29mL/min) | 76 | — |
| 0.88%Pd/ACF[ | 776.07mmol/(gPd·min)-1 (7.4mL/min) | 20 | — | ||
| 0.5%Pt/10%TiO2-Al2O3[ | 固定床 | T=400℃, 2g催化剂, MCH 0.2mL/min+N230mL/min+H2 30mL/min | — | 93 | 99 |
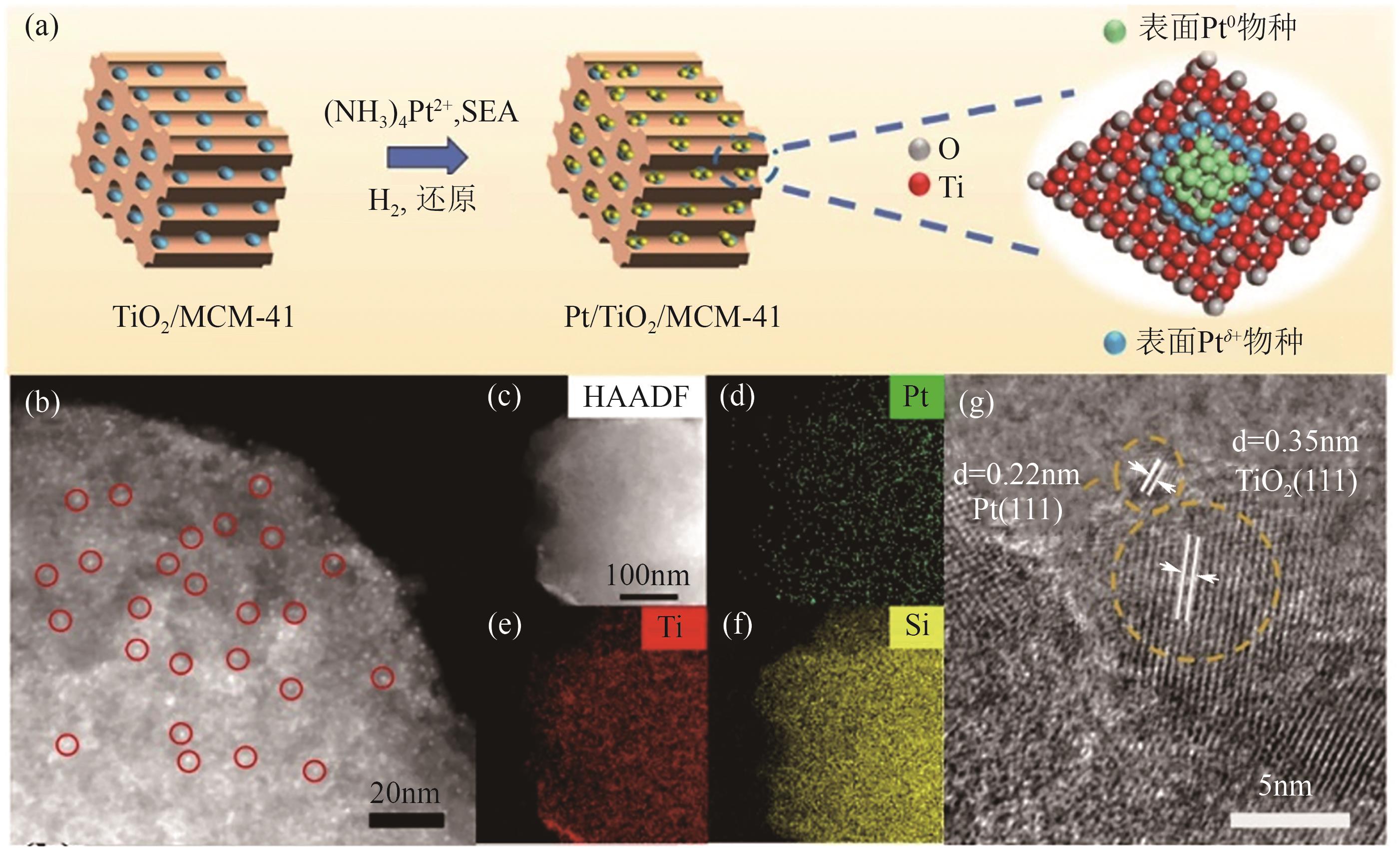
| 1.0%Pt/0.5TiO2/M41[ | 固定床 | T=310℃, 1g催化剂, MCH 0.1mL/min | — | 88 | 99.5 |
| 1%Pt/La0.7Y0.3NiO3[ | 脉冲反应器 | T=350℃, 0.3g催化剂, MCH脉冲进料 | 45.76mmol/(gPt·min)-1 | — | 约100 |
| 1%Pt/La2O3[ | 24.08mmol/(gPt·min)-1 | — | — | ||
| 1%Pt/AC3[ | 固定床 | T=300℃, 50mg催化剂, MCH 0.03mL/min + Ar 5.3mL/min | — | 88 | >99 |
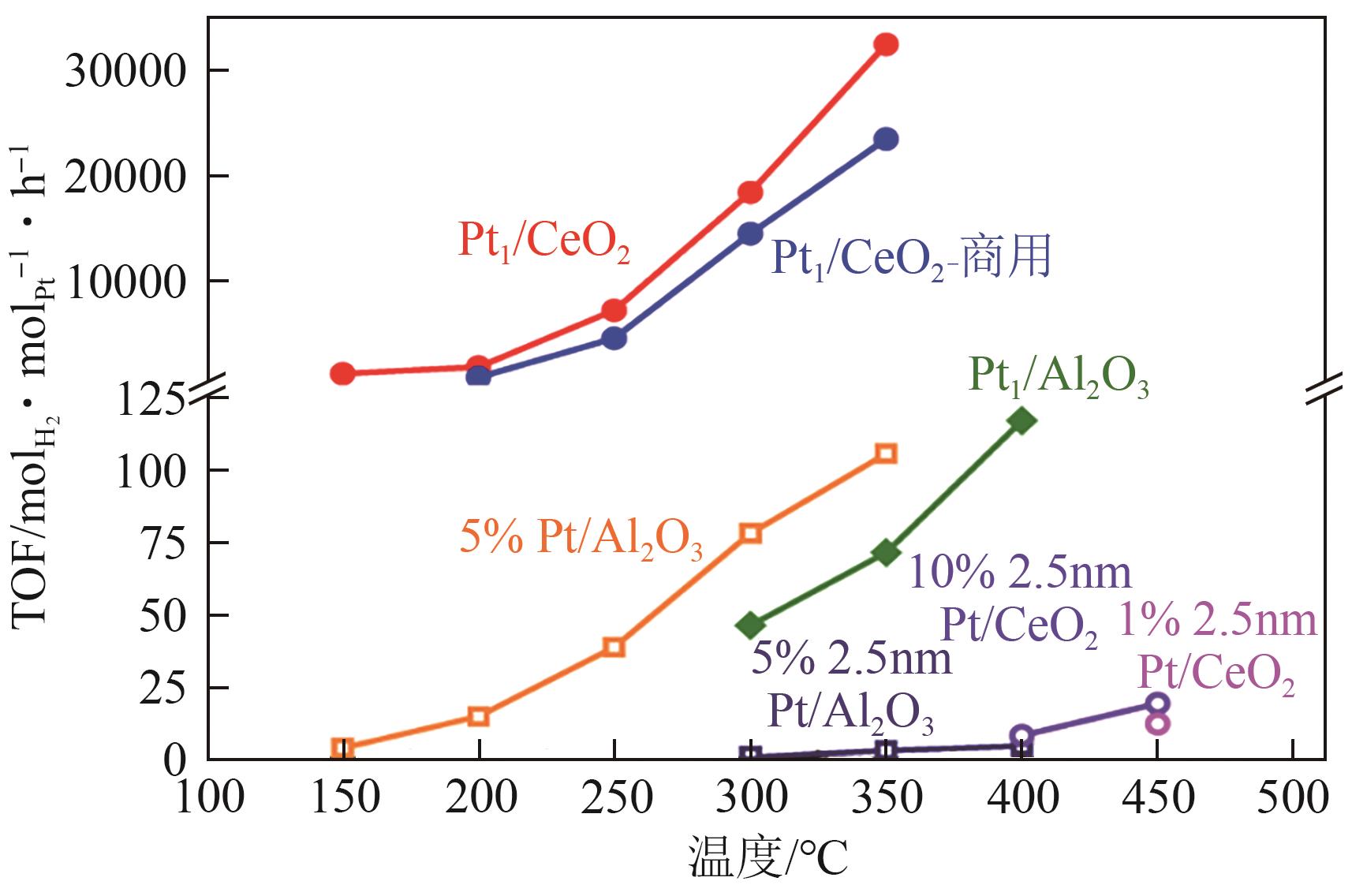
| Pt1/CeO2[ | 固定床 | T=350℃, 0.1 g催化剂, CYH 0.1mL/min+ N2 30mL/min | 2774.7mmol/(gPt·min)-1 [32477mol/(molPt·h)-1] | 30 | 100 |
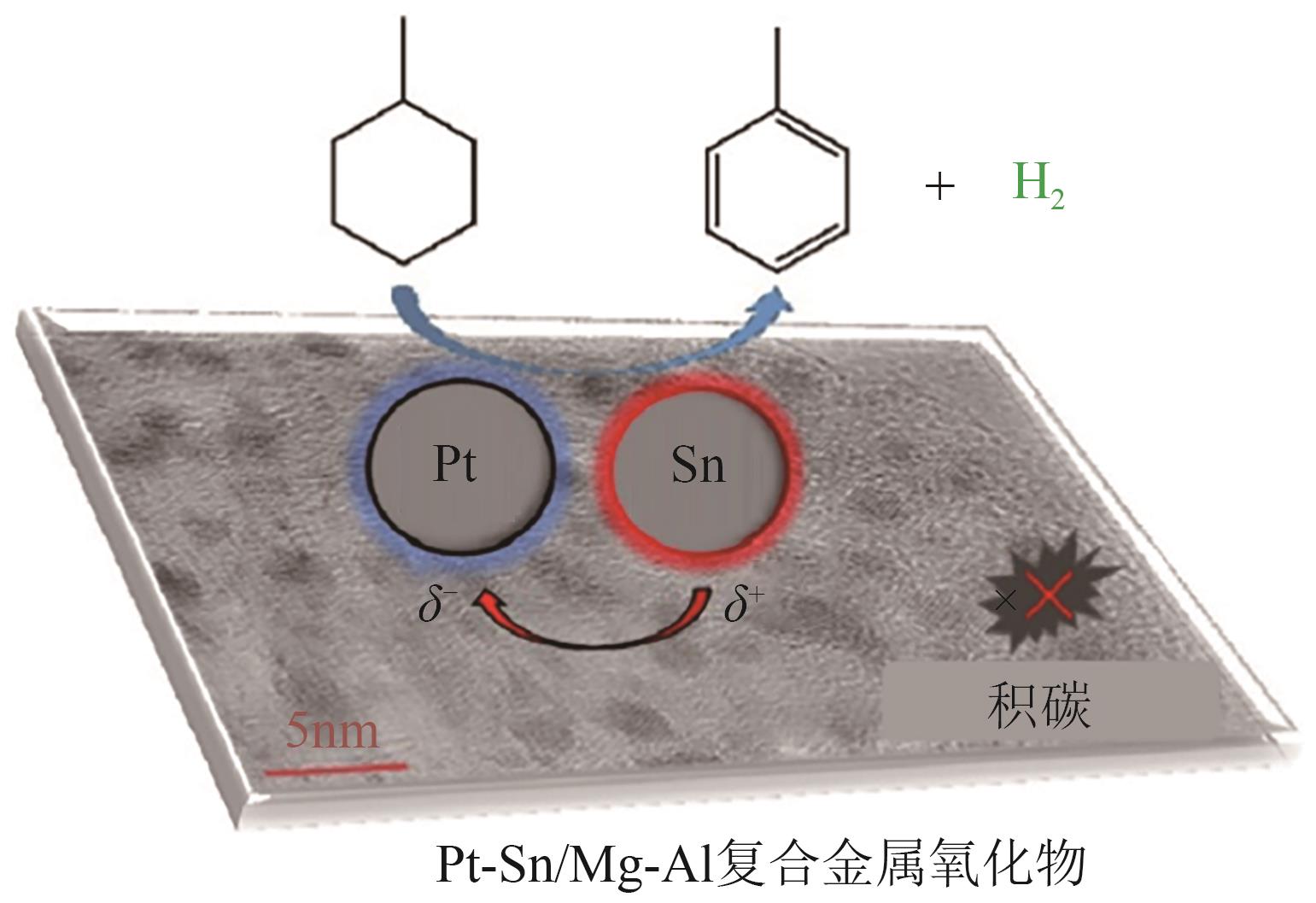
| PtSn/Mg-Al[ | 固定床 | T=350℃, 0.5g催化剂, MCH 0.1mL/min | 262.1mmol/(gPt·min)-1 | 90.5 | — |
| Pt/S-1[ | 固定床 | T=350℃, MCH 4.6h-1 WHSV, H2 15mL/min | 82.43mmol/(gPt·min)-1 | 17.66 | — |
| Pt-Cu/S-1[ | T=350℃, MCH 4.6h-1 WHSV, H2 15mL/min | 288.9mmol/(gPt·min)-1 | 59.35 | — | |
| T=400℃, MCH 4.6h-1 WHSV, H2 15mL/min | 445.3mmol/(gPt·min)-1 | 92.26 | — |
表1 贵金属催化剂甲基环己烷/环己烷脱氢性能汇总
| 催化剂种类 | 反应器 | 反应条件 | 释氢速率① | 转化率/% | 选择性/% |
|---|---|---|---|---|---|
| 0.7%Pt/ACF[ | 固定床 | T=300℃, 0.004mmolmet, MCH 4mL/min+N2 5mL/min | 1659.14mmol/(gPt·min)-1 (29mL/min) | 76 | — |
| 0.88%Pd/ACF[ | 776.07mmol/(gPd·min)-1 (7.4mL/min) | 20 | — | ||
| 0.5%Pt/10%TiO2-Al2O3[ | 固定床 | T=400℃, 2g催化剂, MCH 0.2mL/min+N230mL/min+H2 30mL/min | — | 93 | 99 |
| 1.0%Pt/0.5TiO2/M41[ | 固定床 | T=310℃, 1g催化剂, MCH 0.1mL/min | — | 88 | 99.5 |
| 1%Pt/La0.7Y0.3NiO3[ | 脉冲反应器 | T=350℃, 0.3g催化剂, MCH脉冲进料 | 45.76mmol/(gPt·min)-1 | — | 约100 |
| 1%Pt/La2O3[ | 24.08mmol/(gPt·min)-1 | — | — | ||
| 1%Pt/AC3[ | 固定床 | T=300℃, 50mg催化剂, MCH 0.03mL/min + Ar 5.3mL/min | — | 88 | >99 |
| Pt1/CeO2[ | 固定床 | T=350℃, 0.1 g催化剂, CYH 0.1mL/min+ N2 30mL/min | 2774.7mmol/(gPt·min)-1 [32477mol/(molPt·h)-1] | 30 | 100 |
| PtSn/Mg-Al[ | 固定床 | T=350℃, 0.5g催化剂, MCH 0.1mL/min | 262.1mmol/(gPt·min)-1 | 90.5 | — |
| Pt/S-1[ | 固定床 | T=350℃, MCH 4.6h-1 WHSV, H2 15mL/min | 82.43mmol/(gPt·min)-1 | 17.66 | — |
| Pt-Cu/S-1[ | T=350℃, MCH 4.6h-1 WHSV, H2 15mL/min | 288.9mmol/(gPt·min)-1 | 59.35 | — | |
| T=400℃, MCH 4.6h-1 WHSV, H2 15mL/min | 445.3mmol/(gPt·min)-1 | 92.26 | — |
| 催化剂种类 | 反应器 | 反应条件 | 释氢速率 | 转化率/% | 选择性/% |
|---|---|---|---|---|---|
| Ni/Al2O3[ | 固定床 | T=350℃, 20mg催化剂, GHSV=210084mL/(g·h)-1, MCH∶H2∶Ar=1.4∶42.9∶56.7 | — | 36.2 | 66.9 |
| Ag/Al2O3[ | — | 0.3 | 93.8 | ||
| Sn/Al2O3[ | — | 0.9 | 46.6 | ||
| Zn/Al2O3[ | — | 0.1 | 95.9 | ||
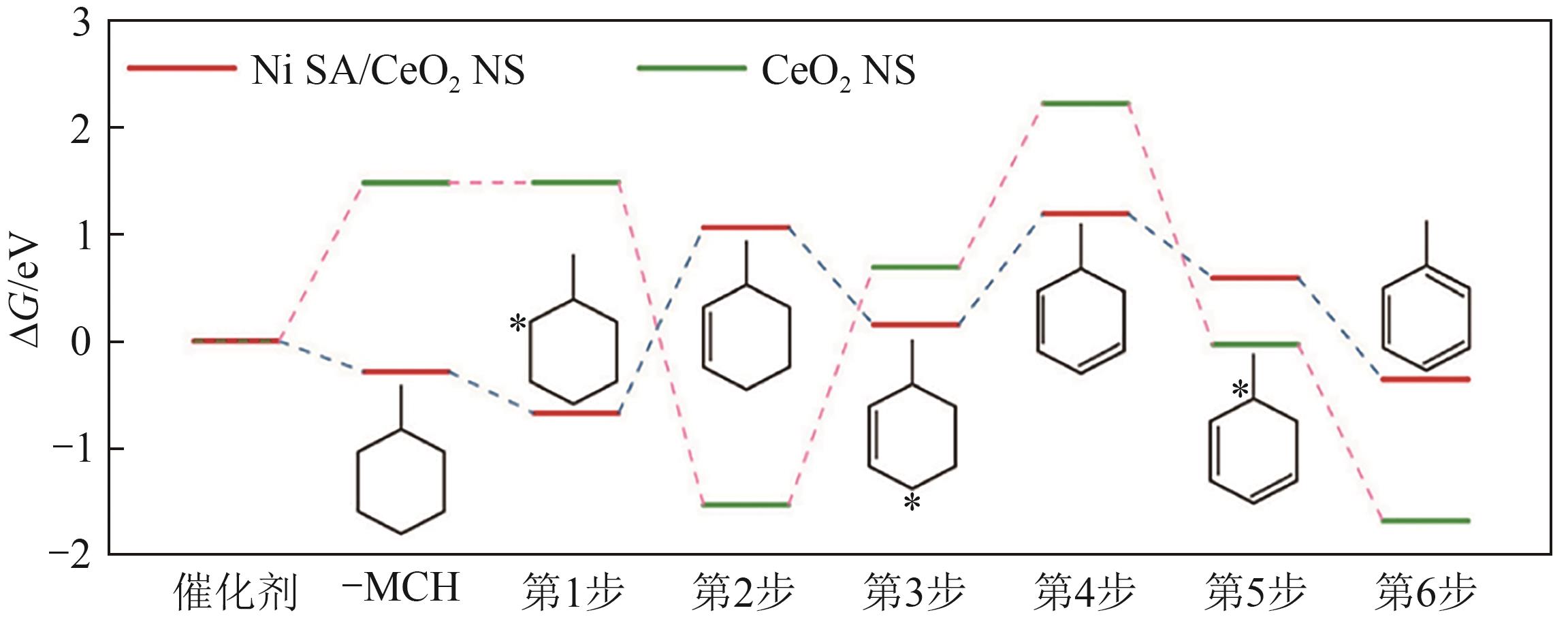
| Ni SA/CeO2 NS[ | 固定床 | T=400℃, MCH, 10mg催化剂 | 2755.8 mmol/(g·h)-1 (45.93mmol/(g·min)-1) | — | >95 |
| Ni80Cu20-SiO2[ | 固定床 | T=275℃, 0.5g催化剂, MCH 12mL/h+H2 200mL/min+N2 200mL/min | — | 75~80 | 89 |
| 8%Ni-2%Cu/ACC[ | 脉冲反应器 | T=350℃, MCH脉冲进料 | 227.23mmol/(g·h)-1 (39.45mmol/(gmet·min)-1) | 25.78 | — |
| Ni-Cu/γ-Al2O3-ZrO2[ | 固定床 | T=450℃, MCH LSHV=10h-1 | 4.92mmol/(g·h)-1 | 82.6 | 98.2 |
| Ni0.85Cu0.15/SiO2[ | 固定床 | T=350℃, GHSV=12000mL·g-1·h-1, CYH∶H2=1∶25 | — | 94.9 | 99.5 |
| Ni-Zn/Al2O3[ | 固定床 | T=350℃, 20mg催化剂, GHSV=210084mL/(g·h)-1, MCH∶H2∶Ar=1.4∶42.9∶56.7 | — | 32.2 | 96.6 |
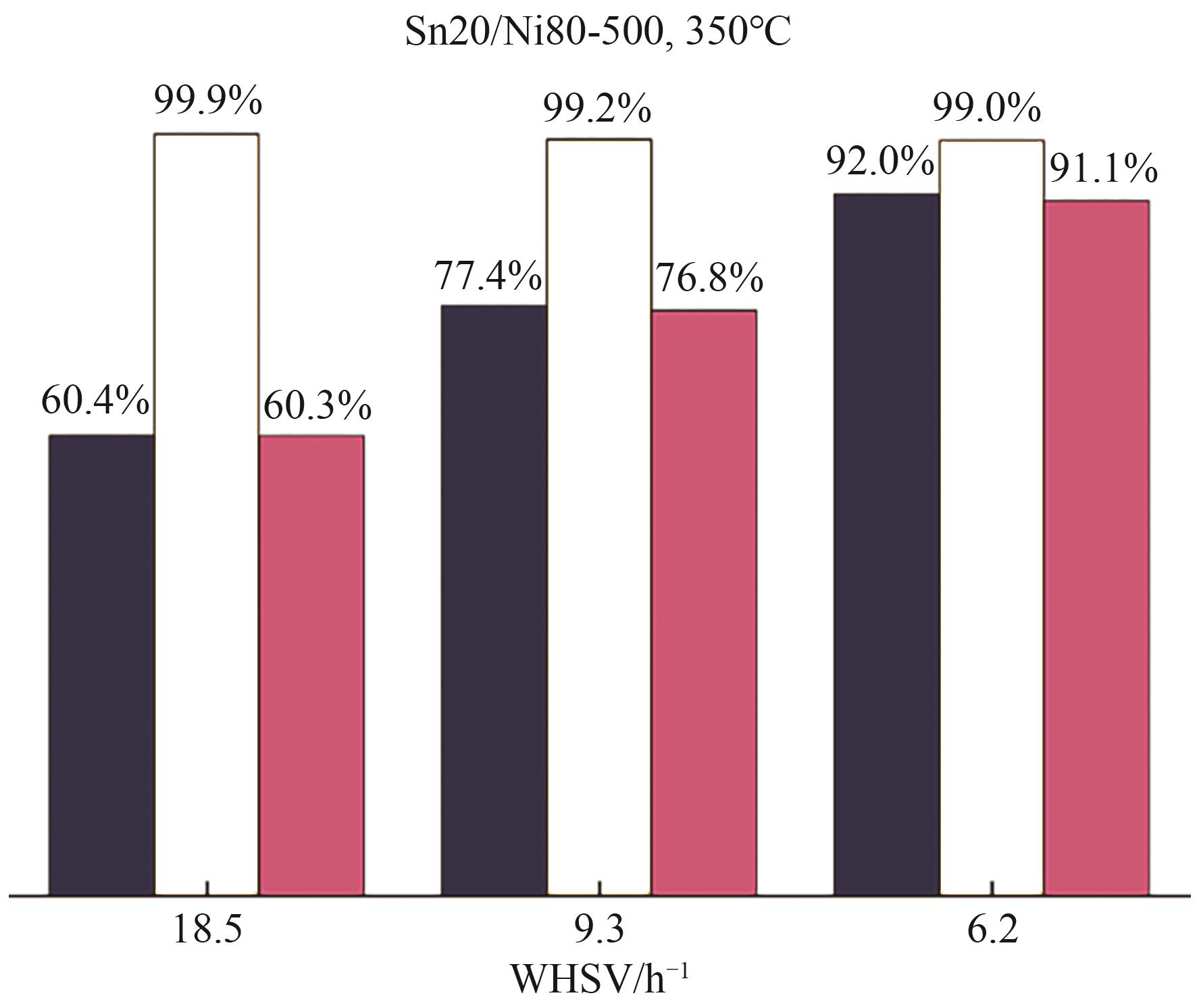
| NiSn/SiO2[ | 固定床 | T=350℃, 0.5g催化剂, MCH, WHSV=6.2h-1 | — | 92 | 99 |
| Ni3Sn/SiO2[ | 固定床 | T=400℃, 0.05g催化剂, CYH 3.7kPa | — | 56 | 99 |
| 2Cu/SBA-15[ | 固定床 | T=350℃, 0.15g催化剂, GHSV=12000mL/(g·h)-1, CYH∶H2=1∶25 | 3223.88 mmol/(g·h)-1 (5578mol/(molCu·h)-1) | 7.5 | 100 |
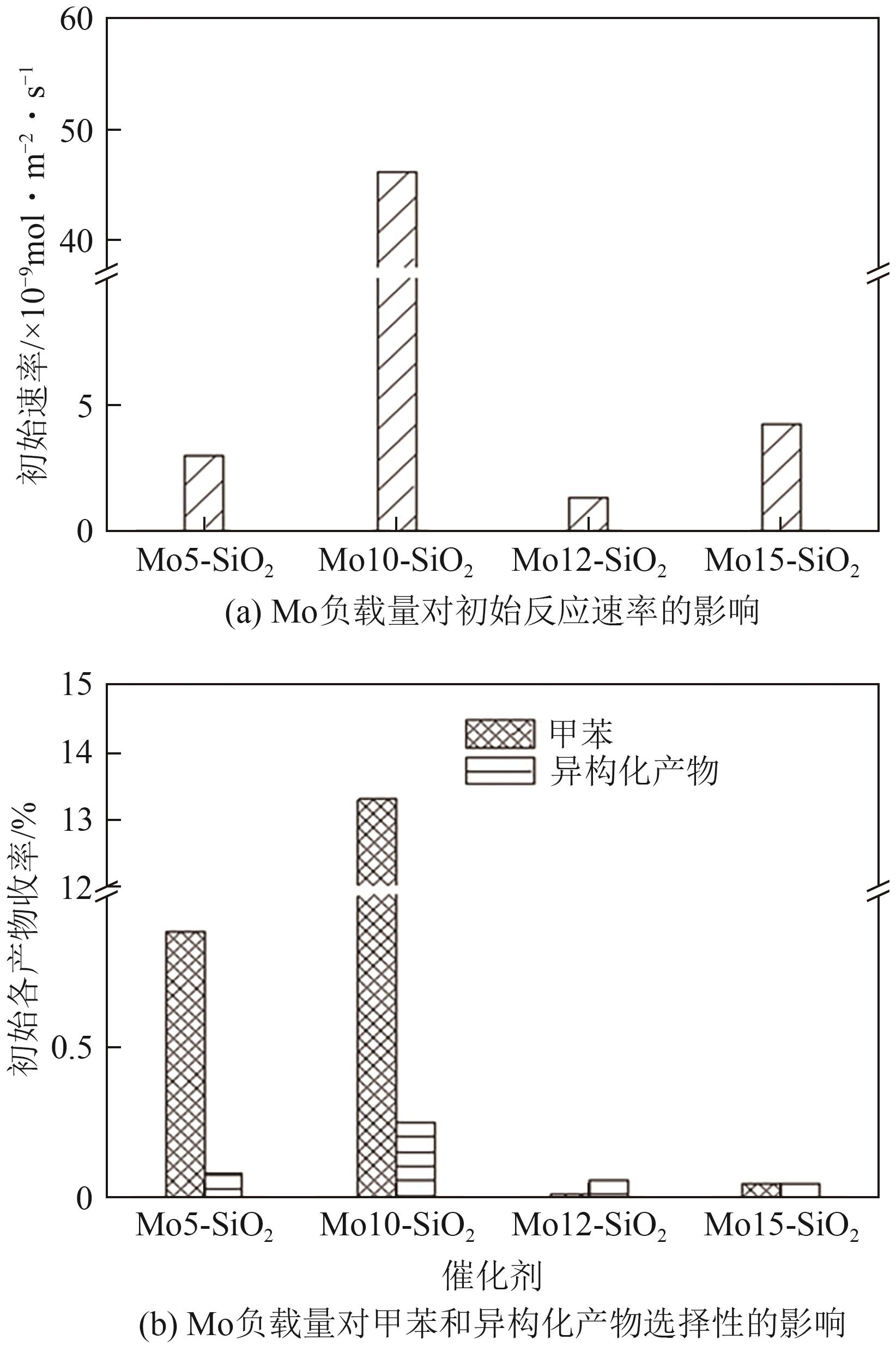
| Mo10-SiO2[ | 固定床 | T=400℃, 0.1g催化剂, WHSV=92.4h-1, MCH∶H2=1∶250 | — | — | 90 |
表2 非贵金属催化剂甲基环己烷/环己烷脱氢性能汇总
| 催化剂种类 | 反应器 | 反应条件 | 释氢速率 | 转化率/% | 选择性/% |
|---|---|---|---|---|---|
| Ni/Al2O3[ | 固定床 | T=350℃, 20mg催化剂, GHSV=210084mL/(g·h)-1, MCH∶H2∶Ar=1.4∶42.9∶56.7 | — | 36.2 | 66.9 |
| Ag/Al2O3[ | — | 0.3 | 93.8 | ||
| Sn/Al2O3[ | — | 0.9 | 46.6 | ||
| Zn/Al2O3[ | — | 0.1 | 95.9 | ||
| Ni SA/CeO2 NS[ | 固定床 | T=400℃, MCH, 10mg催化剂 | 2755.8 mmol/(g·h)-1 (45.93mmol/(g·min)-1) | — | >95 |
| Ni80Cu20-SiO2[ | 固定床 | T=275℃, 0.5g催化剂, MCH 12mL/h+H2 200mL/min+N2 200mL/min | — | 75~80 | 89 |
| 8%Ni-2%Cu/ACC[ | 脉冲反应器 | T=350℃, MCH脉冲进料 | 227.23mmol/(g·h)-1 (39.45mmol/(gmet·min)-1) | 25.78 | — |
| Ni-Cu/γ-Al2O3-ZrO2[ | 固定床 | T=450℃, MCH LSHV=10h-1 | 4.92mmol/(g·h)-1 | 82.6 | 98.2 |
| Ni0.85Cu0.15/SiO2[ | 固定床 | T=350℃, GHSV=12000mL·g-1·h-1, CYH∶H2=1∶25 | — | 94.9 | 99.5 |
| Ni-Zn/Al2O3[ | 固定床 | T=350℃, 20mg催化剂, GHSV=210084mL/(g·h)-1, MCH∶H2∶Ar=1.4∶42.9∶56.7 | — | 32.2 | 96.6 |
| NiSn/SiO2[ | 固定床 | T=350℃, 0.5g催化剂, MCH, WHSV=6.2h-1 | — | 92 | 99 |
| Ni3Sn/SiO2[ | 固定床 | T=400℃, 0.05g催化剂, CYH 3.7kPa | — | 56 | 99 |
| 2Cu/SBA-15[ | 固定床 | T=350℃, 0.15g催化剂, GHSV=12000mL/(g·h)-1, CYH∶H2=1∶25 | 3223.88 mmol/(g·h)-1 (5578mol/(molCu·h)-1) | 7.5 | 100 |
| Mo10-SiO2[ | 固定床 | T=400℃, 0.1g催化剂, WHSV=92.4h-1, MCH∶H2=1∶250 | — | — | 90 |
| [1] | 曹湘洪. 炼油行业碳达峰碳中和的技术路径[J]. 炼油技术与工程, 2022, 52(1): 1-10. |
| CAO Xianghong. Technical route of carbon dioxide emissions peak and carbon neutrality in oil refining industry[J]. Petroleum Refinery Engineering, 2022, 52(1): 1-10. | |
| [2] | BOURANE Abdennour, ELANANY Mohamed, PHAM Thang V, et al. An overview of organic liquid phase hydrogen carriers[J]. International Journal of Hydrogen Energy, 2016, 41(48): 23075-23091. |
| [3] | KHALIL Munawar, KADJA Grandprix T M, ILMI Moh Mualliful. Advanced nanomaterials for catalysis: Current progress in fine chemical synthesis, hydrocarbon processing, and renewable energy[J]. Journal of Industrial and Engineering Chemistry, 2021, 93: 78-100. |
| [4] | TAKEICHI Nobuhiko, SENOH Hiroshi, YOKOTA Tomoyuki, et al. “Hybrid hydrogen storage vessel”, a novel high-pressure hydrogen storage vessel combined with hydrogen storage material[J]. International Journal of Hydrogen Energy, 2003, 28(10): 1121-1129. |
| [5] | 中国石化集团经济技术研究院有限公司. 中国氢能产业展望报告[M]. 北京: 中国石化出版社, 2023: 117-118. |
| Sinopec Economics & Development Research Institute Company Limited. China hydrogen energy industry outlook report[M]. Beijing: Sinopec Press, 2023: 117-118. | |
| [6] | ALGHAMDI Huda S, Ahsan ALI, AJEEBI Afnan M, et al. Catalysts for liquid organic hydrogen carriers (LOHCs): Efficient storage and transport for renewable energy[J]. The Chemical Record, 2024, 24(11): e202400082. |
| [7] | GEMECHU Desalegn Nigatu, MOHAMMED Ahmed Mustefa, REDI Mesfin, et al. First principles-based approaches for catalytic activity on the dehydrogenation of liquid organic hydrogen carriers: A review[J]. International Journal of Hydrogen Energy, 2023, 48(85): 33186-33206. |
| [8] | TAN Ruike, JI Qing, LING Yanni, et al. Advances in liquid organic hydrogen carriers: Developing efficient dehydrogenation strategies[J]. Chemical Communications, 2024, 60(63): 8186-8203. |
| [9] | GAO Jiaojiao, LI Ning, ZHANG Dongqiang, et al. The progress of research based on methylcyclohexane dehydrogenation technology: A review[J]. International Journal of Hydrogen Energy, 2024, 85: 865-880. |
| [10] | 每日氢能:广东公交车全换氢电/中石化旗下加氢站得到大连补贴/545家氢能相关企业今年注册等12月12 |
| 日)[EB/OL]. (2024-12-13). . | |
| Hydrogen Daily : All Guangdong buses switch to hydrogen electricity/Sinopec’s hydrogen filling stations get subsidies from Dalian/545 hydrogen-related companies register this year, etc. (Dec 12) [EB/OL]. (2024-12-13). . | |
| [11] | 36氪. 专注以甲苯为载体的有机液储运氢技术,理谷新能源完成数千万元Pre-A轮融资| 36碳首发[EB/OL]. (2024-07-11). . |
| [36] | Krypton. Focusing on toluene-based organic liquid hydrogen storage and transport technology, Ligli New Energy completes tens of millions of yuan of Pre-A round of financing|36 Carbon Premiere[EB/OL]. (2024-07-11). . |
| [12] | 中国化学:旗下科研院研发MCH(甲基环己烷)储运氢技术,可实现氢能的长距离安全输送[EB/OL]. (2024-10-16). . |
| ChemChina: Its research institute develops MCH (methylcyclohexane) hydrogen storage and transport technology, which can realise long-distance safe delivery of hydrogen energy[EB/OL]. (2024-10-16). . | |
| [13] | ZHAO Wei, CHIZALLET Céline, SAUTET Philippe, et al. Dehydrogenation mechanisms of methyl-cyclohexane on γ-Al2O3 supported Pt13: Impact of cluster ductility[J]. Journal of Catalysis, 2019, 370: 118-129. |
| [14] | CHEN Fengtao, HUANG Yanping, MI Chengjing, et al. Density functional theory study on catalytic dehydrogenation of methylcyclohexane on Pt(111)[J]. International Journal of Hydrogen Energy, 2020, 45(11): 6727-6737. |
| [15] | RAO Nihal, LELE Ashish K, PATWARDHAN Ashwin W. Optimization of liquid organic hydrogen carrier (LOHC) dehydrogenation system[J]. International Journal of Hydrogen Energy, 2022, 47(66): 28530-28547. |
| [16] | 童凤丫, 孙清, 邵一凡, 等. Ni对Pt/γ-Al2O3催化甲基环己烷脱氢性能的影响[J]. 化工进展, 2016, 35(S2): 183-186. |
| TONG Fengya, SUN Qing, SHAO Yifan, et al. Effect of Ni on the catalytic performance of Pt/γ-Al2O3 in methylcyclohexane dehydrogenation[J]. Chemical Industry and Engineering Progress, 2016, 35(S2): 183-186. | |
| [17] | CROMWELL D K, VASUDEVAN P T, PAWELEC B, et al. Enhanced methylcyclohexane dehydrogenation to toluene over Ir/USY catalyst[J]. Catalysis Today, 2016, 259: 119-129. |
| [18] | ZHANG Xiaotong, HE Ning, LIN Long, et al. Study of the carbon cycle of a hydrogen supply system over a supported Pt catalyst: Methylcyclohexane-toluene-hydrogen cycle[J]. Catalysis Science & Technology, 2020, 10(4): 1171-1181. |
| [19] | 郑修新, 赵甲, 孙国方, 等. 萘加氢催化剂的研究进展[J]. 化工进展, 2015, 34(5): 1295-1299. |
| ZHENG Xiuxin, ZHAO Jia, SUN Guofang, et al. Research progress in catalysts for the hydrogenation of naphthalene[J]. Chemical Industry and Engineering Progress, 2015, 34(5): 1295-1299. | |
| [20] | 张雨宸, 张耀远, 吴芹, 等. 丙烷脱氢用高稳定性Pt基催化剂研究进展[J]. 化工进展, 2022, 41(9): 4733-4753. |
| ZHANG Yuchen, ZHANG Yaoyuan, WU Qin, et al. Advances in high stable Pt based catalysts for propane dehydrogenation[J]. Chemical Industry and Engineering Progress, 2022, 41(9): 4733-4753. | |
| [21] | DENG Yuchen, GUO Yu, JIA Zhimin, et al. Few-atom Pt ensembles enable efficient catalytic cyclohexane dehydrogenation for hydrogen production[J]. Journal of the American Chemical Society, 2022, 144(8): 3535-3542. |
| [22] | TIEN Pham Dung, SATOH Tetsuya, MIURA Masahiro, et al. Continuous hydrogen evolution from cyclohexanes over platinum catalysts supported on activated carbon fibers[J]. Fuel Processing Technology, 2008, 89(4): 415-418. |
| [23] | ROUABAH D, FRAISSARD J. Pt-Au/Al2O3 catalysts: Preparation, characterization, and dehydrogenation activity[J]. Journal of Catalysis, 1993, 144(1): 30-37. |
| [24] | SEKINE Yasushi, HIGO Takuma. Recent trends on the dehydrogenation catalysis of liquid organic hydrogen carrier (LOHC): A review[J]. Topics in Catalysis, 2021, 64(7): 470-480. |
| [25] | 张滋长, 余洪蒽, 张茜, 等. 液相有机氢载体脱氢催化剂的设计原理及机理调控研究进展[J]. 中国科学: 化学, 2023, 53(6): 974-991. |
| ZHANG Zichang, YU Hongen, ZHANG Ng, et al. Research progress in design principles and mechanism regulation of dehydrogenation catalysts for liquid organic hydrogen carriers[J]. Scientia Sinica Chimica), 2023, 53(6): 974-991. | |
| [26] | YANG Xue, SONG Ye, CAO Tiantian, et al. The double tuning effect of TiO2 on Pt catalyzed dehydrogenation of methylcyclohexane[J]. Molecular Catalysis, 2020, 492: 110971. |
| [27] | XU Ying, XUE Kang, AI Minhua, et al. Tunable Pt δ+ /Pt0 sites by highly dispersed defected TiO2 for efficient catalytic methylcyclohexane dehydrogenation[J]. Chemical Engineering Journal, 2024, 496: 154192. |
| [28] | SHUKLA Anshu A, GOSAVI Priti V, PANDE Jayshri V, et al. Efficient hydrogen supply through catalytic dehydrogenation of methylcyclohexane over Pt/metal oxide catalysts[J]. International Journal of Hydrogen Energy, 2010, 35(9): 4020-4026. |
| [29] | 李晓芸, 马丁, 包信和. 不同活性炭上Pt催化剂的分散性及其在甲基环己烷脱氢反应中的催化性能[J]. 催化学报, 2008, 29(3): 259-263. |
| LI Xiaoyun, MA Ding, BAO Xinhe. Dispersion of Pt catalysts supported on activated carbon and their catalytic performance in methylcyclohexane dehydrogenation[J]. Chinese Journal of Catalysis, 2008, 29(3): 259-263. | |
| [30] | CHEN Luning, VERMA Pragya, HOU Kaipeng, et al. Reversible dehydrogenation and rehydrogenation of cyclohexane and methylcyclohexane by single-site platinum catalyst[J]. Nature Communications, 2022, 13(1): 1092. |
| [31] | YAN Jing, WANG Weiyan, MIAO Lei, et al. Dehydrogenation of methylcyclohexane over PtSn supported on MgAl mixed metal oxides derived from layered double hydroxides[J]. International Journal of Hydrogen Energy, 2018, 43(19): 9343-9352. |
| [32] | HOU Zhaoyin, CHEN Ping, FANG Heliang, et al. Production of synthesis gas via methane reforming with CO2 on noble metals and small amount of noble-(Rh-) promoted Ni catalysts[J]. International Journal of Hydrogen Energy, 2006, 31(5): 555-561. |
| [33] | KOSAKA Misato, HIGO Takuma, Shuhei OGO, et al. Low-temperature selective dehydrogenation of methylcyclohexane by surface protonics over Pt/anatase-TiO2 catalyst[J]. International Journal of Hydrogen Energy, 2020, 45(1): 738-743. |
| [34] | SEBASTIAN Oshin, NAIR Sharanya, TACCARDI Nicola, et al. Stable and selective dehydrogenation of methylcyclohexane using supported catalytically active liquid metal solutions-Ga52Pt/SiO2 SCALMS[J]. ChemCatChem, 2020, 12(18): 4533-4537. |
| [35] | Carmen ROMÁN-MARTÍNEZ M, MACIÁ-AGULLÓ Juan A, VILELLA I Ma Julieta, et al. State of Pt in dried and reduced PtIn and PtSn catalysts supported on carbon[J]. The Journal of Physical Chemistry C, 2007, 111(12): 4710-4716. |
| [37] | WANG Nailiang, QIU Jiane, WU Zhaowei, et al. Effect of microwave calcination on catalytic properties of Pt/MgAl(Sn)O x catalyst in cyclohexane dehydrogenation to cyclohexene[J]. Applied Catalysis A: General, 2015, 503: 62-68. |
| [38] | ALHUMAIDAN Faisal, TSAKIRIS Dimos, CRESSWELL David, et al. Hydrogen storage in liquid organic hydride: Selectivity of MCH dehydrogenation over monometallic and bimetallic Pt catalysts[J]. International Journal of Hydrogen Energy, 2013, 38(32): 14010-14026. |
| [39] | ALHUMAIDAN Faisal, CRESSWELL David, GARFORTH Arthur. Hydrogen storage in liquid organic hydride: Producing hydrogen catalytically from methylcyclohexane[J]. Energy & Fuels, 2011, 25(10): 4217-4234. |
| [40] | WANG Feng, YANG Yunquan, WANG Weiyan. Efficient hydrogen production by catalytic dehydrogenation of methylcyclohexane over Ni-Pt/nano-film alumina catalyst[J]. Advanced Materials Research, 2014, 881/882/883: 315-323. |
| [41] | MI Chengjing, HUANG Yanping, CHEN Fengtao, et al. Density functional theory study on dehydrogenation of methylcyclohexane on Ni-Pt(111)[J]. International Journal of Hydrogen Energy, 2021, 46(1): 875-885. |
| [42] | HE Zhe, LI Kailang, CHEN Tianxiang, et al. High-purity hydrogen production from dehydrogenation of methylcyclohexane catalyzed by zeolite-encapsulated subnanometer platinum-iron clusters[J]. Nature Communications, 2025, 16(1): 92. |
| [43] | 赵世栋, 周路阳, 商红岩, 等. 有机液相储氢载体脱氢催化剂的制备及催化性能[J]. 中国石油大学学报(自然科学版), 2024, 48(4): 215-223. |
| ZHAO Shidong, ZHOU Luyang, SHANG Hongyan, et al. Preparation and catalytic performance of dehydrogenation catalyst in organic liquid phase hydrogen storage carrier[J]. Journal of China University of Petroleum (Edition of Natural Science), 2024, 48(4): 215-223. | |
| [44] | AL-SHAIKHALI Anaam H, JEDIDI Abdesslem, CAVALLO Luigi, et al. Non-precious bimetallic catalysts for selective dehydrogenation of an organic chemical hydride system[J]. Chemical Communications, 2015, 51(65): 12931-12934. |
| [45] | LV Cuncai, LOU Pingping, CHANG Jiarong, et al. Nickel single atoms/cerium oxide hybrid for hydrogen production via solar-heating catalytic dehydrogenation of methyl cyclohexane[J]. Journal of Power Sources, 2023, 559: 232674. |
| [46] | GULYAEVA Yuliya K, ALEKSEEVA BYKOVA Maria V, ERMAKOV Dmitry Yu, et al. High-loaded nickel based sol-gel catalysts for methylcyclohexane dehydrogenation[J]. Catalysts, 2020, 10(10): 1198. |
| [47] | PATIL Shubhangi P, PANDE Jayshri V, BINIWALE Rajesh B. Non-noble Ni-Cu/ACC bimetallic catalyst for dehydrogenation of liquid organic hydrides for hydrogen storage[J]. International Journal of Hydrogen Energy, 2013, 38(35): 15233-15241. |
| [48] | 李功华, 熊果, 赵欣, 等. Ni-Cu/γ-Al2O3-ZrO2催化剂的制备及其脱氢性能[J]. 石油化工, 2016, 45(7): 798-804. |
| LI Gonghua, XIONG Guo, ZHAO Xin, et al. Preparation of Ni-Cu/γ- Al2O3-ZrO2 catalyst and its activity in dehydrogenation of methylcyclohexane[J]. Petrochemical Technology, 2016, 45(7): 798-804. | |
| [49] | XIA Zhijun, LU Hanfeng, LIU Huayan, et al. Cyclohexane dehydrogenation over Ni-Cu/SiO2 catalyst: Effect of copper addition[J]. Catalysis Communications, 2017, 90: 39-42. |
| [50] | KOSKIN Anton P, STEPANENKO Sergey A, ALEKSEEVA (BYKOVA) Maria V, et al. The origin of extraordinary selectivity in dehydrogenation of methylcyclohexane over Ni-Sn-based catalysts[J]. Chemical Engineering Journal, 2023, 476: 146629. |
| [51] | ONDA Ayumu, KOMATSU Takayuki, YASHIMA Tatsuaki. Characterizations and catalytic properties of fine particles of Ni-Sn intermetallic compounds supported on SiO2 [J]. Journal of Catalysis, 2004, 221(2): 378-385. |
| [52] | XIA Zhijun, LIU Huayan, LU Hanfeng, et al. High selectivity of cyclohexane dehydrogenation for H2 evolution over Cu/SBA-15 catalyst[J]. Catalysis Letters, 2017, 147(6): 1295-1302. |
| [53] | BOUFADEN N, AKKARI R, PAWELEC B, et al. Dehydrogenation of methylcyclohexane to toluene over partially reduced Mo-SiO2 catalysts[J]. Applied Catalysis A: General, 2015, 502: 329-339. |
| [54] | DALMON J A, MARTIN G A. Hydrogenolysis of C2H6, C3H8 and n-C4H10 over silica-supported nickel-copper catalysts[J]. Journal of Catalysis, 1980, 66(1): 214-221. |
| [55] | WU Huixin, WANG Hai, LV Yating, et al. Ultra-small metallic nickel nanoparticles on dealuminated zeolite for active and durable catalytic dehydrogenation[J]. Angewandte Chemie International Edition, 2025, 64(8): e202420306. |
| [56] | FUENTES Edgardo Meza, COSTA FARO Arnaldo DA, DE FREITAS SILVA Tatiana, et al. A comparison between copper and nickel-based catalysts obtained from hydrotalcite-like precursors for WGSR[J]. Catalysis Today, 2011, 171(1): 290-296. |
| [57] | ARDIYANTI A R, KHROMOVA S A, VENDERBOSCH R H, et al. Catalytic hydrotreatment of fast-pyrolysis oil using non-sulfided bimetallic Ni-Cu catalysts on a δ-Al2O3 support[J]. Applied Catalysis B: Environmental, 2012, 117: 105-117. |
| [58] | LI P, LIU J, NAG N, et al. In situ preparation of Ni-Cu/TiO2 bimetallic catalysts[J]. Journal of Catalysis, 2009, 262(1): 73-82. |
| [59] | MINYUKOVA T P, SIMENTSOVA I I, KHASIN A V, et al. Dehydrogenation of methanol over copper-containing catalysts[J]. Applied Catalysis A: General, 2002, 237(1/2): 171-180. |
| [60] | AL-SHAIKHALI Anaam H, JEDIDI Abdesslem, ANJUM Dalaver H, et al. Kinetics on NiZn bimetallic catalysts for hydrogen evolution via selective dehydrogenation of methylcyclohexane to toluene[J]. ACS Catalysis, 2017, 7(3): 1592-1600. |
| [61] | MENG Junchi, ZHOU Feng, MA Huixia, et al. A review of catalysts for methylcyclohexane dehydrogenation[J]. Topics in Catalysis, 2021, 64(7): 509-520. |
| [1] | 李红伟, 许涵侨, 赵燕, 刘耀宗, 滕志君, 季东, 李贵贤. 铂基催化剂电催化甲醇氧化研究进展与展望[J]. 化工进展, 2025, 44(6): 3443-3456. |
| [2] | 孔肖阳, 刘振涛, 邹予桐, 王丹丹, 段爱军, 徐春明, 王喜龙. 多环芳烃加氢裂化制BTX催化剂研究进展[J]. 化工进展, 2025, 44(6): 3468-3485. |
| [3] | 石秀顶, 王永全, 曾静, 苏畅, 洪俊明. 纳米管状Co-N-C活化过碳酸盐降解四环素[J]. 化工进展, 2025, 44(6): 3041-3052. |
| [4] | 谢武强, 张岭, 贺杠, 蒋里锋, 郑晰瑞, 张和鹏. CoTBrPP-PTAB-Cu电催化还原CO2制甲烷[J]. 化工进展, 2025, 44(6): 3093-3100. |
| [5] | 姚如伟, 宋乐音, 牛琴琴, 李聪明. Na-S双助剂修饰铁基催化剂催化CO2加氢制C2+醇[J]. 化工进展, 2025, 44(6): 3154-3162. |
| [6] | 陈少伟, 陈奕, 牛江奇, 刘天奇, 黄建国, 陈焕浩, 范晓雷. 介质阻挡放电等离子体催化反应器研究进展及应用展望[J]. 化工进展, 2025, 44(6): 3175-3189. |
| [7] | 王家慧, 李培雅, 杨福胜, 王斌, 方涛. 有机液态储氢载体甲基环己烷脱氢研究进展[J]. 化工进展, 2025, 44(6): 3208-3223. |
| [8] | 张莹, 郑雪梅, 马爱元, 田时泓. 聚乙烯常规及微波催化热解产物分布特征的研究进展[J]. 化工进展, 2025, 44(6): 3224-3237. |
| [9] | 刘威, 侯雪兰, 杨贵东. 氢-氨绿色循环研究进展与展望[J]. 化工进展, 2025, 44(5): 2625-2641. |
| [10] | 丁阿静, 周巧巧, 顾学红. 膜反应器中杨木催化气化制清洁合成气[J]. 化工进展, 2025, 44(5): 2716-2723. |
| [11] | 何志勇. 分步脱羟/脱碳催化剂实现高效裂解甲醇制氢[J]. 化工进展, 2025, 44(5): 2724-2732. |
| [12] | 范晓娅, 赵镇, 彭强. 电催化二氧化碳和硝酸根共还原合成尿素研究进展[J]. 化工进展, 2025, 44(5): 2856-2869. |
| [13] | 苏俊杰, 刘苏, 周海波, 刘畅, 张琳, 王仰东, 谢在库. 用于CO2加氢直接制低碳烯烃的InZr/SAPO-34双功能催化剂[J]. 化工进展, 2025, 44(5): 2870-2878. |
| [14] | 汪柯, 胡登, 王星博, 孙楠楠, 魏伟. Fe x Co y Ca3Al双功能材料用于CO2捕集-转化一体化制合成气[J]. 化工进展, 2025, 44(5): 2888-2897. |
| [15] | 马梓轩, 施瑞晨, 刘明杰, 杨莹杰, 宋子瑜, 梅晓鹏, 高晓峰, 洪龙城, 姚思宇, 张治国, 任其龙. 环烷烃催化制氢反应器的设计与性能优化: 前沿进展与挑战[J]. 化工进展, 2025, 44(5): 2919-2937. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||