化工进展 ›› 2025, Vol. 44 ›› Issue (6): 3208-3223.DOI: 10.16085/j.issn.1000-6613.2024-2009
• 专栏:化工过程强化 • 上一篇
有机液态储氢载体甲基环己烷脱氢研究进展
王家慧1( ), 李培雅1, 杨福胜1,2, 王斌1,2(
), 李培雅1, 杨福胜1,2, 王斌1,2( ), 方涛1,2(
), 方涛1,2( )
)
- 1.西安交通大学化工学院,陕西能源化工过程强化重点实验室,新能源系统与装备工程研究中心,陕西 西安 710049
2.陕西氢易能源科技有限公司,陕西 西安 712000
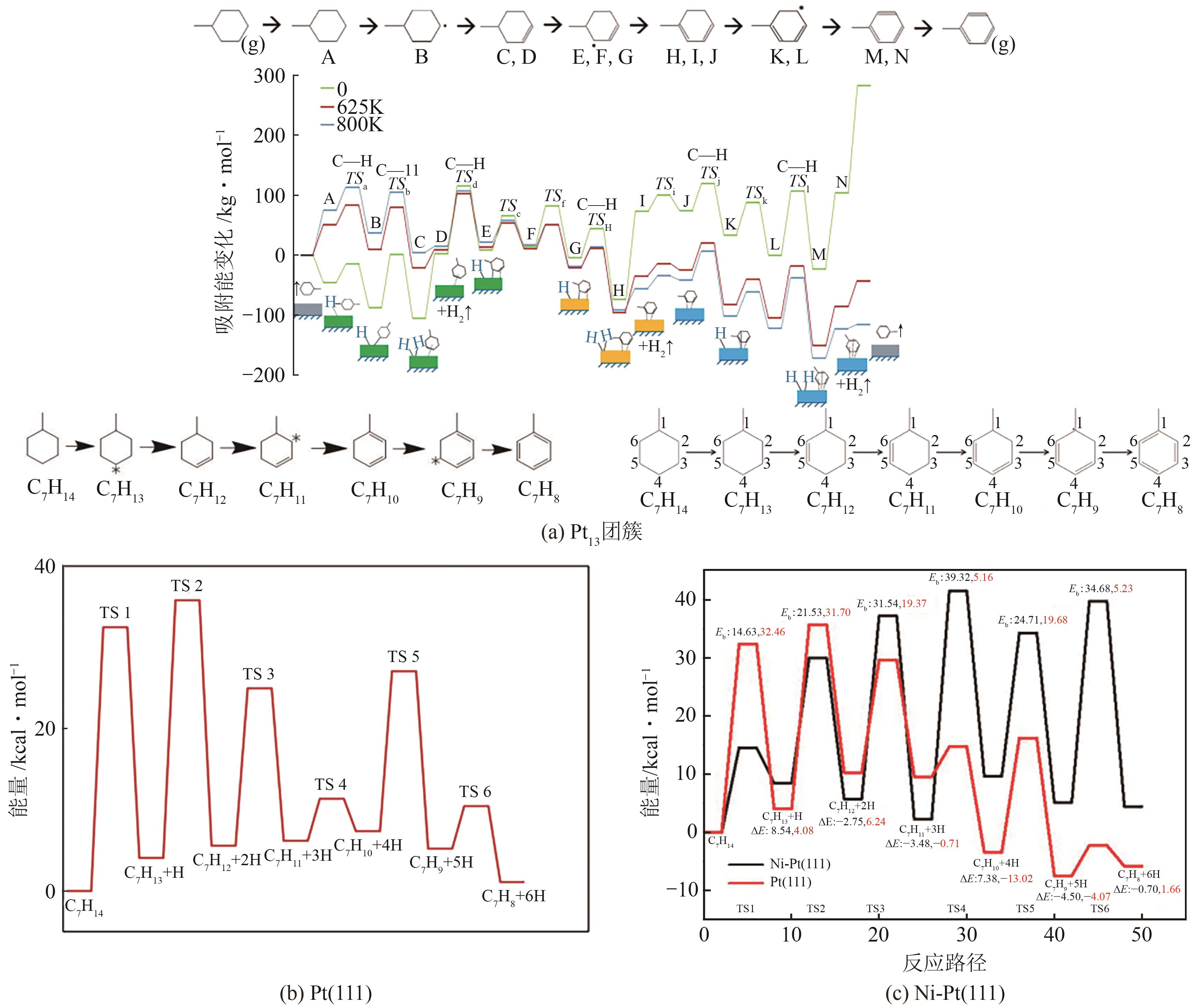
-
收稿日期:2024-12-10修回日期:2025-03-01出版日期:2025-06-25发布日期:2025-07-08 -
通讯作者:王斌,方涛 -
作者简介:王家慧(2000—),女,博士研究生,研究方向为有机液态储氢。E-mail:jh_wang@stu.xjtu.edu.cn。 -
基金资助:陕西省重点研发计划(2024GX-ZDCYL-04-05);陕西省重点研发计划(2024CY2-GJHX-14);陕西省青年科技新星项目(2024ZC-KJXX-073);西安市英才计划(XAYC240010);陕西省自然科学基础研究计划(2022JZ-07);西安市科技计划(23ZCKCGZH0005);中国博士后科学基金会(2024M752588);咸阳市重大科技成果转化落地专项(L2023-ZDKJ-QCY-CGLD-GY-007)
Research progress on the dehydrogenation of methylcyclohexane as a liquid organic hydrogen carrier
WANG Jiahui1( ), LI Peiya1, YANG Fusheng1,2, WANG Bin1,2(
), LI Peiya1, YANG Fusheng1,2, WANG Bin1,2( ), FANG Tao1,2(
), FANG Tao1,2( )
)
- 1.Shaanxi Key Laboratory of Energy Chemical Process Intensification, Engineering Research Center of New Energy System Engineering and Equipment, School of Chemical Engineering, Xi’an Jiaotong University, Xi’an 710049, Shaanxi, China
2.Shaanxi Hydrotransformer Energy Technology Co. , Ltd. , Xi’an 712000, Shaanxi, China
-
Received:2024-12-10Revised:2025-03-01Online:2025-06-25Published:2025-07-08 -
Contact:WANG Bin, FANG Tao
摘要:
氢能作为一种清洁、无污染的替代能源而受到广泛关注,而储氢技术是制约其发展的关键。甲基环己烷(MCH)-甲苯-氢气系统(MTH)作为一种有机液态储氢体系,由于其安全性高和成本低而具有大规模利用的潜力。甲基环己烷脱氢过程作为MTH体系发展的瓶颈,反应条件苛刻、能耗高,理解其反应机理、寻找高性能的催化剂并对反应进行优化是其研究的重点。本文从机理出发,对甲基环己烷脱氢动力学、催化剂设计以及反应过程强化的研究现状进行总结,重点阐述目前贵金属Pt基催化剂和非贵金属Ni基催化剂的研究进展,探讨了通过载体调控、处理方法改进、助剂添加等策略提升催化剂性能的方法。同时,对反应器设计和操作条件调整实现过程强化的相关研究进行了总结。本文旨在为MTH体系的进一步发展提供理论指导和技术支持,并在此基础上对未来发展方向进行展望。
中图分类号:
引用本文
王家慧, 李培雅, 杨福胜, 王斌, 方涛. 有机液态储氢载体甲基环己烷脱氢研究进展[J]. 化工进展, 2025, 44(6): 3208-3223.
WANG Jiahui, LI Peiya, YANG Fusheng, WANG Bin, FANG Tao. Research progress on the dehydrogenation of methylcyclohexane as a liquid organic hydrogen carrier[J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3208-3223.
| 催化剂 | 动力学模型 | 主要结论 | 文献 |
|---|---|---|---|
Pt/Al2O3, Pt-Re/Al2O3, | 幂律模型/Hougen-Watson模型 | 反应阶数与分压有关,不同的催化剂反应速率控制步骤会偏移 | [ |
Pt/Al2O3, Pt-Re/Al2O3, | Langmuir-Hinshelwood模型 | 同时分析了主反应速率和催化剂失活速率,甲基环己烯脱氢为甲基环己二烯是主反应的控速步骤 | [ |
Pt/γ-Al2O3, Pt-Re/γ-Al2O3, Pt-Pd/γ-Al2O3 | 幂律模型/非Langmuir和非竞争性的Horiuti-Polanyi模型 | 对甲基环己烷的近零级依赖性和对氢气的级阶依赖性,引入长期失活模型 | [ |
| Pt/γ-Al2O3 | 幂律模型 | 幂律模型参数(如反应阶次、活化能和动力学速率常数)取决于操作条件 | [ |
| Pt/beta 沸石 | 幂律模型 | 对甲基环己烷来说是零级动力学,简单的幂律模型不适用于甲基环己烷脱氢 | [ |
| Pt/Al2O3 | 幂律模型/LHHW模型/HP模型 | 基于单位点或双位点表面反应动力学的LHHW模型拟合效果更佳 | [ |
| Pt-Sn/Al2O3 | 经验模型 | 考虑3种吸附形式的形成,为正向和反向反应路线的实验数据提供充分描述 | [ |
| Pt-Se/TiO2 | 单位点LHHW模型 | 通过动力学分析确定了Se的促进作用 | [ |
表1 甲基环己烷的脱氢动力学
| 催化剂 | 动力学模型 | 主要结论 | 文献 |
|---|---|---|---|
Pt/Al2O3, Pt-Re/Al2O3, | 幂律模型/Hougen-Watson模型 | 反应阶数与分压有关,不同的催化剂反应速率控制步骤会偏移 | [ |
Pt/Al2O3, Pt-Re/Al2O3, | Langmuir-Hinshelwood模型 | 同时分析了主反应速率和催化剂失活速率,甲基环己烯脱氢为甲基环己二烯是主反应的控速步骤 | [ |
Pt/γ-Al2O3, Pt-Re/γ-Al2O3, Pt-Pd/γ-Al2O3 | 幂律模型/非Langmuir和非竞争性的Horiuti-Polanyi模型 | 对甲基环己烷的近零级依赖性和对氢气的级阶依赖性,引入长期失活模型 | [ |
| Pt/γ-Al2O3 | 幂律模型 | 幂律模型参数(如反应阶次、活化能和动力学速率常数)取决于操作条件 | [ |
| Pt/beta 沸石 | 幂律模型 | 对甲基环己烷来说是零级动力学,简单的幂律模型不适用于甲基环己烷脱氢 | [ |
| Pt/Al2O3 | 幂律模型/LHHW模型/HP模型 | 基于单位点或双位点表面反应动力学的LHHW模型拟合效果更佳 | [ |
| Pt-Sn/Al2O3 | 经验模型 | 考虑3种吸附形式的形成,为正向和反向反应路线的实验数据提供充分描述 | [ |
| Pt-Se/TiO2 | 单位点LHHW模型 | 通过动力学分析确定了Se的促进作用 | [ |
| 催化剂 | 反应器 | 催化剂用量 | 测试条件 | 反应结果 | 文献 |
|---|---|---|---|---|---|
| Pt/La0.7Y0.3NiO3 | 喷雾脉冲反应器 | 300mg | 350℃,频率0.33Hz,脉宽10ms | 45.26 mmol/(gmet·min) | [ |
| Pt/TiO2 | 固定床反应器 | 50mg | 350℃,MCH∶Ar∶N2=6.4∶20∶5 | X=97% | [ |
| 0.55Pt/TiO2-Al2O3 | 固定床反应器 | 10mL | 310℃,H2/MCH=0.713,LHSV=1.5h-1 | X=95% | [ |
| 0.5Pt/TiO2-Al2O3 | 固定床反应器 | 2000mg | 400℃,MCH∶N2∶H2=0.2∶30∶30 | X=93.2% | [ |
| Pt/0.5TiO2/M41 | 固定床反应器 | 1000mg | 310℃,MCH=0.1mL·min | X=88% | [ |
| Pt/CeO2-S | 固定床反应器 | 300mg | 350℃,MCH∶N2=0.05∶15 | X=78.3% | [ |
| Pt/CeO2-SiO2 | 固定床反应器 | 500mg | 340℃,LHSV=2.45~2.5mL/(gcat·h) | X=100% | [ |
| Pt-1B/Al2O3 | 固定床反应器 | 100mg | 350℃,MCH∶N2=0.05∶5 | X=81.5% | [ |
| 0.5Pt/Mg-Al-O | 固定床反应器 | 500mg | 300℃,MCH=0.1mL/min | X=92% | [ |
| 0.4Pt/Ce14-Mg-Al-O | 固定床反应器 | 500mg | 350℃,MCH=0.1mL/min | X=98.5% | [ |
| 3Pt/Co3-Al-O | 固定床反应器 | 300mg | 330℃,MCH∶N2=0.1∶35 | X=90% | [ |
| 0.5Pt/MA | 固定床反应器 | 200mg | 350℃,MCH=0.1mL/min | X=80.2% | [ |
| 0.25Pt/SC-CNT | 固定床反应器 | 500mg | 315℃,MCH=5mL/min(气体) | X=95% | [ |
| Pt/AC | 固定床反应器 | 50mg | 300℃,MCH=0.03mL/min,Ar/MCH=3 | X=88% | [ |
| Pt/CB | 固定床反应器 | 554mg | 300℃,MCH∶N2=0.03∶5 | X=95% | [ |
| 0.2Pt/CNTs | 固定床反应器 | 300mg | 300℃,MCH=0.03mL/min | X=28.6% | [ |
| 3.34Pt/CN | 间歇反应器 | 120mg | 180℃,MCH=420μL | X=99% | [ |
| 0.2Pt/GAC-S | 固定床反应器 | 300mg | 300℃,MCH=0.03mL/min | X=63% | [ |
| 0.2Pt/PTC-S | 固定床反应器 | 300mg | 300℃,MCH=0.03mL/min | X=84.3% | [ |
| 3Pt/HAC | 固定床反应器 | 300mg | 330℃,MCH∶N2=0.05∶35 | X=91% | [ |
| Pt/CF-GL | 固定床反应器 | 300mg | 350℃,MCH∶N2=0.05∶15 | X=96.9% | [ |
| 0.68Pt(acac)2/CS | 固定床反应器 | 554mg | 320℃,MCH∶N2=0.03∶5 | X=97% | [ |
| 3.1Pt/SBA | 固定床反应器 | 50mg | 315℃,WHSV=27.1h-1 | X=95% | [ |
| Pt/KIT-6 | 固定床反应器 | 1000mg | 300℃,LHSV=3.6mL/(gcat·h) | X=98.5% | [ |
表2 单金属Pt基催化剂用于MCH脱氢
| 催化剂 | 反应器 | 催化剂用量 | 测试条件 | 反应结果 | 文献 |
|---|---|---|---|---|---|
| Pt/La0.7Y0.3NiO3 | 喷雾脉冲反应器 | 300mg | 350℃,频率0.33Hz,脉宽10ms | 45.26 mmol/(gmet·min) | [ |
| Pt/TiO2 | 固定床反应器 | 50mg | 350℃,MCH∶Ar∶N2=6.4∶20∶5 | X=97% | [ |
| 0.55Pt/TiO2-Al2O3 | 固定床反应器 | 10mL | 310℃,H2/MCH=0.713,LHSV=1.5h-1 | X=95% | [ |
| 0.5Pt/TiO2-Al2O3 | 固定床反应器 | 2000mg | 400℃,MCH∶N2∶H2=0.2∶30∶30 | X=93.2% | [ |
| Pt/0.5TiO2/M41 | 固定床反应器 | 1000mg | 310℃,MCH=0.1mL·min | X=88% | [ |
| Pt/CeO2-S | 固定床反应器 | 300mg | 350℃,MCH∶N2=0.05∶15 | X=78.3% | [ |
| Pt/CeO2-SiO2 | 固定床反应器 | 500mg | 340℃,LHSV=2.45~2.5mL/(gcat·h) | X=100% | [ |
| Pt-1B/Al2O3 | 固定床反应器 | 100mg | 350℃,MCH∶N2=0.05∶5 | X=81.5% | [ |
| 0.5Pt/Mg-Al-O | 固定床反应器 | 500mg | 300℃,MCH=0.1mL/min | X=92% | [ |
| 0.4Pt/Ce14-Mg-Al-O | 固定床反应器 | 500mg | 350℃,MCH=0.1mL/min | X=98.5% | [ |
| 3Pt/Co3-Al-O | 固定床反应器 | 300mg | 330℃,MCH∶N2=0.1∶35 | X=90% | [ |
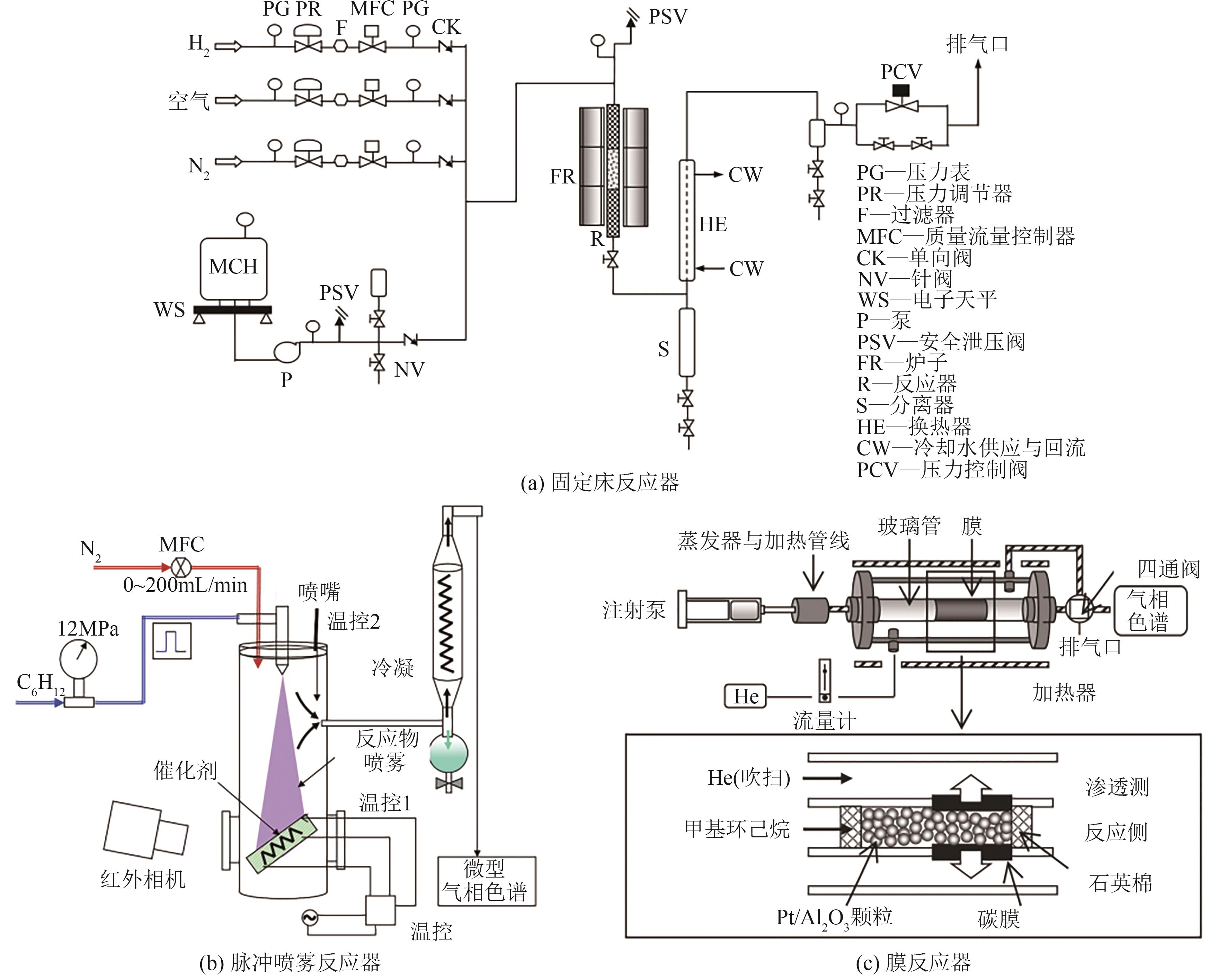
| 0.5Pt/MA | 固定床反应器 | 200mg | 350℃,MCH=0.1mL/min | X=80.2% | [ |
| 0.25Pt/SC-CNT | 固定床反应器 | 500mg | 315℃,MCH=5mL/min(气体) | X=95% | [ |
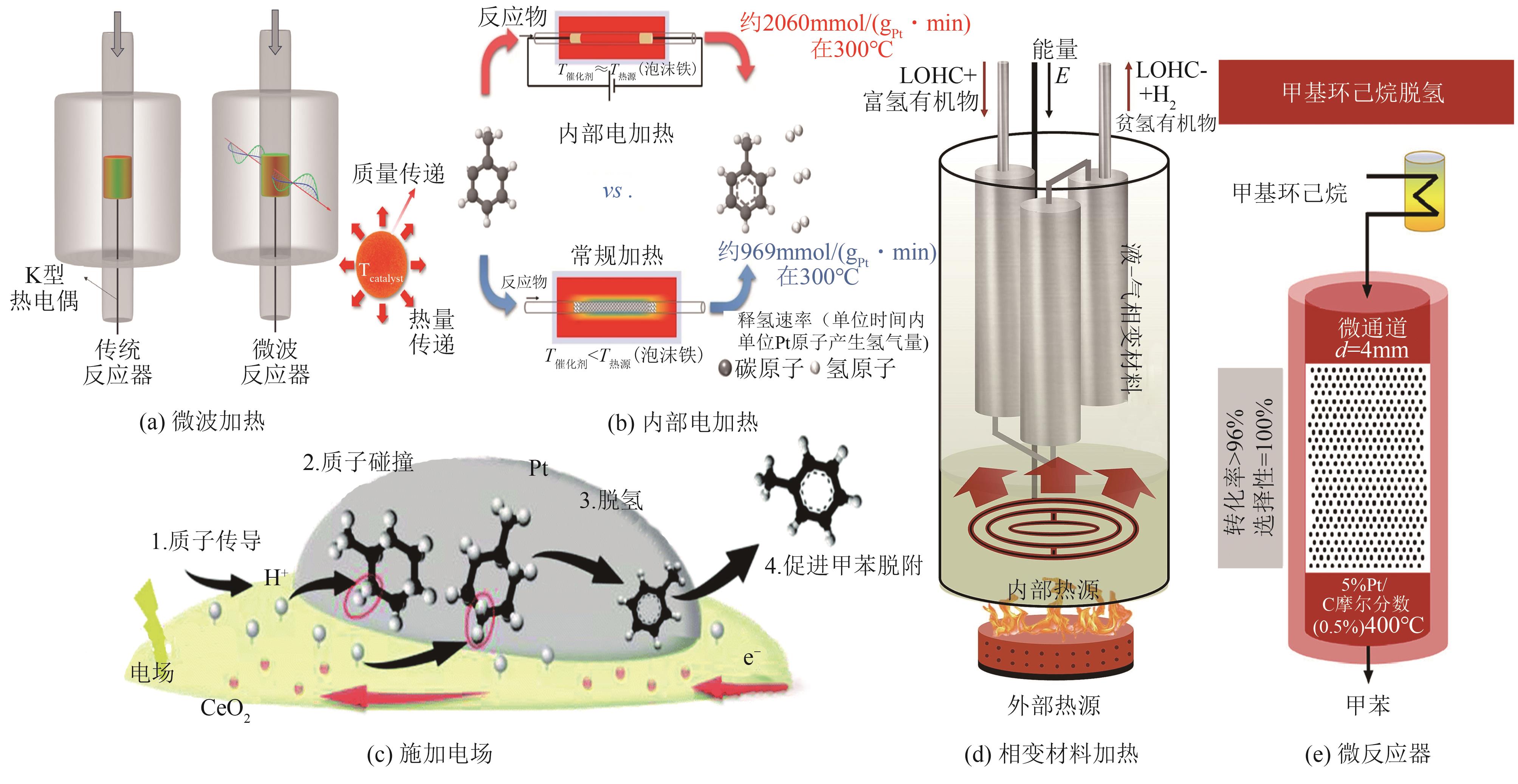
| Pt/AC | 固定床反应器 | 50mg | 300℃,MCH=0.03mL/min,Ar/MCH=3 | X=88% | [ |
| Pt/CB | 固定床反应器 | 554mg | 300℃,MCH∶N2=0.03∶5 | X=95% | [ |
| 0.2Pt/CNTs | 固定床反应器 | 300mg | 300℃,MCH=0.03mL/min | X=28.6% | [ |
| 3.34Pt/CN | 间歇反应器 | 120mg | 180℃,MCH=420μL | X=99% | [ |
| 0.2Pt/GAC-S | 固定床反应器 | 300mg | 300℃,MCH=0.03mL/min | X=63% | [ |
| 0.2Pt/PTC-S | 固定床反应器 | 300mg | 300℃,MCH=0.03mL/min | X=84.3% | [ |
| 3Pt/HAC | 固定床反应器 | 300mg | 330℃,MCH∶N2=0.05∶35 | X=91% | [ |
| Pt/CF-GL | 固定床反应器 | 300mg | 350℃,MCH∶N2=0.05∶15 | X=96.9% | [ |
| 0.68Pt(acac)2/CS | 固定床反应器 | 554mg | 320℃,MCH∶N2=0.03∶5 | X=97% | [ |
| 3.1Pt/SBA | 固定床反应器 | 50mg | 315℃,WHSV=27.1h-1 | X=95% | [ |
| Pt/KIT-6 | 固定床反应器 | 1000mg | 300℃,LHSV=3.6mL/(gcat·h) | X=98.5% | [ |
| 催化剂 | 反应器 | 催化剂用量 | 测试条件 | 反应结果 | 文献 |
|---|---|---|---|---|---|
| 0.1K-0.6Pt/Al2O3 | 固定床反应器 | 10cm3 | 320℃,LHSV=2.0h-1 | X=95% | [ |
| 0.2K-0.5PtE/θ-Al2O3 | 固定床反应器 | 500mg | 320℃,LHSV=2.0h-1 | X=99.99% | [ |
| 20Ni-0.5Pt/Al2O3 | 固定床反应器 | 500mg | 250~330℃,MCH∶N2=0.015∶5 | X=97% | [ |
| 5Pt-10Mo/SiO2 | 固定床反应器 | 100mg | 400℃,WHSV=92.4h-1 H2/MCH=250 | 1.5mmol/(gmet·min) | [ |
| Pt-1.4Mn/Al2O3 | 固定床反应器 | 50mg | 350℃,MCH∶Ar∶N2=6.4∶20∶5 | X≈90% | [ |
| 2Pt-2Sn/Al2O3 | 固定床反应器 | 10mg | 300℃,MCH(气体)∶He=1.6∶98.4 | X=23.6% | [ |
| 2Pt-5Ir/Mg-Al-O | 固定床反应器 | 500mg | 350℃,MCH=0.1mL/min | X=99.9% | [ |
| 2Pt-0.5Sn/Mg-Al-O | 固定床反应器 | 500mg | 300℃,MCH=0.1mL/min | X=90.5% | [ |
| 0.5Zn-Pt/Al2O3 | 固定床反应器 | 20mg | 350℃,MCH∶N2=0.043∶32.8 | X≈78% | [ |
| Pt-2Ga2O3/Al2O3 | 固定床反应器 | 400mg | 320℃,MCH=0.067mL/min | X=91.4% | [ |
| 0.4Pt-0.4Cu/S-1 | 固定床反应器 | 1000mg | 400℃,MCH=0.1mL/min | X=92% | [ |
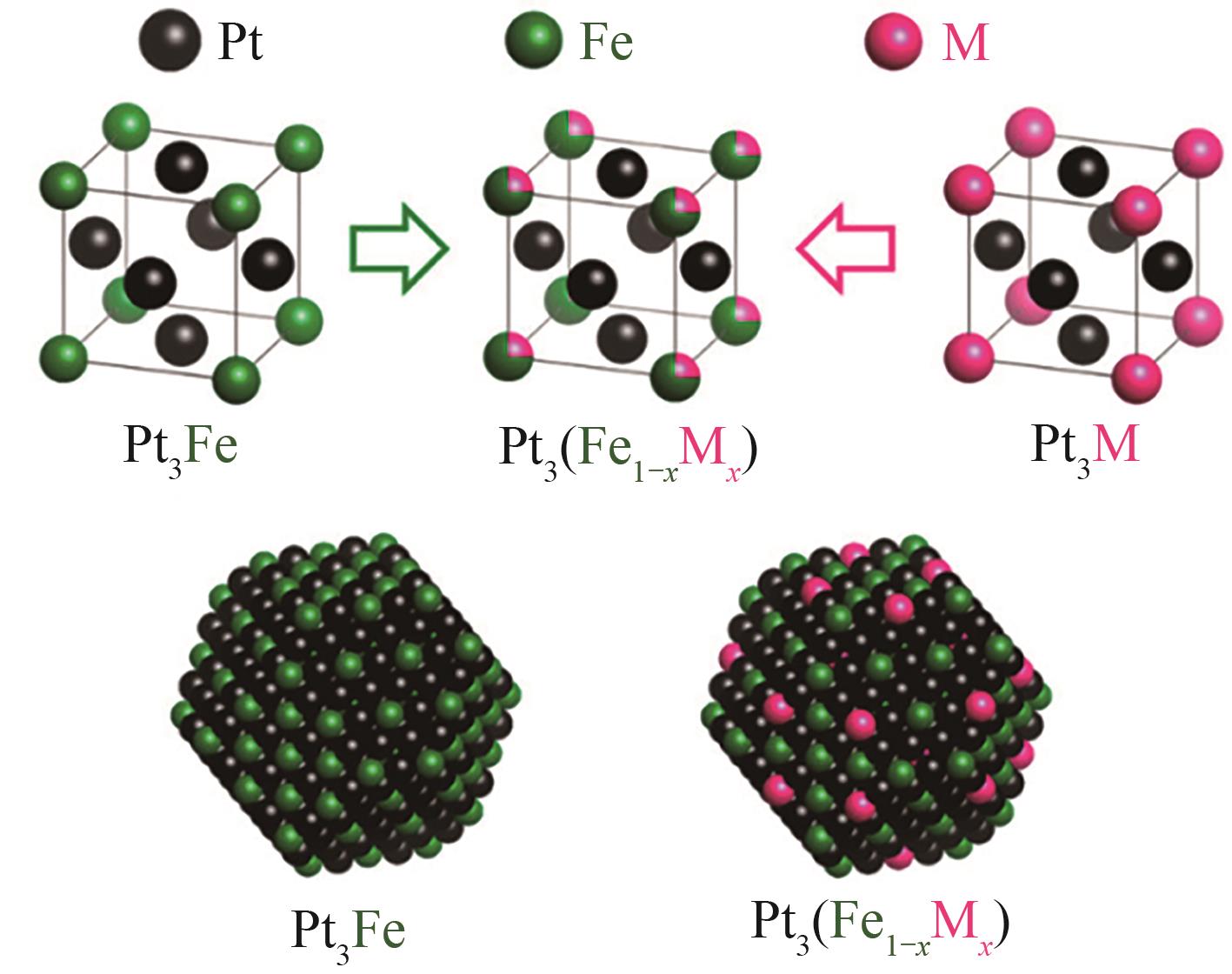
| 3Pt3(Fe0.75Zn0.25)/SiO2 | 固定床反应器 | 10mg | 350℃,MCH(气体)∶He=1.55∶23.45 | X=78.9% | [ |
| Pt-0.2Se/TiO2 | 固定床反应器 | 30mg | 280℃,MCH∶N2=0.6∶39.4 | X=66.4% | [ |
表3 添加金属/助剂对甲基环己烷脱氢的影响
| 催化剂 | 反应器 | 催化剂用量 | 测试条件 | 反应结果 | 文献 |
|---|---|---|---|---|---|
| 0.1K-0.6Pt/Al2O3 | 固定床反应器 | 10cm3 | 320℃,LHSV=2.0h-1 | X=95% | [ |
| 0.2K-0.5PtE/θ-Al2O3 | 固定床反应器 | 500mg | 320℃,LHSV=2.0h-1 | X=99.99% | [ |
| 20Ni-0.5Pt/Al2O3 | 固定床反应器 | 500mg | 250~330℃,MCH∶N2=0.015∶5 | X=97% | [ |
| 5Pt-10Mo/SiO2 | 固定床反应器 | 100mg | 400℃,WHSV=92.4h-1 H2/MCH=250 | 1.5mmol/(gmet·min) | [ |
| Pt-1.4Mn/Al2O3 | 固定床反应器 | 50mg | 350℃,MCH∶Ar∶N2=6.4∶20∶5 | X≈90% | [ |
| 2Pt-2Sn/Al2O3 | 固定床反应器 | 10mg | 300℃,MCH(气体)∶He=1.6∶98.4 | X=23.6% | [ |
| 2Pt-5Ir/Mg-Al-O | 固定床反应器 | 500mg | 350℃,MCH=0.1mL/min | X=99.9% | [ |
| 2Pt-0.5Sn/Mg-Al-O | 固定床反应器 | 500mg | 300℃,MCH=0.1mL/min | X=90.5% | [ |
| 0.5Zn-Pt/Al2O3 | 固定床反应器 | 20mg | 350℃,MCH∶N2=0.043∶32.8 | X≈78% | [ |
| Pt-2Ga2O3/Al2O3 | 固定床反应器 | 400mg | 320℃,MCH=0.067mL/min | X=91.4% | [ |
| 0.4Pt-0.4Cu/S-1 | 固定床反应器 | 1000mg | 400℃,MCH=0.1mL/min | X=92% | [ |
| 3Pt3(Fe0.75Zn0.25)/SiO2 | 固定床反应器 | 10mg | 350℃,MCH(气体)∶He=1.55∶23.45 | X=78.9% | [ |
| Pt-0.2Se/TiO2 | 固定床反应器 | 30mg | 280℃,MCH∶N2=0.6∶39.4 | X=66.4% | [ |
| 催化剂 | 反应器 | 催化剂用量 | 测试条件 | 反应结果 | 文献 |
|---|---|---|---|---|---|
| NiZn0.6/Al2O3 | 固定床反应器 | 20mg | 350℃,H2∶MCH=42.9∶1.37(压力比) | S=96.6%,X=32.2% | [ |
| 8Ni-2Cu/ACC | 喷雾脉冲反应器 | — | 350℃,MCH=0.32mL/min | 39.45mmol/(gmet·min) | [ |
| 2Si-3Ni/SiO2 | 固定床反应器 | 100mg | 350℃,MCH(气体)∶He=0.84∶20 | S≈89%,X≈45% | [ |
| 20Ni/SiO2 | 固定床反应器 | 1000mg | 380~440℃ | X=90% | [ |
| p80Ni-20Cu/SiO2 | 固定床反应器 | 500mg | 325℃,MCH∶H2∶Ar=0.2∶100∶100 | S≈83%,X≈70% | [ |
| p80Ni-20Cu/SiO2 | 固定床反应器 | 500mg | 275℃,MCH∶H2∶Ar=0.2∶100∶100 | S=89%,X=80% | [ |
| 85Ni-15Zn/SiO2 | 固定床反应器 | 500mg | 350℃,MCH∶H2∶Ar=0.2∶100∶100 | Y=80% | [ |
| 80Ni-20Sn/SiO2 | 固定床反应器 | 500mg | 350℃,MCH∶H2∶Ar=0.2∶100∶100 | S=99.9%,X=60.4% | [ |
| Ni20AlO | 固定床反应器 | 600mg | 450℃,MCH∶H2=0.06∶50 | S=84.6%,X=77.4% | [ |
| Ni20/TiO2 | 固定床反应器 | 500mg | 375℃,WHSV=1.9h-1,H2=10mL/min | S=96.5%,X=86.5% | [ |
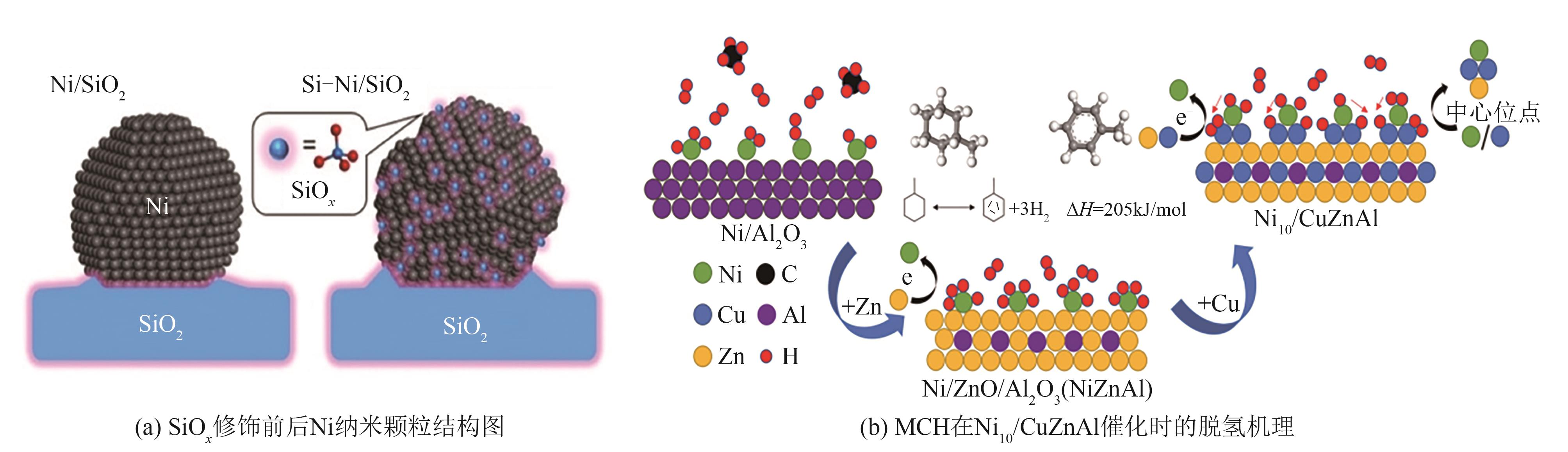
| Ni10/CuZnAl | 固定床反应器 | 500mg | 350℃,MCH=0.05mL/min,载气比为4 | S=99.9%,X=54.3% | [ |
表4 非贵金属催化剂
| 催化剂 | 反应器 | 催化剂用量 | 测试条件 | 反应结果 | 文献 |
|---|---|---|---|---|---|
| NiZn0.6/Al2O3 | 固定床反应器 | 20mg | 350℃,H2∶MCH=42.9∶1.37(压力比) | S=96.6%,X=32.2% | [ |
| 8Ni-2Cu/ACC | 喷雾脉冲反应器 | — | 350℃,MCH=0.32mL/min | 39.45mmol/(gmet·min) | [ |
| 2Si-3Ni/SiO2 | 固定床反应器 | 100mg | 350℃,MCH(气体)∶He=0.84∶20 | S≈89%,X≈45% | [ |
| 20Ni/SiO2 | 固定床反应器 | 1000mg | 380~440℃ | X=90% | [ |
| p80Ni-20Cu/SiO2 | 固定床反应器 | 500mg | 325℃,MCH∶H2∶Ar=0.2∶100∶100 | S≈83%,X≈70% | [ |
| p80Ni-20Cu/SiO2 | 固定床反应器 | 500mg | 275℃,MCH∶H2∶Ar=0.2∶100∶100 | S=89%,X=80% | [ |
| 85Ni-15Zn/SiO2 | 固定床反应器 | 500mg | 350℃,MCH∶H2∶Ar=0.2∶100∶100 | Y=80% | [ |
| 80Ni-20Sn/SiO2 | 固定床反应器 | 500mg | 350℃,MCH∶H2∶Ar=0.2∶100∶100 | S=99.9%,X=60.4% | [ |
| Ni20AlO | 固定床反应器 | 600mg | 450℃,MCH∶H2=0.06∶50 | S=84.6%,X=77.4% | [ |
| Ni20/TiO2 | 固定床反应器 | 500mg | 375℃,WHSV=1.9h-1,H2=10mL/min | S=96.5%,X=86.5% | [ |
| Ni10/CuZnAl | 固定床反应器 | 500mg | 350℃,MCH=0.05mL/min,载气比为4 | S=99.9%,X=54.3% | [ |
| [1] | MARKIEWICZ M, ZHANG Y Q, BÖSMANN A, et al. Environmental and health impact assessment of liquid organic hydrogen carrier (LOHC) systems-challenges and preliminary results[J]. Energy & Environmental Science, 2015, 8(3): 1035-1045. |
| [2] | PREUSTER Patrick, PAPP Christian, WASSERSCHEID Peter. Liquid organic hydrogen carriers (LOHCs): Toward a hydrogen-free hydrogen economy[J]. Accounts of Chemical Research, 2017, 50(1): 74-85. |
| [3] | TEICHMANN Daniel, ARLT Wolfgang, WASSERSCHEID Peter. Liquid organic hydrogen carriers as an efficient vector for the transport and storage of renewable energy[J]. International Journal of Hydrogen Energy, 2012, 37(23): 18118-18132. |
| [4] | OZAKI Makoto, TOMURA Shigeo, OHMURA Ryo, et al. Comparative study of large-scale hydrogen storage technologies: Is hydrate-based storage at advantage over existing technologies?[J]. International Journal of Hydrogen Energy, 2014, 39(7): 3327-3341. |
| [5] | PARK Sanghyoun, NASEEM Mujahid, LEE Sangyong. Experimental assessment of perhydro-dibenzyltoluene dehydrogenation reaction kinetics in a continuous flow system for stable hydrogen supply[J]. Materials, 2021, 14(24): 7613. |
| [6] | 张林海, 丁学强, 张新, 等. 储氢技术研究现状及进展[J]. 中外能源, 2024, 29(4): 17-27. |
| ZHANG Linhai, DING Xueqiang, ZHANG Xin, et al. Research status and progress in hydrogen storage technologies[J]. Sino-Global Energy, 2024, 29(4): 17-27. | |
| [7] | KWAK Yeonsu, KIRK Jaewon, MOON Seongeun, et al. Hydrogen production from homocyclic liquid organic hydrogen carriers (LOHCs): Benchmarking studies and energy-economic analyses[J]. Energy Conversion and Management, 2021, 239: 114124. |
| [8] | YU Shengping, ZENG Qun, YANG Shengyong, et al. A first-principles study of methylcyclohexane adsorption on Pt4 clusters[J]. Journal of Physics B: Atomic, Molecular and Optical Physics, 2010, 43(18): 185101. |
| [9] | ZHAO Wei, CHIZALLET Céline, SAUTET Philippe, et al. Dehydrogenation mechanisms of methyl-cyclohexane on γ-Al2O3 supported Pt13: Impact of cluster ductility[J]. Journal of Catalysis, 2019, 370: 118-129. |
| [10] | MI Chengjing, HUANG Yanping, CHEN Fengtao, et al. Density functional theory study on dehydrogenation of methylcyclohexane on Ni-Pt(111)[J]. International Journal of Hydrogen Energy, 2021, 46(1): 875-885. |
| [11] | CHEN Fengtao, HUANG Yanping, MI Chengjing, et al. Density functional theory study on catalytic dehydrogenation of methylcyclohexane on Pt(111)[J]. International Journal of Hydrogen Energy, 2020, 45(11): 6727-6737. |
| [12] | OUMA Cecil N M, OBODO Kingsley O, MODISHA Phillimon M, et al. Si, P, S and Se surface additives as catalytic activity boosters for dehydrogenation of methylcyclohexane to toluene—A liquid organic hydrogen carrier system: Density functional theory insights[J]. Materials Chemistry and Physics, 2022, 279: 125728. |
| [13] | MANABE Shota, YABE Tomohiro, NAKANO Atsushi, et al. Theoretical investigation on structural effects of Pt-Mn catalyst on activity and selectivity for methylcyclohexane dehydrogenation[J]. Chemical Physics Letters, 2018, 711: 73-76. |
| [14] | ONYEBUCHI OBODO Kingsley, NAPHTALY MORO OUMA Cecil, BESSARABOV Dmitri. Modified Pt (211) and (311) surfaces towards the dehydrogenation of methylcyclohexane to toluene: A density functional theory study[J]. Applied Surface Science, 2022, 584: 152590. |
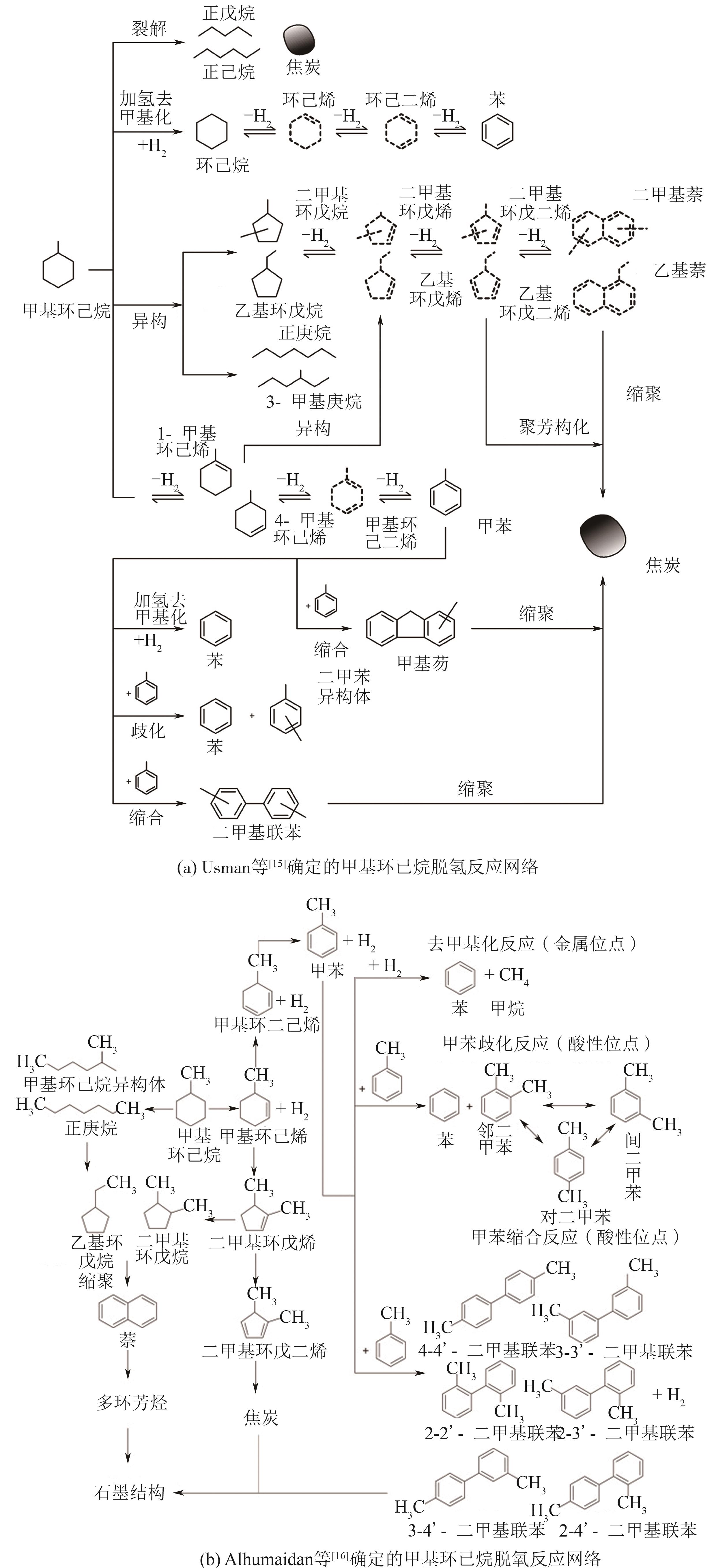
| [15] | USMAN M R, CRESSWELL D L, GARFORTH A A. By-products formation in the dehydrogenation of methylcyclohexane[J]. Petroleum Science and Technology, 2011, 29(21): 2247-2257. |
| [16] | ALHUMAIDAN Faisal, TSAKIRIS Dimos, CRESSWELL David, et al. Hydrogen storage in liquid organic hydride: Selectivity of MCH dehydrogenation over monometallic and bimetallic Pt catalysts[J]. International Journal of Hydrogen Energy, 2013, 38(32): 14010-14026. |
| [17] | VAN TRIMPONT P A, MARIN G B, FROMENT G F. Kinetics of methylcyclohexane dehydrogenation on sulfided commercial platinum/alumina and platinum-rhenium/alumina catalysts[J]. Industrial & Engineering Chemistry Fundamentals, 1986, 25(4): 544-553. |
| [18] | CHAI Maorong, KAWAKAMI Koei. Kinetic model and simulation for catalyst deactivation during dehydrogenation of methylcyclohexane over commercial Pt-, PtRe- and presulfided PtRe-Al2O3 catalysts[J]. Journal of Chemical Technology & Biotechnology, 1991, 51(3): 335-345. |
| [19] | ALHUMAIDAN Faisal, CRESSWELL David, GARFORTH Arthur. Kinetic model of the dehydrogenation of methylcyclohexane over monometallic and bimetallic Pt catalysts[J]. Industrial & Engineering Chemistry Research, 2011, 50(5): 2509-2522. |
| [20] | ALHUMAIDAN Faisal, CRESSWELL David, GARFORTH Arthur. Long-term deactivation of supported Pt catalysts in the dehydrogenation of methylcyclohexane to toluene[J]. Industrial & Engineering Chemistry Research, 2010, 49(20): 9764-9770. |
| [21] | USMAN Muhammad R, ASLAM Rabya. Dehydrogenation of methylcyclohexane for on-board hydrogen use: Initial rate kinetics over 1.0wt% Pt/γ-Al2O3 [J]. Arabian Journal for Science and Engineering, 2014, 39(2): 615-620. |
| [22] | USMAN Muhammad R, CRESSWELL David L, GARFORTH Arthur A. Dehydrogenation of methylcyclohexane: Parametric sensitivity of the power law kinetics[J]. International Scholarly Research Notices, 2013, 2013: 152893. |
| [23] | USMAN Muhammad R, ALOTAIBI Faisal M, ASLAM Rabya. Dehydrogenation-hydrogenation of methylcyclohexane-toluene system on 1.0wt% Pt/zeolite beta catalyst[J]. Progress in Reaction Kinetics and Mechanism, 2015, 40(4): 353-366. |
| [24] | USMAN Muhammad, CRESSWELL David, GARFORTH Arthur. Detailed reaction kinetics for the dehydrogenation of methylcyclohexane over Pt catalyst[J]. Industrial & Engineering Chemistry Research, 2012, 51(1): 158-170. |
| [25] | AKRAM Muhammad Sarfraz, MUNIR Dureem, USMAN Muhammad Rashid. Associative adsorption kinetics: A novel kinetic model for the dehydrogenation of methylcyclohexane[J]. Progress in Reaction Kinetics and Mechanism, 2014, 39(4): 404-417. |
| [26] | AKRAM Muhammad S, ASLAM Rabya, ALHUMAIDAN Faisal S, et al. An exclusive kinetic model for the methylcyclohexane dehydrogenation over alumina-supported Pt catalysts[J]. International Journal of Chemical Kinetics, 2020, 52(7): 415-449. |
| [27] | LOZHKIN A D, ISKHAKOVA L D, MILOVICH F O, et al. Kinetics of hydrogen and toluene production from methylcyclohexane in the presence of a PtSn/Al2O3 catalyst[J]. Kinetics and Catalysis, 2024, 65(3): 280-297. |
| [28] | OSHIMA Kazumasa, ITO Hiroya, YAMAMOTO Tsuyoshi, et al. Kinetics analysis of methylcyclohexane dehydrogenation over Se-modified Pt/TiO2 catalysts[J]. Journal of Chemical Engineering of Japan, 2024, 57(1): 2301533. |
| [29] | SHI Lei, DENG Gaoming, LI Wencui, et al. Al2O3 nanosheets rich in pentacoordinate Al3+ ions stabilize Pt-Sn clusters for propane dehydrogenation[J]. Angewandte Chemie International Edition, 2015, 54(47): 13994-13998. |
| [30] | WANG Yaoxin, WANG Jiandian, ZHENG Ping, et al. Boosting selectivity and stability on Pt/BN catalysts for propane dehydrogenation via calcination & reduction-mediated strong metal-support interaction[J]. Journal of Energy Chemistry, 2022, 67: 451-457. |
| [31] | SHUKLA Anshu A, GOSAVI Priti V, PANDE Jayshri V, et al. Efficient hydrogen supply through catalytic dehydrogenation of methylcyclohexane over Pt/metal oxide catalysts[J]. International Journal of Hydrogen Energy, 2010, 35(9): 4020-4026. |
| [32] | NAGATAKE Satoshi, HIGO Takuma, Shuhei OGO, et al. Dehydrogenation of methylcyclohexane over Pt/TiO2 catalyst[J]. Catalysis Letters, 2016, 146(1): 54-60. |
| [33] | SUGIURA Yukihiro, NAGATSUKA Tomomi, KUBO Kouichi, et al. Dehydrogenation of methylcyclohexane over Pt/TiO2-Al2O3 catalysts[J]. Chemistry Letters, 2017, 46(11): 1601-1604. |
| [34] | YANG Xue, SONG Ye, CAO Tiantian, et al. The double tuning effect of TiO2 on Pt catalyzed dehydrogenation of methylcyclohexane[J]. Molecular Catalysis, 2020, 492: 110971. |
| [35] | XU Ying, XUE Kang, AI Minhua, et al. Tunable Pt δ +/Pt0 sites by highly dispersed defected TiO2 for efficient catalytic methylcyclohexane dehydrogenation[J]. Chemical Engineering Journal, 2024, 496: 154192. |
| [36] | ZHANG Qianlin, ZHANG Zhao, MA Yueer, et al. Morphological regulation of Pt/CeO2 and its catalytic dehydrogenation of methylcyclohexane in fixed bed reactor[J]. International Journal of Hydrogen Energy, 2024, 83: 1338-1348. |
| [37] | JANG Munjeong, CHOI Subin, KIM Yoondo, et al. Effect of CeO2 redox properties on the catalytic activity of Pt-CeO x over irreducible SiO2 support for methylcyclohexane (MCH) dehydrogenation[J]. Applied Surface Science, 2023, 627: 157134. |
| [38] | WU Xu, LU Huaiqian, XIAO Yong, et al. Acid site introduced by Al3+ penta and boron in Pt/Al2O3 catalyst for dehydrogenation of methylcyclohexane[J]. International Journal of Hydrogen Energy, 2022, 47(82): 34955-34962 |
| [39] | WU Kui, CHEN Fengtao, WANG Feng, et al. Preparation of Pt supported on mesoporous Mg-Al oxide catalysts for efficient dehydrogenation of methylcyclohexane[J]. International Journal of Hydrogen Energy, 2021, 46(50): 25513-25519. |
| [40] | WANG Weiyan, MIAO Lei, WU Kui, et al. Hydrogen evolution in the dehydrogenation of methylcyclohexane over Pt/CeMgAlO catalysts derived from their layered double hydroxides[J]. International Journal of Hydrogen Energy, 2019, 44(5): 2918-2925. |
| [41] | MIAO Lei, YAN Jing, WANG Weiyan, et al. Dehydrogenation of methylcyclohexane over Pt supported on Mg-Al mixed oxides catalyst: The effect of promoter Ir[J]. Chinese Journal of Chemical Engineering, 2020, 28(9): 2337-2342. |
| [42] | YAN Jing, WANG Weiyan, MIAO Lei, et al. Dehydrogenation of methylcyclohexane over PtSn supported on MgAl mixed metal oxides derived from layered double hydroxides[J]. International Journal of Hydrogen Energy, 2018, 43(19): 9343-9352. |
| [43] | REN Wenchen, WANG Huanxi, ZHANG Qianlin, et al. Dehydrogenation of methylcyclohexane over layered bimetallic hydroxide-derived Pt/Co-Al-O catalysts[J]. International Journal of Hydrogen Energy, 2024, 88: 771-778. |
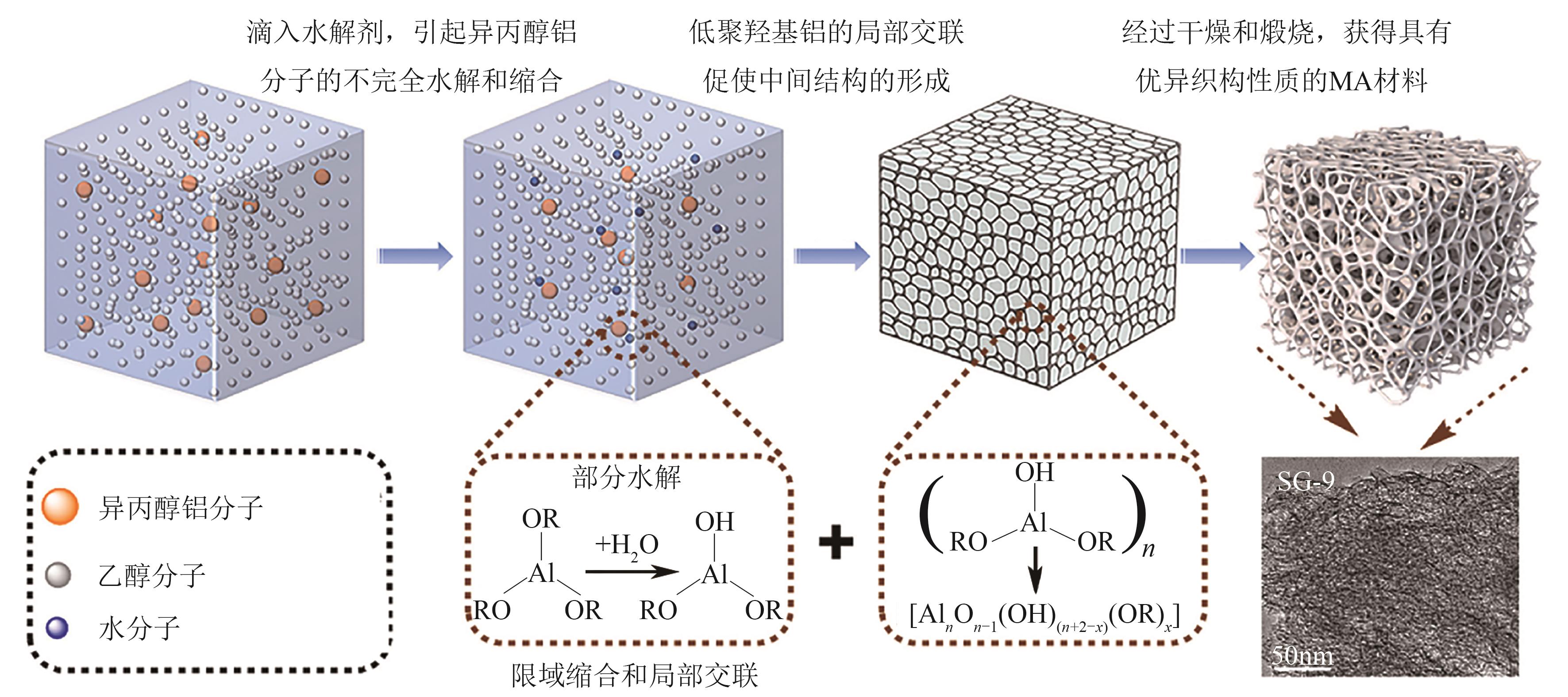
| [44] | LI Changxu, YAN Beibei, PAN Dahai, et al. Efficient and stable dehydrogenation of methylcyclohexane over Pt supported on mesoporous alumina with excellent textural properties[J]. International Journal of Hydrogen Energy, 2024, 74: 297-306. |
| [45] | KWAK Yeonsu, LEE Yujin, MOON Seongeun, et al. Scalable atomic-layer tailoring of abundant oxide supports unlocks superior interfaces for low-metal-loading dehydrogenation[J]. Angewandte Chemie International Edition, 2024, 64(9): e202417598. |
| [46] | WANG Yuguo, SHAH Naresh, HUFFMAN Gerald P. Pure hydrogen production by partial dehydrogenation of cyclohexane and methylcyclohexane over nanotube-supported Pt and Pd catalysts[J]. Energy & Fuels, 2004, 18(5): 1429-1433. |
| [47] | LI Xiaoyun, MA Ding, BAO Xinhe. Dispersion of Pt catalysts supported on activated carbon and their catalytic performance in methylcyclohexane dehydrogenation[J]. Chinese Journal of Catalysis, 2008, 29(3): 259-263. |
| [48] | ZHANG Cui, LIANG Xiuqing, LIU Shuangxi. Hydrogen production by catalytic dehydrogenation of methylcyclohexane over Pt catalysts supported on pyrolytic waste tire char[J]. International Journal of Hydrogen Energy, 2011, 36(15): 8902-8907. |
| [49] | YE Hongli, LIU Shuangxi, HUANG Dongmei, et al. Fabrication of carbon nanotubes derived from waste tire pyrolytic carbon and their application in the dehydrogenation of methylcyclohexane to produce hydrogen[J]. C—Journal of Carbon Research, 2023, 9(4): 121. |
| [50] | WANG Jian, FAN Shiguang, XU Xuan, et al. Catalytic dehydrogenation of methylcyclohexane by Pt nanoparticles supported on nitrogen doped carbon[J]. E3S Web of Conferences, 2020, 194: 01030. |
| [51] | YE Hongli, LIU Shuangxi, ZHANG Cui, et al. Dehydrogenation of methylcyclohexane over Pt-based catalysts supported on functional granular activated carbon[J]. RSC Advances, 2021, 11(47): 29287-29297. |
| [52] | YE Hongli, WANG Tianci, LIU Shuangxi, et al. Fabrication of Pt-loaded catalysts supported on the functionalized pyrolytic activated carbon derived from waste tires for the high performance dehydrogenation of methylcyclohexane and hydrogen production[J]. Catalysts, 2022, 12(2): 211. |
| [53] | DAI Xiaomin, ZHAO Xinkai, GUO Huan, et al. Platinum-based hierarchical activated carbon for methylcyclohexane dehydrogenation in a fixed bed reactor[J]. International Journal of Hydrogen Energy, 2025, 97: 1068-1076. |
| [54] | CHEN Wei, ZHANG Zhao, MA Yueer, et al. Pt-based carbon fiber catalytic dehydrogenation of methylcyclohexane in a fixed-bed reactor[J]. Energy & Fuels, 2025, 39(5): 2834-2842. |
| [55] | OUYANG S C, WANG L W, DU X W, et al. In situ synthesis of highly-active Pt nanoclusters via thermal decomposition for high-temperature catalytic reactions[J]. RSC Advances, 2016, 6(55): 49777-49781. |
| [56] | CHEN Aibing, ZHANG Weiping, LI Xiaoyun, et al. One-pot encapsulation of Pt nanoparticles into the mesochannels of SBA-15 and their catalytic dehydrogenation of methylcyclohexane[J]. Catalysis Letters, 2007, 119(1): 159-164. |
| [57] | Chang-Il AHN, KWAK Yeonsu, KIM Ah-Reum, et al. Dehydrogenation of homocyclic liquid organic hydrogen carriers (LOHCs) over Pt supported on an ordered pore structure of 3-D cubic mesoporous KIT-6 silica[J]. Applied Catalysis B: Environment and Energy, 2022, 307: 121169. |
| [58] | WU Yiqing, LI Yuanyuan, YU Xinbin, et al. Insights into size effects of Pt/Al2O3 catalysts on hydrogen production from methylcyclohexane dehydrogenation[J]. Catalysis Science & Technology, 2024, 14(7): 1791-1801. |
| [59] | CHEN Chenxu, CAO Jingpei, JIANG Wei, et al. Effect of Pt particle size on methylcyclohexane dehydrogenation over Pt/Al2O3 catalysts[J]. Fuel, 2024, 360: 130607. |
| [60] | MA Zixuan, YANG Yingjie, SONG Ziyu, et al. Fine-tuning Pt nanoparticle and coordination for enhanced catalytic efficiency in microwave-assisted methylcyclohexane dehydrogenation over Pt/Al2O3 catalysts[J]. Fuel, 2024, 378: 132851. |
| [61] | ZULAEHAH Siti, SAPUTRA Eqwar, JONUARTI Riri, et al. A first principles study on the catalytic performance of methylcyclohexane dehydrogenation on a monoatomic catalyst[J]. Surface and Interface Analysis, 2024, 56(5): 241-248. |
| [62] | CHEN Luning, VERMA Pragya, HOU Kaipeng, et al. Reversible dehydrogenation and rehydrogenation of cyclohexane and methylcyclohexane by single-site platinum catalyst[J]. Nature Communications, 2022, 13(1): 1092. |
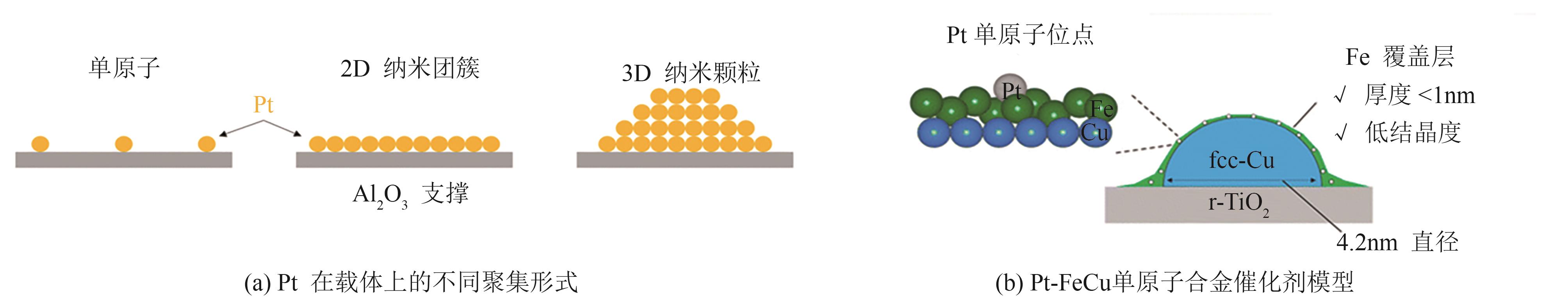
| [63] | Akira ODA, ICHIHASHI Kosei, YAMAMOTO Yuta, et al. Pt single atom alloyed sub-1 nm thick Fe overlayer on supported Cu nanoparticles for methylcyclohexane dehydrogenation[J]. Journal of Materials Chemistry A, 2024, 12(34): 22655-22667. |
| [64] | SONG Mingxia, ZHANG Rongrong, ZHANG Bofeng, et al. Dynamic stable Pt13 clusters anchored on isolated ZnO x nanorafts for efficient cycloparaffin dehydrogenation[J]. Applied Catalysis B: Environment and Energy, 2025, 363: 124787. |
| [65] | HE Zhe, LI Kailang, CHEN Tianxiang, et al. High-purity hydrogen production from dehydrogenation of methylcyclohexane catalyzed by zeolite-encapsulated subnanometer platinum-iron clusters[J]. Nature Communications, 2025, 16(1): 92. |
| [66] | OKADA Yoshimi, SASAKI Eiji, WATANABE Eiji, et al. Development of dehydrogenation catalyst for hydrogen generation in organic chemical hydride method[J]. International Journal of Hydrogen Energy, 2006, 31(10): 1348-1356. |
| [67] | HAN Seungmok, Chang-Il AHN, SHIM Byeong Jo, et al. Synergistic structural and electronic influences of Pt bead catalysts on dehydrogenation activity for liquid organic hydrogen carriers[J]. Chemical Engineering Journal, 2024, 487: 150446. |
| [68] | WANG Feng, YANG Yunquan, WANG Weiyan. Efficient hydrogen production by catalytic dehydrogenation of methylcyclohexane over Ni-Pt/nano-film alumina catalyst[J]. Advanced Materials Research, 2014, 881/882/883: 315-323. |
| [69] | BOUFADEN N, AKKARI R, PAWELEC B, et al. Dehydrogenation of methylcyclohexane to toluene over partially reduced silica-supported Pt-Mo catalysts[J]. Journal of Molecular Catalysis A: Chemical, 2016, 420: 96-106. |
| [70] | BOUFADEN N, AKKARI R, PAWELEC B, et al. Dehydrogenation of methylcyclohexane to toluene over partially reduced Mo-SiO2 catalysts[J]. Applied Catalysis A: General, 2015, 502: 329-339. |
| [71] | BOUFADEN N, PAWELEC B, FIERRO J L G, et al. Hydrogen storage in liquid hydrocarbons: Effect of platinum addition to partially reduced Mo-SiO2 catalysts[J]. Materials Chemistry and Physics, 2018, 209: 188-199. |
| [72] | NAKANO Atsushi, MANABE Shota, HIGO Takuma, et al. Effects of Mn addition on dehydrogenation of methylcyclohexane over Pt/Al2O3 catalyst[J]. Applied Catalysis A: General, 2017, 543: 75-81. |
| [73] | KAZUMASA Murata, NATSUKI Kurimoto, YUTA Yamamoto, et al. Structure-property relationships of Pt-Sn nanoparticles supported on Al2O3 for the dehydrogenation of methylcyclohexane[J]. ACS Applied Nano Materials, 2021, 4(5): 4532-4541. |
| [74] | MORI Kousuke, KANDA Yasuharu, UEMICHI Yoshio. Dehydrogenation of methylcyclohexane over zinc-containing platinum/alumina catalysts[J]. Journal of the Japan Petroleum Institute, 2018, 61(6): 350-356. |
| [75] | YUE Yufan, LIU Xiaohui, SHAKOURI Mohsen, et al. Active and stable Pt-Ga2O3/Al2O3 catalyst for dehydrogenation of methylcyclohexane[J]. Catalysis Today, 2024, 433: 114688. |
| [76] | ZHANG Xiaotong, HE Ning, LIN Long, et al. Study of the carbon cycle of a hydrogen supply system over a supported Pt catalyst: Methylcyclohexane-toluene-hydrogen cycle[J]. Catalysis Science & Technology, 2020, 10(4): 1171-1181. |
| [77] | NAKAYA Yuki, MIYAZAKI Masayoshi, YAMAZOE Seiji, et al. Active, selective, and durable catalyst for alkane dehydrogenation based on a well-designed trimetallic alloy[J]. ACS Catalysis, 2020, 10(9): 5163-5172. |
| [78] | ITO Hiroya, OSHIMA Kazumasa, YAMAMOTO Tsuyoshi, et al. Improved catalytic stability of Pt/TiO2 catalysts for methylcyclohexane dehydrogenation via selenium addition[J]. International Journal of Hydrogen Energy, 2022, 47(91): 38635-38643. |
| [79] | AL-SHAIKHALI Anaam H, JEDIDI Abdesslem, CAVALLO Luigi, et al. Non-precious bimetallic catalysts for selective dehydrogenation of an organic chemical hydride system[J]. Chemical Communications, 2015, 51(65): 12931-12934. |
| [80] | PATIL Shubhangi P, PANDE Jayshri V, BINIWALE Rajesh B. Non-noble Ni-Cu/ACC bimetallic catalyst for dehydrogenation of liquid organic hydrides for hydrogen storage[J]. International Journal of Hydrogen Energy, 2013, 38(35): 15233-15241. |
| [81] | Hyungwon HAM, SIMANULLANG Wiyanti F, KANDA Yasuharu, et al. Silica-decoration boosts Ni catalysis for (de)hydrogenation: Step-abundant nanostructures stabilized by silica[J]. ChemCatChem, 2021, 13(5): 1306-1310. |
| [82] | YOLCULAR S. Organic chemical hydride dehydrogenation over nickel catalysts supported with SiO2 for hydrogen recovery[J]. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 2016, 38(14): 2031-2034. |
| [83] | GULYAEVA Yuliya, BYKOVA Maria Alekseeva, BULAVCHENKO Olga, et al. Ni-Cu high-loaded sol-gel catalysts for dehydrogenation of liquid organic hydrides: Insights into structural features and relationship with catalytic activity[J]. Nanomaterials, 2021, 11(8): 2017. |
| [84] | GULYAEVA YULIYA K, ALEKSEEVA BYKOVA MARIA V, YU Ermakov Dmitry, et al. High-loaded nickel based sol-gel catalysts for methylcyclohexane dehydrogenation[J]. Catalysts, 2020, 10(10): 1198. |
| [85] | ALEKSEEVA (BYKOVA) Maria V, GULYAEVA Yuliya K, BULAVCHENKO Olga A, et al. Promoting effect of Zn in high-loading Zn/Ni-SiO2 catalysts for selective hydrogen evolution from methylcyclohexane[J]. Dalton Transactions, 2022, 51(15): 6068-6085. |
| [86] | KOSKIN Anton P, STEPANENKO Sergey A, ALEKSEEVA (BYKOVA) Maria V, et al. The origin of extraordinary selectivity in dehydrogenation of methylcyclohexane over Ni-Sn-based catalysts[J]. Chemical Engineering Journal, 2023, 476: 146629. |
| [87] | WANG Dongliang, LEI Qian, LI Hongwei, et al. Preparation of a novel NiAlO composite oxide catalyst for the dehydrogenation of methylcyclohexane[J]. Catalysts, 2022, 12(9): 958. |
| [88] | GAO Jiaojiao, LI Ning, ZHANG Dongqiang, et al. Ni x /TiO2 catalysts for enhancing the selectivity of methylcyclohexane dehydrogenation[J]. Molecular Catalysis, 2024, 560: 114148. |
| [89] | MENG Junchi, YUAN Xingzhou, YAN Yang, et al. Supported metal clusters: Ni x /CuZnAl catalysts effectively improve the performance of hydrogen evolution from methylcyclohexane dehydrogenation[J]. Journal of the Taiwan Institute of Chemical Engineers, 2023, 144: 104719. |
| [90] | BINIWALE Rajesh B, KARIYA N, YAMASHIRO H, et al. Heat transfer and thermographic analysis of catalyst surface during multiphase phenomena under spray-pulsed conditions for dehydrogenation of cyclohexane over Pt catalysts[J]. The Journal of Physical Chemistry B, 2006, 110(7): 3189-3196. |
| [91] | HIROTA Yuichiro, ISHIKADO Akinori, UCHIDA Yoshiaki, et al. Pore size control of microporous carbon membranes by post-synthesis activation and their use in a membrane reactor for dehydrogenation of methylcyclohexane[J]. Journal of Membrane Science, 2013, 440: 134-139. |
| [92] | ACHARYA Durga, Derrick NG, XIE Zongli. Recent advances in catalysts and membranes for MCH dehydrogenation: A mini review[J]. Membranes, 2021, 11(12): 955. |
| [93] | GAO Botao, GUO Shenghui, HOU Ming, et al. Study on a potential hydrogen storage system: Microwave-activated methylcyclohexane dehydrogenation[J]. International Journal of Hydrogen Energy, 2024, 79: 619-629. |
| [94] | WANG Wenhan, CUI Guoqing, YAN Cunji, et al. Boosting methylcyclohexane dehydrogenation over Pt-based structured catalysts by internal electric heating[J]. Nano Research, 2023, 16(10): 12215-12222. |
| [95] | KANG Dong Gyun, KWAK Yeonsu, MOON Seongeun, et al. Thermally manageable and scalable reactor configurations towards efficient hydrogen release from liquid organic hydrogen carriers[J]. Energy Conversion and Management, 2024, 307: 118345. |
| [96] | TAKISE Kent, SATO Ayaka, MURAKAMI Kota, et al. Irreversible catalytic methylcyclohexane dehydrogenation by surface protonics at low temperature[J]. RSC Advances, 2019, 9(11): 5918-5924. |
| [97] | Susana PÉREZ-GIL, Sergio SANTOS-MORENO, GARCÍA Cristina Diñeiro, et al. Process intensification in the continuous dehydrogenation of methylcyclohexane to toluene[J]. Chemical Engineering and Processing—Process Intensification, 2024, 203: 109904. |
| [98] | ARORA Deepali, RICHARDS Matt, ZHU Yutong, et al. A multipass catalytic reactor insert for continuous hydrogen generation from methylcyclohexane[J]. Chemical Engineering and Processing—Process Intensification, 2024, 201: 109822. |
| [99] | HAMAYUN Muhammad Haris, MAAFA Ibrahim M, HUSSAIN Murid, et al. Simulation study to investigate the effects of operational conditions on methylcyclohexane dehydrogenation for hydrogen production[J]. Energies, 2020, 13(1): 206. |
| [100] | HAMAYUN Muhammad Haris, HUSSAIN Murid, MAAFA Ibrahim M, et al. Integration of hydrogenation and dehydrogenation system for hydrogen storage and electricity generation-simulation study[J]. International Journal of Hydrogen Energy, 2019, 44(36): 20213-20222. |
| [101] | MAQBOOL Muhammad Anas, KHAN Javed, HAMAYUN Muhammad Haris, et al. Optimal retrofitting of MCH-toluene dehydrogenation system: Energy and technoeconomic analysis[J]. Energy Conversion and Management, 2023, 286: 117049. |
| [102] | FUKUNAGA Akihiko, KATO Asami, HARA Yuki, et al. Dehydrogenation of methylcyclohexane using solid oxide fuel cell—A smart energy conversion[J]. Applied Energy, 2023, 348: 121469. |
| [103] | LI Longquan, VELLAYANI ARAVIND Purushothaman, WOUDSTRA Theo, et al. Assessing the waste heat recovery potential of liquid organic hydrogen carrier chains[J]. Energy Conversion and Management, 2023, 276: 116555. |
| [104] | TSANG Fanlok, KARIMI Iftekhar A, FAROOQ Shamsuzzaman. Low-emissions hydrogen from MCH dehydrogenation: Integration with LNG regasification[J]. Energy Conversion and Management, 2024, 321: 119012. |
| [1] | 姚如伟, 宋乐音, 牛琴琴, 李聪明. Na-S双助剂修饰铁基催化剂催化CO2加氢制C2+醇[J]. 化工进展, 2025, 44(6): 3154-3162. |
| [2] | 陈少伟, 陈奕, 牛江奇, 刘天奇, 黄建国, 陈焕浩, 范晓雷. 介质阻挡放电等离子体催化反应器研究进展及应用展望[J]. 化工进展, 2025, 44(6): 3175-3189. |
| [3] | 石秀顶, 王永全, 曾静, 苏畅, 洪俊明. 纳米管状Co-N-C活化过碳酸盐降解四环素[J]. 化工进展, 2025, 44(6): 3041-3052. |
| [4] | 谢武强, 张岭, 贺杠, 蒋里锋, 郑晰瑞, 张和鹏. CoTBrPP-PTAB-Cu电催化还原CO2制甲烷[J]. 化工进展, 2025, 44(6): 3093-3100. |
| [5] | 范晓娅, 赵镇, 彭强. 电催化二氧化碳和硝酸根共还原合成尿素研究进展[J]. 化工进展, 2025, 44(5): 2856-2869. |
| [6] | 苏俊杰, 刘苏, 周海波, 刘畅, 张琳, 王仰东, 谢在库. 用于CO2加氢直接制低碳烯烃的InZr/SAPO-34双功能催化剂[J]. 化工进展, 2025, 44(5): 2870-2878. |
| [7] | 汪柯, 胡登, 王星博, 孙楠楠, 魏伟. Fe x Co y Ca3Al双功能材料用于CO2捕集-转化一体化制合成气[J]. 化工进展, 2025, 44(5): 2888-2897. |
| [8] | 马梓轩, 施瑞晨, 刘明杰, 杨莹杰, 宋子瑜, 梅晓鹏, 高晓峰, 洪龙城, 姚思宇, 张治国, 任其龙. 环烷烃催化制氢反应器的设计与性能优化: 前沿进展与挑战[J]. 化工进展, 2025, 44(5): 2919-2937. |
| [9] | 于安峰, 吴倩, 杨哲, 罗云, 王宇辰, 刘欢. 绿氢储输过程安全及材料失效机理研究进展[J]. 化工进展, 2025, 44(5): 2972-2983. |
| [10] | 鲍婕, 余攀结, 马永德, 张宏伟, 蔡镇平, 曹彦宁, 黄宽, 江莉龙. Cu-ZrO2催化材料的制备及其棕榈酸甲酯加氢制脂肪醇性能[J]. 化工进展, 2025, 44(5): 2997-3008. |
| [11] | 朱慧红, 刘璐, 刘鹏, 李贺, 杨涛, 王继锋, 侯栓弟, 彭冲, 赵毅毅, 潘云翔. 劣质渣油加氢催化剂构筑及其催化性能提升机制[J]. 化工进展, 2025, 44(5): 3009-3016. |
| [12] | 谢静雯, 孟仪方, 叶文杰, 王华磊, 魏东芝. 半理性设计提高短链醇脱氢酶在(S)-1-(4-氟苯基)乙醇合成中的应用[J]. 化工进展, 2025, 44(5): 2515-2523. |
| [13] | 王水众, 宋国勇. 木质素选择性氢解制备高功能化单酚及其高值利用[J]. 化工进展, 2025, 44(5): 2535-2540. |
| [14] | 丁阿静, 周巧巧, 顾学红. 膜反应器中杨木催化气化制清洁合成气[J]. 化工进展, 2025, 44(5): 2716-2723. |
| [15] | 何志勇. 分步脱羟/脱碳催化剂实现高效裂解甲醇制氢[J]. 化工进展, 2025, 44(5): 2724-2732. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||