化工进展 ›› 2025, Vol. 44 ›› Issue (6): 3154-3162.DOI: 10.16085/j.issn.1000-6613.2024-1984
• 专栏:化工生态环境前沿交叉新技术 • 上一篇
Na-S双助剂修饰铁基催化剂催化CO2加氢制C2+醇
姚如伟1,2( ), 宋乐音1,2, 牛琴琴1,2, 李聪明1,2(
), 宋乐音1,2, 牛琴琴1,2, 李聪明1,2( )
)
- 1.太原理工大学省部共建煤基能源清洁高效利用国家重点实验室,山西 太原 030024
2.太原理工大学化学与化工学院,山西 太原 030024
-
收稿日期:2024-12-05修回日期:2025-01-16出版日期:2025-06-25发布日期:2025-07-08 -
通讯作者:姚如伟,李聪明 -
作者简介:姚如伟(1993—),女,讲师,硕士生导师。研究方向为二氧化碳催化利用。E-mail:yaoruwei@tyut.edu.cn。 -
基金资助:国家自然科学基金(22202150);山西省基础研究计划(202203021212232)
Na-S co-modified iron catalysts for CO2 hydrogenation to C2+ alcohols
YAO Ruwei1,2( ), SONG Yueyin1,2, NIU Qinqin1,2, LI Congming1,2(
), SONG Yueyin1,2, NIU Qinqin1,2, LI Congming1,2( )
)
- 1.State Key Laboratory of Clean and Efficient Coal Utilization, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
2.College of Chemical Engineering and Technology, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
-
Received:2024-12-05Revised:2025-01-16Online:2025-06-25Published:2025-07-08 -
Contact:YAO Ruwei, LI Congming
摘要:
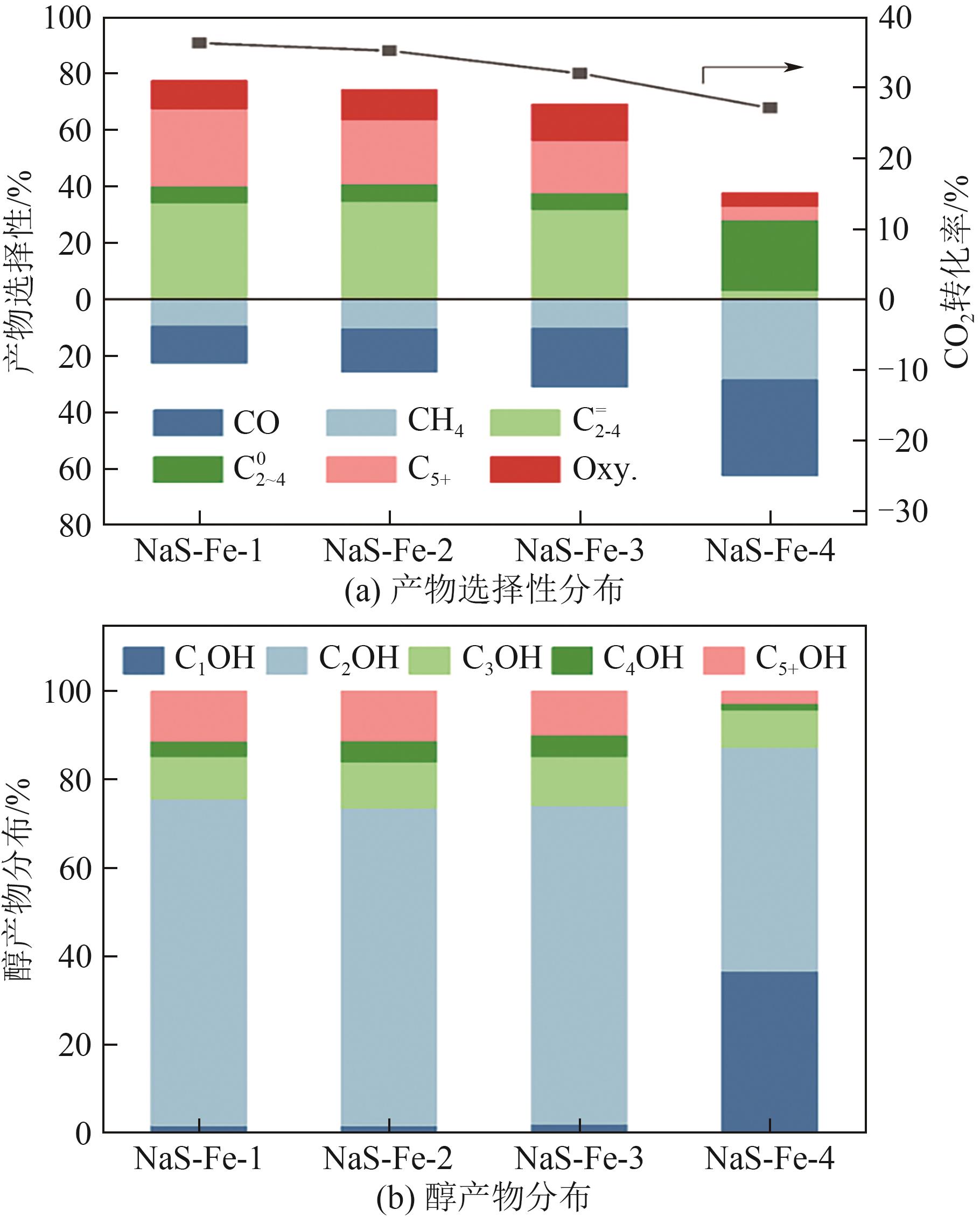
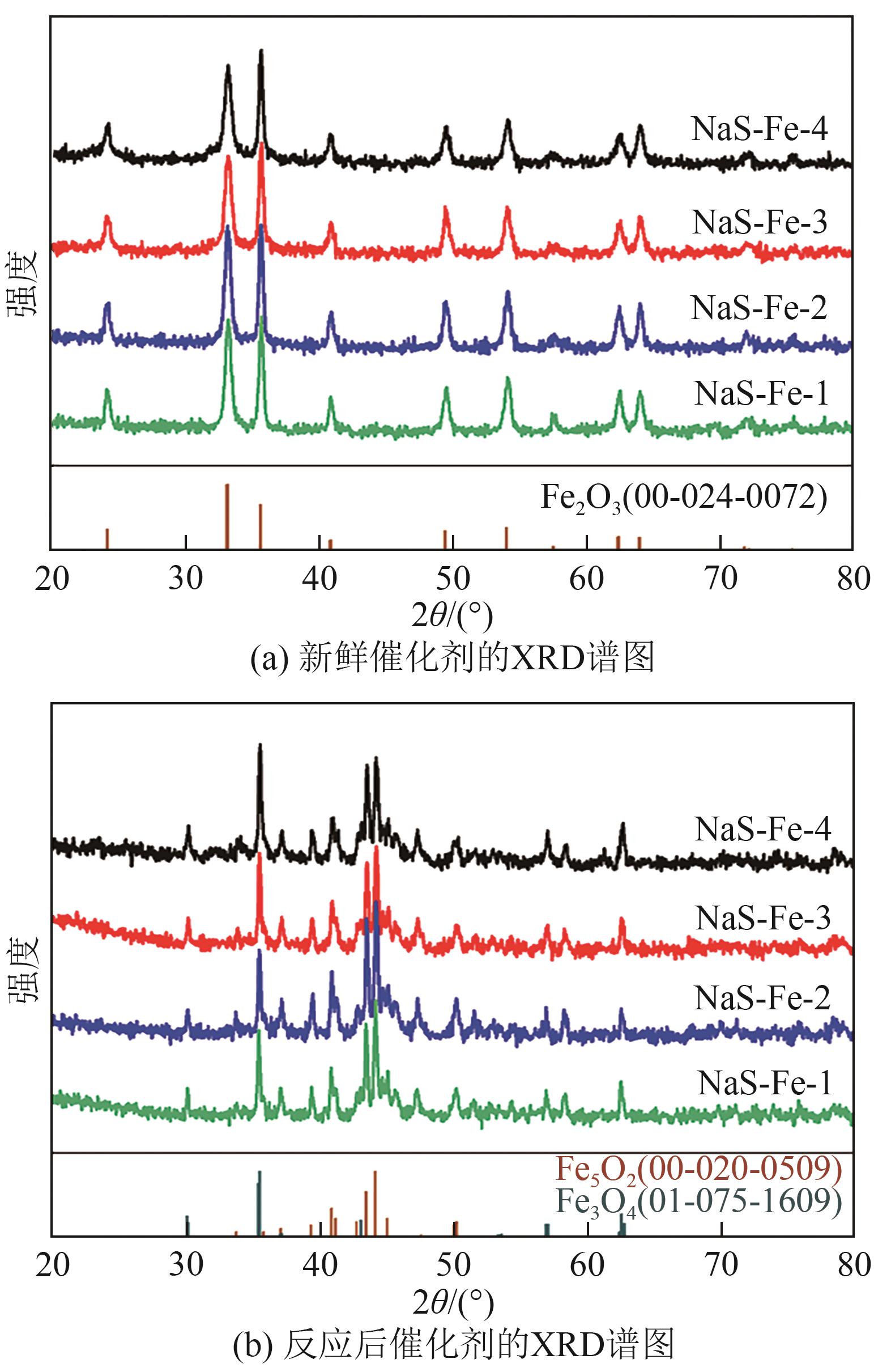
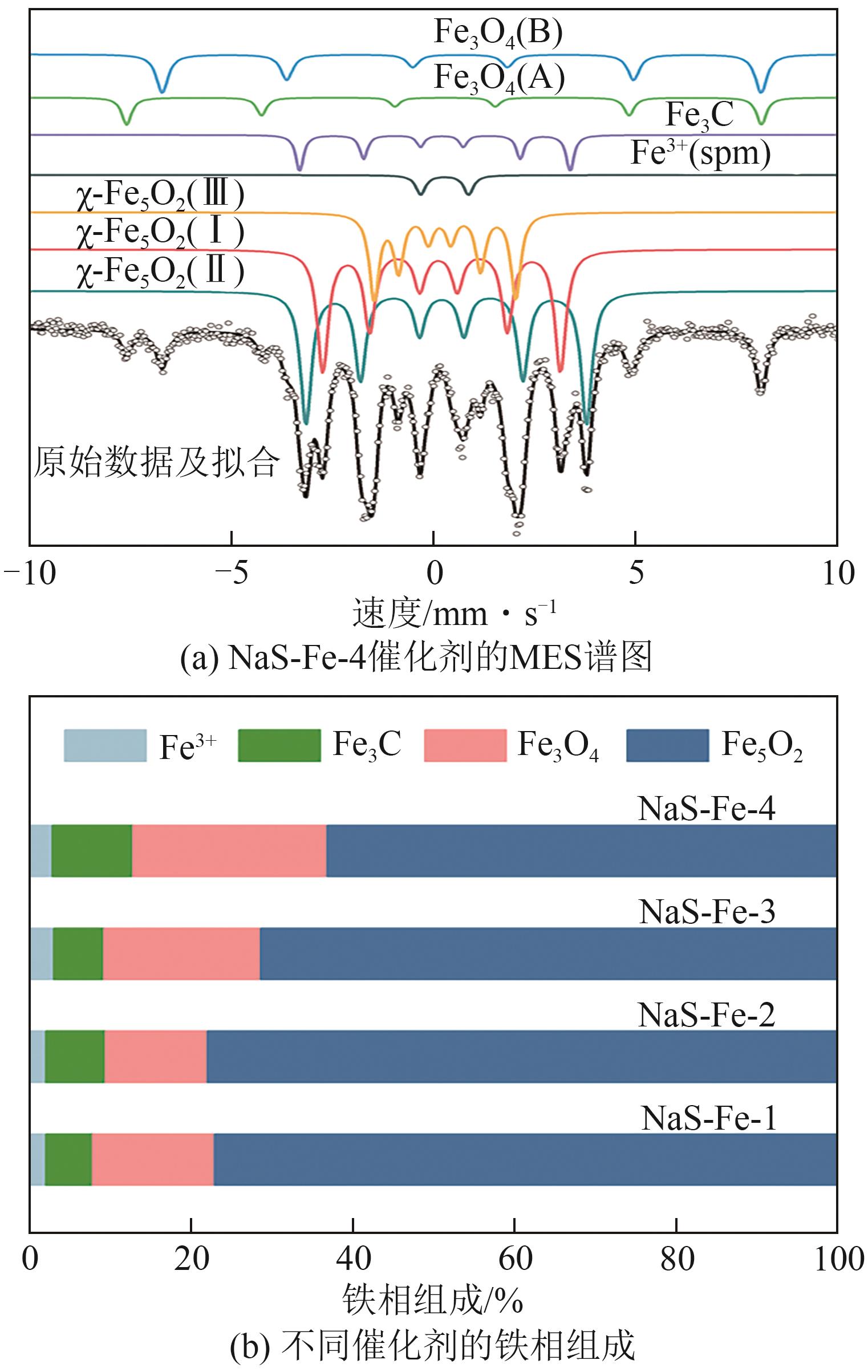
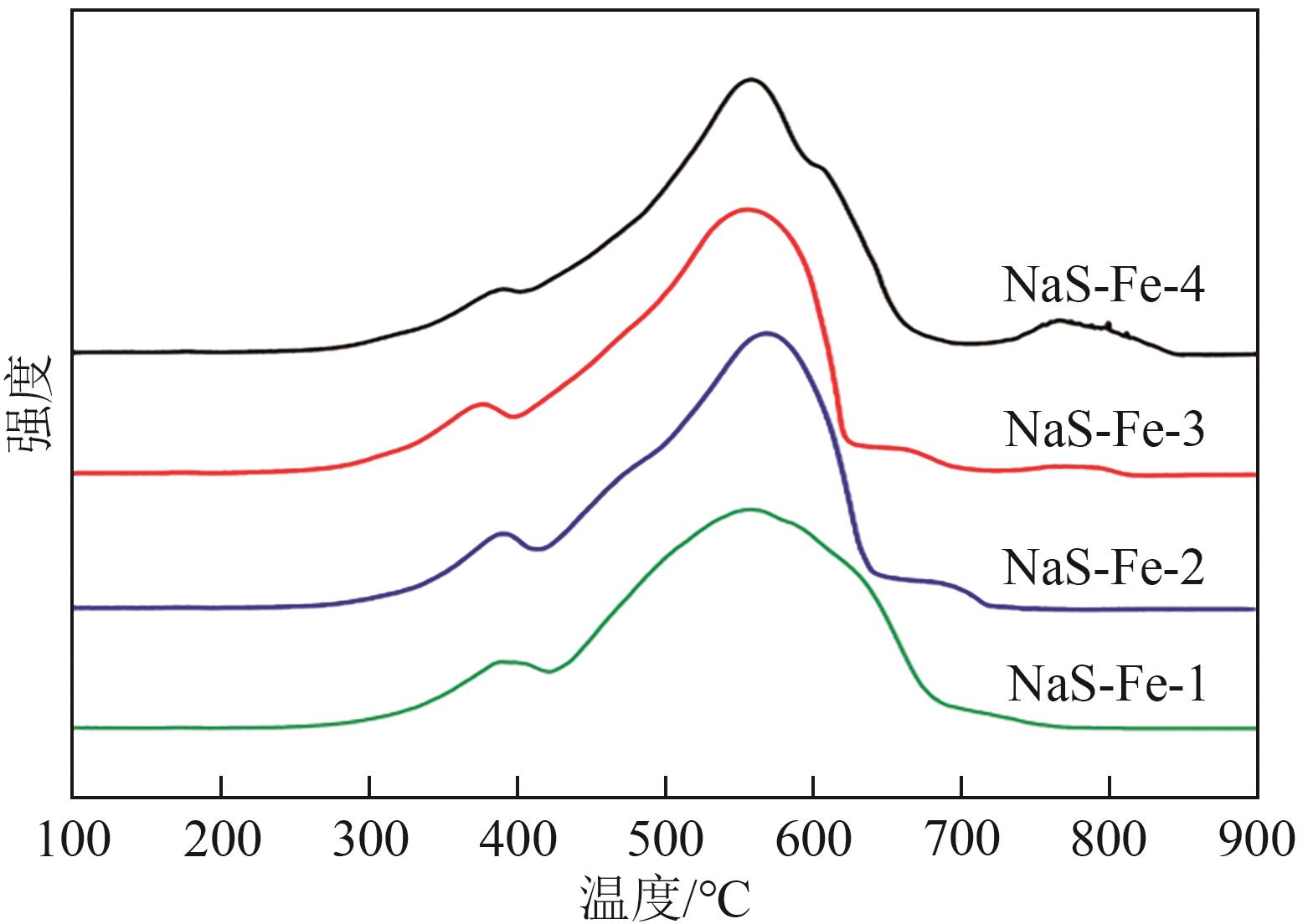
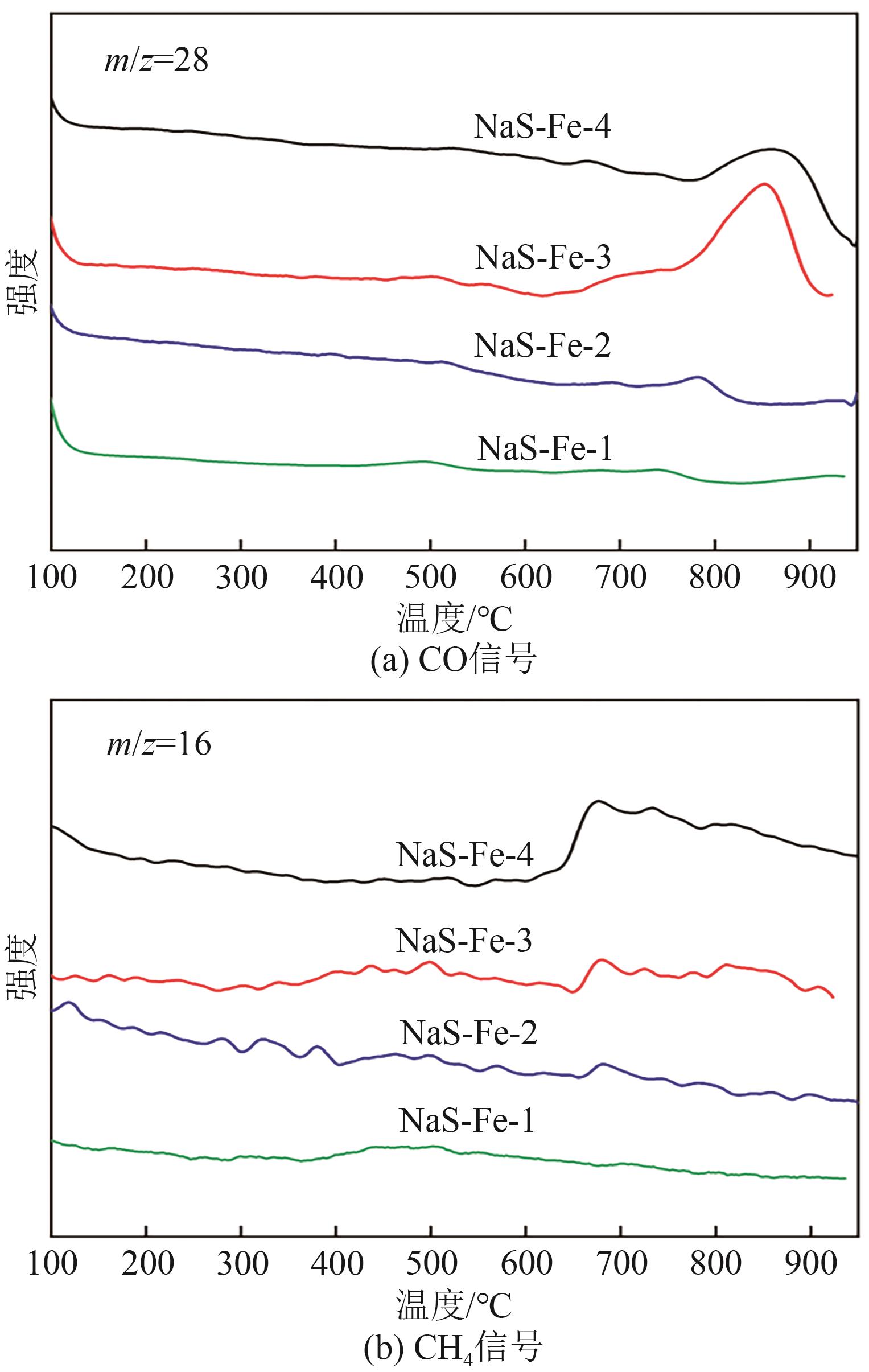
利用CO2加氢合成燃料或高值化学品是控制碳排放、实现能源可持续发展的有效途径。采用沉淀法制备了Na和S共修饰的铁基催化剂,并探究了助剂含量对催化性质及CO2加氢合成C2+醇反应性能的影响。结果表明,Na-S双助剂可通过电子效应调变活性相的组成及其铁位点的电子性质,从而改变催化剂对反应气及CO中间体的活化能力,最终影响反应活性与产物分布。适量的Na和S改性有助于平衡铁基催化剂上CO解离与非解离吸附的能力,促进烷基物种与活性*CO的偶联,进而促进C2+醇的合成。NaS-Fe-3催化剂可在32%的CO2转化率下实现12.8%的C2+醇选择性,改变了传统对于单铁催化剂难以有效催化C2+醇合成的认识。这种通过双助剂改性调控催化性质的策略,为催化剂设计与反应路径调控提供了新的思路。
中图分类号:
引用本文
姚如伟, 宋乐音, 牛琴琴, 李聪明. Na-S双助剂修饰铁基催化剂催化CO2加氢制C2+醇[J]. 化工进展, 2025, 44(6): 3154-3162.
YAO Ruwei, SONG Yueyin, NIU Qinqin, LI Congming. Na-S co-modified iron catalysts for CO2 hydrogenation to C2+ alcohols[J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3154-3162.
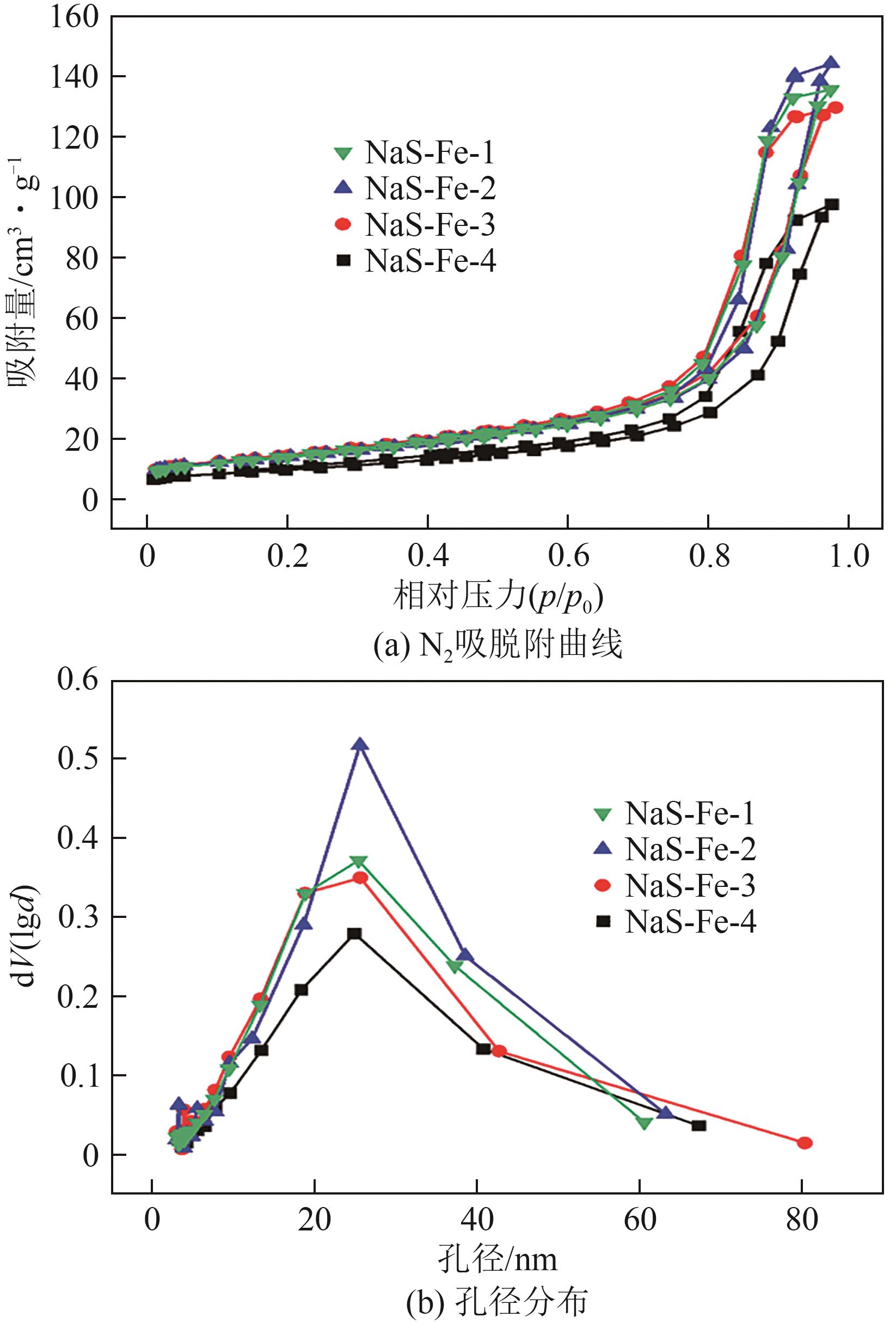
| 催化剂 | Na质量分数/% | S质量分数/% | 比表面积/m2·g-1 | 总孔容/cm3·g-1 | 微孔孔容/cm3·g-1 |
|---|---|---|---|---|---|
| NaS-Fe-1 | 1.0 | 0.04 | 49.7 | 0.21 | 0 |
| NaS-Fe-2 | 1.4 | 0.1 | 50.3 | 0.22 | 0 |
| NaS-Fe-3 | 2.4 | 0.6 | 51.8 | 0.20 | 0 |
| NaS-Fe-4 | 6.0 | 3.0 | 35.0 | 0.15 | 0 |
表1 不同催化剂的物化性质参数
| 催化剂 | Na质量分数/% | S质量分数/% | 比表面积/m2·g-1 | 总孔容/cm3·g-1 | 微孔孔容/cm3·g-1 |
|---|---|---|---|---|---|
| NaS-Fe-1 | 1.0 | 0.04 | 49.7 | 0.21 | 0 |
| NaS-Fe-2 | 1.4 | 0.1 | 50.3 | 0.22 | 0 |
| NaS-Fe-3 | 2.4 | 0.6 | 51.8 | 0.20 | 0 |
| NaS-Fe-4 | 6.0 | 3.0 | 35.0 | 0.15 | 0 |
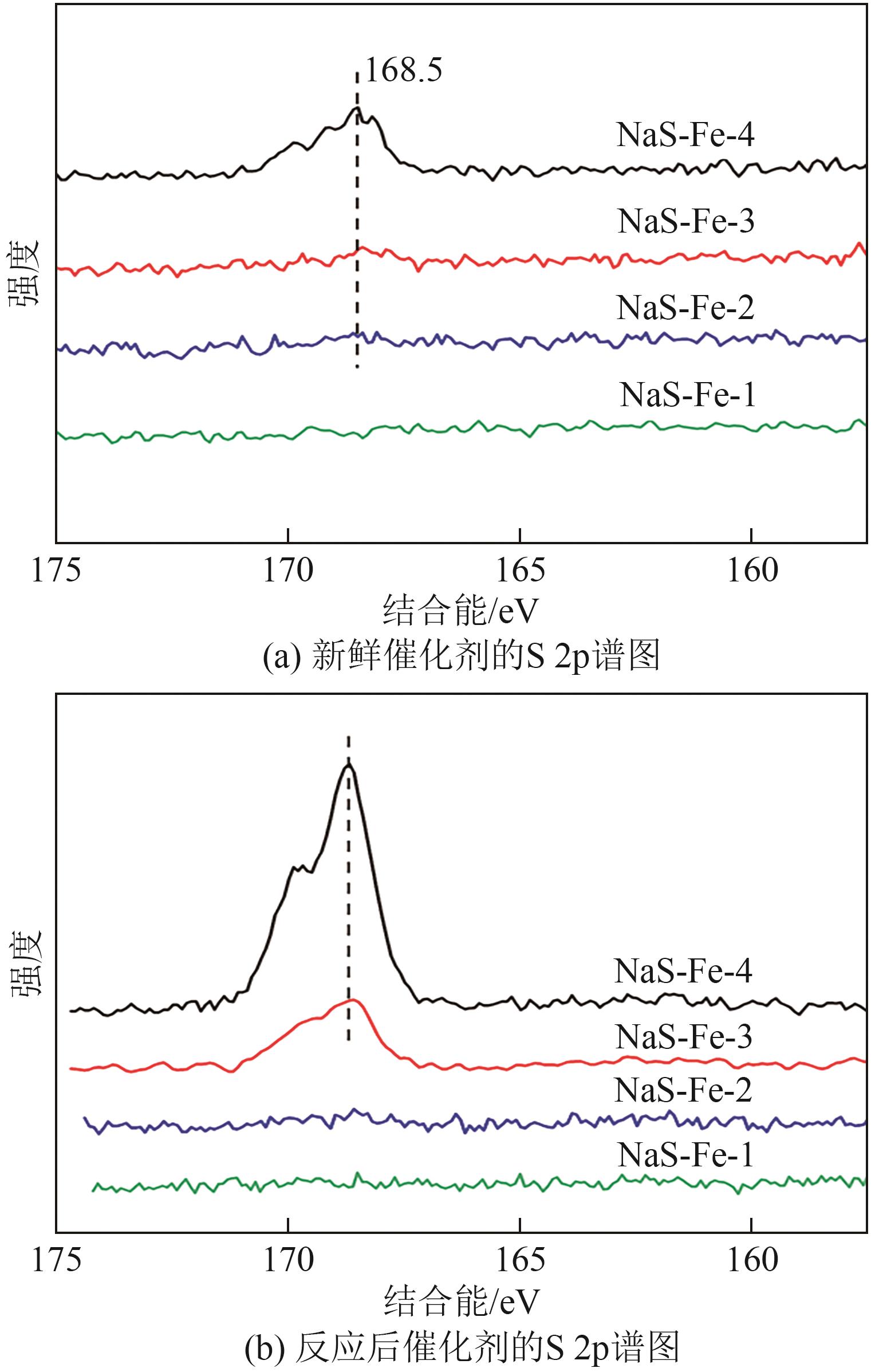
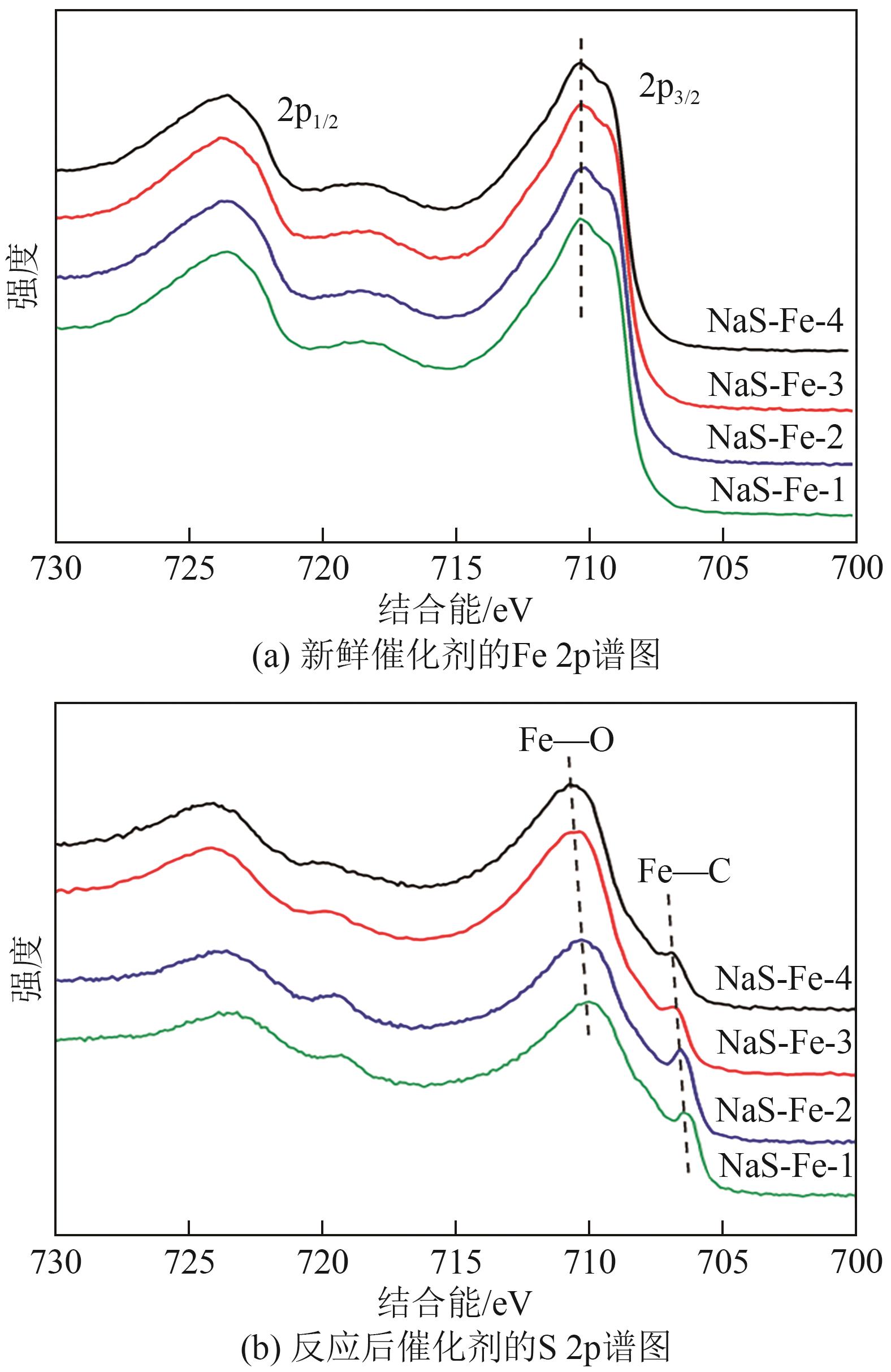
| 催化剂 | 新鲜催化剂中表面元素摩尔比/% | 反应后催化剂中表面元素摩尔比/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fe | S | Na | O | C | Fe | S | Na | O | C | |
| NaS-Fe-1 | 29.45 | 0.41 | 6.59 | 51.85 | 11.70 | 11.85 | 0.45 | 14.61 | 40.54 | 32.55 |
| NaS-Fe-2 | 28.91 | 0.49 | 6.76 | 51.58 | 12.26 | 11.61 | 0.51 | 14.93 | 41.03 | 31.92 |
| NaS-Fe-3 | 29.55 | 0.44 | 5.92 | 51.86 | 12.23 | 14.49 | 1.05 | 13.37 | 45.13 | 25.96 |
| NaS-Fe-4 | 28.73 | 0.67 | 5.14 | 51.85 | 13.61 | 12.61 | 2.76 | 14.19 | 45.25 | 25.19 |
表2 XPS表面元素分析结果
| 催化剂 | 新鲜催化剂中表面元素摩尔比/% | 反应后催化剂中表面元素摩尔比/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fe | S | Na | O | C | Fe | S | Na | O | C | |
| NaS-Fe-1 | 29.45 | 0.41 | 6.59 | 51.85 | 11.70 | 11.85 | 0.45 | 14.61 | 40.54 | 32.55 |
| NaS-Fe-2 | 28.91 | 0.49 | 6.76 | 51.58 | 12.26 | 11.61 | 0.51 | 14.93 | 41.03 | 31.92 |
| NaS-Fe-3 | 29.55 | 0.44 | 5.92 | 51.86 | 12.23 | 14.49 | 1.05 | 13.37 | 45.13 | 25.96 |
| NaS-Fe-4 | 28.73 | 0.67 | 5.14 | 51.85 | 13.61 | 12.61 | 2.76 | 14.19 | 45.25 | 25.19 |
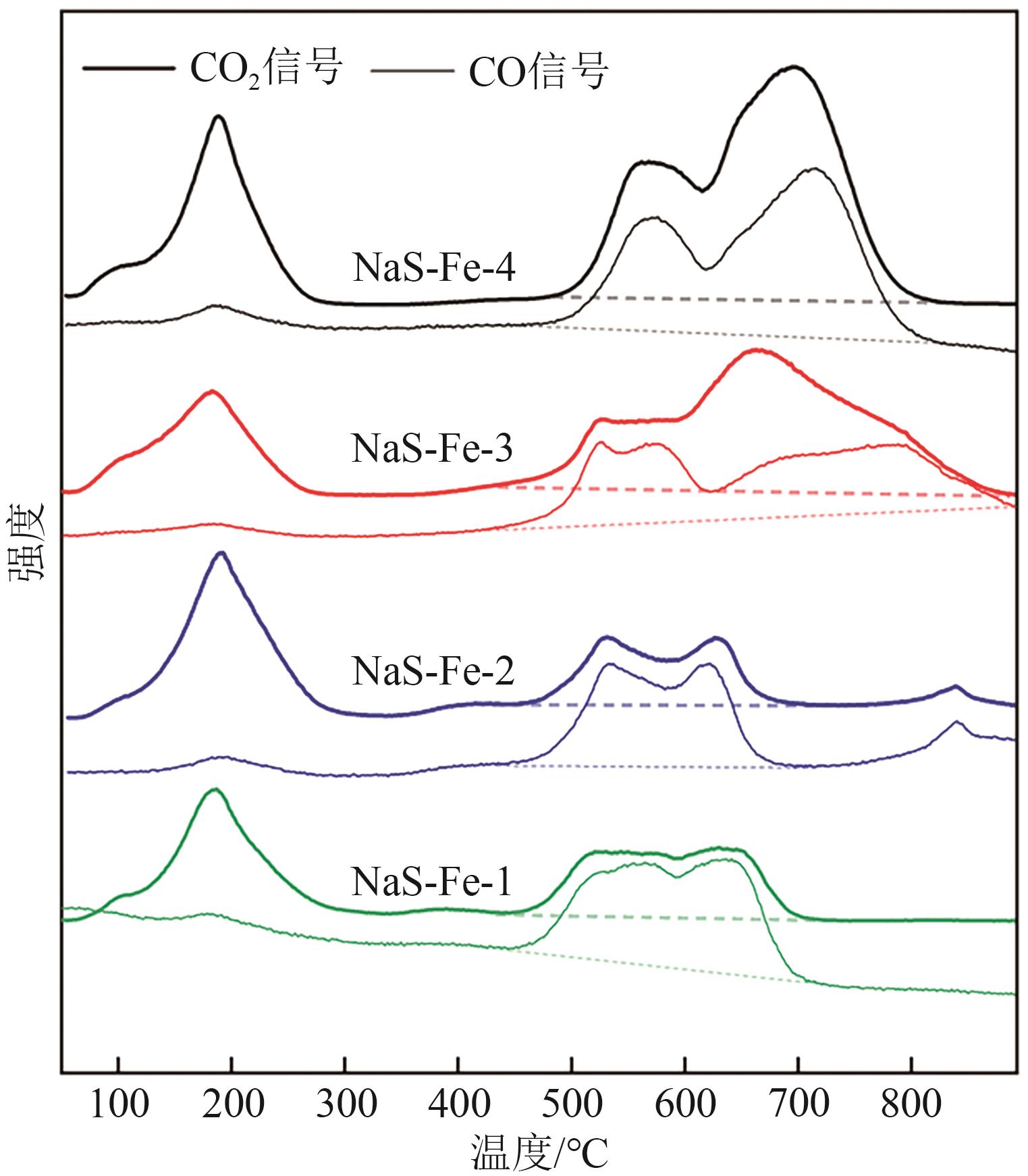
| 催化剂 | CO2峰面积 | CO峰面积 | 峰面积比CO/CO2 |
|---|---|---|---|
| NaS-Fe-1 | 39.6 | 62.0 | 1.57 |
| NaS-Fe-2 | 33.0 | 45.6 | 1.38 |
| NaS-Fe-3 | 100 | 72.8 | 0.73 |
| NaS-Fe-4 | 132.9 | 94.6 | 0.71 |
表3 CO2-TPD谱图中高温脱附区量化结果
| 催化剂 | CO2峰面积 | CO峰面积 | 峰面积比CO/CO2 |
|---|---|---|---|
| NaS-Fe-1 | 39.6 | 62.0 | 1.57 |
| NaS-Fe-2 | 33.0 | 45.6 | 1.38 |
| NaS-Fe-3 | 100 | 72.8 | 0.73 |
| NaS-Fe-4 | 132.9 | 94.6 | 0.71 |
| [1] | 赵锦波, 卞凤鸣. CO2化学转化基础与应用研究进展[J]. 化工进展, 2022, 41(S1): 524-535. |
| ZHAO Jinbo, BIAN Fengming. Progress on basis and application of CO2 chemical conversion technologies[J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 524-535. | |
| [2] | 闫帅, 杨海平, 陈应泉, 等. CO2光热催化还原研究进展[J]. 化工学报, 2022, 73(10): 4298-4310. |
| YAN Shuai, YANG Haiping, CHEN Yingquan, et al. Recent advances in photothermal catalysis of CO2 reduction[J]. CIESC Journal, 2022, 73(10): 4298-4310. | |
| [3] | WANG Menglin, LUO Lei, WANG Chuanhao, et al. Heterogeneous catalysts toward CO2 hydrogenation for sustainable carbon cycle[J]. Accounts of Materials Research, 2022, 3(6): 565-571. |
| [4] | REN Yingyu, YANG Yusen, WEI Min. Recent advances on heterogeneous non-noble metal catalysts toward selective hydrogenation reactions[J]. ACS Catalysis, 2023, 13(13): 8902-8924. |
| [5] | GAO Jiajian, SHIONG Choo Sze Simon, LIU Yan. Reduction of CO2 to chemicals and fuels: Thermocatalysis versus electrocatalysis[J]. Chemical Engineering Journal, 2023, 472: 145033. |
| [6] | 曾壮, 李柯志, 苑志伟, 等. CO/CO2加氢制低碳醇改性费托合成催化剂研究进展[J]. 化工进展, 2024, 43(6): 3061-3079. |
| ZENG Zhuang, LI Kezhi, YUAN Zhiwei, et al. Advances in modified Fischer-Tropsch synthesis catalysts for CO/CO2 hydrogenation to higher alcohols[J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3061-3079. | |
| [7] | Chi Hung VO, Javier PÉREZ-RAMÍREZ, FAROOQ Shamsuzzaman, et al. Prospects of producing higher alcohols from carbon dioxide: A process system engineering perspective[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(36): 11875-11884. |
| [8] | 孔祥宇, 谢亮, 王延民, 等. CO2的捕集及资源化利用[J]. 化工进展, 2022, 41(3): 1187-1198. |
| KONG Xiangyu, XIE Liang, WANG Yanmin, et al. CO2 capture and resource utilization[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1187-1198. | |
| [9] | SHENG Yao, POLYNSKI Mikhail V, ESWARAN Mathan K, et al. A review of mechanistic insights into CO2 reduction to higher alcohols for rational catalyst design[J]. Applied Catalysis B: Environmental, 2024, 343: 123550. |
| [10] | CHEN Gaofeng, SYZGANTSEVA Olga A, SYZGANTSEVA Maria A, et al. Hydrophobic dual metal silicate nanotubes for higher alcohol synthesis[J]. Applied Catalysis B: Environmental, 2023, 334: 122840. |
| [11] | DU Pengfei, FAKIR Abdellah Ait EL, ZHAO Shirun, et al. Ethanol synthesis via catalytic CO2 hydrogenation over multi-elemental KFeCuZn/ZrO2 catalyst[J]. Chemical Science, 2024, 15(38): 15925-15934. |
| [12] | ZHANG Qian, WANG Sen, SHI Xuerong, et al. Conversion of CO2 to higher alcohols on K-CuZnAl/Zr-CuFe composite[J]. Applied Catalysis B: Environment and Energy, 2024, 346: 123748. |
| [13] | YANG Haiyan, DANG Yaru, CUI Xu, et al. Selective synthesis of olefins via CO2 hydrogenation over transition-metal-doped iron-based catalysts[J]. Applied Catalysis B: Environmental, 2023, 321: 122050. |
| [14] | WANG Yanqiu, ZHOU Ying, ZHANG Xinxin, et al. PdFe alloy-Fe5C2 interfaces for efficient CO2 hydrogenation to higher alcohols[J]. Applied Catalysis B: Environment and Energy, 2024, 345: 123691. |
| [15] | LIU Tangkang, XU Di, SONG Mengyang, et al. K-ZrO2 interfaces boost CO2 hydrogenation to higher alcohols[J]. ACS Catalysis, 2023, 13(7): 4667-4674. |
| [16] | ZHANG Qian, WANG Sen, GENG Rui, et al. Hydrogenation of CO2 to higher alcohols on an efficient Cr-modified CuFe catalyst[J]. Applied Catalysis B: Environmental, 2023, 337: 123013. |
| [17] | 胡文德, 王仰东, 王传明. 合成气直接催化转化制低碳烯烃研究进展[J]. 化工进展, 2022, 41(9): 4754-4766. |
| HU Wende, WANG Yangdong, WANG Chuanming. Research progress on the direct catalytic conversion of syngas to light olefins[J]. Chemical Industry and Engineering Progress, 2022, 41(9): 4754-4766. | |
| [18] | YANG Qingxin, KONDRATENKO Evgenii V. From understanding of catalyst functioning toward controlling selectivity in CO2 hydrogenation to higher hydrocarbons over Fe-based catalysts[J]. Accounts of Materials Research, 2024, 5(11): 1314-1328. |
| [19] | YAO Ruwei, WEI Jian, GE Qingjie, et al. Monometallic iron catalysts with synergistic Na and S for higher alcohols synthesis via CO2 hydrogenation[J]. Applied Catalysis B: Environmental, 2021, 298: 120556. |
| [20] | WEI Jian, GE Qingjie, YAO Ruwei, et al. Directly converting CO2 into a gasoline fuel[J]. Nature Communications, 2017, 8: 15174. |
| [21] | LI Siwei, YANG Jinghe, SONG Chuqiao, et al. Iron carbides: Control synthesis and catalytic applications in CO x hydrogenation and electrochemical HER[J]. Advanced Materials, 2019, 31(50): 1901796. |
| [22] | CHOI Yo Han, JANG Youn Jeong, PARK Hunmin, et al. Carbon dioxide Fischer-Tropsch synthesis: A new path to carbon-neutral fuels[J]. Applied Catalysis B: Environmental, 2017, 202: 605-610. |
| [23] | SUN Yanan, WU Yimin, SHAN Honghong, et al. Studies on the promoting effect of sulfate species in catalytic dehydrogenation of propane over Fe2O3/Al2O3 catalysts[J]. Catalysis Science & Technology, 2015, 5(2): 1290-1298. |
| [24] | MCCUE Alan J, ANDERSON James A. Sulfur as a catalyst promoter or selectivity modifier in heterogeneous catalysis[J]. Catalysis Science & Technology, 2014, 4(2): 272-294. |
| [25] | PAALANEN Pasi P, WECKHUYSEN Bert M. Carbon pathways, sodium-sulphur promotion and identification of iron carbides in iron-based Fischer-Tropsch synthesis[J]. ChemCatChem, 2020, 12(17): 4202-4223. |
| [26] | ZHU Jie, WANG Peng, ZHANG Xiaoben, et al. Dynamic structural evolution of iron catalysts involving competitive oxidation and carburization during CO2 hydrogenation[J]. Science Advances, 2022, 8(5): eabm3629. |
| [27] | AHMED Sheraz, IRSHAD Muhammad, YOON Wonjoong, et al. Evaluation of MgO as a promoter for the hydrogenation of CO2 to long-chain hydrocarbons over Fe-based catalysts[J]. Applied Catalysis B: Environmental, 2023, 338: 123052. |
| [28] | QIN Chuan, DU Yixiong, WU Ke, et al. Facet-Controlled Cu-doped and K-promoted Fe2O3 nanosheets for efficient CO2 hydrogenation to liquid hydrocarbons[J]. Chemical Engineering Journal, 2023, 467: 143403. |
| [29] | WEI Jian, SUN Jian, WEN Zhiyong, et al. New insights into the effect of sodium on Fe3O4-based nanocatalysts for CO2 hydrogenation to light olefins[J]. Catalysis Science & Technology, 2016, 6(13): 4786-4793. |
| [30] | NAJARI Sara, SAEIDI Samrand, András SÁPI, et al. Synergistic enhancement of CO2 hydrogenation to C5+ hydrocarbons using mixed Fe5C2 and Na-Fe3O4 catalysts: Effects of oxide/carbide ratio, proximity, and reduction[J]. Chemical Engineering Journal, 2024, 485: 149787. |
| [31] | LI Tingzhen, YANG Yong, TAO Zhichao, et al. Effect of sulfate on an iron manganese catalyst for Fischer-Tropsch synthesis[J]. Journal of Natural Gas Chemistry, 2007, 16(4): 354-362. |
| [32] | XU Jingdong, CHANG Zeying, ZHU Kongtao, et al. Effect of sulfur on α-Al2O3-supported iron catalyst for Fischer-Tropsch synthesis[J]. Applied Catalysis A: General, 2016, 514: 103-113. |
| [33] | GAO Jie, JIANG Qian, LIU Yuefeng, et al. Probing the enhanced catalytic activity of carbon nanotube supported Ni-LaO x hybrids for the CO2 reduction reaction[J]. Nanoscale, 2018, 10(29): 14207-14219. |
| [34] | YANG Qingxin, KONDRATENKO Vita A, PETROV Sergey A, et al. Identifying performance descriptors in CO2 hydrogenation over iron-based catalysts promoted with alkali metals[J]. Angewandte Chemie International Edition, 2022, 61(22): e202116517. |
| [35] | QI Xingzhen, LIN Tiejun, AN Yunlei, et al. Regulating oxygen vacancies for enhanced higher oxygenate synthesis via syngas[J]. ACS Catalysis, 2023, 13(17): 11566-11579. |
| [36] | IRSHAD Muhammad, CHUN Hee-Joon, KHAN Muhammad Kashif, et al. Synthesis of n-butanol-rich C3+ alcohols by direct CO2 hydrogenation over a stable Cu-Co tandem catalyst[J]. Applied Catalysis B: Environmental, 2024, 340: 123201. |
| [37] | WANG Yang, WANG Wenhang, HE Ruosong, et al. Carbon-based electron buffer layer on ZnO x -Fe5C2-Fe3O4 boosts ethanol synthesis from CO2 hydrogenation[J]. Angewandte Chemie International Edition, 2023, 62(46): e202311786. |
| [38] | JIANG Feng, ZHANG Min, LIU Bing, et al. Insights into the influence of support and potassium or sulfur promoter on iron-based Fischer-Tropsch synthesis: Understanding the control of catalytic activity, selectivity to lower olefins, and catalyst deactivation[J]. Catalysis Science & Technology, 2017, 7(5): 1245-1265. |
| [39] | MILLER Douglass G, MOSKOVITS Martin. A study of the effects of potassium addition to supported iron catalysts in the Fischer-Tropsch reaction[J]. The Journal of Physical Chemistry, 1988, 92(21): 6081-6085. |
| [40] | XU Di, DING Mingyue, HONG Xinlin, et al. Selective C2+ alcohol synthesis from direct CO2 hydrogenation over a Cs-promoted Cu-Fe-Zn catalyst[J]. ACS Catalysis, 2020, 10(9): 5250-5260. |
| [41] | 陈永杰, 邢小芳, 王阳, 等. Fe基CO2加氢制C2+醇催化剂研究进展[J]. 燃料化学学报(中英文), 2024, 52(11): 1580-1593. |
| CHEN Yongjie, XING Xiaofang, WANG Yang, et al. Advances in Fe-based catalysts for the hydrogenation of CO2 to C2+ alcohols[J]. Journal of Fuel Chemistry and Technology, 2024, 52(11): 1580-1593. |
| [1] | 石秀顶, 王永全, 曾静, 苏畅, 洪俊明. 纳米管状Co-N-C活化过碳酸盐降解四环素[J]. 化工进展, 2025, 44(6): 3041-3052. |
| [2] | 谢武强, 张岭, 贺杠, 蒋里锋, 郑晰瑞, 张和鹏. CoTBrPP-PTAB-Cu电催化还原CO2制甲烷[J]. 化工进展, 2025, 44(6): 3093-3100. |
| [3] | 吴孟勤, 王佳瑶, 徐友强, 王钰. 化学-生物级联转化CO2合成单细胞蛋白研究进展[J]. 化工进展, 2025, 44(5): 2429-2440. |
| [4] | 王媛媛, 张翀, 韩双艳, 邢新会. 毕赤酵母利用甲醇生产重组蛋白技术的研究进展[J]. 化工进展, 2025, 44(5): 2441-2450. |
| [5] | 姚陆, 马增新, 张聪, 杨松, 邢新会. 甲基杆菌有效同化工业甲醇和植物甲醇的研究进展[J]. 化工进展, 2025, 44(5): 2451-2462. |
| [6] | 盛华康, 张博, 申晓林, 孙新晓, 王佳, 袁其朋. 微生物合成白藜芦醇及其衍生物[J]. 化工进展, 2025, 44(5): 2463-2474. |
| [7] | 谢静雯, 孟仪方, 叶文杰, 王华磊, 魏东芝. 半理性设计提高短链醇脱氢酶在(S)-1-(4-氟苯基)乙醇合成中的应用[J]. 化工进展, 2025, 44(5): 2515-2523. |
| [8] | 钟家伟, 谭涛, 谢君, 陈勇. 生物质高值能源转换技术[J]. 化工进展, 2025, 44(5): 2524-2528. |
| [9] | 聂红, 习远兵, 葛泮珠, 丁石, 张登前. 可持续航空燃料生产路线与展望——以中石化石科院为例[J]. 化工进展, 2025, 44(5): 2529-2534. |
| [10] | 陈彦君, 戴杰, 单军强, 张思欣, 计磊, 朱晨杰, 应汉杰. 我国纤维素乙醇的研究进展和发展趋势[J]. 化工进展, 2025, 44(5): 2541-2562. |
| [11] | 魏志强, 孙丽丽. 工业富碳气体发酵制备燃料乙醇技术现状与挑战[J]. 化工进展, 2025, 44(5): 2563-2576. |
| [12] | 王嘉, 孙丹卉, 乔一凡, 范秀方, 赵立东, 贺雷, 陆安慧. 乙醇催化转化制高值化学品研究进展[J]. 化工进展, 2025, 44(5): 2587-2597. |
| [13] | 朱俊英, 荣峻峰, 宗保宁. 螺旋藻固碳和作为饲用蛋白原料可行性分析[J]. 化工进展, 2025, 44(5): 2705-2715. |
| [14] | 丁阿静, 周巧巧, 顾学红. 膜反应器中杨木催化气化制清洁合成气[J]. 化工进展, 2025, 44(5): 2716-2723. |
| [15] | 何志勇. 分步脱羟/脱碳催化剂实现高效裂解甲醇制氢[J]. 化工进展, 2025, 44(5): 2724-2732. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||