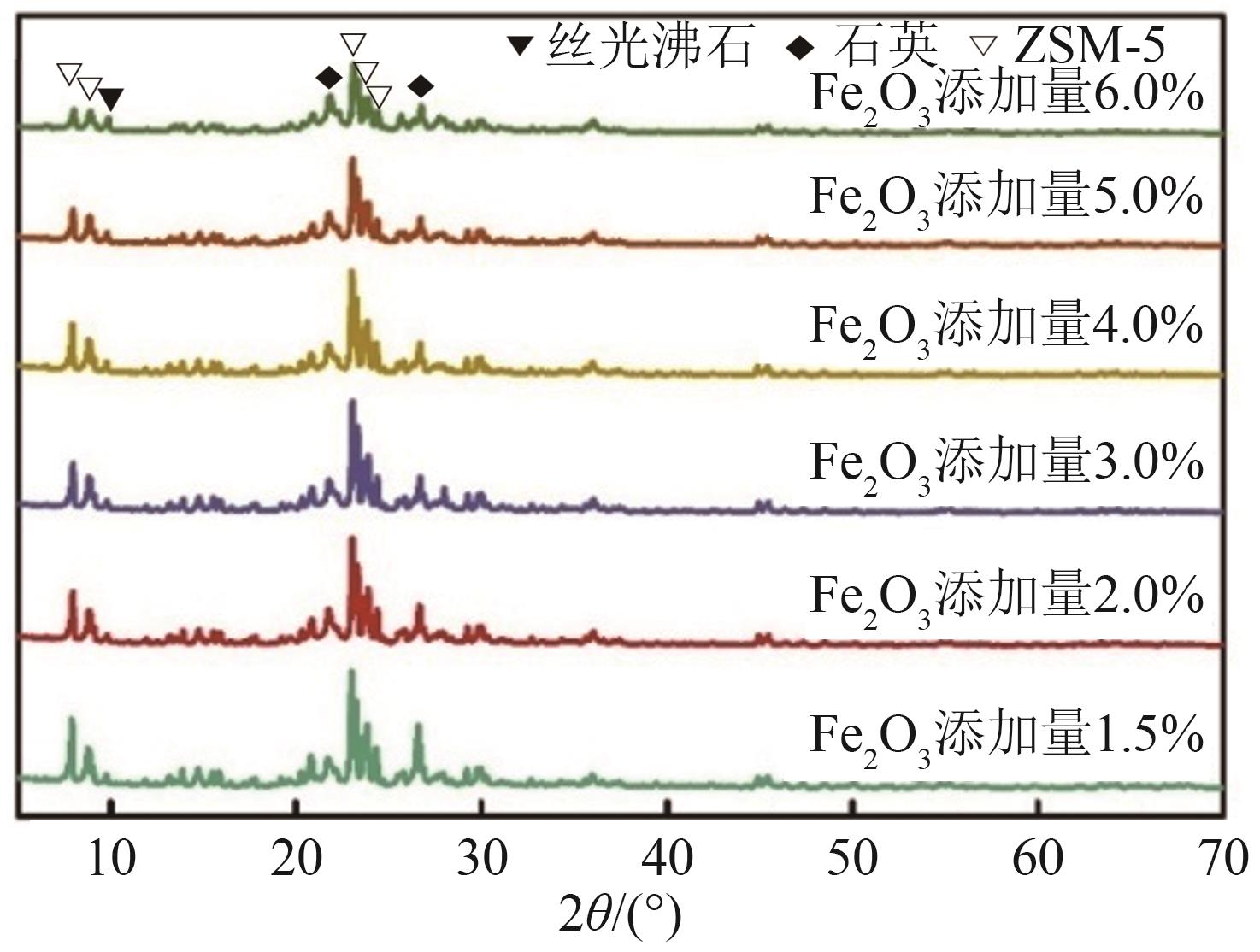
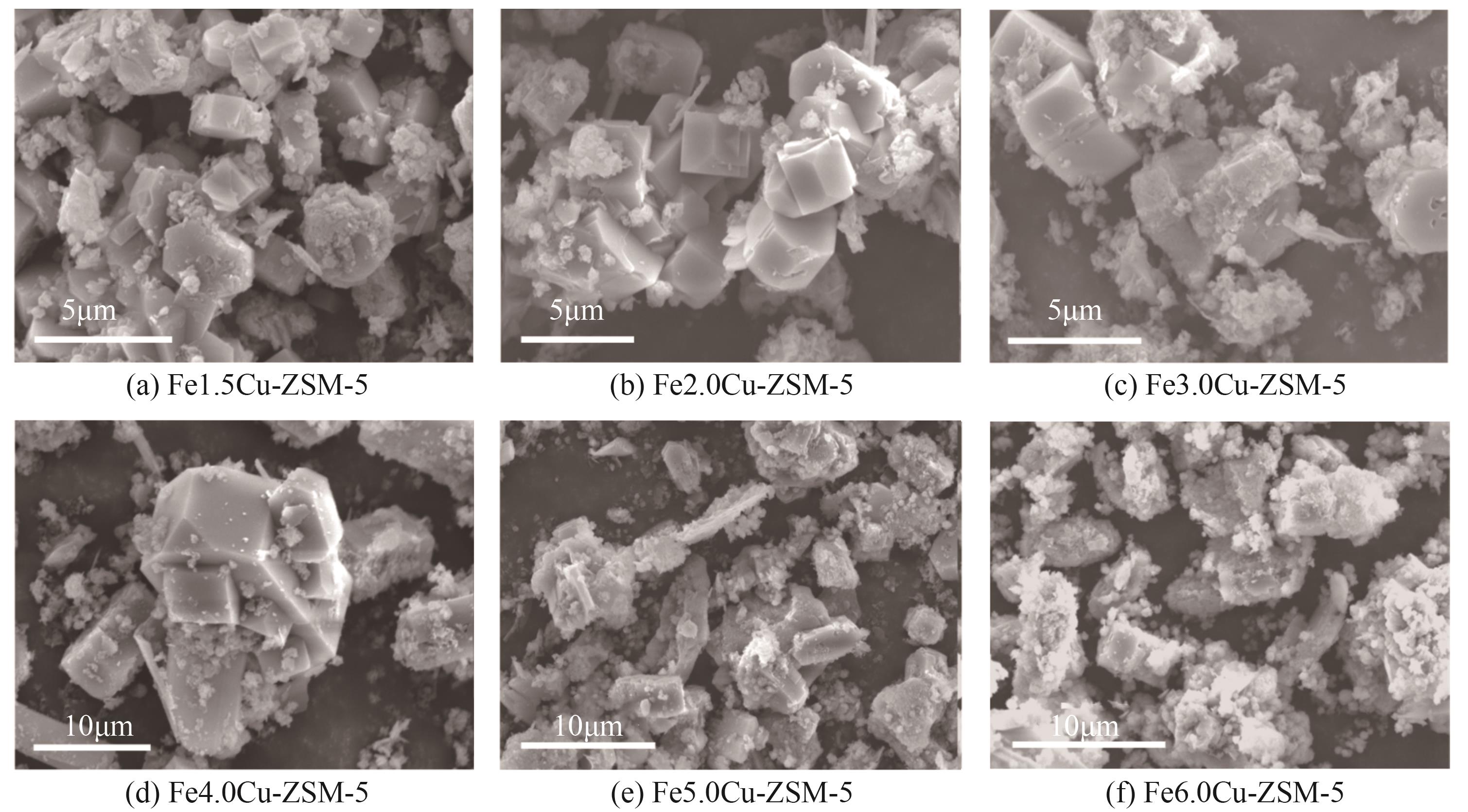
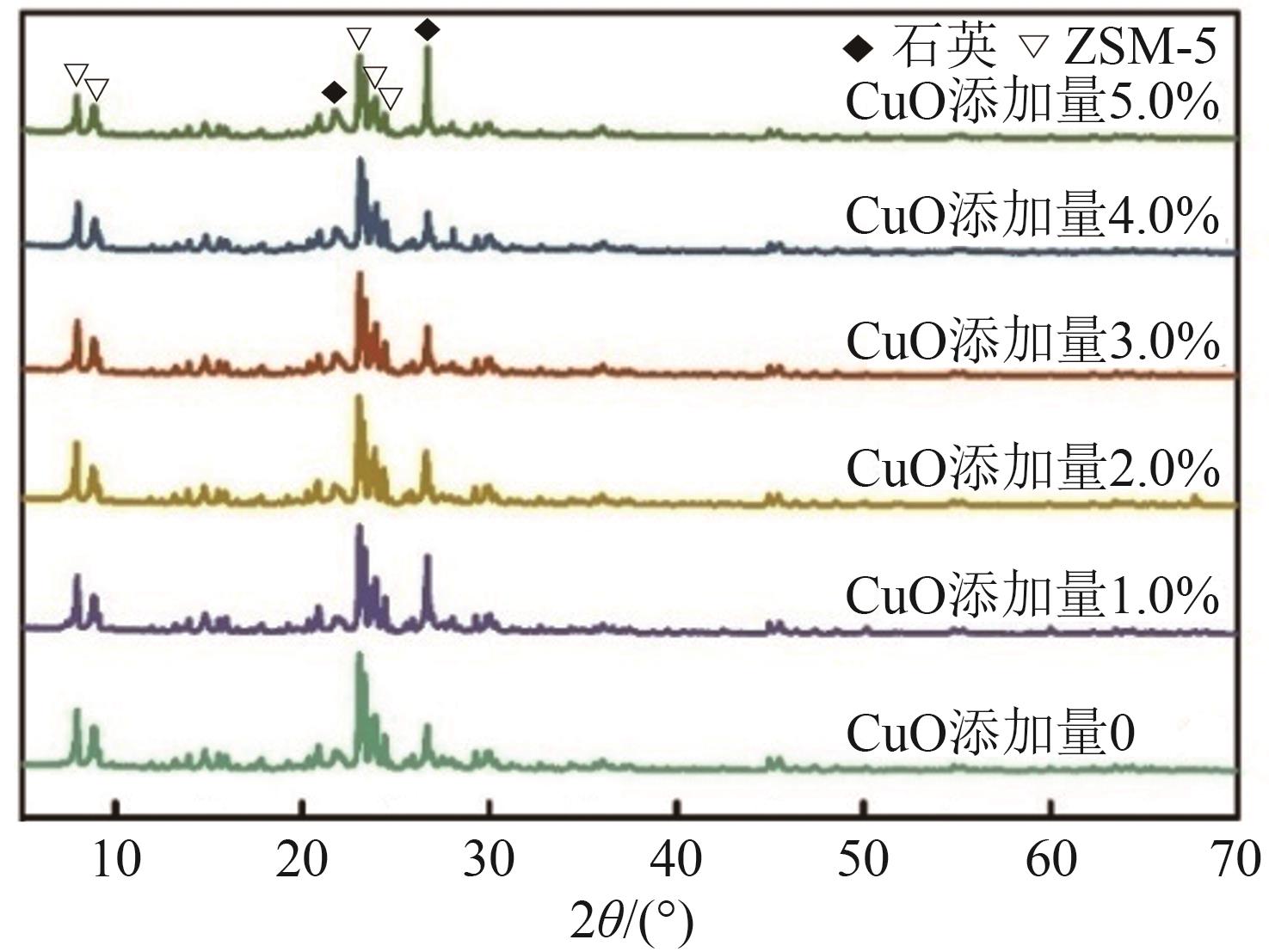
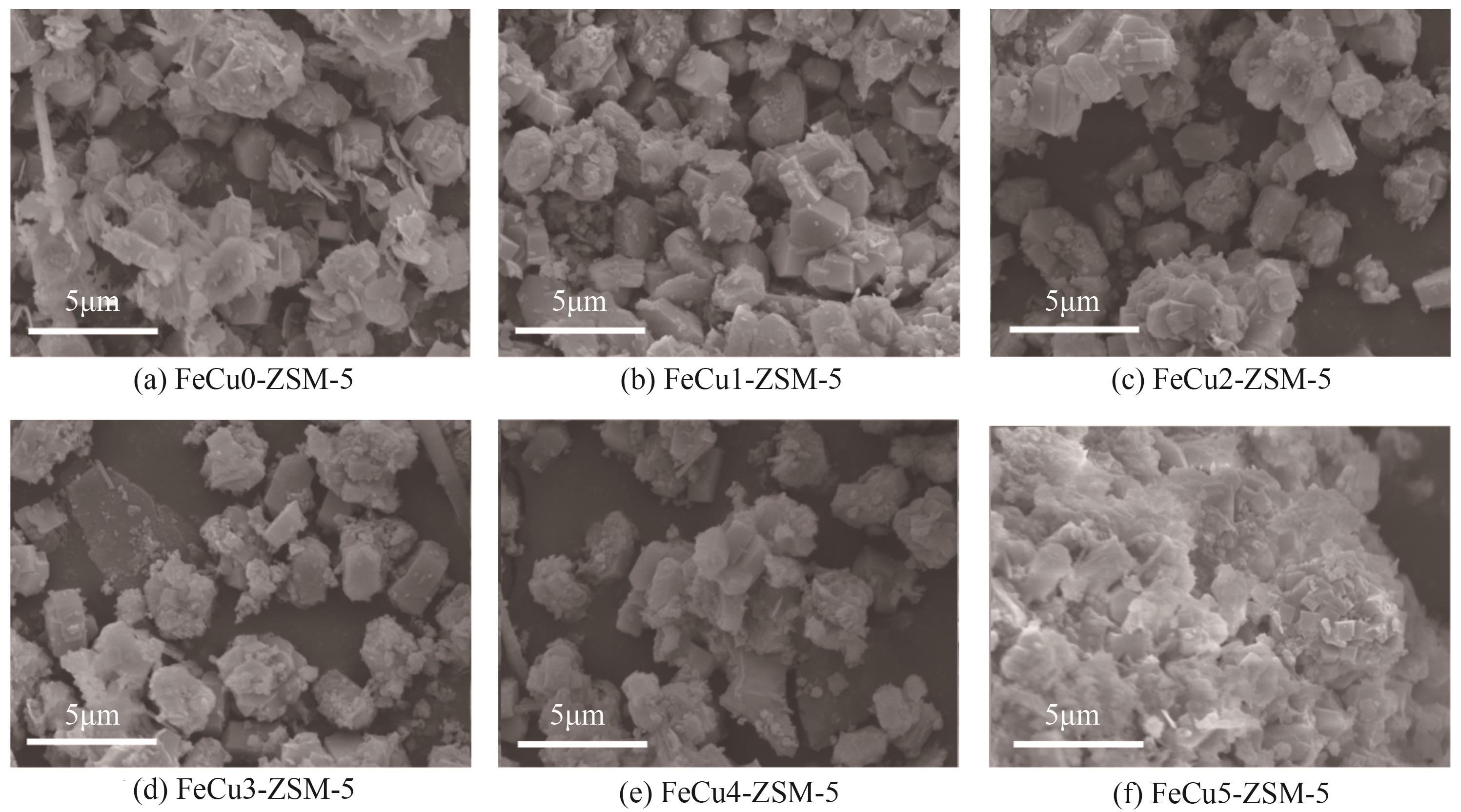
化工进展 ›› 2025, Vol. 44 ›› Issue (6): 3017-3030.DOI: 10.16085/j.issn.1000-6613.2024-1142
• 专栏:化工生态环境前沿交叉新技术 •
FeCu-ZSM-5分子筛的绿色合成及其NH3-SCR性能
徐景东1,2( ), 刘奔3(
), 刘奔3( ), 汪雪琴3, 董鹏4, 席志祥1,2, 徐人威1,2, 岳源源3,4(
), 汪雪琴3, 董鹏4, 席志祥1,2, 徐人威1,2, 岳源源3,4( )
)
- 1.中化石油化工研究院(泉州)有限责任公司,福建 泉州 362100
2.中化泉州石化有限公司,福建 泉州 362103
3.福州大学化工学院,福建 福州 350106
4.清源创新实验室,福建 泉州 362801
-
收稿日期:2024-07-17修回日期:2024-09-29出版日期:2025-06-25发布日期:2025-07-08 -
通讯作者:岳源源 -
作者简介:徐景东(1984—),男,博士,研究方向为炼化催化技术。E-mail:xujingdong01@sinochem.com
刘奔(1995—),男,博士,研究方向为工业催化。E-mail:1794569717@qq.com。 -
基金资助:国家自然科学基金(22322803);国家自然科学基金(22178059);国家自然科学基金(U23A20113);中化泉州能源科技有限责任公司技术开发合作项目(ZHQZKJ-19-F-ZS-0076)
Green synthesis and NH3-SCR performance of FeCu-ZSM-5 zeolite
XU Jingdong1,2( ), LIU Ben3(
), LIU Ben3( ), WANG Xueqin3, DONG Peng4, XI Zhixiang1,2, XU Renwei1,2, YUE Yuanyuan3,4(
), WANG Xueqin3, DONG Peng4, XI Zhixiang1,2, XU Renwei1,2, YUE Yuanyuan3,4( )
)
- 1.Sinochem Petroleum Chemical Research Institute(Quanzhou) Co. , Ltd. , Quanzhou 362100, Fujian, China
2.Sinochem Quanzhou Petrochemical Co. , Ltd. , Quanzhou 362103, Fujian, China
3.College of Chemical Engineering, Fuzhou University, Fuzhou 350106, Fujian, China
4.Qingyuan Innovation Laboratory, Quanzhou 362801, Fujian, China
-
Received:2024-07-17Revised:2024-09-29Online:2025-06-25Published:2025-07-08 -
Contact:YUE Yuanyuan
摘要:
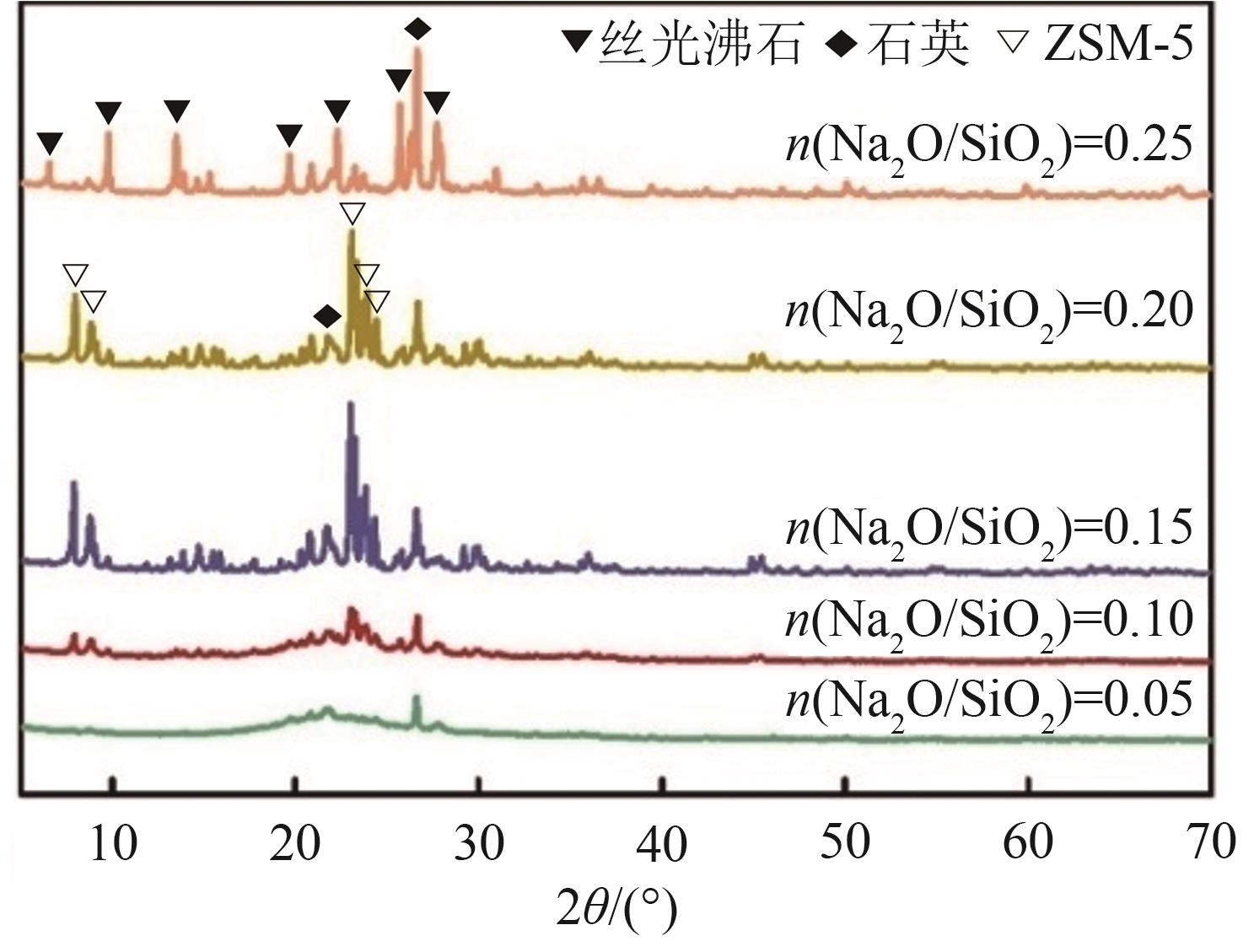
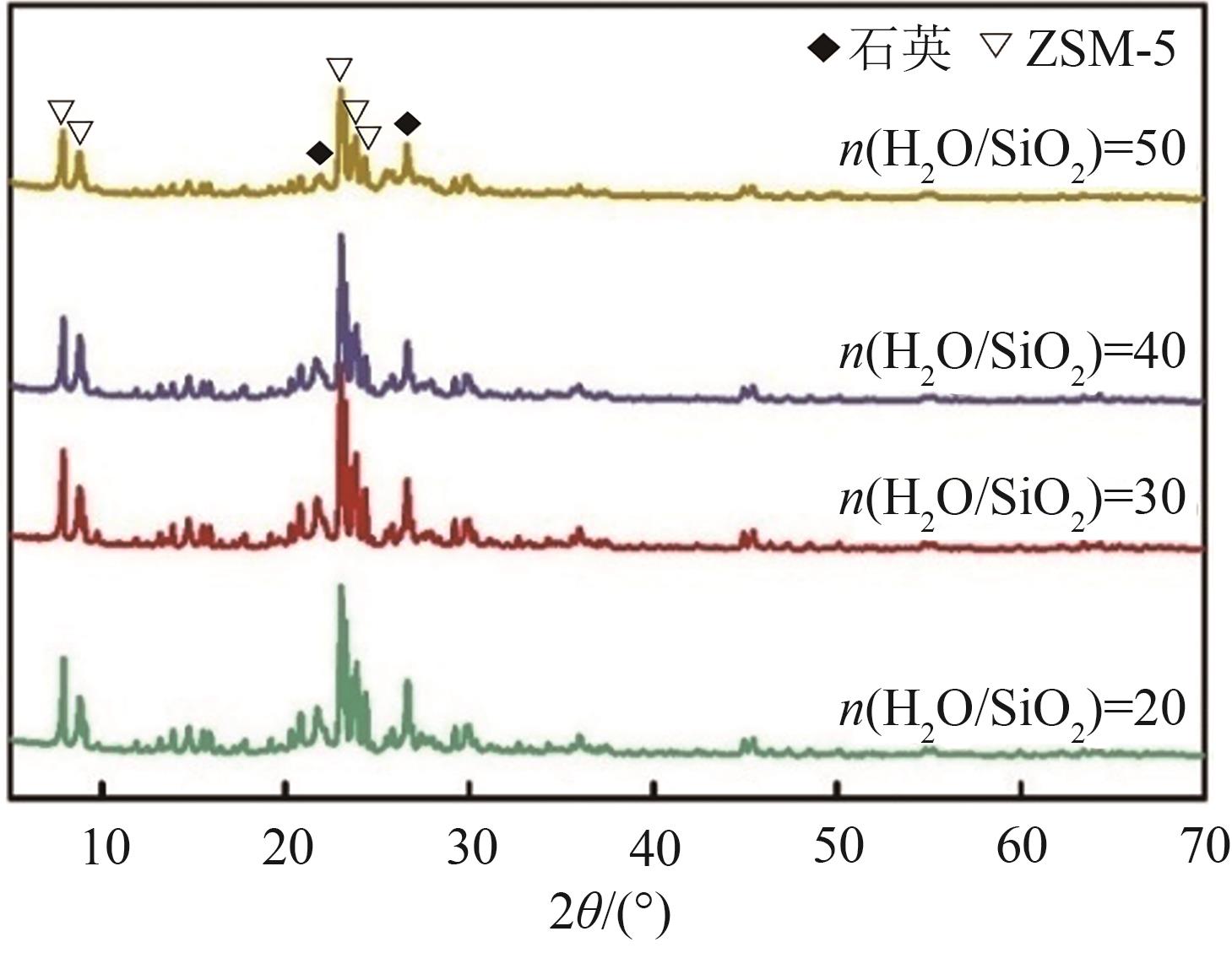
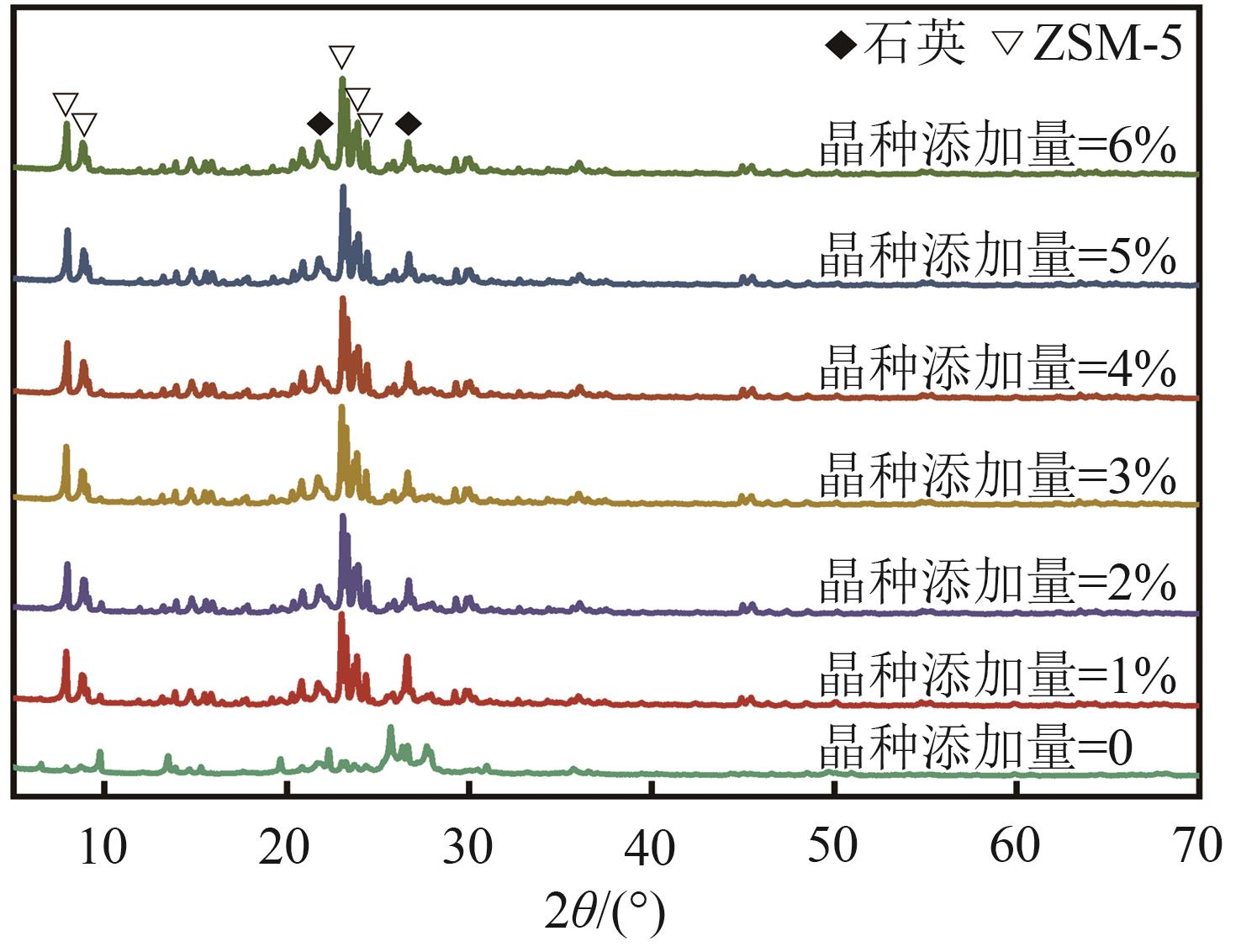
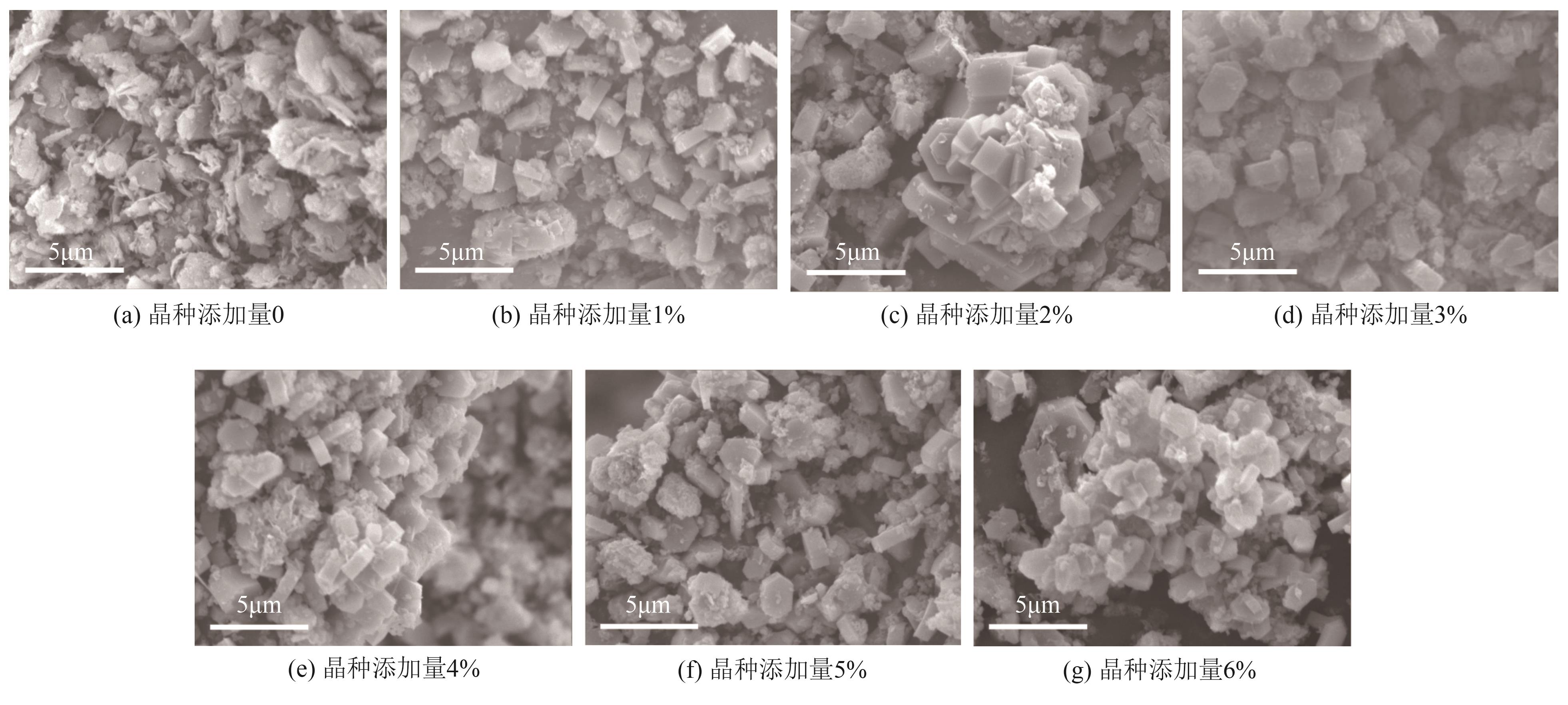
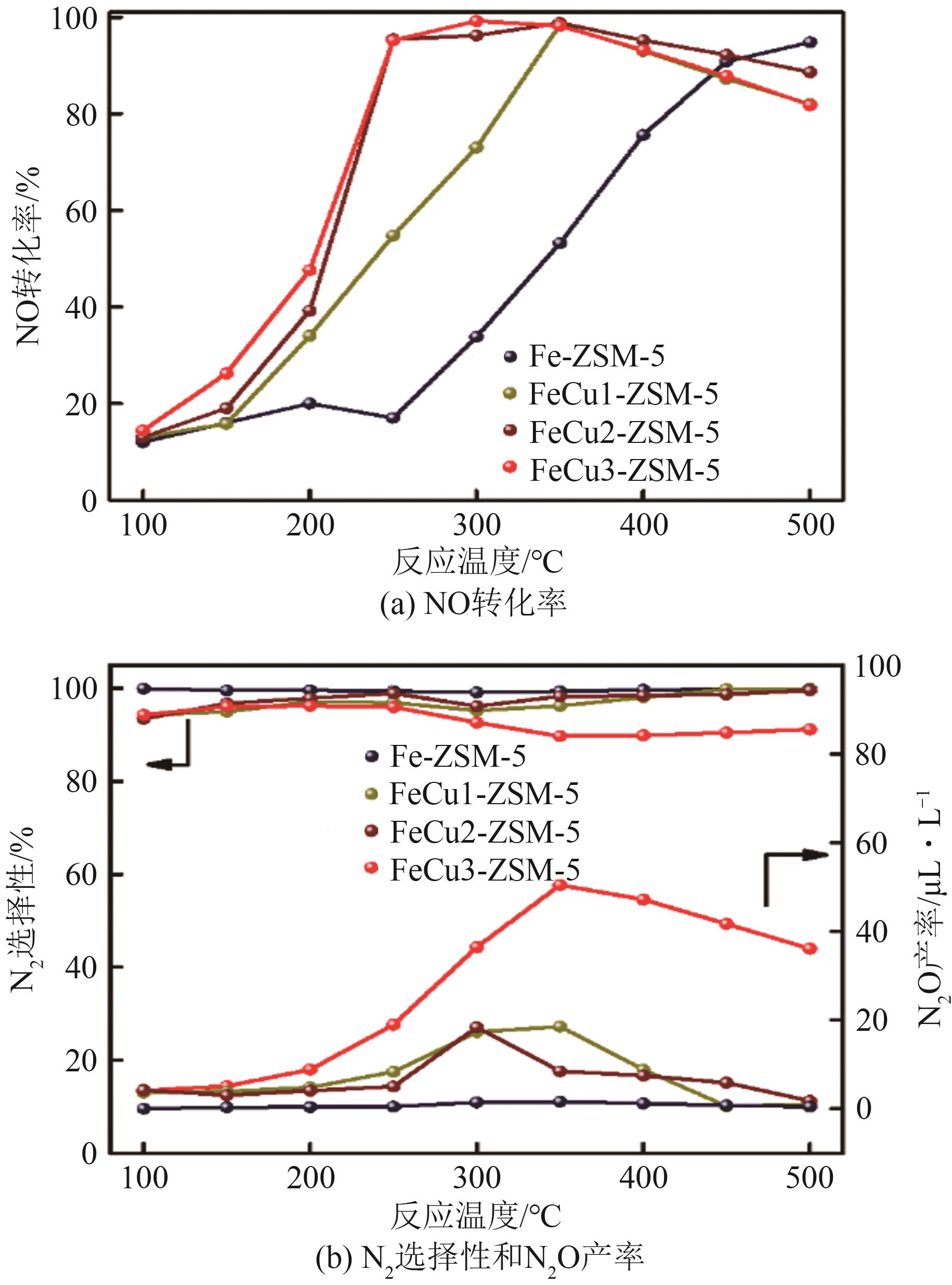
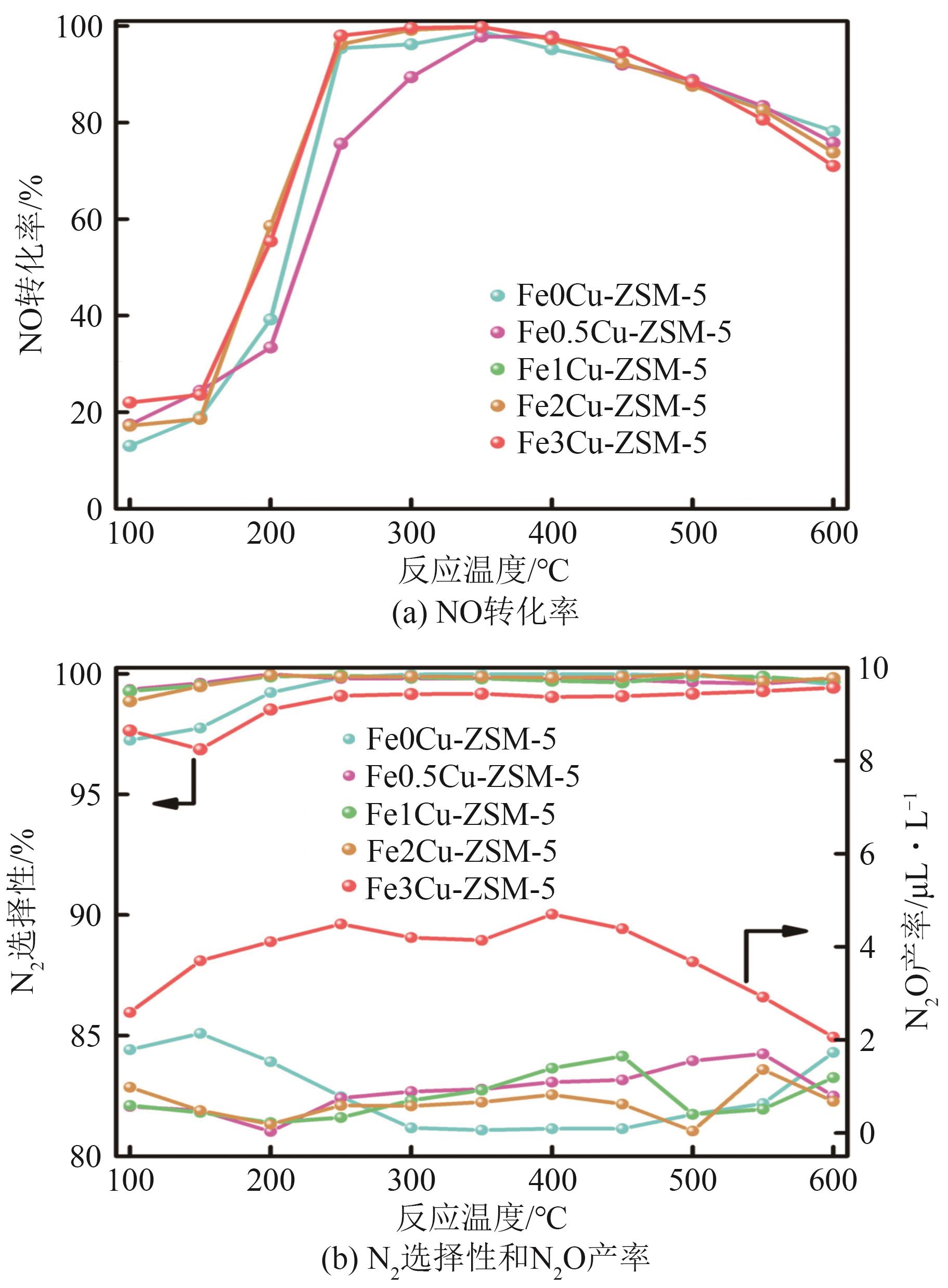
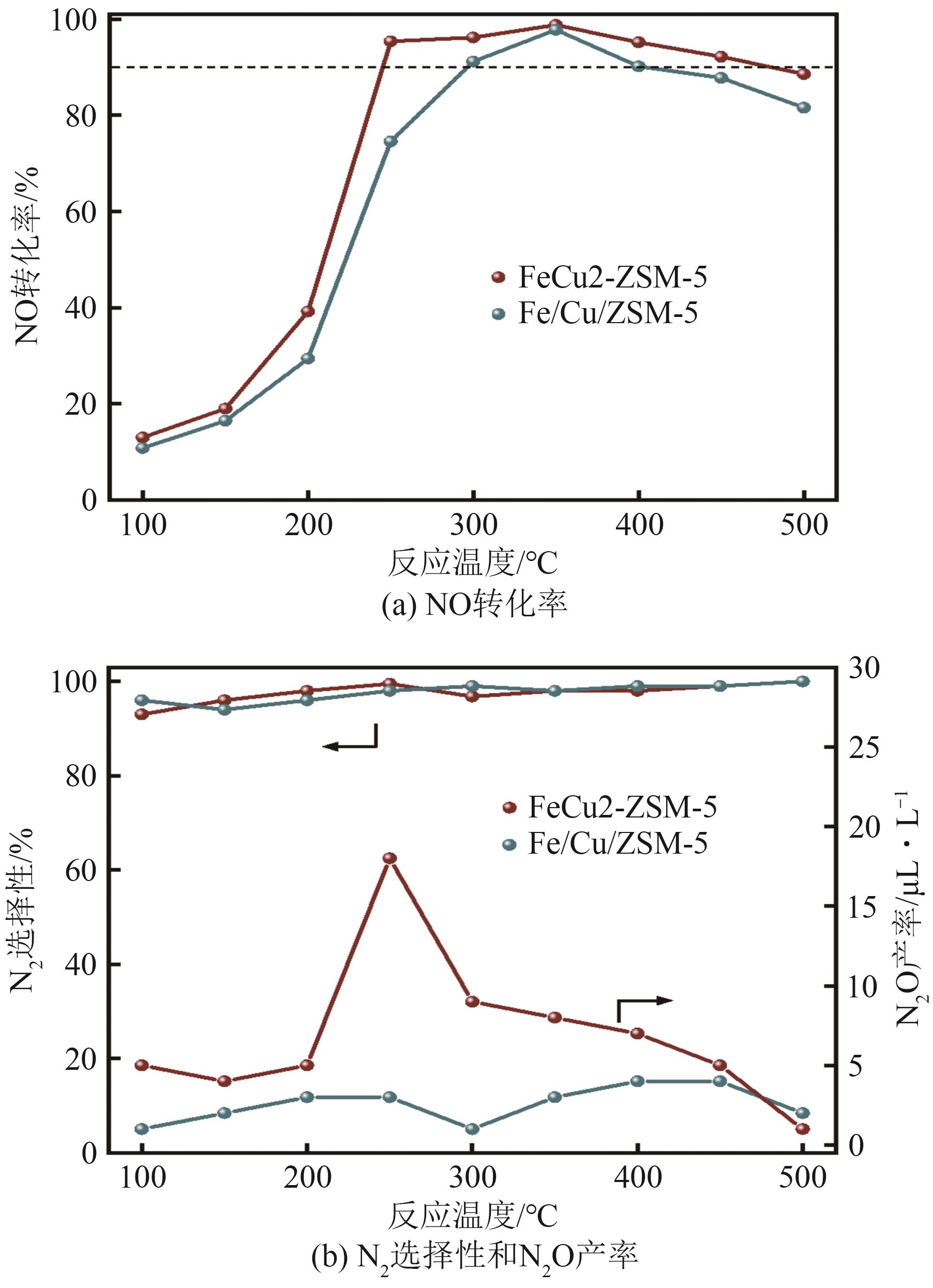
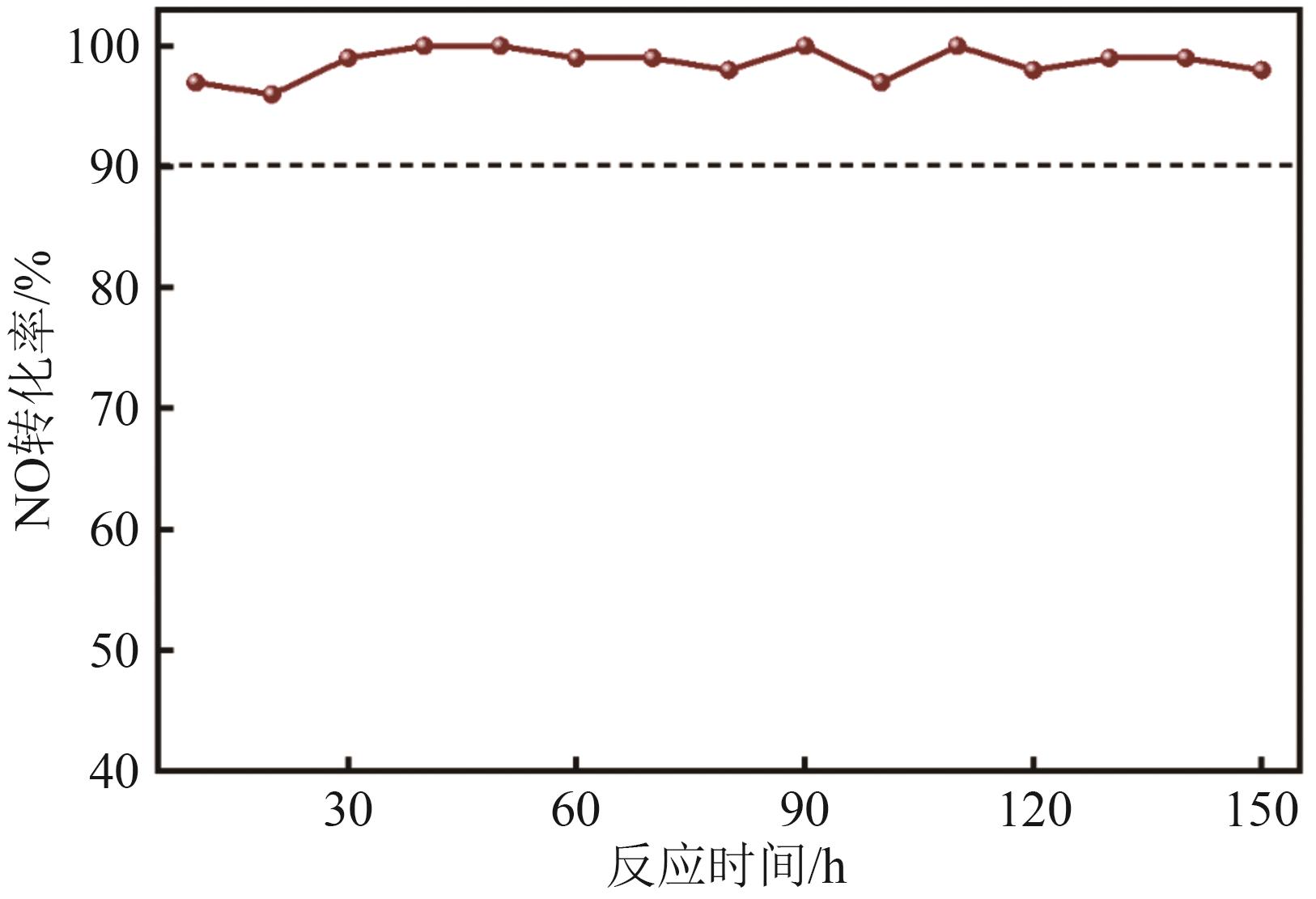
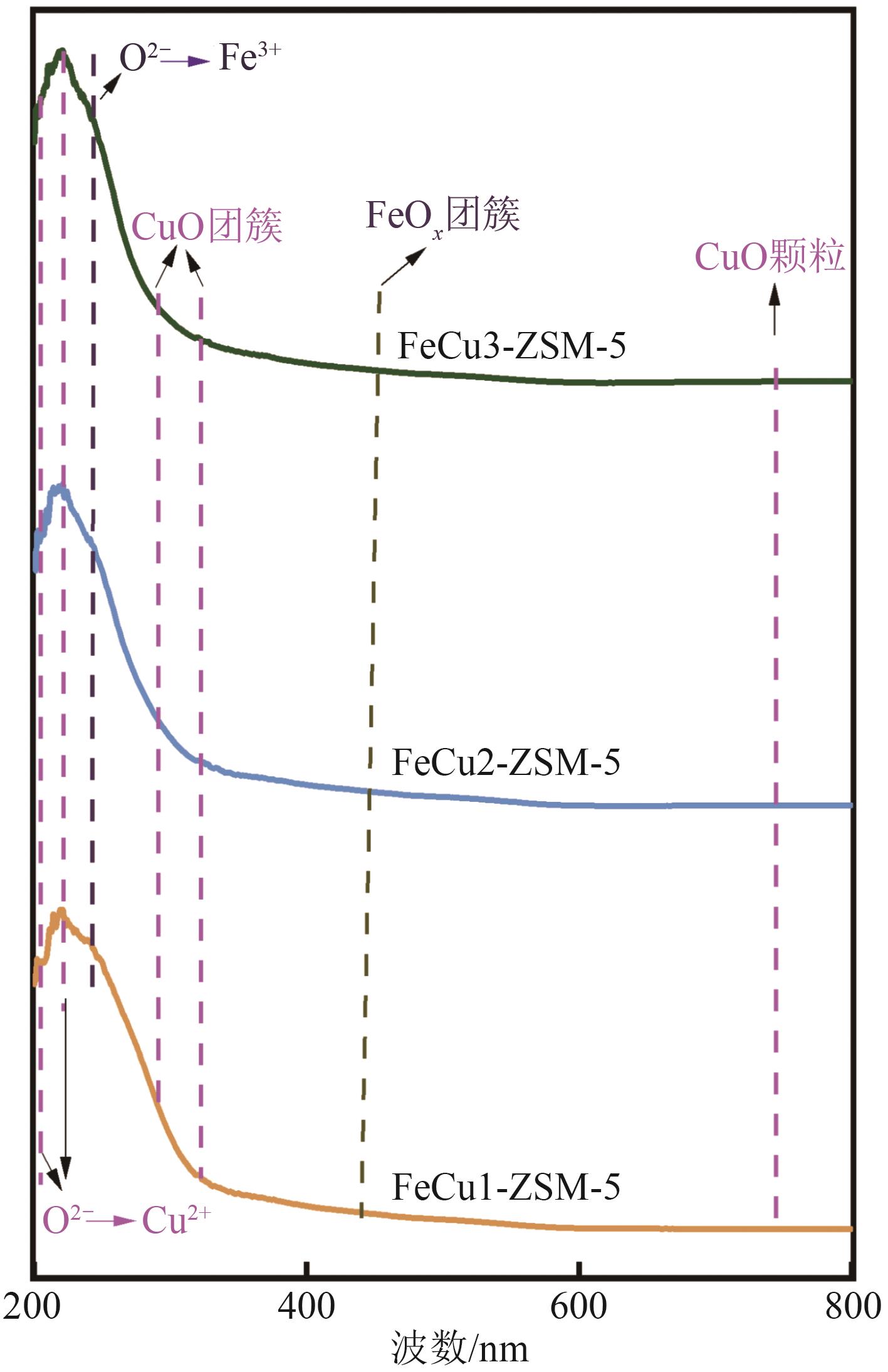
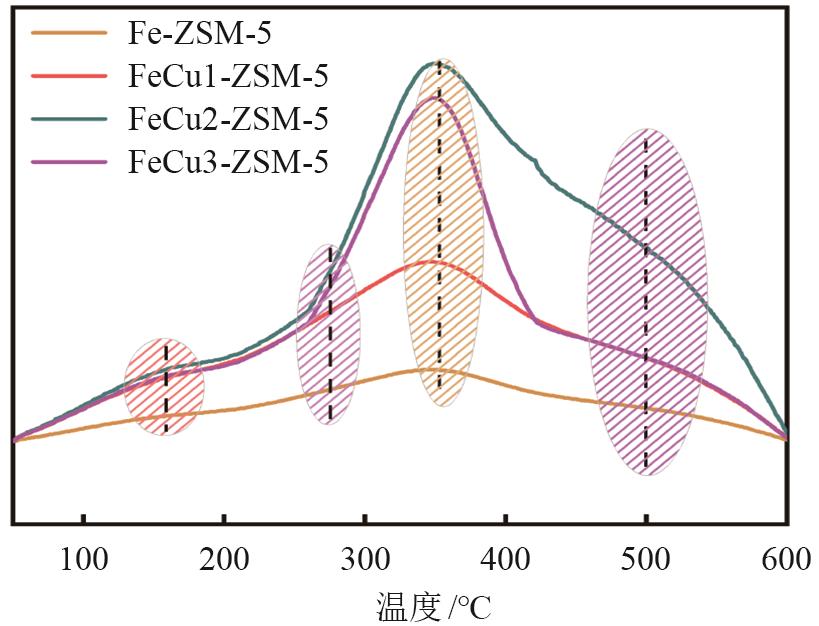
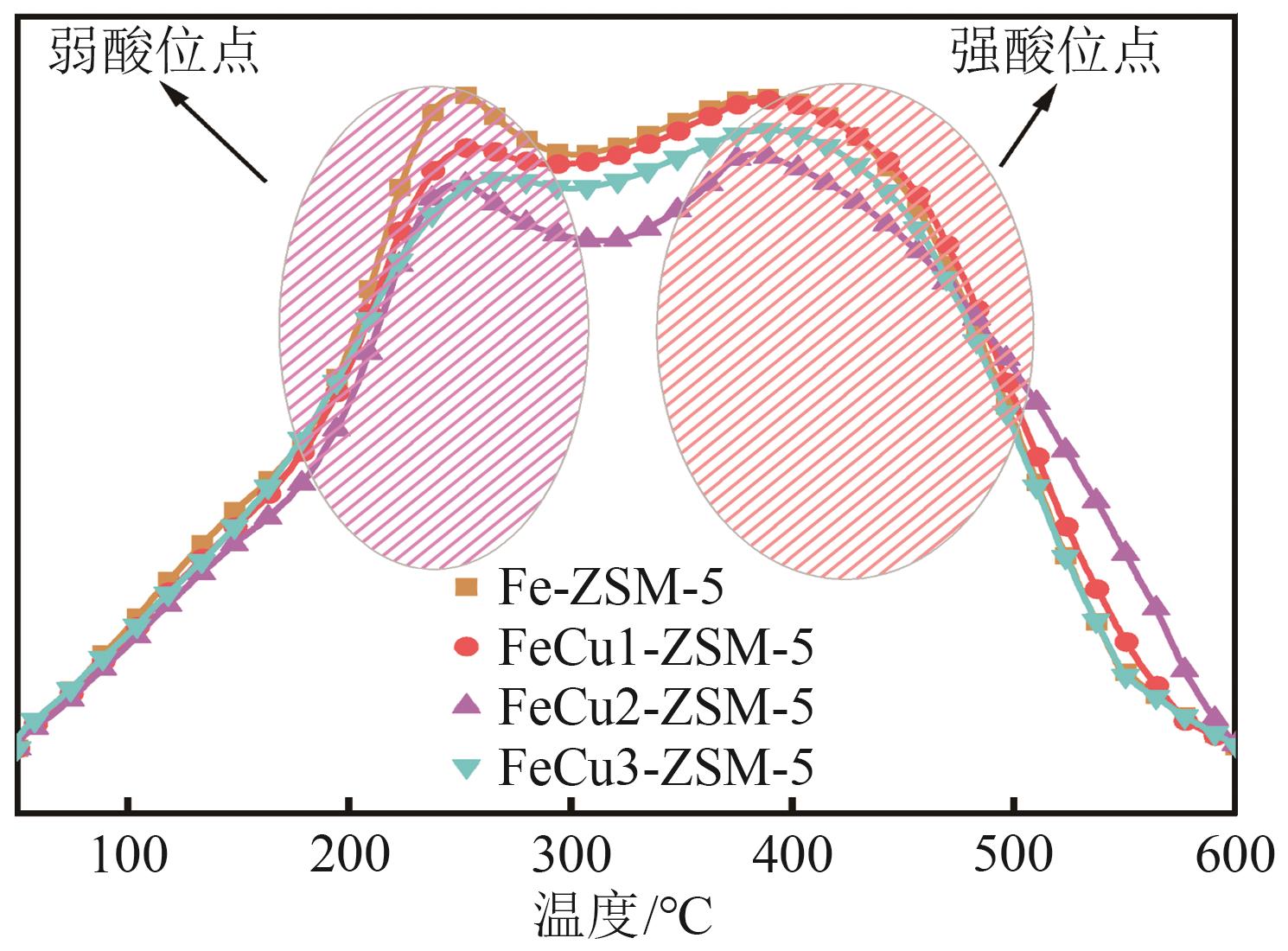
FeCu-ZSM-5杂双金属分子筛因在NH3选择催化还原(NH3-SCR)反应中表现出互补优势和协同效应而展现出广阔的应用前景,然而,其制备面临着原料和能源消耗高及污染严重的问题。基于此,开展了以天然矿物为原料无模板剂绿色制备等级孔FeCu-ZSM-5分子筛新工艺的合成研究。在合成过程中以天然矿物为全部硅铝铁源,无外加任何有机模板剂,采用晶种导向合成等级孔FeCu-ZSM-5分子筛。系统考察合成过程关键影响因素,进而确定了最佳的合成条件。考察所制得的等级孔FeCu-ZSM-5分子筛的NH3-SCR性能,并结合一系列表征手段探究等级孔FeCu-ZSM-5分子筛的构效关系。结果表明,Cu的加入可以显著提高FeCu-ZSM-5分子筛的低温脱硝活性,从而拓宽其温窗。Cu的引入不影响FeCu-ZSM-5的拓扑结构,但可对Fe和Cu在分子筛中的位置和分布、分子筛的还原性和酸性进行调控。较高占比的骨架Fe和孤立Cu2+、良好的氧化还原能力和适宜的酸性共同促使FeCu2-ZSM-5分子筛呈现最优异的脱硝性能。
中图分类号:
引用本文
徐景东, 刘奔, 汪雪琴, 董鹏, 席志祥, 徐人威, 岳源源. FeCu-ZSM-5分子筛的绿色合成及其NH3-SCR性能[J]. 化工进展, 2025, 44(6): 3017-3030.
XU Jingdong, LIU Ben, WANG Xueqin, DONG Peng, XI Zhixiang, XU Renwei, YUE Yuanyuan. Green synthesis and NH3-SCR performance of FeCu-ZSM-5 zeolite[J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3017-3030.
| 组成/% | 累托土/% | 硅藻土/% |
|---|---|---|
| Na2O | 1.03 | 0.40 |
| MgO | 0.23 | 0.34 |
| Al2O3 | 37.2 | 3.22 |
| SiO2 | 43.2 | 92.14 |
| P2O5 | 3.05 | — |
| SO3 | 0.07 | 0.70 |
| K2O | 1.03 | 0.53 |
| CaO | 7.05 | 0.85 |
| TiO2 | 5.94 | 0.16 |
| Fe2O3 | 0.51 | 1.53 |
表1 累托土及硅藻土的化学组成(质量分数)
| 组成/% | 累托土/% | 硅藻土/% |
|---|---|---|
| Na2O | 1.03 | 0.40 |
| MgO | 0.23 | 0.34 |
| Al2O3 | 37.2 | 3.22 |
| SiO2 | 43.2 | 92.14 |
| P2O5 | 3.05 | — |
| SO3 | 0.07 | 0.70 |
| K2O | 1.03 | 0.53 |
| CaO | 7.05 | 0.85 |
| TiO2 | 5.94 | 0.16 |
| Fe2O3 | 0.51 | 1.53 |
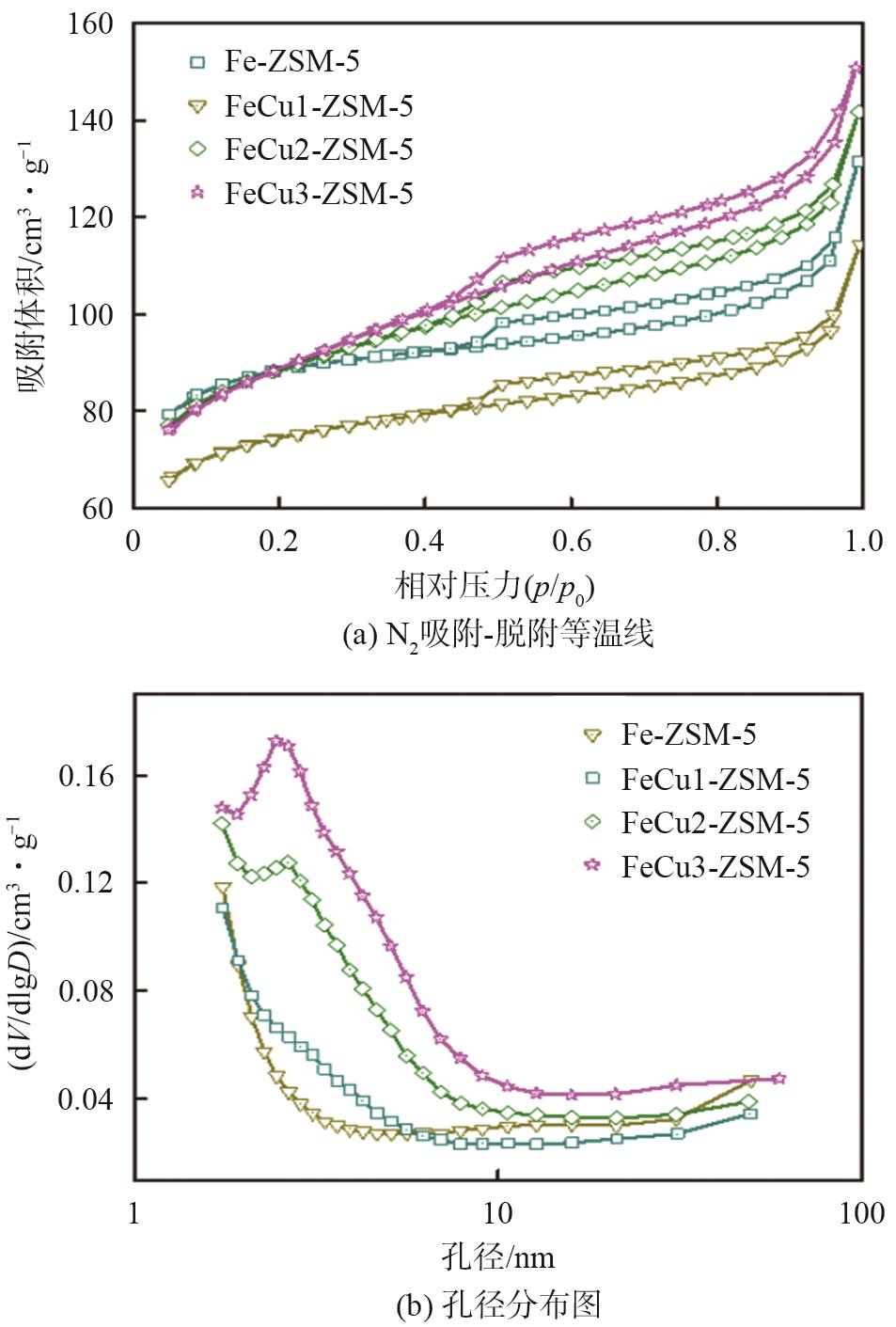
| 样品 | SBET/m2·g-1 | Smicro/m2·g-1 | Vtotal/cm3·g-1 | Vmeso/cm3·g-1 |
|---|---|---|---|---|
| Fe-ZSM-5 | 275 | 197 | 0.17 | 0.07 |
| FeCu1-ZSM-5 | 234 | 153 | 0.15 | 0.07 |
| FeCu2-ZSM-5 | 283 | 158 | 0.19 | 0.12 |
| FeCu3-ZSM-5 | 289 | 136 | 0.20 | 0.13 |
表2 FeCux-ZSM-5分子筛的织构参数
| 样品 | SBET/m2·g-1 | Smicro/m2·g-1 | Vtotal/cm3·g-1 | Vmeso/cm3·g-1 |
|---|---|---|---|---|
| Fe-ZSM-5 | 275 | 197 | 0.17 | 0.07 |
| FeCu1-ZSM-5 | 234 | 153 | 0.15 | 0.07 |
| FeCu2-ZSM-5 | 283 | 158 | 0.19 | 0.12 |
| FeCu3-ZSM-5 | 289 | 136 | 0.20 | 0.13 |
| 样品 | Fe2O3/% | CuO/% |
|---|---|---|
| Fe-ZSM-5 | 2.49 | — |
| FeCu1-ZSM-5 | 2.57 | 1.11 |
| FeCu2-ZSM-5 | 2.22 | 2.01 |
| FeCu3-ZSM-5 | 2.39 | 3.74 |
表3 由XRF测得的FeCux-ZSM-5分子筛中的Fe和Cu质量分数
| 样品 | Fe2O3/% | CuO/% |
|---|---|---|
| Fe-ZSM-5 | 2.49 | — |
| FeCu1-ZSM-5 | 2.57 | 1.11 |
| FeCu2-ZSM-5 | 2.22 | 2.01 |
| FeCu3-ZSM-5 | 2.39 | 3.74 |
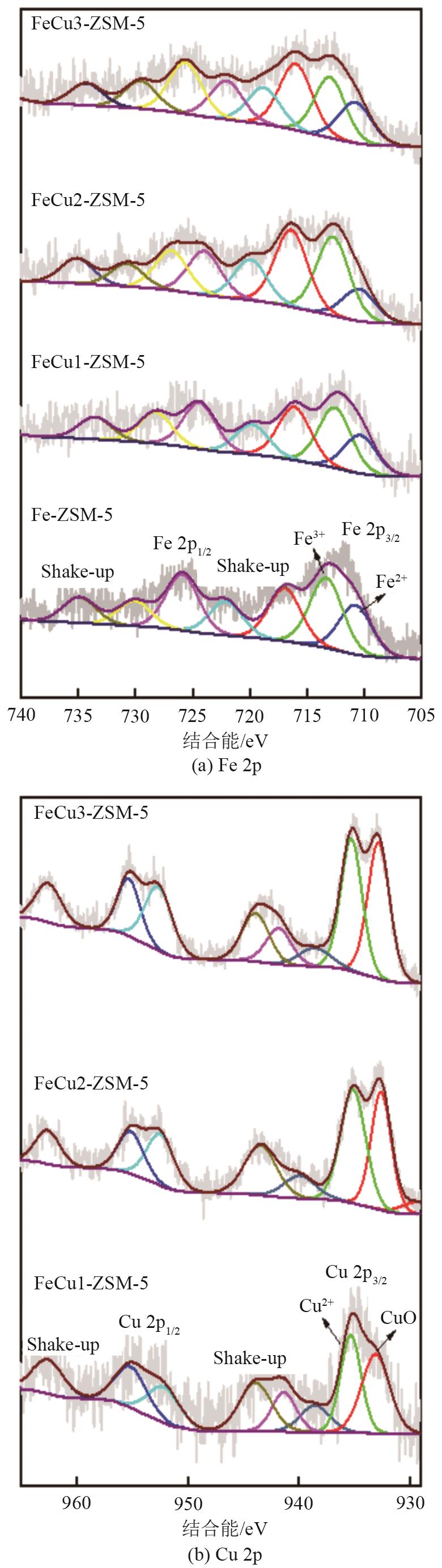
| 样品 | Fe物种 | Cu物种 | ||
|---|---|---|---|---|
| Fe2+/% | Fe3+/% | CuO/% | Cu2+/% | |
| Fe-ZSM-5 | 41.31 | 58.69 | — | — |
| FeCu1-ZSM-5 | 38.86 | 61.14 | 52.57 | 47.43 |
| FeCu2-ZSM-5 | 28.95 | 71.05 | 45.65 | 54.35 |
| FeCu3-ZSM-5 | 41.81 | 58.19 | 51.45 | 48.55 |
表4 FeCux-ZSM-5分子筛的XPS定量结果
| 样品 | Fe物种 | Cu物种 | ||
|---|---|---|---|---|
| Fe2+/% | Fe3+/% | CuO/% | Cu2+/% | |
| Fe-ZSM-5 | 41.31 | 58.69 | — | — |
| FeCu1-ZSM-5 | 38.86 | 61.14 | 52.57 | 47.43 |
| FeCu2-ZSM-5 | 28.95 | 71.05 | 45.65 | 54.35 |
| FeCu3-ZSM-5 | 41.81 | 58.19 | 51.45 | 48.55 |
| 组成 | SiO2/% | Al2O3/% | Na2O/% | n(SiO2)/n(Al2O3) |
|---|---|---|---|---|
| Fe-ZSM-5 | 88.41 | 7.23 | 0.37 | 20.77 |
| FeCu1-ZSM-5 | 87.57 | 7.15 | 0.41 | 20.83 |
| FeCu2-ZSM-5 | 87.55 | 6.94 | 0.38 | 21.44 |
| FeCu3-ZSM-5 | 85.39 | 6.92 | 0.35 | 20.97 |
表5 由XRF测得的FeCux-ZSM-5分子筛中的Si、Al及Na含量
| 组成 | SiO2/% | Al2O3/% | Na2O/% | n(SiO2)/n(Al2O3) |
|---|---|---|---|---|
| Fe-ZSM-5 | 88.41 | 7.23 | 0.37 | 20.77 |
| FeCu1-ZSM-5 | 87.57 | 7.15 | 0.41 | 20.83 |
| FeCu2-ZSM-5 | 87.55 | 6.94 | 0.38 | 21.44 |
| FeCu3-ZSM-5 | 85.39 | 6.92 | 0.35 | 20.97 |
| [2] | 邹润, 董霄, 矫义来, 等. 等级孔分子筛的可控合成、扩散研究及催化应用[J]. 高等学校化学学报, 2021, 42(1): 74-100. |
| ZOU Run, DONG Xiao, JIAO Yilai, et al. Controllable synthesis, diffusion study and catalysis of hierarchical zeolites[J]. Chemical Journal of Chinese Universities, 2021, 42(1): 74-100. | |
| [3] | HARTMANN Martin, THOMMES Matthias, SCHWIEGER Wilhelm. Hierarchically-ordered zeolites: A critical assessment[J]. Advanced Materials Interfaces, 2021, 8(4): 2001841. |
| [4] | SUN Minghui, ZHOU Jian, HU Zhiyi, et al. Hierarchical zeolite single-crystal reactor for excellent catalytic efficiency[J]. Matter, 2020, 3(4): 1226-1245. |
| [5] | Javier PÉREZ-RAMÍREZ, CHRISTENSEN Claus H, EGEBLAD Kresten, et al. Hierarchical zeolites: Enhanced utilisation of microporous crystals in catalysis by advances in materials design[J]. Chemical Society Reviews, 2008, 37(11): 2530-2542. |
| [6] | CHOI Minkee, NA Kyungsu, KIM Jeongnam, et al. Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts[J]. Nature, 2009, 461(7261): 246-249. |
| [7] | PLANK Charles J, ROSINSKI Edward J, RUBIN Mae K. Crystalline zeolite and method of preparing same: US04016245[P]. 1977-04-05. |
| [8] | LI Junhua, CHANG Huazhen, MA Lei, et al. Low-temperature selective catalytic reduction of NO x with NH3 over metal oxide and zeolite catalysts—A review[J]. Catalysis Today, 2011, 175(1): 147-156. |
| [9] | Feng BIN, SONG Chonglin, Gang LYU, et al. Selective catalytic reduction of nitric oxide with ammonia over zirconium-doped copper/ZSM-5 catalysts[J]. Applied Catalysis B: Environmental, 2014, 150: 532-543. |
| [10] | CHEN Zhen, FAN Chi, PANG Lei, et al. One-pot synthesis of high performance Cu-SAPO-18 catalyst for NO reduction by NH3-SCR: Influence of silicon content on the catalytic properties of Cu-SAPO-18[J]. Chemical Engineering Journal, 2018, 348: 608-617. |
| [11] | ZHANG Tao, CHANG Huazhen, YOU Yanchen, et al. Excellent activity and selectivity of one-pot synthesized Cu-SSZ-13 catalyst in the selective catalytic oxidation of ammonia to nitrogen[J]. Environmental Science & Technology, 2018, 52(8): 4802-4808. |
| [12] | YUE Yuanyuan, LIU Haiyan, YUAN Pei, et al. From natural aluminosilicate minerals to hierarchical ZSM-5 zeolites: A nanoscale depolymerization-reorganization approach[J]. Journal of Catalysis, 2014, 319: 200-210. |
| [13] | LI Tiesen, LIU Haiyan, FAN Yu, et al. Synthesis of zeolite Y from natural aluminosilicate minerals for fluid catalytic cracking application[J]. Green Chemistry, 2012, 14(12): 3255-3259. |
| [14] | YUE Yuanyuan, KANG Ying, BAI Yu, et al. Seed-assisted, template-free synthesis of ZSM-5 zeolite from natural aluminosilicate minerals[J]. Applied Clay Science, 2018, 158: 177-185. |
| [15] | ITABASHI Keiji, KAMIMURA Yoshihiro, IYOKI Kenta, et al. A working hypothesis for broadening framework types of zeolites in seed-assisted synthesis without organic structure-directing agent[J]. Journal of the American Chemical Society, 2012, 134(28): 11542-11549. |
| [16] | KIM Shin Dong, Shi Hyun NOH, SEONG Kyeong Hak, et al. Compositional and kinetic study on the rapid crystallization of ZSM-5 in the absence of organic template under stirring[J]. Microporous and Mesoporous Materials, 2004, 72(1/2/3): 185-192. |
| [17] | PAN Feng, LU Xuchen, WANG Yun, et al. Synthesis and crystallization kinetics of ZSM-5 without organic template from coal-series kaolinite[J]. Microporous and Mesoporous Materials, 2014, 184: 134-140. |
| [18] | Jan PŘECH, STROSSI PEDROLO Debora R, MARCILIO Nilson R, et al. Core-shell metal zeolite composite catalysts for in situ processing of Fischer-Tropsch hydrocarbons to gasoline type fuels[J]. ACS Catalysis, 2020, 10(4): 2544-2555. |
| [19] | YANG Guangpeng, DU Xuesen, RAN Jingyu, et al. Irregular influence of alkali metals on Cu-SAPO-34 catalyst for selective catalytic reduction of NO x with ammonia[J]. Journal of Hazardous Materials, 2020, 387: 122007. |
| [20] | FRITZ A., PITCHON V. The current state of research on automotive lean NO x catalysis[J]. Applied Catalysis B: Environmental, 1997, 13(1): 1-25. |
| [21] | BUSCA Guido, LIETTI Luca, RAMIS Gianguido, et al. Chemical and mechanistic aspects of the selective catalytic reduction of NO x by ammonia over oxide catalysts: A review[J]. Applied Catalysis B: Environmental, 1998, 18(1/2): 1-36. |
| [22] | YUE Yuanyuan, LIU Haiyan, ZHOU Yanni, et al. Pure-phase zeolite beta synthesized from natural aluminosilicate minerals and its catalytic application for esterification[J]. Applied Clay Science, 2016, 126: 1-6. |
| [23] | YUE Yuanyuan, LIU Haiyan, YUAN Pei, et al. One-pot synthesis of hierarchical FeZSM-5 zeolites from natural aluminosilicates for selective catalytic reduction of NO by NH3 [J]. Scientific Reports, 2015, 5: 9270. |
| [24] | MOHAN Sooraj, DINESHA P, KUMAR Shiva. NO x reduction behaviour in copper zeolite catalysts for ammonia SCR systems: A review[J]. Chemical Engineering Journal, 2020, 384: 123253. |
| [25] | BRANDENBERGER Sandro, Oliver KRÖCHER, TISSLER Arno, et al. The state of the art in selective catalytic reduction of NO x by ammonia using metal-exchanged zeolite catalysts[J]. Catalysis Reviews, 2008, 50(4): 492-531. |
| [26] | YUE Yuanyuan, LIU Ben, Nangui LYU, et al. Direct synthesis of hierarchical FeCu-ZSM-5 zeolite with wide temperature window in selective catalytic reduction of NO by NH3 [J]. ChemCatChem, 2019, 11(19): 4744-4754. |
| [27] | SHEN Qun, ZHANG Lingyun, WU Minfang, et al. High-silica nanoflower hierarchical Fe-MFI with excellent catalytic performance for N2O decomposition[J]. Materials Research Bulletin, 2017, 87: 1-5. |
| [28] | ANUNZIATA Oscar A, BELTRAMONE Andrea R, JURIC Zoran, et al. Fe-containing ZSM-11 zeolites as active catalyst for SCR of NO x Part I. Synthesis, characterization by XRD, BET and FTIR and catalytic properties[J]. Applied Catalysis A: General, 2004, 264(1): 93-101. |
| [29] | HAN Lina, ZHAO Xiaoge, YU Huafeng, et al. Preparation of SSZ-13 zeolites and their NH3-selective catalytic reduction activity[J]. Microporous and Mesoporous Materials, 2018, 261: 126-136. |
| [30] | HU Xiaoqian, YANG Ming, FAN Dequan, et al. The role of pore diffusion in determining NH3 SCR active sites over Cu/SAPO-34 catalysts[J]. Journal of Catalysis, 2016, 341: 55-61. |
| [31] | ANDONOVA Stanislava, VOVK Evgeny, Jonas SJÖBLOM, et al. Chemical deactivation by phosphorous under lean hydrothermal conditions over Cu/BEA NH3-SCR catalysts[J]. Applied Catalysis B: Environmental, 2014, 147: 251-263. |
| [32] | WEN Chengyan, WANG Chenguang, CHEN Lungang, et al. Effect of hierarchical ZSM-5 zeolite support on direct transformation from syngas to aromatics over the iron-based catalyst[J]. Fuel, 2019, 244: 492-498. |
| [33] | HAN Zeyu, ZHANG Feng, ZHAO Xinhong. Green energy-efficient synthesis of Fe-ZSM-5 zeolite and its application for hydroxylation of phenol[J]. Microporous and Mesoporous Materials, 2019, 290, 109679. |
| [34] | PORNRATTANAPIMOLCHAI Choompoonut, WORATHANAKUL Patcharin. Physicochemical properties and different sequence of metal loading (CuFe) over nanoporous of SUZ-4 zeolite[J]. Journal of Nanoscience and Nanotechnology, 2013, 13(4): 3110-3114. |
| [35] | YIN Chengyang, CHENG Peifu, LI Xiang, et al. Selective catalytic reduction of nitric oxide with ammonia over high-activity Fe/SSZ-13 and Fe/one-pot-synthesized Cu-SSZ-13 catalysts[J]. Catalysis Science & Technology, 2016, 6(20): 7561-7568. |
| [36] | GURGUL Jacek, Kazimierz ŁĄTKA, HNAT Izabela, et al. Identification of iron species in FeSiBEA by DR UV-vis, XPS and Mössbauer spectroscopy: Influence of Fe content[J]. Microporous and Mesoporous Materials, 2013, 168: 1-6. |
| [37] | XU Li, SHI Chuan, CHEN Bingbing, et al. Improvement of catalytic activity over Cu-Fe modified Al-rich beta catalyst for the selective catalytic reduction of NO x with NH3 [J]. Microporous and Mesoporous Materials, 2016, 236: 211-217. |
| [38] | LIU Xiaojiao, LI Yonghong, ZHANG Ranran. Ammonia selective catalytic reduction of NO over Ce-Fe/Cu-SSZ-13 catalysts[J]. RSC Advances, 2015, 5(104): 85453-85459. |
| [39] | Beñat PEREDA-AYO, DE LA TORRE Unai, Manuel ROMERO-SÁEZ, et al. Influence of the washcoat characteristics on NH3-SCR behavior of Cu-zeolite monoliths[J]. Catalysis Today, 2013, 216: 82-89. |
| [40] | PUTLURU Siva Sankar Reddy, SCHILL Leonhard, JENSEN Anker Degn, et al. Selective catalytic reduction of NO x with NH3 on Cu-, Fe-, and Mn-zeolites prepared by impregnation: Comparison of activity and hydrothermal stability[J]. Journal of Chemistry, 2018, 2018(1): 8614747. |
| [41] | METKAR Pranit S, HAROLD Michael P, BALAKOTAIAH Vemuri. Selective catalytic reduction of NO x on combined Fe- and Cu-zeolite monolithic catalysts: Sequential and dual layer configurations[J]. Applied Catalysis B: Environmental, 2012, 111: 67-80. |
| [42] | SCHWIDDER M, Santhosh KUMAR M, BRÜCKNER A, et al. Active sites for NO reduction over Fe-ZSM-5 catalysts[J]. Chemical Communications, 2005(6): 805-807. |
| [43] | LONG Richard Q, YANG Ralph T. Superior Fe-ZSM-5 catalyst for selective catalytic reduction of nitric oxide by ammonia[J]. Journal of the American Chemical Society, 1999, 121(23): 5595-5596. |
| [44] | ZHANG Yiwei, ZHOU Yuming, HUANG Li, et al. Structure and catalytic properties of the Zn-modified ZSM-5 supported platinum catalyst for propane dehydrogenation[J]. Chemical Engineering Journal, 2015, 270: 352-361. |
| [45] | DOU Baojuan, Gang LYU, WANG Chang, et al. Cerium doped copper/ZSM-5 catalysts used for the selective catalytic reduction of nitrogen oxide with ammonia[J]. Chemical Engineering Journal, 2015, 270: 549-556. |
| [46] | PANG Lei, FAN Chi, SHAO Lina, et al. The Ce doping Cu/ZSM-5 as a new superior catalyst to remove NO from diesel engine exhaust[J]. Chemical Engineering Journal, 2014, 253: 394-401. |
| [47] | TAO Haixiang, YANG Hong, LIU Xiaohui, et al. Highly stable hierarchical ZSM-5 zeolite with intra- and inter-crystalline porous structures[J]. Chemical Engineering Journal, 2013, 225: 686-694. |
| [48] | TEMPELMAN Christiaan H L, DE RODRIGUES Victor O, VAN ECK Ernst R H, et al. Desilication and silylation of Mo/HZSM-5 for methane dehydroaromatization[J]. Microporous and Mesoporous Materials, 2015, 203: 259-273. |
| [49] | BOUKOUSSA Bouhadjar, AOUAD Nafissa, HAMACHA Rachida, et al. Key factor affecting the structural and textural properties of ZSM-5/MCM-41 composite[J]. Journal of Physics and Chemistry of Solids, 2015, 78: 78-83. |
| [50] | YUE Chuanjun, GAN Miaomiao, GU Liping, et al. In situ synthesized nano-copper over ZSM-5 for the catalytic dehydration of glycerol under mild conditions[J]. Journal of the Taiwan Institute of Chemical Engineers, 2014, 45(4): 1443-1448. |
| [51] | LI Rui, LI Zhibin, CHEN Liqiang, et al. Synthesis of MnNi-SAPO-34 by a one-pot hydrothermal method and its excellent performance for the selective catalytic reduction of NO by NH3 [J]. Catalysis Science & Technology, 2017, 7(21): 4984-4995. |
| [52] | SUTTIPAT Duangkamon, YUTTHALEKHA Thittaya, WANNAPAKDEE Wannaruedee, et al. Tunable acid-base bifunction of hierarchical aluminum-rich zeolites for the one-pot tandem deacetalization-henry reaction[J]. ChemPlusChem, 2019, 84(10): 1503-1507. |
| [53] | WANG Qinqin, ZHU Mingyuan, ZHANG Haiyang, et al. Enhanced catalytic performance of Zr-modified ZSM-5-supported Zn for the hydration of acetylene to acetaldehyde[J]. Catalysis Communications, 2019, 120: 33-37. |
| [54] | TIEULI Sebastiano, Päivi MÄKI-ARVELA, PEURLA Markus, et al. Hydrodeoxygenation of isoeugenol over Ni-SBA-15: Kinetics and modelling[J]. Applied Catalysis A: General, 2019, 580: 1-10. |
| [55] | GHANI N N M, JALIL A A, TRIWAHYONO S, et al. Tailored mesoporosity and acidity of shape-selective fibrous silica beta zeolite for enhanced toluene co-reaction with methanol[J]. Chemical Engineering Science, 2019, 193: 217-229. |
| [1] | BONILLA Griselda, Isabel DÍAZ, TSAPATSIS Michael, et al. Zeolite (MFI) crystal morphology control using organic structure-directing agents[J]. Chemistry of Materials, 2004, 16(26): 5697-5705. |
| [1] | 薛立新, 董永平, 陈梦瑶, 高从堦. 十二烷基硫酸钠(SDS)和强碱(NaOH)对聚酰胺复合纳滤膜的协同调控机理[J]. 化工进展, 2025, 44(4): 2225-2237. |
| [2] | 李灏, 孙昱楠, 李健, 陶俊宇, 程占军, 颜蓓蓓, 陈冠益. 陈腐垃圾与原生垃圾共气化特性[J]. 化工进展, 2025, 44(1): 525-537. |
| [3] | 周媚, 曾浩桀, 卢俊宁, 蒲婷, 刘宝玉. 等级孔分子筛构筑及扩散过程强化研究进展[J]. 化工进展, 2024, 43(1): 76-86. |
| [4] | 唐婷, 周文凤, 王志, 朱晨杰, 许敬亮, 庄伟, 应汉杰, 欧阳平凯. 多酶共固定化技术在糖类催化中的研究进展[J]. 化工进展, 2022, 41(5): 2636-2648. |
| [5] | 孙小康, 冯宇, 李国梅, 刘妙青, 卢建军. 盐酸为氯源“一锅法”绿色高效合成α-氯噻吩[J]. 化工进展, 2020, 39(S2): 305-311. |
| [6] | 刘清, 邓真宁, 滑熠龙, 招国栋. 纳米铁的绿色合成及其在环境中的应用研究进展[J]. 化工进展, 2020, 39(5): 1950-1963. |
| [7] | 栗童,仲兆平,张波. 纤维素与多氢原料共热解的协同效应[J]. 化工进展, 2019, 38(9): 4044-4051. |
| [8] | 叶榆,张凯伦,霍地. 绿茶水热还原制备微纳米金属铜[J]. 化工进展, 2019, 38(03): 1487-1493. |
| [9] | 黄传峰, 韩磊, 霍鹏举, 刘树伟, 程秋香. 煤油共炼残渣与榆林煤共热解特性及半焦性质[J]. 化工进展, 2018, 37(S1): 57-62. |
| [10] | 唐思哲, 胡家秀, 赵健, 柯伟, 王维斌. 新型无机复配缓蚀剂对钠基膨润土中Q235钢的缓蚀作用[J]. 化工进展, 2018, 37(12): 4806-4813. |
| [11] | 严平, 占昌朝, 曹小华, 谢宝华, 徐常龙, 张旭. 原位合成H4SiW12O40@C协同UV/H2O2降解罗丹明B模拟废水[J]. 化工进展, 2015, 34(3): 872-878. |
| [12] | 付友思, 吴又多, 陈丽杰. Zn2+、Ca2+和Mn2+对丙酮丁醇发酵的协同影响[J]. 化工进展, 2015, 34(10): 3719-3724. |
| [13] | 冯茹森, 蒲迪, 周洋, 陈俊华, 寇将, 姜雪, 郭拥军. 混合型烷醇酰胺组成对油/水动态界面张力的影响[J]. 化工进展, 2015, 34(08): 2955-2960. |
| [14] | 马缓,齐暑华,张帆,史金玲. 复合填料/聚丙烯酸酯导电压敏胶的制备与性能[J]. 化工进展, 2014, 33(07): 1791-1795. |
| [15] | 薛晓军,贾广信,何俊辉,李婷. 二甲醚与合成气反应制乙醇的热力学计算与分析[J]. 化工进展, 2014, 33(05): 1160-1163. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||