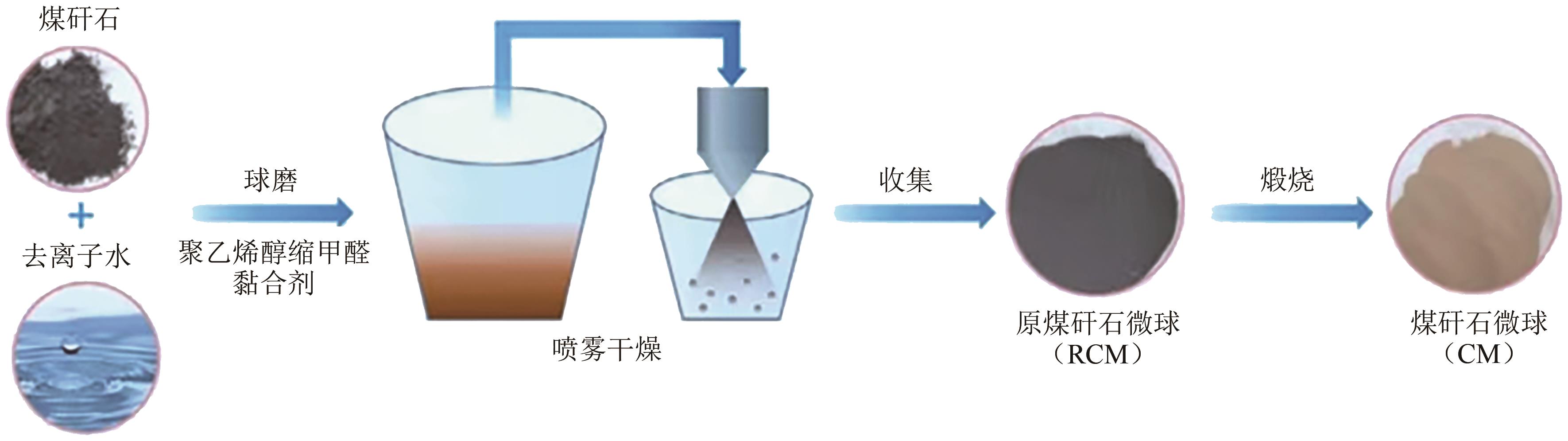
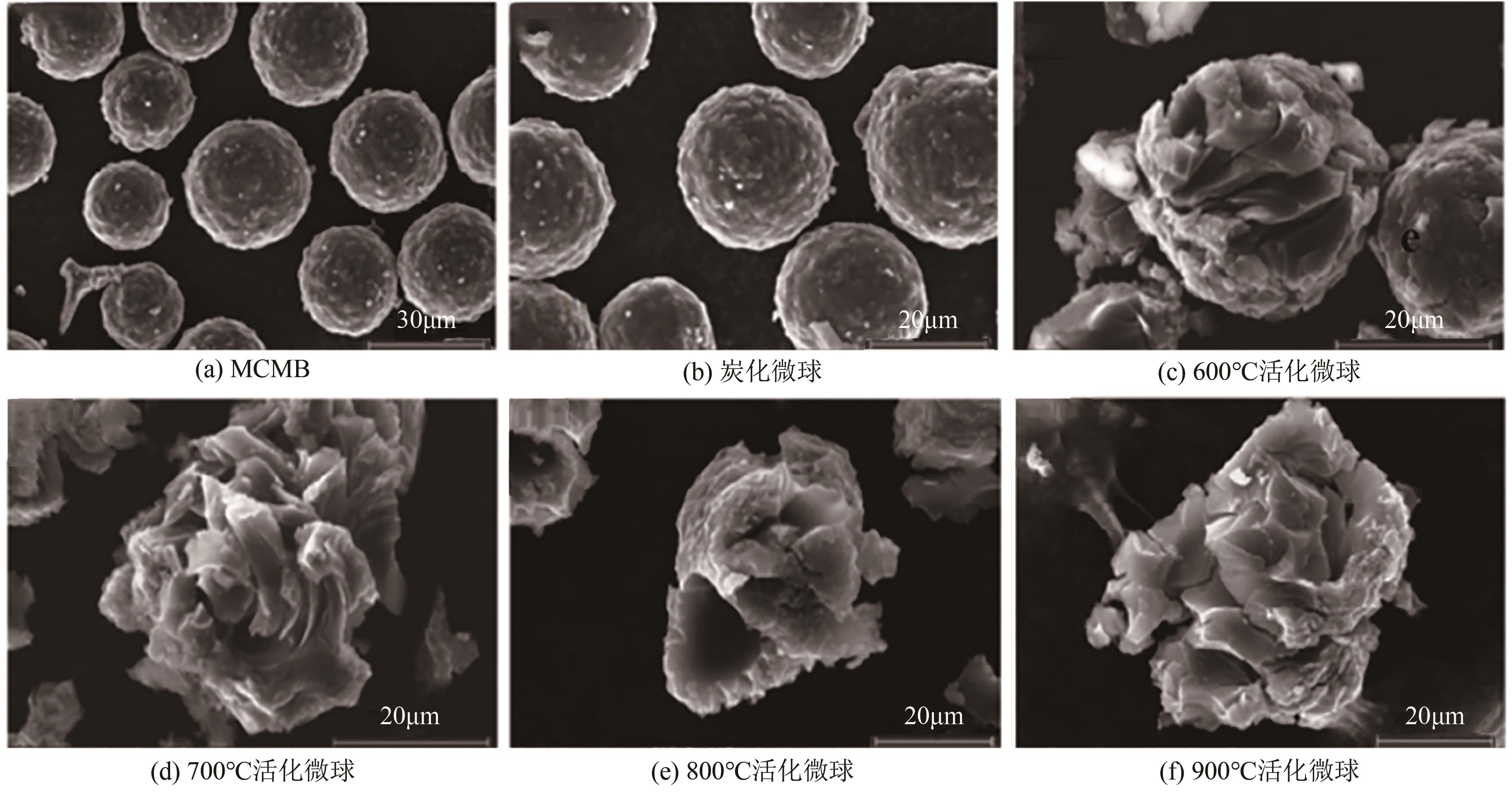
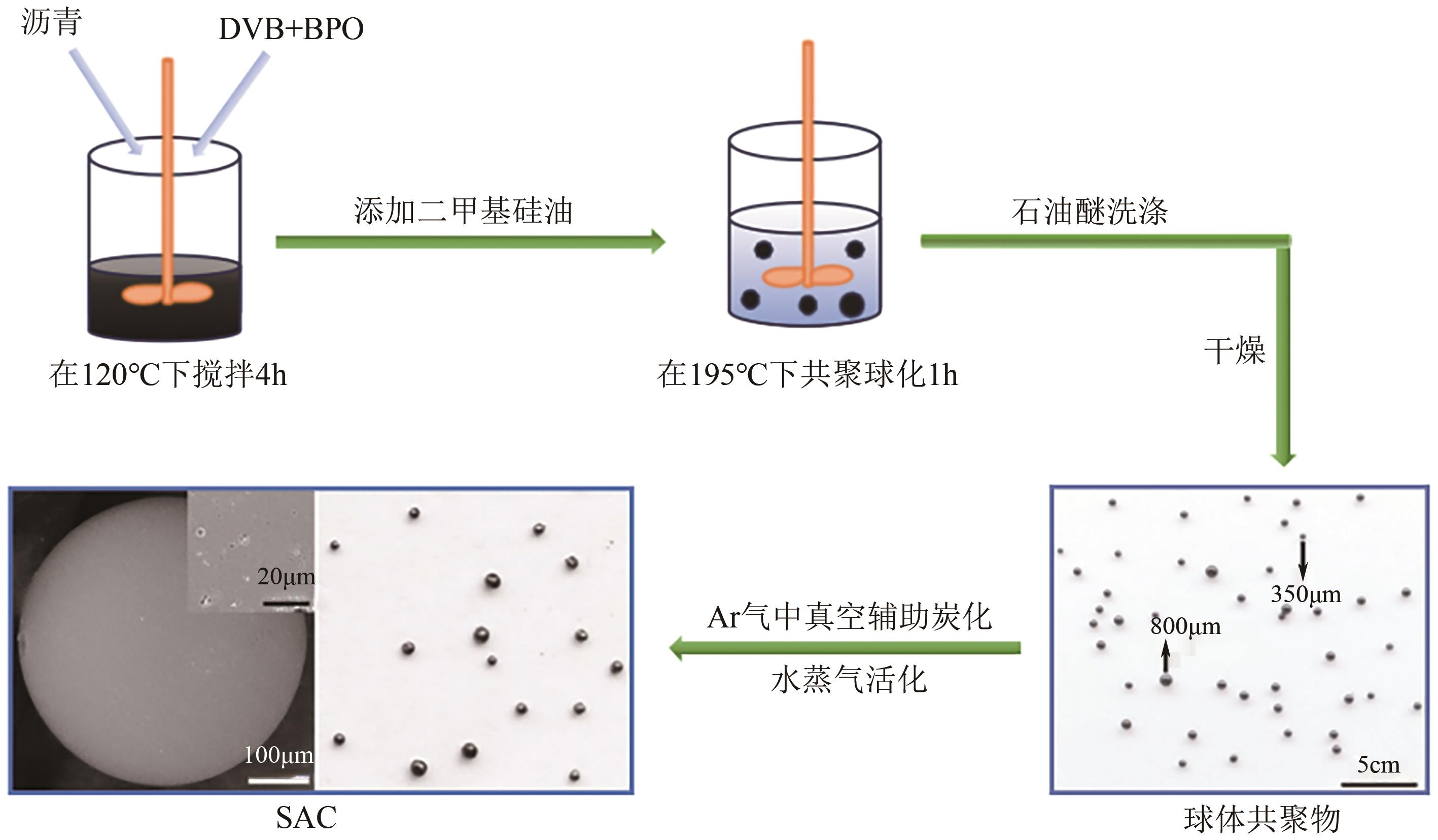
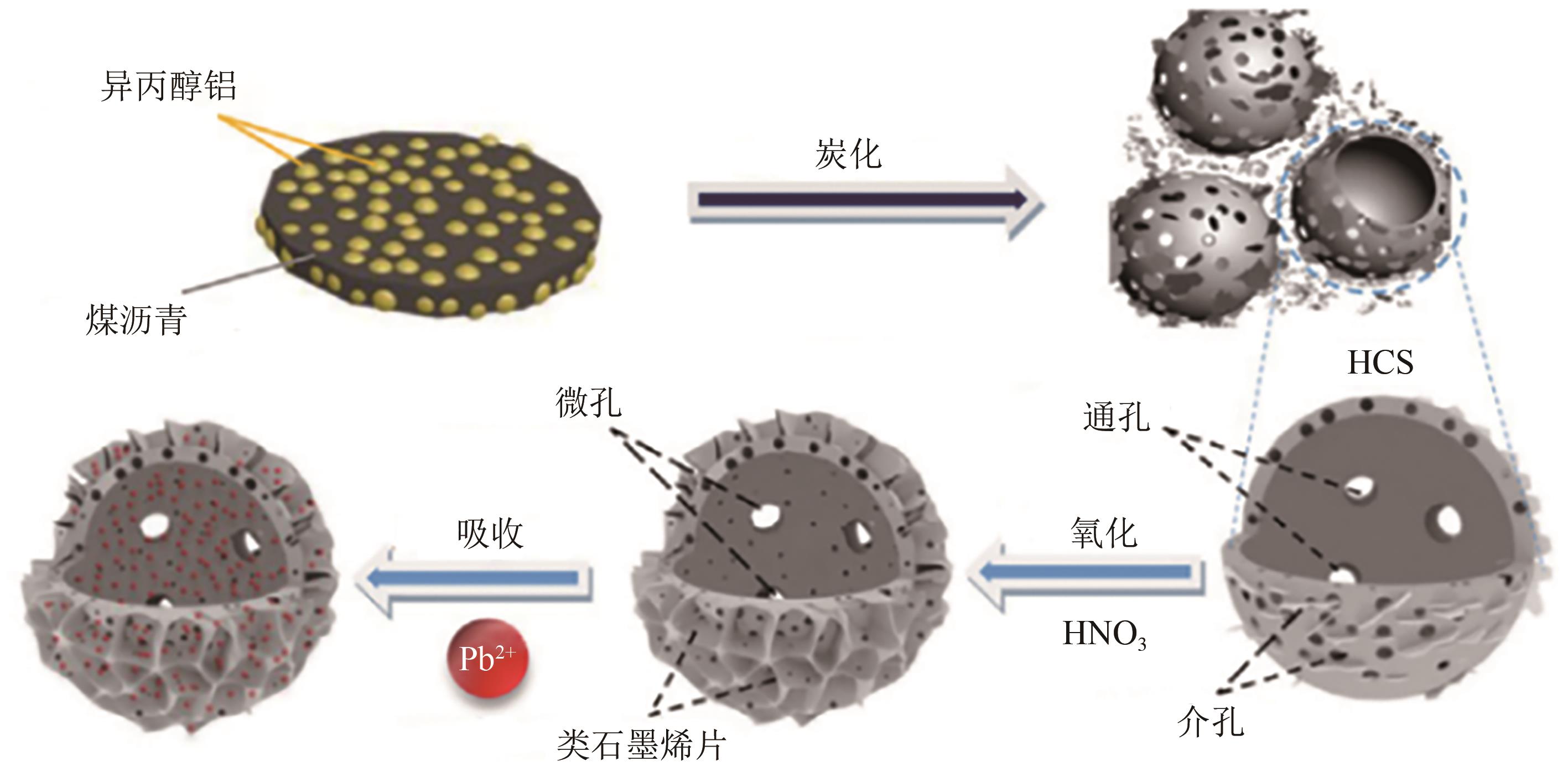
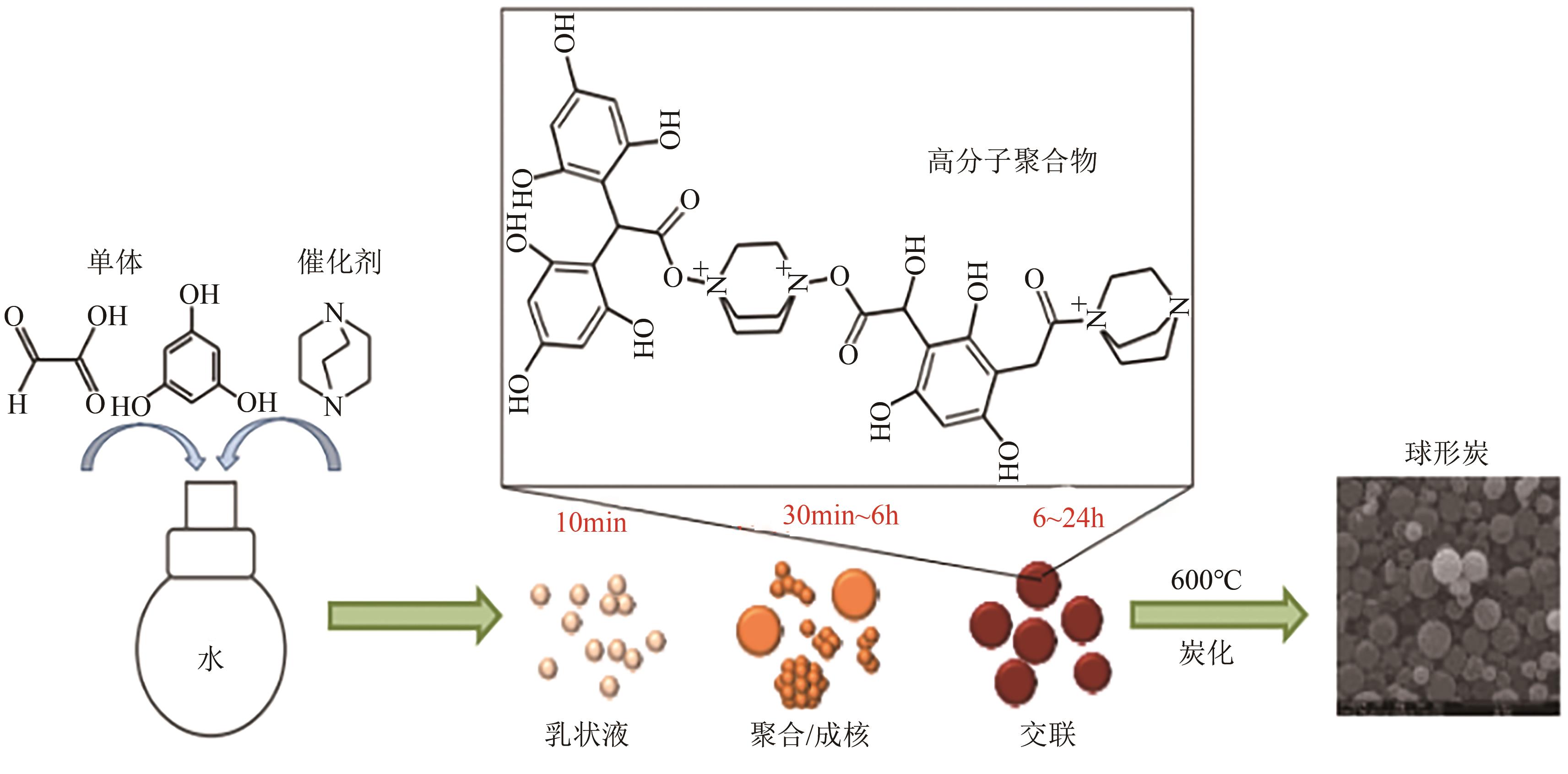
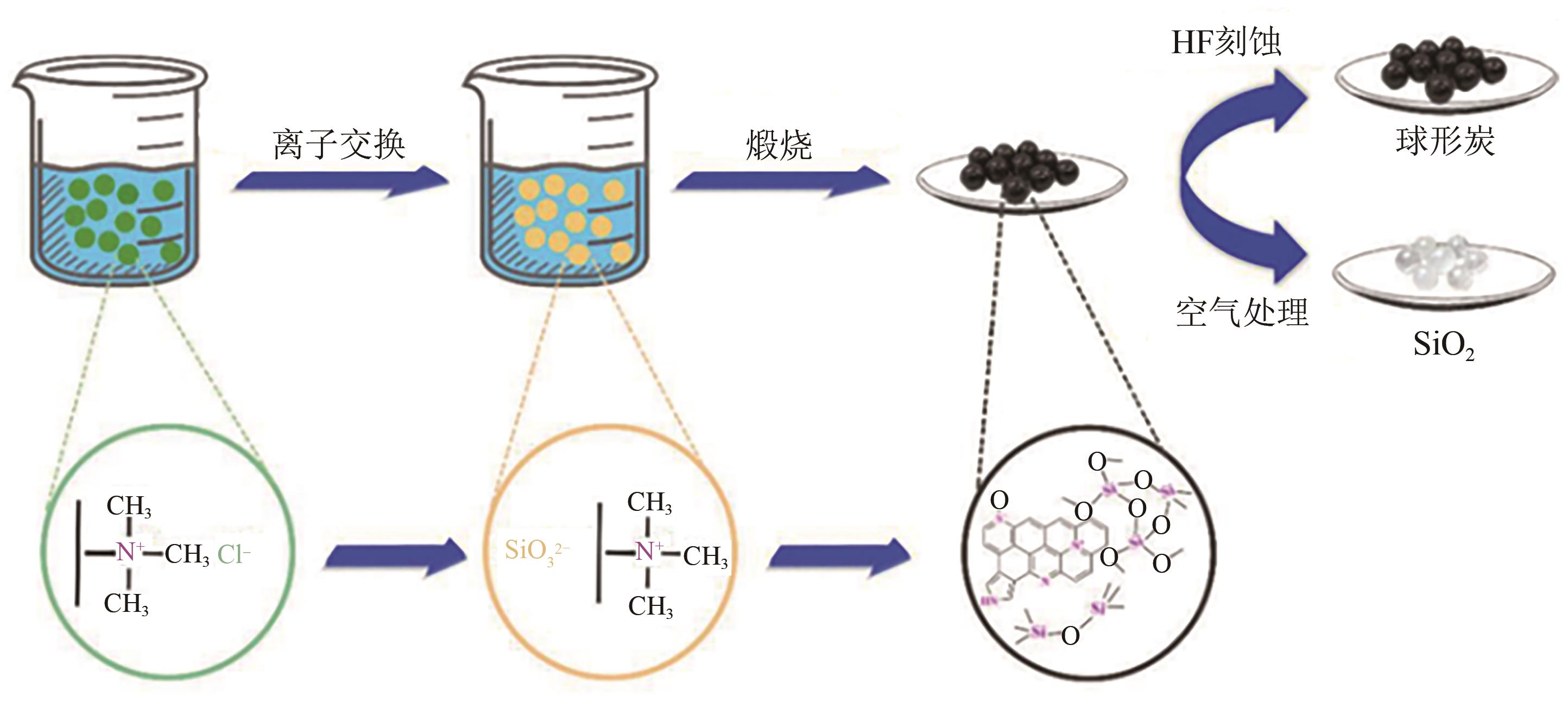
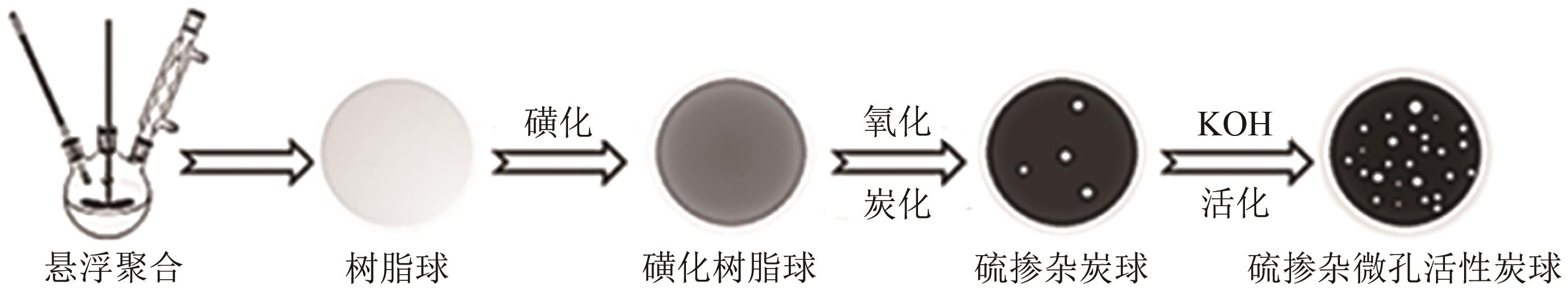
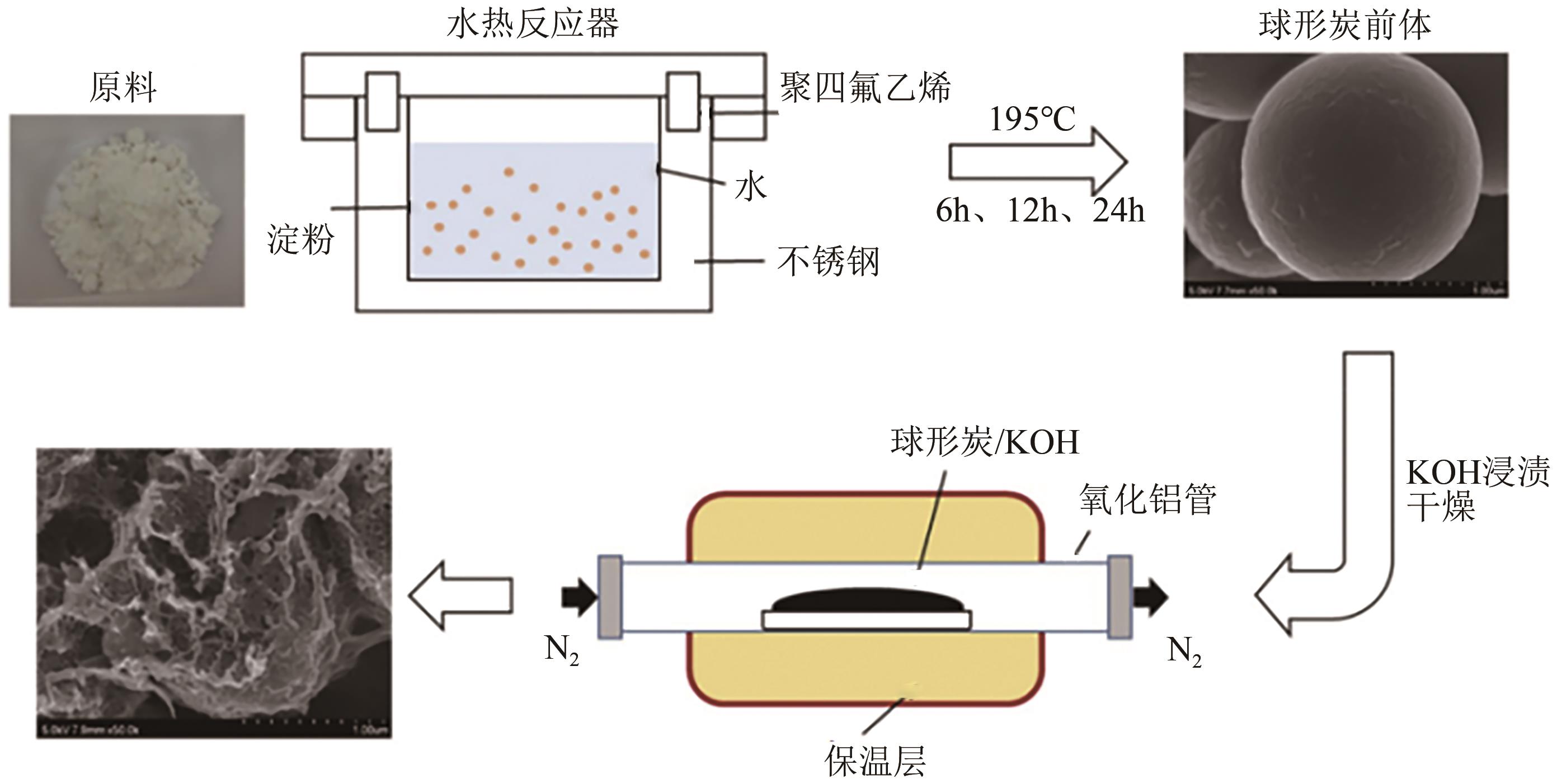
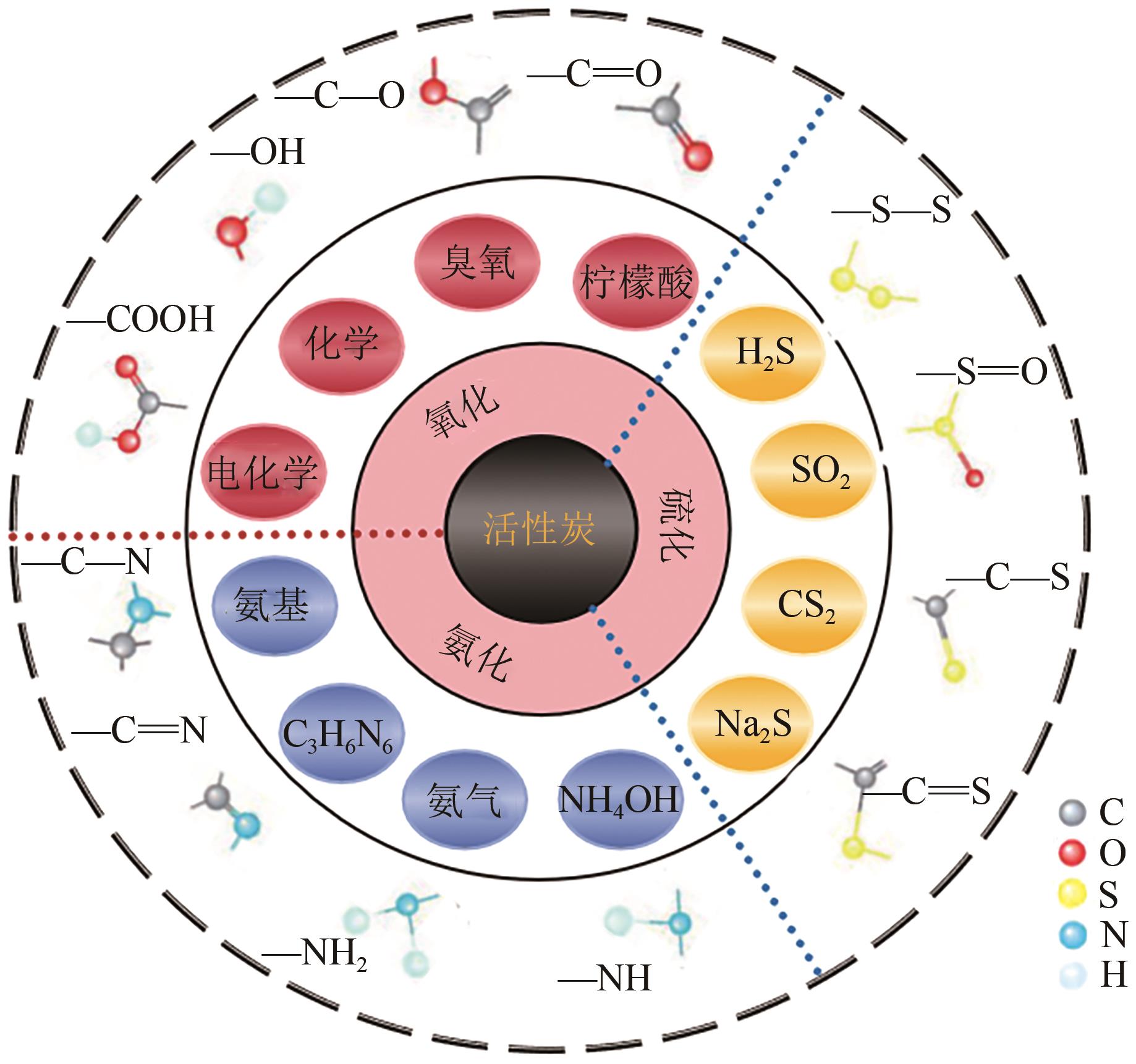
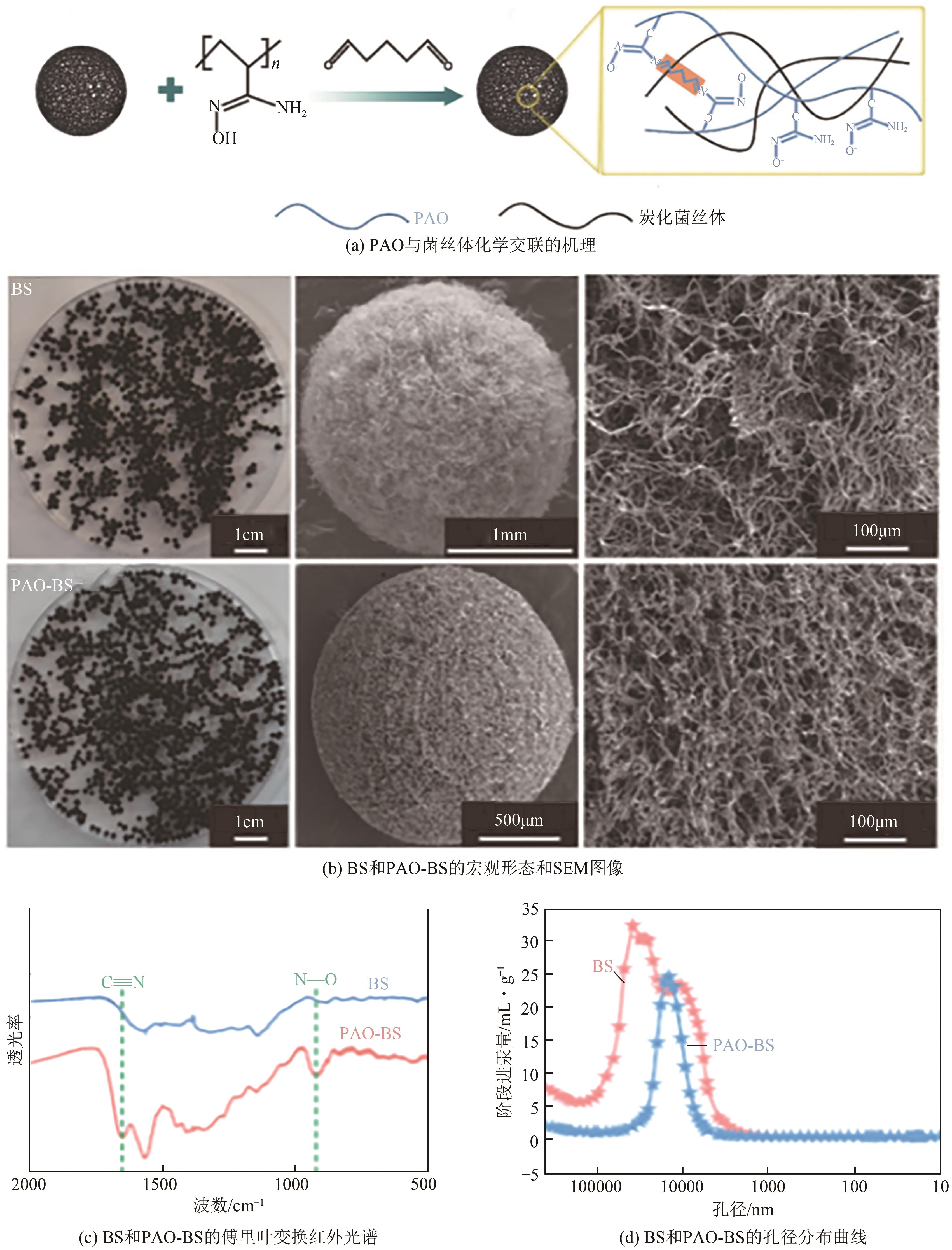
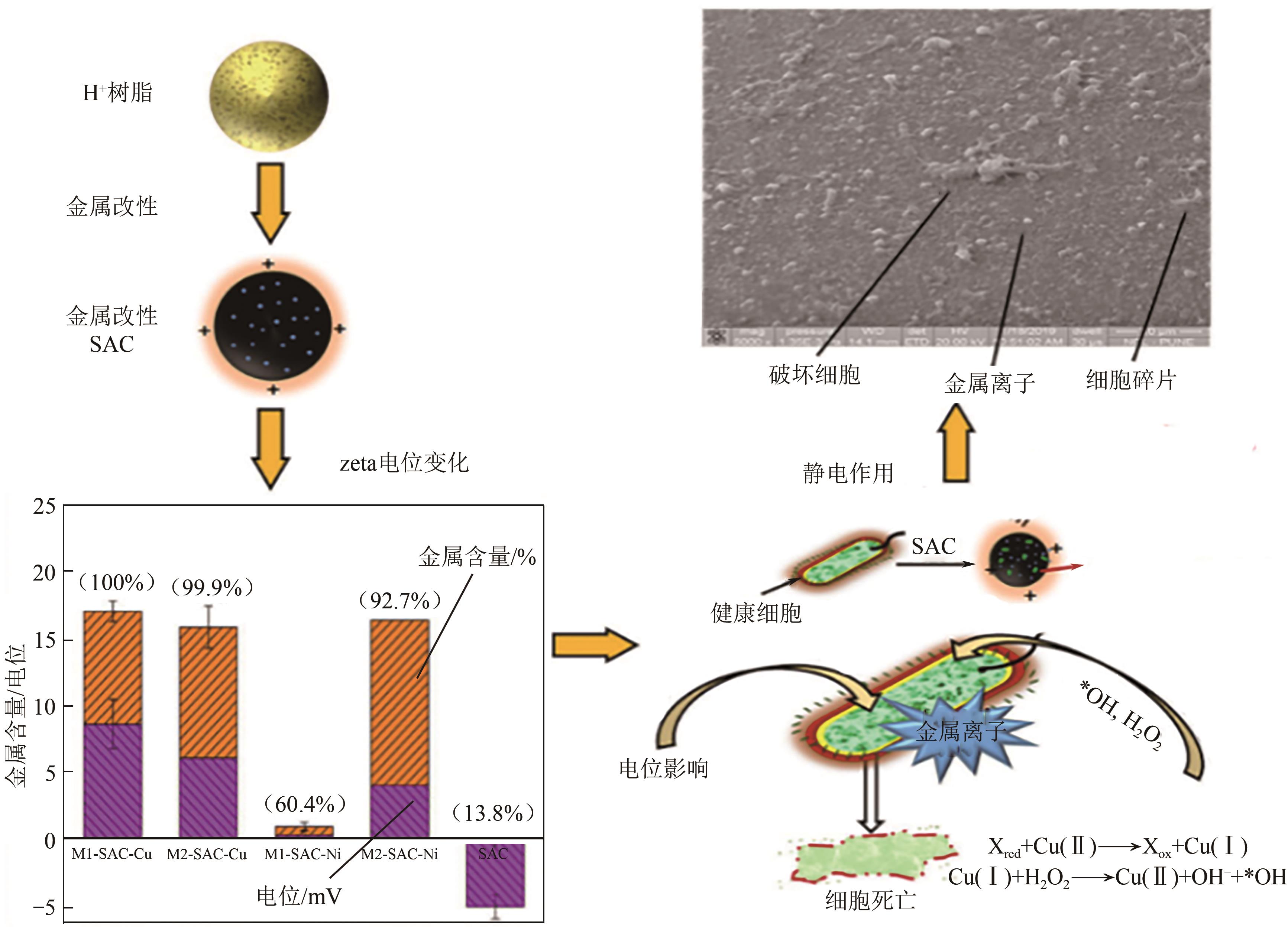
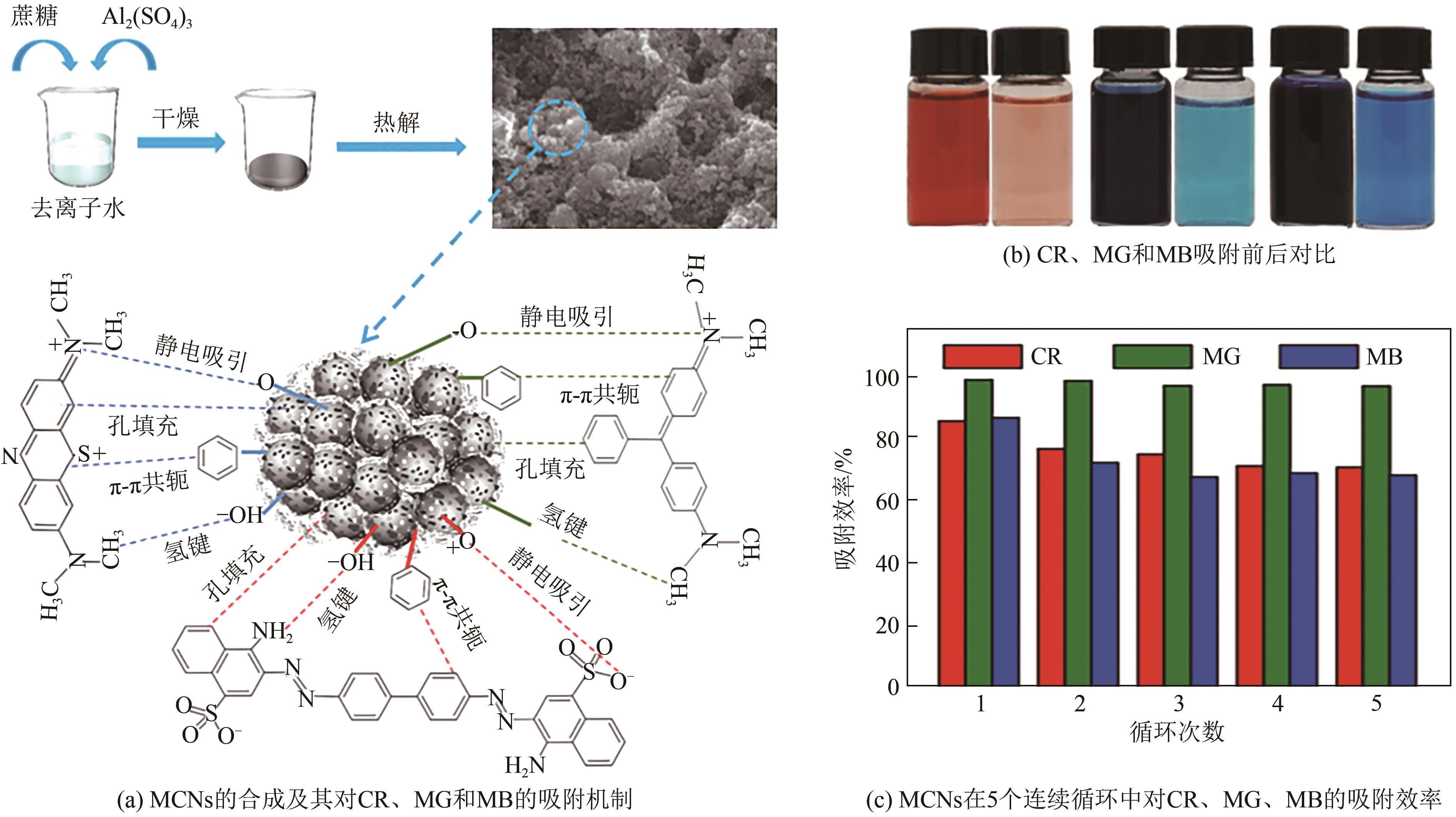
化工进展 ›› 2025, Vol. 44 ›› Issue (4): 2081-2101.DOI: 10.16085/j.issn.1000-6613.2024-0556
球形活性炭的制备、改性及应用研究进展
黄娇1( ), 朱亚明1,2(
), 朱亚明1,2( ), 岳佳兴1, 王莹2,3, 程俊霞1(
), 岳佳兴1, 王莹2,3, 程俊霞1( ), 赵雪飞1(
), 赵雪飞1( )
)
- 1.辽宁科技大学化工学院,辽宁 鞍山 114051
2.辽宁省先进煤焦化技术重点实验室,辽宁 鞍山 114051
3.鞍山兴德材料科技股份有限公司,辽宁 鞍山 114100
-
收稿日期:2024-04-03修回日期:2024-06-05出版日期:2025-04-25发布日期:2025-05-07 -
通讯作者:朱亚明,程俊霞,赵雪飞 -
作者简介:黄娇(2000—),女,硕士研究生,研究方向为沥青基新型炭材料。E-mail:huangjiao1025@163.com。 -
基金资助:国家自然科学基金(22208138);辽宁省自然科学基金(2021-MS-306);辽宁省先进煤焦化技术重点实验室开发课题(2023KFKT-01)
Advances in the preparation, modification and application of spherical activated carbon
HUANG Jiao1( ), ZHU Yaming1,2(
), ZHU Yaming1,2( ), YUE Jiaxing1, WANG Ying2,3, CHENG Junxia1(
), YUE Jiaxing1, WANG Ying2,3, CHENG Junxia1( ), ZHAO Xuefei1(
), ZHAO Xuefei1( )
)
- 1.College of Chemical Engineering, University of Science and Technology Liaoning, Anshan 114051, Liaoning, China
2.The Key Laboratory of Liaoning Province for Advanced Coking Technology, Anshan 114051, Liaoning, China
3.Anshan Xingde Material Technology Co. Ltd. , Anshan 114100, Liaoning, China
-
Received:2024-04-03Revised:2024-06-05Online:2025-04-25Published:2025-05-07 -
Contact:ZHU Yaming, CHENG Junxia, ZHAO Xuefei
摘要:
随着“双碳”目标的深入推进,低碳、低排放的生活方式已被大众普遍认可,因此炭材料的多元化、清洁利用受到广泛关注。球形活性炭(SAC)作为活性炭的一个重要分支,其凭借球形度高、孔隙结构发达、颗粒分布均匀、流动阻力小、机械强度高等优点,在气体捕获、污水净化、能量储存、化学防护、催化等领域具有良好的应用前景。然而,部分前体在制备SAC时存在工艺烦琐、耗能高、耗时长、易产生副产物等问题。为优化SAC的设计思路与制备工艺,本文以SAC在不同领域中的应用发展为导向,综述了SAC的前体选择、制备工艺、改性措施及其在各应用领域的研究成果和进展,并对未来SAC的应用前景进行展望,为实现SAC的多功能、规模化、绿色低碳发展,推动其前体的清洁、高附加值利用提供参考。
中图分类号:
引用本文
黄娇, 朱亚明, 岳佳兴, 王莹, 程俊霞, 赵雪飞. 球形活性炭的制备、改性及应用研究进展[J]. 化工进展, 2025, 44(4): 2081-2101.
HUANG Jiao, ZHU Yaming, YUE Jiaxing, WANG Ying, CHENG Junxia, ZHAO Xuefei. Advances in the preparation, modification and application of spherical activated carbon[J]. Chemical Industry and Engineering Progress, 2025, 44(4): 2081-2101.
| 前体 | 活化剂 | 活化条件 | 比表面积/m2·g-1 | 孔容/cm3·g-1 | 微孔体积/cm3·g-1 | 介孔体积/cm3·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 聚苯乙烯 | 水蒸气 | 840℃,150min | 1749 | 0.97 | 0.69 | 0.28 | [ |
| CO2 | 880℃,150min | 2276 | 1.11 | 0.85 | 0.26 | ||
| 石油沥青 | 水蒸气 | 840℃,360min | 1880 | 0.77 | — | — | [ |
| CO2 | 880℃,1440min | 2586 | 0.97 | — | — | ||
| 石松孢子 | CO2 | 900℃,360min | 3053 | 1.43 | 0.83 | 0.59 | [ |
| 乙基纤维素 | 空气 | 900℃,60min | 1180 | 0.62 | 0.4 | 0.21 | [ |
| 沥青 | KOH | 850℃,360min | 1738.36 | 0.91 | — | — | [ |
| 苯乙烯、二乙烯基苯 | KOH | 800℃,60min | 1631 | 0.771 | 0.598 | — | [ |
| 二乙烯基苯 | ZnCl2 | 800℃,120min | 891 | 0.489 | 0.315 | 0.174 | [ |
| 马铃薯淀粉 | KOH | 900℃,60min | 1579.4 | — | — | — | [ |
| 风化煤腐殖酸 | KOH | 800℃,120min | 2034 | 1.24 | 0.45 | 0.64 | [ |
表1 不同物理和化学活化制备的SAC物理性质
| 前体 | 活化剂 | 活化条件 | 比表面积/m2·g-1 | 孔容/cm3·g-1 | 微孔体积/cm3·g-1 | 介孔体积/cm3·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 聚苯乙烯 | 水蒸气 | 840℃,150min | 1749 | 0.97 | 0.69 | 0.28 | [ |
| CO2 | 880℃,150min | 2276 | 1.11 | 0.85 | 0.26 | ||
| 石油沥青 | 水蒸气 | 840℃,360min | 1880 | 0.77 | — | — | [ |
| CO2 | 880℃,1440min | 2586 | 0.97 | — | — | ||
| 石松孢子 | CO2 | 900℃,360min | 3053 | 1.43 | 0.83 | 0.59 | [ |
| 乙基纤维素 | 空气 | 900℃,60min | 1180 | 0.62 | 0.4 | 0.21 | [ |
| 沥青 | KOH | 850℃,360min | 1738.36 | 0.91 | — | — | [ |
| 苯乙烯、二乙烯基苯 | KOH | 800℃,60min | 1631 | 0.771 | 0.598 | — | [ |
| 二乙烯基苯 | ZnCl2 | 800℃,120min | 891 | 0.489 | 0.315 | 0.174 | [ |
| 马铃薯淀粉 | KOH | 900℃,60min | 1579.4 | — | — | — | [ |
| 风化煤腐殖酸 | KOH | 800℃,120min | 2034 | 1.24 | 0.45 | 0.64 | [ |
| 前体 | 活化剂 | 比表面积/m2·g-1 | 孔容/cm3·g-1 | 吸附质 | 吸附温度/℃ | 吸附模型 | 吸附容量 | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 间苯二酚-甲醛树脂 | — | 1531.29 | 3.527 | 苯 | 25 | — | 21.363mmol/g | [ |
| 间苯二酚-甲醛树脂 | — | 2200.7 | 1.28 | 甲苯 | 25 | Langmuir-Freundlich | 8.64mmol/g | [ |
| 竹焦油 | CO2 | 189 | 0.10 | 甲苯 | 50 | Langmuir | 29mg/g | [ |
| 芒果籽壳 | KOH | 2218 | 1.7755 | 丙酮 | 30 | — | 472m/g | [ |
| 聚苯乙烯树脂 | KOH | 1566 | 1.05 | 二苯并噻吩 | 25 | Freundlich | 105mg/g | [ |
| 聚苯乙烯树脂 | 水蒸气 | 1555 | 1.29 | 二苯并噻吩 | 25 | Freundlich | 102mg/g | |
| 煤焦油沥青 | 水蒸气 | 1573 | 0.68 | 二苯并噻吩 | 25 | Freundlich | 93mg/g | |
| 间甲酚、甲醛 | 水蒸气 | 1501 | 0.72 | 二苯并噻吩 | 25 | Langmuir-Freundlich | 21.83mg/g | [ |
| 木质素磺酸钠 | KOH | 3402 | 2.46 | 二氯甲烷 | 25 | — | 181mg/g | [ |
| 烟煤 | — | 415~983 | 0.22~0.38 | CO2 | 0 | — | 3.19~4.97mmol/g | [ |
| 酚醛树脂 | 水蒸气 | 1128 | 0.45 | CO2 | 25 | Langmuir | 0.79mmol/g | [ |
| 酚醛树脂 | KOH | 1171 | 0.50 | CO2 | 25 | Langmuir | 1.25mmol/g | |
| 聚磷腈 | — | 653 | 0.32 | CO2 | 0 | — | 4.3mg/g | [ |
| 香烟烟蒂 | H3PO4 | 1406 | — | NH3 | 25 | — | 35.9mg/g | [ |
表2 不同前体制备SAC的物理性质及气相吸附效果
| 前体 | 活化剂 | 比表面积/m2·g-1 | 孔容/cm3·g-1 | 吸附质 | 吸附温度/℃ | 吸附模型 | 吸附容量 | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 间苯二酚-甲醛树脂 | — | 1531.29 | 3.527 | 苯 | 25 | — | 21.363mmol/g | [ |
| 间苯二酚-甲醛树脂 | — | 2200.7 | 1.28 | 甲苯 | 25 | Langmuir-Freundlich | 8.64mmol/g | [ |
| 竹焦油 | CO2 | 189 | 0.10 | 甲苯 | 50 | Langmuir | 29mg/g | [ |
| 芒果籽壳 | KOH | 2218 | 1.7755 | 丙酮 | 30 | — | 472m/g | [ |
| 聚苯乙烯树脂 | KOH | 1566 | 1.05 | 二苯并噻吩 | 25 | Freundlich | 105mg/g | [ |
| 聚苯乙烯树脂 | 水蒸气 | 1555 | 1.29 | 二苯并噻吩 | 25 | Freundlich | 102mg/g | |
| 煤焦油沥青 | 水蒸气 | 1573 | 0.68 | 二苯并噻吩 | 25 | Freundlich | 93mg/g | |
| 间甲酚、甲醛 | 水蒸气 | 1501 | 0.72 | 二苯并噻吩 | 25 | Langmuir-Freundlich | 21.83mg/g | [ |
| 木质素磺酸钠 | KOH | 3402 | 2.46 | 二氯甲烷 | 25 | — | 181mg/g | [ |
| 烟煤 | — | 415~983 | 0.22~0.38 | CO2 | 0 | — | 3.19~4.97mmol/g | [ |
| 酚醛树脂 | 水蒸气 | 1128 | 0.45 | CO2 | 25 | Langmuir | 0.79mmol/g | [ |
| 酚醛树脂 | KOH | 1171 | 0.50 | CO2 | 25 | Langmuir | 1.25mmol/g | |
| 聚磷腈 | — | 653 | 0.32 | CO2 | 0 | — | 4.3mg/g | [ |
| 香烟烟蒂 | H3PO4 | 1406 | — | NH3 | 25 | — | 35.9mg/g | [ |
| 前体 | 比表面积/m2·g-1 | 孔容/cm3·g-1 | pH | 动力学模型 | 吸附模型 | 吸附容量 | 参考文献 |
|---|---|---|---|---|---|---|---|
| PBSAC | 1436 | — | 6~8 | 准一级 | Langmuir | U4+-96% | [ |
| 菌丝体 | — | — | 6 | 准二级 | Langmuir | U4+-211.35mg/g | [ |
| 柑橘果胶/AC | 344.343 | 0.389 | 5~6 | 准二级 | Langmuir | Pb2+-279.33mg/g,95.5% | [ |
| 稻壳/陈皮 | 287 | 0.282 | 高pH | 准二级 | Jovanovic | Ni2+-5.18mg/g | [ |
| 戊聚糖 | 13.67 | 0.026 | — | — | Freundlich | Pb2+-380.1mg/g | [ |
| 戊聚糖 | 13.67 | 0.026 | — | — | Langmuir | Cd2+-100.8mg/g | |
| 柳叶 | 294.32 | 0.7638(中) | 2 | 准二级 | Langmuir | Cu2+-12.07mg/g | [ |
| 柳叶 | 294.32 | 0.7638(中) | 4 | 准二级 | Langmuir | Zn2+-30.48mg/g | |
| 柳叶 | 294.32 | 0.7638(中) | 3 | 准二级 | Langmuir | Cr6+-372.46mg/g | |
| 蔗糖/硫酸铝 | 449.25 | 0.57 | 4~10 | 准二级 | Langmuir | CR-1726.60mg/g | [ |
| 蔗糖/硫酸铝 | 449.25 | 0.57 | 2~7 | 准二级 | Langmuir | MG-980.55mg/g | |
| 蔗糖/硫酸铝 | 449.25 | 0.57 | 3~10 | 准二级 | Langmuir | MB-708.82mg/g | |
| 煤焦油 | 1374 | 2.54 | — | — | Langmuir | 直接黑38(DB38)-294mg/g | [ |
| 间苯二酚/正硅酸乙酯 | 1481 | 2.55 | — | 准二级 | Langmuir | 甲基橙(MO)-329mg/g | [ |
| 间苯二酚/正硅酸乙酯 | 1481 | 2.55 | — | 准二级 | Langmuir | 品红(FB)-489mg/g | |
| 间苯二酚/正硅酸乙酯 | 1481 | 2.55 | — | 准二级 | Langmuir | MB-791mg/g | |
| 间苯二酚甲醛树脂 | 371 | 0.56 | 4~10 | 准二级 | Langmuir | RhB-19.9mg/g | [ |
| 废弃离子交换树脂 | 2047 | 1.56 | 3~7 | 准二级 | Langmuir | 四环素-701mg/g | [ |
| 葡萄糖 | 1292 | 0.704 | 2~11 | Elovich | Langmuir | PRC-286mg/g | [ |
| 间苯二酚甲醛树脂 | — | — | — | 准一级 | Langmuir | 环丙沙星-99.6% | [ |
| 树脂 | 904 | 0.37 | 3 | 准二级 | Langmuir | 乙酸-97.5% | [ |
表3 不同前体制备SAC的物理性质及液相吸附效果
| 前体 | 比表面积/m2·g-1 | 孔容/cm3·g-1 | pH | 动力学模型 | 吸附模型 | 吸附容量 | 参考文献 |
|---|---|---|---|---|---|---|---|
| PBSAC | 1436 | — | 6~8 | 准一级 | Langmuir | U4+-96% | [ |
| 菌丝体 | — | — | 6 | 准二级 | Langmuir | U4+-211.35mg/g | [ |
| 柑橘果胶/AC | 344.343 | 0.389 | 5~6 | 准二级 | Langmuir | Pb2+-279.33mg/g,95.5% | [ |
| 稻壳/陈皮 | 287 | 0.282 | 高pH | 准二级 | Jovanovic | Ni2+-5.18mg/g | [ |
| 戊聚糖 | 13.67 | 0.026 | — | — | Freundlich | Pb2+-380.1mg/g | [ |
| 戊聚糖 | 13.67 | 0.026 | — | — | Langmuir | Cd2+-100.8mg/g | |
| 柳叶 | 294.32 | 0.7638(中) | 2 | 准二级 | Langmuir | Cu2+-12.07mg/g | [ |
| 柳叶 | 294.32 | 0.7638(中) | 4 | 准二级 | Langmuir | Zn2+-30.48mg/g | |
| 柳叶 | 294.32 | 0.7638(中) | 3 | 准二级 | Langmuir | Cr6+-372.46mg/g | |
| 蔗糖/硫酸铝 | 449.25 | 0.57 | 4~10 | 准二级 | Langmuir | CR-1726.60mg/g | [ |
| 蔗糖/硫酸铝 | 449.25 | 0.57 | 2~7 | 准二级 | Langmuir | MG-980.55mg/g | |
| 蔗糖/硫酸铝 | 449.25 | 0.57 | 3~10 | 准二级 | Langmuir | MB-708.82mg/g | |
| 煤焦油 | 1374 | 2.54 | — | — | Langmuir | 直接黑38(DB38)-294mg/g | [ |
| 间苯二酚/正硅酸乙酯 | 1481 | 2.55 | — | 准二级 | Langmuir | 甲基橙(MO)-329mg/g | [ |
| 间苯二酚/正硅酸乙酯 | 1481 | 2.55 | — | 准二级 | Langmuir | 品红(FB)-489mg/g | |
| 间苯二酚/正硅酸乙酯 | 1481 | 2.55 | — | 准二级 | Langmuir | MB-791mg/g | |
| 间苯二酚甲醛树脂 | 371 | 0.56 | 4~10 | 准二级 | Langmuir | RhB-19.9mg/g | [ |
| 废弃离子交换树脂 | 2047 | 1.56 | 3~7 | 准二级 | Langmuir | 四环素-701mg/g | [ |
| 葡萄糖 | 1292 | 0.704 | 2~11 | Elovich | Langmuir | PRC-286mg/g | [ |
| 间苯二酚甲醛树脂 | — | — | — | 准一级 | Langmuir | 环丙沙星-99.6% | [ |
| 树脂 | 904 | 0.37 | 3 | 准二级 | Langmuir | 乙酸-97.5% | [ |
| 1 | 康飞宇, 贺艳兵, 李宝华, 等. 炭材料在能量储存与转化中的应用[J]. 新型炭材料, 2011, 26(4): 246-254. |
| KANG Feiyu, HE Yanbing, LI Baohua, et al. Carbon for energy storage and conversion[J]. New Carbon Materials, 2011, 26(4): 246-254. | |
| 2 | NI Mei, ZHOU Lei, LIU Yancen, et al. Advances in the synthesis and applications of porous carbon materials[J]. Frontiers in Chemistry, 2023, 11: 1205280. |
| 3 | 刘于斯, 马超, 王开学, 等. 面向电化学储能的多孔炭材料[J]. 新型炭材料, 2023, 38(1): 1-17. |
| LIU Yusi, MA Chao, WANG Kaixue, et al. Recent advances in porous carbons for electrochemical energy storage[J]. New Carbon Materials, 2023, 38(1): 1-17. | |
| 4 | WANG Xiaohong, CHENG Hairong, YE Guangzheng, et al. Key factors and primary modification methods of activated carbon and their application in adsorption of carbon-based gases: A review[J]. Chemosphere, 2022, 287: 131995. |
| 5 | SAEIDI Navid, LOTFOLLAHI Mohammad Nader. Effects of powder activated carbon particle size on activated carbon monolith’s properties[J]. Materials and Manufacturing Processes, 2016, 31(12): 1634-1638. |
| 6 | KIM Jiyun, KWON Woong, BAI Byong Chol, et al. Recycling of cotton clothing into activated carbon fibers[J]. Carbon Letters, 2022, 32(5): 1315-1327. |
| 7 | YOON Seong-Ho, PARK Yang-Duk, MOCHIDA Isao. Preparation of carbonaceous spheres from suspensions of pitch materials[J]. Carbon, 1992, 30(5): 781-786. |
| 8 | LAN Jingming, WANG Baoying, BO Chunmiao, et al. Progress on fabrication and application of activated carbon sphere in recent decade[J]. Journal of Industrial and Engineering Chemistry, 2023, 120: 47-72. |
| 9 | TRIPATHI Nagesh K. Porous carbon spheres: Recent developments and applications[J]. AIMS Materials Science, 2018, 5(5): 1016-1052. |
| 10 | MA Liang, HE Mengya, FU Pengbo, et al. Adsorption of volatile organic compounds on modified spherical activated carbon in a new cyclonic fluidized bed[J]. Separation and Purification Technology, 2020, 235: 116146. |
| 11 | ZHANG Yang, YU Baojun, ZHANG Jie, et al. Design and preparation of lignin-based hierarchical porous carbon microspheres by high efficient activation for electric double layer capacitors[J]. ChemElectroChem, 2018, 5(15): 2142-2149. |
| 12 | ZHAO Ruihua, WANG Hui, GAO Na, et al. Hollow hemispherical carbon microspheres with Mo2C nanoparticles synthesized by precursor design: Effective noble metal-free catalysts for dehydrogenation[J]. Small Methods, 2020, 4(1): 1900597. |
| 13 | ZHANG Yang, YU Baojun, ZHANG Jie, et al. Design and preparation of lignin-based hierarchical porous carbon microspheres by high efficient activation for electric double layer capacitors[J]. Chem Electro Chem, 2018,5(15): 2142-2149. |
| 14 | ZHANG Changming, SONG Wen, ZHANG Xiaochao, et al. Synthesis and evaluation of activated carbon spheres with copper modification for gaseous elemental mercury removal[J]. Journal of Porous Materials, 2019, 26(3): 693-703. |
| 15 | ZHAO Can, GE Lichao, Longhui MAI, et al. Review on coal-based activated carbon: Preparation, modification, application, regeneration, and perspectives[J]. Energy & Fuels, 2023, 37(16): 11622-11642. |
| 16 | TIAN Hongyu, PAN Jian, ZHU Deqing, et al. Innovative one-step preparation of activated carbon from low-rank coals activated with oxidized pellets[J]. Journal of Cleaner Production, 2021, 313: 127877. |
| 17 | LIU Zhichang, LING Licheng, QIAO Wenming, et al. Effect of hydrogen on the mesopore development of pitch-based spherical activated carbon containing iron during activation by steam[J]. Carbon, 1999, 37(12): 2063-2066. |
| 18 | HUANG Chen-Chia, CHEN Yizuo. Electrochemical performance of supercapacitors with KOH activated mesophase carbon microbead electrodes[J]. Journal of the Taiwan Institute of Chemical Engineers, 2013, 44(4): 611-616. |
| 19 | 冯仲军. 沥青基球形活性炭对亚甲基蓝的吸附特性[J]. 广州化工, 2021, 49(20): 45-49. |
| FENG Zhongjun. Adsorption properties of pitch-based spherical activated carbon for methylene blue[J]. Guangzhou Chemical Industry, 2021, 49(20): 45-49. | |
| 20 | LIU Zhichang, LING Licheng, QIAO Wenming, et al. Preparation of pitch-based spherical activated carbon with developed mesopore by the aid of ferrocene[J]. Carbon, 1999, 37(4): 663-667. |
| 21 | LIN Yinhe, FENG Li, LI Xuhao, et al. Study on ultrasound-assisted oxidative desulfurization for crude oil[J]. Ultrasonics Sonochemistry, 2020, 63: 104946. |
| 22 | MARSH Harry, Francisco RODRÍGUEZ-REINOSO. Activated carbon[M]//Activated Carbon. Amsterdam: Elsevier, 2006. |
| 23 | WANG Dalin, CHEN Mingming, WANG Chengyang, et al. Synthesis of carbon microspheres from urea formaldehyde resin[J]. Materials Letters, 2011, 65(7): 1069-1072. |
| 24 | WANG Qin, LIANG Xiaoyi, ZHANG Rui, et al. Preparation of polystyrene-based activated carbon spheres and their adsorption of dibenzothiophene[J]. New Carbon Materials, 2009, 24(1): 55-60. |
| 25 | ZHU Zhaolian, LI Aimin, ZHONG Sheng, et al. Preparation and characterization of polymer-based spherical activated carbons with tailored pore structure[J]. Journal of Applied Polymer Science, 2008, 109(3): 1692-1698. |
| 26 | Won-Chun OH, KIM Jong-Gyu, KIM Hyuk, et al. Preparation of spherical activated carbon and their physicochemical properties[J]. Journal of the Korean Ceramic Society, 2009, 46(6): 568-573. |
| 27 | YENISOY-KARAKAŞ S, AYGÜN A, GÜNEŞ M, et al. Physical and chemical characteristics of polymer-based spherical activated carbon and its ability to adsorb organics[J]. Carbon, 2004, 42(3): 477-484. |
| 28 | GRIFFITHS Anthony, BOYALL Sarah L, Pia MÜLLER, et al. MOF-based heterogeneous catalysis in continuous flow via incorporation onto polymer-based spherical activated carbon supports[J]. Nanoscale, 2023, 15(44): 17910-17921. |
| 29 | ROMERO-ANAYA A J, OUZZINE M, LILLO-RÓDENAS M A, et al. Spherical carbons: Synthesis, characterization and activation processes[J]. Carbon, 2014, 68: 296-307. |
| 30 | SANKAR S, SARAVANAN S, AHMED Abu Talha Aqueel, et al. Spherical activated-carbon nanoparticles derived from biomass green tea wastes for anode material of lithium-ion battery[J]. Materials Letters, 2019, 240: 189-192. |
| 31 | KIZILDUMAN Berna Koçer, TURHAN Yasemin, Mehmet DOĞAN. Mesoporous carbon spheres produced by hydrothermal carbonization from rice husk: Optimization, characterization and hydrogen storage[J]. Advanced Powder Technology, 2021, 32(11): 4222-4234. |
| 32 | CHENG Feng, LI Xiuwei. Preparation and application of biochar-based catalysts for biofuel production[J]. Catalysts, 2018, 8(9): 346. |
| 33 | 郭旭青, 冯翀, 刘甜甜, 等. 毫米级球形活性炭的制备及应用研究进展[J]. 能源化工, 2023, 44(3): 12-18. |
| GUO Xuqing, FENG Chong, LIU Tiantian, et al. Preparation and application progress of millimeter-scale spherical activated carbon[J]. Energy Chemical Industry, 2023, 44(3): 12-18. | |
| 34 | QIU Jieshan, LI Yongfeng, WANG Yunpeng, et al. A novel form of carbon micro-balls from coal[J]. Carbon, 2003, 41(4): 767-772. |
| 35 | ZHENG Qingxin, MORIMOTO Masato, FOUQUET Thierry, et al. Effect of hydrothermal conditions on production of coal organic microspheres[J]. Fuel, 2018, 234: 1301-1312. |
| 36 | AN Mei, GUO Tuo, GUO Qingjie. Facile preparation of coal-based ultramicroporous carbon microspheres for selective CO2 capture[J]. Carbon Resources Conversion, 2024, 7(3): 100205. |
| 37 | WANG Lu, LIU Jingjing, RONG Yedong, et al. Novel design of microsphere adsorbent for efficient heavy metals adsorption[J]. International Journal of Applied Ceramic Technology, 2020, 17(5): 2228-2239. |
| 38 | YAN Lidong, FANG Yilin, DENG Jianfeng, et al. Preparation and characterization of mesocarbon microbeads by the co-polycondensation of high-temperature coal tar pitch and coal pyrolytic extracts[J]. Materials, 2022, 15(15): 5136. |
| 39 | 龚鑫, 刘小冬, 温福山, 等. 中间相炭微球乳化-聚合法制备及电化学性能[J]. 化工进展, 2022, 41(5): 2379-2388. |
| GONG Xin, LIU Xiaodong, WEN Fushan, et al. Preparation and electrochemical performance of mesocarbon microbeads derived from emulsion-polymerization method[J]. Chemical Industry and Engineering Progress, 2022, 41(5): 2379-2388. | |
| 40 | CHEN Lei, FAN Xiaohua, JIANG Zhao, et al. Observation of “in-contact” characteristics of Brooks-Taylor mesophase spheres obtained by high-temperature centrifugation[J]. Carbon, 2016, 103: 421-424. |
| 41 | PHICIATO Phiciato, THAHA Yudi Nugraha, DARSONO Nono, et al. Production of monodisperse spherical mesophase from coal tar pitch by solvent extraction[J]. Carbon Letters, 2023, 33(6): 1669-1677. |
| 42 | ISHII Chiaki, KANEKO Katsumi. Anomalous magnetism of activated carbon having ultra high surface area[J]. Tanso, 2000, 2000(193): 218-222. |
| 43 | 李枢相, 高源, 夏晶, 等. KOH活化中间相炭微球及其在超级电容器中电化学性能的研究[J]. 炭素技术, 2023, 42(3): 48-52. |
| LI Shuxiang, GAO Yuan, XIA Jing, et al. Preparation of KOH activated mesocarbon microbeads and the electrochemical properties in supercapacitors[J]. Carbon Techniques, 2023, 42(3): 48-52. | |
| 44 | SUN Shuai, WANG Lei, WANG Chengyang, et al. Vacuum-assisted synthesis of spherical activated carbon using coal tar pitch with low softening point by copolymerization[J]. Vacuum, 2018, 158: 215-217. |
| 45 | 焦桂萍, 陈发旺, 陈红香, 等. 沥青基球形活性炭成球工艺研究[J]. 舰船科学技术, 2014, 36(8): 133-136. |
| JIAO Guiping, CHEN Fawang, CHEN Hongxiang, et al. Research on the sphericizing technique of pitch-based spherical activated carbon[J]. Ship Science and Technology, 2014, 36(8): 133-136. | |
| 46 | LIANG Aihua, XU Tinghao, LIOU Sin, et al. Silicon single walled carbon nanotube-embedded pitch-based carbon spheres prepared by a spray process with modified antisolvent precipitation for lithium ion batteries[J]. Energy & Fuels, 2021, 35(11): 9705-9713. |
| 47 | IBRAHIM MOHAMMED Mohammed, ISMAEEL IBRAHIM Raheek, MAHMOUD Luma Hussein, et al. Characteristics of carbon nanospheres prepared from locally deoiled asphalt[J]. Advances in Materials Science and Engineering, 2013, 2013(1): 356769. |
| 48 | ZHANG Xialan, WANG Weiqiang, LUO Shiyuan, et al. Preparation of discrete cage-like oxidized hollow carbon spheres with vertically aligned graphene-like nanosheet surface for high performance Pb2+ absorption[J]. Journal of Colloid and Interface Science, 2019, 553: 484-493. |
| 49 | WANG Yang, CHEN Fenghua, HAN Weijian, et al. Carbothermal synthesis of zirconium carbide hollow microspheres from polyzirconoxane and phenolic resin by spray drying[J]. Ceramics International, 2022, 48(2): 2793-2801. |
| 50 | LIU Jingjing, SUN Nannan, SUN Chenggong, et al. Spherical potassium intercalated activated carbon beads for pulverised fuel CO2 post-combustion capture[J]. Carbon, 2015, 94: 243-255. |
| 51 | ZOU Zhimin, JIANG Chunhai. Solvothermal polycondensation of novolacs phenolic-resin to nanopowders and their derived activated nanocarbons as efficient adsorbents for water cleaning[J]. Journal of Porous Materials, 2017, 24(6): 1555-1564. |
| 52 | LIU Jian, QIAO Shizhang, LIU Hao, et al. Extension of the Stöber method to the preparation of monodisperse resorcinol-formaldehyde resin polymer and carbon spheres[J]. Angewandte Chemie International Edition, 2011, 50(26): 5947-5951. |
| 53 | MAETZ Amandine, DELMOTTE Luc, MOUSSA Georges, et al. Facile and sustainable synthesis of nitrogen-doped polymer and carbon porous spheres[J]. Green Chemistry, 2017, 19(9): 2266-2274. |
| 54 | LIGUORI Francesca, Carmen MORENO-MARRODAN, BARBARO Pierluigi. Metal nanoparticles immobilized on ion-exchange resins: A versatile and effective catalyst platform for sustainable chemistry[J]. Chinese Journal of Catalysis, 2015, 36(8): 1157-1169. |
| 55 | KIM Tae Gyun, LEE Dong Won, LEE Chang Ha, et al. Investigation on hydrogen storage capacity of spherical activated carbons from ion exchange resins[J]. Korean Journal of Chemical Engineering, 2023, 40(10): 2463-2471. |
| 56 | WANG Jing, FAN Sisi, DUAN Hongmin, et al. Anion exchange resin based porous carbon spheres for the catalytic 1,2-dehydrochlorination of dichloroethane[J]. The Journal of Physical Chemistry C, 2021, 125(44): 24422-24428. |
| 57 | SUN Yahui, ZHAO Jianghong, WANG Jianlong, et al. Sulfur-doped millimeter-sized microporous activated carbon spheres derived from sulfonated poly(styrene-divinylbenzene) for CO2 capture[J]. The Journal of Physical Chemistry C, 2017, 121(18): 10000-10009. |
| 58 | ZHU Zhaolian, LI Aimin, ZHONG Sheng, et al. Preparation and characterization of polymer-based spherical activated carbons with tailored pore structure[J]. Journal of Applied Polymer Science, 2008, 109(3): 1692-1698. |
| 59 | KIM Yong Il, LEE Yun Jung, YOO Jungjoon, et al. High-capacitance activated bio-carbons with controlled pore size distribution for sustainable energy storage[J]. Journal of Power Sources, 2019, 438: 226969. |
| 60 | HAO Sufen, ZHANG Qian, WANG Yu, et al. Preparation and adsorption properties of green sustainable biomass carbon microspheres[J]. Industrial & Engineering Chemistry Research, 2022, 61(30): 11249-11261. |
| 61 | 马玉柱, 周聪, 于宝军, 等. 生物质基球形活性炭的制备及其电化学性能[J]. 储能科学与技术, 2016, 5(6): 855-860. |
| MA Yuzhu, ZHOU Cong, YU Baojun, et al. Study on preparation and electrochemical properties of biomass-derived spherical activated carbon[J]. Energy Storage Science and Technology, 2016, 5(6): 855-860. | |
| 62 | ROMERO-ANAYA A J, LILLO-RÓDENAS M A, LINARES-SOLANO A. Spherical activated carbons for low concentration toluene adsorption[J]. Carbon, 2010, 48(9): 2625-2633. |
| 63 | JIN Yiyi, TIAN Kuan, WEI Lu, et al. Hierarchical porous microspheres of activated carbon with a high surface area from spores for electrochemical double-layer capacitors[J]. Journal of Materials Chemistry A, 2016, 4(41): 15968-15979. |
| 64 | SUN Peipei, ZHANG Kaitao, SHANG Shibin, et al. Sustainable production of activated carbon spheres from ethyl cellulose[J]. RSC Advances, 2016, 6(98): 95656-95662. |
| 65 | WU Juncheng, WANG Jianlong, GUAN Taotao, et al. Adsorption and decolorization of hydrogenated coal tar on resin-based activated carbon spheres[J]. New Carbon Materials, 2021, 36(4): 843-850. |
| 66 | GANG Daniel, UDDIN AHMAD Zaki, LIAN Qiyu, et al. A review of adsorptive remediation of environmental pollutants from aqueous phase by ordered mesoporous carbon[J]. Chemical Engineering Journal, 2021, 403: 126286. |
| 67 | HAN Weijie, WANG Xugen, ZHU Mingyuan, et al. Melamine modification of spherical activated carbon and its effects on acetylene hydrochlorination[J]. Journal of Wuhan University of Technology-Mater Sci Ed, 2014, 29(6): 1147-1151. |
| 68 | LI Tong, ZHANG Jianjun, LI Chongxing, et al. Nitrogen and phosphorous co-doped hierarchical meso-microporous carbon nanospheres with extraordinary lithium storage for high-performance lithium-ion capacitors[J]. Science China Materials, 2022, 65(9): 2363-2372. |
| 69 | HASSAN M, GONDAL M A, CEVIK E, et al. High performance pliable supercapacitor fabricated using activated carbon nanospheres intercalated into boron nitride nanoplates by pulsed laser ablation technique[J]. Arabian Journal of Chemistry, 2020, 13(8): 6696-6707. |
| 70 | YANG Xiaodong, WAN Yongshan, ZHENG Yulin, et al. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: A critical review[J]. Chemical Engineering Journal, 2019, 366: 608-621. |
| 71 | WANG Yue, CAO Meng, PENG Qin, et al. Polyamidoxime-loaded biochar sphere with high water permeability for fast and effective recovery of uranium from seawater[J]. Journal of Water Process Engineering, 2023, 55: 104205. |
| 72 | MANE M B, BHANDARI V M. Developing spherical activated carbons from polymeric resins for removal of contaminants from aqueous and organic streams[J]. International Journal of Environmental Science and Technology, 2022, 19(10): 10021-10040. |
| 73 | CHANDRA JOSHI Harish, DUTTA Dhiraj, GAUR Nisha, et al. Silver-doped active carbon spheres and their application for microbial decontamination of water[J]. Heliyon, 2022, 8(4): e09209. |
| 74 | DONG Ning, WANG Ze, WANG Jun, et al. Preparation of CPVC-based activated carbon spheres and insight into the adsorption-desorption performance for typical volatile organic compounds[J]. Environmental Pollution, 2024, 343: 123177. |
| 75 | WU Liangyi, YANG Liuchun. A novel micro-sphere activated carbon synthesized from waste cigarette butts for ammonia adsorption[J]. Waste Management, 2023, 168: 396-405. |
| 76 | CHEN Guanyu, YANG Xiaobing, MA Yuansheng, et al. Structurally controllable hollow carbon spheres for gaseous benzene adsorption[J]. Journal of Environmental Chemical Engineering, 2023, 11(1): 109182. |
| 77 | LI Jinjin, CHENG Tangying, MA Xiuwei, et al. Toluene and water vapor adsorption characteristics and selectivity on hydrophobic resin-based activated carbon[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2022, 642: 128604. |
| 78 | HUANG Yingpin, Hsing-Cheng HSI, LIU Szu-Chen. Preparation of spherical activated phenol-formaldehyde beads from bamboo tar for adsorption of toluene[J]. Journal of the Air & Waste Management Association, 2013, 63(8): 977-983. |
| 79 | DE ANDRADE Robson C, MENEZES Rodrigo S Gonzaga, FIUZA-JR Raildo A, et al. Activated carbon microspheres derived from hydrothermally treated mango seed shells for acetone vapor removal[J]. Carbon Letters, 2021, 31(4): 779-793. |
| 80 | LI Hongqiang, HE Xiaojun, WU Tingting, et al. Synthesis, modification strategies and applications of coal-based carbon materials[J]. Fuel Processing Technology, 2022, 230: 107203. |
| 81 | LIU Shuang, WU Shubin, CHENG Hao, et al. Sodium lignosulfonate derived hierarchical porous carbon spheres for VOC removal and supercapacitors[J]. Industrial Crops and Products, 2022, 179: 114657. |
| 82 | WANG Yahuan, WANG Minghuan, WANG Zhiwei, et al. Tunable-quaternary (N, S, O, P)-doped porous carbon microspheres with ultramicropores for CO2 capture[J]. Applied Surface Science, 2020, 507: 145130. |
| 83 | BOUSSOUGA Youssef-Amine, JOSEPH James, STRYHANYUK Hryhoriy, et al. Adsorption of uranium(Ⅵ) complexes with polymer-based spherical activated carbon[J]. Water Research, 2024, 249: 120825. |
| 84 | ZHANG Xialan, WANG Xin, CHENG Ting, et al. A novel mesoporous carbon nanospheres-based adsorbent material with desirable performances for dyes removal[J]. Journal of Molecular Liquids, 2023, 390: 123091. |
| 85 | TRAN Hai Nguyen, TOMUL Fatma, HOANG HA Nguyen THI, et al. Innovative spherical biochar for pharmaceutical removal from water: Insight into adsorption mechanism[J]. Journal of Hazardous Materials, 2020, 394: 122255. |
| 86 | LAISHRAM Devika, KUMAR Divya, KANT Vishav, et al. Activated hollow and solid carbon spheres for enhanced removal efficiency of pharmaceutical pollutants and heavy metals in water[J]. Water, Air, & Soil Pollution, 2022, 233(10): 404. |
| 87 | WU Huiling, WEI Huangzhao, YANG Xu, et al. Spherical activated carbons derived from resin-microspheres for the adsorption of acetic acid[J]. Journal of Environmental Chemical Engineering, 2023, 11(2): 109394. |
| 88 | WANG Risi, LI Ya, SHUAI Xixiang, et al. Pectin/activated carbon-based porous microsphere for Pb2+ adsorption: Characterization and adsorption behaviour[J]. Polymers, 2021, 13(15): 2453. |
| 89 | DIAZ DE TUESTA Jose L, ROMAN Fernanda F, MARQUES Vitor C, et al. Performance and modeling of Ni(Ⅱ) adsorption from low concentrated wastewater on carbon microspheres prepared from tangerine peels by FeCl3-assisted hydrothermal carbonization[J]. Journal of Environmental Chemical Engineering, 2022, 10(5): 108143. |
| 90 | WU Qiong, LI Wei, LIU Shouxin. Carboxyl-rich carbon microspheres prepared from pentosan with high adsorption capacity for heavy metal ions[J]. Materials Research Bulletin, 2014, 60: 516-523. |
| 91 | QU Jiao, ZHANG Qian, XIA Yunsheng, et al. Synthesis of carbon nanospheres using fallen willow leaves and adsorption of rhodamine B and heavy metals by them[J]. Environmental Science and Pollution Research International, 2015, 22(2): 1408-1419. |
| 92 | ZHANG Chen, ZHANG Weihao, YU Moxin, et al. Synthesis of hollow porous carbon nanospheres from coal tar for adsorption of direct black 38 dye[J]. Journal of Porous Materials, 2017, 24(5): 1289-1293. |
| 93 | CHEN Aibing, LI Yuetong, YU Yifeng, et al. Synthesis of mesoporous carbon nanospheres for highly efficient adsorption of bulky dye molecules[J]. Journal of Materials Science, 2016, 51(14): 7016-7028. |
| 94 | SALEH Tawfik A, Islam ALI. Synthesis of polyamide grafted carbon microspheres for removal of rhodamine B dye and heavy metals[J]. Journal of Environmental Chemical Engineering, 2018, 6(4): 5361-5368. |
| 95 | LI Aiduo, CHEN Zhiwei, MA Cheng, et al. Development of resin based spherical activated carbon with high performance derived from waste ion-exchange resin and its high effective removal of tetracycline[J]. ChemistrySelect, 2023, 8(33): e202301279. |
| 96 | CAI Xinyu, XIAO Yan, SUN Wei, et al. Electrochemical performance of symmetric supercapacitors under compression: Size effects of activated carbon spheres[J]. International Journal of Energy Research, 2022, 46(9): 12871-12884. |
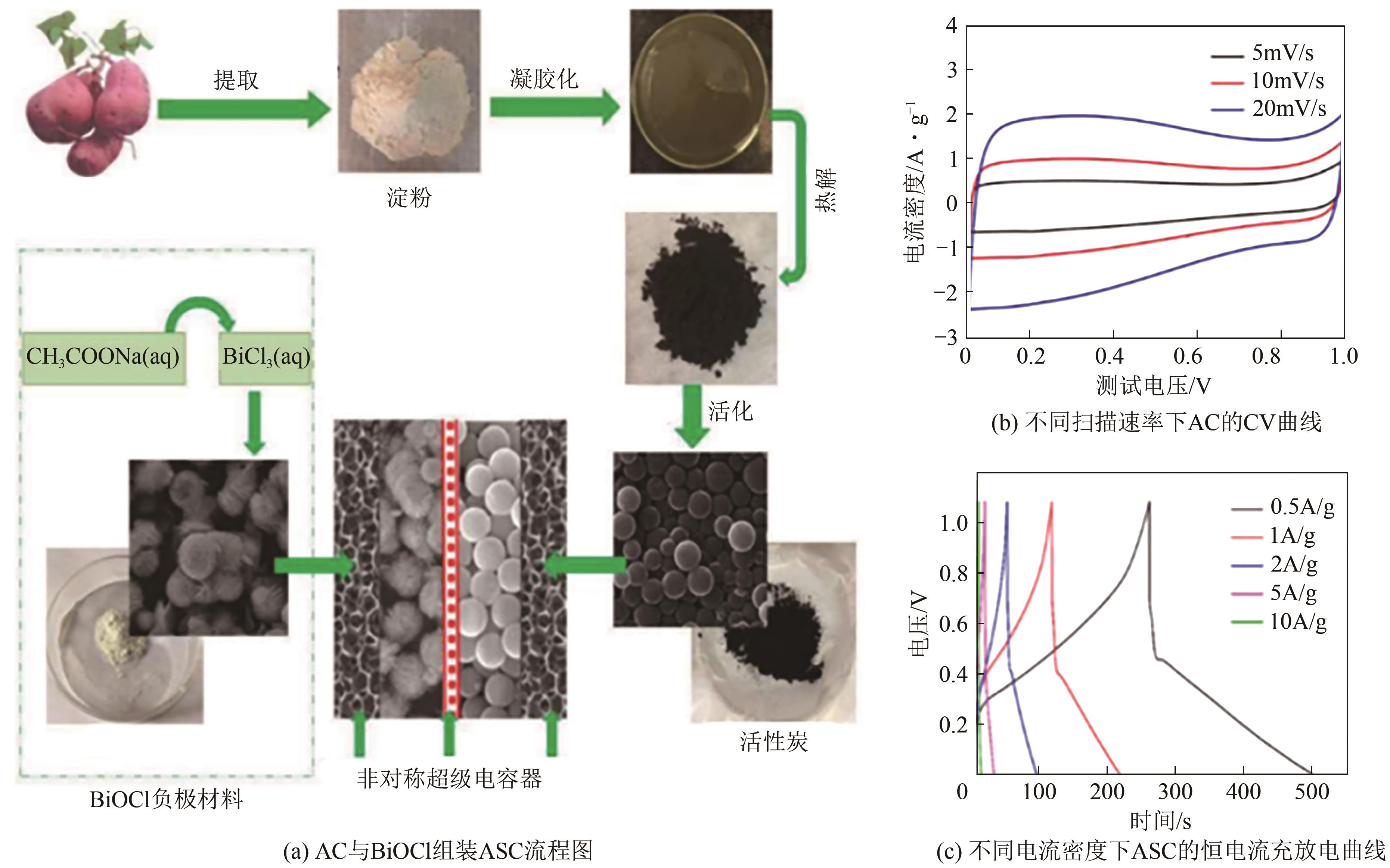
| 97 | HONG Weifeng, WANG Luo, LIU Kun, et al. Asymmetric supercapacitor constructed by self-assembled camellia-like BiOCl and activated carbon microspheres derived from sweet potato starch[J]. Journal of Alloys and Compounds, 2018, 746: 292-300. |
| 98 | 宋燕, 乔文明, 凌立成, 等. 球状活性炭及其应用进展[J]. 炭素, 1999(1): 3-6. |
| SONG Yan, QIAO Wenming, LING Licheng, et al. Recent research and development on spherical activated carbon[J]. Carbon, 1999(1): 3-6. | |
| 99 | JIANG Jinhua, GAO Qiuming, ZHENG Zhoujun, et al. Enhanced room temperature hydrogen storage capacity of hollow nitrogen-containing carbon spheres[J]. International Journal of Hydrogen Energy, 2010, 35(1): 210-216. |
| 100 | ZLOTEA Claudia, CUEVAS Fermin, Valérie PAUL-BONCOUR, et al. Size-dependent hydrogen sorption in ultrasmall Pd clusters embedded in a mesoporous carbon template[J]. Journal of the American Chemical Society, 2010, 132(22): 7720-7729. |
| 108 | SATHE Manisha, SHARMA Pushpendra K, SINGH Virendra K, et al. Chemical protection studies of activated carbon spheres based permeable protective clothing against sulfur mustard, a chemical warfare agent[J]. Defence Science Journal, 2019, 69(6): 577-584. |
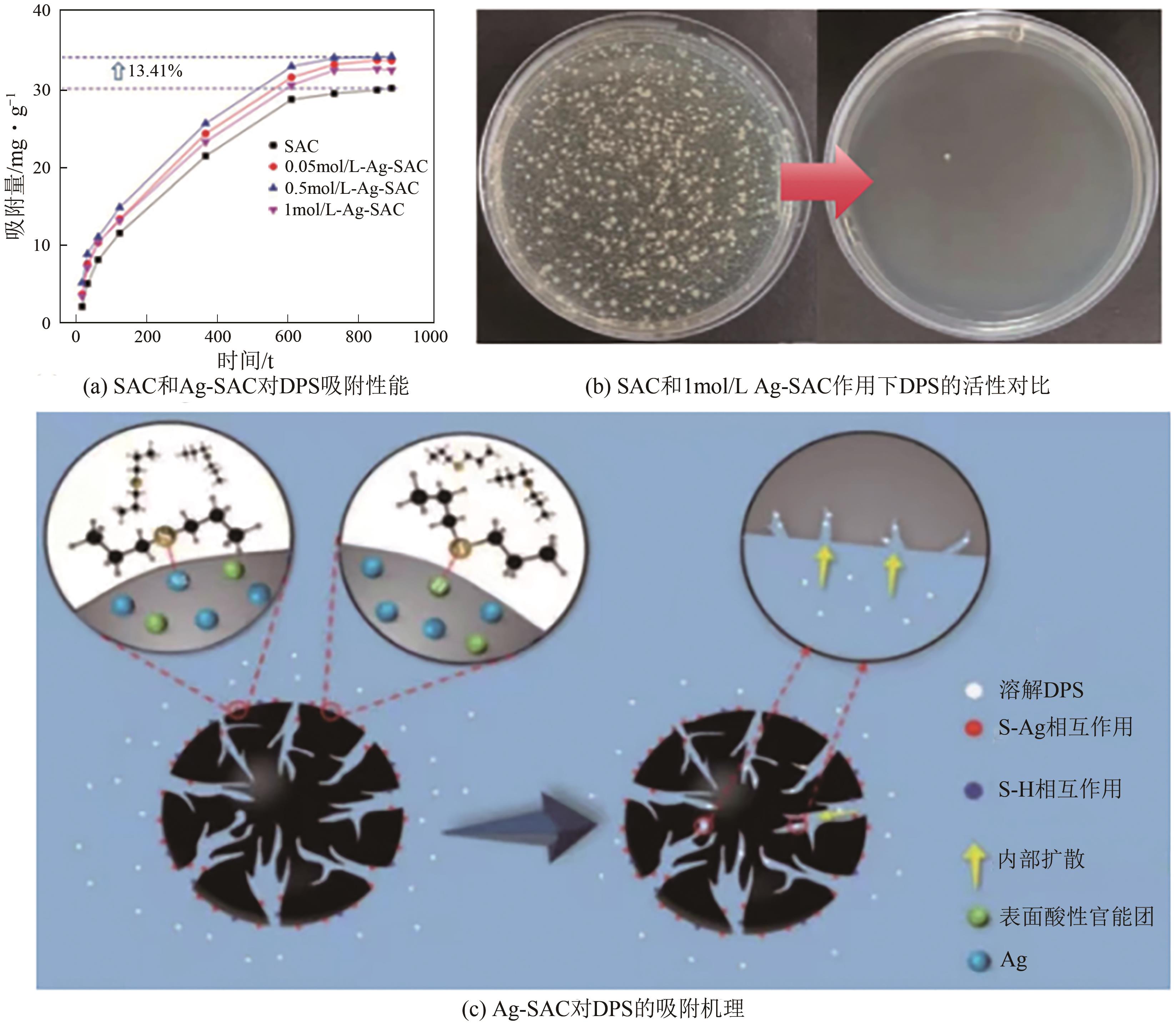
| 109 | YANG Zhilian, ZHANG Tianhao, REN Jiarui, et al. Nano-silver functionalized spherical activated carbon with enhanced dipropyl sulfide adsorption capacity and antibacterial properties[J]. RSC Advances, 2022, 12(16): 9933-9943. |
| 110 | YATZIDIS H A. A convenient haemoperfusion microaparatus over charcoal for the treatment of endogenous and exogenous intoxication; Its use as an effective artificial kidney[C]//Proc Eur Dial Transpl Assoc. 1964, 1: 83-87. |
| 111 | GUO Limin, ZHANG Lingxia, ZHANG Jiamin, et al. Hollow mesoporous carbon spheres—An excellent bilirubin adsorbent[J]. Chemical Communications, 2009(40): 6071-6073. |
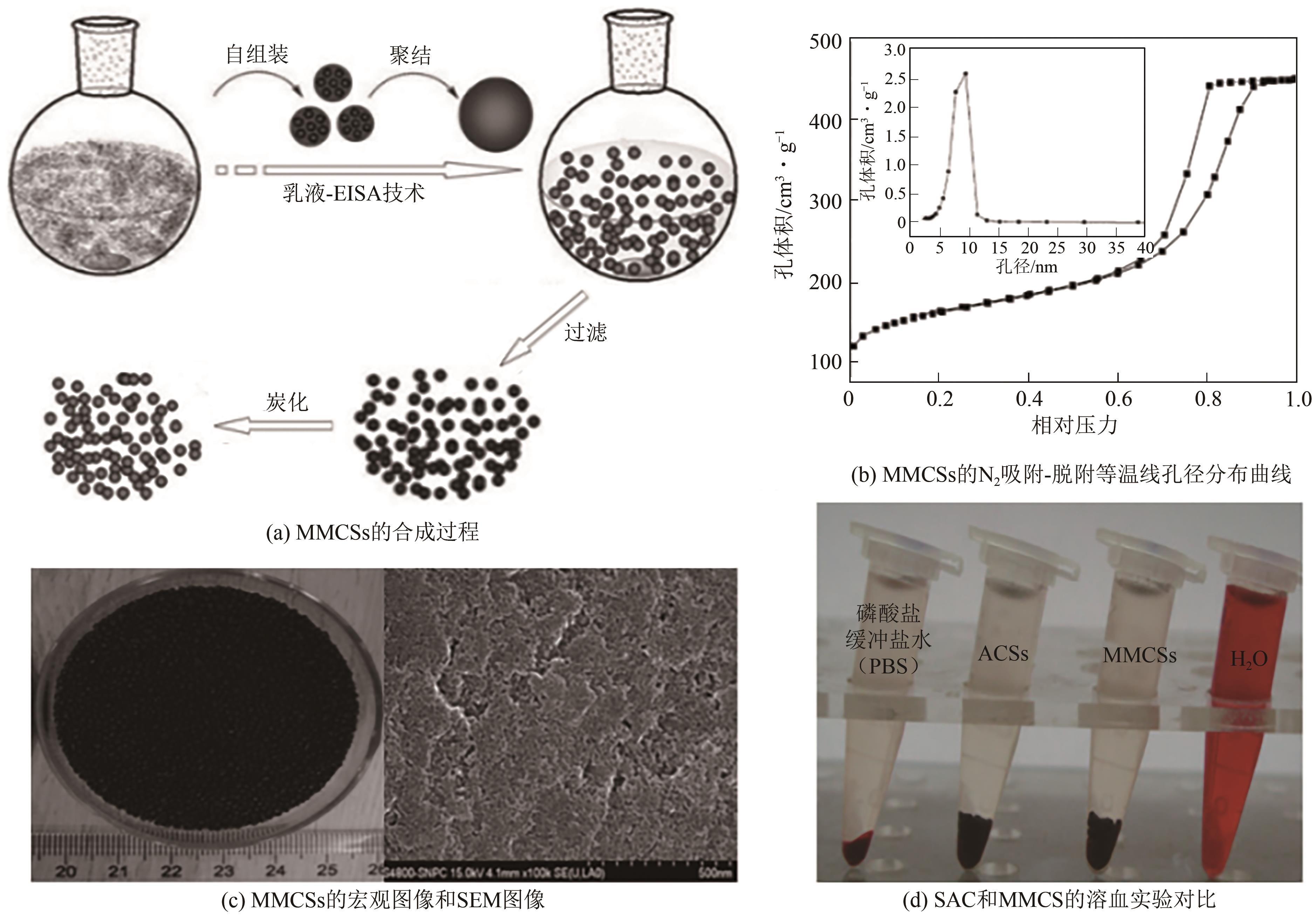
| 112 | GUO Limin, ZHANG Jiamin, HE Qianjun, et al. Preparation of millimetre-sized mesoporous carbon spheres as an effective bilirubin adsorbent and their blood compatibility[J]. Chemical Communications, 2010, 46(38): 7127-7129. |
| 113 | HOWELL C A, SANDEMAN S R, ZHENG Y, et al. New dextran coated activated carbons for medical use[J]. Carbon, 2016, 97: 134-146. |
| 114 | WU Ziling, ZHANG Yongzheng, WANG Yanli, et al. Novel oral spherical activated carbon with ultrahigh specific surface area for adsorption of endogenous toxins[J]. Materials Letters, 2023, 340: 134178. |
| 101 | BACA Martyna, CENDROWSKI Krzysztof, BANACH Paweł, et al. Effect of Pd loading on hydrogen storage properties of disordered mesoporous hollow carbon spheres[J]. International Journal of Hydrogen Energy, 2017, 42(52): 30461-30469. |
| 102 | WANG Yu, DING Zhenmin, LI Xinjun, et al. Improved hydrogen storage properties of MgH2 by nickel@nitrogen-doped carbon spheres[J]. Dalton Transactions, 2020, 49(11): 3495-3502. |
| 103 | MUNOZ Macarena, KOLB Veronika, LAMOLDA Ana, et al. Polymer-based spherical activated carbon as catalytic support for hydrodechlorination reactions[J]. Applied Catalysis B: Environmental, 2017, 218: 498-505. |
| 104 | SCHNEIDER Martin Johannes, LIJEWSKI Martin, WOELFEL René, et al. Continuous gas-phase hydroaminomethylation using supported ionic liquid phase catalysts[J]. Angewandte Chemie International Edition, 2013, 52(27): 6996-6999. |
| 105 | QI Xueyan, CHEN Weifeng, ZHANG Jinli. Highly effective carbon-supported gold-ionic liquid catalyst for acetylene hydrochlorination[J]. RSC Advances, 2019, 9(38): 21931-21938. |
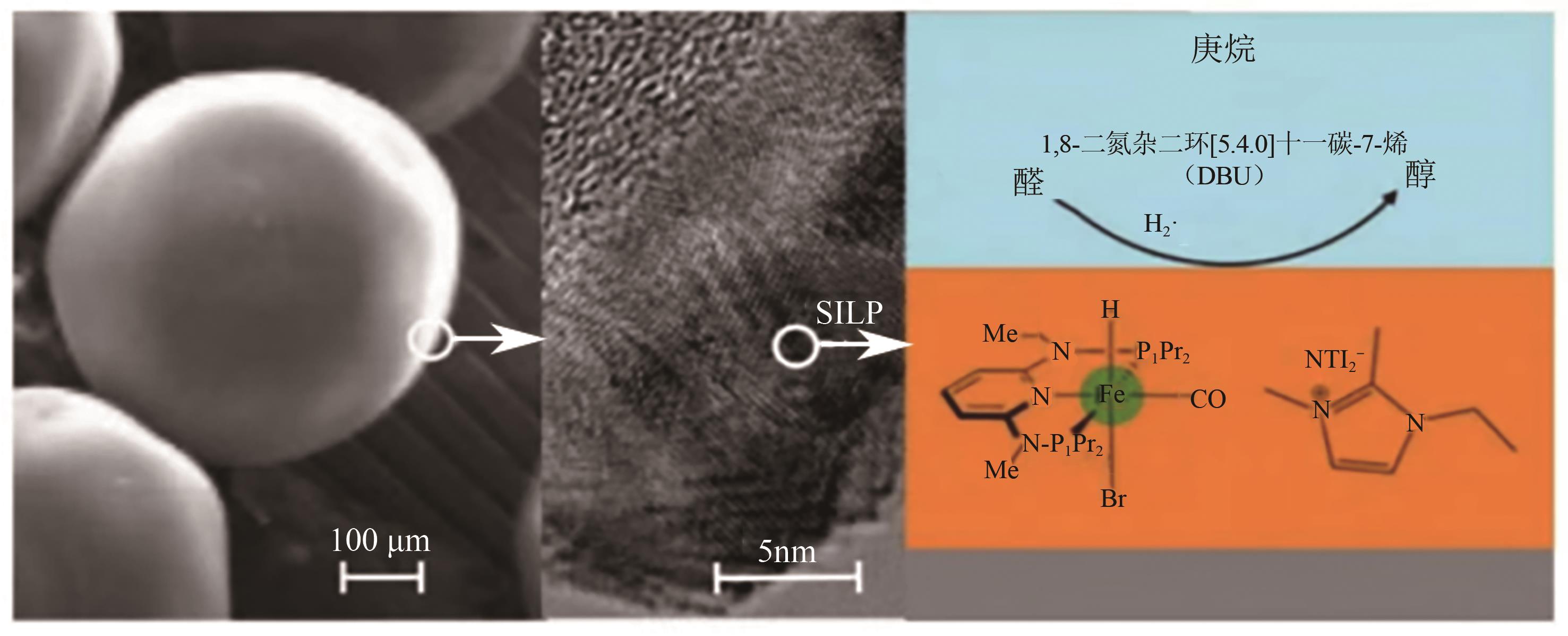
| 106 | Rafael CASTRO-AMOEDO, CSENDES Zita, Julian BRÜNIG, et al. Carbon-based SILP catalysis for the selective hydrogenation of aldehydes using a well-defined Fe(Ⅱ) PNP complex[J]. Catalysis Science & Technology, 2018, 8(18): 4812-4820. |
| 107 | LU Annie Xi, MCENTEE Monica, BROWE Matthew A, et al. MOFabric: Electrospun nanofiber mats from PVDF/UiO-66-NH2 for chemical protection and decontamination[J]. ACS Applied Materials & Interfaces, 2017, 9(15): 13632-13636. |
| [1] | 甄文超, 韩文佳, 卢成帅, 戎旭辉, 陈鲁正, 娄江. 聚丙烯酸酯基柔性传感器的研究进展[J]. 化工进展, 2025, 44(3): 1520-1532. |
| [2] | 郭沛, 崔灿灿, 孔德洁, 黄晟. “双碳”背景下固态锂电池用硫化物固态电解质的发展趋势[J]. 化工进展, 2024, 43(9): 5193-5206. |
| [3] | 舒岗韦, 林钰程, 张为宏, 赵世强, 郑晓阳, 常春. 木糖生物炼制与高值化应用研究进展[J]. 化工进展, 2024, 43(7): 3798-3811. |
| [4] | 龚雪梅, 蒋军, 王超, 梅长彤. 纳米纤维素疏水改性及其功能化应用研究进展[J]. 化工进展, 2024, 43(6): 3187-3198. |
| [5] | 罗芬, 杨晓琪, 段方麟, 李小江, 吴亮, 徐铜文. 双极膜研究进展及应用展望[J]. 化工进展, 2024, 43(1): 145-163. |
| [6] | 翟霖晓, 崔怡洲, 李成祥, 石孝刚, 高金森, 蓝兴英. 微气泡发生器的研究与应用进展[J]. 化工进展, 2024, 43(1): 111-123. |
| [7] | 毛善俊, 王哲, 王勇. 基团辨识加氢:从概念到应用[J]. 化工进展, 2023, 42(8): 3917-3922. |
| [8] | 孙旭东, 赵玉莹, 李诗睿, 王琦, 李晓健, 张博. 我国地方性氢能发展政策的文本量化分析[J]. 化工进展, 2023, 42(7): 3478-3488. |
| [9] | 徐国彬, 刘洪豪, 李洁, 郭家奇, 王琪. ZnO量子点水性喷墨荧光墨水制备及性能[J]. 化工进展, 2023, 42(6): 3114-3122. |
| [10] | 金涌, 程易, 白丁荣, 张晨曦, 魏飞. 中国流态化技术研发史略[J]. 化工进展, 2023, 42(6): 2761-2780. |
| [11] | 李建雄, 耿爽, 胡树坚, 周明. 脂质体递送系统功能结构设计与应用研究进展[J]. 化工进展, 2023, 42(4): 2003-2012. |
| [12] | 司银芳, 胡语婕, 张凡, 董浩, 佘跃惠. 生物合成氧化锌纳米颗粒材料及其抗菌应用[J]. 化工进展, 2023, 42(4): 2013-2023. |
| [13] | 吴恒, 李银龙, 晏刚, 熊通, 张浩, 陶骙. 蒸气压缩制冷/热泵系统中的气液分离技术[J]. 化工进展, 2023, 42(3): 1129-1142. |
| [14] | 陈邦富, 欧阳平, 李宇涵, 段有雨, 董帆. ZnSn(OH)6 基纳米材料在环境光催化中的应用[J]. 化工进展, 2023, 42(2): 756-764. |
| [15] | 李卫东, 李逸龙, 滕霖, 尹鹏博, 黄鑫, 李加庆, 罗宇, 江莉龙. “双碳”目标下的氨能技术与经济性研究进展[J]. 化工进展, 2023, 42(12): 6226-6238. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||