化工进展 ›› 2024, Vol. 43 ›› Issue (9): 5193-5206.DOI: 10.16085/j.issn.1000-6613.2023-1903
• 资源与环境化工 • 上一篇
“双碳”背景下固态锂电池用硫化物固态电解质的发展趋势
- 1.燕山大学公共管理学院,河北 秦皇岛 066004
2.燕山大学环境与化学工程学院,河北 秦皇岛 066004
-
收稿日期:2023-10-28修回日期:2023-12-26出版日期:2024-09-15发布日期:2024-09-30 -
通讯作者:黄晟 -
作者简介:郭沛(1982—),男,副研究员,博士研究生,研究方向为能源环境政策与治理。E-mail:guopei@ysu.edu.cn。
Development trend of sulfide solid electrolytes for solid-state lithium batteries in the context of “dual carbon goals”
GUO Pei1( ), CUI Cancan2, KONG Dejie2, HUANG Sheng1(
), CUI Cancan2, KONG Dejie2, HUANG Sheng1( )
)
- 1.School of Public Administration, Yanshan University, Qinhuangdao 066004, Hebei, China
2.School of Environmental and Chemical Engineering, Yanshan University, Qinhuangdao 066004, Hebei, China
-
Received:2023-10-28Revised:2023-12-26Online:2024-09-15Published:2024-09-30 -
Contact:HUANG Sheng
摘要:
随着我国实施碳达峰、碳中和战略,电动汽车和储能成为实施该战略的重要抓手。锂离子电池作为电动汽车和储能的核心技术,近年来取得极大发展。目前的锂离子电池主要采用液态电解质,其安全性及能量密度面临瓶颈,难以满足电动汽车和储能的应用需求。硫化物全固态锂电池采用无机硫化物固态电解质取代目前广泛应用的液态电解质,有望回避液态电解质易燃易爆的安全问题。同时,基于硫化物电解质的高离子电导率,使硫化物全固态锂电池表现出优异的倍率性能。本文从硫化物电解质的分类与结构入手,首先介绍了硫化物电解质的发展历史,然后讨论了玻璃态、晶体硫化物电解质的结构特点、离子传输机制与电化学性能,接着介绍了硫化物电解质的三种不同合成方法以及其获得的硫化物电解质的电化学性能,最后总结了硫化物电解质的空气稳定性、界面稳定性等决定其产业化应用的关键性能。本文拟为硫化物电解质下一步的研究方向提供建议,有利于促进全固态锂电池产业化应用及我国“双碳”战略目标实现。
中图分类号:
引用本文
郭沛, 崔灿灿, 孔德洁, 黄晟. “双碳”背景下固态锂电池用硫化物固态电解质的发展趋势[J]. 化工进展, 2024, 43(9): 5193-5206.
GUO Pei, CUI Cancan, KONG Dejie, HUANG Sheng. Development trend of sulfide solid electrolytes for solid-state lithium batteries in the context of “dual carbon goals”[J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5193-5206.
| 电解质 | 优点 | 缺点 | 离子电导率/S·cm-1 |
|---|---|---|---|
| 玻璃态电解质 | 在全固态电池中利用率很高,合成简单 | 离子电导率相对较低 | <10-3 |
| 玻璃-陶瓷态电解质 | 离子电导率较高 | 合成步骤复杂 | >10-3 |
| Thio-LISICON型电解质 | 合成简单,热稳定性好,电子电导率小 | 离子电导率相对较低 | <10-3 |
| Li11-x M2-x P1+x S12(M=Ge, Sn, Si)结构 | 离子电导率高 | 成本较高,空气稳定性差 | >10-3 |
| 硫银锗矿型电解质 | 电化学稳定性好 | 空气稳定性较差 | >10-3 |
表1 不同结构硫化物电解质优、缺点和离子电导率的比较
| 电解质 | 优点 | 缺点 | 离子电导率/S·cm-1 |
|---|---|---|---|
| 玻璃态电解质 | 在全固态电池中利用率很高,合成简单 | 离子电导率相对较低 | <10-3 |
| 玻璃-陶瓷态电解质 | 离子电导率较高 | 合成步骤复杂 | >10-3 |
| Thio-LISICON型电解质 | 合成简单,热稳定性好,电子电导率小 | 离子电导率相对较低 | <10-3 |
| Li11-x M2-x P1+x S12(M=Ge, Sn, Si)结构 | 离子电导率高 | 成本较高,空气稳定性差 | >10-3 |
| 硫银锗矿型电解质 | 电化学稳定性好 | 空气稳定性较差 | >10-3 |
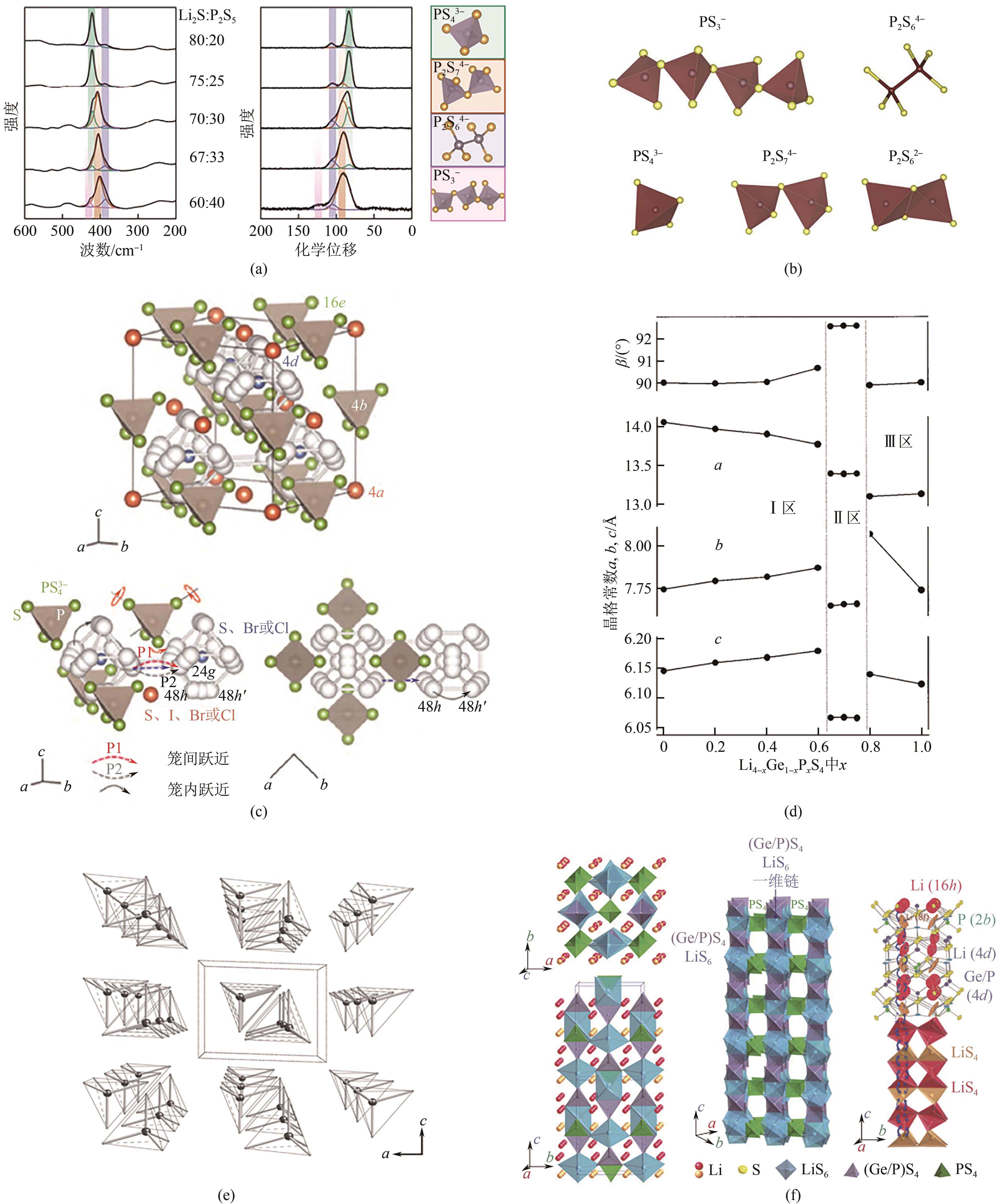
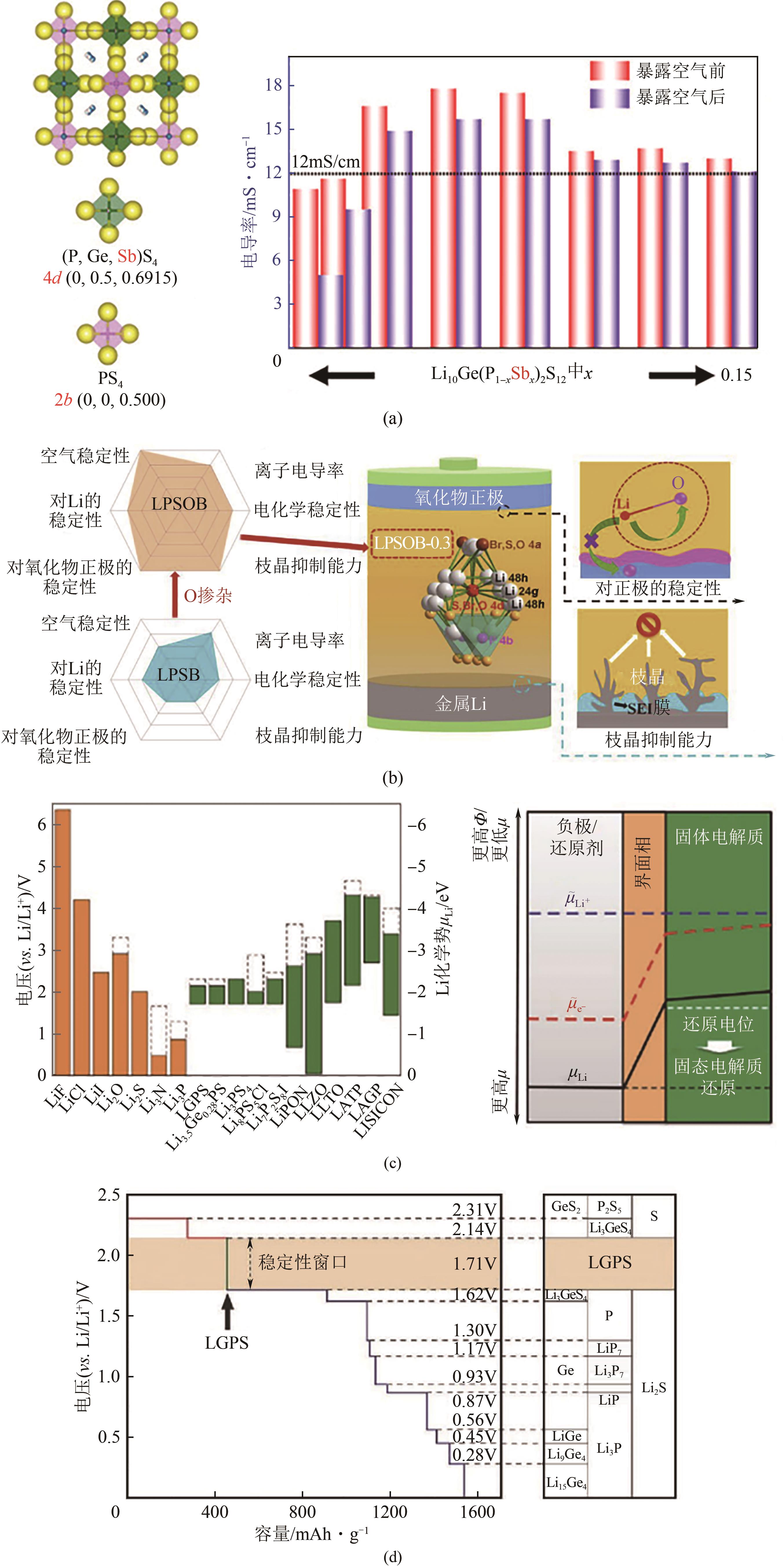
图2 不同Li2S含量的xLi2S-(100倍)P2S5的拉曼和31P轨道自旋核磁共振(MAS-NMR)谱(a);在Li2S-P2S5二元体系中产生的不同晶体相(b);Li6PS5X (X=Ⅰ)的晶体结构,锂的三种不同跃迁机制(c);(1-x) Li4GeS4-x -xLi3PS4固溶体的三个区域(1Å=0.1nm)(d);Li4SiS4的晶体结构(e);Li10GeP2S12的晶体结构(f)
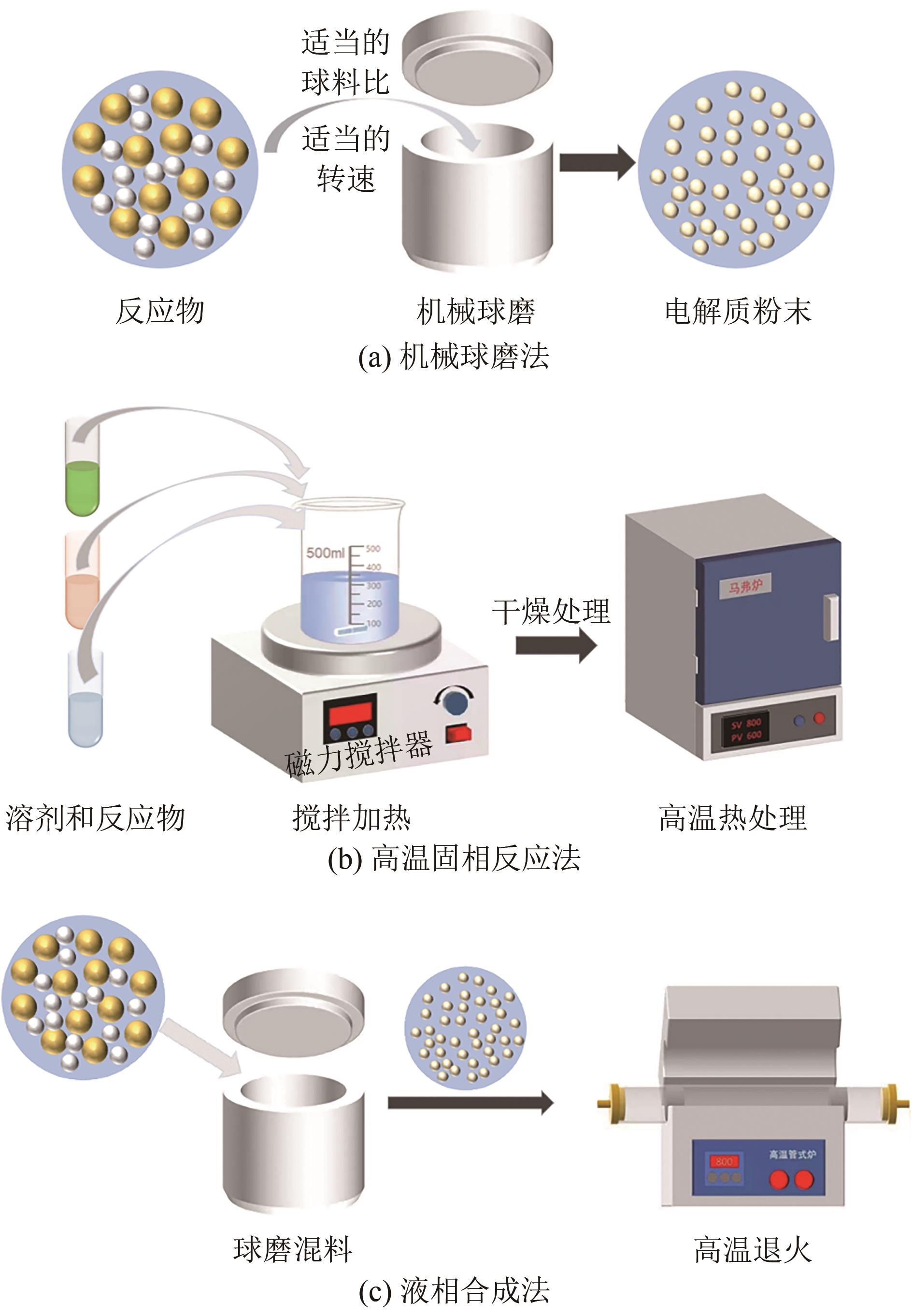
| 合成方法 | 操作步骤 | 离子电导率 /S·cm-1 | 操作环境 | 生产规模 |
|---|---|---|---|---|
| 机械球磨 | 复杂 | <10-3 | 友好 | 较小 |
| 机械球磨后退火 | 复杂 | >10-3 | 友好 | 较小 |
| 高温固相合成 | 简单 | >10-3 | 友好 | 较大 |
| 液相合成 | 复杂 | <10-4 | 苛刻 | 较大 |
表2 不同硫化物电解质合成方法的优缺点
| 合成方法 | 操作步骤 | 离子电导率 /S·cm-1 | 操作环境 | 生产规模 |
|---|---|---|---|---|
| 机械球磨 | 复杂 | <10-3 | 友好 | 较小 |
| 机械球磨后退火 | 复杂 | >10-3 | 友好 | 较小 |
| 高温固相合成 | 简单 | >10-3 | 友好 | 较大 |
| 液相合成 | 复杂 | <10-4 | 苛刻 | 较大 |
| 1 | KANNO Ryoji, MURAYAMA Masahiro. Lithium ionic conductor Thio-LISICON: The Li2S-GeS2-P2S5 system[J]. Journal of the Electrochemical Society, 2001, 148(7): A742. |
| 2 | MIZUNO F, HAYASHI A, TADANAGA K, et al. New, highly ion-conductive crystals precipitated from Li2S-P2S5 glasses[J]. Advanced Materials, 2005, 17(7): 918-921. |
| 3 | DEISEROTH Hans-Jörg, KONG Shiao-Tong, ECKERT Hellmut, et al. Li6PS5X: A class of crystalline Li-rich solids with an unusually high Li+ mobility[J]. Angewandte Chemie International Edition, 2008, 47(4): 755-758. |
| 4 | KAMAYA Noriaki, HOMMA Kenji, YAMAKAWA Yuichiro, et al. A lithium superionic conductor[J]. Nature Materials, 2011, 10: 682-686. |
| 5 | KATO Yuki, HORI Satoshi, SAITO Toshiya, et al. High-power all-solid-state batteries using sulfide superionic conductors[J]. Nature Energy, 2016, 1(4): 16030. |
| 6 | IWASAKI Rui, HORI Satoshi, KANNO Ryoji, et al. Weak anisotropic lithium-ion conductivity in single crystals of Li10GeP2S12 [J]. Chemistry of Materials, 2019, 31(10): 3694-3699. |
| 7 | LIANG Jianwen, CHEN Ning, LI Xiaona, et al. Li10Ge(P1- x Sb x )2S12 lithium-ion conductors with enhanced atmospheric stability[J]. Chemistry of Materials, 2020, 32(6): 2664-2672. |
| 8 | LU Pushun, LIU Lilu, WANG Shuo, et al. Superior all-solid-state batteries enabled by a gas-phase-synthesized sulfide electrolyte with ultrahigh moisture stability and ionic conductivity[J]. Advanced Materials, 2021, 33(32): e2100921. |
| 9 | LU Pushun, XIA Yu, SUN Guochen, et al. Realizing long-cycling all-solid-state Li-In||TiS2 batteries using Li6+ x M x As1- x S5I (M=Si, Sn) sulfide solid electrolytes[J]. Nature Communications, 2023, 14: 4077. |
| 10 | MANTHIRAM Arumugam, YU Xingwen, WANG Shaofei. Lithium battery chemistries enabled by solid-state electrolytes[J]. Nature Reviews Materials, 2017, 2(4): 16103. |
| 11 | UJIIE Satoshi, HAYASHI Akitoshi, TATSUMISAGO Masahiro. Structure, ionic conductivity and electrochemical stability of Li2S-P2S5-LiI glass and glass-ceramic electrolytes[J]. Solid State Ionics, 2012, 211: 42-45. |
| 12 | CHRISTIAN Dietrich, WEBER Dominik A, SEAN Culver, et al. Synthesis, structural characterization, and lithium ion conductivity of the lithium thiophosphate Li2P2S6 [J]. Inorganic Chemistry, 2017, 56(11): 6681-6687. |
| 13 | YAMANE Hisanori, SHIBATA Masatoshi, SHIMANE Yukio, et al. Crystal structure of a superionic conductor, Li7P3S11 [J]. Solid State Ionics, 2007, 178(15/16/17/18): 1163-1167. |
| 14 | MERCIER R, J-P MALUGANI, FAHYS B, et al. Structure du tetrathiophosphate de lithium[J]. Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry, 1982, 38(7): 1887-1890. |
| 15 | KONG Shiao Tong, Ozgül GÜN, KOCH Barbara, et al. Structural characterisation of the Li argyrodites Li7PS6 and Li7PSe6 and their solid solutions: Quantification of site preferences by MAS-NMR spectroscopy[J]. Chemistry, 2010, 16(17): 5138-5147. |
| 16 | MERCIER R, MALUGANI J P, FAHYS B, et al. Synthese, structure cristalline et analyse vibrationnelle de l’hexathiohypodiphosphate de lithium Li4P2S6 [J]. Journal of Solid State Chemistry, 1982, 43(2): 151-162. |
| 17 | TATSUMISAGO Masahiro. Glassy materials based on Li2S for all-solid-state lithium secondary batteries[J]. Solid State Ionics, 2004, 175(1/2/3/4): 13-18. |
| 18 | ITO Seitaro, NAKAKITA Moeka, AIHARA Yuichi, et al. A synthesis of crystalline Li7P3S11 solid electrolyte from 1,2-dimethoxyethane solvent[J]. Journal of Power Sources, 2014, 271: 342-345. |
| 19 | KANNO Ryoji, HATA Takayuki, KAWAMOTO Yoji, et al. Synthesis of a new lithium ionic conductor, Thio-LISICON-lithium germanium sulfide system[J]. Solid State Ionics, 2000, 130(1/2): 97-104. |
| 20 | MURAYAMA Masahiro, KANNO Ryoji, KAWAMOTO Yoji, et al. Structure of the Thio-LISICON, Li4GeS4 [J]. Solid State Ionics, 2002, 154/155: 789-794. |
| 21 | KAIB Thomas, HADDADPOUR Sima, KAPITEIN Manuel, et al. New lithium chalcogenidotetrelates, LiChT: Synthesis and characterization of the Li+-conducting tetralithium ortho-Sulfidostannate Li4SnS4 [J]. Chemistry of Materials, 2012, 24(11): 2211-2219. |
| 22 | SAHU Gayatri, LIN Zhan, LI Juchuan, et al. Air-stable, high-conduction solid electrolytes of arsenic-substituted Li4SnS4 [J]. Energy & Environmental Science, 2014, 7(3): 1053-1058. |
| 23 | HORI Satoshi, KATO Masahiko, SUZUKI Kota, et al. Phase diagram of the Li4GeS4-Li3PS4 quasi-binary system containing the superionic conductor Li10GeP2S12 [J]. Journal of the American Ceramic Society, 2015, 98(10): 3352-3360. |
| 24 | MURAYAMA Masahiro, KANNO Ryoji, IRIE Michihiko, et al. Synthesis of new lithium ionic conductor Thio-LISICON—Lithium silicon sulfides system[J]. Journal of Solid State Chemistry, 2002, 168(1): 140-148. |
| 25 | MINAFRA Nicolò, CULVER Sean P, LI Cheng, et al. Influence of the lithium substructure on the diffusion pathways and transport properties of the Thio-LISICON Li4Ge1- x Sn x S4 [J]. Chemistry of Materials, 2019, 31(10): 3794-3802. |
| 26 | KUHN Alexander, DUPPEL Viola, LOTSCH Bettina V. Tetragonal Li10GeP2S12 and Li7GePS8-exploring the Li ion dynamics in LGPS Li electrolytes[J]. Energy & Environmental Science, 2013, 6(12): 3548-3552. |
| 27 | BHANDARI Arihant, BHATTACHARYA Jishnu. Origin of fast ion conduction in Li10GeP2S12, a superionic conductor[J]. The Journal of Physical Chemistry C, 2016, 120(51): 29002-29010. |
| 28 | Shyue Ping ONG, MO Yifei, RICHARDS William Davidson, et al. Phase stability, electrochemical stability and ionic conductivity of the Li10±1MP2X12 (M=Ge, Si, Sn, Al or P, and X=O, S or Se) family of superionic conductors[J]. Energy & Environmental Science, 2013, 6(1): 148-156. |
| 29 | BRON Philipp, JOHANSSON Sebastian, ZICK Klaus, et al. Li10SnP2S12: An affordable lithium superionic conductor[J]. Journal of the American Chemical Society, 2013, 135(42): 15694-15697. |
| 30 | BRON Philipp, DEHNEN Stefanie, ROLING Bernhard. Li10Si0.3Sn0.7P2S12—A low-cost and low-grain-boundary-resistance lithium superionic conductor[J]. Journal of Power Sources, 2016, 329: 530-535. |
| 31 | KUHN Alexander, Jürgen KÖHLER, LOTSCH Bettina V. Single-crystal X-ray structure analysis of the superionic conductor Li10GeP2S12 [J]. Physical Chemistry Chemical Physics, 2013, 15(28): 11620-11622. |
| 32 | SUN Yulong, SUZUKI Kota, HARA Kosuke, et al. Oxygen substitution effects in Li10GeP2S12 solid electrolyte[J]. Journal of Power Sources, 2016, 324: 798-803. |
| 33 | HORI Satoshi, SUZUKI Kota, HIRAYAMA Masaaki, et al. Lithium superionic conductor Li9.42Si1.02P2.1S9.96O2.04 with Li10GeP2S12-type structure in the Li2S-P2S5-SiO2 pseudoternary system: Synthesis, electrochemical properties, and structure-composition relationships[J]. Frontiers in Energy Research, 2016, 4: 38. |
| 34 | BAI Yang, ZHAO Yanbiao, LI Weidong, et al. New insight for solid sulfide electrolytes LSiPSI by using Si/P/S as the raw materials and I doping[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(15): 12930-12937. |
| 35 | HAYASHI Akitoshi, NISHIO Yusuke, KITAURA Hirokazu, et al. Novel technique to form electrode-electrolyte nanointerface in all-solid-state rechargeable lithium batteries[J]. Electrochemistry Communications, 2008, 10(12): 1860-1863. |
| 36 | HANGHOFER I, BRINEK M, EISBACHER S L, et al. Substitutional disorder: Structure and ion dynamics of the argyrodites Li6PS5Cl, Li6PS5Br and Li6PS5I[J]. Physical Chemistry Chemical Physics: PCCP, 2019, 21(16): 8489-8507. |
| 37 | RAYAVARAPU Prasada Rao, SHARMA Neeraj, PETERSON Vanessa K, et al. Variation in structure and Li+-ion migration in argyrodite-type Li6PS5X (X=Cl, Br, I) solid electrolytes[J]. Journal of Solid State Electrochemistry, 2012, 16(5): 1807-1813. |
| 38 | ZHANG Zhuoran, ZHANG Jianxing, JIA Huanhuan, et al. Enhancing ionic conductivity of solid electrolyte by lithium substitution in halogenated Li-argyrodite[J]. Journal of Power Sources, 2020, 450: 227601. |
| 39 | PECHER Oliver, KONG Shiao-Tong, GOEBEL Thorsten, et al. Atomistic characterisation of Li+ mobility and conductivity in Li7- x PS6- x I x argyrodites from molecular dynamics simulations, solid-state NMR, and impedance spectroscopy[J]. Chemistry: A European Journal, 2010, 16(28): 8347-8354. |
| 40 | ZHANG Zhuoran, SUN Yulong, DUAN Xianbao, et al. Design and synthesis of room temperature stable Li-argyrodite superionic conductors via cation doping[J]. Journal of Materials Chemistry A, 2019, 7(6): 2717-2722. |
| 41 | MINAFRA Nicolò, CULVER Sean P, KRAUSKOPF Thorben, et al. Effect of Si substitution on the structural and transport properties of superionic Li-argyrodites[J]. Journal of Materials Chemistry A, 2018, 6(2): 645-651. |
| 42 | TATSUMISAGO M, MINAMI T. Lithium ion conducting glasses prepared by rapid quenching[J]. Materials Chemistry and Physics, 1987, 18(1/2): 1-17. |
| 43 | OHTOMO Takamasa, HAYASHI Akitoshi, TATSUMISAGO Masahiro, et al. Characteristics of the Li2O-Li2S-P2S5 glasses synthesized by the two-step mechanical milling[J]. Journal of Non-Crystalline Solids, 2013, 364: 57-61. |
| 44 | TATSUMISAGO M, HAMA S, HAYASHI A, et al. New lithium ion conducting glass-ceramics prepared from mechanochemical Li2S-P2S5 glasses[J]. Solid State Ionics, 2002, 154/155: 635-640. |
| 45 | PHUC Nguyen Huu Huy, MORIKAWA Kei, TOTANI Mitsuhiro, et al. Chemical synthesis of Li3PS4 precursor suspension by liquid-phase shaking[J]. Solid State Ionics, 2016, 285: 2-5. |
| 46 | BOULINEAU Sylvain, COURTY Matthieu, TARASCON Jean-Marie, et al. Mechanochemical synthesis of Li-argyrodite Li6PS5X (X=Cl, Br, I) as sulfur-based solid electrolytes for all solid state batteries application[J]. Solid State Ionics, 2012, 221: 1-5. |
| 47 | SEINO Yoshikatsu, Tsuyoshi OTA, TAKADA Kazunori, et al. A sulphide lithium super ion conductor is superior to liquid ion conductors for use in rechargeable batteries[J]. Energy & Environmental Science, 2014, 7(2): 627-631. |
| 48 | HOMMA Kenji, YONEMURA Masao, KOBAYASHI Takeshi, et al. Crystal structure and phase transitions of the lithium ionic conductor Li3PS4 [J]. Solid State Ionics, 2011, 182(1): 53-58. |
| 49 | KUHN Alexander, GERBIG Oliver, ZHU Changbao, et al. A new ultrafast superionic Li-conductor: Ion dynamics in Li11Si2PS12 and comparison with other tetragonal LGPS-type electrolytes[J]. Physical Chemistry Chemical Physics: PCCP, 2014, 16(28): 14669-14674. |
| 50 | YU Chuang, GANAPATHY Swapna, DE KLERK Niek J J, et al. Unravelling Li-ion transport from picoseconds to seconds: Bulk versus interfaces in an argyrodite Li6PS5Cl-Li2S all-solid-state Li-ion battery[J]. Journal of the American Chemical Society, 2016, 138(35): 11192-11201. |
| 51 | LIU Zengcai, FU Wujun, PAYZANT Andrew, et al. Anomalous high ionic conductivity of nanoporous β‑Li3PS4 [J]. J. Am. Chem. Soc, 2013, 135: 975-978. |
| 52 | CALPA Marcela, ROSERO-NAVARRO Nataly Carolina, MIURA Akira, et al. Instantaneous preparation of high lithium-ion conducting sulfide solid electrolyte Li7P3S11 by a liquid phase process[J]. RSC Advances, 2017, 7(73): 46499-46504. |
| 53 | XU R C, XIA X H, YAO Z J, et al. Preparation of Li7P3S11 glass-ceramic electrolyte by dissolution-evaporation method for all-solid-state lithium ion batteries[J]. Electrochimica Acta, 2016, 219: 235-240. |
| 54 | YAO Xiayin, LIU Deng, WANG Chunsheng, et al. High-energy all-solid-state lithium batteries with ultralong cycle life[J]. Nano Letters, 2016, 16(11): 7148-7154. |
| 55 | WANG Yuxing, LU Dongping, BOWDEN Mark, et al. Mechanism of formation of Li7P3S11 solid electrolytes through liquid phase synthesis[J]. Chemistry of Materials, 2018, 30(3): 990-997. |
| 56 | TERAGAWA Shingo, Keigo ASO, TADANAGA Kiyoharu, et al. Liquid-phase synthesis of a Li3PS4 solid electrolyte using N-methylformamide for all-solid-state lithium batteries[J]. Journal of Materials Chemistry A, 2014, 2(14): 5095-5099. |
| 57 | CHOI Sunho, Jiu ANN, Jiyae DO, et al. Application of rod-like Li6PS5Cl directly synthesized by a liquid phase process to sheet-type electrodes for all-solid-state lithium batteries[J]. Journal of the Electrochemical Society, 2018, 166(3): A5193-A5200. |
| 58 | ZHOU Laidong, PARK Kern-Ho, SUN Xiaoqi, et al. Solvent-engineered design of argyrodite Li6PS5X (X=Cl, Br, I) solid electrolytes with high ionic conductivity[J]. ACS Energy Letters, 2019, 4(1): 265-270. |
| 59 | YUBUCHI So, HAYASHI Akitoshi, TATSUMISAGO Masahiro. Liquid-phase synthesis of argyrodite-type Li6PS5Br solid electrolyte with high lithium-ion conductivity[J]. Materials Science, 2016, MA2016-02: 3982. |
| 60 | PEARSON Ralph G. Hard and soft acids and bases[J]. Journal of the American Chemical Society, 1963, 85(22): 3533-3539. |
| 61 | LU Pushun, WU Dengxu, CHEN Liquan, et al. Air stability of solid-state sulfide batteries and electrolytes[J]. Electrochemical Energy Reviews, 2022, 5(3): 3. |
| 62 | HAYASHI Akitoshi, MURAMATSU Hiromasa, OHTOMO Takamasa, et al. Improvement of chemical stability of Li3PS4 glass electrolytes by adding M x O y (M=Fe, Zn, and Bi) nanoparticles[J]. Journal of Materials Chemistry A, 2013, 1(21): 6320-6326. |
| 63 | OHTOMO Takamasa, HAYASHI Akitoshi, TAYSUMISAGO Masahiro,et al. All-solid-state batteries with Li2O-Li2S-P2S5 glass electrolytes synthesized by two-step mechanical milling [J]. Journal of Solid State Electrochemistry, 2013, 17: 2551-2557. |
| 64 | ZHANG Zhixia, ZHANG Long, YAN Xinlin, et al. All-in-one improvement toward Li6PS5Br-Based solid electrolytes triggered by compositional tune[J]. Journal of Power Sources, 2019, 410/411: 162-170. |
| 65 | LIU Gaozhan, XIE Dongjiu, WANG Xuelong, et al. High air-stability and superior lithium ion conduction of Li3+3 x P1- x Zn x S4- x O x by aliovalent substitution of ZnO for all-solid-state lithium batteries[J]. Energy Storage Materials, 2019, 17: 266-274. |
| 66 | WU Jinghua, SHEN Lin, ZHANG Zhihua, et al. All-solid-state lithium batteries with sulfide electrolytes and oxide cathodes[J]. Electrochemical Energy Reviews, 2021, 4(1): 101-135. |
| 67 | WU Jinghua, LIU Sufu, HAN Fudong, et al. Lithium/sulfide all-solid-state batteries using sulfide electrolytes[J]. Advanced Materials, 2021, 33(6): e2000751. |
| 68 | TREVEY James, JANG Jum Suk, JUNG Yoon Seok, et al. Glass-ceramic Li2S-P2S5 electrolytes prepared by a single step ball billing process and their application for all-solid-state lithium-ion batteries[J]. Electrochemistry Communications, 2009, 11:1830-1833. |
| 69 | KANNO R, MURAYAMA M, New Lithium ionic conductor, Thio-LISICON, and its application to all solid-state ceramic battery[J]. Journal of the Japanese Association for Crystal Growth, 2004, 46(1):9-15. |
| 70 | YAMAMOTO Hidekazu, MACHIDA Nobuya, SHIGEMATSU Toshihiko. A mixed-former effect on lithium-ion conductivities of the Li2S-GeS2-P2S5 amorphous materials prepared by a high-energy ball-milling process[J]. Solid State Ionics, 2004, 175(1/2/3/4): 707-711. |
| 71 | ZHU Yizhou, HE Xingfeng, MO Yifei. Origin of outstanding stability in the lithium solid electrolyte materials: Insights from thermodynamic analyses based on first-principles calculations[J]. ACS Applied Materials & Interfaces, 2015, 7(42): 23685-23693. |
| 72 | HAN Fudong, ZHU Yizhou, HE Xingfeng, et al. Electrochemical stability of Li10GeP2S12 and Li7La3Zr2O12 solid electrolytes[J]. Advanced Energy Materials, 2016, 6(8): 1501590. |
| 73 | YU Chuang, VAN EIJCK Lambert, GANAPATHY Swapna, et al. Synthesis, structure and electrochemical performance of the argyrodite Li6PS5Cl solid electrolyte for Li-ion solid state batteries[J]. Electrochimica Acta 2016, 215: 93-99. |
| 74 | ZHENG Bizhu, LIU Xiangsi, ZHU Jianping, et al. Unraveling (electro)-chemical stability and interfacial reactions of Li10SnP2S12 in all-solid-state Li batteries[J]. Nano Energy 2020, 67:104252. |
| 75 | LI Guoyao, WU Shaoping, ZHENG Hongpeng, et al. Sn-O dual-substituted chlorine-rich argyrodite electrolyte with enhanced moisture and electrochemical stability[J]. Advanced Functional Materials, 2023, 33:2211805. |
| [1] | 高玉李, 王红秋, 黄格省, 鲜楠莹, 师晓玉. 全固态锂电池的产业化和技术研究进展[J]. 化工进展, 2024, 43(9): 4767-4778. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||