化工进展 ›› 2024, Vol. 43 ›› Issue (9): 5250-5261.DOI: 10.16085/j.issn.1000-6613.2023-1272
• 资源与环境化工 • 上一篇
焙烧镁铝水滑石脱除厌氧消化沼气中CO2的效果及机制
吴宇琦1( ), 李江涛1, 丁建智2, 宋秀兰1, 苏冰琴1
), 李江涛1, 丁建智2, 宋秀兰1, 苏冰琴1
- 1.太原理工大学土木工程学院,山西 太原 030024
2.煤炭工业太原设计研究院集团有限公司,山西 太原 030000
-
收稿日期:2023-07-23修回日期:2023-11-18出版日期:2024-09-15发布日期:2024-09-30 -
通讯作者:吴宇琦 -
作者简介:吴宇琦(1988—),女,博士,讲师,研究方向为有机固废厌氧发酵。E-mail:wuyuqi@tyut.edu.cn。 -
基金资助:山西省基础研究计划(自由探索类)青年项目(202103021223098);山西省高等学校科技创新项目(2021L035);太原理工大学2022年度校基金(2022QN088)
Calcined Mg/Al hydrotalcites for CO2 removal in anaerobic digestion biogas: Performances and mechanisms
WU Yuqi1( ), LI Jiangtao1, DING Jianzhi2, SONG Xiulan1, SU Bingqin1
), LI Jiangtao1, DING Jianzhi2, SONG Xiulan1, SU Bingqin1
- 1.College of Civil Engineering, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
2.Taiyuan Design Research Institute for Coal Industry, Taiyuan 030000, Shanxi, China
-
Received:2023-07-23Revised:2023-11-18Online:2024-09-15Published:2024-09-30 -
Contact:WU Yuqi
摘要:
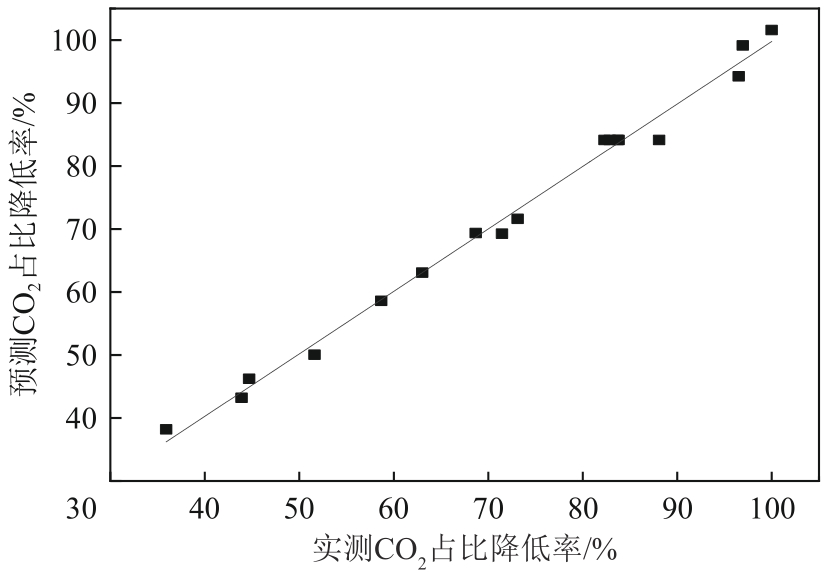
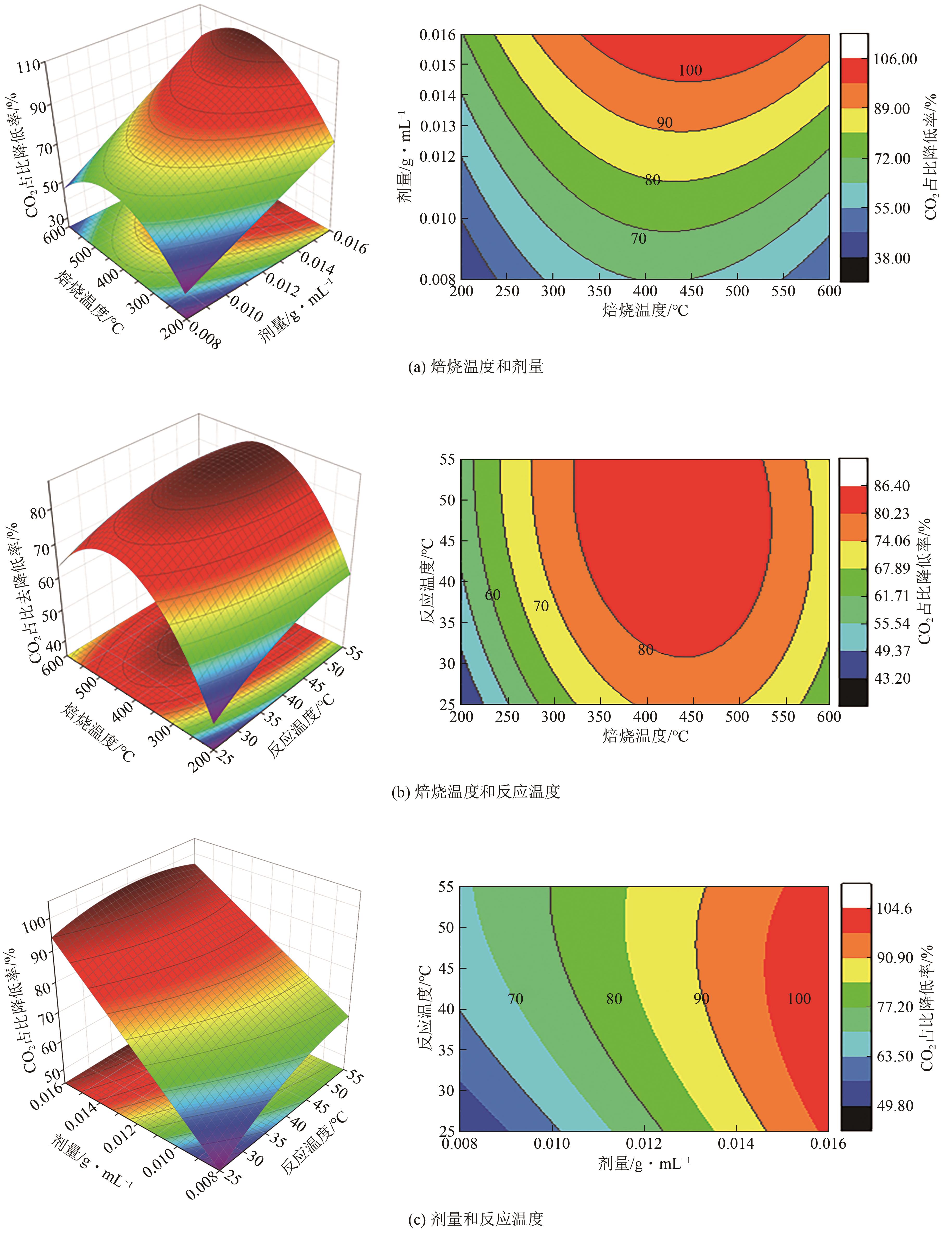
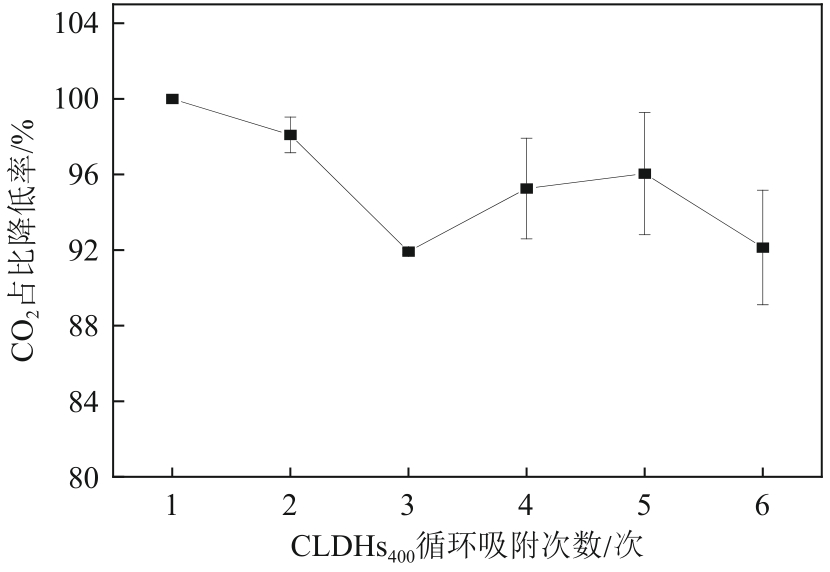
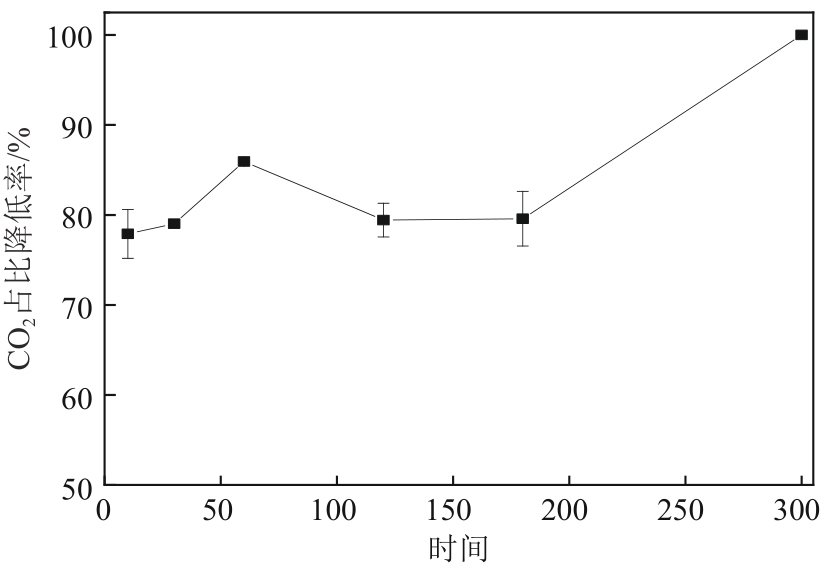
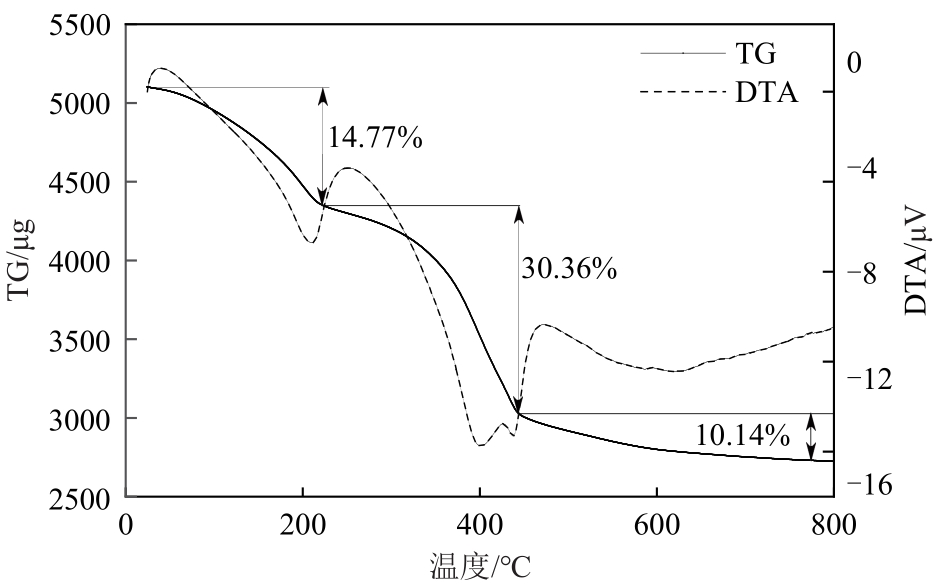
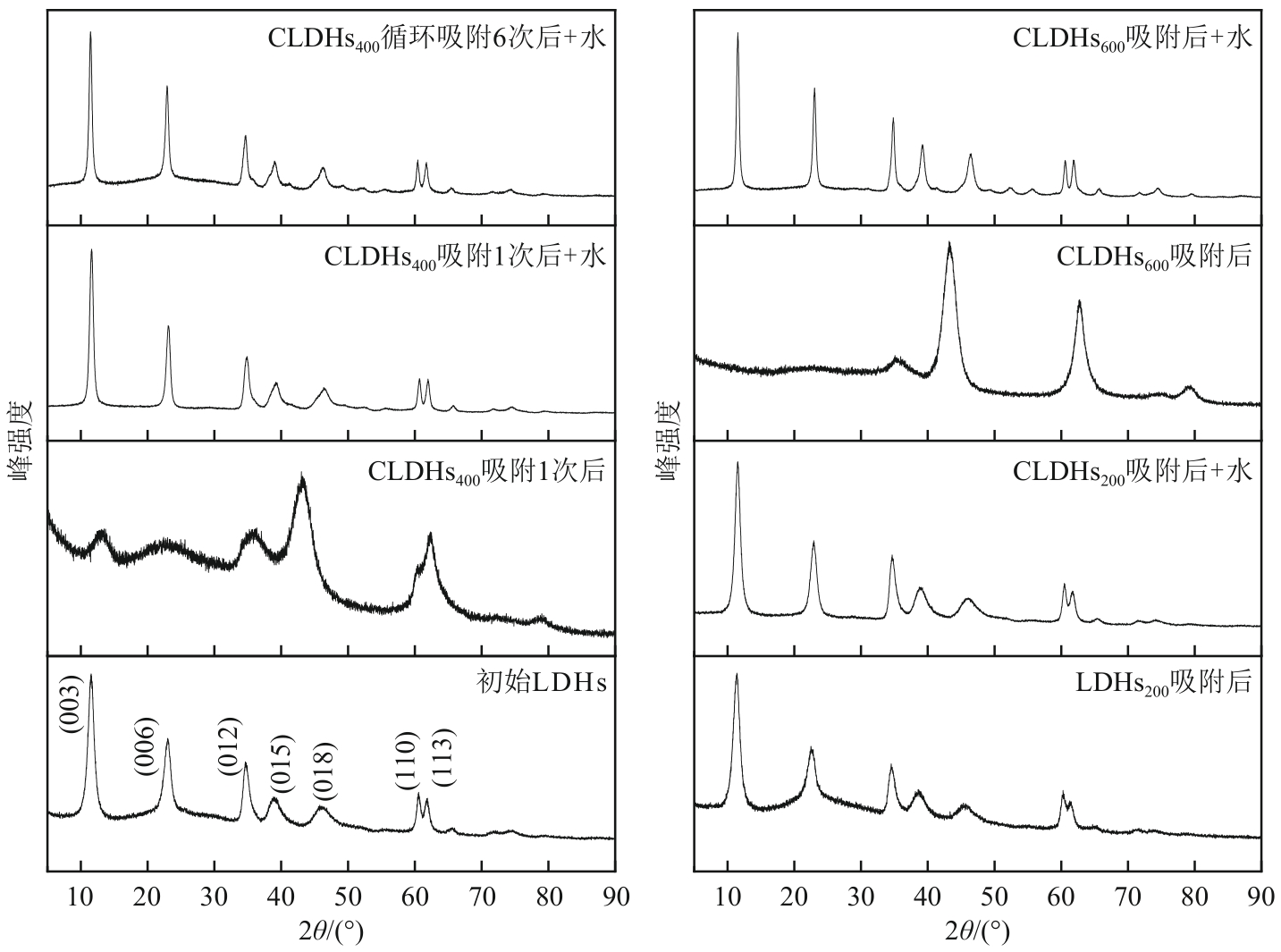
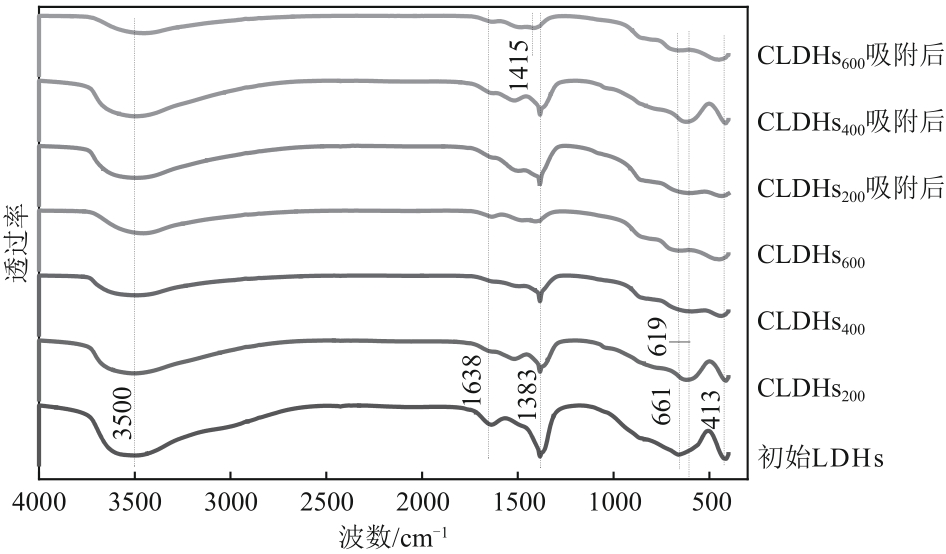
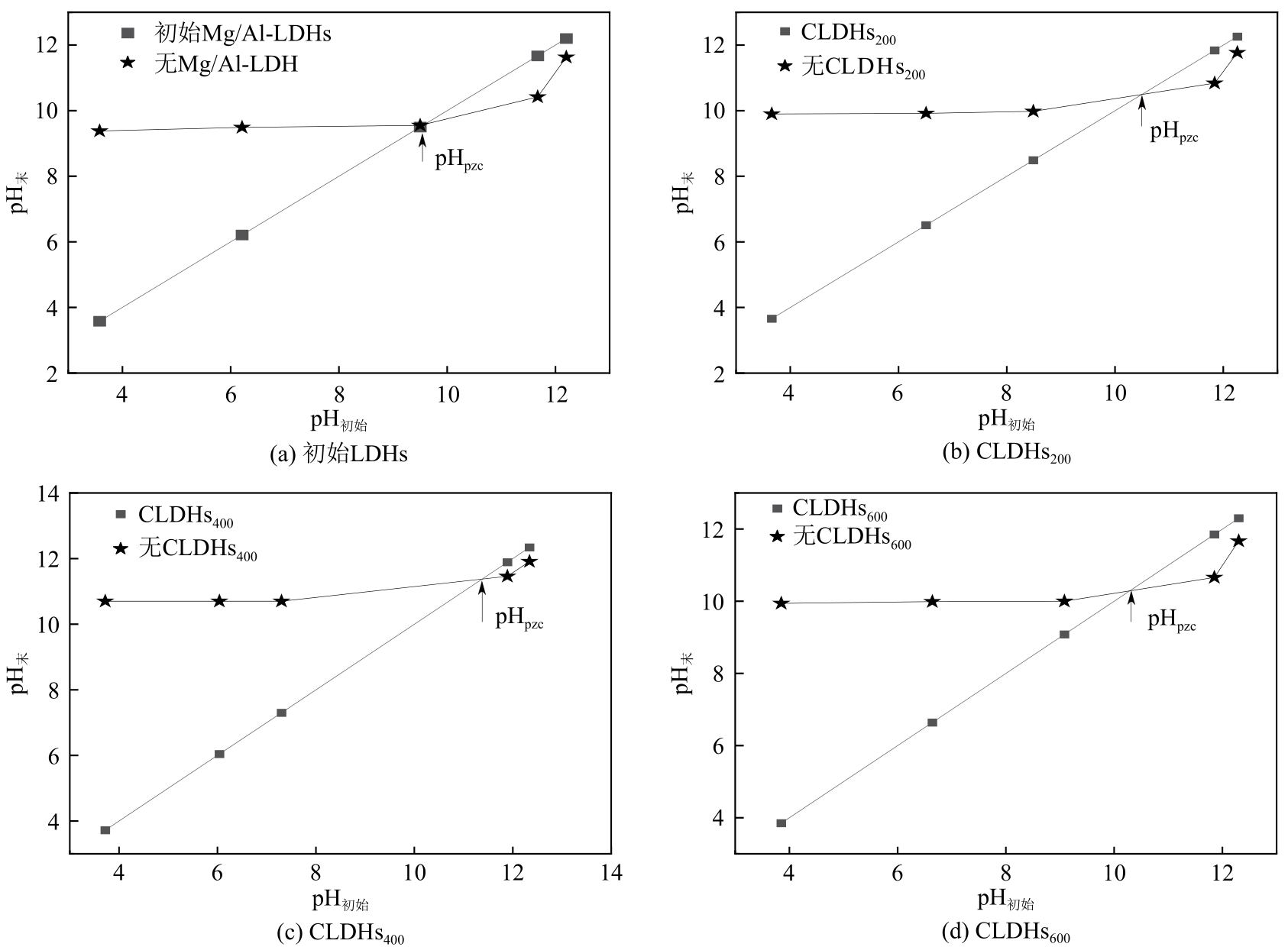
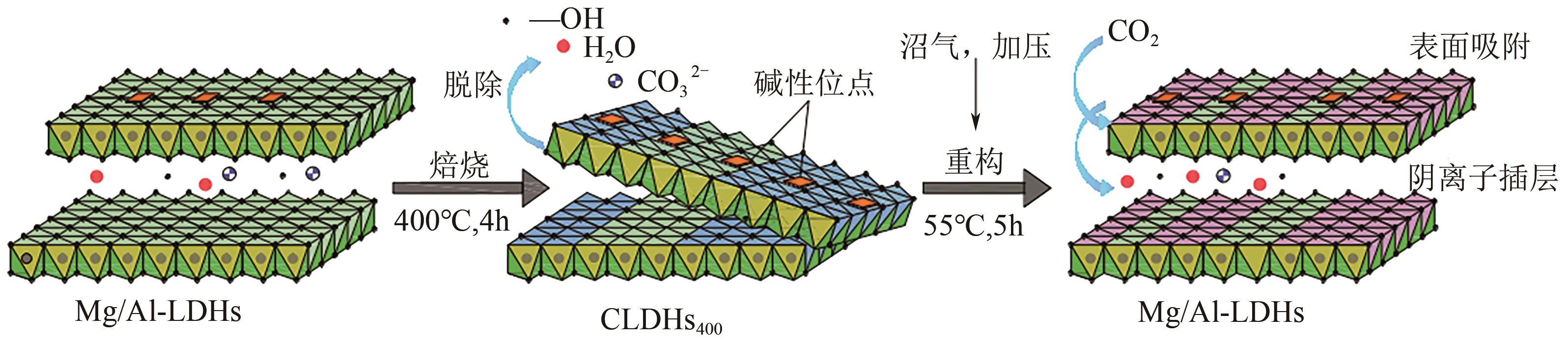
厌氧消化沼气中含有的CO2会降低沼气利用价值,高效去除CO2是沼气利用领域的研究热点。焙烧镁铝水滑石具有良好的CO2吸附性能,本文利用响应面法的Box-Behnken模型优化CO2吸附条件,考察吸附剂剂量、焙烧温度和反应温度对吸附效果的影响;通过吸附材料表征、吸附过程测定分析吸附作用机制。实验结果表明,吸附剂剂量、焙烧温度和反应温度均对CO2吸附有显著影响,最适宜的吸附条件为:投加量0.016g/mL、400℃焙烧、55℃反应。在此条件下,沼气中的CO2体积分数从23.51%降至0,CO2吸附容量为0.625mmol/g,CH4回收率为94.7%,经过6次循环吸附后,吸附剂仍具有良好的再生性能。吸附过程及吸附材料的X射线衍射、扫描电子显微镜、傅里叶变换红外光谱、比表面积、等电点及孔结构测试结果表明,焙烧镁铝水滑石的吸附过程十分迅速,表面碱性位点吸附及层间阴离子插层作用同时促进了CO2吸附。
中图分类号:
引用本文
吴宇琦, 李江涛, 丁建智, 宋秀兰, 苏冰琴. 焙烧镁铝水滑石脱除厌氧消化沼气中CO2的效果及机制[J]. 化工进展, 2024, 43(9): 5250-5261.
WU Yuqi, LI Jiangtao, DING Jianzhi, SONG Xiulan, SU Bingqin. Calcined Mg/Al hydrotalcites for CO2 removal in anaerobic digestion biogas: Performances and mechanisms[J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5250-5261.
| 因素 | 编码变量 | 各水平编码取值 | |||
|---|---|---|---|---|---|
| 编码值 | 非编码值 | -1 | 0 | 1 | |
| 焙烧温度/℃ | x1 | X1 | 200 | 400 | 600 |
| 反应温度/℃ | x2 | X2 | 25 | 40 | 55 |
| 剂量/g·mL-1 | x3 | X3 | 0.008 | 0.012 | 0.016 |
表1 Box-Behnken实验设计的因素与水平
| 因素 | 编码变量 | 各水平编码取值 | |||
|---|---|---|---|---|---|
| 编码值 | 非编码值 | -1 | 0 | 1 | |
| 焙烧温度/℃ | x1 | X1 | 200 | 400 | 600 |
| 反应温度/℃ | x2 | X2 | 25 | 40 | 55 |
| 剂量/g·mL-1 | x3 | X3 | 0.008 | 0.012 | 0.016 |
| 编号 | x1 | x2 | x3 | 吸附后沼气体积分数/% | CO2占比降低率/% | CO2吸附容量 /mmol·g-1 | ||
|---|---|---|---|---|---|---|---|---|
| CH4 | CO2 | 实测值 | 预测值 | |||||
| 1 | 0 | 0 | 0 | 59.35 | 2.80 | 88.09 | 84.14 | 0.723 |
| 2 | -1 | 1 | 0 | 55.85 | 9.72 | 58.67 | 58.60 | 0.537 |
| 3 | 0 | 0 | 0 | 58.68 | 3.81 | 83.79 | 84.14 | 0.760 |
| 4 | -1 | -1 | 0 | 54.56 | 13.19 | 43.89 | 43.20 | 0.480 |
| 5 | 1 | -1 | 0 | 56.42 | 8.70 | 63.01 | 63.08 | 0.600 |
| 6 | 1 | 1 | 0 | 56.78 | 7.37 | 68.67 | 69.36 | 0.637 |
| 7 | 0 | 0 | 0 | 58.83 | 3.84 | 83.67 | 84.14 | 0.730 |
| 8 | -1 | 0 | 1 | 58.47 | 6.32 | 73.12 | 71.61 | 0.495 |
| 9 | 0 | 0 | 0 | 57.79 | 4.03 | 82.86 | 84.14 | 0.730 |
| 10 | -1 | 0 | -1 | 53.49 | 15.07 | 35.92 | 38.19 | 0.645 |
| 11 | 0 | -1 | 1 | 60.66 | 0.72 | 96.94 | 99.14 | 0.610 |
| 12 | 1 | 0 | 1 | 59.46 | 0.82 | 96.51 | 94.24 | 0.608 |
| 13 | 0 | 1 | -1 | 59.63 | 6.71 | 71.46 | 69.26 | 0.980 |
| 14 | 1 | 0 | -1 | 53.99 | 13.00 | 44.70 | 46.21 | 0.730 |
| 15 | 0 | 0 | 0 | 58.37 | 4.16 | 82.30 | 84.14 | 0.727 |
| 16 | 0 | 1 | 1 | 62.44 | 0 | 100.00 | 100.00 | 0.625 |
| 17 | 0 | -1 | -1 | 55.16 | 11.38 | 51.61 | 50.03 | 0.790 |
表2 Box-Behnken响应面优化实验设计及结果
| 编号 | x1 | x2 | x3 | 吸附后沼气体积分数/% | CO2占比降低率/% | CO2吸附容量 /mmol·g-1 | ||
|---|---|---|---|---|---|---|---|---|
| CH4 | CO2 | 实测值 | 预测值 | |||||
| 1 | 0 | 0 | 0 | 59.35 | 2.80 | 88.09 | 84.14 | 0.723 |
| 2 | -1 | 1 | 0 | 55.85 | 9.72 | 58.67 | 58.60 | 0.537 |
| 3 | 0 | 0 | 0 | 58.68 | 3.81 | 83.79 | 84.14 | 0.760 |
| 4 | -1 | -1 | 0 | 54.56 | 13.19 | 43.89 | 43.20 | 0.480 |
| 5 | 1 | -1 | 0 | 56.42 | 8.70 | 63.01 | 63.08 | 0.600 |
| 6 | 1 | 1 | 0 | 56.78 | 7.37 | 68.67 | 69.36 | 0.637 |
| 7 | 0 | 0 | 0 | 58.83 | 3.84 | 83.67 | 84.14 | 0.730 |
| 8 | -1 | 0 | 1 | 58.47 | 6.32 | 73.12 | 71.61 | 0.495 |
| 9 | 0 | 0 | 0 | 57.79 | 4.03 | 82.86 | 84.14 | 0.730 |
| 10 | -1 | 0 | -1 | 53.49 | 15.07 | 35.92 | 38.19 | 0.645 |
| 11 | 0 | -1 | 1 | 60.66 | 0.72 | 96.94 | 99.14 | 0.610 |
| 12 | 1 | 0 | 1 | 59.46 | 0.82 | 96.51 | 94.24 | 0.608 |
| 13 | 0 | 1 | -1 | 59.63 | 6.71 | 71.46 | 69.26 | 0.980 |
| 14 | 1 | 0 | -1 | 53.99 | 13.00 | 44.70 | 46.21 | 0.730 |
| 15 | 0 | 0 | 0 | 58.37 | 4.16 | 82.30 | 84.14 | 0.727 |
| 16 | 0 | 1 | 1 | 62.44 | 0 | 100.00 | 100.00 | 0.625 |
| 17 | 0 | -1 | -1 | 55.16 | 11.38 | 51.61 | 50.03 | 0.790 |
| 方差来源 | 平方和 | 自由度 | 均方 | F | P | 显著性 |
|---|---|---|---|---|---|---|
| 模型 | 6237.35 | 9 | 693.04 | 94.11 | <0.0001 | ** |
| X1 | 469.56 | 1 | 469.56 | 63.76 | <0.0001 | ** |
| X2 | 234.90 | 1 | 234.90 | 31.90 | 0.0008 | ** |
| X3 | 3316.24 | 1 | 3316.24 | 450.33 | <0.0001 | ** |
| X1X2 | 20.79 | 1 | 20.79 | 2.82 | 0.1368 | |
| X1X3 | 53.36 | 1 | 53.36 | 7.25 | 0.0310 | * |
| X2X3 | 70.48 | 1 | 70.48 | 9.57 | 0.0175 | * |
| X12 | 1948.31 | 1 | 1948.31 | 264.57 | <0.0001 | ** |
| X22 | 69.78 | 1 | 69.78 | 9.48 | 0.0179 | * |
| X32 | 0.02 | 1 | 0.02 | 0.0027 | 0.9601 | |
| 残差 | 51.55 | 7 | 7.36 | |||
| 失拟项 | 30.58 | 3 | 10.19 | 1.94 | 0.2643 | 不显著 |
| 净误差 | 20.97 | 4 | 5.24 | |||
| 总离差 | 6288.89 | 16 | ||||
| R2 | 0.9918 | |||||
| R2校正 | 0.9813 | |||||
| R2预测 | 0.9170 | |||||
| 精密度 | 30.457 |
表3 CO2占比降低率的实验模型方差分析
| 方差来源 | 平方和 | 自由度 | 均方 | F | P | 显著性 |
|---|---|---|---|---|---|---|
| 模型 | 6237.35 | 9 | 693.04 | 94.11 | <0.0001 | ** |
| X1 | 469.56 | 1 | 469.56 | 63.76 | <0.0001 | ** |
| X2 | 234.90 | 1 | 234.90 | 31.90 | 0.0008 | ** |
| X3 | 3316.24 | 1 | 3316.24 | 450.33 | <0.0001 | ** |
| X1X2 | 20.79 | 1 | 20.79 | 2.82 | 0.1368 | |
| X1X3 | 53.36 | 1 | 53.36 | 7.25 | 0.0310 | * |
| X2X3 | 70.48 | 1 | 70.48 | 9.57 | 0.0175 | * |
| X12 | 1948.31 | 1 | 1948.31 | 264.57 | <0.0001 | ** |
| X22 | 69.78 | 1 | 69.78 | 9.48 | 0.0179 | * |
| X32 | 0.02 | 1 | 0.02 | 0.0027 | 0.9601 | |
| 残差 | 51.55 | 7 | 7.36 | |||
| 失拟项 | 30.58 | 3 | 10.19 | 1.94 | 0.2643 | 不显著 |
| 净误差 | 20.97 | 4 | 5.24 | |||
| 总离差 | 6288.89 | 16 | ||||
| R2 | 0.9918 | |||||
| R2校正 | 0.9813 | |||||
| R2预测 | 0.9170 | |||||
| 精密度 | 30.457 |
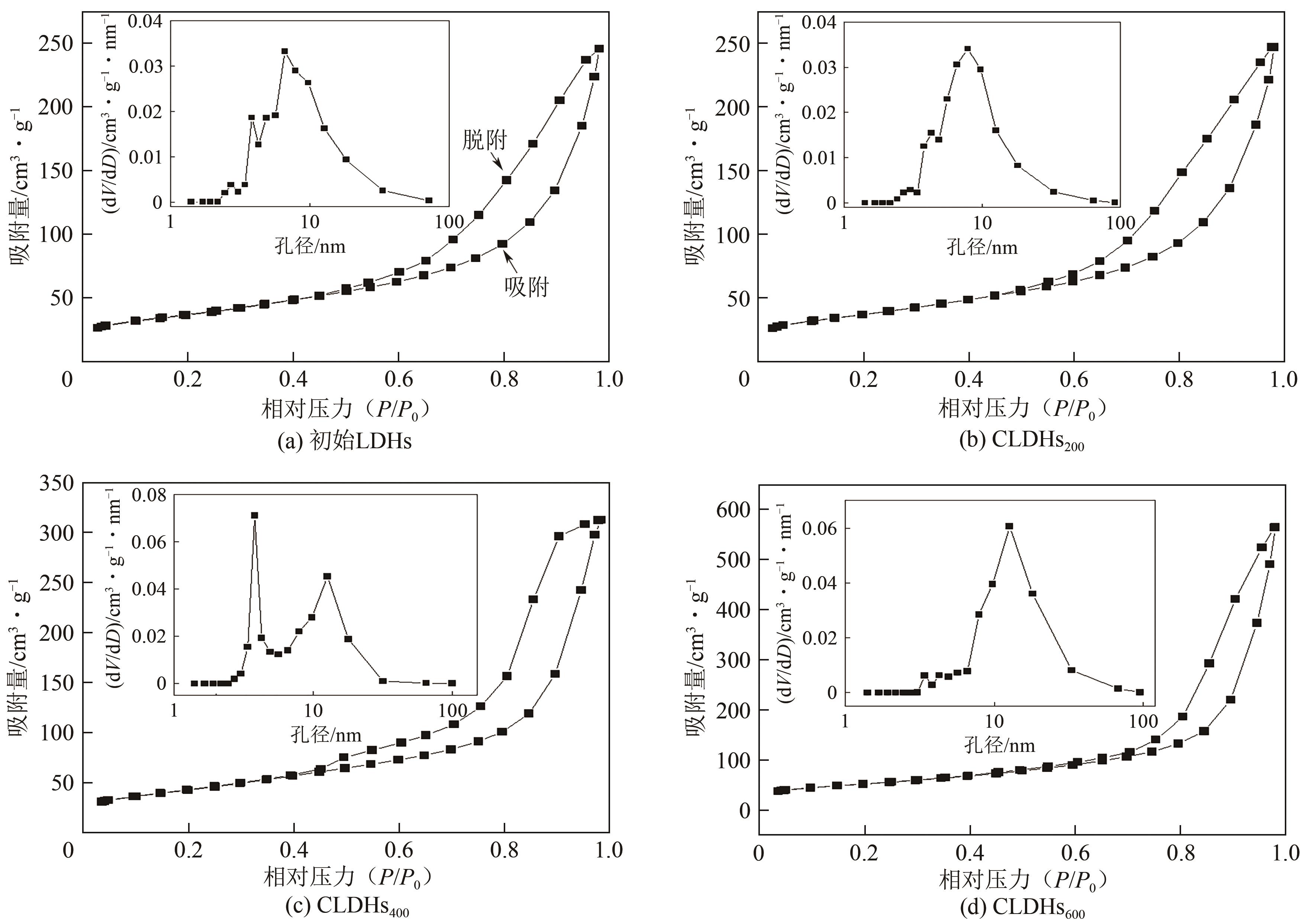
| 样品 | 比表面积/m2·g-1 | 总孔容/cm3·g-1 | 平均孔径/nm |
|---|---|---|---|
| 初始LDHs | 130.32 | 0.380 | 11.66 |
| CLDHs200 | 130.92 | 0.382 | 11.66 |
| CLDHs400 | 153.24 | 0.483 | 12.61 |
| CLDHs600 | 184.98 | 0.873 | 18.87 |
表4 不同吸附剂的BET比表面积和孔结构参数
| 样品 | 比表面积/m2·g-1 | 总孔容/cm3·g-1 | 平均孔径/nm |
|---|---|---|---|
| 初始LDHs | 130.32 | 0.380 | 11.66 |
| CLDHs200 | 130.92 | 0.382 | 11.66 |
| CLDHs400 | 153.24 | 0.483 | 12.61 |
| CLDHs600 | 184.98 | 0.873 | 18.87 |
| 1 | ANDRIAMANOHIARISOAMANANA Fetraj, IHARA Ikko, YOSHIDA Gen, et al. Comparative effects of ferric hydroxide and (semi) conductive iron oxides on the anaerobic digestion of oxytetracycline-contaminated dairy manure[J]. Journal of Environmental Management, 2022, 310: 114731. |
| 2 | LU Tiedong, ZHANG Junya, LI Ping, et al. Enhancement of methane production and antibiotic resistance genes reduction by ferrous chloride during anaerobic digestion of swine manure[J]. Bioresource Technology, 2020, 298: 122519. |
| 3 | WU Yuqi, SONG Kang. Anaerobic co-digestion of waste activated sludge and fish waste: Methane production performance and mechanism analysis[J]. Journal of Cleaner Production, 2021, 279: 123678. |
| 4 | FU Shanfei, CHEN Kaiqiang, SUN Wenxin, et al. Improved methane production of corn straw by the stimulation of calcium peroxide[J]. Energy Conversion and Management, 2018, 164: 36-41. |
| 5 | 包海军. 我国沼气提纯技术及生物天然气产业发展情况[J]. 中国沼气, 2021, 39(1): 54-58. |
| BAO Haijun. Biogas purification technology and development of biogas industry in China[J]. China Biogas, 2021, 39(1): 54-58. | |
| 6 | 韩文彪, 王毅琪, 徐霞, 等. 沼气提纯净化与高值利用技术研究进展[J]. 中国沼气, 2017, 35(5): 57-61. |
| HAN Wenbiao, WANG Yiqi, XU Xia, et al. Progress on purification and high value application of biogas[J]. China Biogas, 2017, 39(1): 54-58. | |
| 7 | 何东发. 厌氧消化技术在造纸废水治理中的应用[J]. 能源研究与利用, 2021(6): 42-45. |
| HE Dongfa. Application of anaerobic digestion technology in papermaking wastewater treatment[J]. Energy Research and Utilization, 2021(6): 42-45. | |
| 8 | Ammarali ABD, OTHMAN Mohdroslee, SHAMSUDIN Ilikhairunnisa, et al. Biogas upgrading to natural gas pipeline quality using pressure swing adsorption for CO2 separation over UiO-66: Experimental and dynamic modelling assessment[J]. Chemical Engineering Journal, 2023, 453: 139774. |
| 9 | TABAR Mohammadazadi, HOSSEINI Seyedsaeid, DENAYER Joerifm. A multicolumn vacuum pressure swing adsorption biogas upgrading process for simultaneous CO2 and N2 separation from methane: Exergy and energy analysis[J]. Energy Conversion and Management, 2022, 269: 116060. |
| 10 | 曲伟国, 张磊, 张淑玲. 沼气提纯脱碳工艺探讨[J]. 燃气与热力, 2016, 36(4): 17-21. |
| QU Weiguo, ZHANG Lei, ZHANG Shuling. Discussion on biogas purification and decarbonization process[J]. Gas&Heat, 2016, 36(4): 17-21. | |
| 11 | 周春萍, 张夏卿, 姜哲. 氨基改性过程引入去离子水对层状双氢氧化物CO2吸附性能的影响规律研究[J]. 功能材料, 2014, 22(45): 22021-22025. |
| ZHOU Chunping, ZHANG Xiaqing, JIANG Zhe. Absorption capacity of carbon dioxide on Mg/Al layered double hydroxide with deionized water adding during amine modified process[J]. Journal of Functional Materials, 2014, 22(45): 22021-22025. | |
| 12 | WU Yuqi, WU Zichuan, YANG Chunfan, et al. Layered double hydroxides for phosphorus recovery from lipid-rich waste anaerobic fermentation liquor[J]. Journal of Environmental Management, 2023, 326: 116759. |
| 13 | LUNDEHOJ L, JENSEN H C, WYBRANDT L, et al. Layered double hydroxides for phosphorus recovery from acidified and non-acidified dewatered sludge[J]. Water Research, 2019, 153: 208-216. |
| 14 | 赵雨, 田念, 王家炜, 等. 复合氨基改性Mg-Al LDH的制备及其CO2吸附性能的研究[J].高校化学工程学报, 2018, 32(3): 659-666. |
| ZHAO Yu, TIAN Nian, WANG Jiawei, et al. Synthesis of amine modified Mg-Al LDH and their CO2 adsorption characteristics[J]. Journal of Chemical Engineering of Chinese Universities, 2018, 3(32): 659-666. | |
| 15 | 孔婷婷. 钛锂铝类水滑石/炭复合材料的制备及CO2吸附与光催化研究[D]. 西安: 西安科技大学, 2017. |
| KONG Tingting. Preparation and CO2 adsorption, photocatalysis performance of Ti/Li/Al-LDHs/coke composite[D]. Xi’an: Xi’an University of Science and Technology, 2017. | |
| 16 | 王君雅, 羊莹, 宁平. 碱金属硝酸盐对促进LDH基材料吸附CO2性能的影响[J]. 环境工程学报, 2018, 12(12): 3379-3388. |
| WANG Junya, YANG Ying, NING Ping. Effect of alkali metal nitrates promoted LDH-based material for CO2 sorption performance[J]. Chinese Journal of Environmental Engineering, 2018, 12(12): 3379-3388. | |
| 17 | MAARTEN Everaert, RUBEN Warrinnier, STIJIN Baken, et al. Phosphate-exchanged Mg-Al layered double hydroxides: A new slow release phosphate fertilizer[J]. ACS Sustainable Chemistry&Engineering, 2016, 4, 4280-4287. |
| 18 | 张国臣. 十二烷基磺酸钠插层有机层状双氢氧化物制备及吸附性能研究[D]. 济南: 山东大学, 2012. |
| ZHANG Guochen. Study on preparation and sorption behavior of sodium dodecylsulfonate intercalated organo layered double hydroxide[D]. Jinan: Shandong University, 2012. | |
| 19 | 邓林. 镁铝水滑石基复合材料吸附去除水中铬(Ⅵ)、磷酸盐的研究[D]. 长沙: 湖南大学, 2015. |
| DENG Lin. Study on removal of chromium(Ⅵ) and phosphate from aqueous solution using Mg-Al hydrotalcite-based composites[D]. Changsha: Hunan University, 2015. | |
| 20 | ABED Marwaf, FAISAL Ayadah. Calcium/iron-layered double hydroxides-sodium alginate for removal of tetracycline antibiotic from aqueous solution[J]. Alexandria Engineering Journal, 2023, 63: 127-142. |
| 21 | 孔婷婷, 董羿蘩, 张颖萍, 等. 类水滑石Ti/Li/Al-LDHs的制备及其CO2吸附性能[J]. 燃料化学学报, 2016, 44(8): 1017-1024. |
| KONG Tingting, DONG Yifan, ZHANG Yingping, et al. Preparation of hydrotalcite-like Ti/Li/Al-LDHs and its performance in CO2 adsorption[J]. Journal of Fuel Chemistry and Technology, 2016, 44(8): 1017-1024. | |
| 22 | MA Xiao, YE Jiongjiong, JIANG Li, et al. Alkaline fermentation of waste activated sludge with calcium hydroxide to improve short-chain fatty acids production and extraction efficiency via layered double hydroxides[J]. Bioresource Technology, 2019, 279: 117-123. |
| 23 | KARIM Ansafv, HASSANI Aydin, EGHBALI Paria, et al. Nanostructured modified layered double hydroxides (LDHs)-based catalysts: A review on synthesis, characterization, and applications in water remediation by advanced oxidation processes[J]. Current Opinion in Solid State and Materials Science, 2022, 26(1): 100965. |
| 24 | SHENG Liang, LIU Jianyong, ZHANG Cheng, et al. Pretreating anaerobic fermentation liquid with calcium addition to improve short chain fatty acids extraction via in situ synthesis of layered double hydroxides[J]. Bioresource Technology, 2019, 271: 190-195. |
| 25 | WANG Jian, ZHANG Yan, SI Jiwen, et al. Structural engineering of NiFe-layered double hydroxides and halloysite composites for efficient CO2 capture[J]. Chemical Engineering Journal, 2023, 463: 142502. |
| 26 | REDDY M K R, XU Z P, LU G Q, et al. Layered double hydroxides for CO2 capture: Structure evolution and regeneration[J]. Industrial&Engineering Chemistry Research, 2006, 45: 7504-7509. |
| 27 | ZHENG Dayang, WU Min, ZHENG Eryang, et al. Parallel adsorption of low concentrated ciprofloxacin by a CoFe-LDH modified sludge biochar[J]. Journal of Environmental Chemical Engineering, 2022, 10: 108381. |
| 28 | JIANG Li, LIU Jianyong, ZHANG Cheng, et al. Synthesis of layered double hydroxides with fermentation liquid of organic waste to extract short-chain fatty acids as a biodenitrification carbon source[J]. ACS Sustainable Chemistry&Engineering, 2017, 5: 9095-9101. |
| 29 | ZHOU Tao, WU Shuya, SU Lianghu, et al. Innovative integrated technique for nutrient acquisition: Simultaneous recovery of carbon and nitrogen sources from the anaerobic fermentation liquid of food waste[J]. ACS Sustainable Chemistry&Engineering, 2018, 6: 10944-10951. |
| 30 | ZHANG Weijun, CHENG Haowan, PENG Sainan, et al. Performance and mechanisms of wastewater sludge conditioning with slag-based hydrotalcite-like minerals (Ca/Mg/Al-LDH)[J]. Water Research, 2020, 169: 115265. |
| [1] | 彭程, 徐漪琳, 石钰婧, 张玟, 李宇涛, 王皓冉, 张卫, 占绣萍. 生物炭改性及其对除草剂污染水体和土壤修复的研究进展[J]. 化工进展, 2024, 43(2): 1069-1081. |
| [2] | 郭宇, 佟民心, 吴红梅. 氨基功能化双醛淀粉吸附剂的制备及其对Pb(Ⅱ)的吸附行为[J]. 化工进展, 2023, 42(12): 6589-6599. |
| [3] | 陈勇, 程宁, 杨育兵, 卢凯玲, 罗应, 易慧. 氧化石墨烯插层膨润土复合材料高效吸附碱性紫3染料[J]. 化工进展, 2022, 41(6): 3324-3332. |
| [4] | 樊相汝, 羊依金, 郭旭晶, 张全碧. 软锰矿-含油污泥基活性炭对亚甲基蓝的吸附特性[J]. 化工进展, 2022, 41(12): 6664-6671. |
| [5] | 陈静, 沈艳琴, 姚一军, 胡成蒙, 武海良. 超吸水材料的研究进展[J]. 化工进展, 2022, 41(11): 5925-5935. |
| [6] | 蒋博龙, 史顺杰, 蒋海林, 封鑫, 孙好芬. 金属有机框架材料吸附处理苯酚污水机理研究进展[J]. 化工进展, 2021, 40(8): 4525-4539. |
| [7] | 刘钦, 周新涛, 黄静, 罗中秋, 邵周军, 王路星, 韦宇, 雒云龙. 赤泥吸附重金属离子性能及其机理研究进展[J]. 化工进展, 2021, 40(6): 3455-3465. |
| [8] | 李孟, 李炜, 张帅, 李雨薇, 刘芳, 赵朝成, 王永强. MOF及其复合材料吸附去除VOCs应用研究进展[J]. 化工进展, 2021, 40(1): 415-426. |
| [9] | 肖永厚, 朱科润, 董晓莹, 贺高红. 燃油选择性吸附脱硫的多孔材料研究进展[J]. 化工进展, 2020, 39(6): 2241-2250. |
| [10] | 崔婉莹, 艾恒雨, 张世豪, 魏金枝. 改性吸附剂去除废水中磷的应用研究进展[J]. 化工进展, 2020, 39(10): 4210-4226. |
| [11] | 金晴,徐建,杨自然,王臣辉,胡军,刘洪来. 等离子体辅助快速合成磁性Fe3O4/NaA复合材料及其CO2吸附性能[J]. 化工进展, 2019, 38(9): 4197-4203. |
| [12] | 郭红霞, 南雁, 寇晓晨, 王胜平, 赵玉军, 马新宾. 钙基CO2吸附剂的惰性掺杂和形貌调控研究进展[J]. 化工进展, 2019, 38(01): 457-466. |
| [13] | 钟黄亮, 王春霞, 周广林, 周红军. 基于纳米材料的静态吸附脱硫进展[J]. 化工进展, 2018, 37(07): 2655-2663. |
| [14] | 王旺阳, 刘聪, 袁珮. 吸附法去除环境中多环芳烃的研究进展[J]. 化工进展, 2017, 36(01): 355-363. |
| [15] | 王 颖,张学军,马小丰,田艳红. 聚丙烯腈基活性碳纤维吸附CO2的研究[J]. 化工进展, 2012, 31(04): 852-856. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||