化工进展 ›› 2024, Vol. 43 ›› Issue (8): 4748-4756.DOI: 10.16085/j.issn.1000-6613.2023-1198
• 资源与环境化工 • 上一篇
铈掺杂镧基钙钛矿制备及对水体磷酸盐和植酸的吸附性能
郭长滨1,2,3( ), 李蒙蒙2,3(
), 李蒙蒙2,3( ), 冯梦晗2,3, 原田2,3, 张克强2,3, 罗艳丽1(
), 冯梦晗2,3, 原田2,3, 张克强2,3, 罗艳丽1( ), 王风2,3(
), 王风2,3( )
)
- 1.新疆农业大学资源与环境学院,新疆 乌鲁木齐 830052
2.农业农村部环境保护科研监测所,天津 300191
3.农业农村部大理农业环境科学观测实验站,云南 大理 671004
-
收稿日期:2023-07-14修回日期:2023-09-13出版日期:2024-08-15发布日期:2024-09-02 -
通讯作者:罗艳丽,王风 -
作者简介:郭长滨(1997—),男,硕士研究生,研究方向为农业面源污染防治。E-mail:18234429217@163.com
李蒙蒙(1996—),男,硕士研究生,研究方向为农业面源污染防治。E-mail:lmm201217@163.com。 -
基金资助:国家重点研发计划(2017YFD0800403);云南省基础研究专项面上项目(202101AT070002);中央级公益性科研院所基本科研业务专项(农业农村部环境保护科研监测所)
Preparation of Ce-doped La-based perovskite and its adsorption properties for phosphate and phytic acid in water
GUO Changbin1,2,3( ), LI Mengmeng2,3(
), LI Mengmeng2,3( ), FENG Menghan2,3, YUAN Tian2,3, ZHANG Keqiang2,3, LUO Yanli1(
), FENG Menghan2,3, YUAN Tian2,3, ZHANG Keqiang2,3, LUO Yanli1( ), WANG Feng2,3(
), WANG Feng2,3( )
)
- 1.College of Resources and Environment, Xinjiang Agricultural University, Urumqi 830052, Xinjiang, China
2.Agro-Environmental Protection Institute, Ministry of Agriculture and Rural Affairs, Tianjin 300191, China
3.Dali Agro-Environmental Science Station, Ministry of Agriculture and Rural Affairs, Dali 671004, Yunnan, China
-
Received:2023-07-14Revised:2023-09-13Online:2024-08-15Published:2024-09-02 -
Contact:LUO Yanli, WANG Feng
摘要:
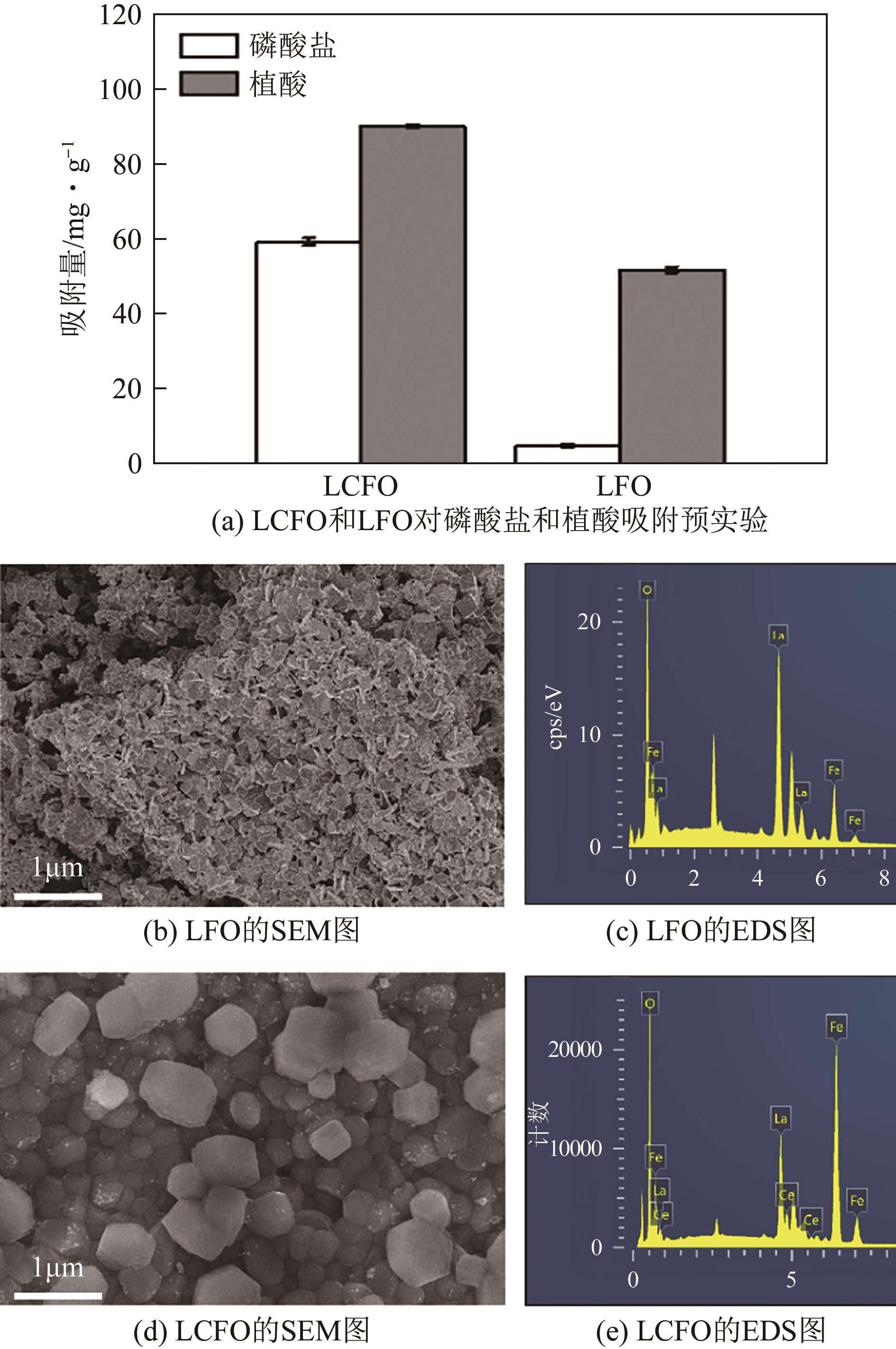
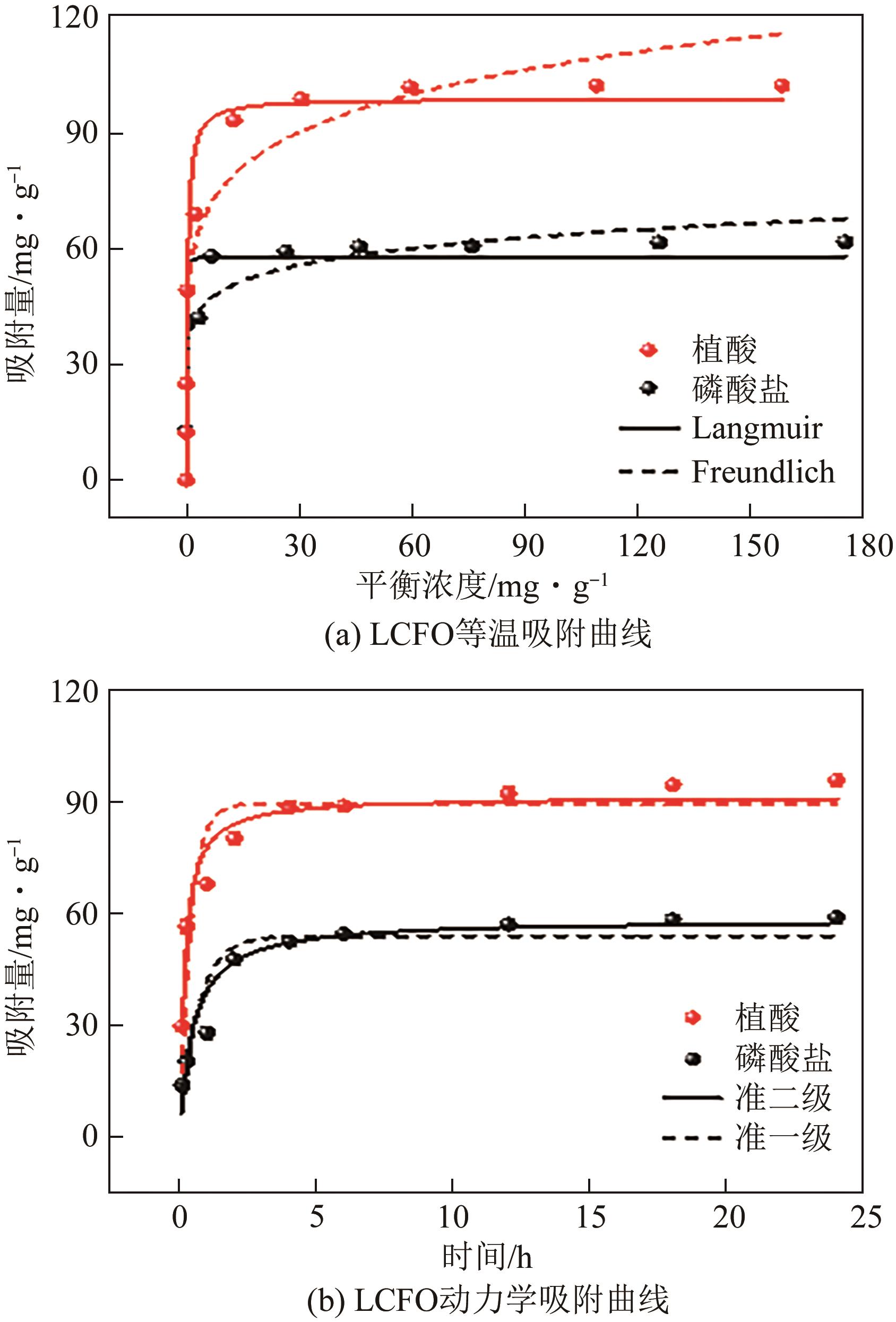
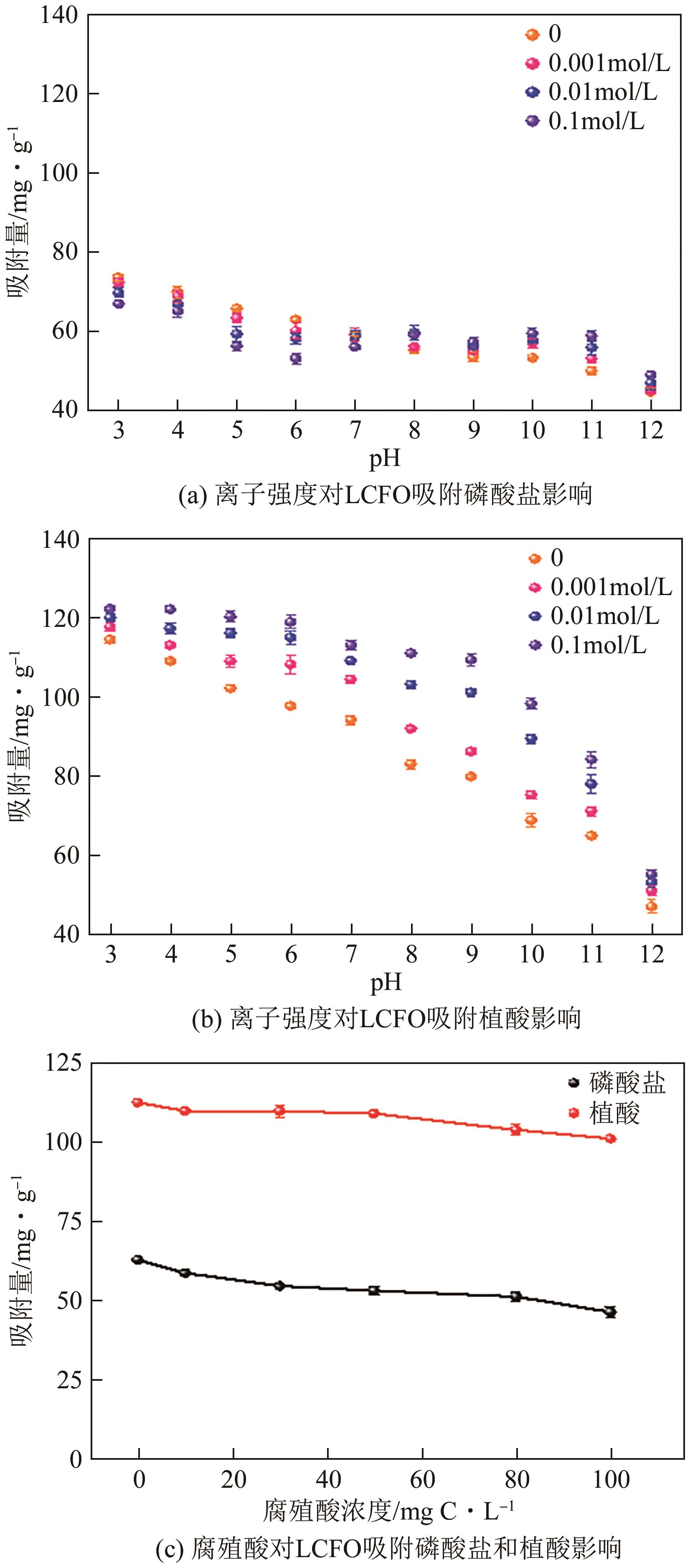
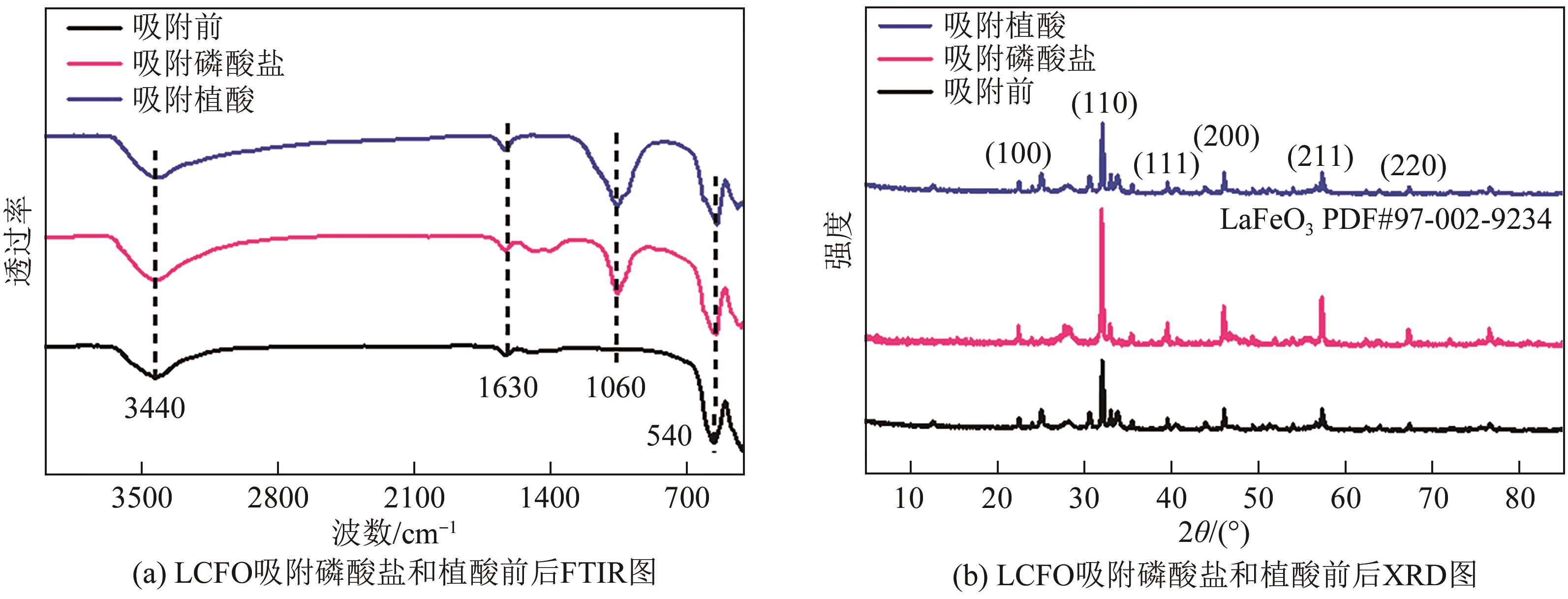
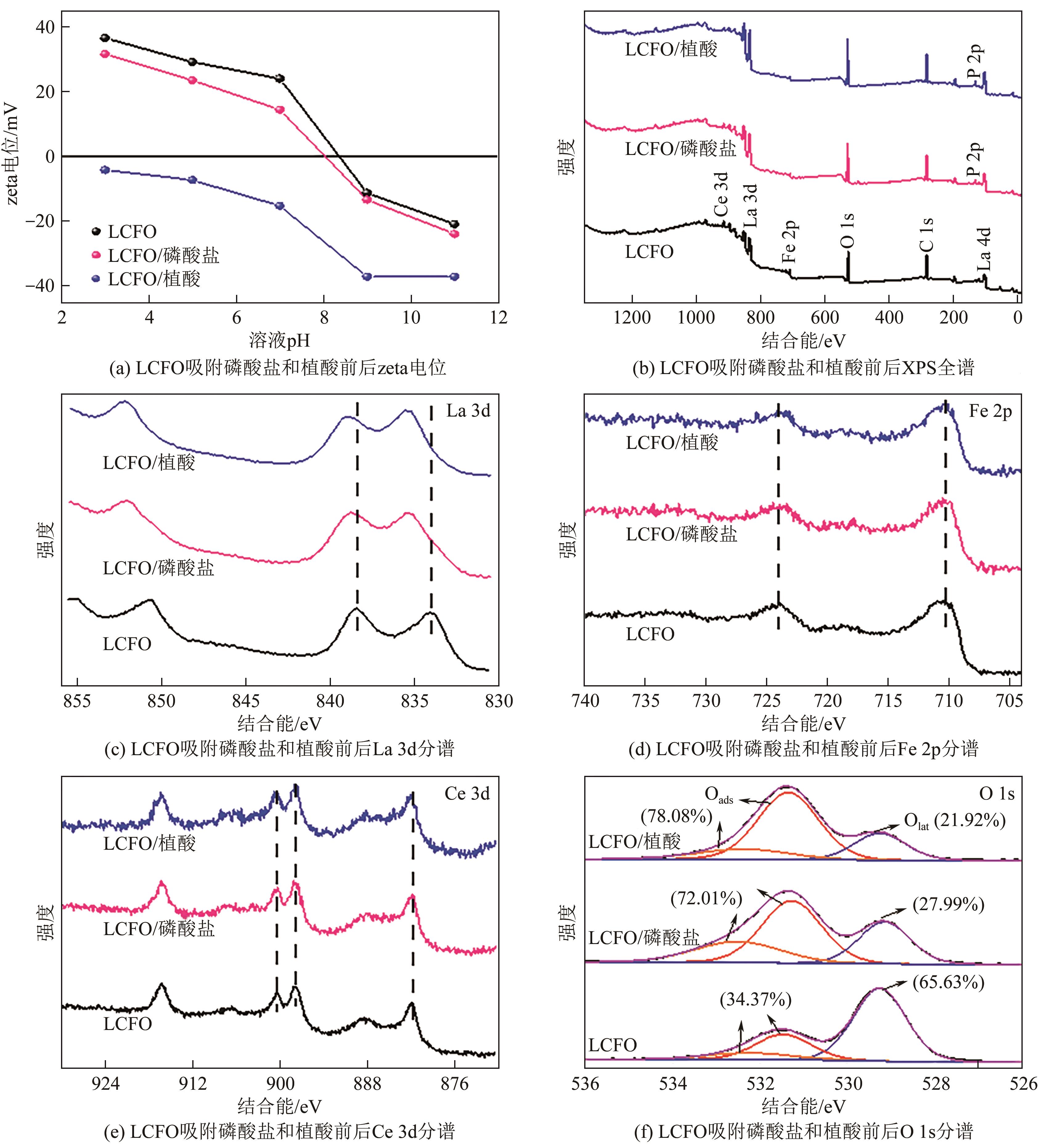
为有效防治水体中的磷酸盐和有机磷污染,利用镧基金属氧化物结构灵活易调控和磷亲和力强等优势,本文在柠檬酸溶胶凝胶法制备的镧铁钙钛矿基础上,在镧位点掺杂铈成功制备了铈镧铁钙钛矿(LCFO),研究了LCFO吸附磷酸盐和植酸的热力学和动力学特征;考察了不同氯离子和腐殖酸根离子等因素对吸附过程的影响;采用扫描电子显微镜-能量色散光谱仪、X射线衍射仪、傅里叶变换红外光谱仪、X射线光电子能谱等表征探究吸附机理。结果表明,LCFO对磷酸盐和植酸吸附能力分别比原始钙钛矿提高了11.56倍和1.74倍,LCFO吸附量分别为57.86mg/g和98.71mg/g;两种形态磷吸附特征均符合准二级动力学方程和Langmuir等温吸附方程,腐殖酸根离子对LCFO对磷酸盐和植酸的吸附作用有一定削弱,但吸附量仍保持在80%以上;通过表征分析表明材料吸附磷酸盐和植酸的机理主要为内层络合。本研究为进一步改善镧铁钙钛矿对无机磷的吸附能力及对有机磷的去除提供参考。
中图分类号:
引用本文
郭长滨, 李蒙蒙, 冯梦晗, 原田, 张克强, 罗艳丽, 王风. 铈掺杂镧基钙钛矿制备及对水体磷酸盐和植酸的吸附性能[J]. 化工进展, 2024, 43(8): 4748-4756.
GUO Changbin, LI Mengmeng, FENG Menghan, YUAN Tian, ZHANG Keqiang, LUO Yanli, WANG Feng. Preparation of Ce-doped La-based perovskite and its adsorption properties for phosphate and phytic acid in water[J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4748-4756.
| 材料 | 比表面积/m2·g-1 | 平均孔径/nm | 孔隙体积/cm3·g-1 |
|---|---|---|---|
| LFO | 1.83 | 7.59 | 0.0034 |
| LCFO | 20.94 | 41.50 | 0.2170 |
表1 LFO和LCFO的理化性质
| 材料 | 比表面积/m2·g-1 | 平均孔径/nm | 孔隙体积/cm3·g-1 |
|---|---|---|---|
| LFO | 1.83 | 7.59 | 0.0034 |
| LCFO | 20.94 | 41.50 | 0.2170 |
| 模型 | 拟合参数 | 磷酸盐 | 植酸 |
|---|---|---|---|
| Langmuir | qmax/mg·g-1 | 57.86 | 98.71 |
| KL/L·mg-1 | 42.49 | 2.76 | |
| R2 | 0.945 | 0.952 | |
| Freundlich | KF/mg·g-1 | 38.28 | 53.98 |
| 1/n | 9.08 | 6.64 | |
| R2 | 0.917 | 0.920 | |
| 准一级动力学 | K1/h-1 | 1.47 | 2.54 |
| qe/mg·g-1 | 54.08 | 89.74 | |
| R2 | 0.87 | 0.82 | |
| 准二级动力学 | K2/g·mg-1·h-1 | 0.03 | 0.06 |
| qe/mg·g-1 | 58.68 | 91.72 | |
| R2 | 0.94 | 0.99 |
表2 LCFO吸附磷酸盐和植酸 Langmuir、Freundlich、准一级动力学和准二级动力学拟合参数
| 模型 | 拟合参数 | 磷酸盐 | 植酸 |
|---|---|---|---|
| Langmuir | qmax/mg·g-1 | 57.86 | 98.71 |
| KL/L·mg-1 | 42.49 | 2.76 | |
| R2 | 0.945 | 0.952 | |
| Freundlich | KF/mg·g-1 | 38.28 | 53.98 |
| 1/n | 9.08 | 6.64 | |
| R2 | 0.917 | 0.920 | |
| 准一级动力学 | K1/h-1 | 1.47 | 2.54 |
| qe/mg·g-1 | 54.08 | 89.74 | |
| R2 | 0.87 | 0.82 | |
| 准二级动力学 | K2/g·mg-1·h-1 | 0.03 | 0.06 |
| qe/mg·g-1 | 58.68 | 91.72 | |
| R2 | 0.94 | 0.99 |
| 吸附剂 | 温度/℃ | pH | 吸附剂投入量/g·L-1 | 磷酸盐吸附量/mg·g-1 | 植酸吸附量/mg·g-1 |
|---|---|---|---|---|---|
| 磁铁矿/氢氧化镧复合材料[ | 25 | 7.0 | 0.2 | 19.3 | — |
| 石膏[ | 25 | 7.0 | 0.75 | — | 2.06 |
| 沸石[ | 25 | 7.0 | 0.75 | — | 1.79 |
| 镧改性生物炭[ | 25 | 7.0 | 0.75 | — | 2.84 |
| α-Al2O3[ | 25 | 5.0 | 25 | 3.64 | 7.01 |
| 勃姆石[ | 25 | 5.0 | 2 | 22.54 | 54.711 |
| LFO(本研究) | 25 | 5.0 | 0.4 | 4.72 | 51.83 |
| LCFO(本研究) | 25 | 5.0 | 0.4 | 57.86 | 97.81 |
表3 磷酸盐和植酸在各种材料上的吸附量比较
| 吸附剂 | 温度/℃ | pH | 吸附剂投入量/g·L-1 | 磷酸盐吸附量/mg·g-1 | 植酸吸附量/mg·g-1 |
|---|---|---|---|---|---|
| 磁铁矿/氢氧化镧复合材料[ | 25 | 7.0 | 0.2 | 19.3 | — |
| 石膏[ | 25 | 7.0 | 0.75 | — | 2.06 |
| 沸石[ | 25 | 7.0 | 0.75 | — | 1.79 |
| 镧改性生物炭[ | 25 | 7.0 | 0.75 | — | 2.84 |
| α-Al2O3[ | 25 | 5.0 | 25 | 3.64 | 7.01 |
| 勃姆石[ | 25 | 5.0 | 2 | 22.54 | 54.711 |
| LFO(本研究) | 25 | 5.0 | 0.4 | 4.72 | 51.83 |
| LCFO(本研究) | 25 | 5.0 | 0.4 | 57.86 | 97.81 |
| 1 | TUAN Vu Ngoc, DINH Trinh Dinh, ZHANG Wenxin, et al. A smart diagnostic tool based on deep kernel learning for on-site determination of phosphate, calcium, and magnesium concentration in a hydroponic system[J]. RSC Advances, 2021, 11(19): 11177-11191. |
| 2 | DU Chuanming, YU Yaohui, JIANG Liudong, et al. Efficient extraction of phosphate from dephosphorization slag by hydrochloric acid leaching[J]. Journal of Cleaner Production, 2022, 332: 130087. |
| 3 | YUAN Mingyao, QIU Shangkai, LI Mengmeng, et al. Adsorption properties and mechanism research of phosphorus with different molecular structures from aqueous solutions by La-modified biochar[J]. Environmental Science and Pollution Research, 2023, 30(6): 14902-14915. |
| 4 | XU Rui, Tao LYU, ZHANG Meiyi, et al. Molecular-level investigations of effective biogenic phosphorus adsorption by a lanthanum/aluminum-hydroxide composite[J]. Science of the Total Environment, 2020, 725: 138424. |
| 5 | AYELE Hailu Sheferaw, ATLABACHEW Minaleshewa. Review of characterization, factors, impacts, and solutions of Lake eutrophication: Lesson for Lake Tana, Ethiopia[J]. Environmental Science and Pollution Research, 2021, 28(12): 14233-14252. |
| 6 | LIU Xuewei, YUAN Zengwei, LIU Xin, et al. Historic trends and future prospects of waste generation and recycling in China’s phosphorus cycle[J]. Environmental Science & Technology, 2020, 54(8): 5131-5139. |
| 7 | BHAGOWATI Biswajit, AHAMAD Kamal Uddin. A review on lake eutrophication dynamics and recent developments in lake modeling[J]. Ecohydrology & Hydrobiology, 2019, 19(1): 155-166. |
| 8 | 陈丽玮, 吴斌程, 王雨航, 等. Fe(Ⅱ)/过一硫酸盐体系降解对氯愈创木酚的研究[J]. 黑龙江大学自然科学学报, 2015, 32(3): 378-383. |
| CHEN Liwei, WU Bincheng, WANG Yuhang, et al. Degradation of 4-chloroguaiacol by Fe(Ⅱ)/peroxymonosulfate Fenton-like oxidation process[J]. Journal of Natural Science of Heilongjiang University, 2015, 32(3): 378-383. | |
| 9 | QIU Fuguo, WANG Juanli, ZHAO Dongye, et al. Adsorption of myo-inositol hexakisphosphate in water using recycled water treatment residual[J]. Environmental Science and Pollution Research, 2018, 25(29): 29593-29604. |
| 10 | CHIAVOLA Agostina, BONGIROLAMI Simona, DI FRANCESCO Giorgia. Technical-economic comparison of chemical precipitation and ion exchange processes for the removal of phosphorus from wastewater[J]. Water Science and Technology, 2020, 81(7): 1329-1335. |
| 11 | DENG Lihua, ZHANG Dehua, KONG Zhaoni, et al. Strong immobilization of phosphate in wastewater onto the surface of MgO-modified industrial hemp-stem-driven biochar by flowerlike crystallization[J]. Industrial & Engineering Chemistry Research, 2020, 59(33): 14578-14586. |
| 12 | LIU Ruiting, CHI Lina, WANG Xinze, et al. Review of metal (hydr)oxide and other adsorptive materials for phosphate removal from water[J]. Journal of Environmental Chemical Engineering, 2018, 6(4): 5269-5286. |
| 13 | WU Zhiying, ZHAO Huan, HU Xiaoya, et al. Tunable porous ferric composite for effective removal of phosphate in water[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2019, 582: 123854. |
| 14 | 罗元, 谢坤, 张克强, 等. 镧(La)改性吸附材料脱除水体磷酸盐研究进展[J]. 化工进展, 2019, 38(11): 5005-5014. |
| LUO Yuan, XIE Kun, ZHANG Keqiang, et al. Research progress on removal phosphate in aqueous solution by lanthanum modified adsorption materials[J]. Chemical Industry and Engineering Progress, 2019, 38(11): 5005-5014. | |
| 15 | LUO Wuhui, HUANG Qidong, ZHANG Xiaomei, et al. Lanthanum/Gemini surfactant-modified montmorillonite for simultaneous removal of phosphate and nitrate from aqueous solution[J]. Journal of Water Process Engineering, 2020, 33: 101036. |
| 16 | LIU Lingyan, ZHANG Chunhong, CHEN Shuangrong, et al. Phosphate adsorption characteristics of La(OH)3-modified, canna-derived biochar[J]. Chemosphere, 2022, 286: 131773. |
| 17 | 罗元, 谢坤, 冯弋洋, 等. 镧改性核桃壳生物炭制备及吸附水体磷酸盐性能[J]. 化工进展, 2021, 40(2): 1121-1129. |
| LUO Yuan, XIE Kun, FENG Yiyang, et al. Preparation of lanthanum modified walnut shell biochar and adsorption of phosphate from aqueous solutions[J]. Chemical Industry and Engineering Progress, 2021, 40(2): 1121-1129. | |
| 18 | LIU Biming, LIU Zhenxue, WU Haixia, et al. Insight into simultaneous selective removal of nitrogen and phosphorus species by lanthanum-modified porous polymer: Performance, mechanism and application[J]. Chemical Engineering Journal, 2021, 415: 129026. |
| 19 | LI Shudan, GUO Meiling, WANG Xiuhua, et al. Fabrication and photocatalytic activity of LaFeO3 ribbon-like nanofibers[J]. Journal of the Chinese Chemical Society, 2020, 67(6): 990-997. |
| 20 | ZHOU Yongjun, Zhe LYU, LI Jingwei, et al. The electronic properties and structural stability of LaFeO3 oxide by niobium doping: A density functional theory study[J]. International Journal of Hydrogen Energy, 2021, 46(13): 9193-9198. |
| 21 | LIU Qingsheng, CHENG Huajin, TU Tao. Experiment and simulation of infrared emissivity properties of doped LaAlO3 [J]. Journal of the American Ceramic Society, 2022, 105(4): 2713-2724. |
| 22 | ZENG Li, PENG Tongjiang, SUN Hongjuan, et al. Fe-doped LaNi1- x Fe x O3 perovskite oxides for enhanced visible-light-driven photocatalytic activity[J]. Journal of Solid State Chemistry, 2021, 297: 122033. |
| 23 | SUN Rong, SHEN Laihong, WANG Shuang, et al. CO conversion over LaFeO3 perovskite during chemical looping processes: Influences of Ca-doping and oxygen species[J]. Applied Catalysis B: Environmental, 2022, 316: 121598. |
| 24 | LI Mengmeng, LUO Yuan, ZHAO Di, et al. Different La/Fe oxide composites for efficient phosphate removal from wastewater: Properties and mechanisms[J]. Journal of Environmental Chemical Engineering, 2022, 10(2): 107329. |
| 25 | GUO Changbin, LI Mengmeng, FENG Menghan, et al. B-site metal modulation of phosphate adsorption properties and mechanism of LaBO3 (B = Fe, Al and Mn) perovskites[J]. Environmental Science and Pollution Research, 2023, 30(25): 66638-66650. |
| 26 | LI Mengmeng, FENG Menghan, GUO Changbin, et al. Green and efficient Al-doped LaFe x Al1- x O3 perovskite oxide for enhanced phosphate adsorption with creation of oxygen vacancies[J]. ACS Applied Materials & Interfaces, 2023, 15(13): 16942-16952. |
| 27 | 杨威, 孙晓蕊, 穆德颖, 等. 铈掺杂纳米氧化锌光催化降解水中抗生素研究[J]. 哈尔滨商业大学学报(自然科学版), 2018, 34(2): 166-169. |
| YANG Wei, SUN Xiaorui, MU Deying, et al. Study on photocatalytic degradation of antibiotics in water by cerium doped nano-zinc oxide[J]. Journal of Harbin University of Commerce (Natural Sciences Edition), 2018, 34(2): 166-169. | |
| 28 | KEERTHANA SP, YUVAKKUMAR R, RAVI G, et al. Fabrication of Ce doped TiO2 for efficient organic pollutants removal from wastewater[J]. Chemosphere, 2022, 293: 133540. |
| 29 | HASSAN Mohamed H, STANTON Robert, SECORA Jeremy, et al. Ultrafast removal of phosphate from eutrophic waters using a cerium-based metal-organic framework[J]. ACS Applied Materials & Interfaces, 2020, 12(47): 52788-52796. |
| 30 | XUE Junbing, WANG Haixia, LI Peng, et al. Efficient reclaiming phosphate from aqueous solution using waste limestone modified sludge biochar: Mechanism and application as soil amendments[J]. Science of the Total Environment, 2021, 799: 149454. |
| 31 | WANG Yajun, LI Jinshou, YUAN Yan, et al. La(OH)3 loaded magnetic nanocomposites derived from sugarcane bagasse cellulose for phosphate adsorption: Characterization, performance and mechanism[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 626: 127060. |
| 32 | LIU Xiaoning, SHEN Feng, QI Xinhua. Adsorption recovery of phosphate from aqueous solution by CaO-biochar composites prepared from eggshell and rice straw[J]. Science of the Total Environment, 2019, 666: 694-702. |
| 33 | XU Suwei, CHEN Ai, ARAI Yuji. Solution 31P NMR investigation of inositol hexakisphosphate surface complexes at the amorphous aluminum oxyhydroxide-water interface[J]. Environmental Science & Technology, 2021, 55(21): 14628-14638. |
| 34 | YAN Yupeng, WAN Biao, LIU Fan, et al. Adsorption-desorption of myo-inositol hexakisphosphate on hematite[J]. Soil Science, 2014, 179(10/11): 476-485. |
| 35 | GIAVENO Cinzia, CELI Luisella, CESSA Raphael Maja Aveiro, et al. Interaction of organic phosphorus with clays extracted from oxisols[J]. Soil Science, 2008, 173(10): 694-706. |
| 36 | ZHOU Aijiao, ZHU Chang, CHEN Wangwei, et al. Phosphorus recovery from water by lanthanum hydroxide embedded interpenetrating network poly (vinyl alcohol)/sodium alginate hydrogel beads[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, 554: 237-244. |
| 37 | SONG Qixuan, HUANG Suzhen, XU Li, et al. Synthesis of magnetite/lanthanum hydroxide composite and magnetite/aluminum hydroxide composite for removal of phosphate[J]. Science of the Total Environment, 2020, 723: 137838. |
| 38 | ZHAO Di, QIU Shangkai, LI Mengmeng, et al. Modified biochar improves the storage capacity and adsorption affinity of organic phosphorus in soil[J]. Environmental Research, 2022, 205: 112455. |
| 39 | 张延一. 植酸和无机磷在(氢)氧化铝表面的吸附—解吸特性与机制[D]. 武汉: 华中农业大学, 2013. |
| ZHANG Yanyi. Adsorption-desorption characteristic and mechanism of IHP and pionto aluminum (oxyhydr) oxides surface[D]. Wuhan: Huazhong Agricultural University, 2013. | |
| 40 | ISLAM Md Aminul, MORTON David W, JOHNSON Bruce B, et al. Adsorption of humic and fulvic acids onto a range of adsorbents in aqueous systems, and their effect on the adsorption of other species: A review[J]. Separation and Purification Technology, 2020, 247: 116949. |
| 41 | LI Xiaodi, KUANG Yue, CHEN Jiabin, et al. Competitive adsorption of phosphate and dissolved organic carbon on lanthanum modified zeolite[J]. Journal of Colloid and Interface Science, 2020, 574: 197-206. |
| 42 | CHENG Peng, LIU Yu, YANG Lei, et al. Phosphate adsorption using calcium aluminate decahydrate to achieve low phosphate concentrations: Batch and fixed-bed column studies[J]. Journal of Environmental Chemical Engineering, 2023, 11(2): 109377. |
| 43 | WANG Jia, WU Liying, LI Jun, et al. Simultaneous and efficient removal of fluoride and phosphate by Fe-La composite: Adsorption kinetics and mechanism[J]. Journal of Alloys and Compounds, 2018, 753: 422-432. |
| 44 | WANG Haiying, HAN Hongjing, SUN Enhao, et al. Production of aryl oxygen-containing compounds by the pyrolysis of bagasse alkali lignin catalyzed by LaM0.2Fe0.8O3 (M = Fe, Cu, Al, Ti)[J]. Energy & Fuels, 2019, 33(9): 8596-8605. |
| 45 | HASSANI Aydin, EGHBALI Paria, MAHDIPOUR Fayyaz, et al. Insights into the synergistic role of photocatalytic activation of peroxymonosulfate by UVA-LED irradiation over CoFe2O4-rGO nanocomposite towards effective Bisphenol A degradation: Performance, mineralization, and activation mechanism[J]. Chemical Engineering Journal, 2023, 453: 139556. |
| 46 | YU Jie, XIANG Chao, ZHANG Gong, et al. Activation of lattice oxygen in LaFe (oxy) hydroxides for efficient phosphorus removal[J]. Environmental Science & Technology, 2019, 53(15): 9073-9080. |
| [1] | 卞维柏, 张睿轩, 潘建明. 无机金属锂离子筛材料制备方法研究进展[J]. 化工进展, 2024, 43(8): 4173-4186. |
| [2] | 王世伟, 王超, 郭琪, 丁红兵. 基于ECT模型修正及算法优化的超音速分离流场图像重建[J]. 化工进展, 2024, 43(8): 4222-4229. |
| [3] | 王嘉, 李文翠, 吴凡, 高新芊, 陆安慧. NiMo/Al2O3催化剂活性组分分布调控及其加氢脱硫应用[J]. 化工进展, 2024, 43(8): 4393-4402. |
| [4] | 谢娟, 贺文, 赵勖丞, 李帅辉, 卢真真, 丁哲宇. 分子动力学模拟在沥青体系中的应用研究进展[J]. 化工进展, 2024, 43(8): 4432-4449. |
| [5] | 郑云香, 高艺伦, 李宴汝, 刘青霖, 张浩腾, 王向鹏. 氨基三乙酸酐改性多孔双网络水凝胶的制备及吸附性能[J]. 化工进展, 2024, 43(8): 4542-4549. |
| [6] | 刘玉灿, 高中鲁, 徐心怡, 纪现国, 张岩, 孙洪伟, 王港. 钙改性水葫芦基生物炭吸附水中敌草隆的效能与机理[J]. 化工进展, 2024, 43(8): 4630-4641. |
| [7] | 胡君杰, 黄兴俊, 雷成, 杨敏, 兰元宵, 罗建洪. 页岩气采出水中小分子有机物的深度处理[J]. 化工进展, 2024, 43(8): 4674-4680. |
| [8] | 武哲, 曲树光, 冯练享, 曾湘楚. 海藻酸钠/微晶纤维素复合水凝胶对水中甲基橙和亚甲基蓝的吸附性能与机理[J]. 化工进展, 2024, 43(8): 4681-4693. |
| [9] | 怀立业, 仲兆平, 杨宇轩. 脱硫石膏转化α-半水石膏的特征及机理:实验与模拟[J]. 化工进展, 2024, 43(8): 4694-4703. |
| [10] | 黄鸿, 欧阳浩民, 杨依静, 李昌霖, 陈烁娜. 硫化零价铁-微生物复合吸附剂对磷酸三(2-氯乙基)酯的吸附-降解机制[J]. 化工进展, 2024, 43(8): 4704-4713. |
| [11] | 丁路, 王培尧, 孔令学, 白进, 于广锁, 李文, 王辅臣. 煤气化过程反应模型研究进展[J]. 化工进展, 2024, 43(7): 3593-3612. |
| [12] | 唐安琪, 魏昕, 丁黎明, 王玉杰, 徐一潇, 刘轶群. 聚酰亚胺气体分离膜的物理老化现象浅析[J]. 化工进展, 2024, 43(7): 3923-3933. |
| [13] | 黄军, 张应娟, 林茵童, 韦雪纯, 吴雨桐, 毋高博, 莫钧麟, 赵祯霞, 赵钟兴. 蚕沙基生物多孔炭的制备及对杀虫单/呋虫胺的协同吸附与缓释性能[J]. 化工进展, 2024, 43(7): 3964-3971. |
| [14] | 张世蕊, 范朕连, 宋慧平, 张丽娜, 高宏宇, 程淑艳, 程芳琴. 粉煤灰负载光催化材料的研究进展[J]. 化工进展, 2024, 43(7): 4043-4058. |
| [15] | 刘克峰, 刘陶然, 蔡勇, 胡雪生, 董卫刚, 周华群, 高飞. 二氧化碳捕集技术研究和工程示范进展[J]. 化工进展, 2024, 43(6): 2901-2914. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||