化工进展 ›› 2024, Vol. 43 ›› Issue (8): 4681-4693.DOI: 10.16085/j.issn.1000-6613.2023-1028
• 资源与环境化工 • 上一篇
海藻酸钠/微晶纤维素复合水凝胶对水中甲基橙和亚甲基蓝的吸附性能与机理
- 1.河池学院广西蚕桑生态学与智能化技术应用重点实验室,广西 河池 546300
2.齐河力厚化工有限公司,山东 德州 251100
-
收稿日期:2023-06-21修回日期:2023-09-16出版日期:2024-08-15发布日期:2024-09-02 -
通讯作者:曾湘楚 -
作者简介:武哲(1993—),女,硕士,研究方向为生物质功能材料。E-mail:hcxywz75@163.com。 -
基金资助:2024年度中国博士后科学基金第75批面上项目(2024M751270);2023年河池市本级财政科技计划(河科AC231107);2023年度广西高校中青年教师科研基础能力提升项目(2024KY0623);河池学院2023年高层次人才科研启动项目(2023GCC015);广西现代蚕桑丝绸协同创新中心2023年开放课题(2023GXCSSC03)
Adsorption performance and mechanism of sodium alginate/microcrystalline cellulose composite hydrogel for aqueous methyl orange and methylene blue
WU Zhe1( ), QU Shuguang2, FENG Lianxiang2, ZENG Xiangchu1(
), QU Shuguang2, FENG Lianxiang2, ZENG Xiangchu1( )
)
- 1.Guangxi Key Laboratory of Sericulture Ecology and Applied Intelligent Technology, Hechi University, Hechi 546300, Guangxi, China
2.Qihe Leahou Chemical Co. , Ltd. , Dezhou 251100, Shandong, China
-
Received:2023-06-21Revised:2023-09-16Online:2024-08-15Published:2024-09-02 -
Contact:ZENG Xiangchu
摘要:
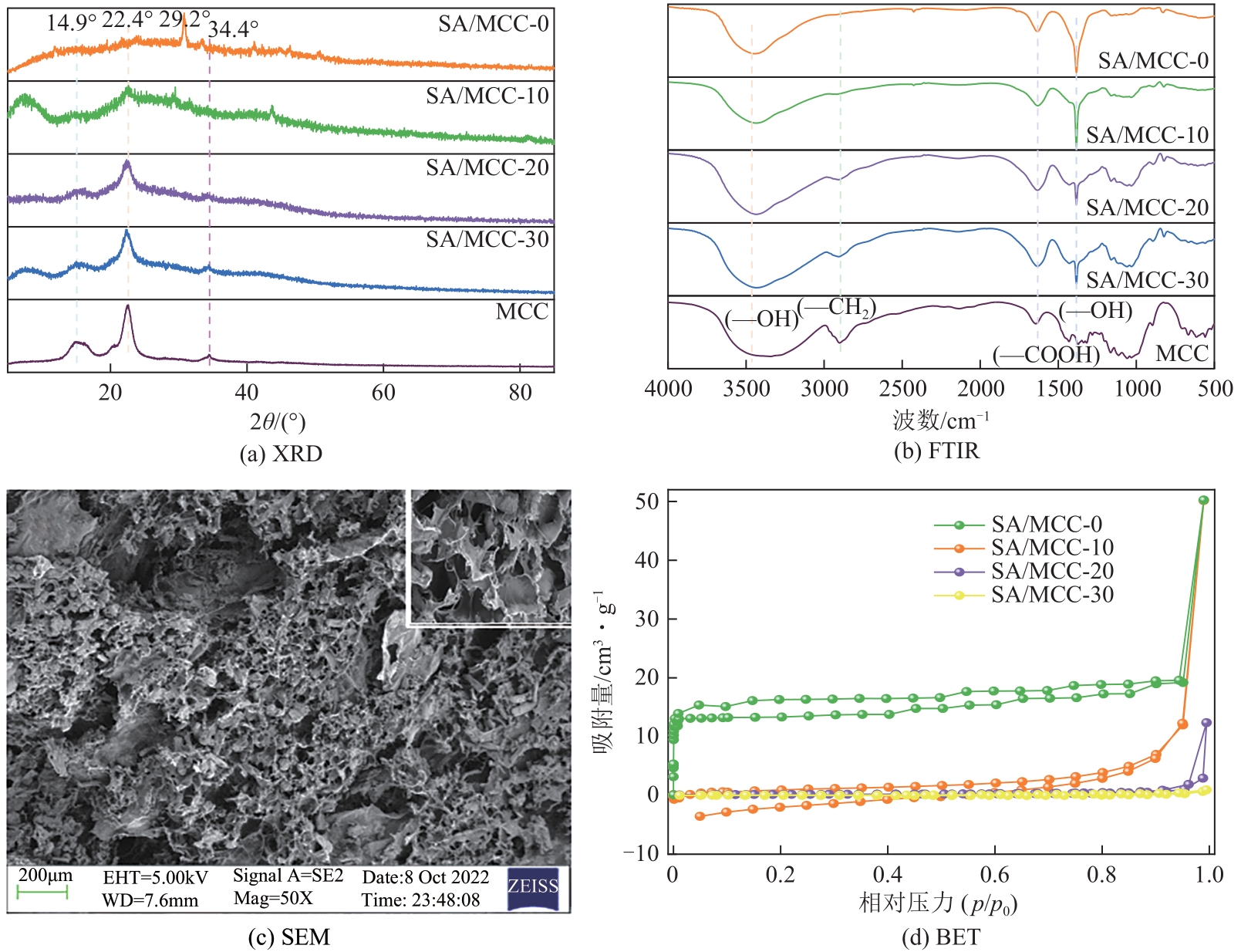
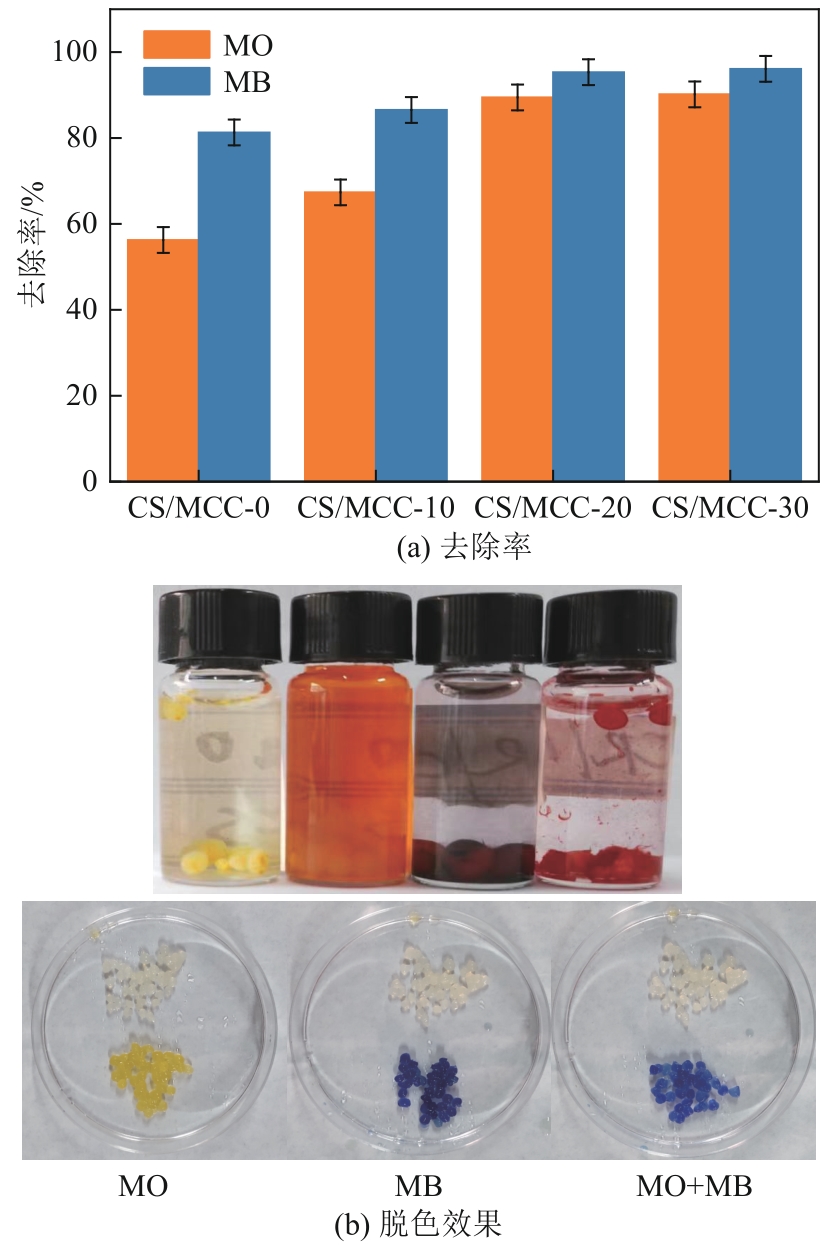
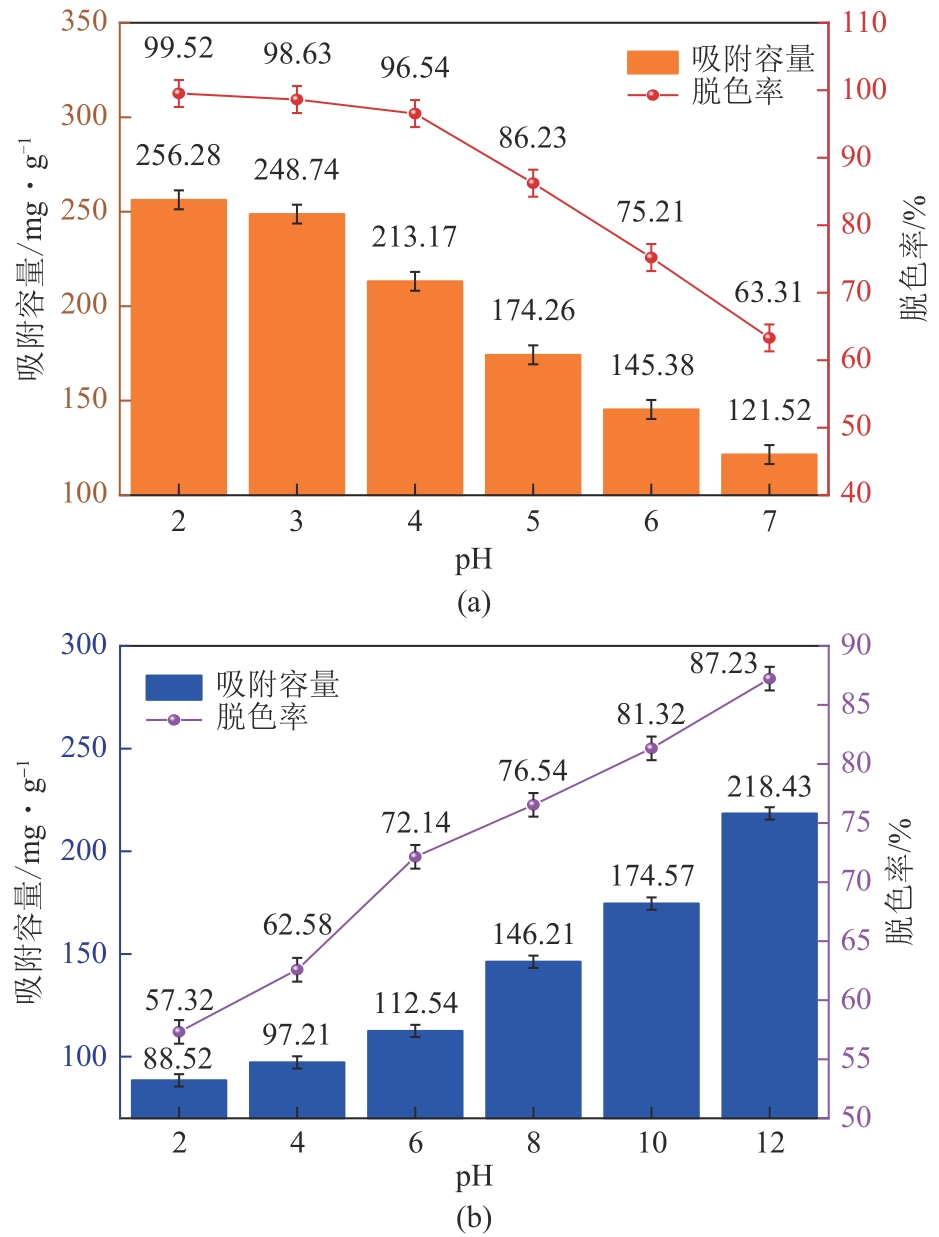
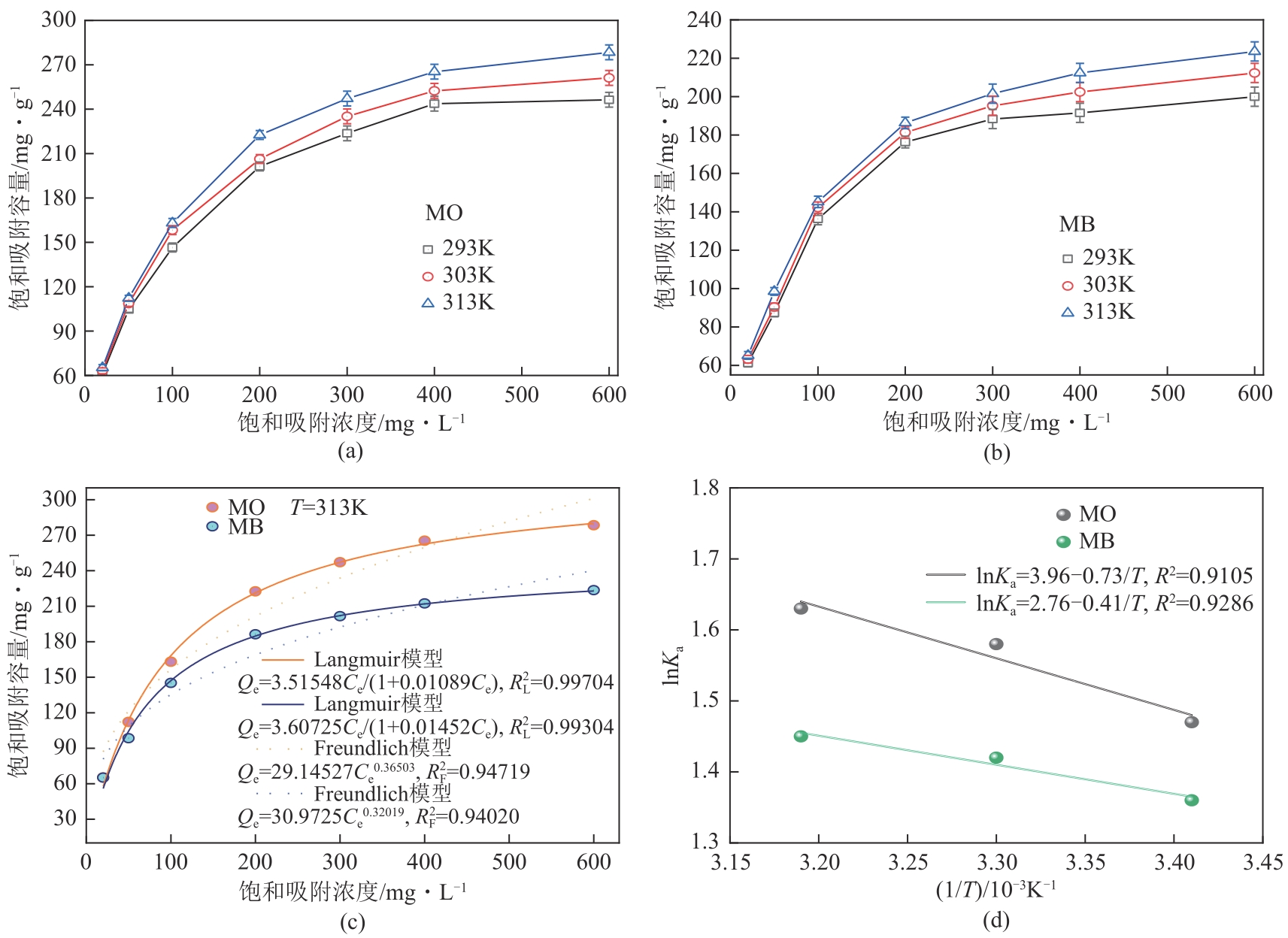
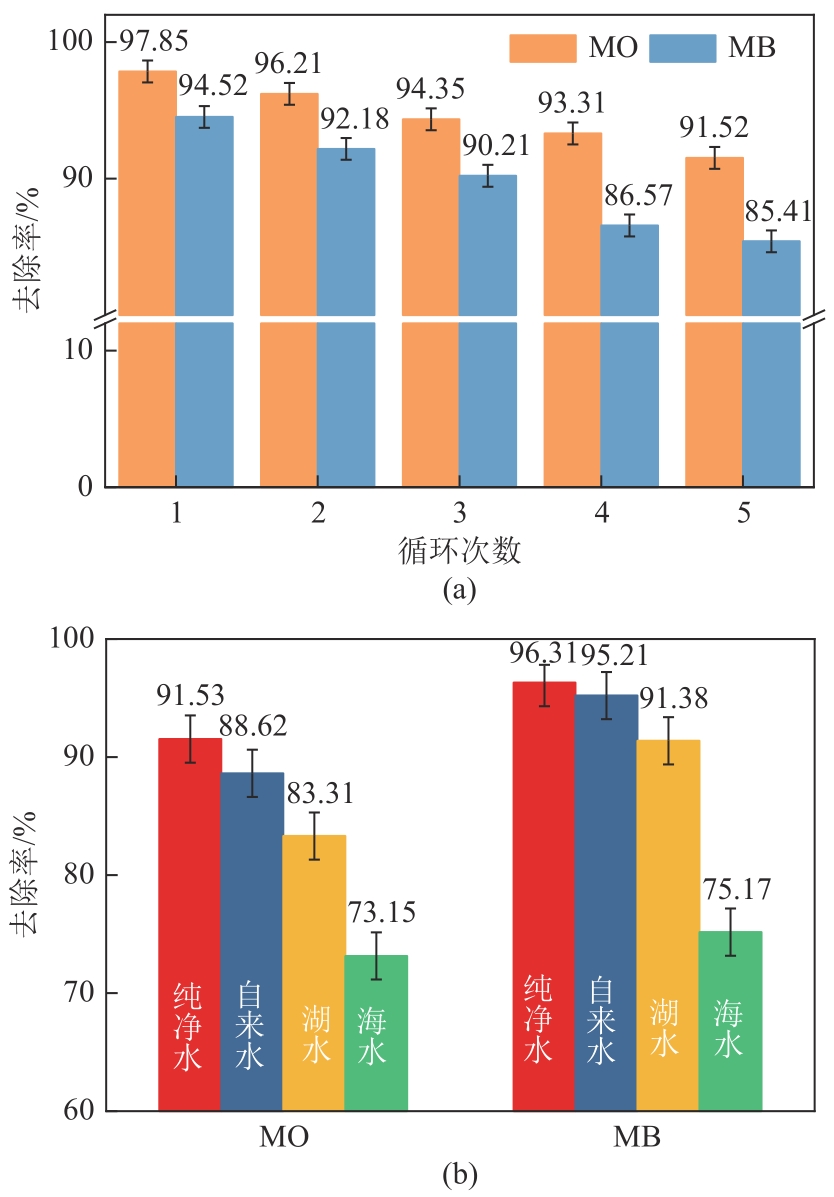
以海藻酸钠(SA)和微晶纤维素(MCC)为原料,合成海藻酸钠/微晶纤维素复合水凝胶(SA/MCC),对制备的复合水凝胶进行结构表征,并研究其对水中甲基橙(MO)和亚甲基蓝(MB)的吸附性能、模型和机理。结果表明,SA/MCC-20去除MO和MB的最佳pH分别为2和12,吸附模型更符合拟二级模型和Langmuir模型,最大吸附容量可达331.25mg/g、253.31mg/g。通过改变水溶液的pH,MO和MB均可在SA/MCC-20表面实现有效的吸附和解吸从而回收利用。经过5次吸附-解吸循环后,在最佳pH下SA/MCC-20对MO和MB的解吸率仍达91.52%和85.41%。SA/MCC对MO和MB具有较强的吸附能力,吸附机理主要包括静电吸引、范德华力、氢键作用、π-π堆积、孔扩散、孔填充等,并以化学吸附为主,物理吸附为辅。
中图分类号:
引用本文
武哲, 曲树光, 冯练享, 曾湘楚. 海藻酸钠/微晶纤维素复合水凝胶对水中甲基橙和亚甲基蓝的吸附性能与机理[J]. 化工进展, 2024, 43(8): 4681-4693.
WU Zhe, QU Shuguang, FENG Lianxiang, ZENG Xiangchu. Adsorption performance and mechanism of sodium alginate/microcrystalline cellulose composite hydrogel for aqueous methyl orange and methylene blue[J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4681-4693.
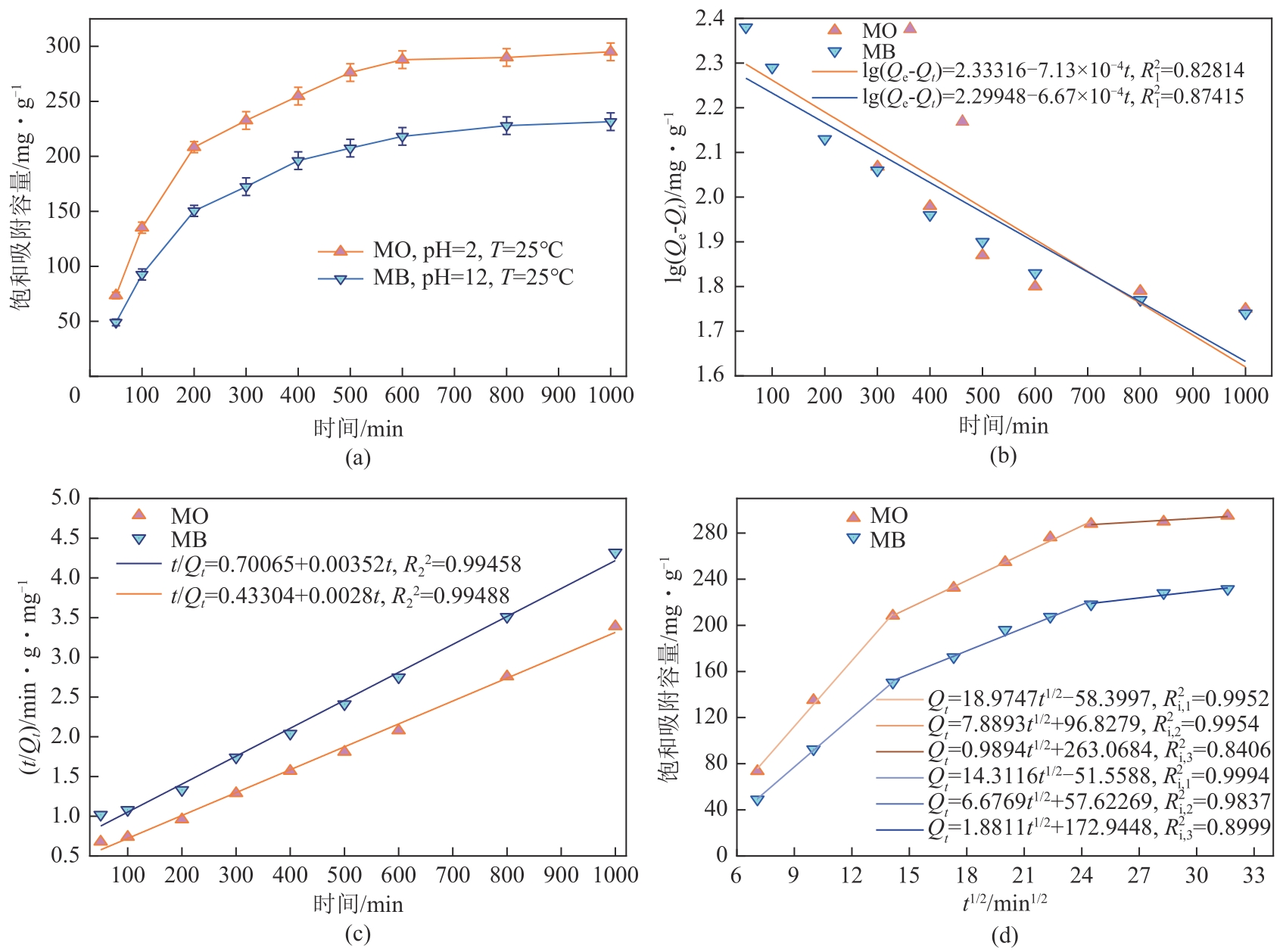
| 染料 | Qe,exp/mg·g-1 | 拟一级动力学模型 | 拟二级动力学模型 | ||||
|---|---|---|---|---|---|---|---|
| Qe,cal/mg·g-1 | k1/min-1 | R12 | Qe,cal/mg·g-1 | k2/g·mg-1·min-1 | R22 | ||
| MO | 287.86 | 217.56 | 1.64×10-3 | 0.82814 | 267.22 | 1.92×10-5 | 0.99488 |
| MB | 218.18 | 198.57 | 1.54×10-3 | 0.87415 | 214.09 | 1.78×10-5 | 0.99458 |
表1 MO和MB的吸附动力学参数
| 染料 | Qe,exp/mg·g-1 | 拟一级动力学模型 | 拟二级动力学模型 | ||||
|---|---|---|---|---|---|---|---|
| Qe,cal/mg·g-1 | k1/min-1 | R12 | Qe,cal/mg·g-1 | k2/g·mg-1·min-1 | R22 | ||
| MO | 287.86 | 217.56 | 1.64×10-3 | 0.82814 | 267.22 | 1.92×10-5 | 0.99488 |
| MB | 218.18 | 198.57 | 1.54×10-3 | 0.87415 | 214.09 | 1.78×10-5 | 0.99458 |
| 染料 | ki,1 | c1 | R2i,1 | ki,2 | c2 | R2i,2 | ki,3 | c3 | R2i,3 |
|---|---|---|---|---|---|---|---|---|---|
| MO | 18.9747 | -58.3997 | 0.9952 | 7.8893 | 96.8279 | 0.9954 | 0.9894 | 263.0684 | 0.8406 |
| MB | 14.3116 | -51.5588 | 0.9994 | 6.6769 | 57.6226 | 0.9837 | 1.8811 | 172.9448 | 0.8999 |
表2 内扩散模型特征参数
| 染料 | ki,1 | c1 | R2i,1 | ki,2 | c2 | R2i,2 | ki,3 | c3 | R2i,3 |
|---|---|---|---|---|---|---|---|---|---|
| MO | 18.9747 | -58.3997 | 0.9952 | 7.8893 | 96.8279 | 0.9954 | 0.9894 | 263.0684 | 0.8406 |
| MB | 14.3116 | -51.5588 | 0.9994 | 6.6769 | 57.6226 | 0.9837 | 1.8811 | 172.9448 | 0.8999 |
| 染料 | Qm,exp | Langmuir模型 | Freundlich模型 | ||||
|---|---|---|---|---|---|---|---|
| Qm,exp/mmol·g-1 | KL/L·mg-1 | RL2 | KF/mg·g-1·L1/n ·mg-1/n | n | RF2 | ||
| MO | 331.25 | 322.82 | 0.01089 | 0.99704 | 29.14527 | 2.73950 | 0.94719 |
| MB | 253.31 | 248.43 | 0.01452 | 0.99304 | 30.9725 | 3.12315 | 0.94020 |
表3 等温模型拟合参数
| 染料 | Qm,exp | Langmuir模型 | Freundlich模型 | ||||
|---|---|---|---|---|---|---|---|
| Qm,exp/mmol·g-1 | KL/L·mg-1 | RL2 | KF/mg·g-1·L1/n ·mg-1/n | n | RF2 | ||
| MO | 331.25 | 322.82 | 0.01089 | 0.99704 | 29.14527 | 2.73950 | 0.94719 |
| MB | 253.31 | 248.43 | 0.01452 | 0.99304 | 30.9725 | 3.12315 | 0.94020 |
| 染料 | T/K | ∆G/kJ·mol-1 | ∆H/kJ·mol-1 | ∆S/kJ·mol-1·K-1 |
|---|---|---|---|---|
| MO | 293 | -3.58 | 6.07 | 32.92 |
| 303 | -3.98 | |||
| 313 | -4.24 | |||
| MB | 293 | -3.31 | 3.41 | 22.95 |
| 303 | -3.58 | |||
| 313 | -3.77 |
表4 吸附热力学参数
| 染料 | T/K | ∆G/kJ·mol-1 | ∆H/kJ·mol-1 | ∆S/kJ·mol-1·K-1 |
|---|---|---|---|---|
| MO | 293 | -3.58 | 6.07 | 32.92 |
| 303 | -3.98 | |||
| 313 | -4.24 | |||
| MB | 293 | -3.31 | 3.41 | 22.95 |
| 303 | -3.58 | |||
| 313 | -3.77 |
| SA/MCC-20 | SA/MCC-20-MO | SA/MCC-MB | SA/MCC-MO/MB | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 吸收峰 | 结合能/eV | 含量/% | 吸收峰 | 结合能/eV | 含量/% | 吸收峰 | 结合能/eV | 含量/% | 吸收峰 | 结合能/eV | 含量/% |
| C 1s | C 1s | ||||||||||
| C—C/C—H | 284.8 | 76.9 | C—C/C—H | 284.8 | 38.1 | C—C/C—H | 284.8 | 69.9 | C—C/C—H | 284.8 | 36.5 |
| C—O | 286.3 | 13.1 | C—O | 286.5 | 40.0 | C—O | 286.4 | 16.8 | C—O | 286.4 | 40.2 |
| C=O | 288.2 | 10.0 | C | 288.3 | 21.5 | C | 288.6 | 13.3 | C | 288.2 | 23.3 |
| O 1s | O 1s | ||||||||||
| C | 531.0 | 48.1 | C | 531.0 | 28.8 | C | 531.0 | 28.8 | C | 531.0 | 27.9 |
| C—O | 532.6 | 26.9 | C-O | 532.1 | 36.5 | C—O | 532.1 | 36.5 | C—O | 532.4 | 44.2 |
| —OH | 533.4 | 22.6 | —OH | 533.3 | 34.7 | —OH | 533.3 | 34.7 | —OH | 533.5 | 27.9 |
| H2O | 536.2 | 2.4 | — | — | — | ||||||
| N 1s | N 1s | ||||||||||
| C | 399.5 | 41.4 | C | 399.5 | 65.8 | C | 399.5 | 32.8 | |||
| C—N | 400.4 | 44.1 | C—N | 400.4 | 19.1 | C—N | 400.3 | 54.6 | |||
| N | 402.3 | 14.5 | N | 401.8 | 15.1 | N | 402.0 | 12.6 | |||
| S 2p | S 2p | ||||||||||
| C—S 2p3/2 | 164.2 | 21.8 | C—S 2p3/2 | 163.6 | 55.1 | C—S 2p3/2 | 164.1 | 46.8 | |||
| C—S 2p1/2 | 165.5 | C—S 2p1/2 | 164.9 | C—S 2p1/2 | 165.4 | ||||||
| SO3- 2p3/2 | 168.4 | 78.2 | SO3- 2p3/2 | 168.0 | 44.9 | SO3- 2p3/2 | 168.3 | 53.2 | |||
| SO3- 2p3/2 | 169.7 | SO3- 2p3/2 | 169.3 | SO3- 2p3/2 | 169.6 | ||||||
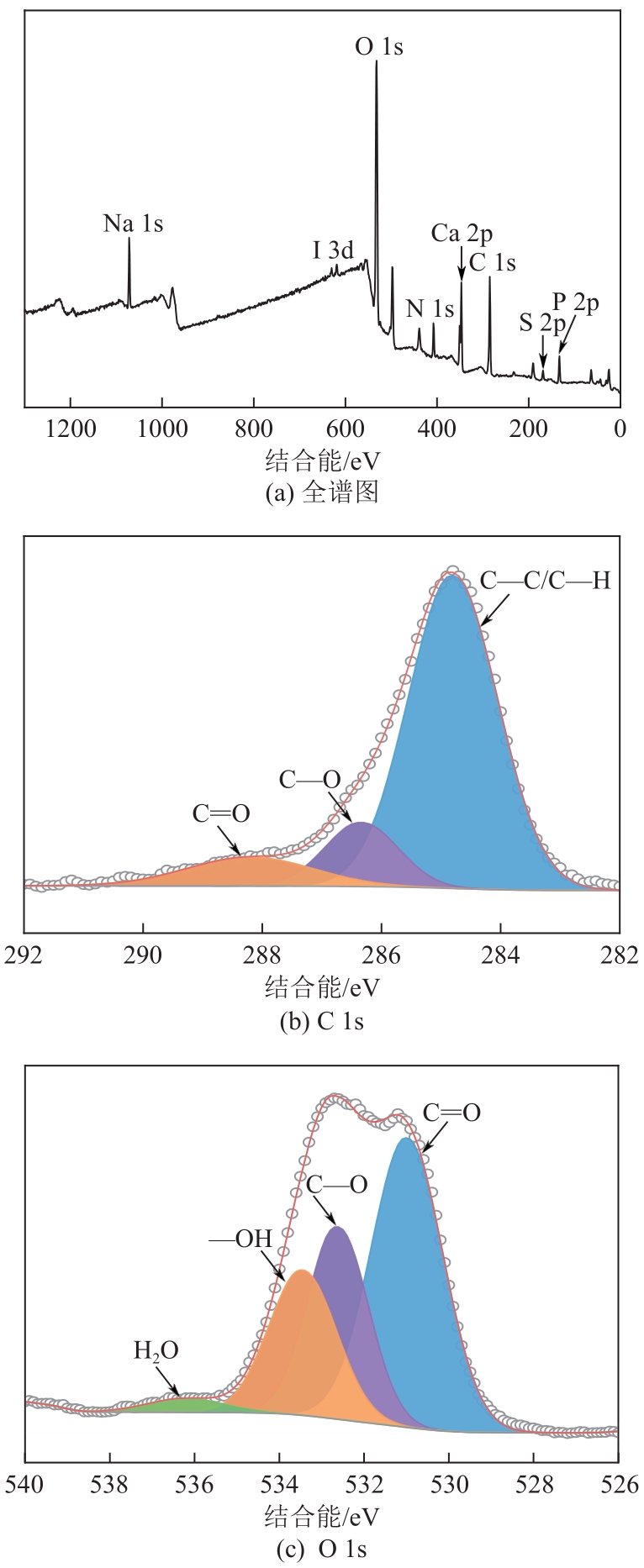
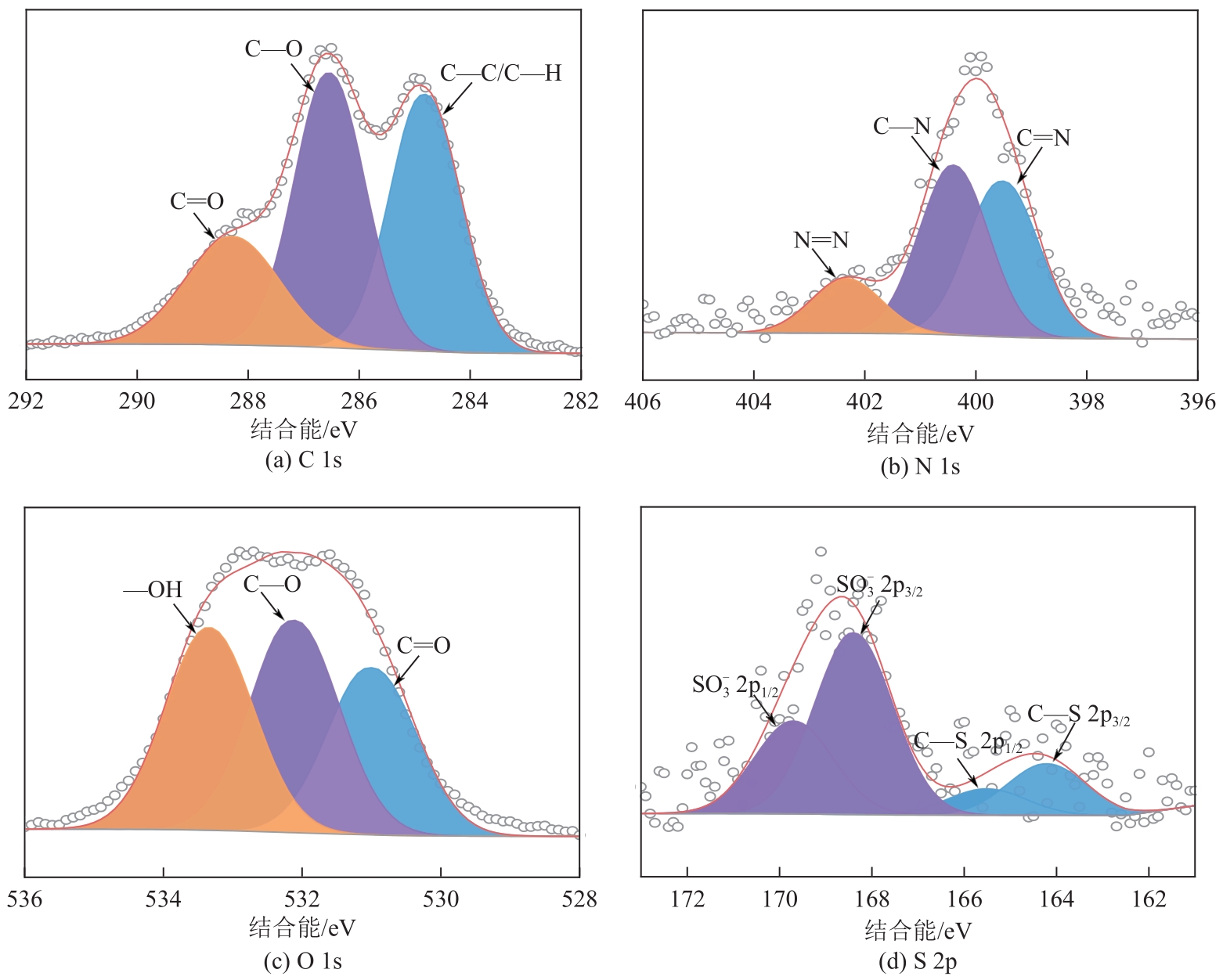
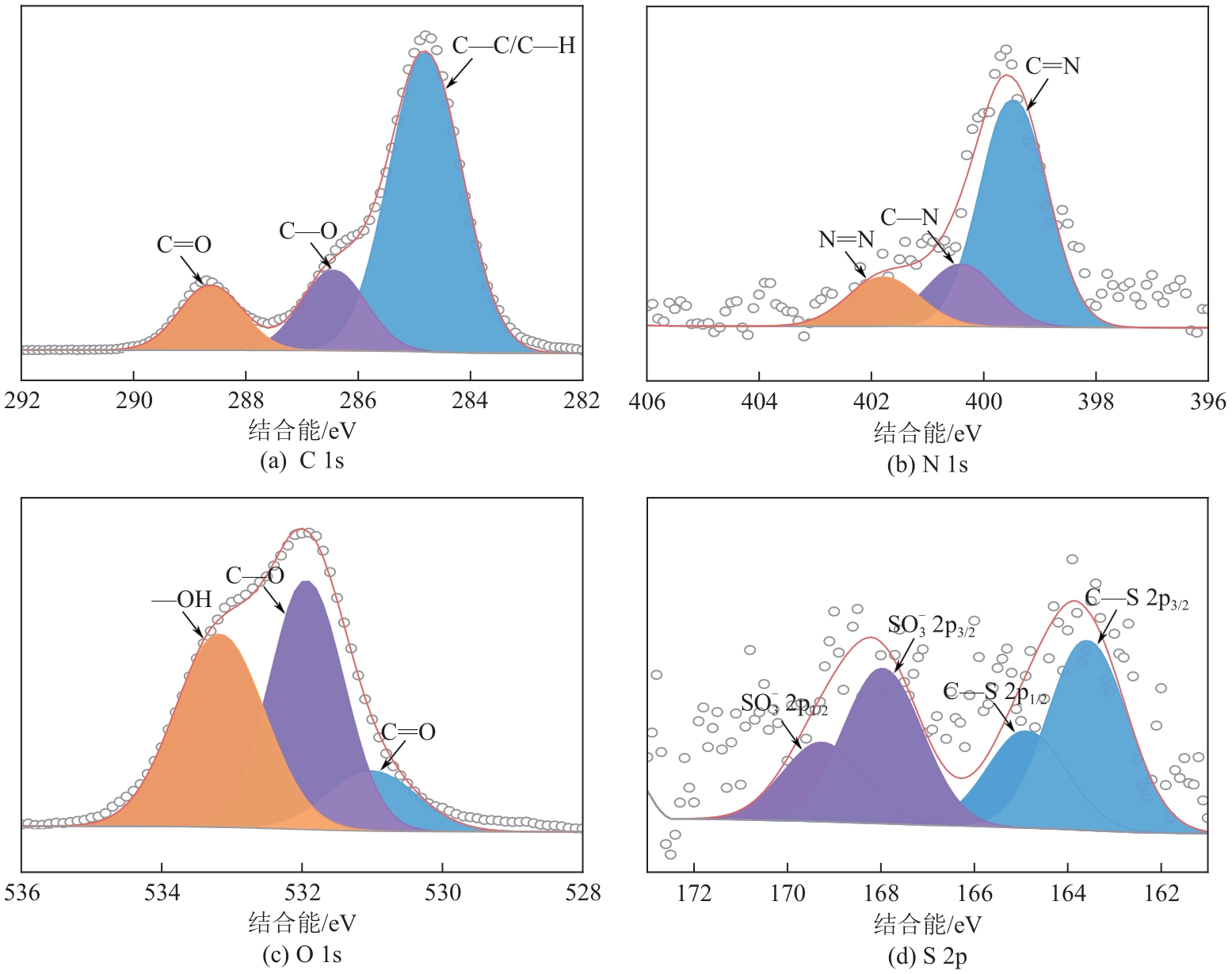
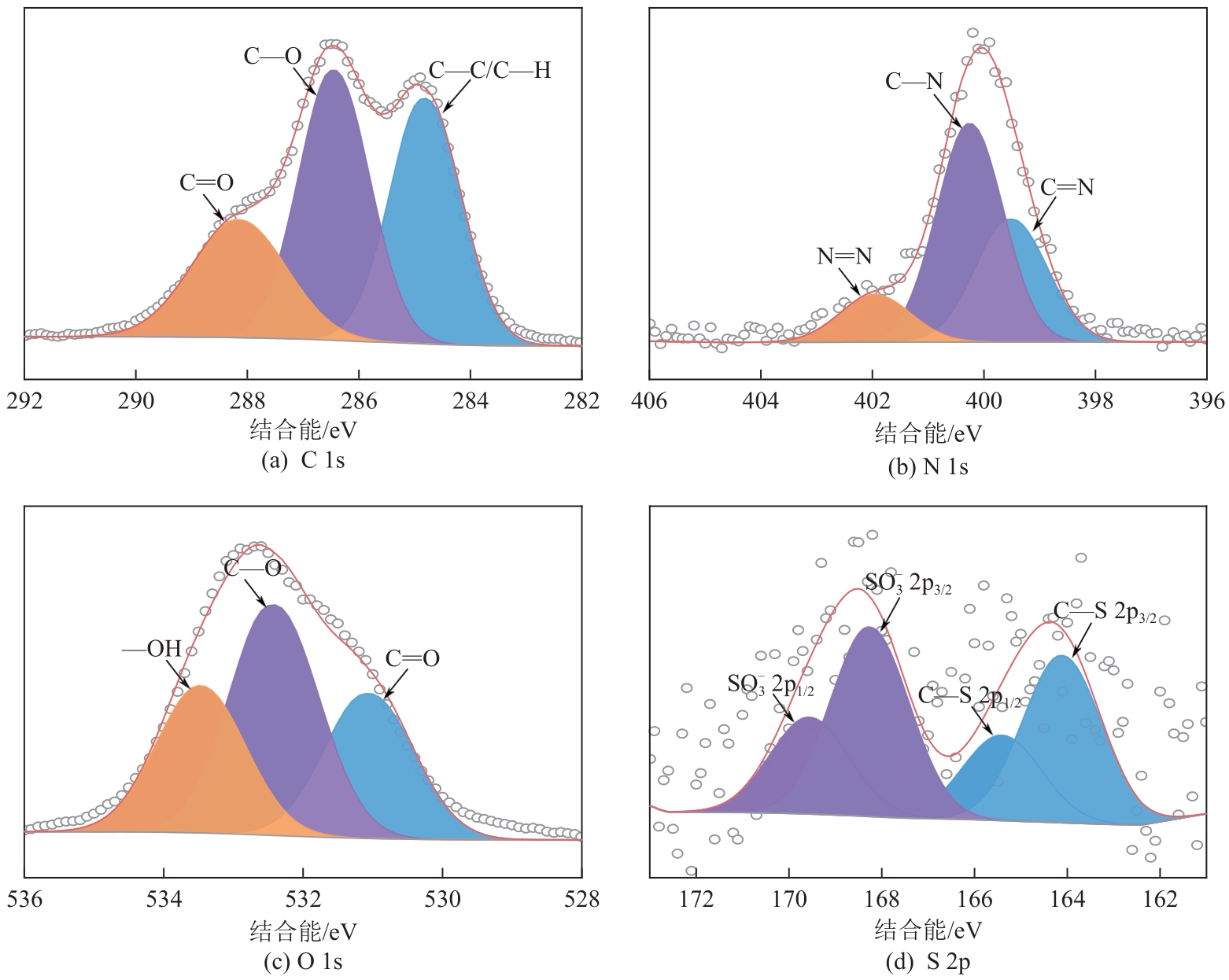
表5 XPS高分辨率谱图
| SA/MCC-20 | SA/MCC-20-MO | SA/MCC-MB | SA/MCC-MO/MB | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 吸收峰 | 结合能/eV | 含量/% | 吸收峰 | 结合能/eV | 含量/% | 吸收峰 | 结合能/eV | 含量/% | 吸收峰 | 结合能/eV | 含量/% |
| C 1s | C 1s | ||||||||||
| C—C/C—H | 284.8 | 76.9 | C—C/C—H | 284.8 | 38.1 | C—C/C—H | 284.8 | 69.9 | C—C/C—H | 284.8 | 36.5 |
| C—O | 286.3 | 13.1 | C—O | 286.5 | 40.0 | C—O | 286.4 | 16.8 | C—O | 286.4 | 40.2 |
| C=O | 288.2 | 10.0 | C | 288.3 | 21.5 | C | 288.6 | 13.3 | C | 288.2 | 23.3 |
| O 1s | O 1s | ||||||||||
| C | 531.0 | 48.1 | C | 531.0 | 28.8 | C | 531.0 | 28.8 | C | 531.0 | 27.9 |
| C—O | 532.6 | 26.9 | C-O | 532.1 | 36.5 | C—O | 532.1 | 36.5 | C—O | 532.4 | 44.2 |
| —OH | 533.4 | 22.6 | —OH | 533.3 | 34.7 | —OH | 533.3 | 34.7 | —OH | 533.5 | 27.9 |
| H2O | 536.2 | 2.4 | — | — | — | ||||||
| N 1s | N 1s | ||||||||||
| C | 399.5 | 41.4 | C | 399.5 | 65.8 | C | 399.5 | 32.8 | |||
| C—N | 400.4 | 44.1 | C—N | 400.4 | 19.1 | C—N | 400.3 | 54.6 | |||
| N | 402.3 | 14.5 | N | 401.8 | 15.1 | N | 402.0 | 12.6 | |||
| S 2p | S 2p | ||||||||||
| C—S 2p3/2 | 164.2 | 21.8 | C—S 2p3/2 | 163.6 | 55.1 | C—S 2p3/2 | 164.1 | 46.8 | |||
| C—S 2p1/2 | 165.5 | C—S 2p1/2 | 164.9 | C—S 2p1/2 | 165.4 | ||||||
| SO3- 2p3/2 | 168.4 | 78.2 | SO3- 2p3/2 | 168.0 | 44.9 | SO3- 2p3/2 | 168.3 | 53.2 | |||
| SO3- 2p3/2 | 169.7 | SO3- 2p3/2 | 169.3 | SO3- 2p3/2 | 169.6 | ||||||
| 1 | YANG Yuxiao, ZHU Junfeng, ZENG Qingzhu, et al. Enhanced activation of peroxydisulfate by regulating pyrolysis temperature of biochar supported nZVI for the degradation of oxytetracycline[J]. Journal of the Taiwan Institute of Chemical Engineers, 2023, 145: 104775. |
| 2 | ZENG Xiangchu, ZHU Junfeng, ZHANG Guanghua, et al. Molecular-level understanding on complexation-adsorption-degradation during the simultaneous removal of aqueous binary pollutants by magnetic composite aerogels[J]. Chemical Engineering Journal, 2023, 468: 143536. |
| 3 | ZENG Xiangchu, ZHANG Guanghua, LI Xiuling, et al. Selective removal of aqueous Hg2+ by magnetic composites sulfur-containing on the hyper-branched surface: Characterization, performance and mechanism[J]. Journal of Environmental Management, 2023, 325: 116621. |
| 4 | ZHANG Zhibin, FU Hiroshi, LI Zheng, et al. Hydrogel materials for sustainable water resources harvesting & treatment: Synthesis, mechanism and applications[J]. Chemical Engineering Journal, 2022, 439: 135756. |
| 5 | FARBOD Ebrahimi, AMIN Sadeghizadeh, FARNAZ Neysan, et al. Fabrication of nanofibers using sodium alginate and poly(vinyl alcohol) for the removal of Cd(Ⅱ) ions from aqueous solutions: Adsorption mechanism, kinetics and thermodynamics[J]. Heliyon, 2019, 5(11): e02941. |
| 6 | ZOU Mingfeng, CHEN Xiangying, LIN Xiajie, et al. Fabrication of magnetic carboxyl-functionalized attapulgite/calcium alginate beads for lead ion removal from aqueous solutions[J]. International Journal of Biological Macromolecules, 2018, 120: 789-800. |
| 7 | ZENG Xiangchu, ZHANG Guanghua, WEN Jia, et al. Simultaneous removal of aqueous same ionic type heavy metals and dyes by a magnetic chitosan/polyethyleneimine embedded hydrophobic sodium alginate composite: Performance, interaction and mechanism[J]. Chemosphere, 2023, 318: 137869. |
| 8 | LI Yangfang, WEN Jia, XUE Zhuangzhuang, et al. Removal of Cr(Ⅵ) by polyaniline embedded polyvinyl alcohol/sodium alginate beads— Extension from water treatment to soil remediation[J]. Journal of Hazardous Materials, 2022, 426: 127809. |
| 9 | ALSWIELEH Abdullah M. Remediation of cationic and anionic dyes from water by histidine modified mesoporous silica[J]. International Journal of Environmental Analytical Chemistry, 2023, 103(5): 1140-1152. |
| 10 | Hazwan HUSSIN M, POHAN, Aqilah Nurul, GARBA, Zaharaddeen N, et al. Physicochemical of microcrystalline cellulose from oil palm fronds as potential methylene blue adsorbents[J]. International Journal of Biological Macromolecules, 2016, 92: 11-19. |
| 11 | ZENG Xiangchu, ZHANG Guanghua, ZHU Junfeng. Selective adsorption of heavy metals from water by a hyper-branched magnetic composite material: Characterization, performance, and mechanism[J]. Journal of Environmental Management, 2022, 314: 907-925. |
| 12 | SUN Haiyan, XU Zhen, GAO Chao. Multifunctional, ultra-flyweight, synergistically assembled carbon aerogels[J]. Advanced Materials, 2013, 25(18): 2554-2560. |
| 13 | LONG Fenglan, NIU Chenggang, TANG Ning, et al. Highly efficient removal of hexavalent chromium from aqueous solution by calcined Mg/Al-layered double hydroxides/polyaniline composites[J]. Chemical Engineering Journal, 2021, 404: 127084. |
| 14 | ZENG Xiangchu, ZHANG Guanghua, ZHU Junfeng, et al. Adsorption of heavy metal ions in water by surface functionalized magnetic composites: A review[J]. Environmental Science: Water Research & Technology, 2022, 8(5): 907-925. |
| 15 | Tian MAI, LI Dandan, CHEN Lei, et al. Collaboration of two-star nanomaterials: The applications of nanocellulose-based metal organic frameworks composites[J]. Carbohydrate Polymers, 2023, 302: 120359. |
| 16 | 刘念, 陈葵, 武斌, 等. 蛋黄-壳介孔磁性炭微球的制备及其对红霉素的高效吸附[J]. 化工进展, 2023, 42(5): 2724-2732. |
| LIU Nian, CHEN Kui, WU Bin, et al. Preparation of yolk-shell mesoporous magnetic carbon microspheres and its efficient adsorption of erythromycin [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2724-2732. | |
| 17 | 曾湘楚, 张光华, 张万斌, 等. 希夫碱改性Fe3O4杂化材料的制备与表征[J]. 精细化工, 2021, 38(9): 1791-1797, 1807. |
| ZENG Xiangchu, ZHANG Guanghua, ZHANG Wanbin, et al. Preparation and characterization of Schiff base modified Fe3O4 hybrid material[J]. Fine Chemicals, 2021, 38(9): 1791-1797, 1807. | |
| 18 | YANG Yuxiao, ZHU Junfeng, ZENG Qingzhu, et al. Activation of persulfate by iron-loaded soybean straw biochar for effcient degradation of dye contaminants: Synthesis, performance, and mechanism[J]. Environmental Progress & Sustainable Energy, 2023, 42(5): e14190. |
| 19 | ALSAMMAN Mohammad T, Julio SÁNCHEZ. Recent advances on hydrogels based on chitosan and alginate for the adsorption of dyes and metal ions from water[J]. Arabian Journal of Chemistry, 2021, 14(12): 103455. |
| 20 | HSINI Abdelghani, NACIRI Yassine, BENAFQIR Mohamed, et al. Facile synthesis and characterization of a novel 1,2,4,5-benzene tetracarboxylic acid doped polyaniline@zinc phosphate nanocomposite for highly efficient removal of hazardous hexavalent chromium ions from water[J]. Journal of Colloid and Interface Science, 2021, 585: 560-573. |
| 21 | GARBA, Zaharaddeen N, LAWAN Ibrahim, ZHOU Weiming, et al. Microcrystalline cellulose (MCC) based materials as emerging adsorbents for the removal of dyes and heavy metals — A review[J]. Science of the Total Environment, 2020, 717: 135070. |
| 22 | Ninad OKE, MOHAN S. Development of nanoporous textile sludge based adsorbent for the dye removal from industrial textile effluent[J]. Journal of Hazardous Materials, 2022, 422: 126864. |
| 23 | ZENG Xiangchu, ZHANG Guanghua, WU Zhe. Preparation and characterization of Schiff-base modified Fe3O4 hybrid material and its selective adsorption for aqueous Hg2+ [J]. Environmental Science and Pollution Research, 2022, 29(20): 30324-30336. |
| 24 | YANG Xiaobing, ZENG Xiangchu, CHEN Hanchun, et al. Coupled homogeneous/heterogeneous Fenton-like system to enhance the synchronized decontamination of aqueous tetracycline and Salmonella typhi [J]. Chemical Engineering Journal, 2024, 483, 148697. |
| 25 | 曾湘楚, 黄红诚, 宋华, 等. 聚乙烯亚胺改性壳聚糖气凝胶对Cr(Ⅵ)和Cu(Ⅱ)的吸附及机理[J]. 华南师范大学学报(自然科学版), 2023, 55(5): 47-58. |
| ZENG Xiangchu, HUANG Hongcheng, SONG Hua, et al. Adsorption and mechanism of Cr(Ⅵ) and Cu(Ⅱ) by polyethyleneimine modified chitosan aerogel[J]. Journal of South China Normal University (Natural Science Edition), 2023, 55(5): 47-58. | |
| 26 | ZENG, Xiangchu, ZHANG Guanghua, SHEN Yiding. Preparation and characterization of a long-chain water-based polyacrylate stripper on paper[J]. Journal of Polymer Research, 2021, 28(7): 241. |
| [1] | 郑云香, 高艺伦, 李宴汝, 刘青霖, 张浩腾, 王向鹏. 氨基三乙酸酐改性多孔双网络水凝胶的制备及吸附性能[J]. 化工进展, 2024, 43(8): 4542-4549. |
| [2] | 张蕾, 杜红英, 冯文浩, 郭军康. 基于二维光热材料的界面太阳能光热蒸发系统优化[J]. 化工进展, 2024, 43(8): 4571-4586. |
| [3] | 刘玉灿, 高中鲁, 徐心怡, 纪现国, 张岩, 孙洪伟, 王港. 钙改性水葫芦基生物炭吸附水中敌草隆的效能与机理[J]. 化工进展, 2024, 43(8): 4630-4641. |
| [4] | 智远, 马吉亮, 陈晓平, 刘道银, 梁财. 流化床喷雾浸渍制备负载型钠基CO2吸附剂脱碳性能[J]. 化工进展, 2024, 43(6): 2961-2967. |
| [5] | 谢蒙蒙, 刘健, 党蕊, 李美馨, 林晓婷, 苏舟, 王洁. 离子导电水凝胶的制备及在柔性电子领域的应用[J]. 化工进展, 2024, 43(6): 3128-3144. |
| [6] | 刘京都, 余关龙, 龙志奇, 周璐, 包璞瑞, 滕骏毅, 杜春艳. 纳米球状LaAlO3的制备及其在酸性条件下的除氟性能[J]. 化工进展, 2024, 43(6): 3199-3208. |
| [7] | 苗诒贺, 王耀祖, 刘雨杭, 朱炫灿, 李佳, 于立军. 添加剂改性固态胺吸附剂用于碳捕集的研究进展[J]. 化工进展, 2024, 43(5): 2739-2759. |
| [8] | 刘萌萌, 秋列维, 万智卫, 李世婧, 许玉雨. 自愈水凝胶的设计原理及应用[J]. 化工进展, 2024, 43(3): 1350-1362. |
| [9] | 董晓涵, 田月, 苏毅. 含钛高炉渣制备复合吸附剂及其铬吸附性能[J]. 化工进展, 2024, 43(3): 1552-1564. |
| [10] | 剧芳. 基于银-硫配位的协同抗菌水凝胶的制备与性能[J]. 化工进展, 2024, 43(2): 1039-1046. |
| [11] | 代洪静, 马学虎, 王四芳. 低中放射性废水处理吸附技术及材料[J]. 化工进展, 2024, 43(1): 529-540. |
| [12] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [13] | 王少凡, 周颖, 郝康安, 黄安荣, 张如菊, 吴翀, 左晓玲. 具有pH响应性的自愈合蓝光水凝胶[J]. 化工进展, 2023, 42(9): 4837-4846. |
| [14] | 张雪伟, 黄亚继, 许月阳, 程好强, 朱志成, 李金壘, 丁雪宇, 王圣, 张荣初. 碱性吸附剂对燃煤烟气中SO3的吸附特性[J]. 化工进展, 2023, 42(7): 3855-3864. |
| [15] | 陆洋, 周劲松, 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛. CeO2/TiO2吸附剂煤气脱汞产物的浸出规律[J]. 化工进展, 2023, 42(7): 3875-3883. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||