化工进展 ›› 2023, Vol. 42 ›› Issue (8): 4264-4274.DOI: 10.16085/j.issn.1000-6613.2022-1832
CuO-CeO2/TiO 2 高效催化CO低温氧化反应性能
李润蕾1( ), 王子彦2, 王志苗1(
), 王子彦2, 王志苗1( ), 李芳1(
), 李芳1( ), 薛伟1, 赵新强1, 王延吉1
), 薛伟1, 赵新强1, 王延吉1
- 1.河北工业大学化工学院,天津 300401
2.中国船舶重工集团公司第七一八研究所,河北 邯郸 056027
-
收稿日期:2022-09-30修回日期:2023-02-04出版日期:2023-08-15发布日期:2023-09-19 -
通讯作者:王志苗,李芳 -
作者简介:李润蕾(1999—),男,硕士研究生,研究方向为绿色催化。E-mail:L530912@163.com。 -
基金资助:国家自然科学基金(U21A20306);河北省自然科学基金(B2022202077)
Efficient catalytic performance of CuO-CeO2/TiO2 for CO oxidation at low-temperature
LI Runlei1( ), WANG Ziyan2, WANG Zhimiao1(
), WANG Ziyan2, WANG Zhimiao1( ), LI Fang1(
), LI Fang1( ), XUE Wei1, ZHAO Xinqiang1, WANG Yanji1
), XUE Wei1, ZHAO Xinqiang1, WANG Yanji1
- 1.School of Chemical Engineering and Technology, Hebei University of Technology, Tianjin 300401, China
2.Purification Equipment Research Institute of CSIC, Handan 056027, Hebei, China
-
Received:2022-09-30Revised:2023-02-04Online:2023-08-15Published:2023-09-19 -
Contact:WANG Zhimiao, LI Fang
摘要:
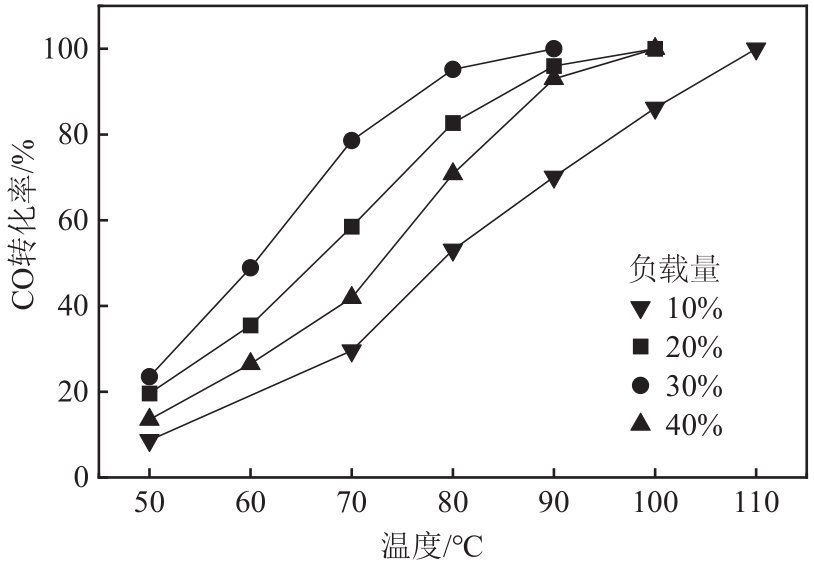
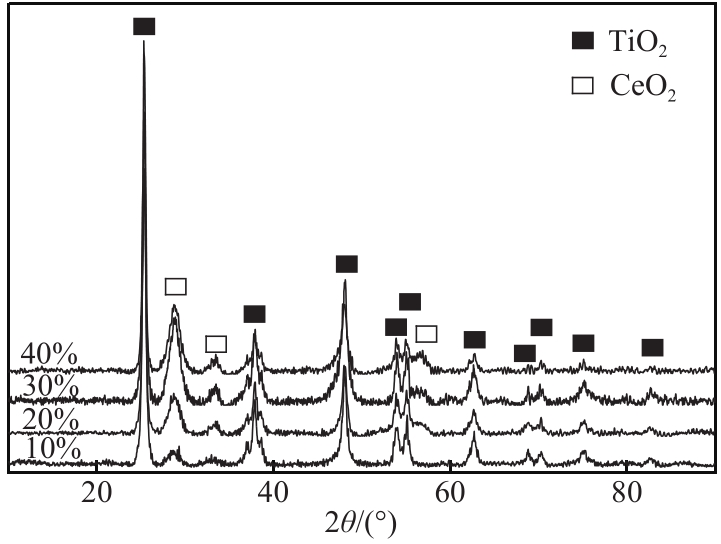
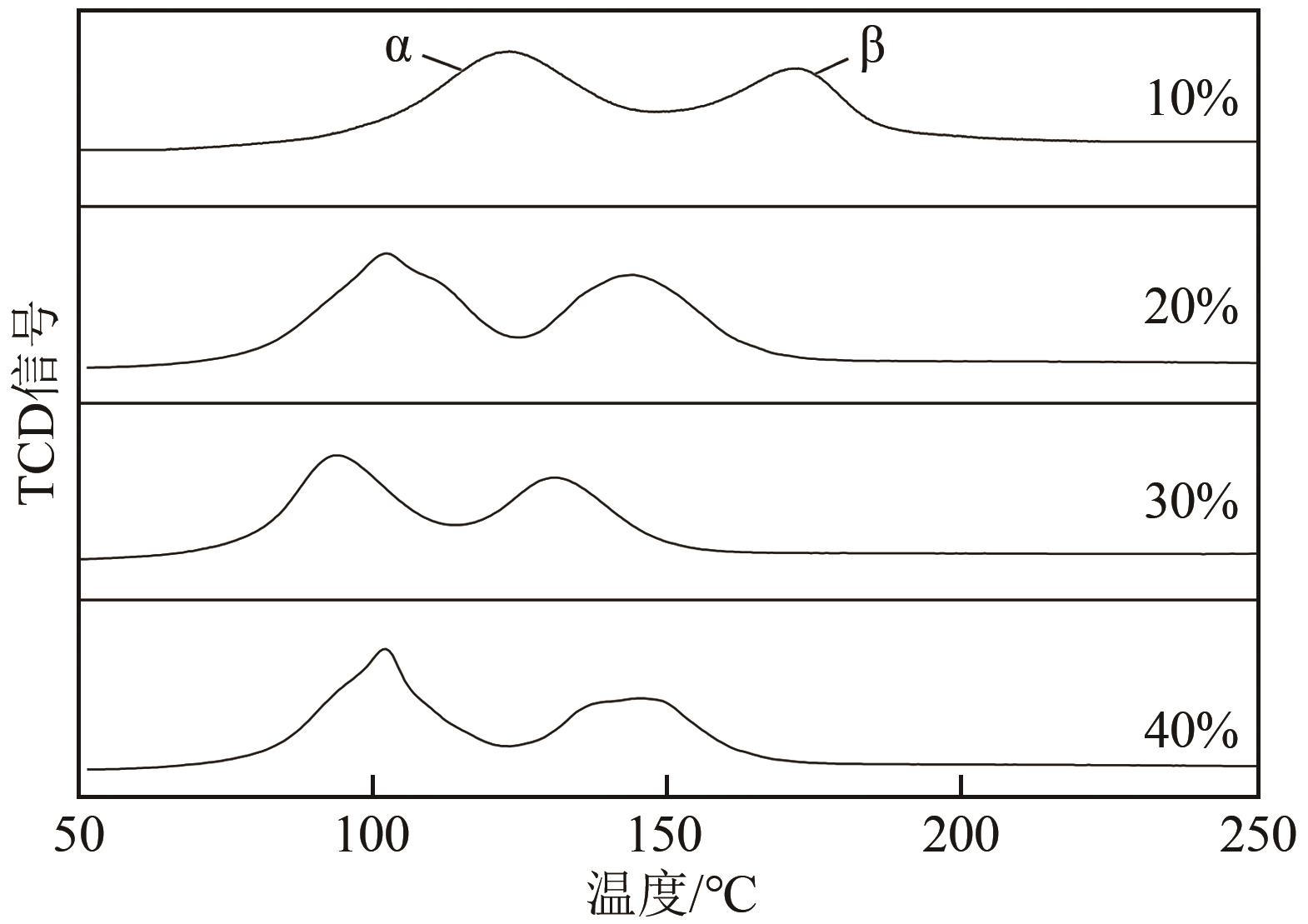
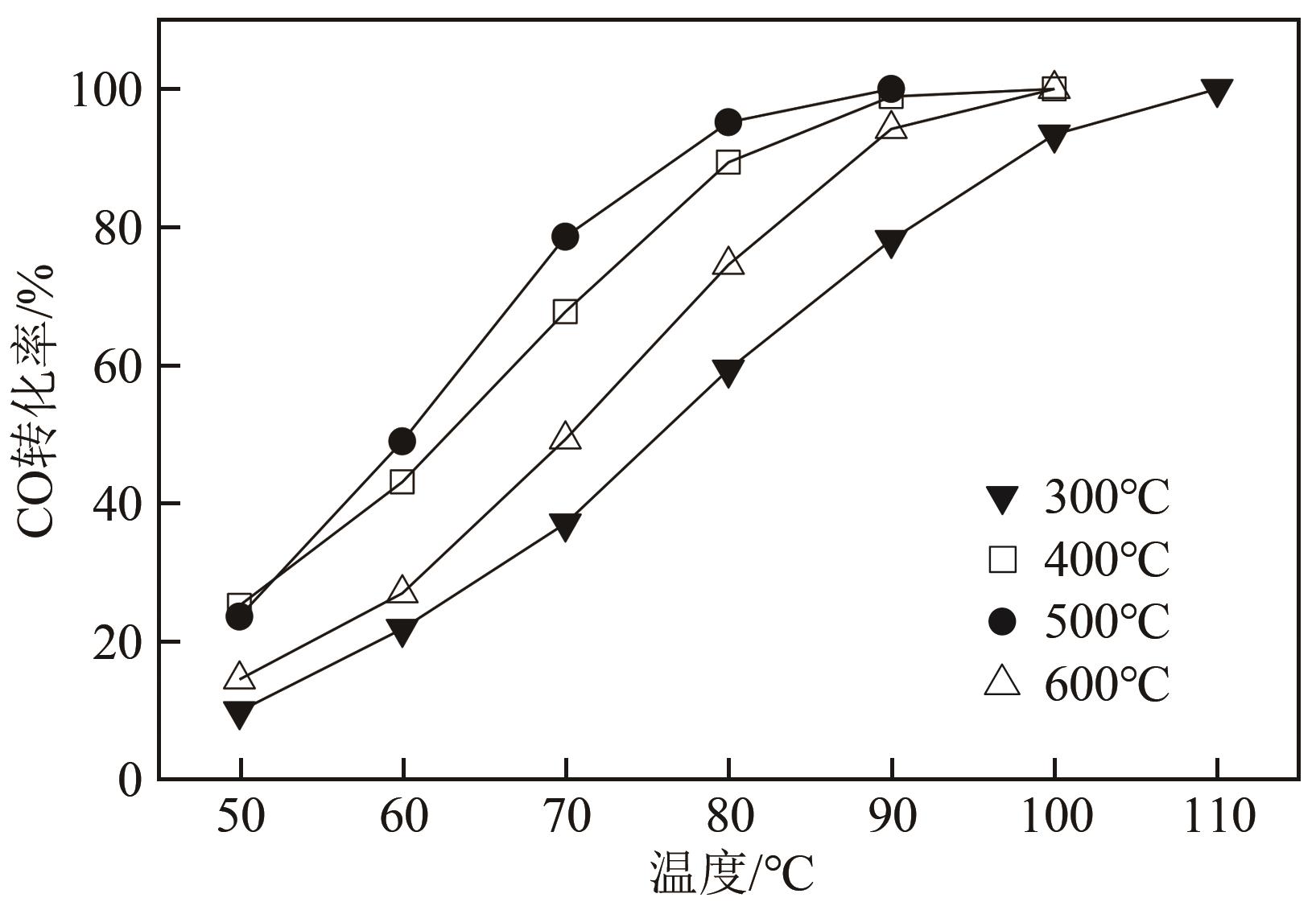
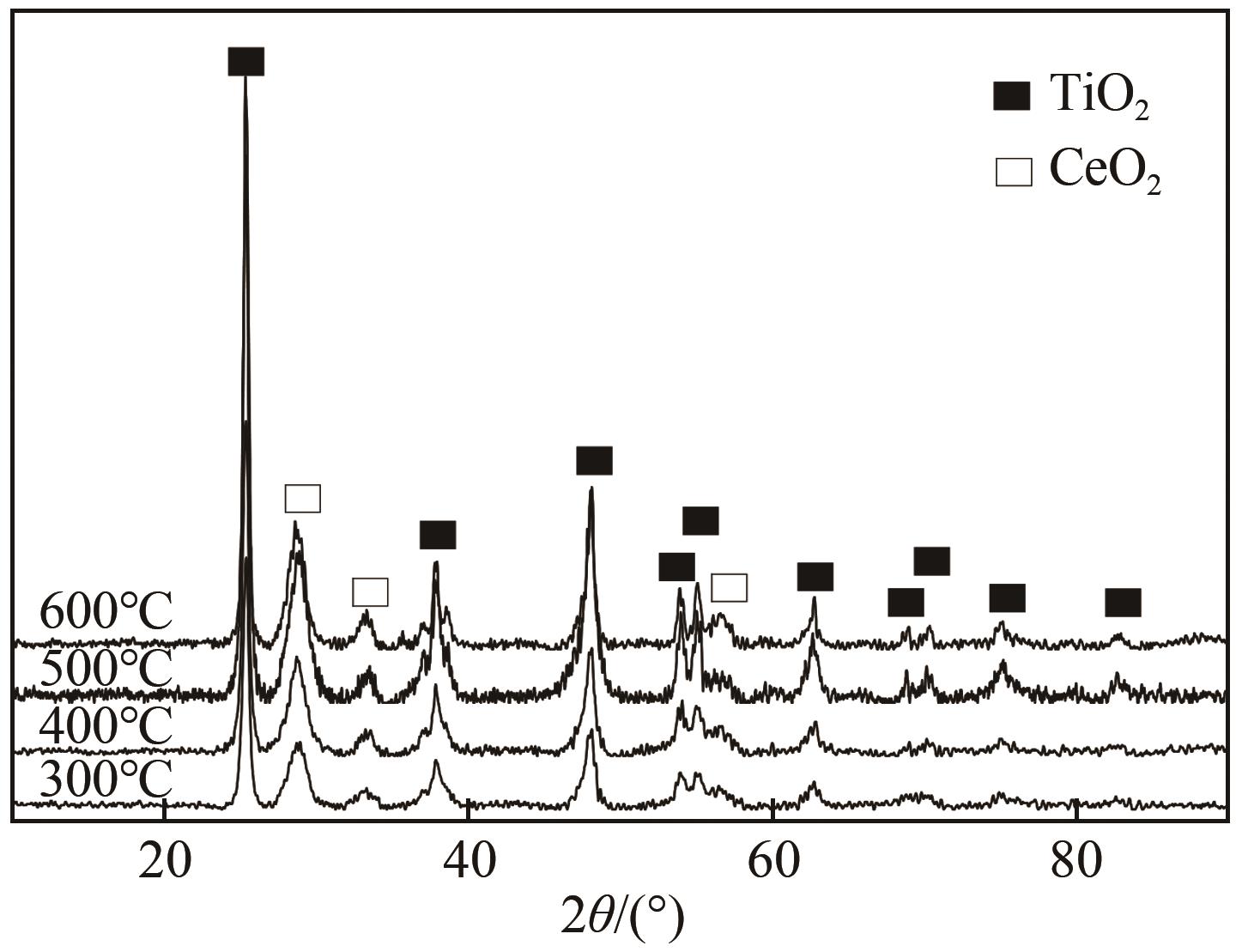
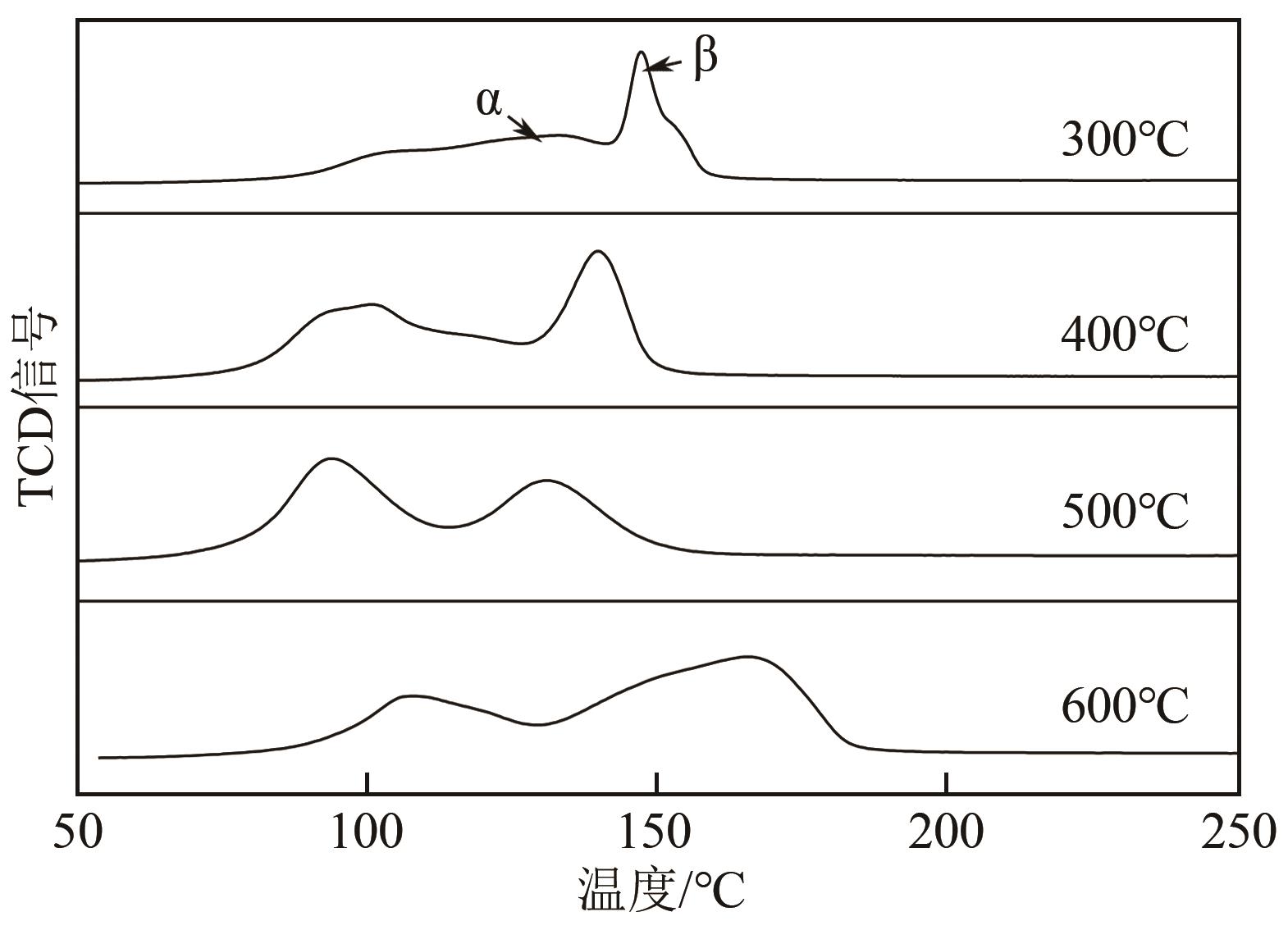
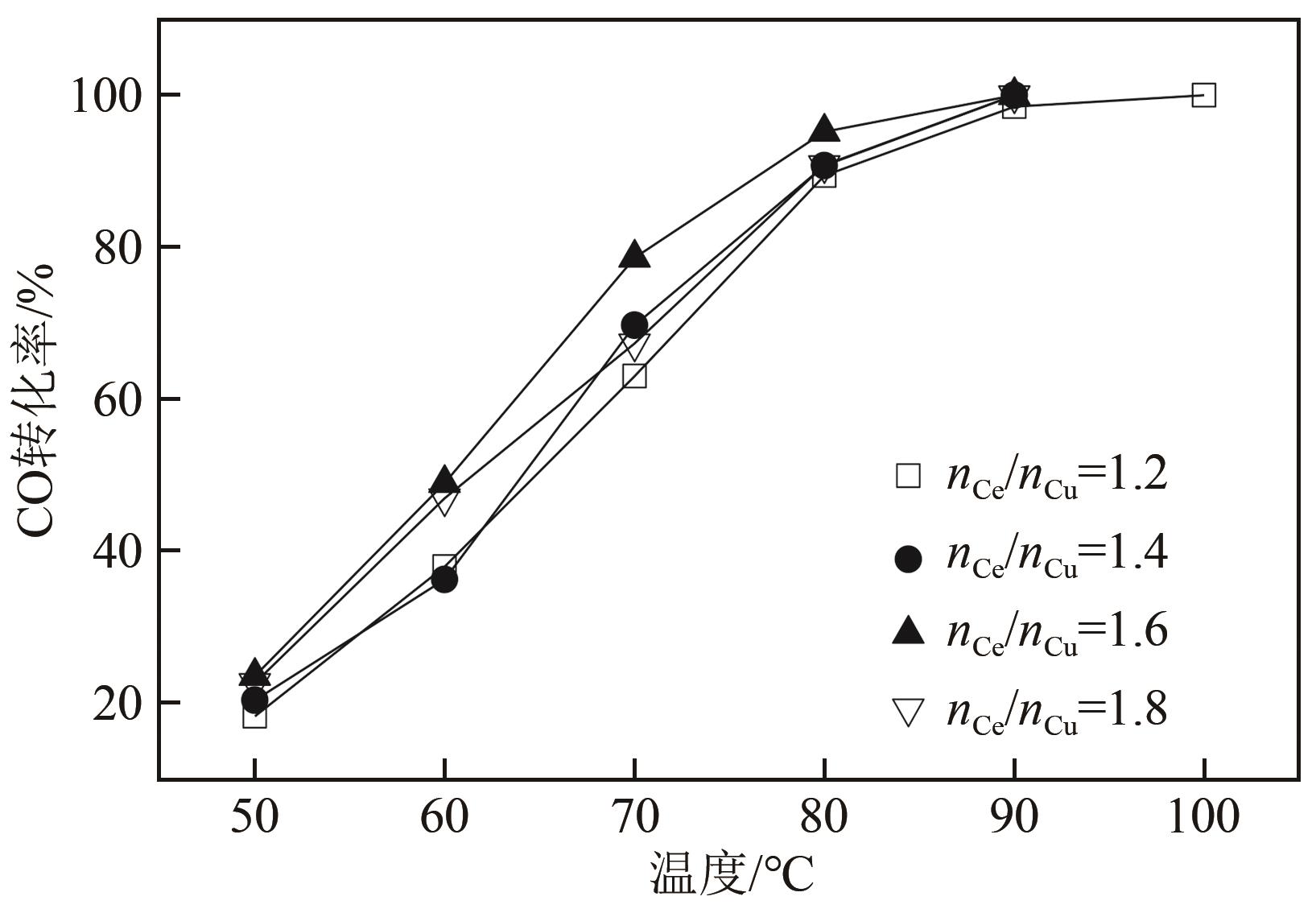
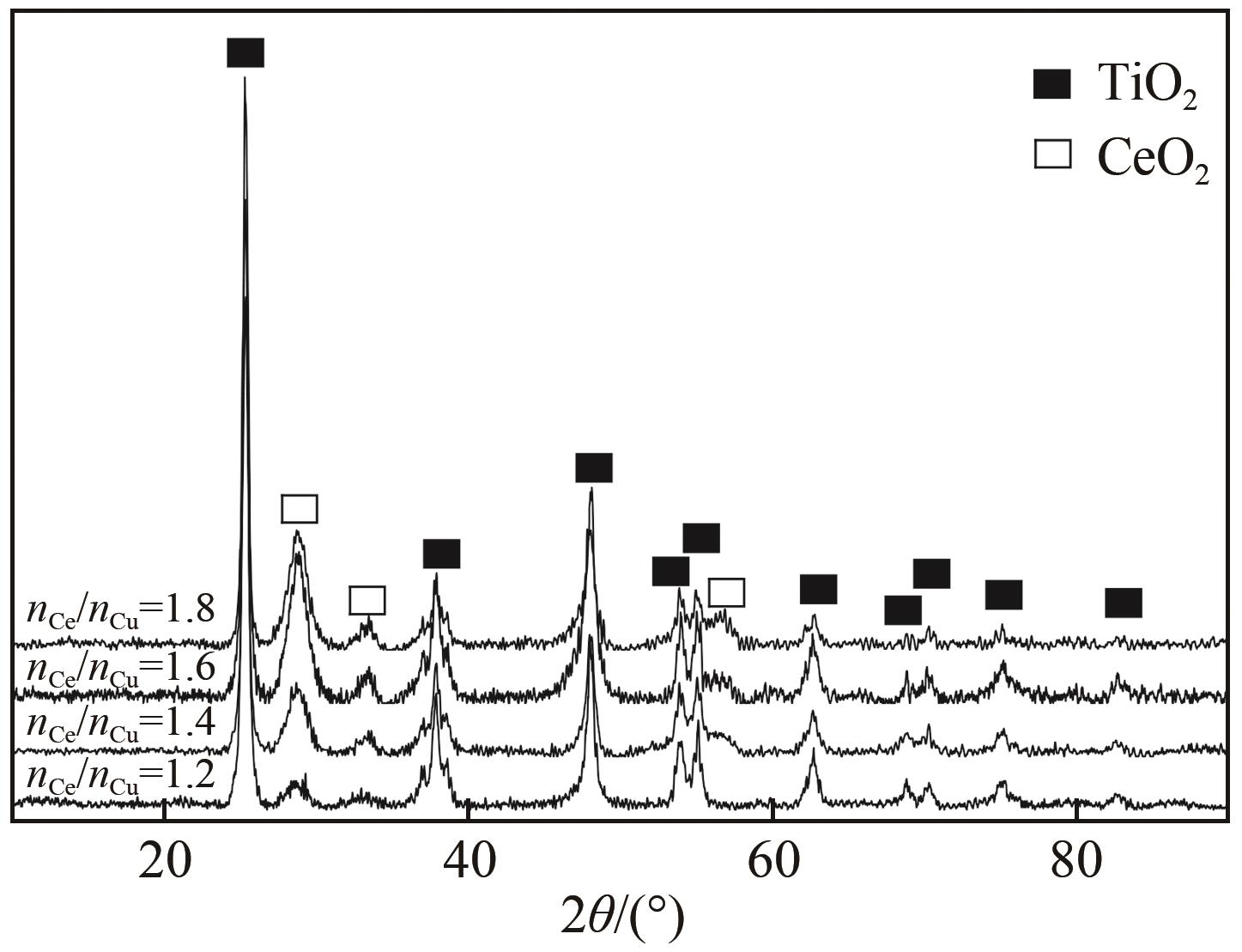
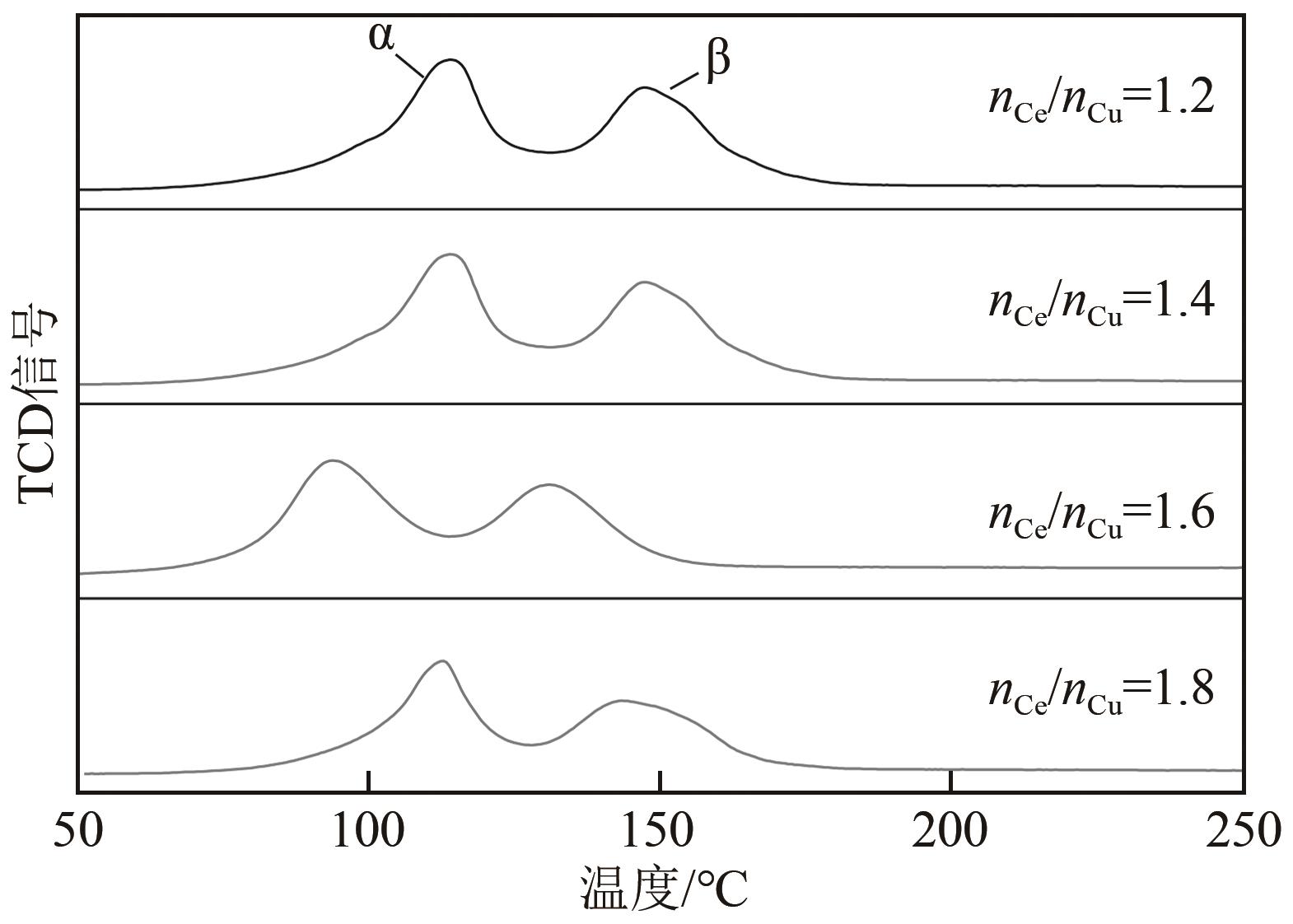
利用等体积浸渍法制备了CuO-CeO2/TiO2催化剂,用于低温CO氧化反应。考察了焙烧温度、Cu-Ce负载量和Ce/Cu摩尔比等对CuO-CeO2/TiO2催化性能的影响,利用XRD、N2等温吸附-脱附、XPS、H2-TPR等方法对催化剂进行了表征。结果表明,当nCe/nCu为1.6,Cu-Ce负载量为30%时,经500℃焙烧所制得催化剂具有最佳催化活性;当空速为24000mL/(g·h),在该催化剂上90℃时CO可完全氧化。表征发现,CuO-CeO2/TiO2催化剂表面较高的Ce3+、Cu+和吸附氧含量有利于催化CO氧化反应。制备条件通过影响催化剂表面的Ce3+、Cu+和吸附氧含量,影响催化剂活性。其中,焙烧温度、Cu-Ce负载量和nCe/nCu均会影响CuO-CeO2/TiO2表面Cu+和吸附氧含量,而催化剂表面Ce3+含量仅受Cu-Ce负载量的影响较大。
中图分类号:
引用本文
李润蕾, 王子彦, 王志苗, 李芳, 薛伟, 赵新强, 王延吉. CuO-CeO2/TiO 2 高效催化CO低温氧化反应性能[J]. 化工进展, 2023, 42(8): 4264-4274.
LI Runlei, WANG Ziyan, WANG Zhimiao, LI Fang, XUE Wei, ZHAO Xinqiang, WANG Yanji. Efficient catalytic performance of CuO-CeO2/TiO2 for CO oxidation at low-temperature[J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4264-4274.
| 编号 | 催化剂参数 | 数值 | |||
|---|---|---|---|---|---|
| 10% | 20% | 30% | 40% | ||
| 1 | CeO2晶粒尺寸/nm | 4.8 | 5.4 | 6.8 | 7.0 |
| 2 | CeO2晶格常数/nm | 0.5406 | 0.5400 | 0.5381 | 0.5389 |
| 3 | SBET/m2·g-1 | 52.2 | 52.5 | 55.6 | 54.1 |
| 4 | 平均孔容/cm3·g-1 | 0.26 | 0.21 | 0.20 | 0.18 |
| 5 | 平均孔径/nm | 15.5 | 14.4 | 11.0 | 10.4 |
| 6 | [Ce3+/(Ce3++Ce4+)]/% | 15.7 | 17.7 | 18.9 | 14.9 |
| 7 | (Isat/Ipp)/% | 30.4 | 29.3 | 27.6 | 29.0 |
| 8 | [Oads/(Oads+Olatt)]/% | 15.1 | 17.4 | 26.6 | 19.6 |
表1 不同负载量CuO-CeO2/TiO2催化剂的物化性质
| 编号 | 催化剂参数 | 数值 | |||
|---|---|---|---|---|---|
| 10% | 20% | 30% | 40% | ||
| 1 | CeO2晶粒尺寸/nm | 4.8 | 5.4 | 6.8 | 7.0 |
| 2 | CeO2晶格常数/nm | 0.5406 | 0.5400 | 0.5381 | 0.5389 |
| 3 | SBET/m2·g-1 | 52.2 | 52.5 | 55.6 | 54.1 |
| 4 | 平均孔容/cm3·g-1 | 0.26 | 0.21 | 0.20 | 0.18 |
| 5 | 平均孔径/nm | 15.5 | 14.4 | 11.0 | 10.4 |
| 6 | [Ce3+/(Ce3++Ce4+)]/% | 15.7 | 17.7 | 18.9 | 14.9 |
| 7 | (Isat/Ipp)/% | 30.4 | 29.3 | 27.6 | 29.0 |
| 8 | [Oads/(Oads+Olatt)]/% | 15.1 | 17.4 | 26.6 | 19.6 |
| 编号 | 催化剂参数 | 数值 | |||
|---|---|---|---|---|---|
| 300℃ | 400℃ | 500℃ | 600℃ | ||
| 1 | CeO2晶粒尺寸/nm | 6.7 | 7.2 | 6.8 | 9.1 |
| 2 | CeO2晶格常数/nm | 0.5399 | 0.5398 | 0.5381 | 0.5376 |
| 3 | SBET/m2·g-1 | 75.5 | 69.8 | 55.6 | 34.0 |
| 4 | 平均孔容/cm3·g-1 | 0.20 | 0.20 | 0.20 | 0.19 |
| 5 | 平均孔径/nm | 9.2 | 9.0 | 11.0 | 17.8 |
| 6 | [Ce3+/(Ce3++Ce4+)]/% | 19.4 | 18.7 | 18.9 | 18.6 |
| 7 | (Isat/Ipp)/% | 29.1 | 30.1 | 27.6 | 29.5 |
| 8 | [Oads/(Oads+Olatt)]/% | 21.0 | 21.9 | 26.6 | 19.8 |
表2 不同焙烧温度CuO-CeO2/TiO2催化剂的物化性质
| 编号 | 催化剂参数 | 数值 | |||
|---|---|---|---|---|---|
| 300℃ | 400℃ | 500℃ | 600℃ | ||
| 1 | CeO2晶粒尺寸/nm | 6.7 | 7.2 | 6.8 | 9.1 |
| 2 | CeO2晶格常数/nm | 0.5399 | 0.5398 | 0.5381 | 0.5376 |
| 3 | SBET/m2·g-1 | 75.5 | 69.8 | 55.6 | 34.0 |
| 4 | 平均孔容/cm3·g-1 | 0.20 | 0.20 | 0.20 | 0.19 |
| 5 | 平均孔径/nm | 9.2 | 9.0 | 11.0 | 17.8 |
| 6 | [Ce3+/(Ce3++Ce4+)]/% | 19.4 | 18.7 | 18.9 | 18.6 |
| 7 | (Isat/Ipp)/% | 29.1 | 30.1 | 27.6 | 29.5 |
| 8 | [Oads/(Oads+Olatt)]/% | 21.0 | 21.9 | 26.6 | 19.8 |
| 编号 | 催化剂参数 | 数值 | |||
|---|---|---|---|---|---|
| nCe/nCu=1.2 | nCe/nCu=1.4 | nCe/nCu=1.6 | nCe/nCu=1.8 | ||
| 1 | CeO2晶粒尺寸/nm | 5.9 | 6.3 | 6.8 | 6.7 |
| 2 | CeO2晶格常数/nm | 0.5389 | 0.5387 | 0.5381 | 0.5382 |
| 3 | SBET/m2·g-1 | 45.4 | 55.4 | 55.6 | 54.2 |
| 4 | 平均孔容/cm3·g-1 | 0.17 | 0.19 | 0.20 | 0.20 |
| 5 | 平均孔径/nm | 13.8 | 10.9 | 11.0 | 10.8 |
| 6 | [Ce3+/(Ce3++Ce4+)]/% | 17.0 | 17.6 | 18.9 | 17.9 |
| 7 | (Isat/Ipp)/% | 30.6 | 27.9 | 27.6 | 29.5 |
| 8 | [Oads/(Oads+Olatt)]/% | 21.9 | 19.2 | 26.6 | 18.6 |
表3 不同nCe/nCu的CuO-CeO2/TiO2催化剂的物化性质
| 编号 | 催化剂参数 | 数值 | |||
|---|---|---|---|---|---|
| nCe/nCu=1.2 | nCe/nCu=1.4 | nCe/nCu=1.6 | nCe/nCu=1.8 | ||
| 1 | CeO2晶粒尺寸/nm | 5.9 | 6.3 | 6.8 | 6.7 |
| 2 | CeO2晶格常数/nm | 0.5389 | 0.5387 | 0.5381 | 0.5382 |
| 3 | SBET/m2·g-1 | 45.4 | 55.4 | 55.6 | 54.2 |
| 4 | 平均孔容/cm3·g-1 | 0.17 | 0.19 | 0.20 | 0.20 |
| 5 | 平均孔径/nm | 13.8 | 10.9 | 11.0 | 10.8 |
| 6 | [Ce3+/(Ce3++Ce4+)]/% | 17.0 | 17.6 | 18.9 | 17.9 |
| 7 | (Isat/Ipp)/% | 30.6 | 27.9 | 27.6 | 29.5 |
| 8 | [Oads/(Oads+Olatt)]/% | 21.9 | 19.2 | 26.6 | 18.6 |
| 催化剂 | 原料气组成及体积分数 | 空速/mL·g-1·h-1 | T/℃ | CO转化率/% | 参考文献 |
|---|---|---|---|---|---|
| CuO-CeO2/TiO2 | 1% CO, 9% O2, N2 | 24000 | 90 | 100 | 本文 |
| Pt/Cr1.3Fe0.7O3 | 1% CO, 1% O2, N2 | 120000 | 80 | 78.8 | [ |
| Ag/CeO2 | 0.2% CO, 1% O2, N2 | 240000 | 230 | 50 | [ |
| Au-CeO2 | 0.05%~1% CO, 10%O2, He | 2650 | 25 | 100 | [ |
| Mn3O4/CeO2 | 1%CO, 4% O2, He | 60000 | 194 | 90 | [ |
| CuO-CeO2/UiO-66 | 1% CO, 3% O2, N2 | 24000 | 160 | 99 | [ |
| Co3O4 NAs-6 | 1% CO, 20% O2, N2 | 10000h-1 | 150 | 100 | [ |
| NiO/CeO2 | 0.6% CO, 1.5% O2, Ar | 60000 | 200 | 99.2 | [ |
| CuO-CeO2/C | 1% CO, 21% O2, He | 45000h-1 | 150 | 100 | [ |
| Au-CeO2/SiO2 | 1% CO, 干空气 | 12000 | 210 | 100 | [ |
| CuaCebFec-PSF | 180mL/m3 CO, 空气 | 7643h-1 | 200 | 100 | [ |
| Cu1/MnO2 | 1% CO, 15% O2, He | 34000 | 80 | 90 | [ |
| CoMn-a | 2% CO, 20% O2, Ar | 60000 | 125 | 100 | [ |
| NiO-CuO | 2% CO, 5% O2, Ar | 60000 | 138 | 100 | [ |
| CuO/CeO2-20 | 0.4% CO, 空气 | 8000 | 110 | 100 | [ |
表4 不同催化剂催化氧化CO的性能表现
| 催化剂 | 原料气组成及体积分数 | 空速/mL·g-1·h-1 | T/℃ | CO转化率/% | 参考文献 |
|---|---|---|---|---|---|
| CuO-CeO2/TiO2 | 1% CO, 9% O2, N2 | 24000 | 90 | 100 | 本文 |
| Pt/Cr1.3Fe0.7O3 | 1% CO, 1% O2, N2 | 120000 | 80 | 78.8 | [ |
| Ag/CeO2 | 0.2% CO, 1% O2, N2 | 240000 | 230 | 50 | [ |
| Au-CeO2 | 0.05%~1% CO, 10%O2, He | 2650 | 25 | 100 | [ |
| Mn3O4/CeO2 | 1%CO, 4% O2, He | 60000 | 194 | 90 | [ |
| CuO-CeO2/UiO-66 | 1% CO, 3% O2, N2 | 24000 | 160 | 99 | [ |
| Co3O4 NAs-6 | 1% CO, 20% O2, N2 | 10000h-1 | 150 | 100 | [ |
| NiO/CeO2 | 0.6% CO, 1.5% O2, Ar | 60000 | 200 | 99.2 | [ |
| CuO-CeO2/C | 1% CO, 21% O2, He | 45000h-1 | 150 | 100 | [ |
| Au-CeO2/SiO2 | 1% CO, 干空气 | 12000 | 210 | 100 | [ |
| CuaCebFec-PSF | 180mL/m3 CO, 空气 | 7643h-1 | 200 | 100 | [ |
| Cu1/MnO2 | 1% CO, 15% O2, He | 34000 | 80 | 90 | [ |
| CoMn-a | 2% CO, 20% O2, Ar | 60000 | 125 | 100 | [ |
| NiO-CuO | 2% CO, 5% O2, Ar | 60000 | 138 | 100 | [ |
| CuO/CeO2-20 | 0.4% CO, 空气 | 8000 | 110 | 100 | [ |
| 1 | 金星, 罗永明, 梅占强, 等. CO催化氧化催化剂改性研究进展[J]. 石油化工, 2019, 48(2): 197-202. |
| JIN Xing, LUO Yongming, MEI Zhanqiang, et al. Research progress on modification of catalytic oxidation catalyst for CO[J]. Petrochemical Technology, 2019, 48(2): 197-202. | |
| 2 | 杨德强, 周庆华. CO低温氧化催化剂研究进展[J]. 化学工程师, 2011, 25(8): 36-38. |
| YANG Deqiang, ZHOU Qinghua. Research progress of low-temperature CO oxidation catalysts[J]. Chemical Engineer, 2011, 25(8): 36-38. | |
| 3 | 梁飞雪, 朱华青, 秦张峰, 等. 一氧化碳低温催化氧化[J]. 化学进展, 2008, 20(10): 1453-1464. |
| LIANG Feixue, ZHU Huaqing, QIN Zhangfeng, et al. Low-temperature catalytic oxidation of carbon monoxide[J]. Progress in Chemistry, 2008, 20(10): 1453-1464. | |
| 4 | YANG N, PATTISSON S, DOUTHWAITE M, et al. Influence of stabilizers on the performance of Au/TiO2 catalysts for CO oxidation[J]. ACS Catalysis, 2021, 11(18): 11607-11615. |
| 5 | LU Rao, HE Lei, WANG Yang, et al. Promotion effects of nickel-doped Al2O3-nanosheet-supported Au catalysts for CO oxidation[J]. Chinese Journal of Catalysis, 2020, 41(2): 350-356. |
| 6 | VALECHHA D, MEGARAJAN S K, AL-FATESH A, et al. Low temperature CO oxidation over a novel nano-structured, mesoporous CeO2 supported Au catalyst[J]. Catalysis Letters, 2019, 149(1): 127-140. |
| 7 | 孟甜甜, 赵世超, 陈朝秋, 等. 原子层沉积制备Pt/CeO2催化剂及其低温CO氧化性能的研究[J]. 现代化工, 2020, 40(10): 184-187. |
| MENG Tiantian, ZHAO Shichao, CHEN Chaoqiu, et al. Preparation of Pt/CeO2 catalyst via atomic layer deposition and its application in oxidation of CO at low temperature[J]. Modern Chemical Industry, 2020, 40(10): 184-187. | |
| 8 | 黄志超, 王际童, 马成, 等. 低负载Pd/Al2O3催化剂的制备及其对CO室温催化性能研究[J]. 工业催化,2021, 29(2): 33-41. |
| HUANG Zhichao, WANG Jitong, MA Cheng, et al. Preparation of Pd/Al2O3 catalyst with low Pd loading amount for CO oxidation at room temperature[J]. Industrial Catalysis, 2021, 29(2): 33-41. | |
| 9 | DEY S, CHANDRA DHAL G. Controlling carbon monoxide emissions from automobile vehicle exhaust using copper oxide catalysts in a catalytic converter[J]. Materials Today Chemistry, 2020, 17: 100282. |
| 10 | ZOU Zhiqiang, MENG Ming, GUO Lihong, et al. Synthesis and characterization of CuO/Ce1- x Ti x O2 catalysts used for low-temperature CO oxidation[J]. Journal of Hazardous Materials, 2009, 163(2/3): 835-842. |
| 11 | GAO Zhiming, GONG Yuanyuan, ZHANG Qiang, et al. Preferential oxidation of CO in excess H2 over the CeO2/CuO catalyst: Effect of initial support[J]. Journal of Energy Chemistry, 2014, 23(4): 475-482. |
| 12 | WANG Cheng, CHENG Qingpeng, WANG Xinlei, et al. Enhanced catalytic performance for CO preferential oxidation over CuO catalysts supported on highly defective CeO2 nanocrystals[J]. Applied Surface Science, 2017, 422: 932-943. |
| 13 | MOBINI S, MESHKANI F, REZAEI M. Supported Mn catalysts and the role of different supports in the catalytic oxidation of carbon monoxide[J]. Chemical Engineering Science, 2019, 197: 37-51. |
| 14 | LUO Jingjie, CHU Wei, XU Huiyuan, et al. Low-temperature CO oxidation over CuO-CeO2/SiO2 catalysts: Effect of CeO2 content and carrier porosity[J]. Journal of Natural Gas Chemistry, 2010, 19(4): 355-361. |
| 15 | QI Lei, YU Qiang, DAI Yue, et al. Influence of cerium precursors on the structure and reducibility of mesoporous CuO-CeO2 catalysts for CO oxidation[J]. Applied Catalysis B: Environmental, 2012, 119/120: 308-320. |
| 16 | SEDMAK G, HOČEVAR S, LEVEC J. Kinetics of selective CO oxidation in excess of H2 over the nanostructured Cu0.1Ce0.9O2- y catalyst[J]. Journal of Catalysis, 2003, 213(2): 135-150. |
| 17 | MARTÍNEZ-ARIAS A, HUNGRÍA A B, MUNUERA G, et al. Preferential oxidation of CO in rich H2 over CuO/CeO2: Details of selectivity and deactivation under the reactant stream[J]. Applied Catalysis B: Environmental, 2006, 65(3/4): 207-216. |
| 18 | JIA Aiping, JIANG Shiyu, LU Jiqing, et al. Study of catalytic activity at the CuO-CeO2 interface for CO oxidation[J]. The Journal of Physical Chemistry C, 2010, 114(49): 21605-21610. |
| 19 | LIU Zhigang, ZHOU Renxian, ZHENG Xiaoming. Influence of preparation methods on CuO-CeO2 catalysts in the preferential oxidation of CO in excess hydrogen[J]. Journal of Natural Gas Chemistry, 2008, 17(2): 125-129. |
| 20 | 孙敬方, 张雷, 葛成艳, 等. 固相浸渍法和湿浸渍法制备CuO/CeO2催化剂及其CO氧化性能的对比研究[J]. 催化学报, 2014, 35(8): 1347-1358. |
| SUN Jingfang, ZHANG Lei, GE Chengyan, et al. Comparative study on the catalytic CO oxidation properties of CuO/CeO2 catalysts prepared by solid state and wet impregnation[J]. Chinese Journal of Catalysis, 2014, 35(8): 1347-1358. | |
| 21 | ZHANG Fang, CHEN Chao, XIAO Weiming, et al. CuO/CeO2 catalysts with well-dispersed active sites prepared from Cu3(BTC)2 metal-organic framework precursor for preferential CO oxidation[J]. Catalysis Communications, 2012, 26: 25-29. |
| 22 | ZHU Chunlan, DING Tong, GAO Wanxian, et al. CuO/CeO2 catalysts synthesized from Ce-UiO-66 metal-organic framework for preferential CO oxidation[J]. International Journal of Hydrogen Energy, 2017, 42(27): 17457-17465. |
| 23 | ZAMARO J M, PÉREZ N C, MIRÓ E E, et al. HKUST-1 MOF: A matrix to synthesize CuO and CuO-CeO2 nanoparticle catalysts for CO oxidation[J]. Chemical Engineering Journal, 2012, 195/196: 180-187. |
| 24 | CHEN Chao, WANG Rui, SHEN Pan, et al. Inverse CeO2/CuO catalysts prepared from heterobimetallic metal-organic framework precursor for preferential CO oxidation in H2-rich stream[J]. International Journal of Hydrogen Energy, 2015, 40(14): 4830-4839. |
| 25 | GONG Xia, WANG Weiwei, FU Xinpu, et al. Metal-organic-framework derived controllable synthesis of mesoporous copper-cerium oxide composite catalysts for the preferential oxidation of carbon monoxide[J]. Fuel, 2018, 229: 217-226. |
| 26 | WANG Yin, YANG Yiqiang, LIU Ning, et al. Sword-like CuO/CeO2 composites derived from a Ce-BTC metal-organic framework with superior CO oxidation performance[J]. RSC Advances, 2018, 8(58): 33096-33102. |
| 27 | GU Chunlei, QI Ran, WEI Ying, et al. Preparation and performances of nanorod-like inverse CeO2-CuO catalysts derived from Ce-1,3,5-benzene tricarboxylic acid for CO preferential oxidation[J]. Reaction Kinetics, Mechanisms and Catalysis, 2018, 124(2): 651-667. |
| 28 | HUANG Jing, WANG Shurong, ZHAO Yingqiang, et al. Synthesis and characterization of CuO/TiO2 catalysts for low-temperature CO oxidation[J]. Catalysis Communications, 2006, 7(12): 1029-1034. |
| 29 | 梁飞雪, 朱华青, 秦张峰, 等. CeO2-TiO2复合氧化物的制备、表征及其对CO氧化的催化性能[J]. 催化学报, 2008, 29(3): 264-268. |
| LIANG Feixue, ZHU Huaqing, QIN Zhangfeng, et al. Preparation and characterization of CeO2-TiO2 composite oxide and its catalytic performance for CO oxidation[J]. Chinese Journal of Catalysis, 2008, 29(3): 264-268. | |
| 30 | LI Hailong, WU Shaokang, LI Liqing, et al. CuO-CeO2/TiO2 catalyst for simultaneous NO reduction and Hg0 oxidation at low temperatures[J]. Catalysis Science & Technology, 2015, 5(12): 5129-5138. |
| 31 | ASTUDILLO J, ÁGUILA G, DÍAZ F, et al. Study of CuO-CeO2 catalysts supported on SiO2 on the low-temperature oxidation of CO[J]. Applied Catalysis A: General, 2010, 381(1/2): 169-176. |
| 32 | REDDY B M, KHAN A, YAMADA Y, et al. Structural characterization of CeO2-MO2 (M = Si4+, Ti4+, and Zr4+) mixed oxides by Raman spectroscopy, X-ray photoelectron spectroscopy, and other techniques[J]. The Journal of Physical Chemistry B, 2003, 107(41): 11475-11484. |
| 33 | DENG Changshun, LI Bin, DONG Lihui, et al. NO reduction by CO over CuO supported on CeO2-doped TiO2: The effect of the amount of a few CeO2 [J]. Physical Chemistry Chemical Physics: PCCP, 2015, 17(24): 16092-16109. |
| 34 | ZHOU Renxian, JIANG Xiaoyuan, MAO Jianxin, et al. Oxidation of carbon monoxide catalyzed by copper-zirconium composite oxides[J]. Applied Catalysis A: General, 1997, 162(1/2): 213-222. |
| 35 | AGUILA G, GUERRERO S, ARAYA P. Effect of the preparation method and calcination temperature on the oxidation activity of CO at low temperature on CuO-CeO2/SiO2 catalysts[J]. Applied Catalysis A: General, 2013, 462/463: 56-63. |
| 36 | XIE Yu, WU Jinfang, JING Guojuan, et al. Structural origin of high catalytic activity for preferential CO oxidation over CuO/CeO2 nanocatalysts with different shapes[J]. Applied Catalysis B: Environmental, 2018, 239: 665-676. |
| 37 | GUO Xiaolin, LI Jing, ZHOU Renxian. Catalytic performance of Manganese doped CuO-CeO2 catalysts for selective oxidation of CO in hydrogen-rich gas[J]. Fuel, 2016, 163: 56-64. |
| 38 | JAMPA S, WANGKAWEE K, TANTISRIYANURAK S, et al. High performance and stability of copper loading on mesoporous ceria catalyst for preferential oxidation of CO in presence of excess of hydrogen[J]. International Journal of Hydrogen Energy, 2017, 42(8): 5537-5548. |
| 39 | ALI S, CHEN L Q, YUAN F L, et al. Synergistic effect between copper and cerium on the performance of Cu x Ce0.5- x Zr0.5 (x = 0.1~0.5) oxides catalysts for selective catalytic reduction of NO with ammonia[J]. Applied Catalysis B: Environmental, 2017, 210: 223-234. |
| 40 | WEN Bin, HE Mingyuan. Study of the Cu-Ce synergism for NO reduction with CO in the presence of O2, H2O and SO2 in FCC operation[J]. Applied Catalysis B: Environmental, 2002, 37(1): 75-82. |
| 41 | ZENG Lei, SONG Wulin, LI Minghui, et al. Catalytic oxidation of formaldehyde on surface of HTiO2/HCTiO2 without light illumination at room temperature[J]. Applied Catalysis B: Environmental, 2014, 147: 490-498. |
| 42 | YANG Shaoxia, ZHU Wanpeng, JIANG Zhanpeng, et al. The surface properties and the activities in catalytic wet air oxidation over CeO2-TiO2 catalysts[J]. Applied Surface Science, 2006, 252(24): 8499-8505. |
| 43 | WANG Ting, XING Jinyuan, ZHU Li, et al. CO oxidation over supported Pt/Cr x Fe2- x O3 catalysts and their good tolerance to CO2 and H2O[J]. Applied Catalysis B: Environmental, 2019, 245: 314-324. |
| 44 | KIBIS L S, SVINTSITSKIY D A, KARDASH T Y, et al. Interface interactions and CO oxidation activity of Ag/CeO2 catalysts: A new approach using model catalytic systems[J]. Applied Catalysis A: General, 2019, 570: 51-61. |
| 45 | JAMPAIAH D, VELISOJU V K, DEVAIAH D, et al. Flower-like Mn3O4/CeO2 microspheres as an efficient catalyst for diesel soot and CO oxidation: Synergistic effects for enhanced catalytic performance[J]. Applied Surface Science, 2019, 473: 209-221. |
| 46 | YU Jihang, YU Jun, WEI Zhecheng, et al. Preparation and characterization of UiO-66-supported Cu-Ce bimetal catalysts for low-temperature CO oxidation[J]. Catalysis Letters, 2019, 149(2): 496-506. |
| 47 | MO Shengpeng, HE Hui, REN Quanming, et al. Macroporous Ni foam-supported Co3O4 nanobrush and nanomace hybrid arrays for high-efficiency CO oxidation[J]. Journal of Environmental Sciences, 2019, 75: 136-144. |
| 48 | 金石山, 张大山, 冯旭浩, 等. Ni含量对NiO/CeO2催化剂催化CO氧化性能的影响[J]. 燃料化学学报, 2022, 50(8): 1034-1040. |
| JIN Shishan, ZHANG Dashan, FENG Xuhao, et al. Effect of Ni content on catalytic oxidation of CO over NiO/CeO2 catalyst[J]. Journal of Fuel Chemistry and Technology, 2022, 50(8): 1034-1040. | |
| 49 | DEREKAYA F, ARASAN N, GÜLDÜR Ç. Effects of preparation method on the characterization and CO oxidation activities of the carbon-supported CuO-CeO2 catalysts[J]. Arabian Journal for Science and Engineering, 2022, 47(5): 6033-6047. |
| 50 | QIAN Kun, Shanshan LYU, XIAO Xiaoyan, et al. Influences of CeO2 microstructures on the structure and activity of Au/CeO2/SiO2 catalysts in CO oxidation[J]. Journal of Molecular Catalysis A: Chemical, 2009, 306(1/2): 40-47. |
| 51 | CHEN Longwen, ZHANG Dong, CHEN Yanwu, et al. Porous stainless-steel fibers supported CuCeFeO x /zeolite catalysts for the enhanced CO oxidation: Experimental and kinetic studies[J]. Chemosphere, 2022, 291: 132778. |
| 52 | JIANG Mingzhu, CHEN Jing, GAO Yanxia, et al. Using the interaction between copper and manganese to stabilize copper single-atom for CO oxidation[J]. Chemistry, 2021, 27(35): 9060-9070. |
| 53 | MOBINI S, REZAEI M, MESHKANI F. One-pot hard template synthesis of mesoporous spinel nanoparticles as efficient catalysts for low temperature CO oxidation[J]. Environmental Science and Pollution Research, 2021, 28(1): 547-563. |
| 54 | RASTEGARPANAH A, LIU Y X, DENG J G, et al. Influence of preparation method on catalytic performance of three-dimensionally ordered macroporous NiO-CuO for CO oxidation[J]. Journal of Solid State Chemistry, 2021, 297: 122091. |
| 55 | CAM T S, OMAROV S O, CHEBANENKO M I, et al. One step closer to the low-temperature CO oxidation over non-noble CuO/CeO2 nanocatalyst: The effect of CuO loading[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105373. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [6] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [7] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [8] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [9] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [10] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [11] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [12] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [13] | 杨建平. 降低HPPO装置反应系统原料消耗的PSE[J]. 化工进展, 2023, 42(S1): 21-32. |
| [14] | 王福安. 300kt/a环氧丙烷工艺反应器降耗减排分析[J]. 化工进展, 2023, 42(S1): 213-218. |
| [15] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||