化工进展 ›› 2023, Vol. 42 ›› Issue (7): 3770-3779.DOI: 10.16085/j.issn.1000-6613.2022-1614
硝酸生产尾气中NO x 和N2O联合脱除技术研究进展
- 北京工业大学区域大气复合污染防治北京市重点实验室,北京 100124
-
收稿日期:2022-09-01修回日期:2023-03-03出版日期:2023-07-15发布日期:2023-08-14 -
通讯作者:樊星 -
作者简介:李佳(1998—),女,硕士研究生,研究方向为大气污染控制。E-mail:591240968@qq.com。 -
基金资助:国家自然科学基金(21707004);北京市自然科学基金(8152011)
Research progress of simultaneous removal of NO x and N2O from the tail gas of nitric acid production
LI Jia( ), FAN Xing(
), FAN Xing( ), CHEN Li, LI Jian
), CHEN Li, LI Jian
- Key Laboratory of Beijing on Regional Air Pollution Control, Beijing University of Technology, Beijing 100124, China
-
Received:2022-09-01Revised:2023-03-03Online:2023-07-15Published:2023-08-14 -
Contact:FAN Xing
摘要:
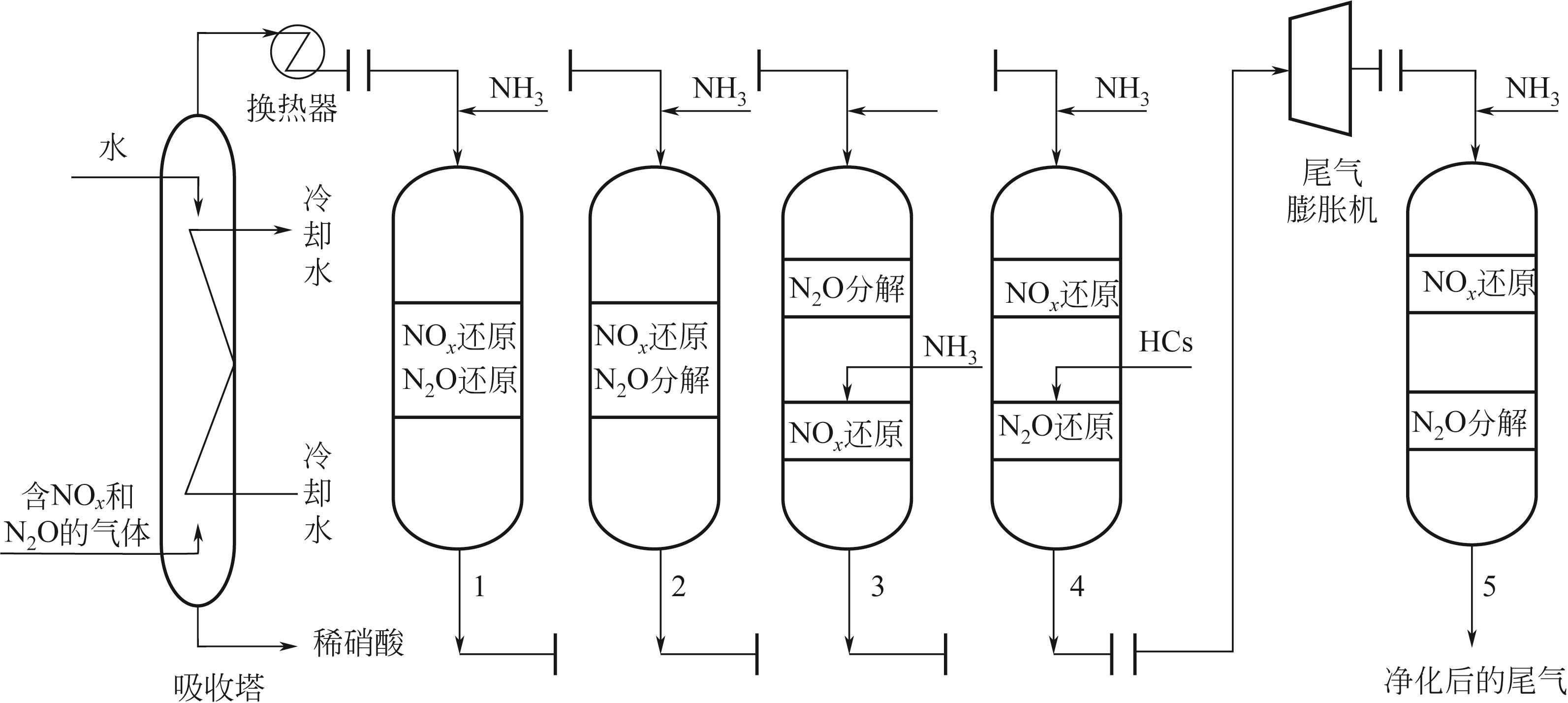
采用氨氧化法生产稀硝酸时会排放NO x (NO和NO2)和N2O等有害气体,可导致光化学烟雾(NO x )、臭氧层消耗(NO x 和N2O)、全球变暖(N2O)等环境问题,减少硝酸生产尾气中NO x 和N2O的排放势在必行。本文在分析硝酸生产过程中NO x 和N2O产生途径及减排措施的基础上,根据国内外研发和应用情况总结了联合脱除硝酸生产尾气中NO x 和N2O的主要技术路线,包括利用SCR催化剂同时催化NO x 和N2O还原和利用复合式催化剂同时催化NO x 还原和N2O分解等一段式工艺以及先催化N2O分解后催化NO x 还原、先催化NO x 还原后催化N2O还原和先催化NO x 还原后催化N2O分解等两段式工艺,分析了不同技术路线的原理、特点及面临的挑战。文中指出了一段式工艺主要存在N2O净化性能有待提升的问题,两段式工艺中先催化NO x 还原后催化N2O分解的工艺在减少还原剂消耗量、促进N2O去除等方面具有优势,未来需围绕同步降低SCR脱硝和N2O分解所需温度、提高催化剂对共存气体(O2、H2O等)的耐受性等方面开展进一步研究。
中图分类号:
引用本文
李佳, 樊星, 陈莉, 李坚. 硝酸生产尾气中NO x 和N2O联合脱除技术研究进展[J]. 化工进展, 2023, 42(7): 3770-3779.
LI Jia, FAN Xing, CHEN Li, LI Jian. Research progress of simultaneous removal of NO x and N2O from the tail gas of nitric acid production[J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3770-3779.
| 尾气组成 | 体积分数/% |
|---|---|
| NO x | 0.01~0.35 |
| N2O | 0.03~0.35 |
| O2 | 1~4 |
| H2O | 0.3~2 |
表1 硝酸生产尾气典型组成[4]
| 尾气组成 | 体积分数/% |
|---|---|
| NO x | 0.01~0.35 |
| N2O | 0.03~0.35 |
| O2 | 1~4 |
| H2O | 0.3~2 |
| 催化剂体系 | 特点 |
|---|---|
| 锰氧化物基 | 低温(<250℃)催化剂,改性后可进一步提高催化剂活性,耐硫性差,高温下N2选择性明显下降 |
| 钒氧化物基 | 中低温(300~400℃)催化剂,耐硫耐水性能优异,操作温度窗口窄,高温下催化剂稳定性和选择性差,主要用于工业尾气脱硝 |
| 铜分子筛 | 中低温(<400℃)催化剂,低温活性和N2选择性优异,高温水热稳定性较差,适用于低硫环境下的SCR脱硝,如汽车尾气脱硝 |
| 铁分子筛 | 高温(>400℃)催化剂,高温活性和N2选择性优异,操作温度窗口宽,水热稳定性好,适用于低硫环境下的SCR脱硝,如电厂尾气脱硝 |
表2 典型NH3-SCR脱硝催化剂的特点[15]
| 催化剂体系 | 特点 |
|---|---|
| 锰氧化物基 | 低温(<250℃)催化剂,改性后可进一步提高催化剂活性,耐硫性差,高温下N2选择性明显下降 |
| 钒氧化物基 | 中低温(300~400℃)催化剂,耐硫耐水性能优异,操作温度窗口窄,高温下催化剂稳定性和选择性差,主要用于工业尾气脱硝 |
| 铜分子筛 | 中低温(<400℃)催化剂,低温活性和N2选择性优异,高温水热稳定性较差,适用于低硫环境下的SCR脱硝,如汽车尾气脱硝 |
| 铁分子筛 | 高温(>400℃)催化剂,高温活性和N2选择性优异,操作温度窗口宽,水热稳定性好,适用于低硫环境下的SCR脱硝,如电厂尾气脱硝 |
| 项目 | 联合脱除工艺 | ||||
|---|---|---|---|---|---|
SCR催化剂同时催化 NO x 和N2O还原 | 复合式催化剂同时催化 NO x 还原和N2O分解 | 先催化N2O分解, 后催化NO x 还原 | 先催化NO x 还原, 后催化N2O还原 | 先催化NO x 还原, 后催化N2O分解 | |
| 工艺类型 | 一段式 | 一段式 | 两段式 | 两段式 | 两段式 |
| 布设位置 | 尾气膨胀机之前 | 尾气膨胀机之前 | 尾气膨胀机之前 | 尾气膨胀机之前 | 尾气膨胀机之后 |
| 工艺特点 | 可同步脱除NO x 和N2O,工艺布置紧凑 | 可同步脱除NO x 和N2O,工艺布置紧凑 | 可利用NO x 对N2O分解的促进作用 | 适用范围广,脱除效率高 | 对硝酸生产工艺无影响,净化装置安装限制小 |
| 开发阶段 | 尚在研究 | 尚在研究 | 已有应用 | 已有应用 | 尚在研究 |
| 催化剂 | Fe-beta | Bi-Ni/V2O5-WO3/TiO2 | Fe-分子筛 | Fe-分子筛 | V2O5/Al2O3(NO x 还原)、改性Co3O4(N2O分解) |
| 反应条件 | 367μL/L NO x +1645μL/L N2O+2012μL/L NH3+1.56% O2+N2(平衡)、空速8200h-1、温度354~373℃ | 250μL/L NO+125μL/L N2O+250μL/L NH3+3% O2+N2(平衡)、空速45000h-1、温度390℃ | Borealis AG硝酸厂、1200t/d、温度425~520℃ | Abu Qir Fertilizer硝酸厂、1830t/d、温度330~520℃ | 700~1500μL/L NO x +900~1200μL/L N2O+700~1500μL/L NH3+1.9%~4.5% O2+N2(平衡)、NO x 还原段空速9664h-1、温度220~240℃、N2O分解段空速7667h-1、温度230~260℃ |
| 脱除效率或排放浓度 | |||||
| NO x | >96% | 90% | 出口浓度5~10μL/L | 出口浓度约1μL/L | >98%(模拟结果) |
| N2O | >96% | 72% | >98% | >99% | >98%(模拟结果) |
| 参考文献 | [ | [ | [ | [ | [ |
| 发展方向 | 改善催化剂低温活性(尤其是催化N2O还原/分解的活性),提高催化剂抗共存气体干扰性 | 拓宽催化剂活性温度窗口 | 降低N2O分解所需温度,提高工艺净化实际尾气的性能 | ||
表3 NO x 和N2O联合脱除工艺性能比较
| 项目 | 联合脱除工艺 | ||||
|---|---|---|---|---|---|
SCR催化剂同时催化 NO x 和N2O还原 | 复合式催化剂同时催化 NO x 还原和N2O分解 | 先催化N2O分解, 后催化NO x 还原 | 先催化NO x 还原, 后催化N2O还原 | 先催化NO x 还原, 后催化N2O分解 | |
| 工艺类型 | 一段式 | 一段式 | 两段式 | 两段式 | 两段式 |
| 布设位置 | 尾气膨胀机之前 | 尾气膨胀机之前 | 尾气膨胀机之前 | 尾气膨胀机之前 | 尾气膨胀机之后 |
| 工艺特点 | 可同步脱除NO x 和N2O,工艺布置紧凑 | 可同步脱除NO x 和N2O,工艺布置紧凑 | 可利用NO x 对N2O分解的促进作用 | 适用范围广,脱除效率高 | 对硝酸生产工艺无影响,净化装置安装限制小 |
| 开发阶段 | 尚在研究 | 尚在研究 | 已有应用 | 已有应用 | 尚在研究 |
| 催化剂 | Fe-beta | Bi-Ni/V2O5-WO3/TiO2 | Fe-分子筛 | Fe-分子筛 | V2O5/Al2O3(NO x 还原)、改性Co3O4(N2O分解) |
| 反应条件 | 367μL/L NO x +1645μL/L N2O+2012μL/L NH3+1.56% O2+N2(平衡)、空速8200h-1、温度354~373℃ | 250μL/L NO+125μL/L N2O+250μL/L NH3+3% O2+N2(平衡)、空速45000h-1、温度390℃ | Borealis AG硝酸厂、1200t/d、温度425~520℃ | Abu Qir Fertilizer硝酸厂、1830t/d、温度330~520℃ | 700~1500μL/L NO x +900~1200μL/L N2O+700~1500μL/L NH3+1.9%~4.5% O2+N2(平衡)、NO x 还原段空速9664h-1、温度220~240℃、N2O分解段空速7667h-1、温度230~260℃ |
| 脱除效率或排放浓度 | |||||
| NO x | >96% | 90% | 出口浓度5~10μL/L | 出口浓度约1μL/L | >98%(模拟结果) |
| N2O | >96% | 72% | >98% | >99% | >98%(模拟结果) |
| 参考文献 | [ | [ | [ | [ | [ |
| 发展方向 | 改善催化剂低温活性(尤其是催化N2O还原/分解的活性),提高催化剂抗共存气体干扰性 | 拓宽催化剂活性温度窗口 | 降低N2O分解所需温度,提高工艺净化实际尾气的性能 | ||
| 1 | ALVES Luís, HOLZ Laura I V, FERNANDES Celina, et al. A comprehensive review of NO x and N2O mitigation from industrial streams[J]. Renewable and Sustainable Energy Reviews, 2022, 155: 111916. |
| 2 | SARAMOK Magdalena, SZYMASZEK Agnieszka, INGER Marek, et al. Modified zeolite catalyst for a NOx selective catalytic reduction process in nitric acid plants[J]. Catalysts, 2021, 11(4): 450. |
| 3 | 环境保护部, 国家质量监督检验检疫总局. 硝酸工业污染物排放标准: [S]. 北京: 中国环境科学出版社, 2011. |
| Ministry of Environmental Protection of the People’s Republic of China, General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Emission standard of pollutants for nitric acid industry: [S]. Beijing: China Environmental Science Press, 2011. | |
| 4 | KONSOLAKIS Michalis. Recent advances on nitrous oxide (N2O) decomposition over non-noble-metal oxide catalysts: Catalytic performance, mechanistic considerations, and surface chemistry aspects[J]. ACS Catalysis, 2015, 5(11): 6397-6421. |
| 5 | ERISMAN Jan Willem, GALLOWAY Jim, SEITZINGER Sybil, et al. Reactive nitrogen in the environment and its effect on climate change[J]. Current Opinion in Environmental Sustainability, 2011, 3(5): 281-290. |
| 6 | 吴玉波, 徐勃, 冯辉, 等. 硝酸装置N2O减排工艺与技术经济分析[J]. 化学工程, 2010, 38(10): 52-55. |
| WU Yubo, XU Bo, FENG Hui, et al. N2O abatement process and techno-economic analysis in nitric acid unit[J]. Chemical Engineering (China), 2010, 38(10): 52-55. | |
| 7 | CHUMACHENKO V A, ISUPOVA L A, IVANOVA YU A, et al. Technologies for simultaneous low-temperature catalytic removal of NO x and N2O from the tail gases of nitric acid plants[J]. Chemistry for Sustainable Development, 2020, 28(2): 203-212. |
| 8 | PÉREZ-RAMı́REZ J, KAPTEIJN F, SCHÖFFEL K, et al. Formation and control of N2O in nitric acid production[J]. Applied Catalysis B: Environmental, 2003, 44(2): 117-151. |
| 9 | ISUPOVA L A, IVANOVA Yu A. Removal of nitrous oxide in nitric acid production[J]. Kinetics and Catalysis, 2019, 60(6): 744-760. |
| 10 | 李璐, 余科, 章小林, 等. 低温硝酸尾气脱硝催化剂的研究[J]. 化肥设计, 2015, 53(3): 11-12, 20. |
| LI Lu, YU Ke, ZHANG Xiaolin, et al. Research on new catalysts for selective catalytic reduction removal of NO x from nitric acid plant flue gas by ammonia[J]. Chemical Fertilizer Design, 2015, 53(3): 11-12, 20. | |
| 11 | Lucie OBALOVÁ. Catalytic decomposition of N2O and NO[J]. Catalysts, 2021, 11(6): 667. |
| 12 | GROVES Michael C E, SASONOW Alexander. Uhde EnviNO x ® technology for NO x and N2O abatement: A contribution to reducing emissions from nitric acid plants[J]. Journal of Integrative Environmental Sciences, 2010, 7(sup1): 211-222. |
| 13 | United States Environmental Protection Agency. Available and emerging technologies for reducing greenhouse gas emissions from the nitric acid production industry[R]. North Carolina: EPA, 2010. |
| 14 | 顾卫荣, 周明吉, 马薇, 等. 选择性催化还原脱硝催化剂的研究进展[J]. 化工进展, 2012, 31(7): 1493-1500. |
| GU Weirong, ZHOU Mingji, MA Wei, et al. Research progress on selective catalytic reduction De-NO x catalysts[J]. Chemical Industry and Engineering Progress, 2012, 31(7): 1493-1500. | |
| 15 | 戴豪波, 杜凯敏, 郑渭建, 等. NH3-SCR脱硝催化剂研究进展[J]. 现代化工, 2021, 41(5): 40-44, 48. |
| DAI Haobo, DU Kaimin, ZHENG Weijian, et al. Research progress on NH3-SCR catalysts[J]. Modern Chemical Industry, 2021, 41(5): 40-44, 48. | |
| 16 | 杜云贵, 杨佳, 沈世玉, 等. 工业钛钨粉制备选择性催化还原催化剂的对比研究[J]. 化工进展, 2014, 33(4): 935-940. |
| DU Yungui, YANG Jia, SHEN Shiyu, et al. Comparative study on SCR catalysts prepared from industrial titanium tungsten powder[J]. Chemical Industry and Engineering Progress, 2014, 33(4): 935-940. | |
| 17 | HUANG Nan, GENG Yang, XIONG Shangchao, et al. The promotion effect of ceria on high vanadia loading NH3-SCR catalysts[J]. Catalysis Communications, 2019, 121: 84-88. |
| 18 | LIU Jixing, DU Yuhao, LIU Jian, et al. Design of MoFe/Beta@CeO2 catalysts with a core-shell structure and their catalytic performances for the selective catalytic reduction of NO with NH3 [J]. Applied Catalysis B: Environmental, 2017, 203: 704-714. |
| 19 | ZHANG Tao, QIU Feng, LI Junhua. Design and synthesis of core-shell structured meso-Cu-SSZ-13@mesoporous aluminosilicate catalyst for SCR of NO x with NH3: Enhancement of activity, hydrothermal stability and propene poisoning resistance[J]. Applied Catalysis B: Environmental, 2016, 195: 48-58. |
| 20 | LIU Zhiming, ZHANG Shaoxuan, LI Junhua, et al. Novel V2O5-CeO2/TiO2 catalyst with low vanadium loading for the selective catalytic reduction of NO x by NH3 [J]. Applied Catalysis B: Environmental, 2014, 158/159: 11-19. |
| 21 | HUANG Jun, HUANG He, JIANG Hongtao, et al. The promotional role of Nd on Mn/TiO2 catalyst for the low-temperature NH3‑SCR of NO x [J]. Catalysis Today, 2019, 332: 49-58. |
| 22 | 卢衍波, 王学海. 硝酸尾气SCR脱硝技术的开发及工业应用[J]. 化工环保, 2019, 39(2): 235-239. |
| LU Yanbo, WANG Xuehai. Development and industrial applicaion of SCR technology for denitration of nitric acide tail gas[J]. Environmental Protection of Chemical Industry, 2019, 39(2): 235-239. | |
| 23 | PENG Yue, LI Junhua, SI Wenzhe, et al. Deactivation and regeneration of a commercial SCR catalyst: Comparison with alkali metals and arsenic[J]. Applied Catalysis B: Environmental, 2015, 168/169: 195-202. |
| 24 | LIANG Quanming, LI Jian, HE Hong, et al. Effects of SO2 and H2O on low-temperature NO conversion over F-V2O5-WO3/TiO2 catalysts[J]. Journal of Environmental Sciences, 2020, 90: 253-261. |
| 25 | INGER Marek, MOSZOWSKI Bartosz, RUSZAK Monika, et al. Two-stage catalytic abatement of N2O emission in nitric acid plants[J]. Catalysts, 2020, 10(9): 987. |
| 26 | 李飞, 黄伟, 李潇, 等. 硝酸或己二酸行业氧化亚氮直接催化分解技术研究进展及现状[J]. 工业催化, 2018, 26(9): 6-10. |
| LI Fei, HUANG Wei, LI Xiao, et al. Research and progress of catalytic decomposition of N2O from nitric acid or adipic acid production[J]. Industrial Catalysis, 2018, 26(9): 6-10. | |
| 27 | 李华波, 许云波, 严会成, 等. 一种用于高温催化分解N2O的催化剂及其制备方法: CN103949264B[P]. 2016-02-24. |
| LI Huabo, XU Yunbo, YAN Huicheng, et al. Catalyst for catalytic decomposition of N2O at high temperature and preparation method thereof: CN103949264B[P]. 2016-02-24. | |
| 28 | 李华波, 许云波, 严会成, 等. 一种用于高温催化分解N2O的催化剂的制备工艺: CN106391037A[P]. 2017-02-15. |
| LI Huabo, XU Yunbo, YAN Huichegn, et al. Preparation process of a catalyst for high-temperature catalytic decomposition of N O: CN106391037A[P]. 2017-02-15. | |
| 29 | LEE Seung-Jae, In-Soo RYU, KIM Byung-Moon, et al. A review of the current application of N2O emission reduction in CDM projects[J]. International Journal of Greenhouse Gas Control, 2011, 5(1): 167-176. |
| 30 | LI Sixuan, ZHANG Chen, LI Jingyu, et al. Direct catalytic decomposition of N2O over Co(x)/RPSA catalysts[J]. Research on Chemical Intermediates, 2019, 45(6): 3601-3616. |
| 31 | Javier PÉREZ-RAMı́REZ, KAPTEIJN Freek, Guido MUL, et al. Ex-framework FeZSM-5 for control of N2O in tail-gases[J]. Catalysis Today, 2002, 76(1): 55-74. |
| 32 | WANG Aiyong, WANG Yilin, WALTER Eric D, et al. Catalytic N2O decomposition and reduction by NH3 over Fe/Beta and Fe/SSZ-13 catalysts[J]. Journal of Catalysis, 2018, 358: 199-210. |
| 33 | WANG Yongzhao, HU Xiaobo, ZHENG Ke, et al. Effect of SnO2 on the structure and catalytic performance of Co3O4 for N2O decomposition[J]. Catalysis Communications, 2018, 111: 70-74. |
| 34 | ZHAO Tianqi, GAO Qiang, LIAO Weiping, et al. Effect of Nd-incorporation and K-modification on catalytic performance of Co3O4 for N2O decomposition[J]. Journal of Fuel Chemistry and Technology, 2019, 47(9): 1120-1128. |
| 35 | WANG Yongzhao, ZHENG Ke, HU Xiaobo, et al. Y2O3 promoted Co3O4 catalyst for catalytic decomposition of N2O[J]. Molecular Catalysis, 2019, 470: 104-111. |
| 36 | LIU Hao, CHEN Jianjun, WANG Ya, et al. Boosting nitrous oxide direct decomposition performance based on samarium doping effects[J]. Chemical Engineering Journal, 2021, 414: 128643. |
| 37 | INGER Marek, KOWALIK Paweł, SARAMOK Magdalena, et al. Laboratory and pilot scale synthesis, characterization and reactivity of multicomponent cobalt spinel catalyst for low temperature removal of N2O from nitric acid plant tail gases[J]. Catalysis Today, 2011, 176(1): 365-368. |
| 38 | YU Haibiao, WANG Xinping, LI Ye. Strong impact of cobalt distribution on the activity for Co3O4/CaCO3 catalyzing N2O decomposition[J]. Catalysis Today, 2020, 339: 274-280. |
| 39 | YU Haibiao, WANG Xinping, WU Xingxing, et al. Promotion of Ag for Co3O4 catalyzing N2O decomposition under simulated real reaction conditions[J]. Chemical Engineering Journal, 2018, 334: 800-806. |
| 40 | ZENG Jie, CHEN Siyu, FAN Zhenhui, et al. Simultaneous selective catalytic reduction of NO and N2O by NH3 over Fe-zeolite catalysts[J]. Industrial & Engineering Chemistry Research, 2020, 59(44): 19500-19509. |
| 41 | BAEK Jeong Hun, LEE Soo Min, PARK Ji Hye, et al. Effects of steam introduction on deactivation of Fe-BEA catalyst in NH3-SCR of N2O and NO[J]. Journal of Industrial and Engineering Chemistry, 2017, 48: 194-201. |
| 42 | COLOMBO Massimo, NOVA Isabella, TRONCONI Enrico, et al. NO/NO2/N2O-NH3 SCR reactions over a commercial Fe-zeolite catalyst for diesel exhaust aftertreatment: Intrinsic kinetics and monolith converter modelling[J]. Applied Catalysis B: Environmental, 2012, 111/112: 106-118. |
| 43 | LEE Seung-Jae, In-Soo RYU, JEON Sang-Goo, et al. Simultaneous catalytic reduction of N2O and NO x for tertiary N2O abatement technology: A field study in a nitric acid production plant[J]. Environmental Progress & Sustainable Energy, 2019, 38(2): 451-456. |
| 44 | ZHAO Li, WANG Hanxiao, XU Mingxin, et al. Simultaneous removal of NO and N2O over commercial V2O5-MoO3/TiO2 catalyst modified with bismuth-nickel oxides[J]. Applied Catalysis A: General, 2021, 625: 118336. |
| 45 | KIM Moon Hyeon, PARK Soo Won. Selective reduction of NO by NH3 over Fe-zeolite-promoted V2O5-WO3/TiO2-based catalysts: Great suppression of N2O formation and origin of NO removal activity loss[J]. Catalysis Communications, 2016, 86: 82-85. |
| 46 | XIA Haian, SUN Keqiang, LIU Zhimin, et al. The promotional effect of NO on N2O decomposition over the bi-nuclear Fe sites in Fe/ZSM-5[J]. Journal of Catalysis, 2010, 270(1): 103-109. |
| 47 | Ariel GUZMÁN-VARGAS, DELAHAY Gérard, Bernard COQ. Catalytic decomposition of N2O and catalytic reduction of N2O and N2O + NO by NH3 in the presence of O2 over Fe-zeolite[J]. Applied Catalysis B: Environmental, 2003, 42(4): 369-379. |
| 48 | HEVIA Miguel A G, Javier PÉREZ-RAMÍREZ. Assessment of the low-temperature EnviNO x ® variant for catalytic N2O abatement over steam-activated FeZSM-5[J]. Applied Catalysis B: Environmental, 2008, 77(3/4): 248-254. |
| 49 | TANG Xiaolong, HAO Jiming, XU Wenguo, et al. Low temperature selective catalytic reduction of NO x with NH3 over amorphous MnO x catalysts prepared by three methods[J]. Catalysis Communications, 2007, 8(3): 329-334. |
| 50 | PU Yijuan, XIE Xinyu, JIANG Wenju, et al. Low-temperature selective catalytic reduction of NO x with NH3 over zeolite catalysts: A review[J]. Chinese Chemical Letters, 2020, 31: 2549-2555. |
| 51 | OHNISHI Chie, ASANO Kimihiro, IWAMOTO Shinji, et al. Alkali-doped Co3O4 catalysts for direct decomposition of N2O in the presence of oxygen[J]. Catalysis Today, 2007, 120(2): 145-150. |
| 52 | STELMACHOWSKI Paweł, MANIAK Gabriela, KOTARBA Andrzej, et al. Strong electronic promotion of Co3O4 towards N2O decomposition by surface alkali dopants[J]. Catalysis Communications, 2009, 10(7): 1062-1065. |
| 53 | MANIAK G, STELMACHOWSKI P, KOTARBA A, et al. Rationales for the selection of the best precursor for potassium doping of cobalt spinel based deN2O catalyst[J]. Applied Catalysis B: Environmental, 2013, 136/137: 302-307. |
| 54 | GRZYBEK Gabriela, STELMACHOWSKI Paweł, GUDYKA Sylwia, et al. Insights into the twofold role of Cs doping on deN2O activity of cobalt spinel catalyst—Towards rational optimization of the precursor and loading[J]. Applied Catalysis B: Environmental, 2015, 168/169: 509-514. |
| 55 | ASANO Kimihiro, OHNISHI Chie, IWAMOTO Shinji, et al. Potassium-doped Co3O4 catalyst for direct decomposition of N2O[J]. Applied Catalysis B: Environmental, 2008, 78(3/4): 242-249. |
| 56 | IVANOVA Yu A, SUTORMINA E F, ISUPOVA I A, et al. Catalytic activity of the oxide catalysts based on Ni0.75Co2.25O4 modified with cesium cations in a reaction of N2O decomposition[J]. Kinetics and Catalysis, 2017, 58(6): 793-799. |
| 57 | IVANOVA Yu A, SUTORMINA E F, ISUPOVA L A, et al. Effect of the composition of Ni x Co3- x O4 (x=0—0.9) oxides on their catalytic activity in the low-temperature reaction of N2O decomposition[J]. Kinetics and Catalysis, 2018, 59(3): 357-362. |
| 58 | ABU-ZIED B M, SOLIMAN S A, ABDELLAH S E. Enhanced direct N2O decomposition over Cu x Co1- x Co2O4 (0.0≤x≤1.0) spinel-oxide catalysts[J]. Journal of Industrial and Engineering Chemistry, 2015, 21: 814-821. |
| 59 | ABU-ZIED B M, SOLIMAN S A, ABDELLAH S E. Effect of substitution degree and the calcination temperature on the N2O decomposition over zinc cobaltite catalysts[J]. Modern Research in Catalysis, 2017, 6(1): 47-64. |
| 60 | ABU-ZIED Bahaa M, Lucie OBALOVÁ, Kateřina PACULTOVÁ, et al. An investigation on the N2O decomposition activity of Mn x Co1- x Co2O4 nanorods prepared by the thermal decomposition of their oxalate precursors[J]. Journal of Industrial and Engineering Chemistry, 2021, 93: 279-289. |
| 61 | YU Haibiao, TURSUN Mamutjan, WANG Xinping, et al. Pb0.04Co catalyst for N2O decomposition in presence of impurity gases[J]. Applied Catalysis B: Environmental, 2016, 185: 110-118. |
| 62 | TURSUN Mamutjan, WANG Xinping, ZHANG Fengfeng, et al. Bi-Co3O4 catalyzing N2O decomposition with strong resistance to CO2 [J]. Catalysis Communications, 2015, 65: 1-5. |
| 63 | Sylwia WÓJCIK, GRZYBEK Gabriela, STELMACHOWSKI Paweł, et al. Bulk, surface and interface promotion of Co3O4 for the low-temperature N2O decomposition catalysis[J]. Catalysts, 2019, 10(1): 41. |
| 64 | MAHAMMADUNNISA S K, AKANKSHA T, KRUSHNAMURTY K, et al. Catalytic decomposition of N2O over CeO2 supported Co3O4 catalysts[J]. Journal of Chemical Sciences, 2016, 128(11): 1795-1804. |
| 65 | 薛莉, 贺泓. Co-M(M=La, Ce, Fe, Mn, Cu, Cr)复合金属氧化物催化分解N2O[J]. 物理化学学报, 2007, 23(5): 664-670. |
| XUE Li, HE Hong. Catalytic decomposition of N2O over Co-M(M=La, Ce, Fe, Mn, Cu, Cr) composite oxide catalysts[J]. Acta Physico-Chimica Sinica, 2007, 23(5): 664-670. | |
| 66 | XIONG Ying, ZHAO Yumei, SHAN Weijun, et al. Potassium promoted Gd0.06Co catalysts for highly efficient catalytic N2O decomposition in presence of impurity gases at low temperature[J]. Chemosphere, 2022, 303: 135257. |
| 67 | XIONG Ying, ZHAO Yumei, QI Xingkun, et al. Strong structural modification of Gd to Co3O4 for catalyzing N2O decomposition under simulated real tail gases[J]. Environmental Science & Technology, 2021, 55(19): 13335-13344. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 张杰, 王放放, 夏忠林, 赵光金, 马双忱. “双碳”目标下SF6排放现状、减排手段分析及未来展望[J]. 化工进展, 2023, 42(S1): 447-460. |
| [6] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [7] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [8] | 赖诗妮, 江丽霞, 李军, 黄宏宇, 小林敬幸. 含碳掺氨燃料的研究进展[J]. 化工进展, 2023, 42(9): 4603-4615. |
| [9] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [10] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [11] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [12] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [13] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [14] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [15] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||